Abstract
The establishment and maintenance of cellular polarity are critical for the development of multicellular organisms. PAR (partitioning-defective) proteins were identified in Caenorhabditis elegans as determinants of asymmetric cell division and polarized cell growth. Recently, vertebrate orthologues of two of these proteins, ASIP/PAR-3 and PAR-6, were found to form a signalling complex with the small GTPases Cdc42/Rac1 and with atypical protein kinase C (PKC). Here we show that ASIP/PAR-3 associates with the tight-junction-associated protein junctional adhesion molecule (JAM) in vitro and in vivo. No binding was observed with claudin-1, -4 or -5. In fibroblasts and CHO cells overexpressing JAM, endogenous ASIP is recruited to JAM at sites of cell–cell contact. Over expression of truncated JAM lacking the extracellular part disrupts ASIP/PAR-3 localization at intercellular junctions and delays ASIP/PAR-3 recruitment to newly formed cell junctions. During junction formation, JAM appears early in primordial forms of junctions. Our data suggest that the ASIP/PAR-3–aPKC complex is tethered to tight junctions via its association with JAM, indicating a potential role for JAM in the generation of cell polarity in epithelial cells.
Keywords: ASIP/PAR-3/cell polarity/JAM/tight junctions
Introduction
The generation of cellular polarity is critical for various cellular functions, such as directed migration, asymmetric cell division and the vectorial transport of molecules. Polarity is best studied in epithelial cells where apical and basolateral surface domains with different lipid and protein compositions can be distinguished (Yeaman et al., 1999). In vertebrate epithelia, the two membrane domains are separated by tight junctions, which act as an intramembrane diffusion barrier (Dragsten et al., 1981) and also as a paracellular seal that prevents diffusion of molecules across the epithelial cell layer. Transmembrane proteins identified so far in tight junctions include occludin (Furuse et al., 1993), the claudin family (Furuse et al., 1998; Morita et al., 1999) and junctional adhesion molecule (JAM) (Martin-Padura et al., 1998; Liu et al., 2000). All of these proteins directly interact with peripheral proteins that contain one or more PDZ domains. Occludin directly associates with the cortical PDZ proteins ZO-1 and ZO-3 (Fanning et al., 1998; Haskins et al., 1998), and claudins bind to ZO-1, ZO-2 and ZO-3 (Itoh et al., 1999). While occludin and the claudins are probably directly involved in the formation of tight junction strands (Tsukita and Furuse, 2000), the role of JAM in the generation of tight junctions and the formation of cell polarity is still unclear. JAM-specific monoclonal antibodies (mAbs) inhibit junction reformation after Ca2+ depletion-induced loss of intercellular junctions, suggesting a role of JAM in the regulation of junction assembly (Liu et al., 2000). JAM directly associates with the PDZ proteins ZO-1 and AF-6 (Bazzoni et al., 2000; Ebnet et al., 2000), and some evidence suggests that JAM can be recruited to intercellular junctions by AF-6 (Ebnet et al., 2000). The mechanisms underlying the recruitment of these proteins to tight junctions, and hence the formation of cell polarity in vertebrate epithelial cells, are unknown.
The establishment of cell polarity has been studied extensively in the nematode Caenorhabditis elegans. The family of PAR (partitioning-defective) proteins regulates anterior/posterior zygote polarity in response to sperm cues (Bowerman and Shelton, 1999). Two PAR proteins, PAR-3 and PAR-6, co-localize with an atypical protein kinase C (PKC-3) at the anterior end of the one-cell embryo. Both PKC-3 and PAR-3 are required for proper asymmetric divisions (Tabuse et al., 1998), and PAR-6 mediates the asymmetric localization of PAR-3 (Watts et al., 1996; Hung and Kemphues, 1999). The correct localization of these three proteins and their functions are mutually interdependent (Tabuse et al., 1998; Hung and Kemphues, 1999).
The first vertebrate homologue of a PAR protein, named ASIP, was identified as an aPKCζ-interacting protein and is the orthologue of PAR-3 (Izumi et al., 1998). ASIP co-localizes with aPKCλ at tight junctions in epithelial cells; overexpression of a dominant-negative mutant of aPKC results in mislocalization of ASIP and other tight junction components, as well as in disruption of the barrier function and epithelial cell polarity (Suzuki et al., 2001). Several reports describe the identification of a complex consisting of the mammalian homologues of PAR-3 and PAR-6, aPKC and the small GTPase Cdc42 (Joberty et al., 2000; Johansson et al., 2000; Lin et al., 2000; Qiu et al., 2000; Yamanaka et al., 2001). All four proteins are detectable in immunoprecipitates of Cdc42 or PAR-6. As reported recently, the Drosophila homologues of PAR-3, PAR-6 and aPKC form a complex, which is required for apical/basal polarity in epithelial and non-epithelial cells (Wodarz et al., 2000; Petronczki and Knoblich, 2001). Taken together, these findings make a strong point for the ASIP/PAR3–PAR-6–aPKC complex as an evolutionarily conserved regulator of cell polarity that plays a central role in the formation or maintenance of tight junctions in vertebrate epithelial cells. How this complex is recruited to tight junctions is not known. There is also no information about the transmembrane proteins at tight junctions that bind any of the components of the complex.
Here we report that ASIP/PAR-3 specifically associates with the tight junction protein JAM. Our results identify JAM as the first transmembrane protein at tight junctions that affects the localization of ASIP/PAR-3 at cell–cell junctions and thus would also have the potential to recruit or tether the signalling complex consisting of ASIP/PAR-3, PAR-6, Rac-1/Cdc42 and aPKC to tight junctions. These findings indirectly suggest a role for JAM in the generation of epithelial cell polarity.
Results
Identification of ASIP as a protein interacting with the cytoplasmic domain of JAM
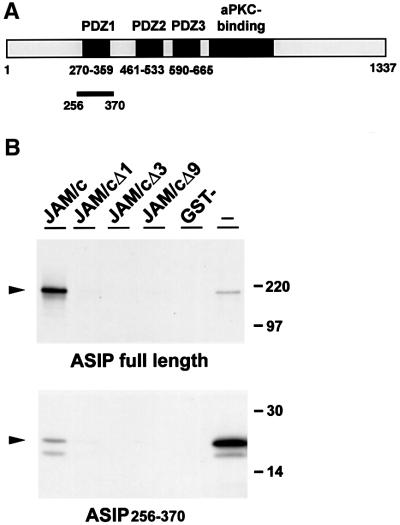
In a yeast two-hybrid screen using the cytoplasmic tail of JAM as bait, performed essentially as described (Ebnet et al., 2000), a cDNA fragment was isolated from a murine embryonic cDNA library (Hollenberg et al., 1995) that corresponds to nucleotides 1023–1371 of the rat ASIP cDNA sequence (Izumi et al., 1998). This fragment reflects amino acid residues 256–370 of the rat ASIP protein and includes the first PDZ domain of ASIP (amino acids 270–359) (Figure 1A). The PDZ domain dependence of the association was confirmed by co-expressing JAM mutants lacking the C-terminal PDZ binding domain with the ASIP fragment in yeast. As summarized in Table I, the association between JAM and ASIP, as assessed by growth on selective medium or by β-galactosidase activity, was abolished after truncating the C-terminal end by one, three or nine amino acid residues. These results indicated that JAM interacts with the first PDZ domain of ASIP through its C-terminal PDZ binding motif.
Fig. 1. ASIP interacts directly with JAM. (A) Schematic organization of ASIP. The PDZ domains are drawn as black boxes; the aPKC- interacting domain is drawn in grey. The black bar indicates the ASIP fragment isolated from the yeast two-hybrid library. (B) Full-length ASIP and the ASIP fragment isolated from the yeast two-hybrid library (ASIP256–370) were generated in vitro in the presence of [35S]methionine and incubated with immobilized GST fusion proteins containing the complete cytoplasmic tail of JAM (JAM/c), the cytoplasmic tail lacking one (JAM/cΔ1), three (JAM/cΔ3) or nine (JAM/cΔ9) C-terminal amino acid residues, or with GST alone (GST-). Both full-length ASIP and the isolated fragment comprising the first PDZ domain of ASIP interact with JAM in a PDZ-domain-dependent manner. The association of full-length ASIP is much stronger than that of the short ASIP fragment. In lanes marked with (–), aliquots of the translation reaction were loaded to indicate the Mr of the generated protein. Both constructs were generated in vitro with comparable efficiencies (data not shown); the different intensities of the signals in the (–) lanes are due to a longer exposure of the gel. The second band at a lower molecular weight represents a degradation product of in vitro generated ASIP.
Table I. Interaction between JAM and ASIP in yeast.
| A | –TL | –THULL |
|||||
|---|---|---|---|---|---|---|---|
| 3-AT (mM) | 0 | 0 | 2.5 | 5 | 7.5 | 10 | 15 |
| JAM/cyt + ASIP | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| JAM/cytΔ1 + ASIP | ++ | + | – | – | – | – | – |
| JAM/cytΔ3 + ASIP | ++ | – | – | – | – | – | – |
| JAM/cytΔ9 + ASIP | ++ | – | – | – | – | – | – |
| VE-cad + p120cas | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| α-cat + VE-cad | ++ | – | – | – | – | – | – |
| B | –TL |
–THULL |
–THULL/7.5 mM 3-AT |
|||
|---|---|---|---|---|---|---|
| Growth | Blue colour | Growth | Blue colour | Growth | Blue colour | |
| JAM/cyt + ASIP | ++ | + | ++ | + | + | + |
| JAM/cytΔ1 + ASIP | ++ | – | + | – | – | n.d. |
| JAM/cytΔ3 + ASIP | ++ | – | – | n.d. | – | n.d. |
| JAM/cytΔ9 + ASIP | ++ | – | – | n.d. | – | n.d. |
| VE-cad + p120cas | ++ | ++ | ++ | ++ | ++ | ++ |
| α-cat + VE-cad | ++ | – | – | n.d. | – | n.d. |
(A) Growth of yeast strains co-expressing the cytoplasmic domain of JAM together with ASIP amino acids 256–370 on selective medium in the absence or presence of 3-AT. Growth of yeast colonies was scored as – (no growth), +/– (colony diameter <0.5 mm), + (colony diameter between 0.5 and 2 mm), ++ (colony diameter >2 mm). The JAM constructs comprised the full-length cytoplasmic tail of JAM (amino acids 261–300, JAM/cyt), or truncation mutants lacking one, three or nine C-terminal amino acid residues (JAM/cytΔ1, JAM/cytΔ3, JAM/cytΔ9, respectively). Yeast strains co-expressing VE-cadherin and p120cas or α-catenin and VE-cadherin were used as positive and negative controls, respectively. (B) X-Gal assay. Blue colour staining was assessed 4 h after placing yeast colonies on filters soaked in β-galactosidase substrate. Colonies were scored as ++ (deep blue staining), + (weak blue staining), and – (no staining). n.d., not done; 3-AT, 3-aminotriazole.
ASIP interacts with JAM through its first PDZ domain
To test whether full-length ASIP and JAM interact biochemically, in vitro glutathione S-transferase (GST) pull-down assays were performed. Full-length ASIP and the ASIP fragment isolated from the yeast two-hybrid library (ASIP256–370) were generated by in vitro translation in the presence of [35S]methionine and incubated with immobilized GST–JAM fusion proteins. Full-length ASIP bound strongly to GST–JAM and the interaction was abolished upon truncating the C-terminus of JAM (Figure 1B), confirming that JAM interacts with ASIP through its C-terminal PDZ domain binding motif. ASIP256–370 also bound to JAM; however, the affinity of this fragment was reduced compared with full-length ASIP (Figure 1B). This raised the possibility that other PDZ domains of ASIP also associate with JAM. When individual recombinant PDZ domains were incubated with GST–JAM, only PDZ domain 1 of ASIP, but not PDZ domains 2 or 3, bound to GST–JAM (Figure 2A). When alone, the first PDZ domain bound with significantly lower affinity to GST–JAM, when compared with a construct containing all three PDZ domains. To further confirm that the first PDZ domain is the predominant JAM binding site we constructed ASIP mutants in which individual PDZ domains were replaced by the PDZ domain present in the secreted form of murine interleukin-16 (IL-16) (Keane et al., 1998). This PDZ domain does not bind ligands (Muhlhahn et al., 1998). Substitution of the first PDZ domain of ASIP completely abolished the binding to GST–JAM (Figure 2B). In contrast, the interaction was retained when the second PDZ domain was substituted by the IL-16 PDZ domain, and was only slightly reduced when the third PDZ domain was substituted. These findings confirmed that ASIP interacts with the C-terminal PDZ binding motif of JAM through its first PDZ domain.
Fig. 2. ASIP interacts with JAM through its first PDZ domain. (A) ASIP fragments comprising all three PDZ domains (PDZ1–3), the first PDZ domain (PDZ1), the second PDZ domain (PDZ2) or the third PDZ domain (PDZ3) were generated in vitro in the presence of [35S]methionine and incubated with immobilized GST fusion proteins as described in the legend to Figure 1. Among individual ASIP PDZ domains, only the first PDZ domain bound to GST–JAM. Note that the interaction is strongly reduced compared with ASIP/PDZ1–3. Right panel: equal amounts of the translation products were loaded, demonstrating that all constructs were generated with comparable efficiencies. (B) GST binding assays with ASIP fragments comprising all three PDZ domains with individual PDZ domains mutated by substitution with the inactive PDZ domain present in the secreted form of IL-16 (mtPDZ1, mtPDZ2, mtPDZ3). Right panel: equal aliquots of the translation reactions were loaded to control for translation efficiencies of the individual constructs. Note that the mutated constructs are generated less efficiently compared with the wild-type PDZ1–3 construct, most likely due to the use of the T3 promoter, as opposed to the T7 promoter used for PDZ1–3. The inactivation of PDZ domain 1 completely abolished the interaction between ASIP and JAM.
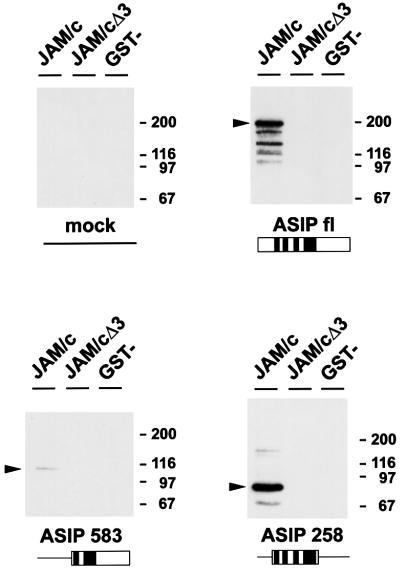
To further confirm the specificity of the interaction between ASIP and JAM, lysates derived from COS-7 cells transiently transfected with expression vectors encoding full-length ASIP, or truncated forms of ASIP encoding amino acids 258–936 (ASIP258) or amino acids 583–1337 (ASIP583), were incubated with GST–JAM immobilized on G–Sepharose beads. The association between JAM and ASIP was assessed by immunoblotting affinity-purified proteins with antibodies against ASIP. Full-length ASIP bound efficiently to GST–JAM, but not to GST–JAMΔ9 or GST alone (Figure 3). ASIP583 which lacks PDZ domains 1 and 2, bound only poorly, whereas ASIP258 containing all three PDZ domains bound with similar efficiency as full-length ASIP. These results indicated that ASIP generated in vivo associates with JAM in vitro, and further confirm that the interaction between ASIP and JAM is predominantly mediated by the first PDZ domain of ASIP.
Fig. 3. JAM associates with ASIP generated in COS-7 cells. COS-7 cells were transiently transfected with either an irrelevant expression vector encoding bacterial alkaline phosphatase (mock), N-terminal T7 tag-fused ASIP expression constructs that encode full-length ASIP (ASIP fl) or deletion mutants comprising amino acid residues 583–1337 (ASIP 583) or 258–936 (ASIP 258). Lysates from transfected cells were incubated with GST–JAM, GST–JAMΔ3 or GST alone, immobilized on G–Sepharose beads. The resulting complexes were analysed by immunoblotting with a mAb directed against the T7 tag. Arrowheads indicate the positions of the recombinant ASIP molecules. Both full-length ASIP and ASIP deletion mutant ASIP 258 were efficiently affinity isolated with GST–JAM, whereas the ASIP mutant lacking the first PDZ domain (ASIP 583) was only poorly affinity isolated.
ASIP interacts with JAM but not other transmembrane components of tight junctions
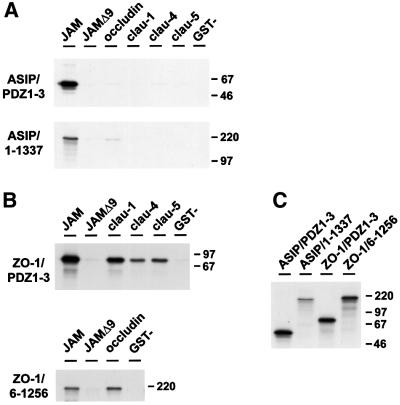
Several transmembrane components have been described to be present in tight junctions. These include occludin (Furuse et al., 1993), the claudins (Furuse et al., 1998) and JAM (Martin-Padura et al., 1998; Liu et al., 2000). Among them, claudins, like JAM, contain C-terminal PDZ domain binding motifs, and interact with ZO-1, ZO-2 and ZO-3 in a PDZ-domain-dependent manner (Itoh et al., 1999). To determine whether ASIP associates exclusively with JAM, we performed GST pull-down assays using the C-terminal cytoplasmic domains of claudin-1, claudin-4, claudin-5 and occludin fused to GST. In contrast to the efficient association of both ASIP/PDZ 1–3 and full-length ASIP with GST–JAM, there was only a very weak association with GST–occludin and no association with GST– claudin-1, –claudin-4 or –claudin-5 (Figure 4A). This specificity was in sharp contrast to ZO-1, which binds to the cytoplasmic domains of all tight junction membrane proteins, including JAM, claudins and occludin (Figure 4B). These findings indicate that among the various tight-junction-associated transmembrane components, ASIP shows high specificity for JAM.
Fig. 4. ASIP interacts exclusively with JAM. (A) ASIP PDZ domains 1–3 (ASIP PDZ1–3) or full-length ASIP (ASIP 1–1337) were transcibed in vitro from the T7 promoter or the T3 promoter, respectively, and translated in the presence of [35S]methionine. Recombinant proteins were incubated with GST fusion proteins containing the cytoplasmic domains of JAM (JAM), the cytoplasmic domain of JAM truncated by nine C-terminal amino acids (JAMΔ9), the C-terminal cytoplasmic domains of occludin (occludin), claudin-1 (clau-1), claudin-4 (clau-4) and claudin-5 (clau-5), or with GST alone. (B) GST fusion proteins described in (A) were incubated with in vitro translated fragments encompassing PDZ-domains 1–3 (ZO-1/PDZ1–3) or amino acids 6–1256 (ZO-1/6–1256) of ZO-1. (C) Equal aliquots of each translation reaction were loaded separately to analyse the translation efficiency. Note that full-length ASIP is generated less efficiently, most likely due to the use of T3 promoter rather than the T7 promoter used for ASIP PDZ1–3. In contrast to ZO-1 fragments, which interacted specifically with all GST fusion proteins, ASIP interacted strongly only with GST–JAM.
ASIP and JAM associate in mammalian epithelial cells
To analyse whether JAM and ASIP associate in mammalian epithelial cells, MDCK II cells were transiently transfected with Flag-tagged JAM and subsequently subjected to a Ca2+ switch to induce new junction formation. Endogenous ASIP was precipitated with ASIP antibodies and the presence of JAM in immunoprecipitates was analysed in western blots using antibodies against the Flag tag. As shown in Figure 5, JAM but not JAMΔ9 co-immunoprecipitated with endogenous ASIP from MDCK II cells. This interaction could only be demonstrated if the cells were lysed in the presence of a cleavable cross-linker (Figure 5, right panels). Similarly, co-immunoprecipitation of JAM with ASIP from confluent MDCK II cells required the presence of a cross-linker (data not shown), suggesting that the association between ASIP and JAM is unstable in lysed cells. Taken together, these findings indicate that ASIP and JAM associate in mammalian epithelial cells, and that the association is induced early after cell contact formation.
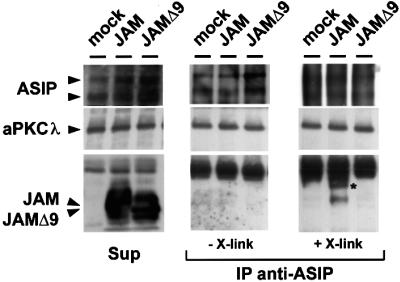
Fig. 5. ASIP and JAM associate in mammalian epithelial cells. MDCK II cells transfected with empty vector, expression vector encoding Flag-JAM or Flag-JAMΔ9 were subjected to a Ca2+ switch for 1 h, then lysed in the presence or absence of a chemical cross-linker. Lysates were incubated with affinity-purified ASIP antibodies, and the resulting immunocomplexes were analysed using the antibodies indicated on the left. To test for the amounts of protein present in each sample, aliquots of each lysate were removed prior to immuno precipitation, and tested for the presence of endogenous ASIP and aPKCλ as well as of transfected JAM (Sup). Co-immunoprecipitations in the absence and presence of the cross-linker are shown in the middle and right panels, respectively. The signals corresponding to the two ASIP isoforms present in MDCK II cells (180 and 150 kDa isoforms) tend to be diffuse in samples subjected to cross-linking, probably because of incomplete cleavage of the cross-linker. The asterisk indicates a high molecular weight band that was specifically detected in immunocomplexes derived from Flag-JAM-expressing cells. Note that Flag-JAM, but not Flag-JAMΔ9, is co-precipitated with ASIP and that this requires the presence of a cross-linker, whereas aPKCλ is co-precipitated with ASIP in all samples.
JAM recruits endogenous ASIP to cell contacts in CHO cells
To address the question of the physiological relevance of the JAM–ASIP association, we used CHO transfectants stably expressing JAM and analysed the expression of endogenous ASIP in these transfectants. As shown in Figure 6A, CHO cells express endogenous ASIP. In heterogeneous CHO cell populations consisting of JAM-transfected and non-transfected cells, ectopically expressed JAM clustered specifically at cell contacts between transfected cells (Figure 6B, arrowheads), but not between transfected and non-transfected cells (Figure 6B, arrows). Endogenous ASIP co-distributed with JAM at sites of cell contact and was absent where JAM was absent. ZO-1 was recruited in a similar way to sites of cell contact by JAM, whereas the heat shock protein HSP-90 was not recruited. We obtained similar results using L cells. JAM-transfected L cells adopted a spindle-like morphology and had a strong tendency to lose ectopic JAM expression upon repeated passaging and, therefore, JAM clusters at cell contact sites were less frequent. Nevertheless, endogenous ASIP co-localized with JAM clusters at cell contact sites (data not shown). Taken together, these findings indicate that JAM is able to recruit ASIP to sites of intercellular contacts, and suggest that JAM could act as an anchor that recruits ASIP to tight junctions in polarized epithelial cells.
Fig. 6. JAM recruits endogenous ASIP to cell–cell contacts in transfected CHO cells. (A) Western blot analysis of ASIP and ZO-1 in CHO and CMT cells. Arrowheads indicate the 100 and 180 kDa isoforms for ASIP and the 220 kDa isoform for ZO-1. (B) Double-label immunofluorescence analysis of CHO cells transfected with JAM. Heterogeneous CHO cell populations consisting of transfected cells and non-transfected cells were simultaneously stained with rat anti-JAM mAb and rabbit pAb directed against ASIP or ZO-1, or mouse mAb directed against HSP-90. Incubation with primary antibodies was followed by biotinylated secondary antibodies and Cy-3-conjugated streptavidin to detect JAM, and with Cy-2-conjugated secondary antibodies to detect ASIP, ZO-1 and HSP-90. In JAM-transfected CHO cells JAM clustered at sites of cell–cell contact between transfected cells (arrowheads), but not between transfected and non-transfected cells (thin arrows), indicating that homophilic interaction is required for junction localization. ASIP and ZO-1 co-localized with JAM at sites of cell–cell contacts. Bar, 5 µm.
Overexpression of the cytoplasmic domain of JAM disturbs the junctional localization of the ASIP–aPKC complex in MDCK II cells
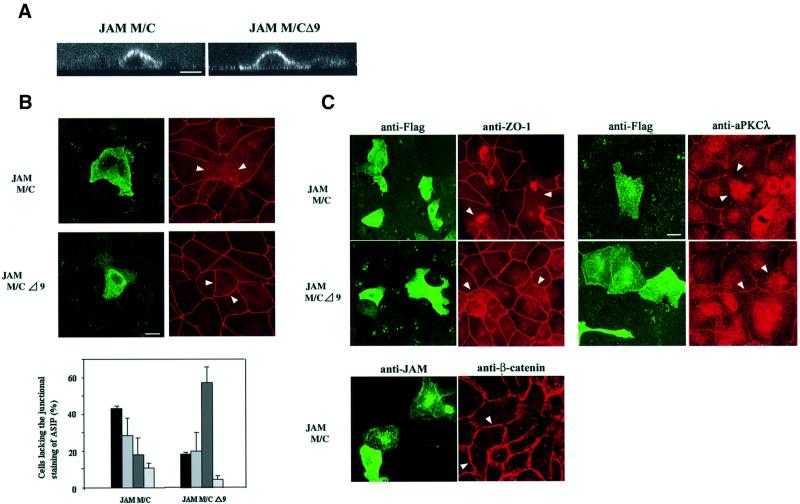
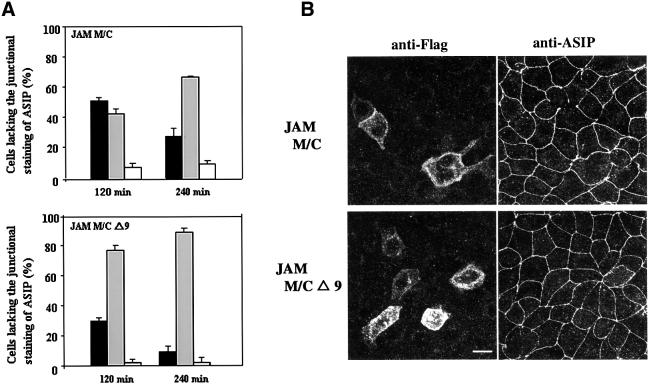
To further demonstrate a role for JAM in tight junction integrity and cell polarization, we transiently overexpressed a JAM mutant consisting of the transmembrane and cytoplasmic domains, but lacking the whole extracellular part (JAM M/C), and analysed the distribution of ASIP in these cells. A similar mutant lacking the C-terminal nine amino acid residues that are required for ASIP interaction (JAM M/CΔ9) was used as a control. As shown in Figure 7A, both mutants localize to the entire cell surface of MDCK II cells, indicating that these mutant proteins are targeted to the plasma membrane but fail to be concentrated at cell–cell contact sites. Overexpression of both mutants had no effect on ASIP distribution when analysed in cells that had reached confluent density (data not shown). However, in cells at sparse (∼50%) density, overexpression of JAM M/C resulted in a loss of ASIP staining at cell junctions in 45% of the cells and ASIP localization was preserved in <20% of the cells (Figure 7B). In contrast, in cells overexpressing JAM M/CΔ9, ASIP localization was preserved in ∼60% of cells and the number of cells lacking ASIP at cell contacts was <20%. Notably, the junctional distribution of ZO-1 and aPKCλ was affected in a similar way in JAM M/C- but not in JAM M/CΔ9-expressing cells (Figure 7C). On the other hand, >95% of JAM M/C-expressing cells showed a normal distribution of β-catenin (Figure 7C), indicating that components of adherens junctions are not affected, and thus ruling out the possiblity that loss of ASIP from junctions results from unspecific effects on cell viability. In cells overexpressing full-length JAM, the redistribution of ASIP was much less pronounced (data not shown). Taken together, these findings suggest a regulatory role for JAM in tight junction biogenesis.
Fig. 7. Overexpression of the cytoplasmic domain of JAM results in the loss of the junctional staining of ASIP in MDCK II cells. Subconfluent MDCK II cells were transiently transfected with expression vectors encoding Flag-tagged mutants of JAM encoding the transmembrane and cytoplasmic domain of JAM, with the complete C-terminus (JAM M/C) or a truncated C-terminus lacking the last nine amino acid residues (JAM M/CΔ9). Cells were immunostained with anti-Flag antibody M2 (A–C) together with anti-ASIP (B), or anti-ZO-1, anti-aPKCλ and anti-β-catenin antibodies (C). (A) Confocal z-sectional views of cells expressing the JAM mutants. Note that both mutants show a similar distribution along the whole cellular surface. (B) JAM-transfected cells appear as bright cells when stained with the anti-FLAG mAb (left panels). Cells overexpressing JAM M/C but not JAM M/CΔ9 lack the junctional staining of ASIP at cell–cell borders (right panels). Based on ASIP localization at cell junctions, cells were classified as complete (complete loss of ASIP, black bars), partial (partial loss, middle grey bars) or intact (intact ASIP staining, dark grey bars). Cells with aberrant shape are represented by light grey bars. Statistical data represent mean values of three independent experiments with a minimum of 200 cells counted for each sample. (C) Cells overexpressing JAM M/C also lack aPKCλ as well as ZO-1 but not β-catenin staining at cell–cell junctions. Note that ZO-1 often forms aggregates in cells expressing JAM M/C but not JAM M/CΔ9. Bars, 15 µm.
Overexpression of dominant-negative mutants of JAM affects the maturation of tight junctions
As mentioned above, the effect of JAM M/C overexpression on the distribution of ASIP, ZO-1 and aPKCλ was less pronounced when cells had reached confluent density. This suggested that JAM M/C can only compete with endogenous JAM for ASIP binding in less polarized cells. To address this possibility, we disrupted cell polarity of JAM M/C-expressing cells in a confluent MDCK II monolayer by replacing regular medium with low Ca2+ medium, and examined the restoration of ASIP distribution after switching back to regular medium (Ca2+ switch). In wild-type MDCK II cells (data not shown) or MDCK II cells overexpressing JAM M/CΔ9 (Figure 8), 80% of the cells already showed normal junctional distribution of ASIP 2 h after the Ca2+ switch. In contrast, under the same conditions >50% of JAM M/C-overexpressing cells showed incomplete junctional staining of ASIP. Interest ingly, 4 h after the Ca2+ switch, this number was reduced to ∼30% with a concomitant increase of cells showing normal ASIP junctional distribution (Figure 8). These findings further confirm that the association of JAM with ASIP occurs early during junction formation and becomes more stable upon junction maturation.
Fig. 8. Overexpression of the C-terminal sequence of JAM retards the restoration of tight junction formation in confluent MDCK II cells after a Ca2+ switch. MDCK II cells were transfected with plasmids encoding the JAM mutants as described in the legend to Figure 7. Having reached confluency, cells were subjected to a Ca2+ switch. Two or four hours after the return to normal Ca2+ concentration, cells were fixed for immunofluorescence analysis. (A) Statistical analysis of cell numbers showing incomplete junctional staining of ASIP. Cells expressing JAM mutants that were identified by anti-Flag immunostaining were categorized into incomplete (black bars) or complete (grey bars) based on their ASIP junctional staining, or aberrant (white bars) based on cell shape. Data represent mean values of three independent experiments with a minimum of 200 cells counted for each sample. (B) Typical immunofluorescent images of cells expressing JAM mutants 2 h after a Ca2+ switch. Cells were stained with anti-Flag and anti-ASIP antibodies as indicated. Bar, 15 µm.
JAM is recruited early into the initial spot-like junctional structures and appears prior to ASIP during junction formation in MTD-1A cells
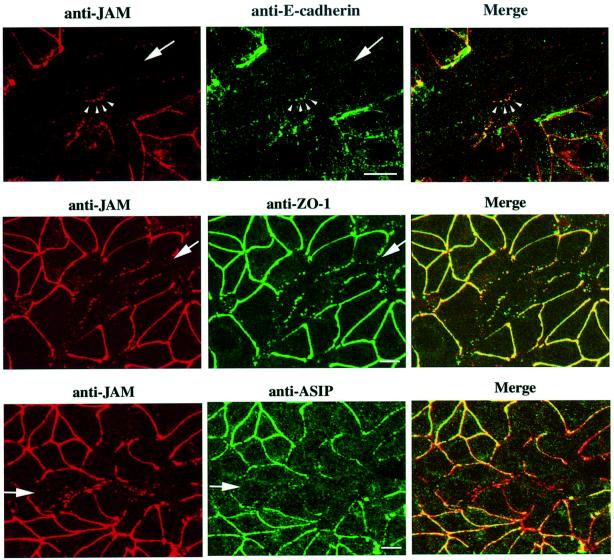
Together with previous findings indicating that JAM can associate with several tight junction proteins, the observation that JAM can recruit ASIP to cell–cell junctions suggests that JAM functions as a regulatory membrane protein that recruits ASIP and possibly other components of the tight junction. As a first step to address this hypothesis, we analysed the order of recruitment of junctional proteins to cell–cell contact regions during wound healing in MTD-1A cells. This cell line has been derived from a mouse mammary tumour and has been shown to be useful in analysing the process of cell polarity formation during wound healing (Yonemura et al., 1995; Ando-Akatsuka et al., 1999). During this process, the initial cell contacts are mediated by thin cellular protrusions characterized by the presence of E-cadherin and α-catenin (Yonemura et al., 1995). ZO-1 is also concentrated at these contact sites, whereas occludin appears at later stages of wound healing (Ando-Akatsuka et al., 1999), making ZO-1 one of the first tight-junction-associated proteins which appears at cell–cell contact sites. As shown in Figure 9 (top panels), JAM co-localizes with E-cadherin at the tips of primordial spot-like protrusions during the initial stage of junction formation. Occludin as well as ASIP and aPKC are not detected at this stage (data not shown). Upon further maturation, JAM co-localizes with ZO-1 along sites of cell–cell contacts (Figure 9, middle panel). Most importantly, ASIP does not co-localize with JAM at sites of immature junctions in the centre of the wound, as can be seen in the image obtained by merging stainings for JAM and ASIP (Figure 9, bottom panel). These results indicate that JAM is recruited to newly formed cell–cell junctions simultaneously with ZO-1 and prior to ASIP, and demonstrate that JAM is indeed available as a transmembrane anchor for the recruitment of ASIP to the junctional complex early during junction formation.
Fig. 9. JAM is recruited into the initial spot-like adherens junctions early during wound healing in MTD-1A cells. Confluent monolayers of MTD-1A cells were scratched manually with an 18G needle, and the wounded regions were allowed to heal for 4–6 h. At initial phases of wound healing, confirmed by phase-contrast microscopy, cells were fixed and processed for double immunostaining with the antibodies indicated at the top of each set of panels. Each photograph represents the projected view of optical sections (0.4 µm) obtained by confocal laser microscopy from the apical to the basal membrane. Large arrows indicate the directions of the healing wounds; small arrowheads indicate the primordial spot-like adherens junction formed at the tip of thin membrane protrusions. JAM co-localizes with E-cadherin and ZO-1 at primordial sites of contact formation where ASIP is absent. Bar, 15 µm.
Discussion
Establishment of cell polarity in epithelial cells of vertebrates as well as of invertebrates depends on several evolutionarily conserved proteins located underneath the plasma membrane in the sub-apical zone of invertebrate epithelia or in tight junctions of vertebrate epithelia (Knust, 2000). The mammalian homologues of four of these proteins, ASIP/PAR-3, PAR-6, aPKC and Cdc42/Rac1 GTPases, form a protein complex that is located at tight junctions of epithelial cells and which also controls cell polarity (Joberty et al., 2000; Johansson et al., 2000; Lin et al., 2000; Qiu et al., 2000; Suzuki et al., 2001). Here we describe the identification of one component of this complex, ASIP/PAR-3, as a protein that specifically interacts with JAM, one of the three transmembrane proteins known to be present at tight junctions. The evidence for this interaction is based on the following: (i) in vitro- and COS-7 cell-generated ASIP/PAR-3 was affinity isolated with a JAM fusion protein; (ii) JAM was co-immunoprecipitated from cell lysates with anti-ASIP/PAR-3 antibodies; (iii) endogenous ASIP was recruited to cell contacts of non-epithelial cells by ectopically expressed JAM; and (iv) regular ASIP expression at epithelial cell contacts was disturbed by overexpression of a dominant-negative mutant of JAM. In summary, our results define JAM as the first transmembrane protein that could serve as an anchor for the ASIP/PAR-3 complex at tight junctions, and point to a possible role of JAM in the regulation of tight junction formation in epithelial cells.
Specific association of ASIP/PAR-3 with JAM
The PDZ-domain-based interaction between JAM and ASIP is highly specific, since we observed binding only to the first of the three PDZ domains of ASIP. More importantly, ASIP/PAR-3 was only found to interact with JAM, not with claudin-1, -4 or -5, and only poorly with occludin. This specificity is remarkable, particularly because both JAM and claudins contain canonical type II PDZ domain binding motifs at the C-terminus (Morita et al., 1999), and both JAM and claudins interact with ZO-1 in a PDZ-domain-dependent manner (Itoh et al., 1999; Ebnet et al., 2000). Claudin-1 and -4 are both expressed in epithelial cells. Although we cannot formally rule out the possibility that ASIP/PAR-3 binds to yet another claudin in other cells, this is not likely since all claudins tested so far behave identically with respect to their binding activities towards ZO-1, ZO-2 and ZO-3 (Itoh et al., 1999). Whether the extremely weak interaction that we observed in vitro between recombinant forms of ASIP/PAR-3 and occludin has any relevance within cells cannot yet be decided. The only other transmembrane protein that would be a potential candidate for a binding partner of ASIP/PAR-3 is ephrin B3, since a partial cDNA clone of ASIP/PAR-3 was isolated from an expression library using a peptide based on the putative PDZ domain binding motif of ephrin B3 (Lin et al., 1999). However, this interaction has not yet been investigated and ephrin B3 has not been reported to be expressed or enriched at epithelial or endothelial tight junctions. Taken together, the evidence indicates that JAM is the first transmembrane protein at tight junctions with the potential to tether ASIP/PAR-3 to tight junctions.
Our in vitro binding studies and the yeast two-hybrid assays clearly suggest that JAM binds to ASIP directly. However, JAM was only co-immunoprecipitated with ASIP from cell lysates if the complex was stabilized by chemical cross-linking, despite the robust stability of the interactions that had been observed in GST pull-down assays. Thus, the in vivo interaction of JAM with ASIP/PAR-3 is destabilized upon cell lysis. It is conceivable that mechanisms that regulate the formation and dissociation of the JAM–ASIP complex in intact cells are influenced upon lysis of intact cells. Understanding such mechanisms might be important for understanding cell contact or junction formation.
JAM can recruit ASIP to sites of cell contact
We found that JAM co-localizes with E-cadherin and ZO-1 at the tips of primordial spot-like protrusions, which appear at a very early stage of cell–cell adhesion. Both E-cadherin and ZO-1 are described to be among the first proteins that appear at the tips of thin cellular protrusions (Yonemura et al., 1995; Ando-Akatsuka et al., 1999) where occludin is absent. ZO-1 gradually separates from E-cadherin during the process of cellular polarization (Ando-Akatsuka et al., 1999), and several lines of evidence suggest that it acts as an anchor to recruit occludin to tight junctions (Van Itallie and Anderson, 1997; Wong and Gumbiner, 1997; Saitou et al., 1998; Mitic et al., 1999). The early appearance of JAM at newly formed cell contacts shows that JAM is available as an anchor for ASIP recruitment. The fact that ASIP is absent at the tips of primordial spot-like protrusions and appears later at sites of cell contact indicates that additional factors may either inhibit the binding of ASIP to JAM at this early stage of cell contact formation or may enhance this interaction at later time points. The alternative explanation, that ASIP appears later than JAM at newly formed cell contacts because it is not expressed at early stages, is unlikely, since we have found that ASIP pre-exists in the cytoplasm in the absence of cell contacts, complexed together with PAR-6 and aPKC (Yamanaka et al., 2001).
In two cell lines, CHO cells and L cell fibroblasts, we could demonstrate that ectopic expression of JAM was sufficient to actively recruit endogenous ASIP/PAR-3 to sites of cell contact. This demonstrates that JAM can trigger recruitment of ASIP to cell contacts even in non-epithelial cells. In combination with our recent finding that contact formation between epithelial cells is accompanied by the recruitment of the pre-existing ternary aPKC–ASIP/PAR-3–PAR-6 complex to cell contacts (Yamanaka et al., 2001), our results suggest that JAM is involved in the recruitment of this complex, which is essential for the formation of tight junctions and cell polarity.
A possible role of JAM in the regulation of tight junction formation
Our studies with a mutated form of JAM lacking the extracellular domain (JAM M/C) allowed us to obtain indirect evidence for a role of JAM in tight junction formation. Overexpressing JAM M/C disrupted the normal localization of the tight junction proteins ASIP, aPKC and ZO-1, while the localization of the adherens junction protein β-catenin at cell contacts was not significantly affected. Since this effect required the presence of the PDZ domain binding motif of JAM, we conclude that the dominant-negative mutant of JAM (which is itself unable to be enriched at tight junctions) mistargeted these proteins and sequestered them from tight junctions. Interestingly, the effect of JAM M/C was observed only in subconfluent but not in fully confluent epithelial cells (Figure 7). Furthermore, in confluent monolayers, the effect could only be detected after Ca2+ depletion in the early phases of a Ca2+-induced regeneration of junctions (Figure 8). This suggests that JAM M/C affects the formation rather than the maintenance of tight junctions. This is similar to the effects we have observed when overexpressing a dominant-negative mutant of aPKC in epithelial cells that disrupts the formation of tight junctions but does not interfere with their stability once they are formed (Suzuki et al., 2001). We assume that the overexpression of JAM M/C interferes with similar processes as the overexpression of aPKC, which would include JAM in the list of proteins important for tight junction formation. Although the interaction of JAM with ASIP is probably central to the observed effects, we cannot exclude a possible involvement of AF-6/afadin or ZO-1.
A role for JAM during the formation of junctions has also been suggested by results obtained with JAM-specific mAbs (Liu et al., 2000). When antibodies against the extracellular domain of JAM were added to epithelial cell cultures during Ca2+ depletion–repletion experiments, the recovery of junctional integrity was strongly reduced and the redistribution of JAM and occludin was disturbed. These observations complement our findings with ectopically expressed JAM M/C, as they indicate that inhibiting the junctional localization of JAM by preventing the homophilic interaction in trans seems to prevent the formation of tight junctions. They also suggest that the homophilic interaction between JAM molecules in trans provide the spatial cues required for the formation of tight junctions after spot-like primordial junctions have been formed by way of E-cadherin-mediated cell adhesion.
Recently, two other members of the Ig-supergene family were identified that are closely related to JAM, and which were named JAM-2 and JAM-3 (Cunningham et al., 2000; Palmeri et al., 2000; Aurrand-Lions et al., 2001). Both proteins are predominantly expressed in endothelial cells of different vascular beds and are absent in epithelial cells (Palmeri et al., 2000; Aurrand-Lions et al., 2001). Interestingly, ectopic expression of JAM-2 in polarized MDCK epithelial cells results in co-localization of JAM-2 with occludin, suggesting that JAM-2 is localized at tight junctions (Aurrand-Lions et al., 2001). The specific functions of different JAM molecules in different cell types still have to be determined. It has been suggested that JAM is involved in the regulation of leukocyte transendothelial migration (Martin-Padura et al., 1998; Del Maschio et al., 1999), and such a role is indirectly supported by the prominent expression of JAM-2 as well as JAM-3/VE-JAM on high endothelial venules (Palmeri et al., 2000; Aurrand-Lions et al., 2001). Our findings suggest that one function of JAM might be the correct targeting of a signalling complex required for the generation of epithelial cell polarity, and thus they point to a more general role of JAM in the regulation of cell polarity formation.
Materials and methods
Cell lines, antibodies and reagents
COS-7 cells, L cells, MDCK II and MTD-1A cells (Yonemura et al., 1995) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine and penicillin/streptomycin (Life Technologies, Germany).
The mAb directed against murine JAM (H2O2-106-7-4) has been described (Malergue et al., 1998). Rabbit polyclonal antibodies (pAbs) against ASIP (C2-3), aPKC (λ1), and JAM were raised in our laboratories (Akimoto et al., 1994; Izumi et al., 1998; Ebnet et al., 2000). Rabbit anti-occludin pAbs, rabbit anti ZO-1 pAbs and mouse anti-ZO-1 mAbs were purchased from Zymed (Zymed Laboratories, Inc., San Francisco, CA). Mouse anti-E-cadherin and anti-β-catenin mAbs were purchased from Transduction Laboratories (Bluegrass-Lexington, KY). Mouse anti-Flag mAb (M2) and mouse anti-T7 tag mAb were purchased from Sigma (Deisenhofen, Germany) and Calbiochem-Novabiochem (Schwalbach, Germany), respectively. Secondary antibodies were purchased from Dianova (Hamburg, Germany).
Expression vectors
JAM bait vectors for expression in yeast, as well as GST–JAM expression vectors, are described elsewhere (Ebnet et al., 2000). Expression vectors encoding GST–claudin-1 (amino acids 188–211), GST–claudin-4 (amino acids 188–210), GST–claudin-5 (amino acids 188–218) and GST– occludin (amino acids 265–521) were generated in pGEX-4T-1 or pGEX-6P-2. Eukaryotic expression vectors encoding full-length ASIP or truncation mutants of ASIP comprising amino acids 583–1337 (ASIP583) or acids 258–936 (ASIP258) have been described (Izumi et al., 1998). For in vitro transcription using T3 polymerase, full-length ASIP was subcloned into pCMV-Tag3 (Stratagene Europe, Amsterdam, The Netherlands). Individual PDZ domains of ASIP (PDZ domain 1, amino acids 256–370; PDZ domain 2, amino acids 448–567; PDZ domain 3, amino acids 561–717) or PDZ domains 1–3 (amino acids 259–717) were cloned in pKE081 (Ebnet et al., 2000). To generate mutant PDZ domain constructs of ASIP, individual PDZ domains of ASIP were replaced by the PDZ domain present in the secreted form of murine IL-16 (amino acids 520–613 of pro-IL-16) (Keane et al., 1998) and cloned into pCMV-Tag3 (Stratagene). N-terminal FLAG-tagged JAM constructs were generated by cloning full-length JAM (amino acids 25–300), JAM M/C (amino acids 231–300), lacking the extracellular domain, or JAM M/CΔ9 (amino acids 231–291), lacking the extracellular domain and the PDZ domain binding motif, in frame with the pre-pro-trypsin leader sequence in pFLAG-CMV1 (Sigma).
Yeast two-hybrid screen and in vitro binding assays
Yeast two-hybrid screening experiments and in vitro binding assays were carried out as described previously (Ebnet et al., 2000).
Transfections
For transient transfection, COS-7 cells were grown to a density of ∼80% confluency and transfected using GeneJammer transfection reagent according to the manufacturer’s instructions (Stratagene). For expression of JAM mutants in MDCK II cells, cells seeded on coverslips were transfected with 1 µg/ml expression plasmids encoding JAM M/C or JAM M/CΔ9 using Lipofectamine Reagent (Gibco BRL), according to the manufacturer’s instructions. When subjected to a Ca2+ switch, the cells were incubated in low Ca2+ (LC) medium containing 5% FCS and 3 µM Ca2+ for 2 h, and then switched back to normal Ca2+ growth medium (NC). Wound healing assays were performed as described (Ando-Akatsuka et al., 1999). For stable transfections, 1 × 107 L cells were transfected with 20 µg of full-length JAM expression vector (pFLAG- CMV-1) and 3 µg of hygromycin-resistance vector (pPGKHyg) by electroporation (0.25 kV, 950 µF), and then cultured in the presence of 900 µg/ml hygromycin B (Calbiochem). Alternatively, 1 × 107 CHO cells were transfected with full-length JAM expression vector (pEF6; Invitrogen) and cultured in the presence of 7 µg/ml blasticidin (Invitrogen). Positive clones were identified by flow cytometry and immunofluorescence analysis.
Immunoprecipitation and western blot analysis
MDCK II cells cultured on 10 cm dishes were transfected with 6 µg of expression vectors encoding Flag-JAM or Flag-JAMΔ9 using Lipofectamine Reagent. Ca2+ switch was performed essentially as described (Suzuki et al., 2001). Eighteen hours after transfection, the medium was replaced with LC medium. After cells had reached ∼80% confluency, LC medium was replaced with regular medium and cells were harvested at appropriate times. Cells were lysed with 500 µl of lysis buffer [50 mM HEPES–NaOH pH 7.5, 1% NP-40, 0.5% deoxycholate, 1 mM EDTA, 1 mM NaF, 1 mM Na3VO4, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM dithiobis (succinimidylpropionate) (DSP)] on ice for 2 h. The cross-linking reaction was stopped by adding 1 M Tris–HCl pH 7.5 to 10 mM final concentration, and NaCl and SDS were added to 150 mM and 0.1% final concentration, respectively. Cell lysates were incubated with affinity-purified anti-ASIP antibodies coupled to Protein G–Sepharose (Amersham Pharmacia Biotech). Immunocomplexes were washed four times with lysis buffer and eluted with SDS sample buffer supplemented with β-mercaptoethanol to 6.5% (v/v). Western blot analysis was performed as described previously (Suzuki et al., 2001).
Immunocytochemistry
Cells grown on coverslips were washed twice with phosphate-buffered saline (PBS) containing 0.9 mM CaCl2 and 0.49 mM MgCl2, and fixed with 2% paraformaldehyde in PBS for 15 min at room temperature. Cells were then permeabilized with PBS containing 0.5% (v/v) Triton X-100 for 10 min and blocked in PBS containing 10% FCS for 1 h at room temperature. Antibody incubations were performed at 37°C for 45 min in buffer containing 10 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.01% (v/v) Tween-20 and 0.1% (w/v) bovine serum albumin. The secondary antibodies used were Alexa488- or Cy-2-conjugated goat anti-rabbit or anti-mouse IgG (Molecular Probes Inc., Eugene, OR, and Dianova), Cy3-conjugated goat anti-mouse, anti-rabbit and anti-rat IgG (Amersham Corp., Arlington Heights, IL, and Dianova). Coverslips were mounted using Vectashield (Vector Laboratories, Burlingame, CA) and analysed by fluorescence microscopy (Axioskop; Zeiss, Germany) or confocal microscopy (µRadiance; Bio-Rad Laboratories, Hercules, CA).
Acknowledgments
Acknowledgements
We thank Dr Michel Aurrand-Lions (Centre Médical Universitaire, Geneva, Switzerland) and Dr Philippe Naquet (INSERM-CNRS, Marseille, France) for providing anti-JAM mAbs, and Dr Jürgen Behrens (Max-Delbrück-Center for Molecular Medicine, Berlin, Germany) for providing reagents and help with the yeast two-hybrid experiments. We would also like to thank Dr Elisabetta Dejana (Istituto Mario Negri, Milan, Italy) for providing us with the JAM cDNA. This work was supported by grants from the Deutsche Forschungs gemeinschaft (K.E. and D.V., VE186/2-2), and from the Japan Society for the Promotion of Science (S.O.).
References
- Akimoto K., Mizuno,K., Osada,S., Hirai,S., Tanuma,S., Suzuki,K. and Ohno,S. (1994) A new member of the third class in the protein kinase C family, PKC λ, expressed dominantly in an undifferentiated mouse embryonal carcinoma cell line and also in many tissues and cells. J. Biol. Chem., 269, 12677–12683. [PubMed] [Google Scholar]
- Ando-Akatsuka Y., Yonemura,S., Itoh,M., Furuse,M. and Tsukita,S. (1999) Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J. Cell Physiol., 179, 115–125. [DOI] [PubMed] [Google Scholar]
- Aurrand-Lions M., Duncan,L., Ballestrem,C. and Imhof,B.A. (2001) JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J. Biol. Chem., 276, 2733–2741. [DOI] [PubMed] [Google Scholar]
- Bazzoni G., Martinez-Estrada,O.M., Orsenigo,F., Cordenonsi,M., Citi,S. and Dejana,E. (2000) Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin and occludin. J. Biol. Chem., 275, 20520–20526. [DOI] [PubMed] [Google Scholar]
- Bowerman B. and Shelton,C.A. (1999) Cell polarity in the early Caenorhabditis elegans embryo. Curr. Opin. Genet. Dev., 9, 390–395. [DOI] [PubMed] [Google Scholar]
- Cunningham S.A., Arrate,M.P., Rodriguez,J.M., Bjercke,R.J., Vanderslice,P., Morris,A.P. and Brock,T.A. (2000) A novel protein with homology to the Junctional Adhesion Molecule: characterization of leukocyte interactions. J. Biol. Chem., 275, 34750–34756. [DOI] [PubMed] [Google Scholar]
- Del Maschio A. et al. (1999) Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to Junctional Adhesion Molecule (JAM). J. Exp. Med., 190, 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragsten P.R., Blumenthal,R. and Handler,J.S. (1981) Membrane asymmetry in epithelia: is the tight junction a barrier to diffusion in the plasma membrane? Nature, 294, 718–722. [DOI] [PubMed] [Google Scholar]
- Ebnet K., Schulz,C.U., Meyer zu Brickwedde,M.K., Pendl,G.G. and Vestweber,D. (2000) Junctional Adhesion Molecule (JAM) interacts with the PDZ domain containing proteins AF-6 and ZO-1. J. Biol. Chem., 275, 27979–27988. [DOI] [PubMed] [Google Scholar]
- Fanning A.S., Jameson,B.J., Jesaitis,L.A. and Anderson,J.M. (1998) The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem., 273, 29745–29753. [DOI] [PubMed] [Google Scholar]
- Furuse M., Hirase,T., Itoh,M., Nagafuchi,A., Yonemura,S. and Tsukita,S. (1993) Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol., 123, 1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Fujita,K., Hiiragi,T., Fujimoto,K. and Tsukita,S. (1998) Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol., 141, 1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins J., Gu,L., Wittchen,E.S., Hibbard,J. and Stevenson,B.R. (1998) ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J. Cell Biol., 141, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg S.M., Sternglanz,R., Cheng,P.F. and Weintraub,H. (1995) Identification of a new family of tissue-specific basic helix–loop–helix proteins with a two-hybrid system. Mol. Cell. Biol., 15, 3813–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T.J. and Kemphues,K.J. (1999) PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development, 126, 127–135. [DOI] [PubMed] [Google Scholar]
- Itoh M., Furuse,M., Morita,K., Kubota,K., Saitou,M. and Tsukita,S. (1999) Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2 and ZO-3, with the COOH termini of claudins. J. Cell Biol., 147, 1351–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y., Hirose,T., Tamai,Y., Hirai,S., Nagashima,Y., Fujimoto,T., Tabuse,Y., Kemphues,K.J. and Ohno,S. (1998) An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J. Cell Biol., 143, 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty G., Petersen,C., Gao,L. and Macara,I.G. (2000) The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nature Cell Biol., 2, 531–539. [DOI] [PubMed] [Google Scholar]
- Johansson A., Driessens,M. and Aspenstrom,P. (2000) The mammalian homologue of the Caenorhabditis elegans polarity protein PAR-6 is a binding partner for the Rho GTPases cdc42 and rac1. J. Cell Sci., 113, 3267–3275. [DOI] [PubMed] [Google Scholar]
- Keane J. et al. (1998) Conservation of structure and function between human and murine IL-16. J. Immunol., 160, 5945–5954. [PubMed] [Google Scholar]
- Knust E. (2000) Control of epithelial cell shape and polarity. Curr. Opin. Genet. Dev., 10, 471–475. [DOI] [PubMed] [Google Scholar]
- Lin D., Gish,G.D., Songyang,Z. and Pawson,T. (1999) The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J. Biol. Chem., 274, 3726–3733. [DOI] [PubMed] [Google Scholar]
- Lin D., Edwards,A.S., Fawcett,J.P., Mbamalu,G., Scott,J.D. and Pawson,T. (2000) A mammalian PAR-3–PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nature Cell Biol., 2, 540–547. [DOI] [PubMed] [Google Scholar]
- Liu Y., Nusrat,A., Schnell,F.J., Reaves,T.A., Walsh,S., Pochet,M. and Parkos,C.A. (2000) Human junction adhesion molecule regulates tight junction resealing in epithelia. J. Cell Sci., 113, 2363–2374. [DOI] [PubMed] [Google Scholar]
- Malergue F., Galland,F., Martin,F., Mansuelle,P., Aurrand-Lions,M. and Naquet,P. (1998) A novel immunoglobulin superfamily junctional molecule expressed by antigen presenting cells, endothelial cells and platelets. Mol. Immunol., 35, 1111–1119. [DOI] [PubMed] [Google Scholar]
- Martin-Padura I. et al. (1998) Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol., 142, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitic L.L., Schneeberger,E.E., Fanning,A.S. and Anderson,J.M. (1999) Connexin–occludin chimeras containing the ZO-binding domain of occludin localize at MDCK tight junctions and NRK cell contacts. J. Cell Biol., 146, 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Furuse,M., Fujimoto,K. and Tsukita,S. (1999) Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl Acad. Sci. USA, 96, 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlhahn P. et al. (1998) Structure of interleukin 16 resembles a PDZ domain with an occluded peptide binding site. Nature Struct. Biol., 5, 682–686. [DOI] [PubMed] [Google Scholar]
- Palmeri D., van Zante,A., Huang,C.C., Hemmerich,S. and Rosen,S.D. (2000) Vascular endothelial junction-associated molecule, a novel member of the immunoglobulin superfamily, is localized to intercellular boundaries of endothelial cells. J. Biol. Chem., 275, 19139–19145. [DOI] [PubMed] [Google Scholar]
- Petronczki M. and Knoblich,J.A. (2001) DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nature Cell Biol., 3, 43–49. [DOI] [PubMed] [Google Scholar]
- Qiu R.G., Abo,A. and Martin,G.S. (2000) A human homolog of the C. elegans polarity determinant PAR-6 links Rac and Cdc42 to PKC ζ signaling and cell transformation. Curr. Biol., 10, 697–707. [DOI] [PubMed] [Google Scholar]
- Saitou M., Fujimoto,K., Doi,Y., Itoh,M., Fujimoto,T., Furuse,M., Takano,H., Noda,T. and Tsukita,S. (1998) Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol., 141, 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A. et al. (2001) Atypical protein kinase C is involved in the evolutionarily conserved PAR protein complex and plays a critical role in establishing epithelia-specific junctional structures. J. Cell Biol., 152, 1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuse Y., Izumi,Y., Piano,F., Kemphues,K.J., Miwa,J. and Ohno,S. (1998) Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development, 125, 3607–3614. [DOI] [PubMed] [Google Scholar]
- Tsukita S. and Furuse,M. (2000) Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J. Cell Biol., 149, 13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie C.M. and Anderson,J.M. (1997) Occludin confers adhesiveness when expressed in fibroblasts. J. Cell Sci., 110, 1113–1121. [DOI] [PubMed] [Google Scholar]
- Watts J.L., Etemad-Moghadam,B., Guo,S., Boyd,L., Draper,B.W., Mello,C.C., Priess,J.R. and Kemphues,K.J. (1996) PAR-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3. Development, 122, 3133–3140. [DOI] [PubMed] [Google Scholar]
- Wodarz A., Ramrath,A., Grimm,A. and Knust,E. (2000) Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol., 150, 1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V. and Gumbiner,B.M. (1997) A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J. Cell Biol., 136, 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T. et al. (2001) PAR-6 regulates aPKC activity in a novel way and mediates cell–cell contact-induced formation of the epithelial junctional complex. Genes Cells, in press. [DOI] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff,K.K., Hansen,M.D. and Nelson,W.J. (1999) Cell polarity: Versatile scaffolds keep things in place. Curr. Biol., 9, R515–R517. [DOI] [PubMed] [Google Scholar]
- Yonemura S., Itoh,M., Nagafuchi,A. and Tsukita,S. (1995) Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J. Cell Sci., 108, 127–142. [DOI] [PubMed] [Google Scholar]