Abstract
A tripartite receptor comprising the external region of the erythropoietin (Epo) receptor, the transmembrane and JAK-binding domains of the gp130 subunit of the interleukin-6 (IL-6) receptor, and a seven amino acid STAT1 recruitment motif (Y440) from the interferon (IFN)-γ receptor, efficiently mediates an IFN-γ-like response. An analogous completely foreign chimeric receptor in which the Y440 motif is replaced with the Y905 motif from gp130 also mediates an IFN-γ-like response, but less efficiently. The IFNGR1 signal-transducing subunit of the IFN-γ receptor is tyrosine phosphorylated through the chimeric receptors and the endogenous IL-6 and OSM receptors. Cross phosphorylation of IFNGR1 is not, however, required for the IFN-γ-like response through the chimeric receptors, nor does it mediate an IFN-γ-like response to IL-6 or OSM. The data argue strongly for modular JAK/STAT signalling and against any rigid structural organization for the ‘pathways’ involved. They emphasize the likely high degree of overlap between the signals generated from disparate JAK–receptor complexes and show that relatively minor changes in such complexes can profoundly affect the response.
Keywords: cytokine/interferon/modular signalling/receptor cross phosphorylation
Introduction
JAK/STAT signalling pathways, first identified as essential in the interferon system, are activated by many cytokines and growth factors (reviewed in Darnell et al., 1994; Ihle and Kerr, 1995). There are four members of the mammalian JAK family of protein tyrosine kinases: JAKs 1–3 and Tyk2, and seven mammalian STAT genes. For the interferons (IFNs) and cytokines, binding of the ligand to the receptor triggers the activation and tyrosine phosphorylation of pre-associated JAKs and the receptors. STATs, predominantly recruited through receptor phosphotyrosine motifs, are phosphorylated, released and, with or without additional factors, migrate to the nucleus to activate transcription (reviewed in Stark et al., 1998). For IFN-γ, JAK/STAT signalling is through JAK1 and JAK2 and, for human cell systems, STAT1. The IFN-γ receptor has two subunits: IFNGR1 and IFNGR2. IFNGR1 is the signal transducing subunit and recruits JAK1. Human IFNGR2 has a short 66 amino acid cytoplasmic domain for which the only known function is the recruitment of JAK2 (Kotenko et al., 1995; Bach et al., 1996). The activation of JAK2 triggers the response (Briscoe et al., 1996). STAT1 is recruited to a phosphotyrosine motif (Y440) towards the C-terminus of IFNGR1. For IFNGR1, only the membrane-proximal JAK1 binding domain and Y440 motif appear essential for an IFN-γ response (Cook et al., 1992; Farrar et al., 1992; Greenlund et al., 1994; Kaplan et al., 1996; reviewed by Bach et al., 1997). For the IL-6 family of cytokines, JAK/STAT signalling is mediated through the common gp130 signal transduction subunit either alone, for IL-6, or in combination with additional highly related subunits as is the case, for example, for Oncostatin M (OSM) (reviewed in Heinrich et al., 1998). In response to ligand, JAKs 1 and 2, Tyk2 and STATs 3 and 1 are all activated. JAK1 and STAT3 play major roles in mediating the response. The cytoplasmic element of the gp130 subunit has six tyrosines, five of which are phosphorylated in response to ligand (Figure 1). Four (Y767, Y814, Y905 and Y915), when phosphorylated, function as docking sites for the STATs (Stahl et al., 1995; Gerhartz et al., 1996; Schmitz et al., 2000a). The role of Y759 appears more complex, being involved in the recruitment of at least SHP2 and, directly or indirectly, SOCS3, Grb2 and the ERK1/2 MAP kinase pathway (Nicholson et al., 2000; Schmitz et al., 2000b; Terstegen et al., 2000).
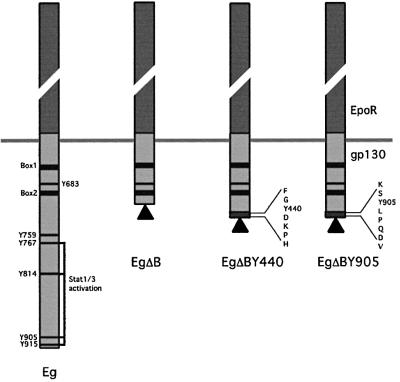
Fig. 1. Schematic representation of the Epo/gp130-based chimeric receptors. All chimeras have the extracellular region of the Epo receptor (dark grey boxes) and the gp130 transmembrane domain. Tyrosine residues in the intracellular region of gp130 are depicted as black lines and box1/2 motifs as black boxes. Flag-tags are indicated by black triangles. Chimeras with the full-length intracellular gp130 (Eg), a truncated gp130 (EgΔB) and a truncated gp130 with added tyrosine motifs from the human IFNGR1 (Y440) or gp130 (Y905), respectively, are shown.
Experiments with mutant cells and, more recently, knock-out mice have established a central role for the JAK/STAT1 pathway in the IFN-γ response (reviewed in Stark et al., 1998). A kinase-negative JAK1 is, however, capable of supporting the JAK/STAT1 pathway but not an antiviral response—data consistent with a requirement for an additional JAK1 kinase-dependent signal(s) for the antiviral response (Briscoe et al., 1996). Indeed, there is increasing evidence for additional JAK-dependent IFN-γ signalling pathways (Discussion).
The STATs are activated at the cell membrane. On activation they translocate to the nucleus through nuclear pores. For STAT1, interaction with importin NPI-1 initiates translocation through the nuclear pore (Sekimoto et al., 1997), but little is known with respect to factors controlling movement of STAT1 from the membrane to the nuclear pore and to what extent, if any, such movement is restricted or governed by additional modification(s), compartmentalization or origin, reflecting, for example, differential membrane distribution of active receptor complexes. Movement through the cytoplasm could, a priori, be ‘hard-wired’ through association with, for example, microtubules, as observed for p53 (Giannakakou et al., 2000), or ‘soft-wired’ by diffusion as in a random walk (reviewed in Teruel and Meyer, 2000).
Against this background it was of interest, both with respect to the requirement for additional signalling and the organization of signalling pathways, to determine to what extent activation of the JAKs and STAT1 through a minimal chimeric receptor could mimic the IFN-γ response. Recent data from a biophysical analysis of the behaviour of STAT1–green fluorescent protein (GFP) are consistent with rapid random movement (‘soft-wiring’) of activated STAT1 in both the cytoplasmic and nuclear compartments of the cell (Lillemeier et al., 2001). Here, a complementary functional analysis establishes that signalling from minimal and completely foreign receptors can yield an unexpectedly complete IFN-γ-like response. Thus, hard-wiring of the IFN-γ response to an element(s) unique to the IFN-γ receptor complex can be excluded. Indeed, taken together, the biophysical and functional data argue strongly for ‘soft-’ rather than ‘hard-wired’ signalling from JAK–receptor complexes and emphasize the likely high degree of overlap between the signals generated from such complexes in response to different ligands.
Results
A tripartite chimeric receptor can efficiently mediate an IFN-γ-like response
Wild-type or mutant human fibrosarcoma cell lines were stably transfected with chimeric constructs based on the erythropoietin (Epo) and IL-6 receptors. All constructs contain the external domains of the Epo receptor and the transmembrane element of gp130 (Figure 1). For the Eg construct, these are combined with the entire cytoplasmic region of gp130. For the EgΔB-based constructs, they are combined with the membrane-proximal JAK-binding domain of gp130 without (EgΔB) or with the seven amino acid (Y440) STAT1 recruitment motif from IFNGR1 to yield EgΔBY440, or an eight amino acid Y905 STATs 1 and 3 recruitment motif from the C-terminus of gp130 to yield EgΔBY905 (Gerhartz et al., 1996). Clones of transfectants showing similar levels of expression of the Flag-tagged receptor chimeras were isolated. To exclude overexpression artefacts, clones showing approximately equivalent levels of Epo-mediated STAT1 activation to those observed through the endogenous IFN-γ receptors were selected and their responses to Epo, IFN-γ, IL-6 and OSM compared (Figures 2A and C, and 9B).
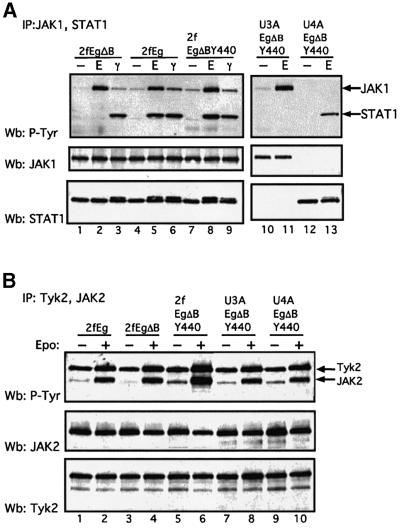
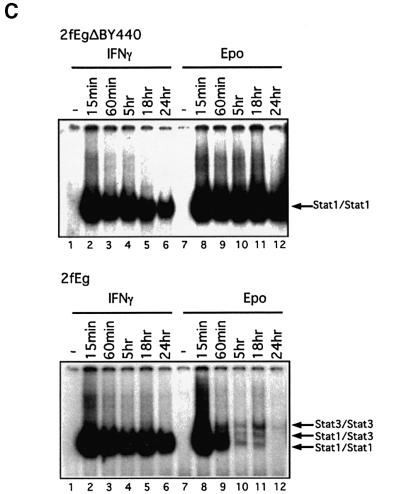
Fig. 2. Comparative activation of the JAK/STAT1 pathway through the Eg, EgΔB and EgΔBY440 receptors and IFN-γ. Wild-type, 2fTGH (2f) and STAT1- or JAK1-negative U3A or U4A cells stably expressing the chimeric receptors were stimulated with either 500 IU/ml IFN-γ or 100 U/ml Epo for 15 min (A and B) or, as indicated (C), prior to lysis (Materials and methods). Tyrosine phosphorylation: immuno precipitates (A) JAK1 and STAT1 or (B) JAK2 and TYK2 were separated by 7.5% SDS–PAGE, and western analysis was carried out with anti-phosphotyrosine antibody (upper panels). Blots were stripped and sequentially probed for (A) JAK1 and STAT1 protein or (B) JAK2 and TYK2 protein, respectively (lower panels). (C) STAT1 and STAT3 activation: EMSAs with an hSIE probe (Materials and methods). Biphasic kinetics for STAT activation in which the level of activated STAT (maximal at ∼15 min) declines over 12–24 h but increases again thereafter are frequently but not invariably observed in these systems. For this, and all other figures, the data presented are representative of at least three independent experiments.
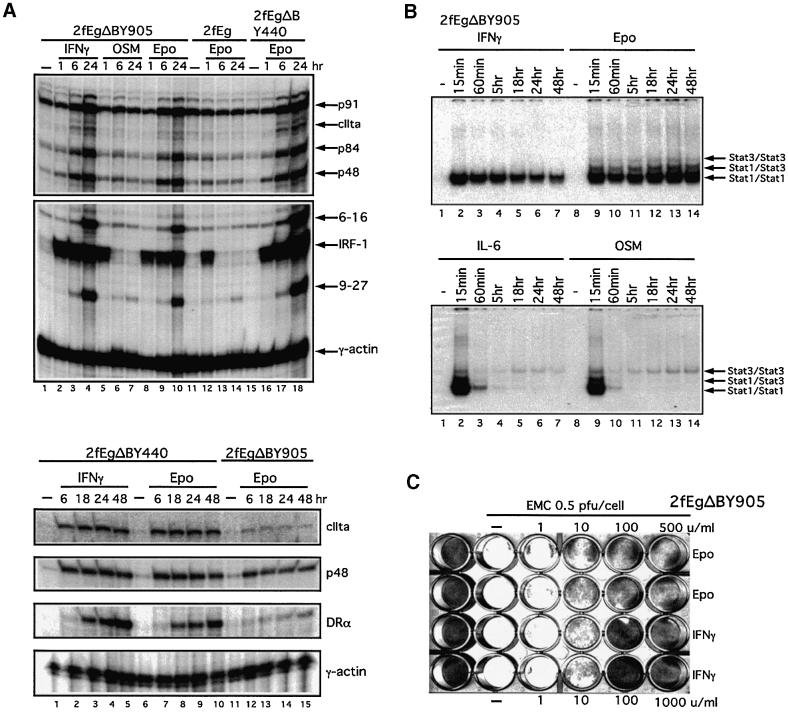
Fig. 9. A completely foreign receptor can mediate IFN-γ-like transcriptional (A), STAT activation (B) and antiviral (C) responses. Wild-type 2fTGH (2f) cells stably expressing the completely foreign EgΔBY905 receptor or, for comparison in (A) the EgΔBY440 receptor, were treated with 1000 IU/ml IFN-γ or 100 U/ml Epo for the times indicated. (A) Aliquots (13 µg) of cytoplasmic RNA were analysed by RNase protection with 9-27, IRF-1, 6-16, p48, CIITA and STAT1 (p91/p84) (A, upper panel) or Class II HLA DRA, p48 and CIITA (A, lower panel) probes, with γ-actin as loading control. (B) STAT activation: lysates were analysed by EMSA with an hSIE probe. (C) Antiviral assays were as in Figure 5.
Activation of the JAK/STAT1 pathway and IFN-γ-like gene expression. Wild-type HT1080-derived 2fTGH cells and mutant U3A and U4A cells lacking STAT1 and JAK1, respectively, stably expressing the chimeric Eg, EgΔB and EgΔBY440 receptors, were assayed for activation of JAK/STAT signalling in response to Epo in comparison with IFN-γ (Figure 2). JAK1, JAK2 and Tyk2, and STATs 1 and 3, are all activated through gp130 in response to IL-6 in wild-type HT1080-based cell lines (Guschin et al., 1995). Consistent with this, for each of the chimeras, all three JAKs were rapidly phosphorylated on tyrosine in response to physiological concentrations of Epo (Figure 2A, lanes 1–9 for JAK1 and Figure 2B, lanes 1–6 for JAK2 and Tyk2). No JAK1 or JAK1 phosphorylation was observed in the JAK1-negative U4A cells (Figure 2A, lanes 12 and 13). STAT1 was tyrosine phosphorylated through EgΔBY440 in all but the STAT1-negative U3A cells (Figure 2A, lanes 10 and 11). Here, no significant recruitment of STAT1 through the JAKs was observed for EgΔB, which lacks all receptor phosphotyrosine STAT recruitment motifs (Figure 2A, lane 2). In electrophoretic mobility shift assays (EMSAs) the kinetics and extent of STAT1 activation by 100 U/ml Epo through the EgΔBY440 chimera were prolonged and comparable to those observed with saturating concentrations (103 IU/ml) of IFN-γ (Figure 2C). In contrast, activation of STAT1 through the Eg chimera is much more transient (Figure 2C) and comparable to that observed with IL-6 or OSM (e.g. Figure 9B; Guschin et al., 1995). (The factors governing the prolonged STAT1 response to IFN-γ through the EgΔBY440 chimera and the relatively low level of STAT3 activation through the Eg, IL-6 and OSM receptors remain to be established.) In similar assays, activation of STAT1 through increasing concentrations of IFN-γ was first observable at 1–10 IU/ml and through Epo at 0.1–1 U/ml, i.e. at very low physiological concentrations. STAT1 Ser727 is constitutively phosphorylated in the HT1080-based cell lines. Nevertheless, clear additional Ser727 phosphorylation was observed in response to both IFN-γ and through the EgΔBY440 receptor (data not presented). Thus, in response to physiological concentrations of Epo, the chimeric receptors activate JAK1, JAK2 and Tyk2, as would be expected for receptors based on the gp130 JAK recruitment site, and EgΔBY440 activates phosphorylation of both Tyr701 and Ser727 residues of STAT1 to levels comparable to and with similar kinetics to those observed with IFN-γ. Consistent with this, Epo signalling through the EgΔBY440 receptor induces the expression of a comparable spectrum of IFN-γ-inducible genes.
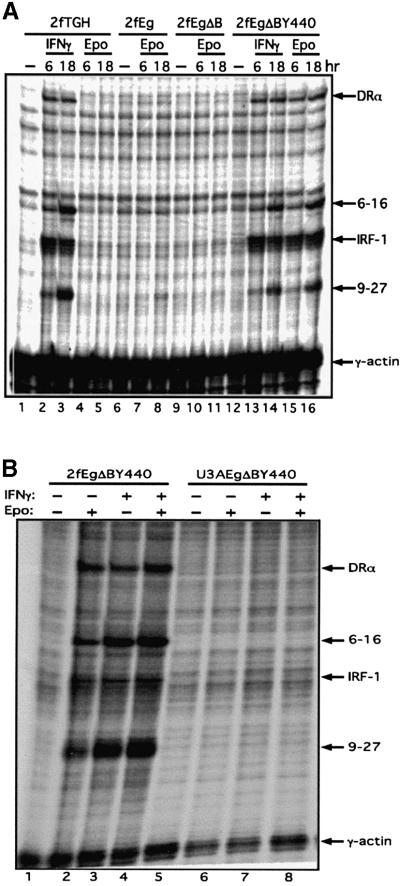
Wild-type and mutant cells, with or without the chimeric receptors, were stimulated with IFN-γ or Epo for 6 and 18 h, the RNA extracted and subjected either to RNase protection assays (Figure 3) for IFN-γ-inducible mRNAs, or to expression profiling using low-density cDNA arrays representing a selection of IFN-γ-inducible genes (Table I; Materials and methods). There is no response to Epo in 2fTGH cells per se (Figure 3A, lanes 4 and 5). For 2fTGH cells expressing the EgΔBY440 receptor, stimulation with IFN-γ or Epo yielded, by RNase protection assay, comparable levels of induction of the 9-27, 6-16, IRF1 and Class II HLA DRA IFN-γ-inducible mRNAs (Figure 3A, lanes 12–16). No such induction was observed through the Eg or EgΔB receptors (Figure 3A, lanes 6–11) or through EgΔBY440 in the STAT1-negative U3A cells (Figure 3B). The induction of IRF1 through the Eg receptor, as for IL-6 and OSM (Guschin et al., 1995; see also lanes 7–9 and 12–14, Figure 9A), is too rapid and transient to be detected here, even at the earlier 6 h time point (Figure 3A, lane 7), despite good STAT activation in parallel EMSA controls (data not presented). On expression profiling, 21 and 38 mRNAs were induced >2-fold in response to IFN-γ at 6 and 18 h, respectively. All of these mRNAs were induced, and 17 (at 6 h) and 31 (at 18 h) were induced >2-fold, in response to Epo through EgΔBY440 (Table I). Of those represented on these arrays, no gene was highly induced only in response to Epo or IFN-γ. In general, the response to Epo was lower. The pattern of induction was, however, similar with those mRNAs more highly induced in response to IFN-γ also more highly induced in response to Epo. It can be concluded that stimulation through the IFN-γ and EgΔBY440 receptors results in a remarkably similar induction for between 30 and 40 IFN-γ-inducible genes.
Fig. 3. The EgΔBY440 receptor mediates an IFN-γ-like transcriptional response (A) that is dependent on STAT1 (B). Wild-type 2fTGH (2f) or STAT1-negative U3A cells, stably transfected with the chimeric receptor as indicated, were stimulated for 6 or 18 h (A) or for 18 h (B) with 500 IU/ml IFN-γ and/or 100 U/ml Epo. Aliquots (13 µg) of cytoplasmic RNA were analysed for inducible gene expression by RNase protection assay using 9-27, IRF-1, 6-16 and Class II HLA DRA as probes, with γ-actin as a loading control (Materials and methods). In parallel EMSA controls, comparable activation of STAT1 was observed in response to Epo in the 2f/Eg and 2f/EgΔBY440 cells.
Table I. Comparison of gene induction in 2fEgΔBY440 cells in response to IFN-γ or Epo.
| Fold induction (6 h stimulation) |
Fold induction (18 h stimulation) |
||||
|---|---|---|---|---|---|
| Gene namea | IFN-γ | Epo | Gene namea | IFN-γ | Epo |
| GBP-1 | 10.98 | 5.87 | GBP-1 | 17.5 | 12.72 |
| HLA-Eb | 5.54 | 3.04 | RING12 | 11.46 | 7.96 |
| TAP1 | 4.69 | 3.81 | Ubiquitin-conjugating enzyme | 9.55 | 5.52 |
| IL-15Rαc | 4.54 | 3.55 | TAP1 | 8.98 | 6.29 |
| 248145 | 4.34 | 2.33 | IL-15Rαc | 7.42 | 6.63 |
| RING12 | 4.04 | 2.66 | HLA-Eb | 7.41 | 4.62 |
| HLA-Eb | 3.89 | 3.18 | CIITA | 6.79 | 3.86 |
| 240703 | 3.24 | 2.75 | 248145 | 5.9 | 4.25 |
| NKC4 | 2.94 | 1.79 | NKC4 | 5.84 | 3.68 |
| CIITA | 2.75 | 2.78 | IFP-53b | 5 | 3.36 |
| Auto Ag SS-A/Ro | 2.72 | 2.46 | STAT1 | 4.74 | 3.51 |
| 364744 | 2.64 | 1.91 | Prot. C13/RING 10 | 4.67 | 4.02 |
| Ubiquitin-conjugating enzyme | 2.63 | 2.28 | MHC I Ag B27 | 4.62 | 4.5 |
| Transthyretin | 2.49 | 1.9 | IFP-53b | 4.55 | 3.55 |
| 247050 | 2.26 | 2.03 | LMP2 | 4.36 | 3.32 |
| 214342 | 2.16 | 1.8 | 247050 | 4.3 | 3.24 |
| Caspase 7 | 2.09 | 2.23 | 240703 | 4.28 | 4.26 |
| STAT1 | 2.07 | 2.69 | RING4 | 4.08 | 2.57 |
| IFP-53b | 2.05 | 2.21 | IP-30 | 4.06 | 2.17 |
| IFP-53b | 2.04 | 2.33 | HLA-Eb | 3.78 | 3.7 |
| IP-30 | 2 | 2.13 | Caspase 8/FLICE/MACH | 3.6 | 2.97 |
| Prot. C13/RING 10 | 1.86 | 1.87 | Nuclear phosphoprotein | 3.5 | 2.81 |
| 256845 | 1.85 | 1.66 | LMP7 | 3.45 | 2.28 |
| Nuclear phosphoprotein | 1.8 | 1.49 | HLA II Ag DP1 | 3.39 | 2.44 |
| BAK1 | 1.76 | 1.72 | IDO | 3.19 | 2.59 |
| NMBD | 1.69 | 1.41 | 364744 | 3.12 | 2.89 |
| IFP-35 | 1.57 | 1.08 | Transthyretin | 3.06 | 2.5 |
| RING4 | 1.49 | 1.76 | BAK1 | 3 | 2.38 |
| ICAM-1 | 1.49 | 1.39 | ICAM-1 | 2.47 | 2.61 |
| IDO | 1.42 | 1.31 | 256845 | 2.35 | 2.15 |
| β-tubulin | 1.42 | 1.08 | Auto Ag SS-A/Ro | 2.33 | 1.96 |
| LMP2 | 1.4 | 1.49 | 214342 | 2.27 | 2.11 |
| 152074 | 1.34 | 1.15 | MHC I HLA-A3 | 2.18 | 1.71 |
| IL-15Rαc | 1.31 | 1.06 | DR-α | 2.16 | 1.42 |
| MHC I Ag B27 | 1.3 | 1.3 | Stannin | 2.15 | 1.84 |
| CG-12 | 1.26 | 1.04 | IRF-2 | 2.06 | 1.58 |
| HLA II Ag DP1 | 1.22 | 1.06 | IFP-35 | 2.03 | 1.84 |
| LMP7 | 1.19 | 1.32 | 9-27 | 2.01 | 1.97 |
aIncluding image ID number for unknown expressed sequence tags.
bFor HLA-E and IFP-53, two image clones corresponding to different regions of the mRNAs were represented on the arrays.
cFor IL-15R, two different image clones were present on the arrays. The basis for the minimal induction observed with one of the clones (1.31 at 6 h and <2 at 18 h) is not known.
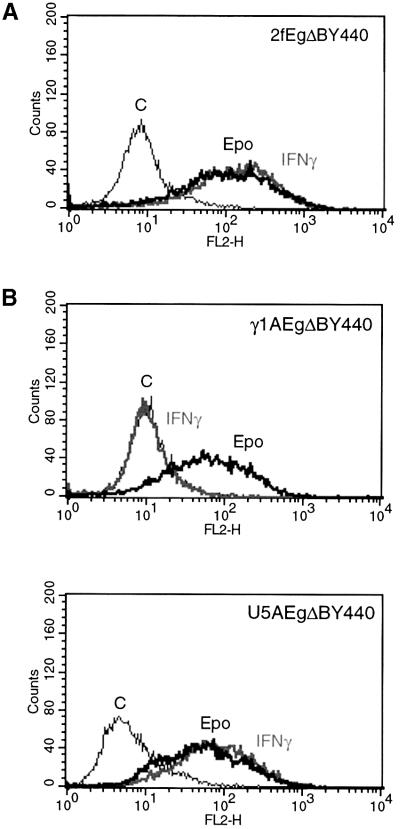
Induction of CIITA and HLA Class II expression. The induction of CIITA and Class II HLAs is unique to IFN-γ. Accordingly, it was of interest to determine whether such induction could also be mediated through the EgΔBY440 receptor. Class II HLA DRA is amongst the mRNAs for which induction is demonstrable by RNase protection (Figure 3A) as is CIITA (cf. Figure 9A). Both CIITA and representatives of the Class II HLAs are amongst the mRNAs for which induction is clearly detected on the cDNA arrays (Table I). On analysis of the 2f/EgΔBY440 cell line by fluorescence-activated cell sorting (FACS), Epo and IFN-γ at saturating concentrations induced comparable levels of cell surface Class II HLA expression (Figure 4A).
Fig. 4. The EgΔBY440 receptor mediates a Class II HLA response in wild-type cells (A) and in cells lacking functional IFN receptors (B). (A) Wild-type (2f) or (B) γ1A (lacking IFNGR2) or U5A (lacking IFNAR2) cells stably expressing the EgΔBY440 receptor were stimulated with 100 U/ml Epo or 1000 IU/ml IFN-γ for 72 h. Class II HLA expression was determined by FACS analysis using phycoerythrin-conjugated antibody to Class II HLA DRA (Materials and methods). Untransfected cells showed no response to Epo (data not presented).
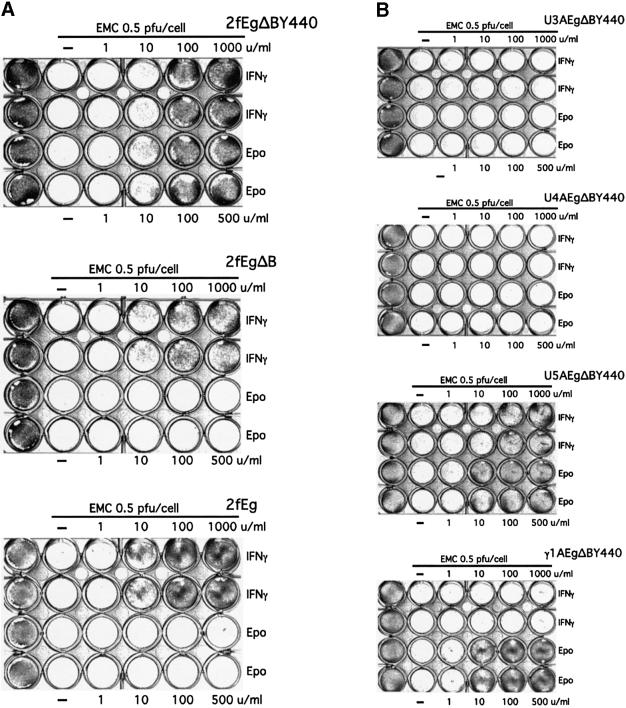
Induction of an antiviral state. It was of particular interest to determine whether an antiviral response comparable to that seen in response to IFN-γ could also be seen through the EgΔBY440 receptor. Comparable antiviral responses to Epo and IFN-γ are indeed observed in the 2f/EgΔBY440 cell line (Figure 5A, top). No such response is observed on Epo stimulation of the Eg or EgΔB receptors (Figure 5A, lower two panels) despite comparable levels of activation of JAK1/STAT1 or JAK1, respectively, in parallel controls. Nor is a response seen through EgΔBY440 in the STAT1- or JAK1-negative U3A and U4A cells (Figure 5B, top two panels).
Fig. 5. The EgΔBY440 receptor mediates an antiviral response (A) that is dependent on STAT1 and JAK1 but not on functional IFN receptors (B). An antiviral response is seen through EgΔBY440 but not Eg or EgΔB stably expressed in wild-type cells (A) and in U5A and γ1A cells lacking IFNAR2 and IFNGR2 receptor subunits, respectively (B, lower two panels). No such response is seen through the EgΔBY440 receptor in STAT1-negative U3A or JAK1-negative U4A cells (B, upper two panels). The different cell lines stably expressing the appropriate chimeras were incubated for 18 h at 37°C with the indicated concentrations of IFN-γ (in duplicate, upper two rows) or Epo (lower two rows), infected where shown with EMC virus (0.5 p.f.u./cell) and stained for live cells 24 h post-infection.
Thus, Epo stimulation through the tripartite EgΔBY440 receptor results in STAT1 activation, IFN-γ-inducible gene induction, Class II HLA expression and an antiviral state comparable to that seen in response to IFN-γ. For convenience of reference, the responses through the different receptors (including EgΔBY905, see below and Figure 9) in wild-type 2fTGH cells and through EgΔBY440 in the various mutant cell lines are summarized in Tables II and III, respectively. Similar results have been obtained with three clones of 2f/EgΔBY440 cells and, less extensively, with the chimeric receptors stably expressed in human lung adenocarcinoma SKLC-7 (e.g. Figures 7 and 8) and A375 melanoma cells.
Table II. Comparison of the responses to Epo of 2fTGH cells stably expressing the chimeric receptors with those induced through the endogenous receptors by IFN-γ and IL-6.
| Receptor | Ligand | JAK activationa | STAT1 activation | IRF-1 mRNA | IFN-γ-inducible mRNAs | Class II HLAb | Antiviral response |
|---|---|---|---|---|---|---|---|
| Eg | Epo | + | + (T)c | + (T)c | – | – | – |
| EgΔB | Epo | + | –d | – | – | – | – |
| EgΔBY440 | Epo | + | + | + | + | + | + |
| EgΔBY905 | Epo | + | + | + | + | + | + |
| IFN-γ | IFN-γ | + | + | + | + | + | + |
| IL-6 | IL-6 | + | + (T)c | + (T)c | – | – | – |
aJAKs1 and 2 for IFN-γ and JAKs1, 2 and Tyk2 for remainder.
bmRNA (RNase protection) for all receptors and cell surface protein (FACS) for EgΔBY440/905, IFN-γ and IL-6.
c (T), transient.
dBarely detectable by EMSA.
Table III. Summary of responses in various mutant cell lines stably expressing EgΔBY440.
| Cell line stably expressing EgΔBY440 | Defect | STAT1 phosphorylation induced by |
Antiviral response induced by |
||||
|---|---|---|---|---|---|---|---|
| IFN-α | IFN-γ | Epo | IFN-α | IFN-γ | Epo | ||
| U1A | Tyk2 | – | + | + | – | + | + |
| U5A | IFNAR2 | – | + | + | – | + | + |
| γ1A | IFNGR2 | + | – | + | + | – | + |
| γ2A | JAK2 | + | – | + | + | – | + |
| 2fTGH | – | + | + | + | + | + | + |
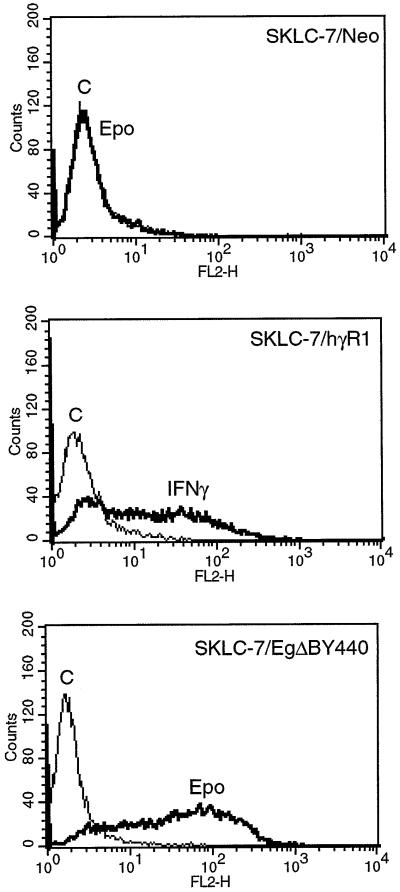
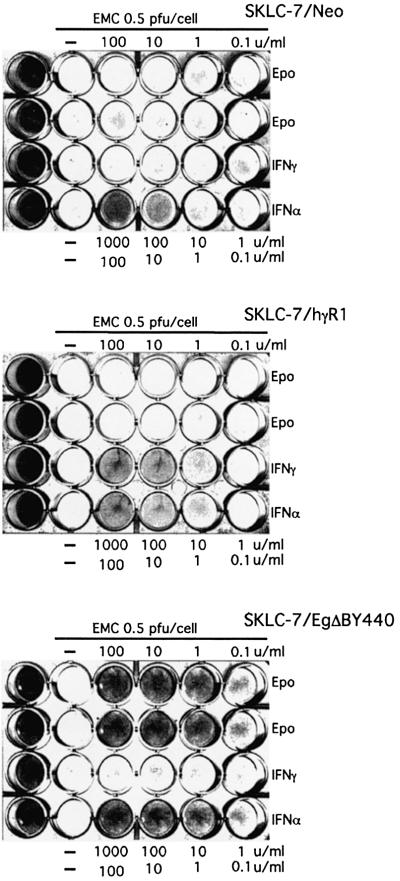
Fig. 7. The induction of Class II HLAs through the chimeric EgΔBY440 receptor is not dependent on IFNGR1. IFNGR1-deficient SKLC-7 cells (SKLC-7/Neo), IFNGR1-complemented SKLC-7 cells (SKLC-7/hγR1) and IFNGR1-deficient SKLC-7 cells stably expressing the chimeric EgΔBY440 receptor (SKLC-7/EgΔBY440) were stimulated with 100 U/ml Epo or 1000 IU/ml IFN-γ for 72 h. Cell surface expression of Class II HLAs was monitored as in Figure 4. There was no response to IFN-γ or Epo in cells not expressing the transfected IFNGR1 or EgΔBY440 receptors, respectively.
Fig. 8. The antiviral response mediated through the EgΔBY440 receptor is not dependent on IFNGR1. IFNGR1-deficient SKLC-7 cells (SKLC-7/Neo), IFNGR1-complemented SKLC-7 cells (SKLC-7/hγR1) and IFNGR1-deficient SKLC-7 cells stably expressing the chimeric EgΔBY440 receptor (SKLC-7/EgΔBY440) were treated with the indicated concentrations of Epo (duplicate rows), IFN-γ or IFN-α for 24 h, infected with EMC virus at 0.5 p.f.u./cell and stained for live cells 4 days post-infection.
The IFN-γ-like response is dependent on STAT1 but not upon signalling through IFN-α/β or -γ receptors
The induction of the IFN-γ-like responses through the EgΔBY440 receptor is rapid and Epo dependent. Nevertheless, it was essential to exclude any possibility that they are mediated through a classical interferon or interferon receptor response. Accordingly, the Epo response through EgΔBY440 was assayed in stably transfected U5A and γ1A cells that lack functional IFNAR2 and IFNGR2 and hence functional IFN-α/β and -γ receptors, respectively. Class II HLA and antiviral responses comparable to those observed in the wild-type 2f/EgΔBY440 cells were observed (Figures 4 and 5). It is reasonable to conclude that the IFN-γ-like response through EgΔBY440 is not dependent upon intact Type I or Type II IFN receptors and does not reflect the secondary activation of classical IFN-α/β or -γ signalling.
The IFN-γ-like response through Y440 is dependent on STAT1 (Figures 2A, 3B and 5B). Although JAKs 1 and 2 and Tyk2 are all similarly activated through gp130 in response to IL-6, JAK1 plays the major role in signalling (Guschin et al., 1995). As expected for a chimeric receptor utilizing the gp130 JAK recruitment domain, all three JAKs are also activated through EgΔBY440 (Figure 2). However, experiments with the mutant U1A and γ2A cell lines that lack Tyk2 and JAK2, respectively, similar to those for U5A (lacking IFNAR2) and γ1A (lacking IFNGR2) above, excluded any requirement for Tyk2 and JAK2 per se and hence for any classical IFN-α/β or -γ responses (Table III). The situation with respect to JAK1 is more complex, as would be expected in light of its major role in signalling through gp130. In the absence of JAK1, lower levels of STAT1 and receptor phosphorylation are observed presumably through JAK2 and Tyk2 (e.g. Figure 2A, lane 13; Guschin et al., 1995). There is, in addition, a reduced transcriptional and no antiviral response to EMC virus (V.Arulampalam, B.Strobl, S.J.Newman and I.M.Kerr, unpublished data) in the absence of JAK1. It will be of interest to determine to what extent these latter defects reflect the reduced quantitative response versus any requirement for additional JAK1-specific signals.
Cross phosphorylation of IFNGR1
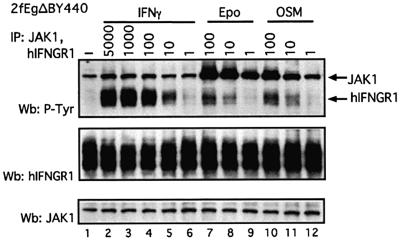
The above data (Figures 4B and 5B; Table III) exclude signalling by Y440 through an intact functional IFN-γ receptor but leave open the possible recruitment of IFNGR1, the signal transduction subunit of the IFN-γ receptor. Consistent with this possibility, IFNGR1 is phosphorylated in response to Epo in the wild-type 2fTGH cells stably expressing EgΔBY440 (Figure 6, lanes 7 and 8). Importantly, IFNGR1 is similarly phosphorylated in a number of cell lines in response to OSM (e.g. Figure 6, lanes 10 and 11) and IL-6 and, to a lesser extent, IFN-α through their endogenous receptors (H.Is’harc, unpublished data). The phosphorylation of IFNGR1 is extremely rapid (within 1 min) and is seen with low to moderate physiological concentrations of Epo, OSM, IL-6 and IFN-α. It is low compared with that observed in response to saturating concentrations of IFN-γ, but assuming it mimics that seen in response to IFN-γ in the context of the natural IFN-γ receptor, it would appear sufficient to mediate a signal (H.Is’harc, B.Strobl and I.M.Kerr, in preparation). Thus, it would appear that substantial cross phosphorylation of IFNGR1 can occur in response to signalling through both EgΔBY440 and endogenous receptors. The significance of this with respect to the EgΔBY440 response was, therefore, investigated in cells lacking IFNGR1.
Fig. 6. Cross phosphorylation of IFNGR1 through the EgΔBY440 and endogenous OSM receptors. Wild-type 2fTGH cells stably transfected with the EgΔBY440 receptor were treated for 15 min at 37°C with the indicated concentrations of IFN-γ, Epo or OSM. Cell lysates were subjected to immunoprecipitation with anti-JAK1 and anti-IFNGR1 antibodies. Immunoprecipitates were separated by 7.5% SDS–PAGE and western blots were performed with anti-phosphotyrosine antibodies. Stripped membranes were reprobed with antibodies to IFNGR1 and JAK1.
The IFN-γ-like response is not dependent on IFNGR1
A mutant cell line lacking IFNGR1 was not amongst those isolated on mutagenesis of HT1080 cells and used throughout the remainder of this work. To date we have been unable to obtain significant expression of the EgΔBY440 receptor in mouse embryo fibroblasts (MEFs) from IFNGR1 knock-out mice. Use, therefore, was made of IFNGR1-negative human SKLC-7 cells for which complementation with IFNGR1 restores the IFN-γ response (Kaplan et al., 1998). When the Epo response in SKLC-7 cells stably expressing EgΔBY440 was compared with the IFN-γ response of the SKLC-7s complemented with IFNGR1, comparable levels of JAK/STAT1 activation were observed similar to those seen (Figure 2) for the HT1080-based cell systems (data not presented). Consistent with this, very similar Class II HLA (Figure 7) and antiviral (Figure 8) responses were obtained in response to Epo and IFN-γ in the SKLC-7/EgΔBY440 and SKLC-7/IFNGR1 cells, respectively. Thus, the IFN-γ-like response mediated through the EgΔBY440 receptor is not dependent on IFNGR1.
A similar IFN-γ-like response can be mediated by a completely foreign receptor (EγΔBY905)
Having excluded a requirement for the complete IFNGR1, it was of interest to determine to what extent the IFN-γ-like response mediated through EgΔBY440 is dependent on a unique contribution from the IFNGR1 Y440 motif. The response mediated by a chimera in which the seven amino acid Y440 STAT1 recruitment motif from the IFNGR1 was replaced by a sequence of eight amino acids encompassing the Y905 STAT recruitment motif of gp130 (EgΔY905, Figure 1) was, therefore, investigated. A number of clones of 2fTGH cells all expressed the stably transfected, flag-tagged EgΔBY905 receptor to slightly lower levels than that observed for the EgΔBY440 receptor in the clone (clone 7) used for the majority of the work here. Consistent with this, activation of the JAK/STAT1 pathway in the best of the 2f/EgΔBY905 clones, although prolonged (Figure 9B), appeared on average 3- to 5-fold lower than that observed for the 2f/EgΔBY440 clone 7 cells. Despite the reduced signal, the response was sufficient to mediate a clear antiviral response (Figure 9C) and cell surface expression of Class II HLA and to detect, by RNase protection assay, clear induction of a test set of IFN-stimulable genes (ISGs), including CIITA and the Class II HLA DRA (Figure 9A, top panel, lanes 8–10, lower panel, lanes 11–15). These latter ISGs are also induced in response to IFN-γ (Figure 9A, lower panel, lanes 1–5) and through the EgΔBY440 receptor (Figure 9A, lower panel, lanes 6–10), but not in response to Eg (Figure 9, upper panel, lanes 11–14), OSM (Figure 9, upper panel, lanes 1 and 5–7) or IL-6. In addition, for the IRF1 RNA, induction through the Y905 and Y440 chimeras shows the prolonged kinetics characteristic of an IFN-γ-type response rather than the very transient induction of this RNA seen with OSM and through the full-length Eg chimera (Figure 9A, upper panel) or with IL-6. On expression profiling using low-density cDNA arrays (as for Table I) the fifteen genes most highly induced by IFN-γ were also induced through the EgΔBY905 chimera but to a substantially lower level, consistent with a quantitatively reduced response to the latter (data not presented). Overall, the responses through the Y440 and Y905 receptors appear very similar but not identical. Whether this reflects purely quantitative or additional qualitative differences (Discussion) remains to be established. Irrespective of this it can be concluded that signalling through the completely foreign ‘Y905’ receptor also mediates an IFN-γ-like response.
Discussion
Classical deletion, mutation and receptor chimera analyses established a set of requirements for the human IFNGR1 subunit and for the human IFN-γ receptor to function in mouse cells (reviewed by Bach et al., 1997; Introduction). These requirements were for the recruitment of JAK2 through the IFNGR2 to trigger the response and for the membrane-proximal JAK1 recruitment domain and C-terminal Y440 motif of IFNGR1 to transduce the signal. Against this background it seemed possible that activation of STAT1 was not only essential but also sufficient to mediate an IFN-γ response. Consistent with this, dimerization of a transiently overexpressed STAT1/oestrogen receptor chimera in response to oestrogen is sufficient to induce the transcriptional activation of a number of IFN-γ ISGs including Class II HLAs (Milocco et al., 1999). On the other hand, there is increasing evidence for additional signalling in response to IFN-γ. Recent experiments in STAT1-negative U3A cells and MEFs have provided direct evidence for JAK/non-STAT1 signalling (Ramana et al., 2000, 2001; Gil et al., 2001). Moreover, STAT activity is modulated by serine phosphorylation. It is likely that MAP kinases are amongst those required for this phosphorylation, particularly for STAT3 (Chung et al., 1997; Goh et al., 1999). The enzymes responsible for STAT1 Ser727 phosphorylation in response to IFN-γ remain, however, to be rigorously identified (Kovarik et al., 1999; Uddin et al., 2000a). For the α/β IFNs, activation of IRS1 and PI3-kinase, requirements for STAT1 methylation (Mowen et al., 2001), and histone phosphorylation (Uddin et al., 2000b) for full transcriptional activation have recently been reported and it would not be unreasonable to expect similar requirements to hold for the response to IFN-γ. Accordingly, although JAK/STAT1 signalling is essential for the IFN-γ response it is clearly complemented by a number of additional signals. To the extent that these additional pathways are required for the induction of multiple ISGs (Table II), Class II HLAs (Figure 4) and an antiviral state (Figure 5), they must either be constitutively active or be activated in response to the chimeric receptors. In the HT1080-based cell lines used here there is constitutive Ser727 phosphorylation of STAT1 and p38 MAP kinase activity. Nevertheless, increases in both, as great or greater than those for IFN-γ, can be seen through the EgΔB chimeras (A.P.Costa-Pereira, B.Strobl and I.M.Kerr, unpublished). Importantly, although ultimately of considerable interest, the precise levels of activation of additional signalling pathways and their roles in response to the chimeric receptors are not central to the major argument here, i.e. it is likely that it is the signals per se not their source, or how they are generated, that determine the outcome.
In fact, the responses to the chimeras likely do not precisely mimic the IFN-γ response. It is possible, for example, that the minor differentials between the responses through the ‘Y905’ and ‘Y440’ receptors are not only quantitative but also reflect differential activation of one or more additional pathways. More particularly, for comparable levels of STAT1 Tyr701 and Ser727 phosphorylation, the transcriptional response to IFN-γ appears consistently ∼3-fold higher than that for the Y440 chimera, suggestive of a possible differential activation of an additional pathway(s) affecting, in this case, the efficiency of transcriptional activation (V.Arulampalam, B.Strobl and I.M.Kerr, unpublished data). That said, although the responses through the chimeras may not precisely mimic those through the endogenous receptors, it is important to emphasize that they are not overexpression artefacts. The levels of JAK/STAT1 activation through the chimeras are not abnormal, they are comparable to those for the endogenous IFN-γ, IL-6 and OSM receptors (Figures 2 and 9) and, most importantly, they are observed with low physiological concentrations of Epo. Moreover, similar results have been obtained for clones of cells expressing substantially lower as well as higher levels of the EgΔBY440 chimera in particular.
The results of recent microarray analyses have emphasized a high degree of overlap in the expression profiles observed in response to different growth factors (Fambrough et al., 1999). Here, the results suggest a high degree of overlap in signalling from the disparate JAK1, JAK2, Tyk2/gp130-based and JAK1, JAK2/IFN-γ receptor-based complexes. It may not be completely inappropriate, therefore, to think in terms of a set of generic JAK/receptor signals that are fine-tuned to give specificity in response to different ligands.
There is increasing evidence for physiologically significant receptor cross recruitment/activation (e.g. Chin et al., 1997; Jubinsky et al., 1997; Yamauchi et al., 1997, 2000; Wu et al., 1997; Qui et al., 1998; von Lindern et al., 2000). It is of particular interest that IFNGR1 is rapidly phosphorylated not only in response to stimulation through the chimeric Y440 receptor but also through the endogenous OSM, IL-6 and IFN-α/β receptors (Figure 6; H.Is'harc and I.M.Kerr, unpublished data). Clearly IFNGR1 is not essential for the IFN-γ-like response through the chimeric receptor (Figures 7 and 8); nor does cross phosphorylation of the IFNGR1 confer an IFN-γ-like response upon OSM or IL-6 (Figures 2 and 9) for which, incidentally, neither a Class II HLA nor an antiviral response is observed (data not presented). One may assume, however, that the cross phosphorylation nevertheless has relevance downstream of the endogenous receptors.
Taniguchi has emphasized a role for autocrine IFN-β in priming the IFN-γ response (Takaoka et al., 2000; Taniguchi and Takaoka, 2001). Neither the activation of STAT1 nor the antiviral responses to IFN-γ or the chimeric Y440 receptor are, however, affected by the absence of a functional IFN-α/β receptor in the human HT1080-based cell systems used here (Figure 5B; A.P.Costa-Pereira, T.M.Williams and I.M.Kerr, in preparation). Accordingly, it appears unlikely that an autocrine loop involving α/β-IFNs, similar to that established in MEFs, plays any role in the IFN-γ-like responses observed in these human cell systems. While there is no need to invoke it, in the absence of a human cell line lacking IFNAR1, a requirement for IFNAR1 for the IFN-γ-like response to the chimeric receptors cannot formally be excluded.
It is intriguing that in contrast to the very similar responses observed through the EgΔBY440 and endogenous IFN-γ receptors, two closely related chimeric receptors yield very different responses. The Eg chimera mediates an IL-6-like response whereas the EgΔBY905 (effectively a deletion mutant of Eg, Figure 1) yields a very different IFN-γ-like response (Figure 9). A priori one might suspect a major role for the gp130 Y759 SHP2 recruitment motif in mediating this differential. Despite slightly prolonged JAK/STAT activation, the response through a comparably expressed Eg receptor bearing a point mutation in this motif (EgY759F) is, however, characteristic of IL-6 not IFN-γ (F.Schaper, B.Strobl, S.J.Newman and I.M.Kerr, unpublished). One obvious difference between the IL-6 family and IFN-γ or chimera-mediated responses is the prolonged STAT1 activation observed for the latter (Figures 2C and 9). A correlation between prolonged STAT1 activation and an antiviral response has previously been noted for signalling through IFNAR2 chimeras (Pattyn et al., 1999). While prolonged STAT1 activation may well be essential to sustain an antiviral response it presumably cannot alone account for the very different spectrum of early transcription mediated through the Eg (or IL-6 family) versus the EgΔBY440/Y905 (or IFN-γ) receptors. The EgΔBY905 and Eg receptors have in common the gp130 JAK-binding domain and the Y905 motif. It is reasonable to assume that for both receptors the former mediates the activation of the JAK/STAT, P-Ser727, p38 and additional pathways, while recruitment of the STATs is predominantly through the Y905 motif. A priori the differences in the response could reflect differences in STAT3 activation, additional signals, either positive or negative, mediated through the fragment of the receptor present in Eg but not EgΔBY905, or altered signalling through differences in the conformation of the JAK/truncated versus ‘intact’ receptor complexes. Irrespective of this, the ability to manipulate easily the relatively small cytoplasmic domain of the chimeric receptors should considerably facilitate the further definition of the differentials in signalling that determine characteristic IFN-γ versus IL-6 responses.
Here it is shown that activation of STAT1, together with whatever additional signals may be necessary, through minimal chimeric receptors, is likely to be as effective in mediating major aspects of an IFN-γ response as the activation of these signals through the natural IFN-γ receptor. In a complementary biophysical approach we have recently shown that STAT1–GFP, with or without activation in response to the IFNs, diffuses freely in the cytoplasm prior to interaction with the nuclear pore and in the nucleoplasm prior to interaction with DNA or protein complexes (Lillemeier et al., 2001). The data do not exclude interaction with additional proteins or mixtures of ‘soft-’ and ‘hard-wiring’ (Lillemeier et al., 2001), but argue against the involvement of non-dynamic scaffolds or ‘pathways’. Taken together the results from these two very different approaches argue strongly for highly flexible ‘soft-’ rather than ‘hard-wiring’ of modular JAK/STAT signalling. In addition, the high degree of similarity in the responses through the gp130/JAK-based chimeras and the IFN-γ receptor–JAK complexes imply a great deal of overlap in the signals emanating from these, on the face of it, very different receptor complexes. This, in turn, suggests that relatively subtle changes in overall signalling may profoundly affect the expression profiles and responses observed.
Materials and methods
Cell culture
Human fibrosarcoma cell lines 2fTGH, U3A, U4A, U5A, γ1A and γ2A (Pellegrini et al., 1989; Müller et al., 1993; Watling et al., 1993) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 2 mM glutamine, 10% heat-inactivated fetal calf serum, 500 U/ml penicillin and 500 µg/ml streptomycin. Resistant cells were maintained in medium containing 250 µg/ml hygromycin (Calbiochem), 400 µg/ml G418 (Life Technologies) and/or 1 µg/ml puromycin (Sigma). SKLC-7/Neo and SKLC-7/hγR1 cells (Kaplan et al., 1998) were cultured in RPMI-1640 medium supplemented with 2 mM glutamine, 10% heat-inactivated fetal calf serum, 500 U/ml penicillin, 500 µg/ml streptomycin, 1 mM sodium pyruvate, 50 µM β-mercaptoethanol and 500 µg/ml G418. A highly purified mixture of human IFNs-α [Wellferon; 1.5 × 108 IU/mg protein (Allen et al., 1982)] was provided by Wellcome Research Laboratories, Beckenham, Kent, UK. Highly purified recombinant IFN-γ (4 × 107 IU/mg protein) and Epo (19.5 × 104 U/mg protein) were generous gifts from G.Adolf, Ernst Boehringer Institut für Arzneimittelforschung, Vienna, Austria and Drs S.K.Sellinger and B.Hilger, Roche Diagnostics, Penzberg, Germany, respectively.
Plasmids and transfected cells
PRcCMVEg/EgΔB/EgΔBY440/EgΔBY905 were derived as described previously (Gerhartz et al., 1996; Schmitz et al., 2000a). For stable transfectants, wild-type 2fTGH and mutant cells (106) were transfected with 10 µg of plasmid using Superfect (Qiagen), selected in 1 mg/ml G418 (Life Technologies) and cloned by limiting dilution. In the case of cells already resistant to G418 (γ1A, γ2A and SKLC-7/Neo), cotransfection of the chimeric receptors was with 0.5 µg of pBABE (a gift from Dr H.Land) and selection with 1 µg/ml puromycin.
Antibodies
Antibodies to JAK1, JAK2 and Tyk2 were from Santa Cruz and to STAT1 from Novacastra. Phosphorylated tyrosines were detected using a mix of PY-20 (Transduction Laboratories) and 4G10 (Upstate Biotechnology) antibodies. Phycoerythrin (PE)-conjugated anti-HLA-DRα was purchased from Becton Dickinson.
Cell lysis, immunoprecipitations, western blotting and EMSAs
Cell lysis was performed on ice using 50 mM Tris pH 8.0, 0.5% NP-40, 10% glycerol, 150 mM NaCl, 1 mM dithiothreitol (DTT), 0.1 mM EDTA, 0.2 mM sodium orthovanadate, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 3 µg/ml aprotinin and 1 µg/ml leupeptin cell debris were removed by centrifugation and whole cell extracts used for EMSA or immunoprecipitations essentially as described previously (Lillemeier et al., 2001).
RNase protection assays
Cytoplasmic RNA was isolated using NP-40 lysis and phenol–chloroform extraction (Porter et al., 1988) and protection assays carried out as previously (Müller et al., 1993). For CIITA the probe was generated from a 350 bp HindIII fragment (nucleotides 2935–3285) from a human CIITA cDNA (from Dr R.Flavell) cloned into pGEM4.
Antiviral assays
Cells seeded into 24-well plates at 2 × 105 cells/well were incubated overnight at 37°C, treated with serial dilutions of ligand for 18 h and challenged with 0.5 p.f.u./cell of EMC virus. Cells were fixed and stained for live cells (Giemsa) 24 h (fibrosarcoma cell lines) or 4 days (SKLC-7 lines) after infection.
Fluorescence-activated cell sorting
Cells treated with medium only, 1000 U/ml IFN-γ or 100 U/ml Epo for 72 h were removed from the plate with buffered EDTA, washed in ice-cold medium, incubated with phycoerythrin-conjugated anti-HLA DRA antibody for 45 min on ice, washed three times with ice-cold phosphate-buffered saline, fixed in 3% paraformaldehyde and analysed on a fluorescence-activated cell scanner (Becton Dickinson).
Expression profiling: macroarray analysis
Total RNA was isolated from cells using Trizol (Life Technologies) according to manufacturer’s instructions, precipitated with 0.3 M LiCl, dissolved in 1 mM sodium citrate pH 6.8, quantified spectrophotometrically and checked by agarose gel electrophoresis. For radioactively labelled cDNA, 25 µg of total RNA were reverse transcribed with 200 U of Superscript II reverse transcriptase (Life Technologies) in the presence of 100 µCi of [33P]dCTP for 1 h at 42°C. The resulting RNA–cDNA hybrid was incubated with 2 U of RNaseH (Life Technologies) for 20 min at 37°C. The 33P-labelled cDNA was heated for 5 min at 65°C and nucleotides and short oligomers removed using G50 columns. The radioactive cDNA was hybridized with 50 µg of human Cot-1 DNA (Life Technologies) for 30 min at 65°C. Preparation of the macroarrays (representing ∼150 known IFN-inducible genes), hybridization of the radioactive cDNAs and scanning of the arrays were carried out essentially as described previously (Aberger et al., 2001; in more detail, J.F.Schlaak, in preparation).
Acknowledgments
Acknowledgements
We are heavily indebted to Drs K.-H.Sellinger and B.Hilger for the erythropoietin and to Dr G.Adolf for the IFN-γ. R.D.S. and K.C.F.S. were supported in part by NIH grant CA43059.
References
- Aberger F., Costa-Pereira,A.P., Schlaak,J.F., Williams,T.M., O’shaughnessy,R.F.L., Hollaus,G., Kerr,I.M. and Frischauf,A.M. (2001) Analysis of gene expression using high density and IFN-γ-specific low-density cDNA arrays. Genomics, in press. [DOI] [PubMed] [Google Scholar]
- Allen G., Fantes,K.H., Burke,D.C. and Morser,J. (1982) Analysis and purification of human lymphoblastoid (Namalwa) interferon using a monoclonal antibody. J. Gen. Virol., 63, 207–212. [DOI] [PubMed] [Google Scholar]
- Bach E.A., Tanner,J.W., Marsters,S., Ashkenazi,A., Aguet,M., Shaw,A.S. and Schreiber,R.D. (1996) Ligand-induced assembly and activation of the γ interferon receptor in intact cells. Mol. Cell. Biol., 16, 3214–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach E.A., Aguet,M. and Schreiber,R.D. (1997) The IFNγ receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol., 15, 563–591. [DOI] [PubMed] [Google Scholar]
- Briscoe J., Rogers,N.C., Witthuhn,B.A., Watling,D., Harpur,A.G., Wilks,A.F., Stark,G.R., Ihle,J.N. and Kerr,I.M. (1996) Kinase-negative mutants of JAK1 can sustain interferon-γ-inducible gene expression but not an antiviral state. EMBO J., 15, 799–809. [PMC free article] [PubMed] [Google Scholar]
- Chin H., Wakao,H., Miyajima,A., Kamiyama,R., Miyasaska,N. and Miura,O. (1997) Erythropoietin induces tyrosine phosphorylation of the interleukin-3 receptor β subunit (βIL3) and recruitment of Stat5 to possible Stat5-docking sites in βIL3. Blood, 89, 4327–4336. [PubMed] [Google Scholar]
- Chung J., Uchida,E., Grammer,T.C. and Blenis,J. (1997) STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol. Cell. Biol., 17, 6508–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.R., Jung,V., Schwartz,B., Wang,P. and Pestka,S. (1992) Structural analysis of the human interferon γ receptor: a small segment of the intracellular domain is specifically required for class I major histocompatibility complex antigen induction and antiviral activity. Proc. Natl Acad. Sci. USA, 89, 11317–11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J.E. Jr, Kerr,I.M. and Stark,G.R. (1994) Jak–STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science, 264, 1415–1421. [DOI] [PubMed] [Google Scholar]
- Fambrough D., McClure,K., Kazlauskas,A. and Lander,E.S. (1999) Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, set of genes. Cell, 97, 727–741. [DOI] [PubMed] [Google Scholar]
- Farrar M.A., Campbell,J.D. and Schreiber,R.D. (1992) Identification of a functionally important sequence in the C terminus of the interferon-γ receptor. Proc. Natl Acad. Sci. USA, 89, 11706–11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhartz C., Heesel,B., Sasse,J., Hemmann,U., Landgraf,C., Schneider-Mergener,J., Horn,F., Heirich,P.C. and Graeve,L. (1996) Differential activation of acute phase response factor/STAT3 and STAT1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. J. Biol. Chem., 271, 12991–12998. [DOI] [PubMed] [Google Scholar]
- Giannakakou P., Sackett,D.L., Ward,Y., Webster,K.R., Blagosklonny, M.V. and Fojo,T. (2000) p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nature Cell Biol., 2, 709–717. [DOI] [PubMed] [Google Scholar]
- Gil M.P., Bohn,E., O’Guin,A.K., Ramana,C.V., Levine,B., Stark,G.R., Virgin,H.W. and Schreiber,R.D. (2001) Biologic consequences of Stat1-independent IFN signaling. Proc. Natl Acad. Sci. USA, 98, 6680–6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh K.C., Haque,S.J. and Williams,B.R.G. (1999) p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J., 18, 5601–5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlund A.C., Farrar,M.A., Viviano,B.L. and Schreiber,R.D. (1994) Ligand-induced IFNγ receptor tyrosine phosphorylation couples the receptor to its signal transduction system (p91). EMBO J., 7, 1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guschin D. et al. (1995) A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J., 14, 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P.C., Behrmann,I., Müller-Newen,G., Schaper,F. and Graeve,L. (1998) Interleukin-6-type cytokine signaling through the gp130/Jak/STAT pathway. Biochem. J., 334, 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J.N. and Kerr,I.M. (1995) Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet., 11, 69. [DOI] [PubMed] [Google Scholar]
- Jubinsky P.T., Krijanovski,O.I., Nathan,D.G., Tavernier,J. and Sieff,C.A. (1997) The β chain of the interleukin-3 receptor functionally associates with the erythropoietin receptor. Blood, 90, 1867–1873. [PubMed] [Google Scholar]
- Kaplan D.H., Greenlund,A.C., Tanner,J.W., Shaw,A.S. and Schreiber,R.D. (1996) Identification of an interferon-γ receptor α chain sequence required for JAK-1 binding. J. Biol. Chem., 271, 9–12. [DOI] [PubMed] [Google Scholar]
- Kaplan D.H., Shankaran,V., Dighe,A.S., Stockert,E., Aguet,M., Old,L.J. and Schreiber,R.D. (1998) Demonstration of an interferon γ-dependent tumor surveillance system in immunocompetent mice. Proc. Natl Acad. Sci. USA, 95, 7556–7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko S.V. et al. (1995) Interaction between the components of the interferon γ receptor complex. J. Biol. Chem., 270, 20915–20921. [DOI] [PubMed] [Google Scholar]
- Kovarik P., Stoiber,D., Eyers,P.A., Menghini,R., Neininger,A., Gaestel,M., Cohen,P. and Decker,T. (1999) Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated protein kinase whereas IFN-γ uses a different signaling pathway. Proc. Natl Acad. Sci. USA, 96, 13956–13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillemeier B.F., Köster,M. and Kerr,I.M. (2001) STAT1 from the cell membrane to the DNA. EMBO J., 20, 2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milocco L.H., Haslam,J.A., Rosen,J. and Seidel,H.M. (1999) Design of conditionally active STATs: insights into STAT activation and gene regulatory function. Mol. Cell. Biol., 19, 2913–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowen K.A., Tang,J., Zhu,W., Schurter,B.T., Shuai,K., Herschman,H.R. and David,M. (2001) Arginine methylation of STAT1 modulates IFNα/β-induced transcription. Cell, 104, 731–741. [DOI] [PubMed] [Google Scholar]
- Müller M. et al. (1993) The protein tyrosine kinase JAK1 complements defects in interferon-α/β and -γ signal transduction. Nature, 366, 129–135. [DOI] [PubMed] [Google Scholar]
- Nicholson S.E. et al. (2000) Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc. Natl Acad. Sci. USA, 97, 6493–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn E., van Ostade,X., Schauvliege,L., Verhee,A., Kalai,M., Vandekerckhove,J. and Tavernier,J. (1999) Dimerisation of the interferon Type I receptor IFNaR2-2 is sufficient for induction of interferon effector genes but not for full antiviral activity. J. Biol. Chem., 274, 34838–34845. [DOI] [PubMed] [Google Scholar]
- Pellegrini S., John,J., Shearer,S., Kerr,I.M. and Stark,G.R. (1989) Use of a selectable marker regulated by α interferon to obtain mutations in the signaling pathway. Mol. Cell. Biol., 9, 4605–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui Y., Ravi,L. and Kung,H.-J. (1998) Requirement of ErbB2 for signalling by interleukin-6 in prostate carcinoma cells. Nature, 393, 83–85. [DOI] [PubMed] [Google Scholar]
- Ramana C.V., Grammatikakis,N., Chernov,M., Nguyen,H., Goh,K.C., Williams,B.R.G. and Stark,G.R. (2000) Regulation of c-myc expression by IFN-γ through STAT1-dependent and independent pathways. EMBO J., 19, 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramana C.V., Gil,M.P., Han,Y., Ransohoff,R.M., Schreiber,R.D. and Stark,G.R. (2001) Stat1-independent regulation of gene expression in response to IFN-γ. Proc. Natl Acad. Sci. USA, 98, 6674–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J., Dahmen,H., Grimm,C., Gendo,C., Müller-Newen,G., Heinrich,P.C. and Schaper,F. (2000a) The cytoplasmic tyrosine motifs in full-length glycoprotein 130 have different roles in IL-6 signal transduction. J. Immunol., 164, 848–854. [DOI] [PubMed] [Google Scholar]
- Schmitz J., Weissenbach,M., Haan,S., Heinrich,P.C. and Schaper,F. (2000b) SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J. Biol. Chem., 275, 12848–12856. [DOI] [PubMed] [Google Scholar]
- Sekimoto T., Imamoto,N., Nakajima,K., Hirano,T. and Yoneda,Y. (1997) Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J., 16, 7067–7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl N., Farruggella,T.J., Boulton,T.G., Zhong,Z., Darnell,J.E.,Jr and Yancopoulos,G.D. (1995) Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science, 267, 1349–1353. [DOI] [PubMed] [Google Scholar]
- Stark G.R., Kerr,I.M., Williams,B.R.G., Silverman,R.H. and Schreiber,R.D. (1998) How cells respond to interferons. Annu. Rev. Biochem., 67, 227–264. [DOI] [PubMed] [Google Scholar]
- Takaoka A., Mitani,Y., Suemori,H., Sato,M., Yokichi,T., Noguchi,S., Tanaka,N. and Taniguchi,T. (2000) Cross talk between interferon-γ and -α/β signaling components in caveolar membrane domains. Science, 288, 2357–2360. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. and Takaoka,A. (2001) A weak signal for strong responses—interferon α/β revisited. Nature Rev. Mol. Cell Biol., 2, 378–386. [DOI] [PubMed] [Google Scholar]
- Terstegen L., Gatsios,P., Bode,J.G., Schaper,F., Heinrich,P.C. and Graeve,L. (2000) The inhibition of interleukin-6-dependent STAT activation by mitogen-activated protein kinases depends on tyrosine 759 in the cytoplasmic tail of glycoprotein 130. J. Biol. Chem., 275, 18810–18817. [DOI] [PubMed] [Google Scholar]
- Teruel M.N. and Meyer,T. (2000) Translocation and reversible localisation of signaling proteins: a dynamic future for signal transduction. Cell, 103, 181–184. [DOI] [PubMed] [Google Scholar]
- Uddin S., Lekmine,F., Sharma,N., Majchrzak,B., Mayer,I., Young,P.R., Bokoch,G.M., Fish,E.N. and Platanias,L.C. (2000a) The Rac1/p38 mitogen-activated protein kinase pathway is required for interferon α-dependent transcriptional activation but not serine phosphorylation of STAT proteins. J. Biol. Chem., 275, 27634–27640. [DOI] [PubMed] [Google Scholar]
- Uddin S., Deb,D., Lekmine,F., Majchrzak,B., Young,P.R., Bokoch,G., Fish,E.N. and Platanias,L.C. (2000b) Activation of the Rac1/p38 MAP kinase pathway by Type I IFNs regulates transcriptional activation via serine phosphorylation of Histone H3. Eur. Cytokine Network, 11, 150. [Google Scholar]
- von Lindern M., Parren-van Amelsvoort,M., van Dijk,T., Deiner,E., van den Akker,E., van Emst-de Vries,S., Willems,P., Beug,H. and Lowenberg,B. (2000) Protein kinase Cα controls erythropoietin receptor signaling. J. Biol. Chem., 275, 34719–34727. [DOI] [PubMed] [Google Scholar]
- Watling D. et al. (1993) Complementation by the protein Tyrosine kinase JAK2 of a mutant cell line defective in the interferon-γ signal transduction pathway. Nature, 366, 166–170. [DOI] [PubMed] [Google Scholar]
- Wu H., Klingmüller,U., Acurio,A., Hsiao,J.G. and Lodish,H.F. (1997) Functional interaction of erythropoietin and stem cell factor receptors is essential for erythroid colony formation. Proc. Natl Acad. Sci. USA, 94, 1806–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T. et al. (1997) Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone. Nature, 390, 91–96. [DOI] [PubMed] [Google Scholar]
- Yamauchi T. et al. (2000) Constitutive tyrosine phosphorylation of ErbB-2 via Jak2 by autocrine secretion of prolactin in human breast cancer. J. Biol. Chem., 275, 33937–33944. [DOI] [PubMed] [Google Scholar]