Abstract
Calcium-dependent protein kinases (CDPKs) comprise a large family of serine/threonine kinases in plants and protozoans. We isolated two related CDPK cDNAs (NtCDPK2 and NtCDPK3) from Nicotiana tabacum. These CDPK transcripts are elevated after race-specific defence elicitation and hypo-osmotic stress. Transiently expressed myc-epitope-tagged NtCDPK2 in Nicotiana benthamiana and N.tabacum leaves showed a rapid transient interconversion to an activated form after elicitation and hypo-osmotic stress. The Avr9 race-specific elicitor caused a more pronounced and sustained response. This transition is due to phosphorylation of the CDPK. Immuno complex kinase assays with epitope-tagged NtCDPK2 showed that stress-induced phosphorylation and interconversion of NtCDPK2 correlates with an increase in enzymatic activity. The function of NtCDPK2 in plant defence was investigated by employing virus-induced gene silencing (VIGS) in N.benthamiana. CDPK-silenced plants showed a reduced and delayed hypersensitive response after race-specific elicitation in a gene-for-gene interaction, and lacked an accompanying wilting phenotype. Silencing correlated with loss of CDPK mRNA, whereas mRNA accumulation of mitogen-activated protein kinase WIPK remained unaltered.
Keywords: CDPK/hypo-osmotic stress/plant defence response/tobacco/VIGS
Introduction
Plants are constantly exposed to changes in their environment and have developed mechanisms to cope with biotic and abiotic stress. Among the earliest cellular responses to such stress stimuli are changes in the cytoplasmic calcium concentration, and a specific calcium signature is often established (McAinsh and Hetherington, 1998). Calcium-dependent protein kinases (CDPKs) may function as a potential sensor that decodes and translates the elevation of calcium concentration into enhanced protein kinase activity and subsequent downstream signalling events (Harmon et al., 2000).
CDPKs are calcium-binding serine/threonine protein kinases. In contrast to calmodulin-dependent kinases, in CDPKs the catalytic kinase domain in the N-terminal half of the protein is directly tethered via an autoinhibitory junction domain to a regulatory calmodulin-like domain, which usually contains four functional EF hands for calcium binding (Satterlee and Sussman, 1998). CDPKs have a highly conserved structure. Isoform-specific differences are mainly restricted to the N-terminal variable domain, which in many CDPKs also includes a fatty acylation site (Hrabak, 2000). Myristoylation and palmitoylation at these sites have been shown for CpCPK1 from zucchini (Ellard-Ivey et al., 1999) and OSCPK2 from rice (Martin and Busconi, 2000), and are necessary for targeting to the membrane.
So far, CDPKs have not been identified in yeast and animal systems. Protein kinase C and calmodulin-dependent kinases are well characterized as major mammalian calcium-dependent signalling molecules, and it has been proposed that CDPKs play the same role in plants (Roberts and Harmon, 1992). CDPKs comprise a large gene family (34 members in Arabidopsis; Harmon et al., 2001). This suggests that individual isoforms have different functions and participate in multiple distinct signalling pathways. However, downstream CDPK-regulated processes remain largely unknown. The challenge that currently faces the CDPK field is not only to allocate defined biological functions to specific CDPK isoforms, but also to integrate CDPK signalling with other signal networks, for example, mitogen-activated protein (MAP) kinase cascades, whose complexity is also beginning to emerge.
Increasing evidence has been provided for CDPKs being involved in environmental stress signalling. CDPK transcript elevation was reported after exposure of Arabidopsis to cold, salt and drought (Urao et al., 1994; Tähtiharju et al., 1997), and remarkably, overexpression of rice OsCDPK7 yielded cold and salt/drought-tolerant rice plants (Saijo et al., 2000). Also, exposure to non-specific elicitors and mechanical wounding were reported to cause an increase of NtCDPK1 transcript in tobacco (Yoon et al., 1999). In a more physiological context, CDPK enzymatic activity has been correlated with osmotic stress (Takahashi et al., 1997) and elicitation (Allwood et al., 1999). However, in only one system so far, by choosing leaf protoplasts as an experimental approach, could the activity of a specific CDPK isoform from Arabidopsis, AtCPK10, be linked with the induction of environmental stress-related promoters after abscisic acid treatment (Sheen, 1996).
The tomato Cf-9 disease resistance gene confers responsiveness in transgenic tobacco to the Avr9 race-specific elicitor (Hammond-Kosack et al., 1998). We have recently identified a 68/70 kDa CDPK from tobacco that becomes biochemically activated in response to Avr9 elicitation in Cf-9 tobacco (Romeis et al., 2000). As demonstrated by in-gel kinase assays and western blot analysis, elicitation caused a phosphorylation-dependent transition from a non-elicited enzyme form into an elicited form, which differed in its electrophoretic mobility. This allowed us to characterize the enzyme in a defence-related biological system after applying an in vivo stimulus. These data suggested that the 68/70 kDa CDPK functions in a signalling pathway to recruit the plant defence.
In this study, we report the cloning of two tobacco CDPK cDNAs, NtCDPK2 and NtCDPK3, which belong to the same subfamily. The cDNAs were isolated from tobacco cell suspension cultures expressing Cf-9 as transgene, which were elicited with Avr9. NtCDPK2 protein was biochemically characterized after expression of a triple-myc-tagged version in Nicotiana benthamiana and Nicotiana tabacum using an Agrobacterium-mediated transient assay. This allowed us to study the NtCDPK2-myc enzyme in response to external stress stimulation, irrespective of the presence of other endogenous CDPKs. NtCDPK2-myc showed a rapid and transient interconversion between two enzyme forms after osmotic stress and race-specific elicitation stimuli, and the activated form showed elevated protein kinase activity.
To address the function of NtCDPK2 and NtCDPK3 in plant defence we employed virus-induced gene silencing (VIGS) in N.benthamiana as a reverse genetics approach (Baulcombe, 1999). VIGS, based on a cosuppression mechanism, was predicted to silence closely related genes. This allowed us to circumvent the problem of gene redundancy in tobacco by potentially silencing the entire NtCDPK2 subfamily. The CDPK-silenced plants were compromised in the Cf-9/Avr9- and Cf-4/Avr4-mediated activation of HR and lacked the concomitant characteristic wilting phenotype.
Our data show that: (i) a CDPK isoform, NtCDPK2, participates in signalling pathways triggered by different external biotic and abiotic stress stimuli; (ii) the phosphorylation-dependent transition between two enzyme forms is correlated with higher enzyme activity. (iii) NtCDPK2 and/or closely related subfamily members are required in the defence-related signal cascade to induce HR triggered by a gene-for-gene interaction.
Results
Isolation of two cDNA clones, NtCDPK2 and NtCDPK3, encoding CDPKs
The activity of protein kinases is frequently regulated by post-translational modifications such as phosphorylation. Changes in protein kinase activity can be accompanied by the transcriptional induction of the corresponding gene. This has been reported for MAP kinases from different plant species in response to cold, drought and wounding, but also after non-specific or race-specific elicitation (for review see Meskiene and Hirt, 2000; Zhang and Klessig, 2000). Biochemical activation followed by transcriptional induction of tobacco WIPK was also observed when Cf-9 tobacco cells or plants were treated with the corresponding race-specific elicitor Avr9 (Romeis et al., 1999). Because a 68/70 kDa CDPK enzyme became phosphorylated and activated in response to a Cf-9/Avr9 elicitation event, we set out to isolate CDPK genes coding for proteins of approximately that size, whose expression was induced in the same experimental system (Durrant et al., 2000). Two full-length cDNAs, designated NtCDPK2 and NtCDPK3, were isolated using an RT–PCR approach with degenerate primers on total RNA from elicited Cf-9 tobacco cells. NtCDPK2 and NtCDPK3 are 88 and 94% identical at nucleotide and amino acid level, respectively. The nucleotide sequence data will appear in DDBJ/EMBL/GenBank under accession Nos AJ344154 and AJ344155. The genes encode proteins of 581 and 578 amino acids with a predicted molecular mass of 64.73 and 64.72 kDa, respectively, which contain a myristoylation motif at the N-terminus. The closest homologs to NtCDPK2 are CpCPK1 from zucchini (90% amino acid identity; DDBJ/EMBL/GenBank accession No. U90262; Ellard-Ivey et al., 1999) and AtCPK2 from Arabidopsis (87% identity; DDBJ/EMBL/GenBank accession No. U38133; Hrabak et al., 1996) (Figure 1). Therefore, both cDNAs from tobacco are members of the same subgroup (Harmon et al., 2000).
Fig. 1. Alignment of the predicted amino acid sequence of tobacco NtCDPK2 with orthologous sequences. NtCDPK2 (Nt) was aligned with CpCPK1 from zucchini (Cp; Ellard-Ivey et al., 1999) and AtCPK1 from Arabidopsis (At; Harper et al., 1993). Amino acids differing from the NtCDPK2 sequence are shaded in black, gaps maximizing the alignment are shown by dashes. The boundaries between the variable, kinase, junction and calmodulin-like domain, as well as the positions of the four EF hands, are shown by arrows and bars. The N-terminal consensus motif for eukaryotic fatty acylation (Nimchuk et al., 2000) is boxed, and numbers at the right indicate amino acid residues.
A genomic Southern blot of BamHI and EcoRV-digested genomic DNA hybridized with the full-length cDNA of NtCDPK2 yielded four cross-reacting bands in N.tabacum (two in Nicotiana sylvestris) (data not shown). Because the NtCDPK2 probe also recognized NtCDPK3, this indicates that NtCDPK2 and NtCDPK3 belong to a small gene subfamily of four members. A gene-specific probe comprising ∼300 bp from the 5′- and 3′-UTRs of NtCDPK3 hybridized with two bands in N.tabacum (one in N.sylvestris) (data not shown). Nicotiana tabacum is an allotetraploid species that is believed to have arisen from hybridization of the diploid species N.sylvestris and Nicotiana tomentosiformis (Lee et al., 1988). The NtCDPK2 and NtCDPK3 alleles may therefore originate from different parental genomes.
Expression patterns of NtCDPK2 and NtCDPK3
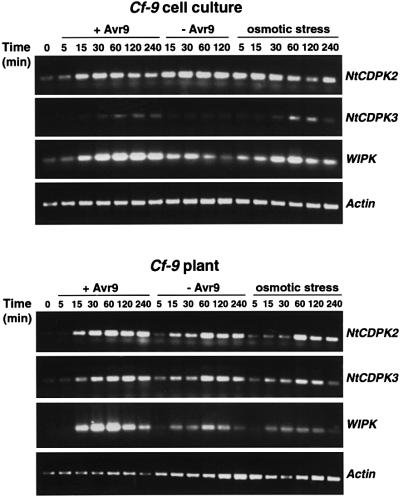
Transcript levels were analyzed in Cf-9 tobacco cell cultures and plants over a time course of 4 h after elicitation with IF(Avr9+) or IF(Avr9–), or after hypo-osmotic stress applied by diluting cells with water or by infiltrating water into leaves. RNA blot analysis with probes from the coding region of NtCDPK2 or NtCDPK3 resulted in cross hybridization between the two isoforms, whereas probes from the 5′- and 3′-UTRs were not sensitive enough to detect a signal (data not shown). Therefore, RT–PCR with gene-specific primers was conducted, using WIPK and actin as controls for an induced and a constitutively expressed gene, respectively (Figure 2).
Fig. 2. Expression patterns of NtCDPK2 and NtCDPK3 after elicitation and hypo-osmotic stress. Cf-9 tobacco cell cultures and plants were treated with IF(Avr9+) or IF(Avr9–), which contains (+Avr9) or does not contain (–Avr9) the Avr9 peptide. Osmotic stress was applied by adding 2 vols of water to cells or by infiltrating water into leaves. At the time points indicated leaf samples were harvested and total RNA was isolated and used for RT–PCR as described in Materials and methods, applying 22, 24 and 22 amplification cycles with specific primers for the NtCDPK2, NtCDPK3 and WIPK gene, respectively (gene indicated on the right). Equal cDNA amounts were controlled by amplification of constitutively expressed actin gene (24 cycles).
In cell cultures, WIPK transcript was induced 30–60 min after dilution or elicitation with IF(Avr9+) but not after treatment with IF(Avr9–) (Figure 2). A similar pattern of transcript accumulation was observed for NtCDPK3, which was induced after hypo-osmotic stress and elicitation, except that the response was slightly delayed (maximal induction at 60–120 min; Figure 2). In contrast, no significant changes in NtCDPK2 transcript accumulation could be observed, irrespective of the inducing stimulus. The transcript appeared to be constitutively expressed, perhaps due to the shaking of the cultures in the dark. The transcript analysis in Figure 2 represents one of three independent experiments showing identical expression patterns.
In plants, an increase in NtCDPK2, NtCDPK3 and WIPK transcript levels could be detected after injecting IF(Avr9+), IF(Avr9–) or just water. This is caused by the mechanical stress of the infiltration procedure (flooding stimulus). Only for WIPK a stronger mRNA increase occurred through the contribution of both stimuli (flooding and specific elicitation), which confirms previous data obtained with RNA blot analysis (Romeis et al., 1999).
Transient expression of NtCDPK2-myc in N.tabacum and N.benthamiana
To characterize NtCDPK2 protein in the context of various stress stimuli a transient transformation system was employed. A tagged version of NtCDPK2 was generated that includes a triple c-myc epitope at its C-terminus. Agrobacterium tumefaciens GV3101 carrying the construct under the control of the constitutive cauliflower mosaic virus 35S promoter (Table I) was infiltrated into tobacco leaves. Western analysis conducted 2 days after infiltration showed the presence of a protein of ∼75 kDa (Figure 3). No myc-tagged protein was detectable in the transformed Agrobacterium strain itself, 1 day after infiltration (data not shown), or in leaves infiltrated with the control vector (Figure 3A). As predicted by the putative myristoylation site, 90% of NtCDPK2-myc was localized in the membrane fraction (data not shown). In N.benthamiana and N.tabacum (Cf-9 tobacco), infiltration of water 2 days after Agrobacterium infiltration caused a rapid and transient interconversion of the expressed kinase from a faster migrating form (∼75 kDa) to a slower migrating form (∼77 kDa). In parallel, a decrease in the western signal intensity could be observed, suggesting that in addition to kinase interconversion, CDPK protein turnover was induced. Because in these experiments NtCDPK2-myc was expressed under the control of the 35S promoter, the activation of overexpressed enzyme may have preferentially induced a degradation pathway. When lower expression levels were achieved, no such decrease could be observed (see Figure 5B). The kinetics of the transition into the 77 kDa form was identical in both cultivars with the maximal shift peaking ∼7.5 min after the inducing stimulus (Figure 3A). Such shift in electrophoretic mobility was previously correlated with an Avr9/Cf-9-mediated elicitation event in tobacco cell cultures, and was proposed to reflect a transition between a non-elicited, less active and an elicited, more active enzyme form (Romeis et al., 2000).
Table I. Agrobacterium tumefaciens GV3101 strains.
| Name | Purpose | Description of binary vector | Literature |
|---|---|---|---|
| NtCDPK2-myc | transient expression | 35S NtCDPK2 full-length gene fused to C-terminal triple c-myc tag in pBIN19 | this study |
| NtCDPK21–380myc | transient expression | 35S truncated NtCDPK2, coding for variable and kinase domain only, fused to triple c-myc in pBIN19 | this study |
| PVX-NtCDPKCLD | silencing | 417 bp of the calmodulin-like domain of NtCDPK2 inserted into PVX genome behind coat protein promoter in pGR106 | this study |
| PVX-GFP | silencing | GFP in pGR106 | Jones et al. (1999) |
| 4/456/Avr4 | HR | 35S Cf-4, 35S Avr4 | Thomas et al. (2000) |
| 4/456/Avr9 | HR (control) | 35S Cf-4, 35S Avr9 | Thomas et al. (2000) |
| 9/456/Avr9 | HR | 35S Cf-9, 35S Avr9 | Thomas et al. (2000) |
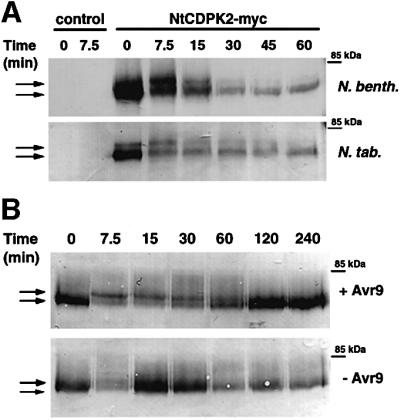
Fig. 3. Transiently expressed NtCDPK2-myc shows an elicitation- and osmotic stress-induced transition between two enzyme forms in N.tabacum (Cf-9 tobacco) and N.benthamiana. In a transient expression assay, A.tumefaciens, carrying the modified NtCDPK2 gene with an in-frame triple-myc tag at the 3′ end under the control of a constitutive promoter (NtCDPK2-myc), or the empty vector (control) was infiltrated in leaves of N.benthamiana or N.tabacum. After 2 days, the injected area was exposed to hypo-osmotic stress by infiltrating water (A). Alternatively, in Cf-9 tobacco, the Agrobacterium-infiltrated area was elicited by injecting IF(Avr9+) or IF(Avr9–), which contains (+Avr9) or does not contain (–Avr9) the Avr9 peptide (B). At the time points indicated, samples were harvested and total solubilized membrane extracts were prepared. Proteins were separated on an SDS gel, transferred onto nitrocellulose, and the blots were subjected to immunodetection using an anti-c-myc antibody. Equal protein amount was confirmed by Ponceau S staining prior to immunodetection.
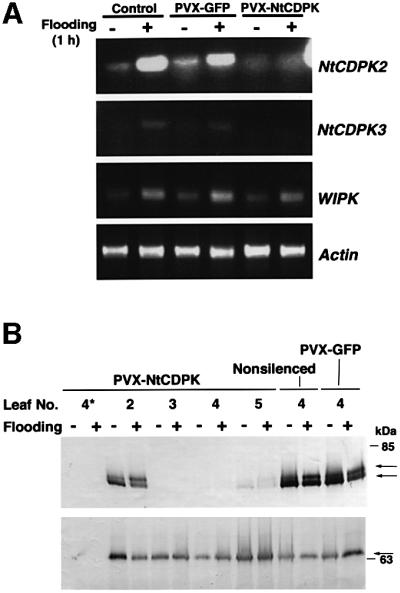
Fig. 5. VIGS of NtCDPK2 in N.benthamiana. Seedlings were inoculated with A.tumefaciens PVX-NtCDPKCLD, containing the silencing construct, in which a 417 bp fragment of the calmodulin-like domain of the NtCDPK2 gene was integrated into the PVX genome, or an unrelated control insert (Jones et al., 1999). After 3 weeks the silencing was accomplished, and if not otherwise stated, the fourth leaf above the inoculated one was then used for further experiments. (A) To determine the degree of silencing and analyze the transcript levels, the silenced fourth leaf of PVX-treated or the equivalent leaf of an untreated plant was exposed to hypo-osmotic stress by infiltrating water. Before (–) and 1 h after the flooding stimulus (+), samples were harvested and analyzed by RT–PCR for the expression of N.benthamina homologs of NtCDPK2, NtCDPK3 and WIPK, as described in the legend to Figure 2. Actin cDNA was amplified as an internal control for equal cDNA amounts. (B) Transient expression of NtCDPK2-myc and its truncated version in CDPK-silenced plants. Different leaves of plants previously inoculated with PVX-NtCDPKCLD or leaf No. 4 of an untreated or PVX-GFP-inoculated control plant were infiltrated with A.tumefaciens NtCDPK2-myc (Table I) as described in the legend to Figure 3 (upper panel) or with NtCDPK21–380-myc lacking the junction and calmodulin-like domain (lower panel; Harper et al., 1994). After two more days, the Agrobacterium-infiltrated area was subjected to hypo-osmotic stress by infiltrating water for 7.5 min. Leaf samples before (–) and after (+) the flooding stimulus were harvested, and extracts were prepared and analyzed by western blotting as described in the legend to Figure 3. The arrows at the right indicate NtCDPK2-myc and its truncated version (lower panel), respectively. Positions of the molecular mass markers are given at the right.
To investigate the response of NtCDPK2-myc to a gene-for-gene-specific elicitation event, Cf-9 tobacco leaves that transiently expressed NtCDPK2-myc protein were infiltrated with IF(Avr9+) or IF(Avr9–). Again, CDPK interconversion from the non-elicited to elicited form was detectable (Figure 3B). In the IF(Avr9+) series this transition was maintained for 60 min before returning to basal level (Figure 3B, upper panel). When IF(Avr9–) was injected (lower panel), only a transient shift at 7.5 min, reminiscent of the hypo-osmotic stress series from Figure 3A, could be observed. These experiments were repeated at least three times with similar results. Thus, NtCDPK2-myc responded to two different external stimuli by adopting an identical mechanism but with altered duration.
Kinase activity of immunoprecipitated NtCDPK2-myc
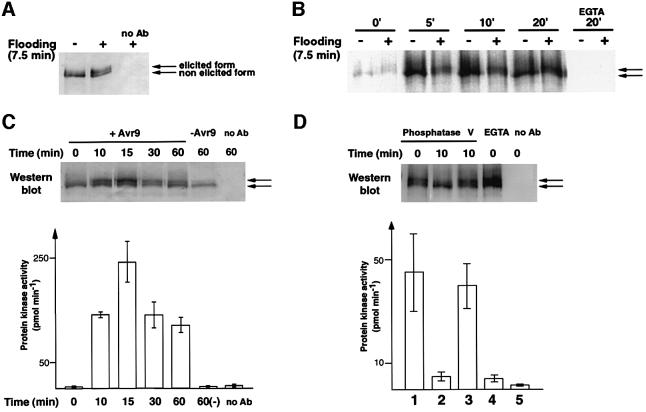
We next studied whether the stimulus-induced transition of NtCDPK2-myc into the elicited form correlated with an increase in enzymatic activity. NtCDPK2-myc was transiently expressed in N.benthamiana, and samples were prepared before and after exposure to a stress stimulus. Both the non-elicited and elicited form of NtCDPK2-myc could be immunoprecipitated from crude solubilized membrane extracts with anti-c-myc antibodies. No myc-tagged protein was observed in the control without specific antibody (Figure 4A).
Fig. 4. Enzymatic activities of immunoprecipitated NtCDPK2-myc. (A) Leaf discs of N.benthamiana that transiently expressed NtCDPK2-myc were harvested before (–) or after (+) infiltration of water. Solubilized membrane extracts were subjected to immunoprecipitation with monoclonal anti-c-myc antibodies, and precipitated proteins were analyzed by western blotting and immunodetected using a polyclonal c-myc antiserum. (B) Immunoprecipitates of NtCDPK2-myc before and after the flooding stimulus were prepared as in (A), and analyzed for CDPK autophosphorylation by incubation with [γ-33P]ATP (92 kBq) for the time indicated in the presence of 1 mM calcium or 5 mM EGTA (last data pair). Samples were separated on an SDS gel and analyzed by autoradiography. The two arrows indicate the non-elicited and elicited enzyme forms. (C) Leaf discs of Cf-9 tobacco that transiently expressed NtCDPK2-myc were harvested over a time course after elicitation with IF(Avr9+) or IF(Avr9–) (–). Solubilized membrane extracts were analyzed by western blotting (upper panel) and subjected to immunocomplex kinase assays with 100 µg/ml syntide-2 as substrate in the presence of 50 µM [γ-33P]ATP (92 kBq) and 1 mM calcium for 5 min at 30°C (lower panel). Supernatants werespotted on phosphocellulose paper and subjected to scintillation counting (phosphorylation of syntide-2; histogram). (D) NtCDPK2-myc was immunoprecipitated from N.benthamiana leaf extracts after a flooding stimulus as described in (A). Aliquots containing the immobilized enzyme were incubated with non-specific λ phosphatase for 10 min where indicated, or with phosphatase in the presence of 50 mM NaF and 10 mM Na3VO4 (lane 3). After separating the phosphatase by washing, immunocomplex kinase assays were conducted as described in (C) with syntide-2 as substrate in the presence of 50 µM [γ-33P]ATP (92 kBq), 1 mM calcium (lanes 1–3 and 5) or 5 mM EGTA (lane 4). Supernatants were analyzed by western blotting (upper panel) as well as spotted on phosphocellulose paper and subjected to scintillation counting (lower panel).
When immunoprecipitated NtCDPK2-myc that had been purified before or after the flooding stimulus was incubated with [γ-33P]ATP, both CDPK forms became phosphorylated (Figure 4B). The reaction was calcium dependent and did not occur in the presence of 5 mM EGTA. Remarkably, even after prolonged reaction time, no change in the distribution of signal intensity between the non-elicited (75 kDa) and elicited (77 kDa) form took place. This suggests that both CDPK forms were able to autophosphorylate in a calcium-dependent manner. However, such in vitro autophosphorylation of isolated, immobilized NtCDPK2-myc alone could not mimic the stress-induced in vivo transition between the non-elicited and elicited CDPK form.
To address whether NtCDPK2-myc in its elicited form is more active, we conducted immunocomplex kinase assays with syntide-2 as artificial substrate. Cf-9 tobacco, transiently expressing NtCDPK2-myc, was elicited with IF(Avr9+) or IF(Avr9–), samples were harvested at different time points, and solubilized membrane fractions were analyzed for NtCDPK2-myc expression (Figure 4C, upper panel) or NtCDPK2-myc kinase activity in immunoprecipitates (lower panel). In parallel with the elicitor-induced transition into the elicited CDPK form, a 200-fold increase in kinase activity could be observed. No phosphorylation activity was detected in the absence of antibody or when the enzyme existed in the non-elicited form [for example, when returned to basal level 60 min after injection with control IF(Avr9–)].
In addition, immunocomplex kinase assays were conducted with transiently expressed NtCDPK2-myc isolated after the flooding stimulus. As shown in Figure 4D, immunoprecipitated NtCDPK2-myc efficiently phosphorylated syntide-2 in the presence of calcium (histogram, bar 1), but not in the presence of EGTA (bar 4) or when no antibody was used (bar 5). Incubation of the immobilized enzyme with a non-specific bacteriophage lambda phosphatase resulted in an interconversion back into the non-elicited form (Figure 4D, upper panel). At the same time, the corresponding immunocomplex kinase activity decreased ∼10-fold (bar 2). In the presence of phosphatase inhibitors, in vitro transition and loss of kinase activity were compromised (lane and bar 3).
The immunocomplex kinase assay experiments were repeated three times with similar results in terms of induction pattern and kinetics. However, the kinase activity values were dependent on the amount of immunoprecipitated CDPK enzyme in each experimental series and were therefore influenced by varying expression levels in different tobacco plants. Our data indicate that the in vivo phosphorylation and interconversion of NtCDPK2-myc from the non-elicited into the elicited form is accompanied by a sustained (10- to 200-fold) increase in enzymatic activity.
VIGS of the NtCDPK2 subfamily
To examine the biological function of NtCDPK2 we employed the recently established VIGS as a gene knockout system (Baulcombe, 1999; Burton et al., 2000; R.Lu and D.C.Baulcombe, personal communication). VIGS is a rapid and transient method using potato virus X vectors that carry 300–500 bp elements from the exon of plant host genes. The silencing mechanism is based on cosuppression between transgene and endogenous gene, likely involving the formation of double-stranded RNA, and should also target close homologs of the gene of interest (∼90% sequence identity). Because NtCDPK2 and NtCDPK3 are very closely related this technique should allow us to silence both genes.
VIGS of the NtCDPK2 gene was conducted in N.benthamiana. Southern analysis of genomic DNA from N.benthamiana revealed two cross hybridizing signals with NtCDPK2 full-length cDNA as probe (data not shown). The silencing construct was generated by inserting a 417 bp cDNA fragment, which comprised most of the calmodulin-like domain of NtCDPK2, into the viral vector. This fragment showed 98% identity on nucleotide level to NtCDPK3, and 96% identity to the corresponding NtCDPK2 orthologous gene from N.benthamiana (DDBJ/EMBL/GenBank accession No. AJ344156; data not shown). Three weeks after the inoculation of seedlings with A.tumefaciens PVX-NtCDPKCLD or PVX-GFP (Table I), plants were analyzed for CDPK and WIPK transcripts before and 1 h after a flooding stimulus (Figure 5A). mRNA levels were determined by RT–PCR using gene-specific primers that amplify a region outside the calmodulin-like domain (Burton et al., 2000). Stress-induced mRNA accumulation of N.benthamiana orthologs to NtCDPK2 and NtCDPK3 could be detected in non-silenced (control) and PVX-GFP-treated plants, but were absent in CDPK-silenced plants. In contrast, the flooding-induced increase of WIPK mRNA was unaltered in all three plants. The actin gene was amplified as control for a constitutively expressed gene.
The degree of VIGS varies with the distance from the leaf used for the onset of silencing, and was reported to be highest on the third to fifth leaf above the originally inoculated one (R.Lu and D.C.Baulcombe, personal communication). Different leaves of silenced and control plants were analyzed for their ability to transiently express NtCDPK2-myc or a truncated version lacking the junction and calmodulin-like domain (Figure 5B), combined with the previously described flooding stimulus (see Figure 3). If effective silencing were established, expression of full-length NtCDPK2-myc, which shares the 417 bp fragment with the PVX-delivered silencing construct, should be prevented. Two days after infiltration of A.tumefaciens NtCDPK2-myc and NtCDPK21–380-myc (Table I), NtCDPK2-myc was expressed in non-silenced and PVX-GFP control plants, and the flooding-induced shift between the non-elicited and elicited CDPK form could be observed (Figure 5B, upper panel). Western blot analysis of CDPK-silenced plants revealed some NtCDPK2-myc expression on leaf No. 2, no signal on leaf No. 3 and No. 4, and again a weak signal on leaf No. 5. Also no band could be seen when the vector control was used (first two lanes, leaf No. 4 marked by an asterisk). In contrast, a truncated NtCDPK2-myc construct, which lacked the domain used for the onset of silencing, was uniformly expressed on leaves in silenced and control plants (Figure 5B, lower panel).
Compared with untreated plants, the PVX-GFP- and PVX-CDPK-infected plants were ∼10 and 5% shorter in stature, and PVX-infected leaves showed a slight mottling caused by the virus.
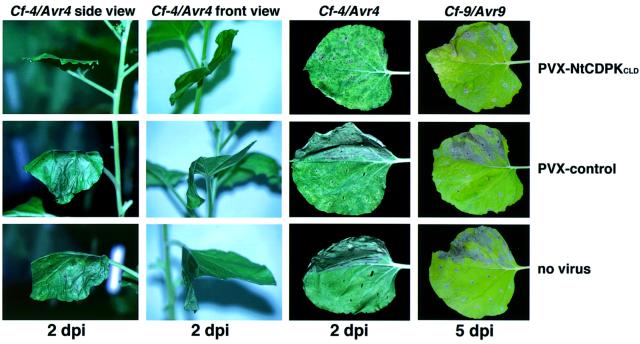
Next we studied the effect of CDPK silencing on defence-related responses. Our biochemical data using western blot and/or in-gel kinase assays demonstrated that elicitation of Cf-9 and Cf-4 tobacco cells with the corresponding Avr9 and Avr4 elicitor resulted in a gene-for-gene-specific CDPK interconversion and activation (Romeis et al., 2000; data not shown). Here we determined the HR-inducing activity in the respective gene-for-gene interactions using an Agrobacterium-mediated transient assay in which the resistance gene (Cf-4 or Cf-9) and the corresponding avirulence gene (Avr4 or Avr9) were co-expressed (Thomas et al., 2000). The necrotic reaction induced by Cf-4/Avr4 or Cf-9/Avr9 could be detected after 2 or 5 days in the upper leaf half of the non-silenced (Figure 6, lower panels) and PVX-control-treated plants (Figure 6, middle panels). In the CDPK-silenced plants, necrotic symptoms in the Cf-4/Avr4 and Cf-9/Avr9 combinations were significantly reduced and delayed, and developed only around the infiltration sites (Figure 6, upper panels). No HR-inducing activity was found when the inactive combination Cf-4/Avr9 was infiltrated (Figure 6, lower leaf halves). This suggests that NtCDPK2 alone or together with subfamily isoforms is required in the signalling cascade to activate HR. In addition, in CDPK-silenced plants the characteristic leaf wilting phenotype was absent (Figure 6, side and front views). This leaf flopping, which happens within a short period of time just before onset of necrosis in the Cf-4/Avr4-treated leaf half, could be observed in non-silenced and PVX control-infected plants. These HR and wilting phenotypes were repeated in several independent experiments over a long period of time. However, when the silencing was incomplete, so that a (less pronounced) increase in CDPK transcripts was still detectable by RT–PCR, gene-for-gene-dependent HR and leaf wilting were no longer affected.
Fig. 6. CDPK-silenced leaves were compromised in gene-for-gene-dependent HR induction. For the onset of silencing, N.benthamiana seedlings were inoculated with A.tumefaciens PVX-NtCDPKCLD (upper row), PVX-GFP (control; middle row) or remained untreated (lower row), as described in the legend to Figure 5. After 3 weeks the fifth leaf (first column) or the fourth leaf (second to fourth column) above the primary infected one was infiltrated with A.tumefaciens 4/456/Avr4 or 9/456/Avr9, as indicated, carrying either Cf-4/Avr4 or Cf-9/Avr9 gene-for-gene combinations on one leaf half (upper half in third and fourth column) or with strain 4/456/Avr9 as control carrying Cf4/Avr9 on the other leaf half (lower half in third and fourth column). Photos of necrosis and wilting phenotype were taken as indicated.
Discussion
A challenge in plant signalling is to understand how cellular responses to environmental stimuli are activated and whether unique, overlapping or redundant signalling pathways are used (Bowler and Fluhr, 2000). Different signalling inputs converge at a single component (scenario 1); conversely, one stimulus can trigger components that are located in different signal cascades (scenario 2). Substantial progress has been made in characterizing the large CDPK gene family from various plant species and it has been shown that CDPKs are multifunctional (Harmon et al., 2000). The future challenge is to address which specific isoforms are integrated in which cellular processes in which cell type and when.
NtCDPK2 and NtCDPK3 function in multiple signalling pathways
Recently, a 68/70 kDa membrane-associated CDPK was identified by its characteristic shift in electrophoretic mobility in in-gel kinase assay and western blot analysis when Cf-9 tobacco cells were treated with the Avr9 elicitor (Romeis et al., 2000), or Cf-4 tobacco cells with Avr4 (A.Ludwig, S.Rivas, J.D.G.Jones and T.Romeis, unpublished). The same shift, although less sustained, could be observed after diluting tobacco cells with 2 vols of water (data not shown). In this paper we have isolated two genes, NtCDPK2 and NtCDPK3, and we conclude that NtCDPK2 codes for that previously characterized 68/70 kDa Avr9/Cf-9-dependent CDPK. Transiently expressed NtCDPK2-myc protein is membrane associated, as predicted by its N-terminal myristoylation motif; it migrates at the correct size (allowing for the increase in molecular mass for the triple-myc tag); and it responds to hypo-osmotic stress and elicitation with reversible interconversion between two enzyme forms. Furthermore, although both genes were transcriptionally activated in response to stress stimuli in Cf-9 cells and plants, more NtCDPK2 than NtCDPK3 message accumulated after stimulation. In particular in Cf-9 cells, NtCDPK2 was constitutively highly expressed so that no further mRNA induction could be triggered by stress stimuli. This suggests that the major part of CDPK signalling in Cf-9 cells was contributed by NtCDPK2.
How can one enzyme be involved in different signalling pathways? Transiently expressed NtCDPK2-myc protein responds to osmotic stress and specific elicitation with a shift from a non-elicited (75 kDa) to an elicited enzyme form (77 kDa). Whereas after gene-for-gene-specific elicitation this transition was complete and sustained for ∼60 min, flooding or treatment with control IF(Avr9–) caused a shorter response, peaking at 7.5 min, and only a portion of the enzyme available participated in the shift. This let us conclude that the enzyme is regulated by altering the extent and duration of the post-translational modification depending on the inducing stimulus.
Protein activation is followed by an increase in transcript level, and maximal mRNA accumulation was observed 60–120 min after hypo-osmotic stress and elicitation. Interestingly, this time course correlates with the CDPK shifting back into its non-elicited form after the Cf-9/Avr9 elicitation event in tobacco cells, as reported previously. Because CDPK activation is accomplished by phosphorylation, CDPK interconversion back to basal level and thus inactivation may be catalyzed by a protein phosphatase. Alternatively, the elicited form could be degraded by proteolysis, and reappearing CDPK in its non-elicited form is attributable to newly synthesized enzyme due to enhanced transcriptional activity. The latter hypothesis is supported by the fact that NtCDPK2 and NtCDPK3 contain a PEST sequence, which is a conserved protein degradation motif, in the N-terminal variable domain (Rechsteiner and Rogers, 1996). Protein degradation also became evident in addition to the mobility shift, when transiently expressed NtCDPK2-myc was challenged by elicitor or flooding (Figure 3). What was described as scenario 1—one isoform participates in different signal cascades—has thus been demonstrated for NtCDPK2 and its derived tagged gene product.
Enzymatic activity of NtCDPK2-myc protein
The fact that NtCDPK2 and NtCDPK3 share 94% amino acid sequence and have an identical N- and C-terminus hampered the design of isoform-discriminating anti-peptide antibodies. To investigate the activation and characteristics of the elicited and non-elicited CDPK without the interference of kinase activities caused by other protein kinases or endogenous CDPKs, tagged NtCDPK2-myc was transiently expressed. Enzymatic properties of the isolated CDPK isoform could then be analyzed in immunoprecipitates reflecting samples taken before or after in vivo stimulation in the biological system. In immunocomplex kinase assays (Figure 4C and D) the elicited form of isolated NtCDPK2-myc showed a 10- to 200-fold higher calcium-dependent enzymatic activity towards syntide-2 due to osmotic stress and elicitation, respectively, than the non-elicited form. This confirms previous studies in which a CDPK ‘shift down’ experiment after phosphatase treatment was shown using crude solubilized membrane extracts from elicited cells (Romeis et al., 2000). Whether NtCDPK2-myc interconversion and activation requires identical intramolecular phosphorylation sites for both stimuli or whether different sites and phosphorylation kinetics are involved will be subject to future studies.
Both forms of purified NtCDPK2-myc were able to autophosphorylate in vitro (Figure 3B); however, CDPK autophosphorylation appears not to be sufficient for the observed shift between the two enzyme forms in vivo, suggesting that different and/or additional phosphorylation sites exist. This let us conclude that upon elicitation or abiotic stress, an upstream kinase is responsible for the phosphorylation and activation of NtCDPK2-myc in the biological system. This is consistent with the fact that the elicitation-induced in vivo transition between the two CDPK forms in Cf-9 cells was not compromised in the presence of inhibitors that would block CDPK activity (Romeis et al., 2000). Whether this kinase is also responsive to multiple stress stimuli or whether branched cascades exist in which more than one enzyme is involved in CDPK activation remains to be shown. The latter scenario is reminiscent of MAP kinase signalling where in a stimulus-specific manner, upstream members of kinase cascades such as MAPKK or MAPKKK become recruited and are responsible for MAP kinase activation (Kiegerl et al., 2000; Kovtun et al., 2000; Liu et al., 2000; for review see Meskiene and Hirt, 2000; Bent, 2001). By investigating CDPKs in transient assays, we therefore established an excellent system that will facilitate studies of the enzymes’ in vivo activation mechanism, the identification of the phosphorylation acceptor sites and the upstream kinase.
Function of NtCDPK2 orthologs and homologs
NtCDPK2 and NtCDPK3 belong to a small gene sub family of four members in the allotetraploid N.tabacum genome. NtCDPK2-myc protein activation and NtCDPK2 transcript levels suggest that this subfamily functions in triggering cellular responses to extracellular stimuli. Is this task restricted to one enzyme, one subfamily or, in line with signalling scenario 2, are homologs from other CDPK subfamilies recruited?
Elicitation of Cf-9 cells with Avr9 resulted in the activation of a 68/70 kDa CDPK, likely to be attributable to NtCDPK2. However, it can not be excluded that NtCDPK3 and the other subfamily members also contributed to the observed signals on western blot and in-gel kinase activities. Activation of a 55 kDa CDPK in response to non-specific elicitation of soybean cell cultures has been suggested (Allwood et al., 1999).
NtCDPK2 is orthologous to AtCPK1 and AtCPK2 from Arabidopsis and CpCPK1 from zucchini and falls into the same group of the CDPK superfamily (Harmon et al., 2000). AtCPK1 is one of the best investigated CDPK isoforms, and detailed biochemical analysis of its enzyme mechanism is available (Harper et al., 1993, 1994; Huang et al., 1996; Vitart et al., 2000). In vitro studies with recombinant AtCPK1 revealed that the enzyme interacted with 14-3-3 proteins (Camoni et al., 1998), was able to activate a tonoplast chloride channel in vitro (Pei et al., 1996), and could phosphorylate and inactivate an endoplasmic reticulum-localized, calmodulin-stimulated calcium pump (Hwang et al., 2000). CpCPK1 was reported to be highly expressed in hypocotyls of dark grown zucchini seedlings, and CpCPK1 protein could be myristoylated in vitro (Ellard-Ivey et al., 1999). However, in vivo function has so far not been elucidated for any of these orthologs.
In response to drought and salt, CDPK transcript accumulation was shown for AtCPK10 and AtCPK11 in Arabidopsis (Urao et al., 1994). The transient expression of AtCPK10 in an active, calcium-independent form in protoplasts activated a cold, dark and osmotic stress response pathway, and mimicked the response to abscisic acid (Sheen, 1996). A salt/drought- and cold-induced increase in CDPK transcript was also reported for OsCDPK7 from rice, and overexpression of the gene resulted in plants more tolerant to these environmental stresses (Saijo et al., 2000). These CDPK isoforms fall into different side branches of the CDPK superfamily (Harmon et al., 2000). So far, only one other tobacco CDPK homolog has been identified, NtCDPK1, which also classifies into a distant subgroup compared with NtCDPK2 and NtCDPK3. Transcript accumulation of NtCDPK1 and/or closely related subfamily members was induced by wounding and salt treatment of leaves, and by exposure to fungal elicitor or chitosan of BY2 tobacco cells (Yoon et al., 1999). Therefore, one stimulus can activate more than one CDPK of more than one subfamily. How CDPK signalling contributed by different isoforms is integrated remains largely unknown. More data are needed on intracellular location, and cell- or tissue-specific expression patterns of CDPK isoforms.
Crosstalk between CDPKs and other signalling pathways?
The post-translational activation of a protein kinase followed by an increase in the transcript level of the corresponding gene, shown here for NtCDPK2, is known for one subclass of MAP kinases, which includes WIPK from tobacco, SAMK from alfalfa, AtMPK3 from Arabidopsis and ERMK from parsley (for review see Meskiene and Hirt, 2000; Zhang and Klessig, 2000; Romeis, 2001). Interestingly, these orthologous enzymes are multifunctional and participate in more than one signal cascade. The likely complexity of the MAP kinase signalling is further increased by the existence of SIPK, SIMK and AtMPK6 orthologs and/or by the AtMPK4 class of MAP kinases that also respond to multiple external stimuli (Ichimura et al., 2000a,b). To study whether the CDPK and MAP kinase pathways are interconnected in an Avr9/Cf-9 interaction we previously applied pharmacological inhibitors. Data with W7, a rather non-specific inhibitor of calcium-dependent enzymes, suggested that in the Avr9/Cf-9 response a calcium-dependent enzyme is located upstream of the MAP kinase activation, which might or might not be a CDPK (Romeis et al., 2000).
In this paper we used VIGS as a reverse genetics approach to silence signalling components that become transiently activated, which allowed us to address that question much more precisely. RT–PCR revealed that the flooding-induced increase in CDPK transcript was absent in the CDPK-silenced leaves, and therefore cross-silencing of both isoforms was obtained in N.benthamiana. On the other hand, the 417 bp fragment of the calmodulin-like domain of NtCDPK2 was <56% identical to calmodulin at the nucleotide level. This excludes the possibility that the observed effects are due to calmodulin cosuppression. In CDPK-silenced leaves both flooding-induced activation of WIPK and SIPK in-gel protein kinase activity towards myelin basic protein and flooding-induced accumulation of WIPK mRNA were unaffected (data not shown). Thus, NtCDPK2 subfamily members appear not to be involved in the activation of WIPK, and these signalling components, despite similiar stress-induced kinase activities and transcript levels, function in distinct pathways.
Function of NtCDPK2 in the plant defence response
To dissect the biological function of CDPKs, loss-of-function genetic analysis is essential, and Arabidopsis mutant lines that carry T-DNA insertions in CDPK isoforms have already been identified (Krysan et al., 1996). However, NtCDPK2 belongs to a gene subfamily in tobacco, and in employing the more conventional gene knock-out methods based on T-DNA or transposon insertions, a loss-of-function might have been compensated by functional homologs, for example, NtCDPK3. VIGS is based on cosuppression, and as confirmed by RT–PCR, closely related subfamily members are silenced.
By using VIGS we could show that plants silenced with the calmodulin-like domain of NtCDPK2 were compromised in generating a Cf-9/Avr9- and Cf-4/Avr4-dependent, defence-related HR, and also lacked an accompanying wilting phenotype. This indicates that NtCDPK2 and NtCDPK3 play a key role in HR induction. Early signal processes that activate an HR in response to a gene-for-gene interaction include changes in ion fluxes and protein kinase activation. An important role for reactive oxygen species such as H2O2 and NO has also been demonstrated (for review see Yang et al., 1997; Richberg et al., 1998; Scheel, 1998; Heath, 2000; Nürnberger and Scheel, 2001). However, the biological target(s) that become phosphorylated by NtCDPK2 are unknown. Although several potential pathogen-related CDPK targets have been discussed in the literature, including H+-ATPase (Schaller and Oecking, 1999), ion channels (see below) or NADPH-oxidase (Blumwald et al., 1998; Romeis et al., 2000), it is unclear at which step CDPK signalling feeds into the HR pathway. The leaf wilting phenotype, which precedes the onset of a macroscopic visible HR and is absent in CDPK-silenced leaves, suggests that NtCDPK2 may be involved in controlling the early changes in ion fluxes. Supraoptimal stomata opening allowing transpirational water loss had been observed in the Cf-9/Avr9 interaction and the loss of turgor and subsequent collapse of epidermal and mesophyll tissue may account for the developing gray necrosis (Hammond-Kosack et al., 1996). In guard cells, calcium has been shown to be a messenger that affects both stomatal opening and stomatal closure, and the outcome very much depends on the specific calcium signature as well as on the availability of the calcium-sensing enzymes (Wang and Wu, 1999). Interestingly, recombinant AtCPK1, a NtCDPK2 ortholog, was shown to activate a guard cell tonoplast anion channel in vitro, and calcium-dependent activation of that channel might be required for stomatal opening (Pei et al., 1996).
In investigating biochemical properties of CDPK isoforms in transient expression assays, studying their biological function with VIGS, and in combining both, we present novel approaches for the research in CDPK signalling that will facilitate future studies towards activation mechanism, the identification of upstream regulatory components, as well as downstream phosphorylation targets in a homologous biological system.
Materials and methods
Tobacco cell culture and plant treatments
Suspension cultures from N.tabacum cv Petite Havana and the derived Cf-9 line were subcultured at 2-weekly intervals and prepared for experiments as described previously (Romeis et al., 2000). For elicitation, cells were challenged with 75 µl of intercellular fluid originating from transgenic tobacco that produces the Avr9 peptide apoplastically [IF(Avr9+)] or with control intercellular fluid [IF(Avr9–)] (Hammond-Kosack et al., 1998). To apply osmotic stress, cells were diluted with 40 ml (twice the culture volume) of water. At the times indicated, cells were harvested by filtration, immediately frozen in liquid nitrogen, and stored at –70°C. Nicotiana tabacum cv Petite Havana, Cf-9 tobacco and N.benthamiana plants were grown in environmentally controlled growth and containment cabinets under a 16 h photoperiod at 22°C and an 8 h dark period at 18°C. Elicitation with IF (Cf-9 tobacco) was conducted on 6-week-old plants, just before the flower bud started to emerge, by infiltrating IF with a syringe in tiny cuts at the lower leaf side and thereby flooding the apoplastic space. The response to osmotic stress (without elicitation component) was investigated after the infiltration of water.
Isolation of CDPK cDNAs
Total RNA was extracted from Cf-9 tobacco cell suspension cultures treated with IF(Avr9+) for 30 min as described (Romeis et al., 1999) and used for reverse transcription with degenerate primers A07 (5′-CCITAYTAYRTIGCICCIGARGT-3′) and WD73 (5′-CCYTTYTK CATCATIGCIACRAAYTC-3′), which bind within conserved regions of the kinase and calcium-binding domain following the protocol from Klimyuk et al. (1993). PCR fragments of ∼750 bp were cloned into pGEMT (Promega) and sequenced. A 750 bp PCR fragment representing the majority of the clones obtained was then used as probe to screen a λZAP cDNA library established from elicited tobacco cells (Durrant et al., 2000). Seven independent clones were found to encode two different CDPK isoforms, NtCDPK2 and NtCDPK3. The longest clones encompassing the entire reading frame were selected for further study.
DNA constructs and seedling infection for VIGS
A 417 bp cDNA fragment encompassing most of the calmodulin-like domain of NtCDPK2 was amplified with phosphorylated primers CK1 (5′-GAAGAAATTGCTGGTCTG-3′) and CK2 (5′-CTTTTTCATCA TGGCGACGAAC-3′), subcloned into pPCR-Script Amp SK(+) (Stratagene), and ligated as a ClaI–NotI fragment into vector pGR106. The fragment is inserted downstream of the duplicated coat protein promoter in the PVX genome, which by itself is integrated into the pGreen binary vector derivative (Baulcombe et al., 1995; Takken et al., 2000; R.Lu and D.C.Baulcombe, personal communication). The construct contained the insert in sense orientation and was designated PVX-NtCDPKCLD. PVX-GFP, a near full-length cDNA coding for the green fluorescent protein in the same vector, was used as PVX control. Both binary plasmids were transformed into strain GV3101, harboring transformation helper plasmid pSup (R.Lu and D.C.Baulcombe, personal communication).
For infection, second leaves of 2- to 3-week-old N.benthamiana seedlings were wounded with a tooth-pick that was streaked over an agar plate with the respective A.tumefaciens GV3101 strain (Table I). After an additional 3–4 weeks the fourth and fifth leaves above the primarily punched leaf of each plant were analyzed for CDPK transcript level and transient expression of NtCDPK2-myc.
To analyze for defence-related phenotypes, the silenced fourth and fifth leaves were infiltrated with A.tumefaciens 4/456/Avr4 or 9/456/Avr9 (expressing transiently Cf-4 and Avr4 or Cf-9 and Avr9; gene-for-gene interaction) on one leaf half, and 4/456/Avr9 (Cf-4 and Avr9; control) on the other leaf half (Thomas et al., 2000). In non-silenced plants, the hypersensitive cell death reaction can be observed after 2 and 5 days in the Cf-4/Avr4 and Cf-9/Avr9 combination, respectively (Wulff et al., 2001).
Transcript levels and RT–PCR
Total RNA from leaf material or cultured cells was isolated with the RNA-Isolator (Genosys). Twenty micrograms of RNA were treated with 5 U of RNase-free DNase and 5 U of RNase inhibitor (both Amersham Pharmacia Biotechnology) for 30 min at 37°C, purified by phenol/chloroform extraction and dissolved in RNase-free water. Two micrograms of DNase-treated RNA were reverse transcribed for 90 min at 42°C in a 20 µl reaction volume containing 1 U of Expand™ Reverse Transcriptase (Roche), 250 µM each dNTP, 30 µM oligo (dT)30M primer, 20 U of RNase inhibitor and 10 mM dithiothreitol (DTT). One microliter of the RT reaction was used for PCR in a 20 µl volume with 1 U of Taq DNA-polymerase (Gibco-BRL), 100 µM each dNTP and 100 ng of each forward (CK11: 5′-ATGGGGAACACTTGTGTTGGACC-3′) and reverse primer (NtCDPK2-specific CK30-2: 5′-CTTAGGCTTTACCG GTCCCTCTTC-3′; NtCDPK3-specific CK30-3: 5′-TTTGGGCTTTTTT GGTTGTTCTTT-3′). PCR conditions were the following: 3 min, 94°C (first cycle); 30 s, 94°C; 30 s, 50°C; 1.5 min, 72°C (22–26 cycles); 10 min, 72°C (last cycle). PCR products were separated on a 1% agarose gel and visualized after EtBr staining. As control for equal cDNA amount in each reaction, a PCR was performed with primers for actin AC1 (5′-ATGGCAGACGGTGAGGATATTCA-3′) and AC2 (5′-GCCTTT GCAATCCACATCTGTTG-3′).
Transient expression of NtCDPK2 in N.benthamiana and N.tabacum
To generate a tagged version of NtCDPK2, the full-length cDNA and a truncated version coding for the variable and kinase domain were amplified by PCR with primers CK-V-Nco-F (5′-AATTCCATGGGG AACACTTGTGTTGGACCA-3′) and CK-CLD-Bam-R (5′-AATTG GATCCAAGTCTTAGAGCCTCTCTAA-3′) or CK-K-Bam-R (5′-AAT TGGATCCCACACCATCAACTTGAACCC-3′), which introduced NcoI and BamHI restriction sites at the 5′ and 3′ ends. The respective 1746 (full-length) or 1140 bp (truncated) fragment was inserted together with a 123 bp BamHI–XbaI fragment coding for an in-frame C-terminal triple c-myc tag (Piedras et al., 2000) into the NcoI and XbaI sites of the vector SLJ4D4 (Jones et al., 1992). The EcoRI–HindIII fragments were then cloned into pBIN19 to yield plasmids pSLJ13791 and pSLJ13801 and the plasmids were electroporated into A.tumefaciens GV3101 (Table I). Overnight cultures were harvested by centrifugation, cells were resuspended in 10 mM MgCl2, 10 mM MES pH 5.6 and 150 µM acetosyringone to an OD of 0.5, incubated for 2–5 h at room temperature, and infiltrated into leaves of 6-week-old N.tabacum (Cf-9 tobacco) or N.benthamiana plants. Two days after Agrobacterium infiltration, leaf discs used for experiments were harvested, immediately frozen in liquid nitrogen and stored at –70°C.
Preparation of protein extracts
Leaf discs (2 cm diameter, 0.5 g wet weight) were ground in liquid nitrogen, thawed in 500 µl of extraction buffer [50 mM Tris–HCl pH 7.5, 5 mM EDTA, 5 mM EGTA, 2 mM DTT, 10 mM NaF, 10 mM Na3VO4, 25 mM β-glycerophosphate, 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF), 2 µg/ml antipain, 2 µg/ml aprotinin and 2 µg/ml leupeptin], and centrifuged at 100 000 g for 25 min at 6°C in a TL100 ultracentrifuge (Beckman). The pellet was resuspended in 250 µl of solubilization buffer (20 mM Tris–HCl pH 7.5, 1 mM MgCl2, 1 mM DTT, 5 mM NaF, 1 mM Na3VO4, 10 mM β-glycerophosphate, 1 mM AEBSF, 2 µg/ml antipain, 2 µg/ml aprotinin, 2 µg/ml leupeptin, 1% Triton X-100) and incubated for 30 min at 6°C with end-over-end rotating. After centrifugation (as above), the crude solubilized membrane extracts were analyzed directly or frozen in liquid nitrogen and stored at –70°C. The protein concentration was determined using the bicinchoninic acid protein assay kit (Pierce) with bovine serum albumin as a standard.
Immunoblotting
Solubilized crude extracts (10 µg of total protein per lane) or immunoprecipitates (see below) were separated on a 10.5% SDS gel, and the proteins were transferred onto nitrocellulose (Amersham) by wet electroblotting (Mini-Protean II system; Bio-Rad). Equal loading of protein was confirmed by Ponceau S staining of the membrane, and the blots were subsequently blocked in TBST buffer (25 mM Tris–HCl pH 7.5, 100 mM NaCl and 0.1% Tween-20) with 5% fat-free milk powder for 30 min at room temperature. For immunodetection, blots were probed with a monoclonal (9E10 epitope; crude extracts) or polyclonal (A14 epitope; immunoprecipitates) anti-c-myc antiserum (both from Santa Cruz) in a 1:3000 dilution in TBST at 6°C overnight. Alkaline phosphatase-conjugated goat anti-mouse (crude extracts) or goat anti-rabbit (immunoprecipitates) IgG (1:3000 dilution; Sigma) was used as secondary antibodies, and the reaction was visualized by hydrolysis of tetrazolium-5-bromo-4-chloro-3-indolyl phosphate as substrate (Sigma).
Immunocomplex protein kinase assay
To determine kinase activity of transiently expressed NtCDPK2-myc, 150 µg of solubilized crude membrane extracts were incubated with 300 µl of solubilization buffer (lacking the detergent and DTT) and 0.25 µg of monoclonal anti-c-myc antibody with end-over-end rotation for 90 min at 6°C. After addition of 50 µl of protein G and a further incubation for 45 min, samples were harvested by centrifugation and washed with solubilization buffer containing 0.3% Triton X-100 (first and second wash), 0.3% Triton X-100 and 1 M NaCl (third wash). Subsequently, samples were aliquoted (5 µl of beads each), washed with kinase buffer and resuspended in 5 µl of kinase buffer (40 mM HEPES pH 7.4, 10 mM MgCl2, 2 mM DTT, 0.1 mM EGTA).
For in vitro interconversion experiments, reactions with NtCDPK2-myc immunoprecipitates/beads were washed once with phosphatase buffer (NEB) and resuspended in 100 µl of the same buffer containing 2 U of non-specific λ phosphatase (NEB) in the absence or presence (control) of 50 mM NaF and 10 mM Na3VO4. After 10 min at 37°C the reaction was stopped by addition of NaF and Na3VO4, and the beads were washed twice to separate from residual (although inhibited) phosphatase and resuspended in 5 µl of kinase buffer.
The kinase reaction was started by addition of 25 µl of reaction mix to yield a final concentration of 10 mM MgCl2, 100 µg/ml syntide-2, 0.925 MBq (2.5 µCi) [γ-33P]ATP (92 TBq/mmol; Amersham Pharmacia Biotech), 50 µM ATP, and either 1 mM CaCl2 or 5 mM EGTA. After incubation for 5 min at 30°C, 20 µl of supernatant were spotted on P81 phosphocellulose paper squares and the incorporatation of phosphate was determined as described (Romeis et al., 2000). A 5 µl aliquot of each sample was analyzed in parallel by SDS–PAGE and immunoblotting.
Acknowledgments
Acknowledgements
We are grateful to Olivier Voinnet, Jack Peart, Rui Lu, Abdelhafid Bendahmane and David Baulcombe for supplying vectors and for advice and discussions concerning gene silencing. We thank Owen Rowland and Wendy Durrant for the cDNA library and Colwyn Thomas for the A.tumefaciens GV3101 strains 4/456/Avr4, 9/456/Avr9 and 4/456/Avr9. We would also like to thank Alice Harmon (Gainesville, FL) for very stimulating discussions and critical reading of the manuscript. Matthew Smoker, Sara Perkins and Mike Hill are gratefully acknowledged for the propagation of cell cultures and the care and maintenance of plants. This work was supported by the Gatsby Charitable Foundation and the Crosstalk in Signalling in Plants project (EC Grant No. HRPN/CT-2000-00093).
References
- Allwood E.G., Davies,D.R., Gerrish,C., Ellis,B.E. and Bolwell,G.P. (1999) Phosphorylation of phenylalanine ammonia-lyase: evidence for a novel protein kinase and identification of the phosphorylated residue. FEBS Lett., 457, 47–52. [DOI] [PubMed] [Google Scholar]
- Baulcombe D.C. (1999) Fast forward genetics based on virus-induced gene silencing. Curr. Opin. Plant Biol., 2, 109–113. [DOI] [PubMed] [Google Scholar]
- Baulcombe D.C., Chapman,S. and Cruz,S.S. (1995) Jellyfish green fluorescent protein as a reporter for virus-infections. Plant J., 7, 1045–1053. [DOI] [PubMed] [Google Scholar]
- Bent A.F. (2001) Plant mitogen-activated protein kinase cascades: negative regulatory roles turn out positive. Proc. Natl Acad. Sci. USA, 98, 784–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Aharon,G.S. and Lam,B.C.H. (1998) Early signal transduction pathways in plant–pathogen interactions. Trends Plant Sci., 3, 342–346. [Google Scholar]
- Bowler C. and Fluhr,R. (2000) The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci., 5, 241–246. [DOI] [PubMed] [Google Scholar]
- Burton R.A., Gibeaut,D.M., Bacic,A., Findlay,K., Roberts,K., Hamilton,A., Baulcombe,D.C. and Fincher,G.B. (2000) Virus-induced silencing of a plant cellulose synthase gene. Plant Cell, 12, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camoni L., Harper,J.F. and Palmgren,M.G. (1998) 14-3-3 proteins activate a plant calcium-dependent protein kinase (CDPK). FEBS Lett., 430, 381–384. [DOI] [PubMed] [Google Scholar]
- Church G.M. and Gilbert,W. (1984) Genomic sequencing. Proc. Natl Acad. Sci. USA, 81, 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant W., Rowland,O., Piedras,P. and Jones,J.D.G. (2000) cDNA-AFLP expression profiling reveals a striking overlap in early gene induction by plant race-specific resistance and by mechanical stress. Plant Cell, 12, 963–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard-Ivey M., Hopkins,R.B., White,T.J. and Lomax,T.L. (1999) Cloning, expression and N-terminal myristoylation of CpCPK1, a calcium-dependent protein kinase from zucchini (Cucurbita pepo L.). Plant Mol. Biol., 39, 199–208. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack K.E., Silverman,P., Raskin,I. and Jones,J.D.G. (1996) Race-specific elicitors of Cladosporium fulvum induce changes in cell morphology and the synthesis of ethylene and salicylic acid in tomato plants carrying the corresponding Cf disease resistance genes. Plant Physiol., 110, 1381–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack K.E., Tang,S.J., Harrison,K. and Jones,J.D.G. (1998) The tomato Cf-9 disease resistance gene functions in tobacco and potato to confer responsiveness to the fungal avirulence gene product Avr9. Plant Cell, 10, 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon A.C., Gribskov,M. and Harper,J.F. (2000) CDPKs—a kinase for every Ca2+ signal? Trends Plant Sci., 5, 154–159. [DOI] [PubMed] [Google Scholar]
- Harmon A.C., Gribskov,M., Gubrium,E. and Harper,J.F. (2001) The CDPK superfamily of protein kinases. New Phytol., 151, 175–183. [DOI] [PubMed] [Google Scholar]
- Harper J.F., Binder,B.M. and Sussman,M.R. (1993) Calcium and lipid regulation of an Arabidopsis protein-kinase expressed in Escherichia coli. Biochemistry, 32, 3282–3290. [DOI] [PubMed] [Google Scholar]
- Harper J.F., Huang,J.F. and Lloyd,S.J. (1994) Genetic identification of an autoinhibitor in CDPK, a protein-kinase with a calmodulin-like domain. Biochemistry, 33, 7267–7277. [DOI] [PubMed] [Google Scholar]
- Heath M.C. (2000) Hypersensitive response-related death. Plant Mol. Biol., 44, 321–334. [DOI] [PubMed] [Google Scholar]
- Hrabak E. (2000) Calcium-dependent protein kinases and their relatives. In Kreis,M. and Walker,J. (eds), Advances in Botanical Research Incorporating Advances in Plant Pathology. Vol. 32. Academic Press Inc., San Diego, CA, pp. 185–223.
- Hrabak E.M., Dickmann,L.J., Satterlee,J.S. and Sussman,M.R. (1996) Characterization of eight new members of the calmodulin-like domain protein kinase gene family from Arabidopsis thaliana. Plant Mol. Biol., 31, 405–412. [DOI] [PubMed] [Google Scholar]
- Huang J.F., Teyton,L. and Harper,J.F. (1996) Activation of a Ca2+-dependent protein kinase involves intramolecular binding of a calmodulin-like regulatory domain. Biochemistry, 35, 13222–13230. [DOI] [PubMed] [Google Scholar]
- Hwang I., Sze,H. and Harper,J.F. (2000) A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proc. Natl Acad. Sci. USA, 97, 6224–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K., Mizoguchi,T., Yoshida,R., Yuasa,T. and Shinozaki,K. (2000a) Various abiotic stresses vapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J., 24, 655–665. [DOI] [PubMed] [Google Scholar]
- Ichimura K., Mizoguchi,T., Yoshida,R., Yuasa,T. and Shinozaki,K. (2000b) Protein phosphorylation and dephosphorylation in environmental stress responses in plants. In Kreis,M. and Walker,J. (eds), Advances in Botanical Research Incorporating Advances in Plant Pathology. Vol. 32. Academic Press Inc., San Diego, CA, pp. 355–377.
- Jones J.D.G., Shlumukov,L., Carland,F., English,J., Scofield,S., Bishop,G. and Harrison,K. (1992) Effective vectors for transformation, expression of heterologous genes, and assaying transposon excision in transgenic plants. Transgenic Res., 1, 285–297. [DOI] [PubMed] [Google Scholar]
- Jones L., Hamilton,A.J., Voinnet,O., Thomas,C.L., Maule,A.J. and Baulcombe,D.C. (1999) RNA–DNA interactions and DNA methylation in post-transcriptional gene silencing. Plant Cell, 11, 2291–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegerl S. et al. (2000) SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell, 12, 2247–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimyuk V.I., Carroll,B.J., Thomas,C.M. and Jones,J.D.G. (1993) Alkali treatment for rapid preparation of plant-material for reliable PCR analysis. Plant J., 3, 493–494. [DOI] [PubMed] [Google Scholar]
- Kovtun Y., Chiu,W.L., Tena,G. and Sheen,J. (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl Acad. Sci. USA, 97, 2940–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan P.J., Young,J.C., Tax,F. and Sussman,M.R. (1996) Identification of transferred DNA insertions within Arabidopsis genes involved in signal transduction and ion transport. Proc. Natl Acad. Sci. USA, 93, 8145–8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.Y., Townsend,J., Tepperman,J., Black,M., Chui,C.F., Mazur,B., Dunsmuir,P. and Bedbrook,J. (1988) The molecular basis of sulfonylurea herbicide resistance in tobacco. EMBO J., 7, 1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.H., Zhang,S.Q. and Klessig,D.F. (2000) Molecular cloning and characterization of a tobacco MAP kinase kinase that interacts with SIPK. Mol. Plant Microbe Interact., 13, 118–124. [DOI] [PubMed] [Google Scholar]
- Martin M.L. and Busconi,L. (2000) Membrane localization of a rice calcium-dependent protein kinase (CDPK) is mediated by myristoylation and palmitoylation. Plant J., 24, 429–435. [DOI] [PubMed] [Google Scholar]
- McAinsh M.R. and Hetherington,A.M. (1998) Encoding specificity in Ca2+ signaling systems. Trends Plant Sci., 3, 32–36. [Google Scholar]
- Meskiene I. and Hirt,H. (2000) MAP kinase pathways: molecular plug-and-play chips for the cell. Plant Mol. Biol., 42, 791–806. [DOI] [PubMed] [Google Scholar]
- Nimchuk Z., Marois,E., Kjemtrup,S., Leister,R.T., Katagiri,F. and Dangl,J.L. (2000) Eukaryotic fatty acylation drives plasma membrane targeting and enhances function of several type III effector proteins from Pseudomonas syringae. Cell, 101, 353–363. [DOI] [PubMed] [Google Scholar]
- Nürnberger T. and Scheel,D. (2001) Signal transmission in the plant immune response. Trends Plant Sci., 6, 372–379. [DOI] [PubMed] [Google Scholar]
- Pei Z.M., Ward,J.M., Harper,J.F. and Schroeder,J.I. (1996) A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J., 15, 6564–6574. [PMC free article] [PubMed] [Google Scholar]
- Piedras P., Rivas,S., Droge,S., Hillmer,S. and Jones,J.D.G. (2000) Functional, c-myc-tagged Cf-9 resistance gene products are plasma membrane localised and glycosylated. Plant J., 21, 529–536. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. and Rogers,S.W. (1996) PEST sequences and regulation by proteolysis. Trends Biochem. Sci., 21, 267–271. [PubMed] [Google Scholar]
- Richberg M.H., Aviv,D.H. and Dangl,J.L. (1998) Dead cells do tell tales. Curr. Opin. Plant Biol., 1, 480–485. [DOI] [PubMed] [Google Scholar]
- Roberts D.M. and Harmon,A.C. (1992) Calcium-modulated proteins—targets of intracellular calcium signals in higher-plants. Annu. Rev. Plant Physiol. Plant Mol. Biol., 43, 375–414. [Google Scholar]
- Romeis T. (2001) Protein kinases in the plant defence response. Curr. Opin. Plant Biol., 4, 807–814. [DOI] [PubMed] [Google Scholar]
- Romeis T., Piedras,P., Zhang,S.Q., Klessig,D.F., Hirt,H. and Jones,J.D.G. (1999) Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell, 11, 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis T., Piedras,P. and Jones,J.D.G. (2000) Resistance gene-dependent activation of a calcium-dependent protein kinase (CDPK) in the plant defense response. Plant Cell, 12, 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y., Hata,S., Kyozuka,J., Shimamoto,K. and Izui,K. (2000) Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J., 23, 319–327. [DOI] [PubMed] [Google Scholar]
- Satterlee J.S. and Sussman,M.R. (1998) Unusual membrane-associated protein kinases in higher plants. J. Membr. Biol., 164, 205–213. [DOI] [PubMed] [Google Scholar]
- Schaller A. and Oecking,C. (1999) Modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses in tomato plants. Plant Cell, 11, 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel D. (1998) Resistance response physiology and signal transduction. Curr. Opin. Plant Biol., 1, 305–310. [DOI] [PubMed] [Google Scholar]
- Sheen J. (1996) Ca2+-dependent protein kinases and stress signal transduction in plants. Science, 274, 1900–1902. [DOI] [PubMed] [Google Scholar]
- Tähtiharju S., Sangwan,V., Monroy,A.F., Dhindsa,R.S. and Borg,M. (1997) The induction of kin genes in cold-acclimating Arabidopsis thaliana. Evidence of a role for calcium. Planta, 203, 442–447. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Isobe,M. and Muto,S. (1997) An increase in cytosolic calcium ion concentration precedes hypoosomotic shock-induced activation of protein kinases in tobacco suspension culture cells. FEBS Lett., 401, 202–206. [DOI] [PubMed] [Google Scholar]
- Takken F.L.W., Luderer,R., Gabriels,S., Westerink,N., Lu,R., deWit,P. and Joosten,M. (2000) A functional cloning strategy, based on a binary PVX-expression vector, to isolate HR-inducing cDNAs of plant pathogens. Plant J., 24, 275–283. [DOI] [PubMed] [Google Scholar]
- Thomas C.M., Tang,S., Hammond-Kosack,K.E. and Jones,J.D.G. (2000) Comparison of the hypersensitive response induced by the tomato Cf-4 and Cf-9 genes in Nicotiana spp. Mol. Plant Microbe Interact., 11, 1155–1166. [DOI] [PubMed] [Google Scholar]
- Urao T., Katagiri,T., Mizoguchi,T., Yamaguchi-Shinozaki,K., Hayashida,N. and Shinozaki,K. (1994) Two genes that encode Ca2+-dependent protein-kinases are induced by drought and high-salt stresses in Arabidopsis thaliana. Mol. Gen. Genet., 244, 331–340. [DOI] [PubMed] [Google Scholar]
- Vitart V., Christodoulou,J., Huang,J.F., Chazin,W.J. and Harper,J.F. (2000) Intramolecular activation of a Ca2+-dependent protein kinase is disrupted by insertions in the tether that connects the calmodulin-like domain to the kinase. Biochemistry, 39, 4004–4011. [DOI] [PubMed] [Google Scholar]
- Wang X.-Q. and Wu,W.-H. (1999) Involvement of calcium-dependent protein kinases in ABA-regulation of stomatal movement. Acta Bot. Sinica, 41, 556–559. [Google Scholar]
- Wulff B.B.H., Thomas,C.M., Smoker,M., Grant,M. and Jones,J.D.G. (2001) Domain swapping and gene shuffling identify sequences required for induction of an Avr-dependent hypersensitive response by the tomato Cf-4 and Cf-9 proteins. Plant Cell, 13, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.O., Shah,J. and Klessig,D.F. (1997) Signal perception and transduction in defense responses. Genes Dev., 11, 1621–1639. [DOI] [PubMed] [Google Scholar]
- Yoon G.M., Cho,H.S., Ha,H.J., Liu,J.R. and Lee,H.S.P. (1999) Characterization of NtCDPK1, a calcium-dependent protein kinase gene in Nicotiana tabacum, and the activity of its encoded protein. Plant Mol. Biol., 39, 991–1001. [DOI] [PubMed] [Google Scholar]
- Zhang S. and Klessig,D.F. (2000) Pathogen-induced MAP kinases in tobacco. In Hirt,H. (ed.), Results and Problems in Cell Differentiation. Springer, Heidelberg, Germany, pp. 65–84. [DOI] [PubMed]