Abstract
The membrane receptors DCC and UNC5H have been shown to be crucial for axon guidance and neuronal migration by acting as receptors for netrin-1. DCC has also been proposed as a dependence receptor inducing apoptosis in cells that are beyond netrin-1 availability. Here we show that the netrin-1 receptors UNC5H (UNC5H1, UNC5H2, UNC5H3) also act as dependence receptors. UNC5H receptors induce apoptosis, but this effect is blocked in the presence of netrin-1. Moreover, we demonstrate that UNC5H receptors are cleaved in vitro by caspase in their intracellular domains. This cleavage may lead to the exposure of a fragment encompassing a death domain required for cell death induction in vivo. Finally, we present evidence that during development of the nervous system, the presence of netrin-1 is crucial to maintain survival of UNC5H- and DCC-expressing neurons, especially in the ventricular zone of the brainstem. Altogether, these results argue for a role of netrin-1 during the development of the nervous system, not only as a guidance cue but as a survival factor via its receptors DCC and UNC5H.
Keywords: apoptosis/DCC/dependence receptor/nervous system development/UNC5H
Introduction
In the developing nervous system, migrating cells and axons are guided to their targets by cues that include members of the netrin, semaphorin, ephrin and slit protein families (Tessier-Lavigne and Goodman, 1996; Cook et al., 1998). In mammals, the netrin family is composed of four members, netrin-1, netrin-2, netrin-3 and β-netrin (Kennedy et al., 1994; Van Raay et al., 1997; Koch et al., 2000). Netrin-1 has been shown to be required for proper development of the nervous system (Serafini et al., 1996) by acting as a chemoattractant or as a chemorepellent (Kennedy et al., 1994; Colamarino and Tessier-Lavigne, 1995). To date, six receptors for netrin-1 have been identified and belong to three different protein families. DCC (Deleted in Colorectal Cancer) and neogenin are type I transmembrane receptors that share sequence similarity with proteins of the NCAM family (Cho and Fearon, 1995; Keino-Masu et al., 1996) while UNC5H1, UNC5H2 and UNC5H3 are type I transmembrane receptors that are similar to the Caenorhabditis elegans UNC-5 (Ackerman et al., 1997; Leonardo et al., 1997). Finally, the adenosine A2b receptor, a member of the adenosine receptor family, has recently been described as a netrin-1 receptor (Corset et al., 2000). Such a diversity of receptors for netrin-1 raised the question of the role of these different proteins in netrin-1-mediated axon/cell guidance. It was recently proposed that DCC expressed in the absence of UNC5H was involved in axon attraction while the dual expression of DCC and UNC5H was involved in netrin-1-mediated axon repulsion (Hong et al., 1999). This effect is probably related to the ability of UNC5H proteins to interact with DCC in the presence of netrin-1. Moreover, it has been extensively described that cyclic nucleotides are crucial second messengers for netrin-1-induced axon guidance (Ming et al., 1997) and we have recently reported that netrin-1-induced cAMP production is mediated via the A2b receptor (Corset et al., 2000).
Conversely, very little is known concerning the signalling of netrin-1 through UNC5H or DCC receptors. A ‘negative’ signal transduction has, however, been suggested for DCC. DCC was indeed described as a dependence receptor (Mehlen et al., 1998) and therefore functionally related to other dependence receptors such as p75NTR, the common neurotrophin receptor, the androgen receptor and RET (Rabizadeh et al., 1993; Ellerby et al., 1999; Bordeaux et al., 2000). Such receptors create cellular states of dependence on their respective ligands by inducing apoptosis when unoccupied by ligand, but inhibiting apoptosis in the presence of ligand (Bredesen et al., 1998). Hence, the expression of DCC induces apoptosis in the absence of netrin-1 (Mehlen et al., 1998; Chen et al., 1999), but the presence of netrin-1 blocks DCC-induced cell death (Mehlen et al., 1998). Further more, DCC was described as a caspase substrate, with the major site of cleavage at Asp1290 (Mehlen et al., 1998). The caspase cleavage of DCC was shown to be required for DCC to exert its pro-apoptotic effect, just as has been shown for the androgen receptor and RET (Ellerby et al., 1999; Bordeaux et al., 2000). Functionally, therefore, DCC serves as a caspase amplifier in the absence of ligand via exposure of a pro-apoptotic domain lying in the N-terminal region of the intracellular domain, proximal to the cleavage site. Thus, the regulation of apoptosis by netrin-1 availability could then represent an alternative function for the couple DCC–netrin-1.
The fact that the other netrin-1 receptors, UNC5H1, UNC5H2 and UNC5H3 display a death domain, a domain usually found within the death receptor family (Hofmann and Tchopp, 1995; Leonardo et al., 1997) led us to evaluate whether UNC5H proteins were also dependence receptors. Here, we show that UNC5H1, UNC5H2 or UNC5H3 expression leads to apoptosis induction while the presence of netrin-1 blocks this effect. We also demonstrate that UNC5H proteins are caspase substrates in vitro and that the caspase cleavage is required for cell death induction. Finally, analysis of the developing brainstem of netrin-1 knockout mice shows that, in the absence of netrin-1, DCC or UNC5H-expressing neurons undergo massive apoptosis, hence suggesting the importance of cell death regulation by the pairs DCC–netrin-1 and UNC5H–netrin-1 during development of the nervous system.
Results
UNC5H receptors are dependence receptors
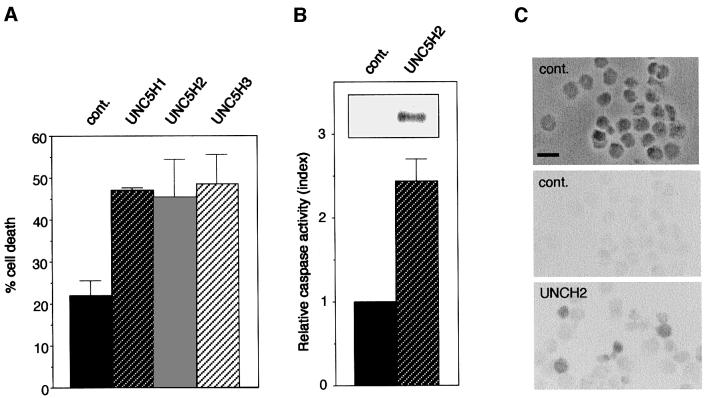
To monitor the effect of UNC5H proteins on cell death, full-length UNC5H1, UNC5H2 and UNC5H3 were transiently expressed in 293T human embryonic kidney (HEK) cells and in immortalized olfactory neuroblasts 13.S.24. As the first evaluation for cell death, populations transfected with a mock vector or with the UNC5H-expressing vectors were analysed for Trypan blue staining. As shown in Figure 1A, massive 293T cell death induction was associated with the transfection of the three UNC5H-expressing constructs. UNC5H-induced cell death was defined as apoptosis since UNC5H2 expression (Figure 1B, inset) induced caspase activation (Figure 1B and not shown) and DNA fragmentation (Figure 1C and not shown) in both 293T and 13.S.24 cells. Hence, the three UNC5H proteins induce apoptosis in the absence of their known ligand netrin-1. We then investigated whether netrin-1 affected UNC5H-induced cell death. Purified netrin-1 was added to 293T cells expressing UNC5H2. As shown in Figure 2, netrin-1 drastically reduces UNC5H2-induced cell death. Similar apoptosis inhibition was observed when netrin-1 was added to UNC5H1 or UNC5H3 transfected cells (not shown). Hence, UNC5H receptors are new members of the dependence receptor family.
Fig. 1. UNC5H receptors induce apoptosis in 293T or 13.S.24 cells. HEK 293T cells or rat olfactory neuroblasts 13.S.24 were transiently transfected as described previously (Bordeaux et al., 2000) with either the pCMV control plasmid (cont.) or the UNC5H1-3 expression plasmids and harvested 48 h after transfection. (A) UNC5H protein expression induces cell death. Cell death was monitored by Trypan blue exclusion as described in Materials and methods. (B) UNC5H2 protein expression induces caspase activation in 293T cells. Caspase-3 activation was measured as described in Materials and methods. Index of relative caspase activity is presented as the ratio between the caspase activity of the sample and that measured in 293T cells transfected with pCMV. Inset: UNC5H2 protein expression was checked by western blot analysis using an anti-HA antibody. (C) UNC5H2 expression induces DNA fragmentation in 13.S.24 cells. TUNEL assays were performed on cytospun 13.S.24 cells as described in Materials and methods. Phase contrast of control cells and TUNEL staining of either control (cont.) or UNC5H2-transfected cells is shown. Scale bar, 10 µm.

Fig. 2. Netrin-1 blocks UNC5H2-induced cell death. 293T cells were transiently transfected with either the pCMV control plasmid (cont.) or the UNC5H2 expression construct. Twenty-four hours after transfection 250 ng/ml of purified netrin was added or not to UNC5H2-transfected cells. Twenty-four hours later, UNC5H2 expression was analysed by western blot using an anti-HA antibody (A), and cell death (B) was monitored by measuring caspase activity as described for Figure 1.
UNC5H receptors are caspase substrates in vitro
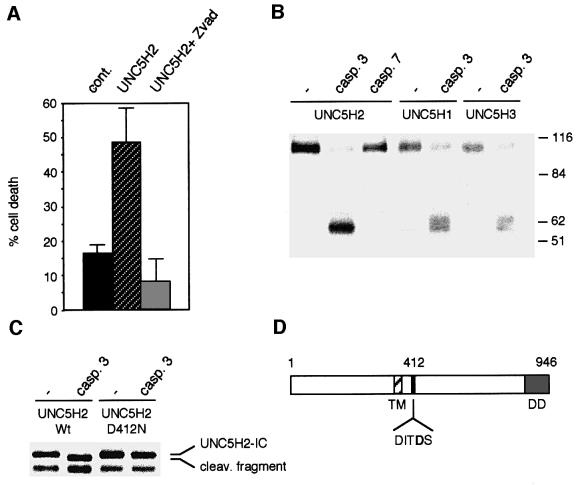
To elucidate the molecular mechanisms of UNC5H-induced cell death, we further analysed the involvement of caspases. UNC5H2 was expressed in the presence of the potent and general caspase inhibitor zVAD-fmk. UNC5H2-induced apoptosis was completely inhibited in the presence of zVAD-fmk (Figure 3A), strongly suggesting the requirement of caspase activity for cell death induction. Such requirement resembles what was observed with DCC (Mehlen et al., 1998; Forcet et al., 2001) and RET (Bordeaux et al., 2000), two dependence receptors that are actually caspase substrates. We therefore analysed the ability of UNC5H proteins to be cleaved by caspases. In vitro translations of the full-length proteins UNC5H1, UNC5H2 and UNC5H3 were then performed and purified active caspase-3 or caspase-7 was incubated with the different UNC5H proteins. It is of interest that the three proteins were cleaved by caspase-3 generating a similar pattern of cleavage while addition of caspase-7 has only a minor effect on the UNC5H2 protein (Figure 3B). Using in vitro translation of the intracellular domain of UNC5H2 (Figure 3C), we concluded that UNC5H2 (and then probably UNC5H1 and UNC5H3) was cleaved in the N-terminal part of the intracellular domain. The caspase cleavage site was mapped by constructing mutants based on preferred P4 and P1′ positions (Thornberry et al., 1997). Mutation of Asp412 to Asn completely suppressed caspase-3 cleavage of UNC5H2-IC (Figure 3C), hence demonstrating that the caspase cleavage site of UNC5H2 is located in position Asp412 with a cleavage site sequence of DITD(S). Interestingly, this sequence appears to be a classic caspase DXXD site (Thornberry et al., 1997) and is conserved in UNC5H1 and UNC5H3 [DVAD(S) and DIID(S), respectively].
Fig. 3. UNC5H proteins are cleaved by caspase. (A) 293T cells were transfected with the UNC5H2 expression plasmid and incubated for 24 h in the presence or not of 20 µM zVAD-fmk. Cell death was then analysed by measuring caspase activity as described for Figure 1. (B) UNC5H proteins are caspase substrates. In vitro translated full-length UNC5H1, UNC5H2 or UNC5H3 were incubated without caspase (–) or with purified caspase-3 (0.3 µM) or caspase-7 (1.4 µM) for 2 h as indicated. An autoradiograph is shown. (C) Mapping of the caspase cleavage site. Similar experiment to that in (B) except that the UNC5H2-IC and mutant UNC5H2-IC D412N were in vitro translated and incubated with caspase-3. (D) Diagram of UNC5H.
The cleavage of a fragment containing a death domain is required for UNC5H2-induced cell death
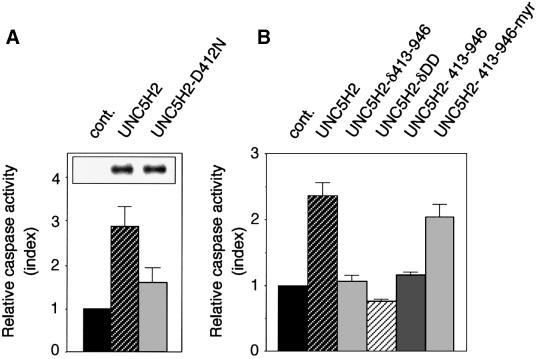
To evaluate the functional importance of the cleavage of UNC5H proteins by caspases, we then expressed the full-length UNC5H2 D412N mutant in 293T cells and apoptosis was assessed by following caspase activation (Figure 4A). Remarkably, the D412N point mutation was sufficient to strongly inhibit UNC5H2 pro-apoptotic activity, hence supporting the view that caspase cleavage of UNC5H proteins is crucial to initiate UNC5H-induced apoptosis. It is however interesting to note that, while the mutation of the caspase site of DCC completely abrogated DCC-induced cell death (Mehlen et al., 1998), the D412N mutation in UNC5H2 did not lead to a complete inhibition of UNC5H2-induced cell death.
Fig. 4. Caspase cleavage and the presence of death domain are required for UNC5H2-induced cell death. (A) 293T cells were transfected with the UNC5H2 or UNC5H2 D412N construct and cell death was then analysed by measuring caspase activity as described for Figure 1. Inset: expression of wild-type UNC5H2 or mutant UNC5H2 D412N was checked by western blot analysis using an anti-HA antibody. (B) 293T cells were transfected with the UNC5H2 expression plasmid, the constructs allowing expression of full-length UNC5H2 deleted from amino acids 413 to 946 (UNC5H2-δ413–946) or from amino acids 857 to 946 (UNC5H2-δDD), or constructs allowing expression of the UNC5H2 fragment from the caspase cleavage (amino acids 413–946) with or without addition of a myristoylation signal. Cell death was then analysed by measuring caspase activity as described for Figure 1.
Since the UNC5H proteins induce apoptosis and are cleaved by caspase, an important step for further cell death induction, it was tempting to speculate that the cleavage allows the presentation of a domain containing the ‘death information’. Along this line, a UNC5H2 mutant deleted of the C-terminal part lying after the Asp412 had no more ability to induce cell death (Figure 4B), indicating that the fragment lying after the caspase cleavage site was required for cell death induction. Expression of this C-terminal fragment (amino acids 413–946) was, however, unable to induce cell death per se unless a myristoylation signal peptide was added. This observation suggests the requirement of sub-membrane localization of this fragment for cell death induction. UNC5H proteins display a death domain in this C-terminal region. Remarkably, deletion of the death domain in UNC5H2 totally abrogates UNC5H2 pro-apoptotic activity (Figure 4B). Thus, taken together these results suggest that in the absence of netrin-1, UNC5H proteins induce cell death via their death domains, which are probably exposed after the caspase cleavage.
The lack of netrin-1 induces the death of DCC- or UNC5H-expressing neurons in vivo
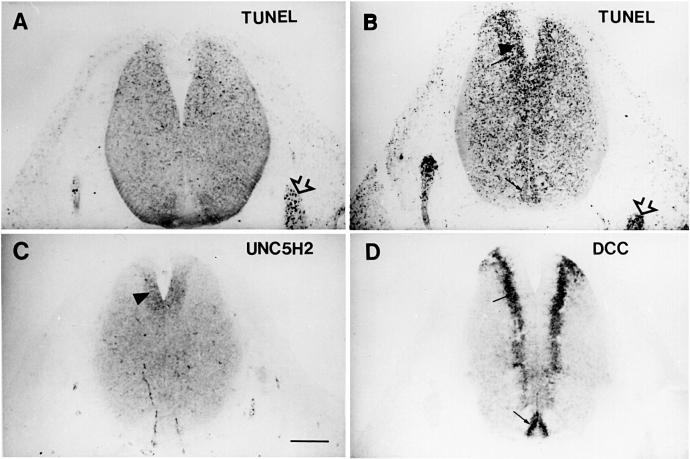
The finding that UNC5H proteins behave similarly to the other netrin-1 receptor DCC strongly supports the hypothesis that netrin-1 is not only a chemotropic molecule but also a survival factor in vitro. It is of interest that netrin-1 knockout mice (Serafini et al., 1996) not only showed major defects in the nervous system due to targeting and migratory problems but also showed fewer cells (Bloch-Gallego et al., 1999; Yee et al., 1999), in particular in the developing brainstem, which may result from increased cell death. To investigate this point further, we analysed whether cells expressing UNC5H or DCC in the developing brainstem of netrin-1 knockout mice undergo apoptosis. Post-mitotic inferior olivary neuron precursors were shown to migrate from the rhombic lips toward the ventral midline through a netrin-1-dependent mechanism (Bloch-Gallego et al., 1999). These cells express UNC5H1 and UNC5H2 when located in the ventricular zone, whereas DCC is expressed in the sub-ventricular zone by post-mitotic neurons in both wild-type and netrin-1 mutant mice (Bloch-Gallego et al., 1999; Figure 5). At E12 in mutant mice deficient for netrin-1 expression, DCC is additionally expressed in the ventricular zone of the basal plate (Figure 5). Remarkably, in netrin-1 –/– mutant mice at E12 and compared with wild-type embryos (Figure 5), TUNEL staining as a marker for apoptosis is strongly enhanced in the whole brainstem, particularly in cells expressing UNC5H2 and/or DCC transcripts. Moreover, the ventricular zone showed a substantial increase in TUNEL reactivity in netrin-1 knockout embryos that is associated only with UNC5H1 and UNC5H2 but not DCC expression, suggesting that netrin-1 regulates UNC5H pro-apoptotic activity. This effect of netrin-1 is not restricted to UNC5H-expressing cells since a similar increase in TUNEL staining can be observed in the sub-ventricular zone and in the rhombic lips of the brainstem where DCC but not UNC5H members are expressed at E12. A similar effect is also observed outside the brainstem since, at E12, the cerebellum, composed of early post-mitotic and migrating neurons expressing DCC and neogenin but not UNC5H members, showed a similar increase in TUNEL reactivity (not shown). Hence, these observations support the notion that both DCC and UNC5H pro-apoptotic activities are regulated by netrin-1 availability in the developing nervous system.
Fig. 5. Netrin-1 as a regulator of survival of UNC5H- or DCC-expressing precerebellar neurons. (A and B) TUNEL-positive cells on sections treated with the Apoptag kit and revealed with the substrate solutions NBT/BCIP (nitroblue tetrazolium/5-bromo-4-chloro-3-indoylphosphate; Roche Diagnostics). At E12, the TUNEL reactivity was higher in homozygous netrin-1 mutant mice (B) than in wild-type embryos (A). (C and D) In situ hybridization at E12 using either a UNC5H2 probe (C) or a DCC probe (D). Note that TUNEL-positive reactivity is increased in the area expressing UNC5H2 (arrowhead in C) and DCC transcripts (arrow in D), which contains, among others, precursors of precerebellar neurons. Sensory ganglions (empty arrows) display numerous TUNEL-positive neurons in both control wild-type (A) and homozygous mutant (B) embryos.
Discussion
Different netrin-1-dependent processes such as axon guid ance and neuronal migration have been shown previously to require DCC and UNC5H as membrane receptors (Keino-Masu et al., 1996; Hong et al., 1999; Yee et al., 1999). Depending on DCC and/or UNC5H expression, netrin-1 was described as either a chemoattractant or a chemorepellent (Hong et al., 1999). Here, we show in vitro as well as in vivo that netrin-1 can also regulate cell survival through its binding to both receptors, suggesting that cell death regulation by the pairs DCC–netrin-1 and UNC5H–netrin-1 may represent an important aspect of the development of the nervous system.
UNC5H proteins as dependence receptors
While DCC and UNC5H proteins were described as netrin-1 receptors respectively five and four years ago, no intracellular signal transduction is known for these receptors. Elegant studies have shown the respective importance of DCC and UNC5H in either the chemoattractant or chemorepellent effect of netrin-1 both in vivo and in vitro (Keino-Masu et al., 1996; Serafini et al., 1996; Ackerman et al., 1997; Hong et al., 1999) but as yet no accumulation of key second messengers or phosphorylation of important kinases has been reported that may indicate the ability of DCC or UNC5H to transduce an ‘informative’ signal in the presence of netrin-1. In the same time it has been shown that (i) netrin-1 displays a ‘positive’ transducing receptor A2b that allows cAMP production in response to netrin-1 binding (Corset et al., 2000) and that (ii) both DCC and UNC5H display a ‘negative’ signal transduction in the absence of netrin-1 (Mehlen et al., 1998; Chen et al., 1999; present study). Indeed, previous studies have shown that DCC is a dependence receptor and therefore functionally related to other dependence receptors such as P75NTR, the common neurotrophin receptor, the androgen receptor and RET (Rabizadeh et al., 1993; Mehlen et al., 1998; Ellerby et al., 1999; Bordeaux et al., 2000). Such receptors create cellular states of dependence on their respective ligands by inducing apoptosis when unoccupied by ligand. Here, we have shown that the three UNC5H proteins are also dependence receptors. Expression of these receptors induced apoptosis in different cultured cell lines while the presence of netrin-1 is sufficient, in both cultured cells and the developing brainstem, to prevent UNC5H-induced cell death.
Caspase cleavage and cell death induction
UNC5H-induced cell death seems to require a cleavage by caspases in its intracellular domain. We have observed that UNC5H1, UNC5H2 and UNC5H3 are all cleaved by caspase-3 in vitro, and, that the mutation of the cleavage site strongly inhibited UNC5H2-induced cell death in vivo. It is of interest that the cleavage site sequence DITD(S) found in UNC5H2 appears to be a classic caspase DXXD site (Thornberry et al., 1997) and is conserved in UNC5H1 and UNC5H3 [DVAD(S) and DIID(S), respectively]. It is then interesting to note that this site is not present in C.elegans UNC-5. However, whether the caspase cleavage and the subsequent cell death induction are absent in nematodes and could represent a characteristic of chordates or mammals remains to be shown. Alterna tively, other proteases may also cleave UNC5H proteins. Along this line, we have observed that while the mutation of the caspase site of DCC completely abrogated DCC-induced cell death (Mehlen et al., 1998), the D412N mutation in UNC5H2 did not lead to a complete inhibition of UNC5H2-induced cell death. Such observations could then suggest that in the absence of UNC5H cleavage, another mechanism—yet inhibitable by the caspase inhibitor zVAD-fmk—leads to moderate cell death induction or that other proteases may also cleave UNC5H. In the latter case, inhibition of the caspase cleavage may not completely abrogate UNC5H cleavage. Along this line, UNC5H1 was also shown to be cleaved by the calpain protease (M.Williams and L.Hinck, submitted). Then, similarly to DCC, RET and AR, UNC5H receptors are cleaved by proteases and particularly by caspases, a mechanism that probably creates a caspase activation amplification loop (i.e. once UNC5H receptors are cleaved, they acquire a pro-apoptotic effect, which then leads directly or indirectly to an increased caspase activation that will allow further cleavage of these receptors). Functionally, therefore, UNC5H receptors serve as caspase amplifiers.
Death domain and apoptosis induction
Since UNC5H proteins are cleaved by protease and more specifically by caspase, an interesting model would be that this cleavage allows the release or the exposure of a fragment that induces cell death. However, while expression of cleavage fragments issued from DCC, RET and AR allows cell death induction (Mehlen et al., 1998; Ellerby et al., 1999; Bordeaux et al., 2000), expression of the UNC5H2 C-terminal fragment lying after Asp412 has no pro-apoptotic activity unless a myristoylation signal peptide is added. This observation then suggests the requirement of a sub-membrane localization of this fragment for cell death induction. Interestingly, both DCC and UNC5H proteins show oligomeric properties (C.Bidaud-Bonod, F.Llambi, S.Castro-Obregon, D.E.Bredesen and P.Mehlen, in preparation), which may explain heterodimeric binding of full-length UNC5H molecules with caspase-cleaved C-terminal fragments. One hypothesis would then be that a heterodimeric complex allows, within membrane proximity, the exposure of the pro-apoptotic fragment lying downstream of the caspase cleavage site.
It is of interest that this pro-apoptotic fragment contains a death domain (Leonardo et al., 1997). Such death domains have been found in various receptors including death receptors Fas and tumour necrosis factor receptor (TNFR) and the dependence receptor p75NTR (Hofmann and Tchoop, 1995; Ashkenazi and Dixit, 1998; Bredesen et al., 1998). They are considered as ‘adaptor’ domains, allowing interaction of these receptors with ‘adaptor’ proteins. Death domains can be divided into two types (i.e. I or II) depending on their ability to homodimerize. Sequence alignment revealed that the UNC5H2 death domain is more related to the type II death domain of p75NTR than to the type I death domain of Fas, hence suggesting that the death domain of UNC5H probably displays no ability to homodimerize (G.del Rio, personal communication). In any case, both Fas and p75NTR death domains have been reported to be crucial for cell death induction. Remarkably, we have observed that the deletion of the UNC5H2 death domain totally abrogates UNC5H2 pro-apoptotic activity. This result is in agreement with the observation of Tessier-Lavigne and collaborators who had to delete the death domain from their constructs to analyse the effect of UNC5H proteins in axon repulsion. Indeed, they reported that the expression of full-length constructs was associated with death of their cultures, which cast doubt on their observations on axon guidance (Hong et al., 1999). Hence, taken together these results suggest that in the absence of netrin-1, UNC5H proteins induce cell death via the requirement of their death domain, which is probably exposed via the caspase cleavage. The role of this death domain is, however, completely unknown. The death domain of Fas allows, via the recruitment of the ‘adaptor’ molecule FADD, the formation of a caspase-activating complex that drives caspase-8 activation (Ashkenazi and Dixit, 1998). It is also interesting to note that DCC, while without a death domain, recruits a caspase-activating complex allowing caspase-3 activation via the interaction of DCC with caspase-9 (Forcet et al., 2001). Whether the UNC5H death domain is involved in another caspase-activating complex through the recruitment of ‘adaptor’ molecules via its death domain needs now to be analysed further.
Netrin-1 as a survival factor
The fact that netrin-1 has been demonstrated as a cue that allows axon outgrowth and orientation and cell migration, together with our finding that netrin-1 receptors DCC and UNC5H induce apoptosis in the absence of this cue, argues for a dual role of netrin-1. Moreover, the observation that during brainstem development, DCC- and UNC5H-expressing neurons are committed to undergo apoptosis in developing mice deficient for netrin-1 expression argues strongly for a role of netrin-1 as a regulator of neuronal survival during the development of the nervous system. Hence, in vivo, the presence of netrin-1 may not be important only for chemoattraction or repulsion (Kennedy et al., 1994; Colamarino and Tessier-Lavigne, 1995) but may also be required for regulating UNC5H and DCC pro-apoptotic activities. Alternatively, the increased TUNEL reactivity in the developing brainstem of netrin-1 knockout mice may be explained as a consequence of pathfinding errors. Thus, in the absence of netrin-1, DCC- or UNC5H-expressing cells may be misrouted and then fail to receive survival signals. However, the death of the inferior olivary neurons at E12 can hardly be interpreted as a consequence of disrupted migration and probably results from a direct effect of netrin-1 on cell death regulation. Indeed, at E12, the onset of migration of inferior olivary neurons has just started and the detection of a late marker of apoptosis such as DNA fragmentation would suggest that the cell death stimulus occurred even 8–12 h earlier (E11.5).
These results then favour the notion that cell death regulation by the receptor–cue pairs of UNC5H–netrin-1 and DCC–netrin-1 per se may represent an important aspect of neuronal guidance during the development of the nervous system. It has been observed recently that during commissural axon migration, a survival control ‘en passant’ was also required to allow accurate pathfinding but this survival stimulus was, in this specific case, independent of netrin-1 (Wang and Tessier-Lavigne, 1999). It is also interesting to note that four of the six known receptors for netrin-1 have been shown to transduce apoptosis in the absence of this cue. Thus, netrin-1, besides its known function as a chemotropic molecule, would emerge as a survival factor. UNC5H or DCC protein expression within specific neurons would then determine not only a guidance property/susceptibility of each neuron—outgrowth, attraction, repulsion—but also the information that this neuron should not extend axons or migrate toward regions in which netrin-1 is not expressed. This ‘death control’ would then represent an additional mechanism for defining migration pathways.
Materials and methods
Cells, transfection procedures, immunoblotting and netrin-1 production
Transient transfections of HEK 293T cells were performed as described previously (Mehlen et al., 1998) according to a modified calcium phosphate procedure. Transient transfections of immortalized olfactory neuroblast 13.S.24 cells (Bordeaux et al., 2000) were performed using Lipofectamine and the manufacturer’s suggested procedure (Life Technology). One-dimensional immunoblots using antibody raised against haemagglutinin (HA) (Roche Diagnostic) were performed as described previously (Mehlen et al., 1998). Netrin-1 was purified from netrin-1-producing 293-EBNA cells according to Serafini et al. (1996).
Site-directed mutagenesis and plasmid constructs
HA-tagged UNC5H2 coding sequence was PCR-amplified from the UNC5H2-pMT21 construct described in Leonardo et al. (1997) and inserted in the TA cloning vector pcDNA3-TOPO (Clontech) using the primers 5′-TGACCATGAGGGCCCGGAGCGGG-3′ and 5′-GCCTATGCATAATCCGGCACATCATACGGATAGCAATCGCCATCAGTGGTC-3′. PSEC-UNC5H1-myc and PSEC-UNC5H3-myc contain, respectively, the UNC5H1 and UNC5H3 coding sequences in pSEC-A vector (Invitrogen). The intracellular domain of UNC5H2, UNC5H2-IC, was PCR-amplified using 5′-TGACCATGGTGTACCGGAGAAACTGC-3′ and 5′-GCCTATGCATAATCCGGCACATCATACGGATAGCAATCGCCATCAGTGGTC-3′ primers and pcDNA3-UNC5H2-HA as template and cloned in pcDNA3-TOPO expression vector. Caspase site mutants UNC5H2 D412N and UNC5H2-IC D412N were obtained by the Quikchange strategy (Stratagene) using 5′-CACGGACATCACTAACTCCTCTGCTGC-3′ and 5′-GCAGCAGAGGAGTTAGTGATGTCCGTG-3′ primers and, respectively, pcDNA3-UNC5H2-HA or pcDNA3-UNC5H2-IC as template. UNC5H2 deleted from amino acids 412 to 946 or from 857 to 946 (death domain deletion) expression constructs were obtained by PCR amplification of pcDNA3-UNC5H2-HA using respectively, 5′-TGACCATGAGGGCCCGGAGCGGG-3′ and 5′-CGTCAGTCAGTGATGTCCGTGTCGAAG-3′ primers or 5′-TGACCATGA GGGCCCGGAGCGGG-3′ and 5′-CGCTATGCATAATCCGGCACATCATACGGATAGAAGGCATAGGGTCCCAGCTG-3′, and subsequent insertion in pcDNA3-TOPO vector. Constructs allowing expression of the UNC5H2 fragment from amino acids 413 to 946 containing a myristoylation signal were made by PCR amplification of pcDNA3-UNC5H2-HA using 5′-CGACCATGGGAAGTAGTAAAAGTAAACCAAAAGATCCAAGTCAACGACGACGAAGTCTAGAATCCTCTGCTGCCCTCACTG-3′ and 5′-GCCTATGCATAATCCGGCACATCA TACGGATAGCAATCGCCATCAGTGGTC-3′ primers; the PCR fragment was then cloned in pcDNA3-TOPO.
In vitro translation and caspase-mediated cleavage reactions
Purified caspases were from PharMingen. In vitro translations and caspase incubation were performed as described previously (Mehlen et al., 1998).
Cell death analysis
Cell death was analysed using the Trypan blue staining procedure (Mehlen et al., 1998). Apoptosis was monitored using the caspase-3 assay that utilizes the Ac-DEVD-AFC substrate (MBL, Japan). This activity was determined according to the manufacturer’s instructions using a Ac-DEVD-CHO pretreated lysate as a negative control. Caspase activation is presented as the ratio between the caspase activity of the sample and that measured in 293T cells transfected with pCMV; for all samples, the background remaining after inhibition by Ac-DEVD-CHO was subtracted. TUNEL staining was also performed on cytospun 13.S.24 cells as described previously (V.Thiemmara, E.Moyse, F.Jourdan and P.Mehlen, submitted).
Cell death analysis in developing netrin-1 knockout embryos
Production, breeding and genotyping of the netrin-1-deficient mice were carried out as described previously (Skarnes et al., 1995; Serafini et al., 1996). For detection of DNA fragmentation on cryosections, a terminal deoxynucleotidyl transferase-mediated dUTP-digoxygenin nick end labelling method (TUNEL assay) was performed as described in Ghoumari et al. (2000) using the Apoptag Kit (Appligene Oncor, Illkirch, France). In situ hybridization analysis was performed as described previously (Bloch-Gallego et al., 1999).
Acknowledgments
Acknowledgements
We wish to thank M.Tessier-Lavigne for critical reading of the manuscript and for the generous gift of UNC5H-expressing constructs. We thank G.del Rio for helpful discussion and L.Granger for excellent technical assistance. We wish to thank X.Ye and E.Danty for critical reading of the manuscript. This work was supported by the Ligue Contre le Cancer (P.M.) and the Association pour la Recherche contre le Cancer (9954; E.B.-G.).
References
- Ackerman S.L. et al. (1997) The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature, 386, 838–842. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A. and Dixit,V.M. (1998) Death receptors: signaling and modulation. Science, 281, 1305–1308. [DOI] [PubMed] [Google Scholar]
- Bloch-Gallego E., Ezan,F., Tessier-Lavigne,M. and Sotelo,C. (1999) Floor plate and netrin-1 are involved in the migration and survival of inferior olivary neurons. J. Neurosci., 19, 4407–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordeaux M.C. et al. (2000) The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschprung disease. EMBO J., 19, 4056–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredesen D.E. et al. (1998) p75NTR and the concept of cellular dependence: seeing how the other half die. Cell Death Differ., 5, 365–371. [DOI] [PubMed] [Google Scholar]
- Chen Y.Q., Hsieh,J.T., Yao,F., Fang,B., Pong,R.C., Cipriano,S.C. and Krepulat,F. (1999) Induction of apoptosis and G2/M cell cycle arrest by DCC. Oncogene, 18, 2747–2754. [DOI] [PubMed] [Google Scholar]
- Cho K.R. and Fearon,E.R. (1995) DCC: linking tumor suppressor genes and altered cell surface interactions in cancer? Curr. Opin. Genet. Dev., 5, 72–78. [DOI] [PubMed] [Google Scholar]
- Colamarino S.A. and Tessier-Lavigne,M. (1995) The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell, 81, 621–629. [DOI] [PubMed] [Google Scholar]
- Cook G., Tannahill,D. and Keynes,R. (1998) Axon guidance to and from choice points. Curr. Opin. Neurobiol., 8, 64–72. [DOI] [PubMed] [Google Scholar]
- Corset V. et al. (2000) Netrin-1-mediated axonal outgrowth and cAMP production requires interaction with the adenosine A2b receptor. Nature, 407, 747–750. [DOI] [PubMed] [Google Scholar]
- Ellerby L.M. et al. (1999) Kennedy’s disease: caspase cleavage of the androgen receptor is a crucial event in cytotoxicity. J. Neurochem., 72, 185–195. [DOI] [PubMed] [Google Scholar]
- Forcet C. et al. (2001) The dependence receptor DCC (Deleted in Colorectal Cancer) defines an alternative mechanism for caspase activation. Proc. Natl Acad. Sci. USA, 98, 3416–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoumari A.M., Wehrle,R., Bernard,O., Sotelo,C. and Dusart,I. (2000) Implication of Bcl-2 and caspase-3 in age-related Purkinje cell death in murine organotypic culture: an in vitro model to study apoptosis. Eur. J. Neurosci., 12, 2935–2949. [DOI] [PubMed] [Google Scholar]
- Hofmann K. and Tchopp,J. (1995) The death domain motif found in Fas (Apo-1) and TNF receptor is present in proteins involved in apoptosis and axonal guidance. FEBS Lett., 371, 321–323. [DOI] [PubMed] [Google Scholar]
- Hong K. et al. (1999) A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell, 97, 927–941. [DOI] [PubMed] [Google Scholar]
- Keino-Masu K. et al. (1996) Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell, 87, 175–185. [DOI] [PubMed] [Google Scholar]
- Kennedy T.E., Serafini,T., de la Torre,J.R. and Tessier-Lavigne,M. (1994) Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell, 78, 425–435. [DOI] [PubMed] [Google Scholar]
- Koch M. et al. (2000) A novel member of the netrin family, β-netrin, shares homology with the β chain of laminin. Identification, expression and functional characterization. J. Cell Biol., 151, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo E.D. et al. (1997) Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature, 386, 833–838. [DOI] [PubMed] [Google Scholar]
- Mehlen P. et al. (1998) The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature, 395, 801–804. [DOI] [PubMed] [Google Scholar]
- Ming G.L. et al. (1997) cAMP-dependent growth cone guidance by netrin-1. Neuron, 19, 1225–1235. [DOI] [PubMed] [Google Scholar]
- Rabizadeh S., Oh,J., Zhong,L.T., Yang,J., Bitler,C.M., Butcher,L.L. and Bredesen,D.E. (1993) Induction of apoptosis by the low-affinity NGF receptor. Science, 261, 345–348. [DOI] [PubMed] [Google Scholar]
- Serafini T. et al. (1996) Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell, 87, 1001–1014. [DOI] [PubMed] [Google Scholar]
- Skarnes W.C., Moss,J.E., Hurtley,S.M. and Beddington,R.S. (1995) Capturing genes encoding membrane and secreted proteins important for mouse development. Proc. Natl Acad. Sci. USA, 92, 6592–6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M. and Goodman,C.S. (1996) The molecular biology of axon guidance. Science, 274, 1123–1133. [DOI] [PubMed] [Google Scholar]
- Thornberry N.A. et al. (1997) Combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem., 272, 17907–17911. [DOI] [PubMed] [Google Scholar]
- Van Raay T.J. et al. (1997) The NTN2L gene encoding a novel human netrin maps to the autosomal dominant polycystic kidney disease region on chromosome 16p13.3. Genomics, 41, 279–282. [DOI] [PubMed] [Google Scholar]
- Wang H. and Tessier-Lavigne,M. (1999) En passant neurotrophic action of an intermediate axonal target in the developing mammalian CNS. Nature, 401, 765–769. [DOI] [PubMed] [Google Scholar]
- Yee K.T., Simon,H.H., Tessier-Lavigne,M. and O’Leary,D.D.M. (1999) Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron, 24, 607–614. [DOI] [PubMed] [Google Scholar]