Abstract
The role of Notch signaling in growth/differentiation control of mammalian epithelial cells is still poorly defined. We show that keratinocyte-specific deletion of the Notch1 gene results in marked epidermal hyperplasia and deregulated expression of multiple differentiation markers. In differentiating primary keratinocytes in vitro endogenous Notch1 is required for induction of p21WAF1/Cip1 expression, and activated Notch1 causes growth suppression by inducing p21WAF1/Cip1 expression. Activated Notch1 also induces expression of ‘early’ differentiation markers, while suppressing the late markers. Induction of p21WAF1/Cip1 expression and early differentiation markers occur through two different mechanisms. The RBP-Jκ protein binds directly to the endogenous p21 promoter and p21 expression is induced specifically by activated Notch1 through RBP-Jκ-dependent transcription. Expression of early differentiation markers is RBP-Jκ-independent and can be induced by both activated Notch1 and Notch2, as well as the highly conserved ankyrin repeat domain of the Notch1 cytoplasmic region. Thus, Notch signaling triggers two distinct pathways leading to keratinocyte growth arrest and differentiation.
Keywords: cell cycle/integrins/keratins/p21WAF1/Cip1/RBP-Jκ–CBF-1
Introduction
The epidermis provides a prototype for self-renewing stratified epithelia, and consists of several cellular layers organized into well defined ‘zones’ of proliferation, and early versus late stages of differentiation. The Notch family of cell surface receptors and their ligands belonging to the Delta or Serrate/Jagged families play a crucial role in cell fate determination and border formation (Artavanis-Tsakonas et al., 1999). A role for Notch signaling in vertebrate epidermal development has been suggested by the specific pattern of expression of its various components, and by the fact that ectopic expression of Notch or its ligand(s) modifys skin appendage formation (Lin et al., 2000 and references therein; Pourquie, 2000). In human epidermis, localized expression of Delta in putative ‘stem cells’ can induce neighboring cells to enter ‘transit amplifying’ but still proliferating populations (Lowell et al., 2000). This is reminiscent of ‘lateral inhibition’, a process whereby negative cross-regulation between Notch and its ligand(s) in equipotent progenitors eventually produces cell populations with divergent cell fates (Artavanis-Tsakonas et al., 1999). On the other hand, in developing murine epidermis, Jagged 2 and Notch1 expression are coincident in the apical aspects of the germinative layer of hair follicles and interfollicular epidermis (Luo et al., 1997). This is consistent with a positive feedback mechanism, similar to that reported in Drosophila (de Celis and Bray, 1997), whereby Notch-dependent upregulation of ligand expression is involved in the coordinate control of differentiation along the same cell lineage.
Whether or not endogenous Notch signaling plays an essential function in epidermal growth/differentiation control remains to be established. Similarly, the direct functional consequences of increased Notch activity in normal keratinocytes, and primary epithelial cells in general, have not been assessed. In the present communication, we show that Notch signaling is a key determinant of the coordinate transition between keratinocyte growth and differentiation both in vivo and in vitro. Notch functions through at least two distinct mechanisms involving the induction of p21WAF1/Cip1 expression and selective control of early versus late differentiation marker expression.
Results
Notch1 is essential for coordination of keratinocyte growth and differentiation in vivo
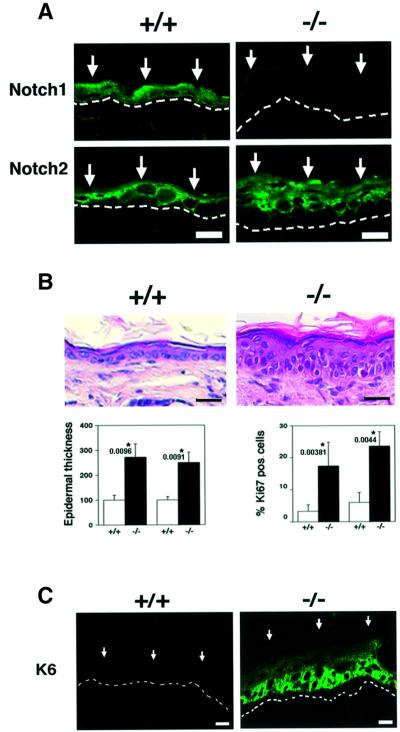
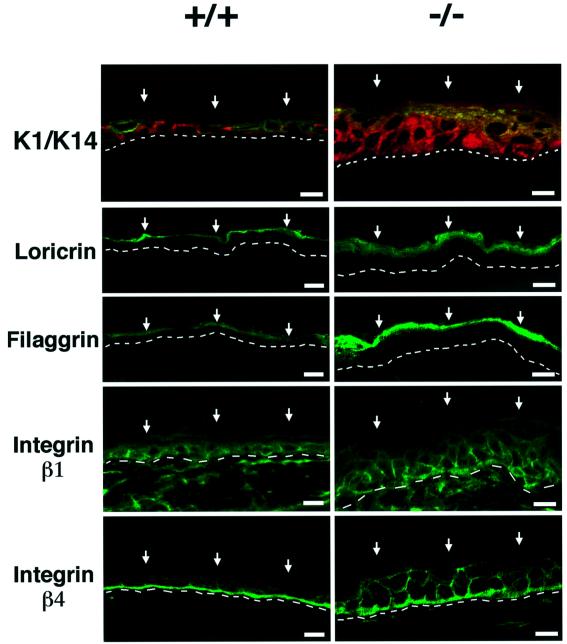
Mouse epidermis provides an excellent model system to study control of epithelial cell growth and differentiation (Dotto, 1999). To test whether Notch signaling plays an essential function in this tissue, we created mice with a keratinocyte-specific conditional deletion of the Notch1 gene. Mice in which the Notch1 gene is flanked by loxP sequences (Radtke et al., 1999) were crossed with transgenic mice with keratinocyte-specific expression of a Cre recombinase gene fused to a mutated estrogen receptor hormone-binding domain, inserted behind a keratin 5 promoter (Indra et al., 1999). Treatment of newborn mice with tamoxifen resulted in the expected deletion of the Notch1 gene selectively in the skin (see Supplementary figure 1 available at The EMBO Journal Online). Immunofluorescence with antibodies against Notch1 and Notch2 proteins showed that both are expressed in the epidermis of wild-type mice. No detectable Notch1 was found in mice with induced deletion of the Notch1 gene, while expression of Notch2 was similar to the controls (Figure 1A). Histological analysis revealed a significant epidermal thickening in the Notch1–/– skin, which was accompanied by an increase in cells expressing Ki67, a marker of proliferation (Figure 1B) as well as induction of keratin 6 (Figure 1C), which is expressed in the inter-follicular epidermis only under pathological or hyper-proliferative conditions (Navarro et al., 1995). Deregulation of the normal balance between growth and differentiation was further indicated by the fact that keratin 14, integrin β1 and integrin β4 lost their prevalent or exclusive localization in keratinocytes of the basal layer, and were found in cells of the upper layers as well (Figure 2). Expression of markers of the intermediate epidermal layers, keratin 1 and involucrin, did not appear to be significantly affected, while expression of the ‘late’ markers loricrin and filaggrin was higher in the Notch1–/– skin than in the control (Figure 2).
Fig. 1. Inducible keratinocyte-specific deletion of the Notch1 gene and resulting epidermal hyperplasia. (A) Immunofluorescence analysis of frozen skin sections from a mouse with an induced deletion of the Notch1 gene (–/–) and littermate control (+/+) with antibodies against the Notch1 and Notch2 proteins. Identical exposure and image capture conditions were used for comparison of the Notch1+/+ and Notch1–/– skins. Images are representative of multiple fields and similar results were obtained in a second independent experiment. Bar: 10 µm. (B) Hematoxylin and eosin staining of the skin of a mouse with an induced deletion of the Notch1 gene (–/–) and littermate control (+/+). Bar: 15 µm. Lower panels show the quantification of epidermal thickness (left) and cells positive by immunohistochemical staining for the Ki67 proliferation marker (right) in two independent sets of control and induced Notch1–/– littermates. Similar results were obtained with a third set of mice. Same regions of mouse back skin were analyzed in each case. Digitally acquired images from a minimum of three independent areas per section were quantified using a computer-assisted program. The observed differences were highly significant by Fisher test; P values are as indicated. (C) Immunofluorescence analysis of frozen skin sections from a mouse with an induced deletion of the Notch1 gene (–/–) and littermate control (+/+) with antibodies against the hyper-proliferative marker keratin 6. Bar: 8 µm.
Fig. 2. Aberrant keratinocyte differentiation marker and integrin expression in the epidermis of mice after induced Notch1 deletion. Frozen skin sections from mice with an induced deletion of the Notch1 gene (–/–) and littermate controls (+/+) were analyzed by immunofluorescence with polyclonal antibodies against the indicated proteins and fluorescein isothiocyanate (FITC)-conjugated secondaries. Double staining of keratin 1 and keratin 14 was carried out by sequential incubation with anti-keratin 14 antibodies followed by biotin-conjugated secondaries and Texas red-conjugated streptavidin, and directly FITC-conjugated antibodies against keratin 1. Identical exposure and image capture conditions were used for comparison of the Notch1+/+ and Notch1–/– skins. Similar results were obtained with two and in some cases three independent sets of mice. Bar: 8 µm.
Endogenous Notch activity increases upon induction of keratinocyte differentiation and is required for this process
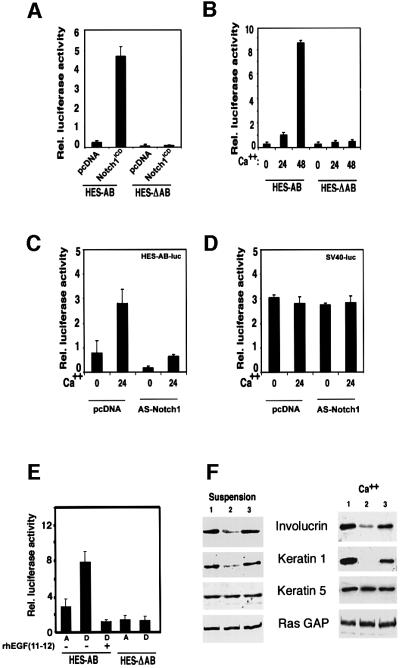
The in vivo alterations described above may reflect a direct role of Notch in keratinocyte growth/differentiation control. One important target of Notch activation is the DNA-binding protein RBP-Jκ, which is converted from a repressor into an activator of transcription by Notch (Hsieh et al., 1996; Kao et al., 1998). As a measure of endogenous Notch activity, keratinocytes were transiently transfected with a reporter plasmid containing the Notch/RBP-Jκ-responsive region of the Hairy and Enhancer of Split homologue-1 (HES-1) promoter (HES-AB) (Jarriault et al., 1995). As expected, HES-AB promoter activity was induced when co-transfected with an expression vector encoding an activated form of Notch1 (Capobianco et al., 1997) (Figure 3A), while activity of a mutated promoter with deletion of the RBP-Jκ binding sites (HES-ΔAB) (Jarriault et al., 1995) remained unaffected. When transfected alone, the activity of the HES-AB reporter was enhanced several fold in keratinocytes induced to differentiate by increased extracellular calcium or by suspension culture, while the activity of the Notch-unresponsive HES-ΔAB mutant promoter did not change (Figure 3B and E). Both basal and calcium-induced activity of the HES-AB promoter were significantly suppressed by co-transfection of a plasmid expressing anti-sense mouse Notch1 cDNA (Figure 3C), which can block the effects of both activated Notch1 and Notch2 on transcription (as assessed by co-transfection experiments with the corresponding expression vectors on both the HES and involucrin promoters; data not shown). Unlike the Notch-responsive HES-AB promoter, activity of an unrelated minimal SV40 promoter was not induced with differentiation and remained unaffected by anti-sense Notch1 expression (Figure 3D). Increased activity of the HES-AB promoter in differentiating keratinocytes was also suppressed by treatment with a soluble recombinant polypeptide, Hurn1EGF11–12, which competes with endogenous Notch for ligand binding (Garces et al., 1997) (Figure 3E). In parallel with this effect, treatment with the Hurn1EGF11–12 peptide suppressed expression of the keratin 1 and involucrin differentiation markers in keratinocytes in suspension culture as well as in response to increased extracellular calcium (Figure 3F). In contrast, levels of keratin 5, a marker of basal keratinocytes constitutively expressed by these cells in culture, and an unrelated signaling protein, ras-GTPase activating protein (GAP), remained unaffected.
Fig. 3. Increased endogenous Notch activity in differentiating keratinocytes and suppressing effects of a Notch ligand competing peptide. (A) Activation of HES-AB promoter activity by activated Notch1. Primary mouse keratinocytes were transfected with luciferase reporter plasmids carrying the HES-AB promoter or the mutated HES-ΔAB promoter with two site-specific deletions that abrogate RBP-Jκ/Notch-dependent transcription (Jarriault et al., 1995), with or without an expression plasmid expressing the activated form of Notch1 (Capobianco et al., 1997). These and all other promoter activity studies are representative of at least three independent experiments. (B) HES-AB promoter activity in growing versus differentiating keratinocytes. Keratinocytes were transfected with the HES-AB or HES-ΔAB reporters, ± high calcium exposure for the last 24 or 48 h of the experiment (72 h after transfection). (C) Reduced activity of HES-AB promoter by anti-sense Notch1 cDNA. Keratinocytes were transfected with the HES-AB reporter with or without an expression plasmid for anti-sense mouse Notch1 cDNA (nucleotides 1–6805 inserted in the reverse orientation in pcDNA3) (AS-Notch1). Cells were either kept under low calcium conditions or exposed to high calcium concentrations for the last 24 h of the experiment. (D) Insensitivity of the SV40 minimal promoter to inhibition by anti-sense Notch1 cDNA. Same experiment as described in (C), except that keratinocytes were transfected with a reporter plasmid carrying a minimal SV40 early promoter. (E) Increased HES-AB promoter activity in keratinocytes induced to differentiate in suspension, and suppressing effects of a Notch ligand competing peptide (Hurn1EGF11–12 peptide). Keratinocytes were transfected with the HES-AB or HES-ΔAB reporters, and either kept under attached growing conditions (A) or induced to differentiate by detachment from the dish (D) for 24 h ± pretreatment with the Hurn1EGF11–12 peptide. (F) Effects of the Hurn1EGF11–12 peptide on keratinocyte differentiation marker expression. Keratinocytes were induced to differentiate by suspension culture (left panel) or high calcium exposure (right panel). Cells received no other treatment (lane 1), or were pretreated with the Hurn1EGF11–12 peptide (lane 2), or mock pretreated (lane 3). Total cell extracts were analyzed by immunoblotting with antibodies against involucrin and keratin 1. The same blots were stripped and sequentially re-probed with antibodies against keratin 5 and ras-GAP as a measure of equal loading conditions. Densitometric quantification of the autoradiographs showed that, relative to the mock-treated controls, involucrin expression was suppressed ∼3-fold by the Hurn1EGF11–12 peptide in both calcium-treated cells and cells brought into suspension. Keratin 1 expression was suppressed ∼10-fold by the Hurn1EGF11–12 peptide in the calcium-treated cells, and ∼3-fold in the cells brought into suspension. Similar results were obtained in two independent experiments.
Activated Notch1 causes keratinocyte growth arrest through increased p21WAF1/Cip1 expression
Withdrawal from the cell cycle and growth arrest are an intrinsic part of the keratinocyte terminal differentiation program (Missero et al., 1995, 1996). To assess whether increased Notch activity is sufficient to induce keratinocyte growth arrest, we employed an adenoviral vector approach, expressing Notch1 in a truncated active form (Ad-Notch1) (Capobianco et al., 1997). Infection of growing keratinocytes with this adenovirus caused strong suppression of DNA synthesis by 24–48 h, while no such effect was observed with a control green fluor escent protein (GFP)-expressing adenovirus (Ad-GFP) (Figure 4A, left panel). Induction of the cyclin-dependent kinase inhibitors p21WAF1/Cip1 and p27Kip1 occurs as one of the early steps in keratinocyte differentiation and contributes to growth arrest of these cells (Missero et al., 1996). Expression of activated Notch1 in keratinocytes under low calcium conditions caused a significant increase of the p21 protein (Figure 4B), to an extent similar to that observed after calcium-induced differentiation (Missero et al., 1996), while p27Kip1 expression was unaffected or slightly reduced. Cyclin-dependent kinase 2 (CDK2) expression also remained constant (Figure 4B). To test whether the growth-suppressing effects of activated Notch1 in keratinocytes are mediated by induction of p21 expression, primary keratinocytes derived from p21 null mice were infected with the Notch1 adenovirus. Unlike wild-type cells, p21 null keratinocytes were completely resistant to Notch1-mediated growth suppression (Figure 4A, right panel).
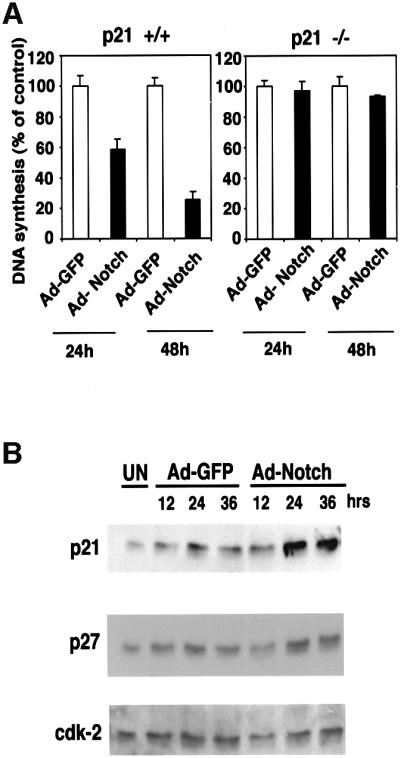
Fig. 4. Growth suppression of keratinocytes by activated Notch1 through a p21WAF1/Cip1-dependent mechanism. (A) DNA synthesis in wild-type and p21-null keratinocytes infected with a control GFP adenovirus (Ad-GFP) or an adenovirus expressing the constitutively active form of Notch1 (Ad-Notch). Primary keratinocytes derived from wild-type mice (left) or mice with a homozygous disruption of the p21WAF1/Cip1 gene in the same genetic background as the controls (right), were pulse-labeled with [3H]thymidine for 1.5 h at the indicated times (h) after adenoviral vector infection, and [3H]thymidine incorporation was determined as described (Missero et al., 1996). All samples were tested in triplicate wells, and standard deviation is indicated. Values are expressed as percentages relative to the adeno-GFP-infected controls. Similar results were observed in two independent experiments. (B) Induction of p21WAF1/Cip1 protein expression by activated Notch1 expression. Total cell extracts were prepared from keratinocytes infected with the GFP control or activated Notch adenoviruses at the indicated times (h) after infection. UN: uninfected control. The same protein amounts (30 µg) were analyzed by 12.5% SDS–PAGE and immunoblotting with monoclonal antibodies against mouse p21WAF1/Cip1 or p27Kip1 proteins. The same blot was stripped and reprobed with anti-CDK2 antibodies. Densitometric quantification of the autoradiographs indicated that p21 expression was induced ∼4-fold in cells infected with the Ad-Notch1 adenovirus versus the Ad-GFP control at both 24 and 36 h after infection. A similar induction of p21 protein expression was observed in two other independent experiments.
Activated Notch1 causes selective modulation of early versus late differentiation markers and suppression of integrin expression
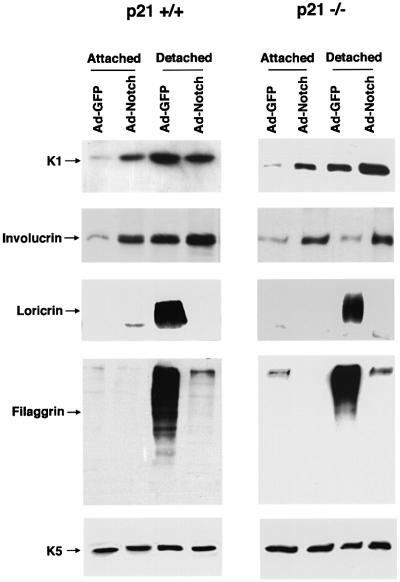
A second aspect of the keratinocyte differentiation program is induction of biochemical markers which can be distinguished between ‘early markers’, whose expression starts in the spinous layer (keratin 1/10, involucrin), and ‘late markers’, which are expressed in the granular layer and beyond (loricrin, filaggrin) (Dlugosz and Yuspa, 1993). Keratinocyte cultures under low calcium conditions consist of two compartments, one of attached and continuously proliferating cells, and the other of spontaneously detached and terminally differentiated cells (Di Cunto et al., 1998). Expression of activated Notch1 by adenoviral infection caused a substantial induction of the early markers keratin 1 and involucrin in the attached keratinocyte population, while levels of the late markers loricrin and filaggrin remained unaffected (Figure 5, left panel). In the detached cell population, which normally expresses elevated levels of all differentiation markers, activated Notch1 caused no further increase of keratin 1 and involucrin, while it significantly suppressed loricrin and filaggrin (Figure 5, left panel).
Fig. 5. Induction of early differentiation markers and suppression of late markers by activated Notch1. Primary keratinocytes from wild-type (left) or p21 null mice (right) with the same genetic background were infected under low calcium conditions with the GFP control or activated Notch1 adenoviruses, and total cell extracts were collected from the attached and spontaneously detached cell populations at 36 h after infection. The same protein amounts (10 µg) were analyzed by 7.5% SDS–PAGE and immunoblotting with antibodies against the various differentiation markers as previously reported (Missero et al., 1996). Filaggrin is synthesized as a high molecular weight precursor, profilaggrin, which is subsequently processed. The diffuse bands correspond to the multiple products of this processing. The same blots were stripped and reprobed with anti-keratin 5 antibodies as control for equal loading conditions. Similar results were obtained in three independent experiments.
Our previous work indicates that p21, besides being involved in differentiation-associated growth arrest, plays an unexpectedly complex role in differentiation which is unlinked to its effects on the cell cycle (Di Cunto et al., 1998). Infection with the Ad-Notch1 virus caused a similar induction of early differentiation markers and a suppression of the late markers in the p21-negative keratinocytes as in the wild-type cells (Figure 5, right panel), indicating that modulation of differentiation marker expression by activated Notch1 is independent of p21 expression.
In parallel with the above effects, activated Notch1 expression resulted in reduced adhesion of keratinocytes to the substratum and caused down-modulation of integrin α3/β1 and α6/β4expression, events that have been closely linked with differentiation (Carroll et al., 1995) (see Supplementary figure 2).
Activated Notch1 causes induction of p21WAF1/Cip1 expression through RBP-Jκ-dependent transcription
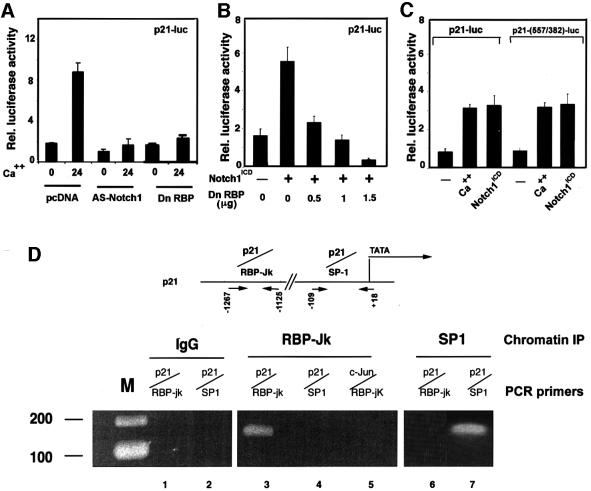
Upregulation of p21 expression in differentiating keratinocytes has been shown to occur at the transcription– promoter activity level (Missero et al., 1995). Increased activity of the p21 promoter in calcium-treated keratinocytes was suppressed by co-transfection with the anti-sense Notch1 cDNA, indicating that endogenous Notch signaling is required for induction of this promoter in differentiation (Figure 6A). Conversely, p21 promoter activity in growing keratinocytes was induced by co-expression with activated Notch1 (Figure 6B). The 2.4 kb region of the human p21 promoter contains a sequence at position –476 that fully matches the consensus binding site of RBP-Jκ (Tun et al., 1994). Induction of this promoter by increased extracellular calcium, as well as activated Notch1, was suppressed by expression of a dominant-negative form of RBP-Jκ (Kato et al., 1997) in a dose-dependent fashion, indicating that a RBP-Jκ-dependent mechanism is involved (Figure 6A and B).
Fig. 6. Induction of p21 expression by activated Notch1 through RBP-Jκ-dependent transcription. (A) Suppression of p21 promoter activity in differentiating keratinocytes by antisense Notch1 cDNA and dominant-negative RBP-Jκ. Keratinocytes were transfected with a reporter plasmid carrying the proximal 2.4 kb region of the human p21 promoter (p21-luc) ± expression vectors for anti-sense mouse Notch1 cDNA or dominant-negative RBP-Jκ (Kato et al., 1997). Cells were kept in low calcium medium or exposed to high calcium for the last 24 h of the experiment (72 h after transfection). (B) RBP-Jκ-dependent induction of p21 promoter activity by activated Notch1. Keratinocytes were transfected with the p21-luc reporter ± an expression vector for activated Notch1 (Notch1ICD) and a vector for the dominant-negative RBP-Jκ mutant in increasing amounts. Empty plasmid vector was also added to ensure that all cells were transfected with the same amount of total DNA. (C) Calcium and Notch1 responsiveness of a minimal p21 promoter region containing the fully conserved RBP-Jκ binding site. Keratinocytes were transfected with the reporters p21-luc, carrying the 2.4 kb p21 promoter, or p21-(557/382)-luc, containing 175 bp of the p21 promoter containing the RBP-Jκ binding site (–557 to –382 position) fused to a minimal promoter (pluc-MCS vector; Stratagene). Cells were either kept under low calcium conditions, exposed to high calcium for 24 h or co-transfected with an expression vector for activated Notch1. (D) Binding of the RBP-Jκ protein to the endogenous p21 promoter as assessed by chromatin immunoprecipitation. Primary keratinocytes under low calcium conditions were processed for chromatin immunoprecipitation with antibodies against the RBP-Jκ or SP1 proteins and affinity-purified IgGs. The immunoprecipitates were analyzed by PCR with oligonucleotide primers specific for the indicated regions of the mouse p21WAF1/Cip1 promoter and for a region of the mouse c-jun promoter containing a canonical RBP-Jκ binding site. Preliminary PCR reactions were carried out with naked DNA to determine optimal conditions for amplification of each DNA.
The transcriptional activity of a 175 bp region of the p21 promoter containing the RBP-Jκ binding sites and fused to a minimal TATA box promoter was induced by activated Notch1 to a similar extent as the intact p21 promoter (Figure 6C). To test whether RBP-Jκ binds to the endogenous p21 promoter, chromatin preparations from mouse primary keratinocytes were cross-linked to cellular DNA and immunoprecipitated with anti-RBP-Jκ antibodies, followed by PCR amplification of specific p21 promoter regions. As shown in Figure 6D, a 142 bp fragment of the mouse p21 promoter containing a fully conserved RBP-Jκ binding site was present in the RBP-Jκ immunoprecipitates but not in immunoprecipitates with antibodies against Sp1 or in the non-immune control. Importantly, a region of the c-jun promoter containing the same RBP-Jκ binding sequence as in the p21 promoter was not found in the RBP-Jκ immunoprecipitates (Figure 6D), pointing to an element of specificity in RBP-Jκ binding beyond its DNA recognition sequence. As expected, the TATA box proximal region of the p21 promoter containing Sp1/Sp3 binding sites was absent in the RBP-Jκ immunoprecipitates and present in immunoprecipitates with antibodies against Sp1 (Figure 6D). Similar results were obtained by chromatin immunoprecipitations of keratinocytes infected with either Ad-GFP or Ad-Notch1 adenoviruses (data not shown), consistent with the fact that Notch activation does not increase binding of RBP-Jκ to the DNA, but converts it from a transcriptional repressor into an activator (Hsieh et al., 1996).
Involucrin expression is induced by both activated Notch1 and Notch2 and the conserved Notch1 ankyrin domain through a RBP-Jκ-independent mechanism
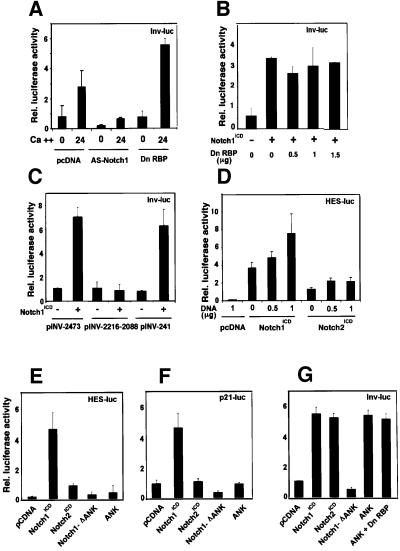
Like p21, differentiation marker genes such as the one for involucrin are induced in keratinocyte differentiation at the level of transcription (Dotto, 1999). Involucrin is already expressed in a small fraction of keratinocytes undergoing spontaneous differentiation under low calcium conditions (Brissette et al., 1996). As with the HES-AB and p21 promoter, involucrin promoter activity was induced in keratinocytes after exposure to high extracellular calcium, it was strongly suppressed by anti-sense Notch1 cDNA under both low and high calcium conditions, and it was induced by constitutively active Notch1 (Figure 7A and B). However, in contrast to the p21 promoter, induction of the involucrin promoter by either high extracellular calcium or activated Notch1 was not suppressed by the concomitant expression of a RBP-Jκ dominant-negative mutant (Figure 7A and B).
Fig. 7. Induction of involucrin expression by activated Notch1 and Notch2 expression and the conserved Notch1 ANK domain. (A) Suppression of involucrin promoter activity in differentiating keratinocytes by expression of anti-sense Notch1 cDNA but not dominant-negative RBP-Jκ. Keratinocytes were transfected with a reporter plasmid (Inv-luc) carrying the human involucrin promoter (Brissette et al., 1996) ± expression vectors for anti-sense mouse Notch1 cDNA or dominant-negative RBP-Jκ. Cells were kept under low calcium conditions or exposed to high calcium for 24 h. (B) Induction of involucrin promoter activity by activated Notch1 independently of RBP-Jκ activity. Keratinocytes were transfected with the Inv-luc reporter ± an expression vector for activated Notch1 (Notch1ICD) and a vector for dominant-negative RBP-Jκ in increasing amounts. Empty plasmid vector was also added to ensure that all cells were transfected with the same amount of total DNA. (C) Identification of the minimal Notch responsive region of involucrin promoter. Primary keratinocytes were transfected with reporter plasmids carrying either 2473, 2116–2088 or 241 bases (Efimova et al., 1998) of the involucrin promoter region, ± an expression vector for activated Notch1 cDNA. (D) Differential induction of the RBP-Jκ responsive HES-AB promoter by activated Notch1 versus Notch2 expression. Keratinocytes were transfected with the HES-AB reporter with increasing amounts of expression vectors for either activated Notch1 or Notch2 proteins (Capobianco et al., 1997). An analogous dose–response experiment was also performed with the involucrin promoter (as in G), which, unlike the HES-AB promoter, showed similar induction by both activated Notch1 and Notch2 (not shown). (E–G) Selective induction of involucrin promoter activity by activated Notch2 and the conserved Notch1 ANK domain. Keratinocytes were transfected with reporter plasmids for the HES-AB (E), p21 (F) and involucrin (G) promoters with or without expression plasmids for the activated cytoplasmic region of Notch1 (Notch1ICD), the same region with an internal deletion of the ANK domain (Notch1-ΔANK), the Notch1 ANK domain alone (ANK) and activated Notch2 (Notch2ICD). The Notch1 ANK domain was also co-expressed with increasing amounts of the vector for dominant-negative RBP-Jκ as in (B); shown is the result with 1.5 µg of transfected plasmid DNA for the mutant RBP-Jκ.
The involucrin promoter that was used for these studies lacks any consensus RBP-Jκ binding sites. Two critical regulatory regions have been identified in this promoter that contribute to induction with differentiation: a distal element containing synergistic AP-1 and Sp1 binding sites, and a TATA box proximal region with a critical AP-1 binding site overlapping with a consensus binding site for ets transcription factors (Efimova et al., 1998). While transcriptional activity of the distal regulatory region was not affected by activated Notch1 expression, activity of the proximal region was induced to a similar extent as the full-length involucrin promoter (Figure 7C).
The Notch1 cytoplasmic region is composed of several domains that share a varying degree of homology with Notch2 (Weinmaster et al., 1992). Expression of the equivalent activated form of Notch2 (Capobianco et al., 1997) in keratinocytes by the same vector induced activity of the HES-AB promoter to a much lesser degree than activated Notch1 (Figure 7D and E) and failed to induce the p21 promoter (Figure 7F). In contrast, activated Notch2 induced involucrin promoter activity as effectively as Notch1 and with a similar dose response (Figure 7G and data not shown).
The most highly conserved region between the Notch1 and Notch2 proteins corresponds to the ankyrin-repeat domain (ANK), which provides a secondary site for RBP-Jκ binding and is not by itself sufficient to induce RBP-Jκ-dependent transcription (Aster et al., 1997; Kurooka et al., 1998). Expression of the Notch1 cytoplasmic region with an internal deletion of the ANK domain failed to induce the HES-1, p21 and involucrin promoters (Figure 7E–G). In contrast, expression of the ANK domain alone devoid of any other upstream or downstream sequences, while not activating the p21 or HES-1 promoters, induced the involucrin promoter to similar levels as those induced by the intact Notch1 and Notch2 cytoplasmic regions, and was also independent of RBP-Jκ function (Figure 7E–G).
Endogenous p21 and early differentiation marker genes are under separate Notch control
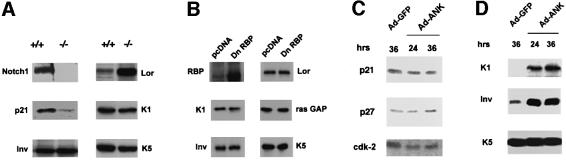
To determine whether the results of the promoter activity studies shown above could be confirmed at the level of the endogenous genes, three complementary approaches were undertaken. In the first, primary keratinocytes from mice homozygous for the Notch1/loxP gene were infected with a GFP control adenovirus or an adenovirus expressing the Cre recombinase to induce deletion of the Notch1 gene (see Supplementary figure 1B). In differentiating keratinocytes harboring the Notch1 deletion, expression of the p21 protein remained at much lower levels than in the controls, while involucrin and keratin 1 were similarly expressed (Figure 8A).
Fig. 8. Separate regulation of endogenous p21 and differentiation markers by Notch signaling. (A) p21 and differentiation marker expression in cultured keratinocytes with induced deletion of the Notch1 gene. Primary keratinocytes derived from mice homozygous for the Notch1/flox gene were infected with GFP- or Cre-expressing adenoviruses and, 4 days after infection, were induced to differentiate by high calcium exposure. Total cell extracts from cells with (+/+) or without the Notch1 gene (–/–) were analyzed by immunoblotting with antibodies against the indicated proteins. Similar results were obtained in a second independent experiment. (B) RBP-Jκ-independent expression of keratinocyte differentiation markers. Keratinocytes were transfected with an expression vector for GFP together with an expression vector for dominant-negative RBP-Jκ or empty vector control. Forty-eight hours after transfection, cells were induced to differentiate by suspension culture as previously described (Di Cunto et al., 1998). Transfected GFP-positive cells were purified by sorting and analyzed by sequential immunoblotting with antibodies against the indicated proteins, including ras-GAP as an unrelated internal control. (C and D) Keratinocytes under growing low calcium conditions were infected with a GFP control adenovirus or an adenovirus expressing the ANK domain of Notch1 (Ad-ANK) for either 24 or 36 h as indicated. Total cell extracts were analyzed by SDS–PAGE and sequential immunoblotting with antibodies against the indicated cell cycle regulatory proteins (C) or differentiation markers (D).
To test whether expression of the latter genes is RBP-Jκ-independent, keratinocytes were transfected with the expression vector for the dominant-negative RBP-Jκ mutant together with a vector for GFP. Cells were induced to differentiate by suspension culture, and the transfected GFP-positive population was purified by sorting. As predicted from the promoter activity studies, immunoblotting of total cell extracts showed that expression of these various differentiation markers was not affected by expression of the RBP-Jκ mutant protein (Figure 8B).
Finally, we constructed a recombinant adenovirus expressing the ANK domain of Notch1. Infection with the ANK-Notch1 adenovirus failed to induce endogenous p21 expression in the attached growing keratinocytes, while it readily induced expression of both the involucrin and keratin 1 differentiation marker genes (Figure 8C and D). No induction of differentiation marker expression is caused by infection with an adenovirus expressing the p16Ink4a protein, which contains an unrelated ankyrin repeat domain (Di Cunto et al., 1998), suggesting that the observed effects are specific for the Notch1 ANK domain.
Discussion
Mice with a keratinocyte-specific deletion of the Notch1 gene were used to assess the relevance of Notch1 signaling in the epidermis in vivo. Although both Notch1 and Notch2 receptors are expressed in mouse epidermis after birth, their function is only partially overlapping. In fact, loss of Notch1 alone caused significant alterations that are consistent with loss of normal growth control in the epidermis and disruption of the well defined border between basal and upper differentiating layers. The increased cell proliferation in the Notch1–/– epidermis reflects the growth inhibitory effects exerted by activated Notch1 in primary keratinocytes in culture. Because of the intrinsically low levels of p21 expression in mouse epidermis, quantitative comparison of expression of this protein in wild-type versus Notch1–/– epidermis could not be achieved. However, significantly reduced p21 levels were found in differentiating keratinocytes with an induced deletion of the Notch1 gene in culture. This is consistent with our reciprocal finding that p21WAF1/Cip1 expression can only be induced by activated Notch1 and not Notch2 expression. Another alteration that is likely to contribute to the hyperproliferation of the Notch1–/– epidermis is the striking upregulation of integrin β1 and β4 expression in keratinocytes of the supra-basal epidermal layers, where expression of these integrins is usually low or undetectable. In fact, alterations similar to the ones found in the Notch1–/– epidermis were previously reported after over-expression of integrin β1 in the supra-basal epidermal layers (Carroll et al., 1995). Deregulated integrin expression in the Notch1–/– epidermis is consistent with the down-modulation of these integrins caused by activated Notch1 in cultured cells (see Supplementary figure 2).
Expression of late differentiation markers was also increased in the Notch1 knockout skin and keratinocytes (Figures 2 and 8A), in parallel with the suppressed expression of these genes by activated Notch1 in culture. In contrast, levels of early markers, such as keratin 1 and involucrin, were not affected by deletion of the Notch1 gene, while they were inhibited by generalized suppression of Notch activity by treatment with a Notch ligand competing peptide. This can be explained by the overlapping function of the Notch1 and Notch2 proteins in induction of the early marker genes, as discussed further below.
Both Notch1 and Notch2 proteins and Jagged-1 and -2 ligands are concomitantly expressed in mouse keratinocytes in culture, in parallel with the expression pattern of these molecules in the epidermis in vivo (Luo et al., 1997; our unpublished observations). This is consistent with a model of a positive feedback loop between Notch and Jagged ligand expression that serves to reinforce Notch activation and coordinate the program of differentiation as keratinocytes migrate from the basal to upper epidermal layers (Luo et al., 1997). In the human system, a Notch/Delta negative feedback mechanism has been proposed to promote the commitment of keratinocyte stem cells to transit amplifying but still proliferating populations (Lowell et al., 2000). A similar mechanism may also operate in mouse skin, although under our conditions we could not detect any signal for Delta expression in the basal layer of murine epidermis, nor in cultured cells under either growing or differentiating conditions.
Irrespective of the positive and/or negative feedback mechanisms involved in amplification of the Notch signal, the initial trigger for Notch activation in differentiating keratinocytes remains to be identified. In cultured keratinocytes induced to differentiate by increased extracellular calcium, we found that endogenous Notch activity increases in parallel with the Notch1 and Notch2 proteins losing their predominant membrane localization and becoming widely re-distributed throughout the cell (Figure 3 and data not shown). Thus, at least in culture, Notch activation could be triggered by a direct calcium-induced conformational change of the extracellular domain of these receptors that affects their ability to bind and/or be activated by ligand(s). However, a similar conformational change may also be triggered when keratinocytes lose contact with the matrix, as induction of Notch activity and suppression by the Notch-ligand competing peptide was also observed when these cells were brought into suspension (Figure 3E and F).
Induction of p21 expression is one of the earliest cell cycle regulatory events underlying differentiation-associated growth arrest (Missero et al., 1995, 1996), and we have shown here that endogenous Notch1 activity is required for induction of this gene in differentiation. The role of Notch signaling in control of p21 expression is unlinked from that in differentiation, as expression of activated Notch1 fails to suppress growth of p21–/– keratinocytes but elicits similar effects on differentiation marker expression as in wild-type cells. Besides being functionally unlinked, induction of early differentiation markers by increased Notch signaling occurs through a distinct biochemical mechanism from that leading to induction of p21. We have shown that the RBP-Jκ protein binds directly to the endogenous p21 promoter, and that both increased extracellular calcium and activated Notch1 induce p21 promoter activity through an RBP-Jκ-dependent mechanism, while activated Notch2 causes no such induction.
In contrast to p21, expression of early differentiation markers is induced by both activated Notch1 and Notch2, and is RBP-Jκ independent. Unlike in other cell types such as Burkitt lymphoma cells (Hsieh et al., 1997), expression of activated Notch2 in keratinocytes causes a much lesser increase of RBP-Jκ-dependent transcription than activated Notch1. An attractive possibility is that RBP-Jκ in keratinocytes is associated with co-repressors (or co-activators) that are specific to this cell type and which can be differentially displaced by the Notch1 versus Notch2 proteins. In contrast to the HES and p21 promoters, Notch2 induced promoter activity of the involucrin gene to a similar extent as Notch1. This common function of the Notch1 and Notch2 proteins can be attributed to their highly conserved ankyrin-rich domain. In fact, the ANK domain of Notch1 failed to induce RBP-Jκ-dependent transcription and p21 expression but could readily induce expression of early differentiation marker genes. This is most likely due to an indirect effect on gene transcription, and an attractive possibility is that the Notch ANK domain can directly interact and/or compete with other proteins involved in negative regulation of differentiation marker genes. In this context, it is relevant to mention that the ANK domain of Notch and an adjacent sequence bind to a number of signaling proteins such as the p50 subunit of NF-κB (Guan et al., 1996) and Deltex (Matsuno et al., 1998). NF-κB is a key transcription factor involved in regulation of many cellular processes, including keratinocyte growth and differentiation (Seitz et al., 1998). By binding the p50 subunit, Notch has been proposed to alter the ratio of p50–p50 versus p65–p50 NF-κB dimers, thereby affecting the selectivity of NF-κB-dependent transcription (Guan et al., 1996). However, by using a battery of NF-κB-responsive promoters we observed no consistent increase of NF-κB transcriptional activity in differentiating or Notch1-expressing keratinocytes (our unpublished observations). Furthermore, NF-κB p65 expression had no inductive effects on either p21 or involucrin promoter activity. Deltex is a cytoplasmic SH3-binding protein that can mediate effects of Notch on transcription which are independent of RBP-Jκ (Matsuno et al., 1998). Induction of the p21 and involucrin promoters by activated Notch1 are suppressed by concomitant Deltex expression (our unpublished observations), pointing to a possible competitive interaction between Notch and Deltex in keratinocyte differentiation control.
At the transcriptional level, we have mapped the Notch-responsive region of the involucrin promoter to a minimal sequence with tandem binding sites for the AP-1 and ets transcription factors (Efimova et al., 1998), suggesting that activity of either factor is under Notch control, possibly in a promoter-specific context. Unlike the early markers, the late differentiation markers are down-modulated rather than induced by increased Notch activity. This is consistent with the fact that control of early versus late differentiation markers can be dissociated both genetically and pharmacologically. For instance, an inactivating mutation of the Whn/FOXN1 gene, encoding a keratinocyte-specific transcription factor responsible for the nude mouse phenotype, results in increased expression of late differentiation markers and suppression of the early ones (Brissette et al., 1996), and similar effects are induced by protein kinase C activation by phorbol ester treatment (Dlugosz and Yuspa, 1993). Thus, Notch signaling is likely to be a critical integrator of signals that control the induction of keratinocyte growth arrest and early versus late stages of differentiation.
Materials and methods
Cells and viruses
Primary keratinocytes were prepared from p21+/+ or p21–/– mice of the same genetic background (Sencar) as described (Missero et al., 1996). The recombinant adenoviruses expressing the constitutively active form of Notch1 and the ANK domain alone were obtained by inserting the corresponding cDNAs [amino acids 1758–2556 and 1852–2125, respectively, of human Notch1 (Capobianco et al., 1997)] into the BamHI–XhoI sites of pAdTrack-CMV, and then recombined into the adenoviral backbone plasmid pAdEasy-1 in bacteria (He et al., 1998). Conditions for adenoviral vector infection were as described (Di Cunto et al., 1998).
Notch ligand competition experiments were performed by trypsinizing keratinocytes and replating them in either fresh medium or medium containing 20 µg/ml of the recombinant protein HurnEGF11–12 (Garces et al., 1997) for 48 h prior to induction of differentiation by increased extracellular calcium (2 mM) or suspension culture (Di Cunto et al., 1998) for an additional 12 h.
Antibodies and immunofluorescence conditions
Rabbit polyclonal antibodies against keratinocyte differentiation markers were raised in our laboratory, while those against the Notch1, Notch2, p21, p27, CDK2 and ras-GAP proteins were from Santa Cruz Biotechnology. Rabbit polyclonal antibodies against mouse CBF1/RBP-Jκ were kindly provided by Dr E.Kieff. Immunoblotting and immunofluorescence conditions were as described (Missero et al., 1996; Foitzik et al., 1999).
Chromatin immunoprecipitation (ChIP) experiments
ChIP experiments were carried out as described (Orlando et al., 1997). After phenol–chloroform extraction and ethanol precipitation, the DNAs recovered from the immunoprecipitated chromatin were analyzed by PCR amplification. Preliminary experiments with naked DNA were carried out to optimize PCR conditions for each target sequence. The promoter for the p21 mouse gene contains a sequence at position –1223 that fully matches the binding site for RBP-Jκ. A 142 bp region surrounding this sequence was amplified using the following forward and reverse primers: ‘CCCCAAACAGAAGGAA’ and ‘CCAGACAGTCCACTAA’. The mouse p21 promoter contains tandemly repeated Sp1 binding sites in the TATA box proximal region. A 128 bp region surrounding this sequence was amplified using the following forward and reverse primers: ‘GTCTGGCGCGGGCTTAGA’ and ‘GTCGAGCTGCCTCCTTAT’. The promoter for the c-jun mouse gene contains a sequence at position –1971 that fully matches the binding site for RBP-Jκ. A 128 bp region surrounding this sequence was amplified using the following forward and reverse primers: ‘GGGATAGTTTCCTCC’ and ‘CGTCCACTAAAC ACCACC’.
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We are grateful to Drs A.Israel, R.Eckert, S.Artavanis-Tsakonas and T.Honjo for their gifts of reporter and expression plasmids, Dr F.Giancotti for providing polyclonal antibodies against integrins β1 and β4, and Dr E.Kieff for antibodies against RBP-Jκ. We thank Drs C.Brisken and P.Stein for critical reading of the manuscript. This work was supported by NIH Grants AR39190, CA16038 and CA73796 to G.P.D., and, in part, by the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Ltd Agreement, by grants from the Swiss National Science Foundation and the Leenaards Foundation to M.A., Krebsforschung Schweiz to F.R., and by a fellowship from the Japan Society for the Promotion of Science to R.O.
References
- Artavanis-Tsakonas S., Rand,M.D. and Lake,R.J. (1999) Notch signaling: cell fate control and signal integration in development. Science, 284, 770–776. [DOI] [PubMed] [Google Scholar]
- Aster J.C., Robertson,E.S., Hasserjian,R.P., Turner,J.R., Kieff,E. and Sklar,J. (1997) Oncogenic forms of NOTCH1 lacking either the primary binding site for RBP-Jκ or nuclear localization sequences retain the ability to associate with RBP-Jκ and activate transcription. J. Biol. Chem., 272, 11336–11343. [DOI] [PubMed] [Google Scholar]
- Brissette J., Li,J., Kamimura,J., Lee,D. and Dotto,G.P. (1996) The product of the mouse nude locus, Whn, regulates the balance between epithelial cell growth and differentiation. Genes Dev., 10, 2212–2221. [DOI] [PubMed] [Google Scholar]
- Capobianco A.J., Zagouras,P., Blaumueller,C.M., Artavanis-Tsakonas,S. and Bishop,J.M. (1997) Neoplastic transformation by truncated alleles of human NOTCH1/TAN1 and NOTCH2. Mol. Cell. Biol., 17, 6265–6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J.M., Romero,M.R. and Watt,F.M. (1995) Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell, 83, 957–968. [DOI] [PubMed] [Google Scholar]
- de Celis J.F. and Bray,S. (1997) Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development, 124, 3241–3251. [DOI] [PubMed] [Google Scholar]
- Di Cunto F., Topley,G., Calautti,E., Hsiao,J., Ong,L., Seth,P.K. and Dotto,G.P. (1998) Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science, 280, 1069–1072. [DOI] [PubMed] [Google Scholar]
- Dlugosz A.A. and Yuspa,S.H. (1993) Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase. J. Cell Biol., 120, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G.P. (1999) Signal transduction pathways controlling the switch between keratinocyte growth and differentiation. Crit. Rev. Oral Biol. Med., 10, 442–457. [DOI] [PubMed] [Google Scholar]
- Efimova T., LaCelle,P., Welter,J.F. and Eckert,R.L. (1998) Regulation of human involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J. Biol. Chem., 273, 24387–24395. [DOI] [PubMed] [Google Scholar]
- Foitzik K., Paus,R., Doetschman,T. and Dotto,G.P. (1999) The TGF-β2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev. Biol., 212, 278–289. [DOI] [PubMed] [Google Scholar]
- Garces C., Ruiz-Hidalgo,M.J., de Mora,J.F., Park,C., Miele,L., Goldstein,J., Bonvini,E., Porras,A. and Laborda,J. (1997) Notch-1 controls the expression of fatty acid-activated transcription factors and is required for adipogenesis. J. Biol. Chem., 272, 29729–29734. [DOI] [PubMed] [Google Scholar]
- Guan E., Wang,J., Laborda,J., Norcross,M., Baeuerle,P.A. and Hoffman,T. (1996) T cell leukemia-associated human Notch/translocation-associated Notch homologue has I κB-like activity and physically interacts with nuclear factor-κB proteins in T cells. J. Exp. Med., 183, 2025–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T.C., Zhou,S., da Costa,L.T., Yu,J., Kinzler,K.W. and Vogelstein,B. (1998) A simplified system for generating recombinant adenoviruses. Proc. Natl Acad. Sci. USA, 95, 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J.J., Henkel,T., Salmon,P., Robey,E., Peterson,M.G. and Hayward,S.D. (1996) Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein–Barr virus EBNA2. Mol. Cell. Biol., 16, 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J.J., Nofziger,D.E., Weinmaster,G. and Hayward,S.D. (1997) Epstein–Barr virus immortalization: Notch2 interacts with CBF1 and blocks differentiation. J. Virol., 71, 1938–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra A.K., Warot,X., Brocard,J., Bornert,J.M., Xiao,J.H., Chambon,P. and Metzger,D. (1999) Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res., 27, 4324–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarriault S., Brou,C., Logeat,F., Schroeter,E.H., Kopan,R. and Israel,A. (1995) Signalling downstream of activated mammalian Notch. Nature, 377, 355–358. [DOI] [PubMed] [Google Scholar]
- Kao H.Y., Ordentlich,P., Koyano-Nakagawa,N., Tang,Z., Downes,M., Kintner,C.R., Evans,R.M. and Kadesch,T. (1998) A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev., 12, 2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Taniguchi,Y., Kurooka,H., Minoguchi,S., Sakai,T., Nomura-Okazaki,S., Tamura,K. and Honjo,T. (1997) Involvement of RBP-J in biological functions of mouse Notch1 and its derivatives. Development, 124, 4133–4141. [DOI] [PubMed] [Google Scholar]
- Kurooka H., Kuroda,K. and Honjo,T. (1998) Roles of the ankyrin repeats and C-terminal region of the mouse notch1 intracellular region. Nucleic Acids Res., 26, 5448–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Leimeister,C., Gessler,M. and Kopan,R. (2000) Activation of the notch pathway in the hair cortex leads to aberrant differentiation of the adjacent hair-shaft layers. Development, 127, 2421–2432. [DOI] [PubMed] [Google Scholar]
- Lowell S., Jones,P., Le Roux,I., Dunne,J. and Watt,F.M. (2000) Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr. Biol., 10, 491–500. [DOI] [PubMed] [Google Scholar]
- Luo B., Aster,J.C., Hasserjian,R.P., Kuo,F. and Sklar,J. (1997) Isolation and functional analysis of a cDNA for human Jagged2, a gene encoding a ligand for the Notch1 receptor. Mol. Cell. Biol., 17, 6057–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K., Eastman,D., Mitsiades,T., Quinn,A.M., Carcanciu,M.L., Ordentlich,P., Kadesch,T. and Artavanis-Tsakonas,S. (1998) Human deltex is a conserved regulator of Notch signalling. Nature Genet., 19, 74–78. [DOI] [PubMed] [Google Scholar]
- Missero C., Calautti,E., Eckner,R., Chin,J., Tsai,L., Livingston,D.M. and Dotto,G.P. (1995) Involvement of the cell cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc. Natl Acad. Sci. USA, 92, 5451–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C., Di Cunto,F., Kiyokawa,H., Koff,A. and Dotto,G.P. (1996) The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev., 10, 3065–3075. [DOI] [PubMed] [Google Scholar]
- Navarro J.M., Casatorres,J. and Jorcano,J.L. (1995) Elements controlling the expression and induction of the skin hyperproliferation-associated keratin K6. J. Biol. Chem., 270, 21362–21367. [DOI] [PubMed] [Google Scholar]
- Orlando V., Strutt,H. and Paro,R. (1997) Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods, 11, 205–214. [DOI] [PubMed] [Google Scholar]
- Pourquie O. (2000) Skin development: delta laid bare. Curr. Biol., 10, R425–R428. [DOI] [PubMed] [Google Scholar]
- Radtke F., Wilson,A., Stark,G., Bauer,M., van Meerwijk,J., MacDonald,H.R. and Aguet,M. (1999) Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity, 10, 547–558. [DOI] [PubMed] [Google Scholar]
- Seitz C.S., Lin,Q., Deng,H. and Khavari,P.A. (1998) Alterations in NF-κB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-κB. Proc. Natl Acad. Sci. USA, 95, 2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun T., Hamaguchi,Y., Matsunami,N., Furukawa,T., Honjo,T. and Kawaichi,M. (1994) Recognition sequence of a highly conserved DNA binding protein RBP-Jκ. Nucleic Acids Res., 22, 965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmaster G., Roberts,V.J. and Lemke,G. (1992) Notch2: a second mammalian Notch gene. Development, 116, 931–941. [DOI] [PubMed] [Google Scholar]