Abstract
G-protein-coupled receptor kinase 2 (GRK2) plays a key role in the regulation of G-protein-coupled receptors (GPCRs). GRK2 expression is altered in several pathological conditions, but the molecular mechanisms that modulate GRK2 cellular levels are largely unknown. We recently have described that GRK2 is degraded rapidly by the proteasome pathway. This process is enhanced by GPCR stimulation and is severely impaired in a GRK2 mutant that lacks kinase activity (GRK2-K220R). In this report, we find that β-arrestin function and Src-mediated phosphorylation of GRK2 are critically involved in GRK2 proteolysis. Overexpression of β-arrestin triggers GRK2-K220R degradation based on its ability to recruit c-Src, since this effect is not observed with β-arrestin mutants that display an impaired c-Src interaction. The presence of an inactive c-Src mutant or of tyrosine kinase inhibitors strongly inhibits co-transfected or endogenous GRK2 turnover, respectively, and a GRK2 mutant with impaired phosphorylation by c-Src shows a markedly retarded degradation. This pathway for the modulation of GRK2 protein stability puts forward a new feedback mechanism for regulating GRK2 levels and GPCR signaling.
Keywords: β-arrestin/c-Src/degradation/GRK2/signal transduction
Introduction
G-protein-coupled receptors (GPCRs) are key regulators of cellular function, growth and differentiation (Luttrell et al., 1999a; Gutkind, 2000). In addition to promoting heterotrimeric G-protein activation, agonist stimulation of GPCRs also triggers receptor phosphorylation by a family of specific G-protein-coupled receptor kinases (GRKs). This phosphorylation event allows the recruitment of cytosolic proteins known as β-arrestins to the receptor signaling complex. β-arrestin binding inhibits interaction of G-proteins with the receptor, thus leading to a loss of receptor signaling to effector proteins, a process termed desensitization. GRK2 is a ubiquitous member of the GRK family, which has an important role in the modulation of a variety of GPCRs (Carman and Benovic, 1998; Pitcher et al., 1998).
Recent evidence indicates that GRK2 and β-arrestins play additional roles in GPCR regulation and signaling. The agonist-induced interaction of β-arrestin with the phosphorylated receptor allows the β-arrestin-mediated recruitment of clathrin, thus triggering receptor internalization, dephosphorylation and recycling (reviewed in Carman and Benovic, 1998; Pitcher et al., 1998). In addition, β-arrestin can interact with the cytosolic tyrosine kinase c-Src and promote its recruitment to the receptor signaling complex (Luttrell et al., 1999b), thus suggesting a key role for GRK2 and β-arrestin function in modulation of mitogen-activated protein kinase (MAPK) cascades by GPCRs. Finally, recent data indicate that GRK2 is also able to phosphorylate non-receptor substrates, such as tubulin or phosducin (Pitcher et al., 1998; Ruiz-Gómez et al., 2000).
GRK2 activity and subcellular localization appear to be tightly controlled by different mechanisms, including interaction with activated receptors, Gβγ subunits of G-proteins, lipids, anchoring proteins, caveolin or calmodulin (reviewed in Pitcher et al., 1998; Ruiz-Gómez et al., 2000). Phosphorylation by other kinases such as PKC, c-Src or MAPK can also promote changes in GRK2 activity (Winstel et al., 1996; Sarnago et al., 1999; Elorza et al., 2000). However, very little is known about the mechanisms that modulate GRK2 cellular levels. This issue is physiologically relevant, since increased or decreased GRK2 expression has been reported in several pathological conditions, such as in congestive heart failure patients (Ungerer et al., 1993), cardiac hypertrophy experimental models (Choi et al., 1997; Rockman et al., 1998), hypertension (Gros et al., 1997), hypothyroidism (Penela et al., 2001) or rheumatoid arthritis (Lombardi et al., 1999, 2001). In this context, we have recently reported that GRK2 is degraded rapidly by the proteasome pathway (Penela et al., 1998). Interestingly, kinase turnover is enhanced upon β2-adrenergic receptor stimulation, thus suggesting a functional relationship between GRK2 activation and its degradation. Consistently, a GRK2 point mutant that lacks kinase activity (GRK2- K220R) displayed a strongly impaired degradation, even under conditions of receptor activation (Penela et al., 1998). In this report, we show that recruitment of β-arrestin and Src-mediated GRK2 phosphorylation on tyrosine residues are critical signals that trigger GRK2 degradation, putting forward a new level of phosphorylation-dependent regulation of GRK2.
Results
As a first step to understand the molecular mechanisms of GRK2 degradation, we explored why the GRK2-K220R point mutant displayed a very retarded degradation compared with the wild-type kinase. GRK2-K220R is a dominant-negative GRK2 mutant which is able to block receptor internalization and desensitization, lacks kinase activity and displays lower affinity towards Gβγ subunits (Kong et al., 1994). Therefore, the impaired degradation of the K220R mutant could be ascribed to its lack of activity or to an altered membrane anchoring. In order to discriminate between these possibilities, we generated geranyl-geranylated forms of both wild-type GRK2 (GRK2-gg) and its mutant (GRK2-K220R gg), which display a similar targeting to the plasma membrane (Inglese et al., 1992, and data not shown), and analyzed their degradation pattern by pulse–chase experiments in transfected HEK-293 cells. Figure 1 shows that the levels of GRK2-K220R gg protein remain unaltered after 3 h of chase, as previously observed with the non-isoprenylated K220R mutant (Penela et al., 1998), whereas wild-type GRK2-gg protein levels rapidly decline, at a rate even higher than that of the unmodified GRK2 (36% of protein remaining after 1 h of chase compared with 54% of the wild-type kinase; see Figure 1). Interestingly, GRK2-gg also has higher kinase activity than the wild-type GRK2 (Inglese et al., 1992, and data not shown). Taken together, our results strongly suggest that GRK2 activity, and not membrane localization per se, is required for its degradation.
Fig. 1. Effect of isoprenylation on GRK2 and GRK2-K220R degradation. HEK-293 cells were transiently transfected with GRK2, or with the geranyl-geranylated forms of the wild-type kinase (GRK2-gg) or the inactive K220R mutant (GRK2-K220R gg), and the turnover of these proteins was assessed by pulse–chase experiments as described in Materials and methods. 35S-labeled proteins immunoprecipitated with the anti-GRK2 antibody AbFP1 were resolved by SDS–PAGE followed by fluorography and densitometry. Data are the mean ± SE of three independent experiments performed in triplicate. A representative gel fluorograph is shown below.
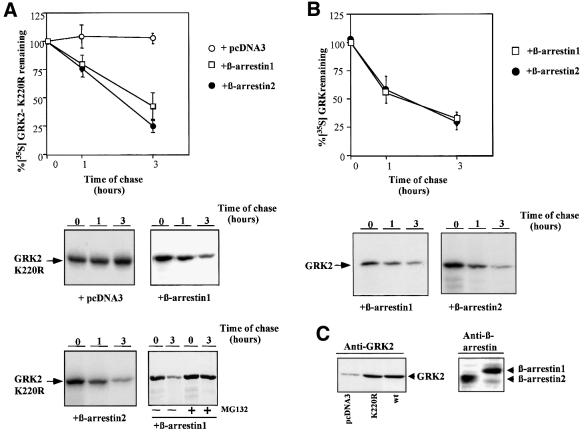
A key functional consequence of GRK2 activity is the recruitment of β-arrestin to the GRK2-phosphorylated receptor. Overexpression of β-arrestin is known to facilitate its phosphorylation-independent interaction with the receptor, in a process enhanced by agonist occupation (Ferguson et al., 1996; Gurevich et al., 1997). Therefore, we explored whether co-transfection of β-arrestin with GRK2-K220R and β2-adrenergic receptor (β2AR) had any effect on the basal degradation of this mutant. Figure 2A shows that overexpression of β-arrestin-1 or β-arrestin-2 to similar levels (∼5-fold over endogenous, right panel in Figure 2C) causes a dramatic change in GRK2-K220R stability, promoting an active degradation pattern with an estimated half-life of ∼150 and 120 min, respectively. This β-arrestin-induced GRK2-K220R proteolysis is blocked in the presence of the proteasome inhibitor MG132 (Figure 2A, lower panel). Upon β2AR stimulation, a modest but reproducible increase in the turnover of GRK2-K220R in the presence of β-arrestin-1 is noted (62 ± 3% of [35S]GRK2-K220R remaining after 1 h in the presence of isoproterenol versus 80 ± 7% in its absence), whereas receptor activation was without effect on the proteolysis of GRK2-K220R alone (Penela et al., 1998). In conclusion, β-arrestin overexpression is able to ‘switch on’ GRK2-K220R degradation, although the rate of protein decay is still diminished with respect to the wild-type kinase by a factor of ∼2-fold. It is worth noting that, at similar levels of GRK2 and K220R mutant expression (10 ± 3-fold over basal, see left panel in Figure 2C), β-arrestin-1 or β-arrestin-2 did not promote significant changes in the basal (compare data in Figure 2B with GRK2 proteolysis in the absence of β-arrestin overexpression in Figure 1) or the agonist-stimulated (data not shown) degradation pattern of wild-type GRK2, indicating that endogenous β-arrestin levels are sufficient to allow basal and receptor-stimulated wild-type GRK2 degradation.
Fig. 2. β-arrestin-1 or β-arrestin-2 overexpression promotes degradation of GRK2-K220R. (A) HEK-293 cells were transiently transfected with GRK2-K220R, β2AR and either β-arrestin-1, β-arrestin-2 or empty vector (pCDNA3), and GRK2-K220R turnover was determined by pulse–chase experiments as detailed in Materials and methods and in Figure 1. Data are the mean ± SE of four or five independent experiments performed in duplicate. A representative gel fluorograph is shown below. The right lower panel shows a similar experiment performed in the presence of the proteasome inhibitor MG132. (B) Similar experiments were performed in cells transiently transfected with wild-type GRK2, β2AR and β-arrestin-1 or β-arrestin-2. Data are the mean ± SE from three or four experiments performed in duplicate, and representative fluorographs are shown below. (C) Lysates from cells transfected with wild-type GRK2 (wt), GRK2-K220R, β-arrestin 1, β-arrestin-2 or empty vector as indicated were subjected to immunoblot analysis with specific GRK2 and β-arrestin antibodies as detailed in Materials and methods.
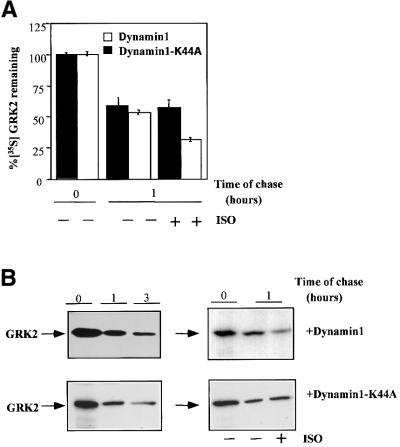
Since GRK2 degradation appeared to be dependent on β-arrestin function, we next explored which of β-arrestin’s cellular roles was relevant for this process. β-arrestins are able to bind clathrin (Goodman et al., 1996), thus triggering the internalization of receptor complexes by the endocytic pathway. Therefore, we tested whether blocking GPCR endocytosis had any effect on GRK2 degradation in HEK-293 cells stably expressing β2AR and GRK2. The presence of hypertonic sucrose, a condition that blocks receptor endocytosis by interfering with interactions between clathrin and adaptor proteins (Hansen et al., 1993), impairs β2AR internalization but does not have a significant effect on GRK2 turnover, in both the absence and presence of isoproterenol stimulation (data not shown). We performed similar experiments in cells transiently co-transfected with β2AR, GRK2 and dynamin-1 or dynamin-1 K44A, a dominant-negative mutant that blocks β2AR endocytosis (data not shown) as a result of impaired fission of the clathrin-coated vesicles (Damke et al., 1994). The presence of either dynamin or its mutant did not affect basal GRK2 degradation (Figure 3), consistent with the notion that internalization per se is not critical for GRK2 proteolysis. It should be noted, however, that the overexpression of the dynamin K44A mutant abrogated the agonist-induced increase in GRK2 degradation, whereas wild-type dynamin was without effect (Figure 3A and B).
Fig. 3. Effect of inhibition of receptor internalization on GRK2 degradation. HEK-293 cells were transiently transfected with GRK2, β2AR and wild-type dynamin-1 or a dynamin K44A mutant, and GRK2 degradation was assessed as in Figure 1 under basal conditions [–ISO and representative gels on the left in (B)] and upon isoproterenol stimulation for 1 h (+ISO). Data in (A) are the mean ± SE of 4–6 independent experiments performed in duplicate. Representative gel fluorographs are shown in (B).
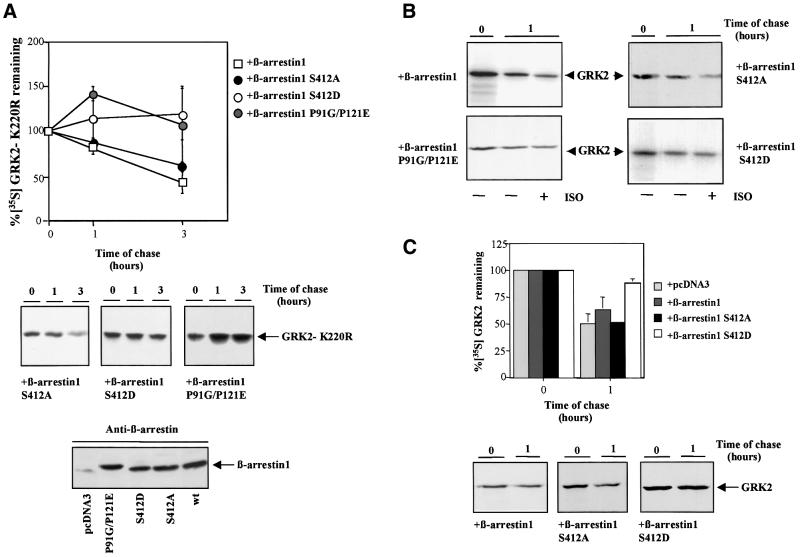
We next explored the involvement of other signaling pathways downstream of β-arrestin function. In this regard, recent reports have shown that β-arrestin can recruit the tyrosine kinase c-Src to the GPCR signaling complex (Luttrell et al., 1999b). In order to test if β-arrestin-mediated Src recruitment was involved in the GRK2 degradation process, we investigated whether β-arrestin mutants differing in their ability to interact with c-Src were able to promote the degradation of the GRK2-K220R mutant. As shown in Figure 4A, the β-arrestin mutants S412D and P91G/P121E, which display an impaired interaction with c-Src (Luttrell et al., 1999b), are unable to promote GRK2-K220R degradation. Conversely, when expressed at similar levels (see lower panel in Figure 4A), the β-arrestin-S412A mutant, which binds to c-Src efficiently (Luttrell et al., 1999b), did elicit GRK2-K220R proteolysis with a pattern similar to that of wild-type β-arrestin-1. These data strongly suggest that β-arrestin-mediated Src recruitment is required for GRK2 turnover. We also tested the effect of the β-arrestin mutants on wild-type GRK2 protein stability in two cells lines with different levels of endogenous β-arrestin expression. In HEK-293 cells, neither of these mutants nor wild-type β-arrestin-1 changed the pattern of basal GRK2 degradation. However, overexpression of the S412D or the P91G/P121E mutants impaired the isoproterenol-induced increase in GRK2 turnover, whereas this component was not modified by similar levels of expression of wild-type β-arrestin or the S412A mutant (Figure 4B). In Cos-7 cells, which display reduced endogenous β-arrestin levels compared with HEK-293 cells (Ménard et al., 1997), the expression of the S412D mutant (but not that of wild-type β-arrestin or the S412A mutant) clearly inhibited the rate of basal GRK2 degradation (Figure 4C). These data are consistent with a role for β-arrestin in wild-type GRK2 degradation. Under our experimental conditions, the dominant-negative effect of the Src binding-defective β-arrestin mutants is detected more clearly in cells with lower levels of endogenous β-arrestin expression or upon GPCR stimulation.
Fig. 4. Effect of β-arrestin-1 mutants on GRK2-K220R degradation. (A) HEK-293 cells were transiently transfected with GRK2-K220R, β2AR and wild-type β-arrestin-1 or the indicated β-arrestin-1 mutants, and the turnover of GRK2-K220R was analyzed as in Figure 2. Data are the mean ± SE of at least four independent experiments performed in duplicate. Representative gel fluorographs are shown below. Cell lysates were also subjected to immunoblot analysis with anti-β-arrestin antibodies as detailed in Materials and methods to assess the expression of the different β-arrestin constructs (lower panel). (B) Similar pulse–chase experiments were performed in cells transfected with wild-type GRK2, β2AR and the indicated β-arrestin-1 constructs, incubated for 1 h in the presence (+) or absence (–) of 10 µM isoproterenol (ISO). The gel fluorographs are representative of three independent experiments. (C) Cos-7 cells were transiently transfected with GRK2, β2AR and β-arrestin-1 mutants or empty vector as indicated, and GRK2 turnover under basal conditions analyzed as in previous figures. Data are the mean ± SE of three independent experiments. Representative gel fluorographs are shown below.
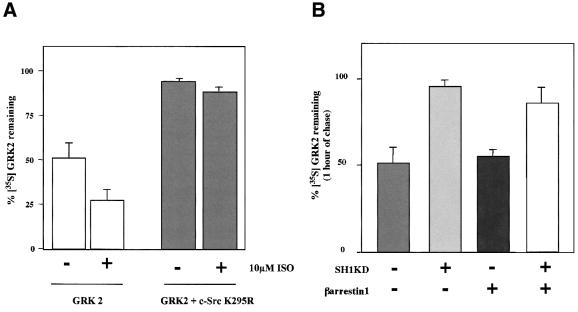
In order to evaluate whether Src activity was indeed playing a role in GRK2 degradation, HEK-293 cells were co-transfected with GRK2 and β2AR in the presence or absence of c-Src K295R, a catalytically inactive mutant of this tyrosine kinase. Comparison of GRK2 degradation after 1 h of chase under the different conditions shown in Figure 5 indicates that inhibition of Src activity leads to a severe impairment of GRK2 turnover under basal conditions (94 ± 2.5% of 35S-labeled kinase remaining) and upon isoproterenol-mediated β2AR stimulation (88 ± 3.5% of labeled protein remaining). Interestingly, the expression of a catalytically inactive (kinase-dead) mutant of the kinase domain of c-Src (SH1-KD), that specifically blocks c-Src–β-arrestin-1 interaction (Miller et al., 2000), strongly impairs GRK2 degradation in both the absence and presence of co-expressed β-arrestin (Figure 5B). These results provide additional evidence for the role of β-arrestin in GRK2 proteolysis and strongly suggest that Src tyrosine kinase activity is a key factor in the modulation of GRK2 stability.
Fig. 5. The inactive c-Src K295R mutant or the specific blockade of β-arrestin–c-Src interaction strongly inhibit GRK2 degradation. (A) HEK-293 cells were transfected with GRK2, β2AR and c-Src K295R or empty vector, and pulse–chase experiments were performed in the absence (–) or presence (+) of isoproterenol (ISO) as indicated, followed by analysis of the remaining 35S-labeled GRK2 as in previous figures. Data are the mean ± SE of three independent experiments. (B) Similar experiments were performed in cells transfected with GRK2, and the indicated combinations of β-arrestin-1 and SH1KD, the kinase-dead mutant of the SH1 catalytic domain of c-Src, that specifically blocks β-arrestin–c-Src interaction (Miller et al., 2000). Data are the mean ± SE of three independent experiments.
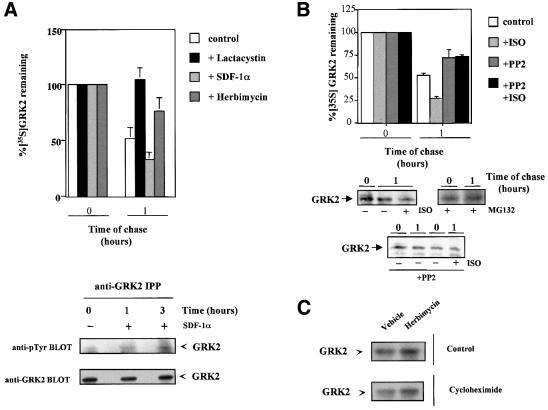
In order to address the potential biological significance of these mechanisms, we investigated the modulation of endogenous GRK2 degradation in two different cell lines. Pulse–chase experiments show the rapid degradation (∼50% proteolysis at 1 h of chase) of GRK2 in either Jurkat T cells or C6 glioma cells (Figures 6A and B, respectively). In both cases, GRK2 proteolysis is strongly decreased by proteasome inhibitors (lactacystin in A or MG132 in B). Activation of endogenous GPCR (CXCR4 chemokine receptors by SDF-1α in Jurkat cells or β-adrenergic receptors by isoproterenol in C6 cells) increases the apparent rate of degradation of endogenous GRK2 (Figure 6A and B). Moreover, the presence of the cytosolic tyrosine kinase inhibitor herbimycin or the c-Src inhibitor PP2 markedly inhibits the degradation of endogenous GRK2 in Jurkat or C6 cells, respectively. Consistently, the presence of a tyrosine kinase inhibitor leads to an increase in steady-state GRK2 expression levels in Jurkat cells, as assessed by immunoblot analysis, both under control conditions and in the presence of cycloheximide to avoid possible interference of transcriptional effects on GRK2 expression (Figure 6C).
Fig. 6. Modulation of endogenous GRK2 degradation in Jurkat and C6 cell lines. (A) The turnover of endogenous GRK2 was assessed by pulse–chase experiments as detailed in Materials and methods in Jurkat T cells chased for 1 h in culture medium (control) or in the presence of the proteasome inhibitor lactacystin, the CXCR4 receptor agonist SDF-1α or the cytosolic tyrosine kinase inhibitor herbimycin as indicated. Data are the mean ± SE of 3–4 independent experiments. In the lower panel, GRK2 immunoprecipitates from cells treated or not with SDF-1α were obtained and subjected to anti-phosphotyrosine analysis as detailed in Materials and methods. After stripping, the presence of GRK2 was analyzed in the same gel with the Ab9 polyclonal antibody. (B) Similar turnover experiments were performed in C6 glioma cells chased for the indicated times in culture medium alone or in the presence of the indicated combinations of the β-adrenergic agonist isoproterenol (ISO), the c-Src tyrosine kinase inhibitor PP2 or the proteasome inhibitor MG132. Data are the mean ± SE of 2–3 independent experiments. Representative gel fluorographs are shown below. (C) Effect of inhibition of cytosolic tyrosine kinases on GRK2 expression levels in Jurkat T cells. Cells were incubated with or without cycloheximide in the presence or absence (vehicle) of the tyrosine kinase inhibitor herbimycin, as detailed in Materials and methods, and GRK2 expression levels determined by immunoblot analysis of cell lysates. Data are representative of two independent experiments.
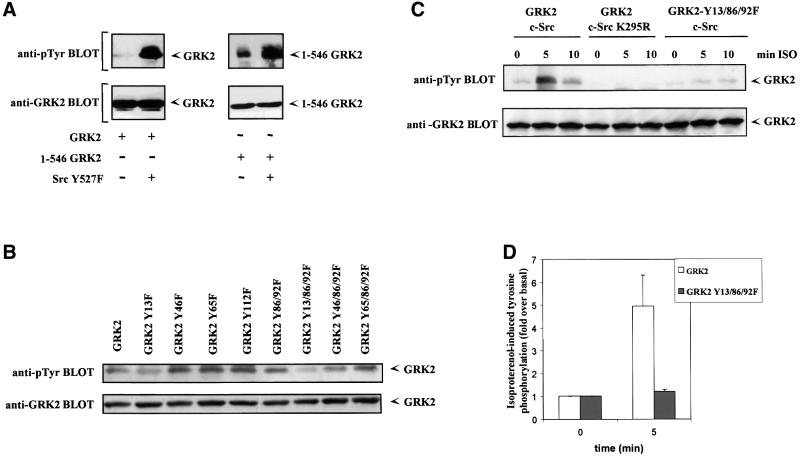
Overall, these results indicate a general role for c-Src or Src-like tyrosine kinases in the modulation of GRK2 degradation in different cell types. This could be a consequence of the direct phosphorylation of GRK2 by c-Src, or an indirect effect caused by c-Src-mediated phosphorylation of other cellular substrates. Interestingly, we have reported previously that GRK2 is a high affinity substrate for c-Src and that agonist stimulation of β2AR leads to a rapid increase in GRK2 phosphorylation on tyrosine residues (Sarnago et al., 1999). Activation of other GPCRs can also promote endogenous GRK2 tyrosine phosphorylation (Figure 6A, lower panel). To address the potential role of GRK2 phosphorylation by Src on its degradation, we set out to localize the phosphorylation sites in order to generate a GRK2 mutant with impaired phosphorylation by this tyrosine kinase. A first approach using a GRK2 deletion construct lacking the last 143 amino acid residues of the C-terminal region of GRK2 (1–546 GRK2) indicated that the latter region is not critical for Src-mediated phosphorylation, since the extent of in situ tyrosine phosphorylation by a co-transfected constitutively active mutant of Src (Src Y527F) is similar to that observed with full-length GRK2 (Figure 7A). The search of the GRK2 sequence for potential consensus tyrosine phosphorylation sites (Zhou et al., 1995) suggested that the phosphorylation sites would be located on the N-terminal domain of GRK2. Therefore, we generated several single and combined tyrosine to phenylalanine mutants at different positions of this GRK2 domain, and tested the level of tyrosine phosphorylation upon co-transfection with the constitutively active c-Src-Y527F mutant. These experiments suggested (Figure 7B) that mutation of residues 86/92 or 13 clearly decreased GRK2 tyrosine phosphorylation, which was particularly low in the triple Y13/86/92F mutant, which was then characterized in more detail. Neither the subcellular localization pattern nor the kinase activity of this mutant towards GPCR (rhodopsin) or soluble substrates (casein) were significantly altered when compared with wild-type GRK2 (data not shown). Interestingly, agonist-induced tyrosine phosphorylation of the Y13/86/92F mutant is blocked. Figure 7C and D shows that in HEK-293 cells transfected with β2AR, stimulation with the β-agonist isoproterenol results in a marked increase in tyrosine phosphorylation of wild-type GRK2, which is maximal (4.9 ± 1.3-fold over non-stimulated control) after 5 min of agonist challenge, similar to previous results of our laboratory in COS-7 cells (Sarnago et al., 1999). Wild-type GRK2 tyrosine phosphorylation in response to GPCR activation is completely blocked upon overexpression of an inactive Src mutant (c-Src K295R, see Figure 6C). On the other hand, the GRK2-Y13/86/92F mutant is very poorly phosphorylated both under basal conditions and upon isoproterenol treatment in the presence of wild-type c-Src (1.23 ± 0.07-fold increase over non-stimulated control at 5 min of incubation, Figure 7C and D), thus indicating that these tyrosine residues are critical for Src-mediated GRK2 phosphorylation.
Fig. 7. Identification of critical tyrosine residues involved in GRK2 phosphorylation by c-Src. (A) Cos-7 cells were transiently transfected with wild-type GRK2 or the GRK2 deletion mutant 1–546, and the constitutively active c-Src Y257F or empty vector as indicated. Immunoprecipitates of GRK2 were analyzed with an anti-phosphotyrosine monoclonal antibody as detailed in Materials and methods (top panels). After stripping, the presence of GRK2 was analyzed in the same gel as in the lower panel of Figure 6A. Gels representative of two independent experiments are shown. (B) Similar experiments were performed in Cos-7 cells transiently transfected with the constitutively active c-Src Y527F mutant and wild-type GRK2 or the indicated tyrosine to phenylalanine mutants of GRK2. Tyrosine phosphorylation of GRK2 (upper panel) was measured by scanner laser densitometry, and the data normalized to the amount of GRK2 protein present in the immunoprecipitates as assessed with a GRK2 antibody (lower panel). A gel representative of two independent experiments is shown. (C) HEK-293 cells transiently transfected with β2AR and the indicated combinations of GRK2, GRK2-Y13/86/92F, wild-type c-Src or an inactive c-Src K295R mutant were stimulated or not (0 min) for the indicated times with 1 µM isoproterenol (ISO) as described in Materials and methods. Tyrosine phosphorylation of GRK2 was determined as in previous panels. Tyrosine phosphorylation levels of GRK2 and the GRK2 Y13/86/92F mutant in the presence of β2AR and wild-type c-Src after 5 min of agonist stimulation were compared in more detail by laser densitometry (D). Non-stimulated controls were taken as the basal condition. Data are the mean ± SE of three independent experiments.
We next tested the degradation pattern of this tyrosine phosphorylation-impaired GRK2 mutant in transfection experiments. Consistent with a key role for Src-mediated phosphorylation in GRK2 turnover, the Y13/86/92F mutant displays an altered degradation, with 84 ± 6% of protein remaining after 1 h of chase, and an estimated half-life >3-fold higher than that obtained for wild-type GRK2 (Figure 8A). Moreover, β2AR activation barely stimulates degradation of the GRK2 mutant (Figure 8B). After 1 h of isoproterenol stimulation, most of GRK2 is proteolyzed (25 ± 3% remaining) whereas 74 ± 3% of the phosphorylation mutant remains unaltered. Taken together, these results clearly indicate that GRK2 phosphorylation by Src is critically involved in GRK2 degradation.
Fig. 8. Impaired degradation of the GRK2 Y13/86/92F tyrosine phosphorylation mutant. HEK-293 cells were transiently transfected with β2AR and GRK2 or GRK2 Y13/86/92F in the absence [(A) and ‘minus’ in (B)] or presence [`plus' in (B)] of isoproterenol (ISO), followed by analysis of the remaining 35S-labeled GRK2 proteins as in Figure 1. Data in all panels are the mean ± SE of three independent experiments performed in duplicate.
Discussion
The data presented provide new insights into the molecular mechanisms modulating GRK2 cellular levels and the functional relationship between GRK2 activation and its degradation. We have found that membrane localization per se is not a limiting step in the impaired degradation process of the inactive GRK2-K220R mutant, and that GRK2 activity appears to be the critical factor leading to GRK2 turnover. In this regard, recent reports have shown that kinase-dead mutants of cytosolic tyrosine kinases (Hakak and Martin, 1999; Harris et al., 1999; Andoniou et al., 2000) or PKCα (Lu et al., 1998) are resistant to degradation, whereas activation of these kinases triggers their rapid ubiquitin-mediated turnover, probably as a result of changes in conformation, phosphorylation or interaction with additional cellular proteins.
The activity of GRK2 promotes the recruitment of β-arrestin to the phosphorylated receptor complex (Carman and Benovic, 1998; Pitcher et al., 1998). Interestingly, we find that overexpression of β-arrestin-1 or β-arrestin-2 can trigger GRK2-K220R degradation. The ability of overexpressed β-arrestins to interact with non-phosphorylated receptors even under basal conditions (Ferguson et al., 1996; Gurevich et al., 1997) would bypass the requirement for GRK2 function, thus resulting in a marked degradation of the GRK2-K220R mutant. Consistently, β-arrestin-2, which displays higher affinity for non-phosphorylated receptors (Gurevich et al., 1995), is slightly more efficient that β-arrestin-1 in the ‘rescue’ of GRK2-K220R degradation. This effect of β-arrestins is similar to that previously reported for the triggering of receptor internalization in the absence of GRK phosphorylation (Ferguson et al., 1996). The fact that the rate of degradation reached is not as rapid as that of wild-type GRK2 could be explained by a less efficient recruitment of β-arrestin and/or the possible facilitatory role of GRK2 phosphorylation of other substrates. In summary, these data suggest that GRK2 activity-dependent recruitment of β-arrestin is involved in GRK2 degradation.
Recent evidence indicates that, besides promoting receptor uncoupling from G-proteins, arrestins function as adaptor molecules that recruit other proteins to the receptor complex. Interaction of β-arrestin with clathrin and related proteins targets the receptor for internalization (Goodman et al., 1996), whereas β-arrestin-mediated recruitment of c-Src appears to be involved in GPCR-dependent dynamin phosphorylation and downstream signaling leading to the activation of MAPK cascades (Ahn et al., 1999; Luttrell et al., 1999a,b). Whereas blocking receptor internalization with sucrose, dynamin K44A or the β-arrestin S412D mutant (Lin et al., 1997) does not alter basal GRK2 turnover in 293 cells, several lines of evidence suggest that β-arrestin-mediated c-Src recruitment and subsequent phosphorylation of GRK2 on tyrosine residues play a key role in GRK2 degradation. First, overexpression of β-arrestin mutants that share an impaired interaction with c-Src (Luttrell et al., 1999b) does not mimic the effect of wild-type β-arrestin-1 in promoting GRK2-K220R degradation. On the other hand, the presence of a specific inhibitor of the β-arrestin–c-Src interaction, the kinase-dead SH1 domain of c-Src (Miller et al., 2000), markedly inhibits wild-type GRK2 proteolysis, and the Src binding-defective β-arrestin mutants retard GRK2 turnover in cells with low levels of endogenous β-arrestin or under conditions of agonist stimulation. Secondly, the presence of a catalytically inactive c-Src mutant strongly inhibits basal and β2AR-stimulated GRK2 degradation. Consistent with these data, the rapid turnover of endogenous GRK2 in Jurkat or C6 cell lines is enhanced upon CXCR4 or β2AR activation, respectively, and kinase degradation is clearly inhibited in the presence of tyrosine kinase inhibitors, leading to increased GRK2 expression levels. Thirdly, a GRK2 mutant (Y13/86/92F), which is very poorly phosphorylated on tyrosine residues by c-Src, displays a retarded proteolysis under both basal and activated conditions.
Our results are consistent with the notion that GRK2-dependent binding of β-arrestin to GPCRs allows the recruitment of c-Src to the receptor signaling complex at the plasma membrane, leading to phosphorylation of GRK2 on tyrosine residues and its targeting for degradation. This model is in agreement with the rapid β-arrestin and c-Src recruitment following β2AR stimulation (Luttrell et al., 1999b), and with the agonist-stimulated phosphorylation of GRK2 by c-Src (Sarnago et al., 1999). Under our basal conditions, β-arrestin recruitment to the plasma membrane would be promoted by the activated state of different endogenous GPCRs and/or by the reported basal activity of overexpressed β2AR (Ruiz-Gómez and Mayor, 1997). In the presence of GPCR agonists, we detect an acceleration of the GRK2 degradation rate (Penela et al., 1998), consistent with a more efficient β-arrestin and c-Src translocation to the receptor complex. Although detailed knowledge of the sequential assembly of these proteins in a multimolecular complex is lacking, and other molecular interactions may participate in c-Src binding to the receptor complex and GRK2 tyrosine phosphorylation (Fan et al., 2001), the proposed model is consistent with the co-immunoprecipitation of β-arrestin and c-Src (Luttrell et al., 1999b), of GRK2 and β-arrestin (Aragay et al., 1998; P.Penela, unpublished observations) and of GRK2 and c-Src (S.Sarnago, unpublished observations). Disruption of the β-arrestin–c-Src interaction with specific mutants or inhibition of the phosphorylation step by dominant-negative Src or a GRK2 mutant lacking critical phosphorylation sites results, as predicted by this model, in a marked reduction in GRK2 degradation.
It is worth noting that GRK2 phosphorylation by Src appears to require an adequate cellular context in order to promote GRK2 turnover, since the constitutively active c-Src Y527F mutant triggers GRK2-K220R tyrosine phosphorylation but not its degradation (data not shown). Thus, β-arrestin seems to favor the proximity of GRK2 and Src to other molecules involved downstream in the degradation process. Similarly, the specific blockade of the interaction between β-arrestin and c-Src with the kinase-dead SH1 Src domain (SH1-KD) inhibits c-Src-mediated phosphorylation of dynamin, without affecting the activity of the kinase towards other substrates (Miller et al., 2000). Interestingly, the SH1-KD construct markedly retards GRK2 degradation. The elucidation of the detailed mechanisms by which c-Src-mediated GRK2 phosphorylation leads to degradation is an important field for future research.
It should be noted, however, that the inhibition, by different means, of GRK2 tyrosine phosphorylation by c-Src does not completely block GRK2 proteolysis, suggesting the occurrence of additional pathways for GRK2 turnover. In this regard, the fact that the presence of dynamin K44A strongly inhibits the agonist-induced increase in GRK2 degradation by mechanisms that appear to be unrelated to receptor internalization suggests that other downstream functions of dynamin (Whistler and von Zastrow, 1999) may cooperate in the GRK2 degradation process, particularly upon marked GPCR stimulation. Currently, the identification of these additional mechanisms is being carried out in our laboratory.
The discovery of a pathway for GRK2 degradation involving β-arrestin and c-Src function emerges as a potentially relevant feedback mechanism for regulating GPCR signaling and may contribute to understanding the alterations in GRK2 levels in several pathophysiological situations. A decrease in GRK2 levels has been reported in different situations characterized by acute stimulation of GPCR signaling pathways, such as the rat perinatal period (P.Penela and F.Mayor,Jr, unpublished observations), or targeted cardiac overexpression of Gαq in transgenic mice (Dorn et al., 2000), a condition expected to result in increased c-Src activation (Nagao et al., 1998). On the other hand, overexpression of c-Src has been reported to potentiate β-adrenergic signaling in murine fibroblasts (Bushman et al., 1990; Moyers et al., 1993). Interestingly, recent evidence indicates a decrease in GRK2 activity and protein levels (but not in mRNA levels) in lymphocytes of rheumatoid arthritis patients and in a rat model of adjuvant arthritis (Lombardi et al., 1999, 2001), suggesting a role for changes in GRK2 stability in chronic inflammation processes. It is tempting to speculate that such alterations could be related to increased β-arrestin and c-Src function in these pathological conditions.
However, the relationship between increased activity of GPCR signaling pathways and GRK2 cellular levels is not straightforward. In fact, an increase in GRK2 mRNA levels and activity has been reported in several pathological and experimental situations characterized by increased stimulation of GPCR and Src pathways (Ungerer et al., 1993; Choi et al., 1997; Gros et al., 1997; Rockman et al., 1998; Ramos-Ruiz et al., 2000). Our data suggest that the final effect of the stimulation of these pathways on GRK2 cellular levels would result from their combined action on both GRK2 gene transcription and protein stability in a given physiological situation. The interplay or ‘balance’ between both effects may lead to marked increases in GRK2 turnover and function without drastic changes in GRK2 cellular levels in physiologically relevant situations such as heart failure or inflammation.
Materials and methods
Materials and plasmids
Cos-7, HEK-293, C6 glioma and Jurkat T cells were from the American Type Culture Collection (Manassas, VA). Culture media and LipofectAMINE were from Life Technologies, Inc. Protein A–Sepharose and isoproterenol were purchased from Sigma. [35S]methionine and [35S]cysteine labeling mixture was from NEN Life Science Products. The cDNAs encoding human pp60c-Src and the constitutively active Y527F pp60c-src were provided by Dr S.Gutkind (NIH, Bethesda), and a dominant-negative c-Src mutant (K295R) by Dr J.Brugge (Harvard Medical School, Boston). The cDNAs of wild-type β-arrestin-1 and β-arrestin-2 were a gift from Dr V.V.Gurevich (Sun Health Research Institute) and were subcloned in our laboratory in the pCDNA3-higro+ plasmid using NotI and ApaI sites. The β-arrestin-1 mutants S412A, S412D and P91G/P121E, and the kinase-dead c-Src catalytic domain (SH1-KD) were generously provided by Dr R.J.Lefkowitz (Duke University, Durham). Wild-type dynamin-1 and a dominant-negative mutant dynamin-1 K44A were obtained from Dr M.G.Caron (Duke University, Durham). The C-terminal truncated mutant GRK2 (1–546) was provided by Dr C.Murga (NIH, Bethesda), and the Y112F mutant by M.C.Jiménez-Sainz of our laboratory. C-terminal geranyl-geranylated GRK2 (GRK2-gg) was constructed using standard recombinant DNA procedures as described (Inglese et al., 1992). The K220R mutation was introduced in the GRK2-gg construct by digestion of the GRK2-K220R plasmid with BalI and XhoI restriction enzymes and subsequent subcloning. The source of other expression plasmids (GRK2, GRK2-K220R, β2AR) was as previously reported (Penela et al., 1998). All other reagents were of the highest grade commercially available.
Site-directed mutagenesis of GRK2
Replacement of specific GRK2 tyrosine residues for phenylalanine was performed by using a ‘bridge’ PCR method. The double mutant GRK2 Y86/92F was generated by sequentially using the external primers upstream (nucleotides 230–256) and downstream (nucleotides 496–516) of the mutation in combination with the mutant oligonucleotides 5′TTC(Tyr86-Phe)GAGGAGATCAAGAAATTC(Tyr92-Phe)GAGAAGCTGGATGGAGAAGAGC3′ and 5′-GAA(Tyr92-Phe)TTTCTTGATCTCCTCGAA(Tyr86-Phe)GAACTCTACC-3′. The amplified product was subcloned into KpnI–BspM sites in pCDNA3-GRK2. The point mutant GRK2-Y13F was generated using a T7 oligonucleotide present in the vector template as external upstream primer and the region encompassing nucleotides 496–516 as external downstream primer. The primer bearing the Y/F mutation was 5′ACGTGAGCTTC(Tyr13-Phe)CTGATGGCCATGGAGAAGAGC3′. The amplified fragment was digested with HindIII–KpnI and subcloned in pCDNA3-GRK2. Similar strategies were used for generating the Y46F and Y65F point mutants, and the fragments containing the mutations were digested with HindIII–BspMII and subcloned in pCDNA3-GRK2. Finally, for generating the triple mutants Y13/86/92F, Y46/86/92F and Y65/86/92F, the primers used were the same as for the single mutations but using the double mutant GRK2 Y86/92F as template. The sequence of all constructs was confirmed using an automated ABI DNA sequencer (Centro de Biología Molecular, Madrid).
Cell culture and transfection
HEK-293, Cos-7 and C6 glioma cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) at 37°C in a humidified 5% CO2 atmosphere. Jurkat cells were maintained in RPMI 1640 medium supplemented with 5% FBS. Plasmid DNA (5–10 µg) was transiently trasfected on 70% confluent monolayers in 100 mm dishes (for phosphotyrosine detection assays) or 60 mm dishes (for 35S-labeling experiments) by using the LipofectAMINE reagent as described (Penela et al., 1998; Sarnago et al., 1999). Empty vector was added as needed to keep the total amount of DNA transfected constant. In each case, expression of wild-type and mutant proteins was analyzed by immunoblotting with specific antibodies (see below) to confirm that similar expression levels were attained. In some experiments, HEK-293 cells stably transfected with GRK2 and β2AR were also used as described (Penela et al., 1998).
Cell treatments and metabolic labeling
For metabolic labeling, cells were kept for 2 h in methionine- and cysteine-free DMEM and then incubated for 10–15 min (or 30–45 min for Jurkat or C6 cells) in this medium supplemented with 250 µCi/ml of [35S]methionine and [35S]cysteine labeling mixture (NEN Life Science Products) as reported (Penela et al., 1998). The plates were washed with phosphate-buffered saline and chased for the desired times in DMEM plus 10% FBS. To block receptor internalization or proteasome activity, respectively, sucrose (250 mM) or the specific inhibitors lactacystin (30 µM; Calbiochem) or MG132 (10 µM; Biomol) were added to the medium 2 h before metabolic labeling and maintained during the chase periods. Cells expressing β2AR were challenged with 10 µM isoproterenol or vehicle during chase periods in DMEM supplemented with 20 mM HEPES pH 7.5 and 1 mM ascorbic acid as previously described (Penela et al., 1998). Endogenous CXCR4 or β-adrenergic receptors in Jurkat or C6 glioma cells were activated with the chemokine SDF-1α (100 nM added every 30 min, PeproTechEC) or 10 µM isoproterenol as detailed above, respectively. When desired, cells were treated with the tyrosine kinase inhibitors PP2 (5 µM; Calbiochem) or herbimycin (1 µM; Calbiochem) 2 or 18 h prior to metabolic labeling, respectively, and then maintained during the chase periods. In some experiments, protein translation was blocked by incubation for 18 h with cycloheximide (18 µM; Calbiochem).
Immunoprecipitation and western blot analysis
Cells were washed and lysed in RIPA buffer as reported (Sarnago et al., 1999). The buffer was supplemented with 1 mM sodium orthovanadate in experiments involving phosphotyrosine analysis. After centrifugation (15 000 g, 15 min), cellular extracts were immunoprecipitated with the specific GRK2 polyclonal antibody AbFP1, followed by incubation with protein A–Sepharose for 1 h as previously described (Elorza et al., 2000). Immunoprecipitates were resolved by 10% SDS–PAGE and the gel was either subjected to fluorography (Amplify; Amersham Pharmacia Biotech) in pulse–chase experiments, or transferred to nitrocellulose membranes to be probed with a specific horseradish peroxidase-conjugated anti-phosphotyrosine monoclonal antibody (PY99-HRP; Santa Cruz Laboratories). After membrane stripping, these blots were reprobed subsequently (dilution 1:1000) with GRK2 Ab9 polyclonal antibody, raised against recombinant GRK2, as described (Sarnago et al., 1999). Lysate aliquots were taken to assess protein overexpression of the different wild-type and mutant constructs transfected in each experiment. GRK2 and its mutants were analyzed with the AbFP1 or Ab9 antibodies; β-arrestin-1 and β-arrestin-2 constructs with the specific Ab186 anti-β-arrestin-1 polyclonal antibody raised in our laboratory (Penela et al., 2001) or the general F4C1 anti-arrestin monoclonal antibody (a gift from Dr L.A.Donoso); dynamin-1 and its K44A mutant with a monoclonal antibody from Transduction Laboratories; and c-Src constructs with the G-D11 monoclonal antibody (Upstate Biotechnology). Blots were developed using a chemiluminescent method (ECL, Amersham). Band density was quantitated by laser densitometric analysis.
Acknowledgments
Acknowledgements
We thank Mrs A.Morales and Mr R.Campos for skillful secretarial and technical assistance, respectively. This work was supported by grants from the European Union (BMH4-98-3566), the Ministry of Science and Technology of Spain (PM98-0020) and Comunidad de Madrid (84/61/98 and 84/10/00). P.P. and A.E. were supported by post-doctoral and pre-doctoral fellowships, respectively, from Comunidad de Madrid, and S.S. was supported by a pre-doctoral fellowship from Fundación Ramón Areces. The Centro de Biología Molecular holds an institutional grant from Fundación Ramón Areces.
References
- Ahn S., Maudsley,S., Luttrell,L.M., Lefkowitz,R.J. and Daaka,Y. (1999) Src-mediated tyrosine phosphorylation of dynamin is required for β2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol. Chem., 274, 1185–1188. [DOI] [PubMed] [Google Scholar]
- Andoniou C.E., Lill,N.L., Thien,C.B., Lupher,M.L.,Jr, Ota,S., Bowtell,D.D., Scaife,R.M., Langdon,W.Y. and Band,H. (2000) The Cbl proto-oncogene product negatively regulates the Src-family tyrosine kinase Fyn by enhancing its degradation. Mol. Cell. Biol., 20, 851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragay A.M., Mellado,M., Frade,J.M.R., Martín,A.M., Jiménez-Sainz, M.C., Martínez,A.C. and Mayor,F.,Jr (1998) MCP-1 induced CCR2B receptor desensitization mediated by the G-protein-coupled receptor kinase 2. Proc. Natl Acad. Sci. USA, 95, 2985–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman W.A., Wilson,L.K., Luttrell,D.K., Moyers,J.S. and Parsons,S.J. (1990) Overexpression of c-src enhances β-adrenergic-induced cAMP accumulation. Proc. Natl Acad. Sci. USA, 87, 7462–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman C.V. and Benovic,J.L. (1998) G-protein-coupled receptors: turn-ons and turn-offs. Curr. Opin. Neurobiol., 8, 335–344. [DOI] [PubMed] [Google Scholar]
- Choi D.-J., Walter,J.K., Hunter,J.J. and Rockman,H.A. (1997) Mechan ism of β-adrenergic receptor desensitization in cardiac hypertrophy is increased β-adrenergic receptor kinase. J. Biol. Chem., 272, 17223–17229. [DOI] [PubMed] [Google Scholar]
- Damke H., Baba,T., Warnock,D.E. and Schmid,S.L. (1994) Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol., 127, 915–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn G.W., Tepe,N.M., Wu,G., Yatani,A. and Liggett,S.B. (2000) Mechanisms of impaired β-adrenergic receptor signaling in G(αq)-mediated cardiac hypertrophy and ventricular dysfunction. Mol. Pharmacol., 57, 278–287. [PubMed] [Google Scholar]
- Elorza A., Sarnago,S. and Mayor,F.,Jr (2000) Agonist-dependent modulation of G-protein-coupled receptor kinase 2 by mitogen-activated protein kinases. Mol. Pharmacol., 57, 778–783. [DOI] [PubMed] [Google Scholar]
- Fan G.-F., Shumay,E., Malbon,C.C. and Wang,H.Y. (2001) c-Src tyrosine kinase binds the β2-adrenergic receptor via phospho-tyr-350, phosphorylates G-protein-linked receptor kinase 2 and mediates agonist-induced receptor desensitization. J. Biol. Chem., 276, 13240–13247. [DOI] [PubMed] [Google Scholar]
- Ferguson S.S.G., Downey,W.E., Colapietro,A.-M., Barak,L.S., Menard, L. and Caron,M.G. (1996) Role of β-arrestin in mediating agonist-promoted G-protein-coupled receptor internalization. Science, 271, 363–366. [DOI] [PubMed] [Google Scholar]
- Goodman O.B. Jr, Krupnick,J.G., Santini,F., Gurevich,V.V., Penn,R.B., Gagnon,A.W., Keen,J.H. and Benovic,J.L. (1996) β-arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature, 383, 447–450. [DOI] [PubMed] [Google Scholar]
- Gros R., Benovic,J.L., Tan,C.M. and Feldman,R.D. (1997) G-protein-coupled receptor kinase activity is increased in hypertension. J. Clin. Invest., 99, 2087–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich V.V., Dion,S.B., Onorato,J.J., Ptasienski,J., Kim,C.M., Sterne-Marr,R., Hosey,M.M. and Benovic,J.L. (1995) Arrestin interactions with G-protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, β2-adrenergic and m2 muscarinic cholinergic receptors. J. Biol. Chem., 270, 720–731 [DOI] [PubMed] [Google Scholar]
- Gurevich V.V., Pals-Rylaarsdam,R., Benovic,J.L., Hosey,M.M. and Onorato,J.J. (1997) Agonist–receptor–arrestin, an alternative ternary complex with high agonist affinity J. Biol. Chem., 272, 28849–28852. [DOI] [PubMed] [Google Scholar]
- Gutkind J.S. (2000) Regulation of mitogen-activated protein kinase signaling networks by G-protein-coupled receptors. Science’s stke. http://www.stke.org/cgi/content/ full/OC_sigtrans; 2000/40/re1 [DOI] [PubMed]
- Hakak Y. and Martin,G.S. (1999) Ubiquitin-dependent degradation of active Src. Curr. Biol., 9, 1039–1042. [DOI] [PubMed] [Google Scholar]
- Hansen S.H., Sandvig,K. and van Deurs,B. (1993) Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium and cytosol acidification. J. Cell Biol., 121, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K.F., Shoji,I., Cooper,E.M., Kumar,S., Oda,H. and Howley,P.M. (1999) Ubiquitin-mediated degradation of active Src tyrosine kinase. Proc. Natl Acad. Sci. USA, 96, 13738–13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese J., Koch,W.J., Caron,M.G. and Lefkowitz,R.J. (1992) Isoprenylation in regulation of signal transduction by G-protein-coupled receptor kinases. Nature, 359, 147–150. [DOI] [PubMed] [Google Scholar]
- Kong G., Penn,R. and Benovic,J.L. (1994) A β-adrenergic receptor kinase dominant negative mutant attenuates desensitization of the β2-adrenergic receptor. J. Biol. Chem., 269, 13084–13087. [PubMed] [Google Scholar]
- Lin F.T., Krueger,K.M., Kendall,H.E., Daaka,Y., Fredericks,Z.L., Pitcher,J.A. and Lefkowitz,R.J. (1997) Clathrin-mediated endo cytosis of the β-adrenergic receptor is regulated by phosphoryl ation/dephosphorylation of β-arrestin1. J. Biol. Chem., 272, 31051–31057. [DOI] [PubMed] [Google Scholar]
- Lombardi M.S. et al. (1999) Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J., 13, 715–725. [DOI] [PubMed] [Google Scholar]
- Lombardi M.S., Kavelaars,A., Cobelens,P.M., Schmidt,R.E., Schedlowski,M. and Heijnen,C.J. (2001) Adjuvant arthritis induces down-regulation of G-protein-coupled receptor kinases in the immune system. J. Immunol., 166, 1635–1640. [DOI] [PubMed] [Google Scholar]
- Lu Z., Liu,D., Hornia,A., Devonish,W., Pagano,M. and Foster,D.A. (1998) Activation of protein kinase C triggers its ubiquitination and degradation. Mol. Cell. Biol., 18, 839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell L.M., Daaka,Y. and Lefkowitz,R.J. (1999a) Regulation of tyrosine kinase cascades by G-protein-coupled receptors, Curr. Opin. Cell Biol., 11, 177–183. [DOI] [PubMed] [Google Scholar]
- Luttrell L.M. et al. (1999b) β-arrestin-dependent formation of β2-adrenergic receptor–src protein kinase complexes. Science, 283, 655–661. [DOI] [PubMed] [Google Scholar]
- Ménard L., Ferguson,S.S.G., Zhang,J., Lin,F.T., Lefkowitz R.J., Caron,M.G. and Barak,L.S. (1997) Synergistic regulation of β2-adrenergic receptor sequestration: intracellular complement of β2-adrenergic receptor kinase and β-arrestin determine kinetics of internalization. Mol. Pharmacol., 51, 800–808. [PubMed] [Google Scholar]
- Miller W.E., Maudsley,S., Ahn,S., Dad Khan,K., Luttrell,L.M. and Lefkowitz,R.J. (2000) β-arrestin1 interacts with the catalytic domain of the tyrosine kinase c-Src. J. Biol. Chem., 275, 11312–11319. [DOI] [PubMed] [Google Scholar]
- Moyers J.S., Bouton,A.H. and Parsons,S.J. (1993) The sites of phosphorylation by protein kinase C and an intact SH2 domain are required for the enhanced response to β-adrenergic agonists in cells overexpressing c-src. Mol. Cell. Biol., 13, 2391–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M., Yamauchi,J., Kaziro,Y. and Itoh,H. (1998) Involvement of protein kinase C and Src family tyrosine kinase in Gαq/11-induced activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. J. Biol. Chem., 273, 22892–22898. [DOI] [PubMed] [Google Scholar]
- Penela P., Ruiz-Gómez,A., Castaño,J.G. and Mayor,F.,Jr (1998) Degradation of the G-protein-coupled receptor kinase 2 by the proteasome pathway. J. Biol. Chem., 273, 35238–35244. [DOI] [PubMed] [Google Scholar]
- Penela P., Barradas,M., Alvarez-Dolado,M., Muñoz,A. and Mayor,F.,Jr (2001) Effect of hypothyroidism on G-protein-coupled receptor kinase 2 (GRK2) expression levels in rat liver, lung and heart. Endocrinology, 142, 987–991. [DOI] [PubMed] [Google Scholar]
- Pitcher J.A., Freedman,N.J. and Lefkowitz,R.J. (1998) G-protein-coupled receptor kinases. Annu. Rev. Biochem., 67, 653–692. [DOI] [PubMed] [Google Scholar]
- Ramos-Ruiz R., Penela,P., Penn,R.B. and Mayor,F.,Jr (2000) Analysis of the human G-protein-coupled receptor kinase 2 (GRK2) gene promoter. Regulation by signal transduction systems in aortic smooth muscle cells. Circulation, 101, 2083–2089. [DOI] [PubMed] [Google Scholar]
- Rockman H.A., Chien,K.R., Choi,D.J., Iaccarino,G., Hunter,J.J., Ross,J.,Jr, Lefkowitz,R.J. and Koch,W.J. (1998) Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc. Natl Acad. Sci. USA, 195, 7000–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gomez A. and Mayor,F.,Jr (1997) β-adrenergic receptor kinase (GRK2) colocalizes with β-adrenergic receptors during agonist-induced receptor internalization. J. Biol. Chem., 272, 9601–9604 [DOI] [PubMed] [Google Scholar]
- Ruiz-Gómez A., Humrich,J., Murga,C., Quitterer,U., Lohse,M.J. and Mayor,F.,Jr (2000) Phosphorylation of phosducin and phosducin-like protein by G-protein-coupled receptor kinase 2 (GRK2). J. Biol. Chem., 275, 29724–29730. [DOI] [PubMed] [Google Scholar]
- Sarnago S., Elorza,A. and Mayor,F.,Jr (1999) Agonist-dependent phosphorylation of the G-protein-coupled receptor kinase 2 (GRK2) by src tyrosine kinase. J. Biol. Chem., 274, 34411–34416. [DOI] [PubMed] [Google Scholar]
- Ungerer M., Bohm,M., Elce,J.S., Erdmann,E. and Lohse,M.J. (1993) Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in the failing human heart. Circulation, 87, 454–463. [DOI] [PubMed] [Google Scholar]
- Whistler J.L. and von Zastrow,M. (1999) Dissociation of functional roles of dynamin in receptor-mediated endocytosis and mitogenic signal transduction. J. Biol. Chem., 274, 24575–24578. [DOI] [PubMed] [Google Scholar]
- Winstel R., Freund,S., Krasel,C., Hoppe,E. and Lohse,M.J. (1996) Protein kinase cross-talk: membrane targeting of the β-adrenergic receptor kinase by protein kinase C. Proc. Natl Acad. Sci. USA, 93, 2105–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. et al. (1995) Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature, 373, 536–539. [DOI] [PubMed] [Google Scholar]