Abstract
A series of ionic quaternary ammonium bromides featuring triazole moieties, QAS-trzBn 4 , QAS-trzPic 4 , and QAS-trzBn 2 Pic 2 , were synthesized via Cu-catalyzed azide–alkyne cycloaddition (CuAAC) between propargyl-based ammonium bromide and benzyl- or 2-picolylazide. X-ray crystallographic analyses of QAS-trzBn 4 and QAS-trzBn 2 Pic 2 revealed strong interactions between Br– ions and both triazolyl H and methylene H atoms (+NCH2), as evidenced by short Br–···H contacts ranging from 2.68 to 3.00 Å. The catalytic activities of these compounds as bifunctional, single-component catalysts for the CO2/epoxide cycloaddition were evaluated under both atmospheric and elevated CO2 pressures. Notably, catalysts containing pyridyl-triazole groups exhibited superior catalytic performances compared with the benzyl-triazole-based catalyst, QAS-trzBn 4 . A substrate scope study using QAS-trzPic 4 under 20 atm of CO2 at 100 °C revealed that electron-deficient epoxide substrates were more active, yielding good to excellent conversions (88–100%) to cyclic carbonates within 6 h. Computational studies identified key binding modes in pyridine-substituted systems that position both the epoxide and CO2 in close proximity. In particular, the QAS-trzPic 4 -CO 2 -epoxide complex is more stabilized than its benzyl derivative, QAS-trzBn 4 -CO 2 -epoxide, due to favorable interactions of CO2 with the pyridyl substituents.


Introduction
Carbon capture and utilization technologies have gained increasing interest due to their potential to mitigate CO2 emissions while transforming this abundant C1 feedstock into value-added chemicals. This approach aligns with the principles of sustainable chemistry by enabling the conversion of CO2, which has often been treated as waste, into useful products. Among the various chemicals that can be derived from CO2, such as alcohols, formic acid, urea, and CO, cyclic carbonates stand out due to their broad applications in green solvents, lithium–ion battery electrolytes, and polymer precursors. −
Traditionally, CO2/epoxide conversion to cyclic carbonates has relied on binary catalytic systems that pair a Lewis-acidic activator with a nucleophilic cocatalyst. ,, The Lewis acidic site, either a metal center or an organic hydrogen-bond donor, activates epoxides, while halide counterions usually from tetrabutylammonium salts (TBA+X–; X = Cl, Br, I) promote epoxide’s ring-opening. , Within these catalyst frameworks, metal-free organocatalytic systems featuring HBDs, such as ureas, phenols, and amide-containing macrocycles, combined with TBA+X– constitute a major class. Meanwhile, bifunctional, single-component catalysts integrate activation and nucleophilic functions within one structure, allowing easy handling and reduced chemical waste. − In addition, when immobilized, they enable more efficient reuse in heterogeneous platforms. ,
Quaternary ammonium halides have previously been investigated as effective bifunctional single-component catalysts for the CO2/epoxide cycloaddition. In 2002, Caló and colleagues demonstrated that NBu4 +X– could catalyze this type of reaction alone. In particular, a 1:1 wt % mixture of tetrabutylammonium bromide (TBABr) and tetrabutylammonium iodide (TBAI) converted styrene oxide (SO) and CO2 (1 atm) into styrene carbonate (SC) with an 83% yield at 120 °C in 4 h. Zhang and co-workers further explored the catalytic conversion of propylene oxide using 1.6 mol % of TBABr or its hydroxyl-functionalized derivative, hydroxyethyltributylammonium bromide (HETBAB), under 2.0 MPa CO2 at 125 °C. With the catalyst TBABr or HETBAB, 74% and 96% conversions of propylene oxide to propylene carbonate with >99% selectivity were achieved at 1 h, respectively. The presence of Lewis acid sites, i.e., hydroxyl functional groups, promotes epoxide activation and enhanced coupling activity. − Recently, our group also reported ionic, triazole-based polymers as efficient and reusable single-component heterogeneous catalysts for CO2/epoxide cycloaddition.
Building on this concept, triazole-based compounds present a promising catalyst platform for CO2/epoxide cycloaddition due to their structural tunability and ability to incorporate functional groups via click chemistry. In particular, we are interested in introducing pyridine into the catalyst framework to enhance CO2-capturing ability. Meanwhile, the quaternary ammonium-triazole moiety provides several advantages: (i) the Lewis basic triazole N atoms contribute to CO2 adsorption, , (ii) triazolyl H atoms may function as Lewis acid sites to activate epoxides, and (iii) the quaternary ammonium moiety creates a sterically hindered cationic environment that allows weak interactions with halide counterions, potentially increasing their nucleophilicity and overall catalytic efficiency.
In this study, we present a new class of quaternary ammonium salts featuring 1,2,3-triazole functional groups with either benzyl (QAS-trzBn 4 ) or 2-picolyl (QAS-trzPic 4 ) substituents. Additionally, we demonstrate a synthetic pathway for a dual-substituted variant, QAS-trzBn 2 Pic 2 . These novel QAS-trz catalysts were evaluated as bifunctional, single-component catalysts for CO2/epoxide cycloaddition with a particular focus on understanding the impact of triazole substituents on catalytic performance.
Experimental Section
Materials, Methods, and Instrumentation
All air-sensitive reactions were carried out in dry glassware under a N2 atmosphere in a glovebox or using standard Schlenk techniques. All reagents, including propargyl bromide (>97% stabilized in MgO) and tripropargylamine, were purchased from TCI or Sigma-Aldrich and used without further purification. Reagent-grade solvents were purchased from LabScan. PhCH2N3 and pyCH2N3 were prepared according to the literature. , Tetrapropargylammonium bromide (TPABr) and the triazole-based compounds, (1-R-1H-1,2,3-triazol-4-yl)methanol [R = PhCH2 (1-Bn), 2-pyCH2 (1-Pic), 1-benzyl-1H-1,2,3-triazole-4-carbaldehyde (2-Bn), and N,N-bis((1-benzyl-1H-1,2,3-triazol-4yl)methyl)prop-2-yn-1-amine (3-Bn), were synthesized following the literature methods.
1H (400 MHz) and 13C{1H} (100 MHz) NMR spectra were acquired in deuterated solvents at room temperature by using a Bruker-Ascend 400 high-resolution nuclear magnetic resonance spectrometer with chemical shifts referenced to residual solvent peaks. Fourier transform-infrared (FT-IR) spectra were collected on a Bruker model Alpha spectrometer from solid samples. High resolution mass spectra (HRMS) of 4-Bn, 4-Pic, QAS-trzR 4 (R = Bn, 2-Pic), and QAS-trzBn 2 Pic 2 in CH3CN were obtained in positive-ion mode on a Bruker micrOTOF II. Elemental analyses were performed using the LECO CHN analyzer model CNH-828. Product yields and percent substrate conversions obtained from catalytic experiments were analyzed by 1H NMR spectroscopy.
Synthesis and Characterization
1-(Pyridin-2-ylmethyl)-1H-1,2,3-triazole-4-carbaldehyde (2-Pic)
A mixture of 1-Pic (7.16 g, 37.6 mmol) and excess MnO2 (49.1 g, 564.6 mmol) in 150 mL of CH3CN was refluxed at 90 °C for 2 days. After it was cooled to room temperature, the suspension was filtered through silica/Celite to remove MnO2. The precipitates were washed with 50 mL of EtOAc and 50 mL of CH2Cl2. The combined filtrates were dried in vacuo to yield 2-Pic as a yellow liquid in 97% yield (6.86 g, 36.5 mmol). 1H NMR (400 MHz, CDCl3): δ 10.14 (s, 1H, CHO), 8.61 (m, 1H, pyH), 8.33 (s, 1H, trzH), 7.73 (t J = 8 Hz, 1H, PyH), 7.30 (d J = 4 Hz, 2H, PyH), 5.71 (s, 2H, CH 2). 13C{1H} NMR (100 MHz, CDCl3): δ 185.0 (CHO), 153.1, 150.1, 147.9, 137.5, 126.2, 123.8, 122.7 (aromatic Cs), 55.7 (pyCH2). ESI-MS (m/z): calcd for C9H8N4O, [M]: 188.07; found, 211.0595 [M + Na]+. FT-IR (cm–1): 1686 (CO).
N,N-Bis((1-(pyridin-2-ylmethyl)-1H-1,2,3-triazol-4yl)methyl)prop-2-yn-1-amine (3-Pic)
To a 50 mL CH2Cl2 solution of 2-Pic (6.86 g, 36.5 mmol) was added propargylamine (0.96 g, 17.4 mmol). The reaction solution was stirred for 24 h at room temperature, after which Na[BH(OAc)3] (11.0 g, 52.1 mmol) was added into the reaction mixture. After another 24 h of stirring, a 1 M aqueous NaOH solution (50 mL) was added, and the reaction mixture was vigorously stirred for 20 min. The product was extracted by CH2Cl2 (3 × 50 mL), upon which the combined CH2Cl2 extracts were dried in anhydrous Na2SO4, filtered, and removed in vacuo to afford the spectroscopically clean product, 3-Pic, obtained as a yellow oil in 90% yield (6.27 g, 15.7 mmol). The product was immediately used in the next step without purification. 1H NMR (400 MHz, CDCl3): δ 8.49 (m, 2H pyH), 7.67 (s, 2H, trzH), 7.59 (dt J = 8, 4 Hz, 2H, pyH), 7.18 (m, 2H, pyH), 7.08 (d J = 4 Hz, 2H, pyH), 5.56 (s, 4H, CH 2py), 5.26 (s, 4H, NCH 2trz), 3.78 (s, 2H, NCH 2C), 2.06 (s, 1H, CH). 13C{1H} NMR (125 MHz, CDCl3): δ 154.4, 149.7, 144.8, 137.3, 123.8, 123.3, 122.2 (aromatic Cs), 78.4 (CCH), 73.8 (CCH), 55.4, 47.8, 41.9 (CH2).
Dipropargyl-bis(4-R-1H-1,2,3-triazolylmethyl)ammonium bromide [R = PhCH2 (4-Bn), 2-pyCH2 (4-Pic)]
To a 20 mL CH2Cl2 solution of 3-Bn or 3-Pic was added excess propargyl bromide. The yellow solution was stirred at room temperature for 4 days, upon which white precipitates were formed. The solid was filtered, washed with CH2Cl2, and dried under vacuum, resulting in a spectroscopically clean product.
4-Bn: 3-Bn (1.5 g, 3.7 mmol) and propargyl bromide (1.79 g, 15.1 mmol) were used as substrates. A white solid identified as 4-Bn was obtained in a 95% yield (1.9 g, 3.7 mmol). 1H NMR (400 MHz, DMSO-d 6): δ 8.57 (s, 2H, trzH), 7.43–7.34 (m, 10H, PhH), 5.70 (s, 4H, CH 2Ph), 4.75 (s, 4H CH2trz), 4.30 (s, 4H, + NCH 2C), 4.07 (s, 2H, CH). 13C{1H} NMR (100 MHz, DMSO-d 6): δ 135.6, 134.4, 129.3, 129.0, 128.5, 128.3 (aromatic Cs), 84.2, 71.7 (CCH), 53.7, 53.3, 49.2 (CH2). ESI-MS (m/z): calcd for C26H26N7, [M+] 436.2244; found, 436.2303 [M+]. FT-IR (cm–1): 3296 (w, Ar–H), 3154 (m, C–H), 2121 (w, CC).
4-Pic: 3-Pic (6.27 g, 15.7 mmol) and propargyl bromide (7.47 g, 62.8 mmol) were used as substrates. The product 4-Pic was obtained as a yellow solid in 70% yield (5.70 g, 11.0 mmol).1H NMR (400 MHz, DMSO-d 6): δ 8.63 (s, 2H, trzH), 8.54 (m, 2H, pyH), 7.85 (m, 2H, pyH), 7.38 (m, 4H, pyH), 5.85 (s, 4H, CH2py), 4.79 (s, 4H, + NCH 2trz), 4.32 (d J = 4 Hz, 4H, + NCH 2C) 4.12 (t J = 4 Hz, 2H, CH). 13C{1H} NMR (100 MHz, DMSO-d 6): δ 154.9, 149.3, 137.9, 134.6, 130.7, 130.4, 123.9, 122.9 (aromatic Cs), 84.6, 72.0 (CCH), 55.0, 54.0, 49.5 (CH2). ESI-MS (m/z): calcd for C24H24N9, [M+] 438.215; found, 438.217 [M+]. FT-IR (cm–1): 3296 (w, Ar–H), 3140 (str, C–H) 2122 (w, CC).
Symmetrical QAS-trzR4 [R = CH2Ph (Bn) and 2-CH2py (Pic)]
To a mixture of TPABr (0.10 g, 0.40 mmol) and RN3 (R = CH2Ph or 2-CH2py, 1.7 mmol) was added 15 mL of CH3OH, followed by CuSO4·5H2O (24.9 mg, 0.10 mmol) and ascorbic acid (30.3 mg, 0.17 mmol) at room temperature. The reaction mixture was stirred for 24 h, upon which it became a homogeneous solution. The solvent was dried under vacuum, after which 30 mL of CH2Cl2 was added. To this solution, EDTA (0.41 g, 1.4 mmol) in 20 mL of 10% aqueous NH4OH solution was added, and the mixture was stirred at room temperature for 8 h. The product was extracted with CH2Cl2 and dried by using anhydrous Na2SO4. Filtration, solvent evaporation, and crystallization afforded the desired product.
QAS-trzBn4
RN3 = PhCH2N3 (0.23 g). After the addition of EDTA, the homogeneous aqueous solution was extracted with 3 × 60 mL CH2Cl2. A layer diffusion of Et2O onto a CH2Cl2 solution of the crude product at room temperature afforded an off-white crystalline solid in 65% yield (0.20 g, 0.26 mmol). 1H NMR (400 MHz, CDCl3): δ 8.64 (s, 4H, trzH), 7.32–7.23 (m, 20H, PhH), 5.62 (s, 8H, trzCH 2Ph), 4.43 (s, 8H, + NCH 2). 13C{1H} NMR (100 MHz, CDCl3): δ 134.9, 134.1, 129.8, 129.1, 128.8, 128.3 (ArC), 54.5 (CH2Ph), 52.8 (+N–CH2). HRMS (m/z): calcd for C40H40N13, [M+] 702.3524; found, 702.3596 [M+]. X-ray fluorescence (XRF) (wt %): Br 100%. Anal. Calcd for C40H40N13Br: C, 61.37; H, 5.15; N, 23.27. Found: C, 61.50; H, 5.16; N, 23.45.
QAS-trzPic4
RN3 = 2-pyCH2N3 (0.24 g). After stirring with the EDTA solution, the addition of 30 mL of CH2Cl2 resulted in white precipitates, which were filtered, washed with 20 mL of CH2Cl2, and dried under reduced pressure. In addition, the combined CH2Cl2 filtrate was concentrated and layered with Et2O at room temperature to induce more product precipitation. The product was obtained as a white solid in a 67% yield (0.21 g, 0.27 mmol). 1H NMR (400 MHz, DMSO-d 6): δ 8.81 (s, 4H, trzH), 8.54 (m, 4H, pyH), 7.84 (dt J = 8, 4 Hz, 4H, pyH), 7.41 (d J = 8 Hz, 4H, pyH) 7.36 (m, 4H, pyH), 5.85 (s, 8H, trzCH 2py), 4.61 (s, 8H, +NCH 2). 13C{1H} NMR (100 MHz, DMSO-d 6): δ 154.5, 149.5, 137.5, 134.9, 130.6, 123.4, 122.5 (ArC) 54.6 (trzCH2py), and 52.1 (+NCH2). ESI-MS (m/z): calcd for C36H36N17, [M+] 706.3334; found, 706.3395 [M+]. XRF (wt %): Br 100%. Anal. Calcd for C36H36N17Br: C, 54.96; H, 4.61; N, 30.27. Found: C, 54.91; H, 4.58; N, 30.32.
Dual-Substituted QAS-trzBn2Pic2
The CuAAC reaction was employed for the synthesis using either (i) 4-Bn and 2-pyCH2N3 or (ii) 4-Pic and PhCH2N3 as reactants. The procedure followed that of QAS-trzR 4 , utilizing CuSO4·5H2O (60.4 mg, 0.24 mmol) and ascorbic acid (46 mg, 0.26 mmol) as the catalyst system. During the workup, a solution of EDTA (0.9 g, 3.4 mmol) in 20 mL of a 10% aqueous NH4OH solution was added to sequester any residual copper. Crystallization by a layer diffusion of Et2O onto a CH3CN solution of the product at room temperature afforded an off-white crystalline solid after several days.
-
(i)
4-Bn (0.50 g, 0.96 mmol) and 2-pyCH2N3 (0.26 g, 1.96 mmol) were stirred in 60 mL CH3OH and 9 mL DMSO. Yield = 43% (0.32 g, 0.41 mmol).
-
(ii)
4-Pic (0.50 g, 0.96 mmol) and PhCH2N3 (0.26 g, 1.96 mmol) were stirred in 30 mL CH3OH. Yield = 87% (0.66 g, 0.84 mmol).
1H NMR (400 MHz, CD3OD): δ 8.87 (s, 2H, trzH), 8.77 (s, 2H, trzH), 8.49 (m, 2H, pyH), 7.86 (m, 2H, pyH), 7.48 (d J = 8 Hz, 2H, pyH), 7.40–7.33 (m, 12H, pyH, and PhH) 5.82 (s, 4H, trzCH 2py), 5.69 (s, 4H, trzCH 2Ph), 4.59 (s, 8H, + NCH2). 13C{1H} NMR (100 MHz, CD3OD): δ 155.4, 150.6, 139.2, 136.8, 136.7, 136.4, 131.9, 131.1, 130.1, 129.7, 129.4, 124.9, 124.2 (ArC), 56.1 (trzCH2py), 55.1 (trzCH2Ph), 53.5, 53.4 (+NCH2). ESI-MS (m/z): calcd for C38H38N15, [M+]: 704.3429; found, 704.3494 [M+]. XRF (wt %): Br 100%. Anal. Calcd for C38H38N15Br: C, 57.50; H, 4.95; N, 26.48. Found: C, 57.62; H, 4.94; N, 26.59.
X-ray Crystallography
Crystal data for 4-Pic, QAS-trzBn 4 , and QAS-trzBn 2 Pic 2 are shown in Table . Single crystals of 4-Pic were obtained via a layer diffusion of Et2O into a CH3OH solution of the product at room temperature to afford colorless crystalline solids after several days. Meanwhile, X-ray quality crystals of QAS-trzBn 4 and QAS-trzBn 2 Pic 2 were grown by the slow diffusion of Et2O into concentrated solutions of the ion compounds in CH2Cl2 (for QAS-trzBn 4 ) and CH3CN (for QAS-trzBn 2 Pic 2 ) at room temperature.
1. Crystallographic Data for 4-Pic, QAS-trzBn 4 , and QAS-trzBn 2 Pic 2 .
| 4-pic | QAS-trzBn4 | QAS-trzBn2Pic2 | |
|---|---|---|---|
| formula | C24H24BrN9 | C40H40BrN13 | C38H38BrN15 |
| formula wt | 518.43 | 782.76 | 784.74 |
| crystal system | monoclinic | monoclinic | monoclinic |
| space group | P21/c | C 2/c | P21/c |
| crystal size/mm | 0.15 × 0.13 × 0.052 | 0.12 × 0.098 × 0.051 | 0.098 × 0.094 × 0.049 |
| a/Å | 6.06650(10) | 51.117(2) | 12.3902(12) |
| b/Å | 21.2650(4) | 15.3307(5) | 29.425(3) |
| c/Å | 18.2518(3) | 10.0195(4) | 10.4638(10) |
| α/deg | 90 | 90 | 90 |
| β/deg | 98.051(2) | 94.423(3) | 98.241(5) |
| γ/deg | 90 | 90 | 90 |
| V/Å3 | 2331.35(7) | 7828.4(5) | 3775.6(6) |
| Z | 4 | 8 | 4 |
| D c/mg m–3 | 1.477 | 1.328 | 1.381 |
| μ/mm–1 | 2.665 | 1.806 | 1.890 |
| F(000) | 1064 | 3248 | 1624 |
| θ range/deg | 3.209–68.479 | 3.010–68.538 | 3.004–68.368 |
| reflns. collected | 4249 | 30261 | 54416 |
| reflns. unique | 3844 | 3662 | 6922 |
| parameters | 308 | 549 | 549 |
| goodness-of-fit on F 2 | 1.035 | 1.037 | 1.029 |
| R 1, wR 2 [I > 2σ(I)] | 0.0350, 0.0876 | 0.0727, 0.1759 | 0.0506, 0.1373 |
| R all, wR 2 [all data] | 0.0385, 0.0900 | 0.1375, 0.2074 | 0.0667, 0.1490 |
| max, min Δρ/e Å–3 | 0.44, −0.39 | 0.65, −0.43 | 0.24, −0.48 |
X-ray crystallographic data of 4-Pic were collected on a Rigaku SuperNova diffractometer with a HyPix3000 detector and CuKα radiation (λCu = 1.54184 Å) at 150 K. The data were reduced, scaled, and integrated using CrysAlisPro (Rigaku, 1.171.40.81a, 2020). An absorption correction was applied as implemented in SCALE3 ABSPACK (Rigaku, 1.171.40.81a, 2020). Meanwhile, data of QAS-trzBn 4 and QAS-trzBn 2 Pic 2 were obtained on Bruker D8 Venture CMOS PHOTON I diffractometer with a microfocus detector and CuKα radiation (λCu = 1.54178 Å) at 298 K. Data reduction was performed using SAINT. All three structures were solved, and the space group was determined by ShelXT using intrinsic phasing and refined by full matrix least-squares minimization on F 2 using ShelXL (version 2018/3) with OLEX2 used as a graphical interface. All non-hydrogen atoms were refined anisotropically, while hydrogen atom positions were calculated geometrically and refined using a riding model. In the case of 4-Pic, the structure was found to be twinned and was refined as a two-component twin in a 71:29 ratio. Disorder of two of the phenyl groups was found in QAS-trzBn 4 and QAS-trzBn 2 Pic 2 , which were modeled in two parts with appropriate restraints. All pictures were created using OLEX2. Crystallographic data for 4-Pic, QAS-trzBn 4 , and QAS-trzBn 2 Pic 2 have been deposited with the Cambridge Crystallographic Data Center, CCDC 2428915, 2429633, and 2429633.
General Procedure for CO2/Epoxide Cycloaddition
1 atm CO2: To a round-bottom flask with a magnetic stir bar was added epoxide (12.8 mmol) and QAS-trzR 4 (50 mg, 0.50 mol %). The balloon was pressurized with 1 bar CO2, and the reaction mixture was heated at 100 °C for a given time, after which the flask was immersed into an ice bath, and CO2 was carefully released. The conversions to cyclic carbonates were determined using 1H NMR spectroscopy (400 MHz, CDCl3).
10 atm CO 2 : To a stainless-steel autoclave equipped with a magnetic stir bar was added epoxide (25.6 mmol) and QAS-trzR 4 (100 mg, 0.50 mol %). Then, the autoclave was sealed, pressurized with 10 atm CO2, and heated at 100 °C. After a given time, it was immersed into an ice bath, and CO2 was carefully released. The conversions to cyclic carbonates were determined using 1H NMR spectroscopy (600 MHz, CDCl3).
Results and Discussion
Symmetrical QAS-trzBn 4 and QAS-trzPic 4
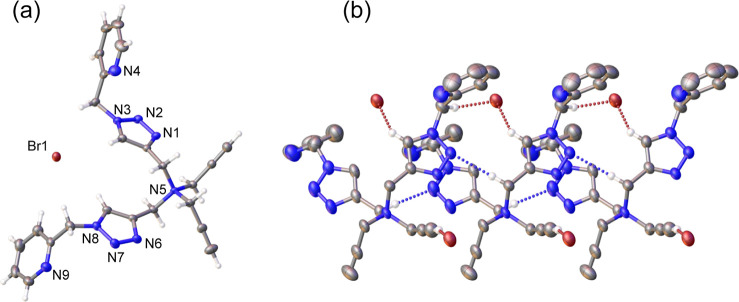
The click reaction between TPABr and 4.4 equiv of ArCH2N3 (Ar = Ph or 2-py), catalyzed by CuSO4·5H2O and ascorbic acid in CH3OH at room temperature, afforded ionic, symmetrical tetra(triazolyl)ammonium bromides, QAS-trzBn 4 and QAS-trzPic 4 , as a white solid (Scheme ). Substituting benzyl with 2-picolyl groups significantly impacted the solubility of the ionic compounds. Specifically, QAS-trzBn 4 dissolves in a broad range of organic solvents, including CHCl3, CH3CN, and CH3OH, whereas QAS-trzPic 4 , is soluble only in highly polar solvents such as DMF and DMSO, exhibiting low solubility in CH3OH, CH3CN, and CH2Cl2. This property may result from extensive intermolecular dipole–dipole interactions between the pyridine N atom and electropositive +NCH 2, as suggested by the crystal structure of its related variant QAS-trzBn 2 Pic 2 (Figure ).
1. Synthesis of QAS-trzR 4 (R = Bn, Pic).
2.
Crystal structures of (a) the asymmetric unit of QAS-trzBn 4 , showing a short H···Br– contact. (b) The asymmetric unit of QAS-trzBn 2 Pic 2 , showing short H···Br– contacts. (c) Two QAS-trzBn 2 Pic 2 molecules, displaying H···Br– and H···Npy contacts. Non-H atoms are represented as thermal ellipsoids at the 30% thermal probability level. For clarity, all H atoms except those involved in short contacts are omitted.
The low solubility of QAS-trzPic 4 facilitated its precipitation as a pure white solid (67% yield) during workup by adding CH2Cl2 after the EDTA treatment. On the other hand, QAS-trzBn 4 was purified by slow Et2O diffusion into a concentrated CH2Cl2 solution at room temperature, yielding crystalline, colorless solids in 65% yield. Notably, despite the presence of SO4 2– during catalysis, XRF and elemental analyses confirmed that the Br– ion was the sole counterion, consistent with proposed molecular formulas. The 1H NMR spectra of QAS-trzBn 4 (CDCl3) and QAS-trzPic 4 (DMSO-d 6) each display three characteristic singlet resonances at δ 8.71, 5.55, 4.63 and δ 8.81, 5.85, 4.61, which are assignable to trzH, trzCH 2Ph, and +NCH 2, respectively (Figures S20 and S24).
Dual-Substituted QAS-trzBn2Pic2
In addition to all identical substituents on the quaternary ammonium compounds, the dual-substituted derivative QAS-trzBn 2 Pic 2 could also be synthesized from the ionic dipropargylbis(triazolylmethyl)ammonium bromide precursors 4-Bn and 4-Pic, whose synthesis is shown in Scheme . It should be mentioned that the neutral benzyl-substituted propargylbis(triazolylmethyl)amine compound 3-Bn and its precursors 1-Bn and 2-Bn have previously been reported by our group. Accordingly, the synthetic methods of related picolyl-substituted compounds 1-Pic, 2-Pic, and 3-Pic, followed those of their benzyl-substituted counterparts.
2. Synthesis of n-Pic ( n = 2–4) and 4-Bn .
The Cu-catalyzed click reaction between equimolar amounts of 2-pyCH2N3 and propargyl alcohol in the presence of 25 mol % CuSO4·5H2O and 45 mol % ascorbic acid in CH3OH at room temperature afforded the triazole product 1-Pic as a yellow solid in 67% yield. Subsequent selective alcohol oxidation using excess MnO2 in refluxing CH3CN, followed by purification via flash column chromatography, yielded the aldehyde product 2-Pic as a yellow oil in 97% yield. The formation of the aldehyde functional group (CHO) was confirmed by a characteristic singlet resonance in the 1H NMR spectrum at δ 10.14 (in CDCl3, Figure S4) and a strong absorption band at 1686 cm–1 in the FT-IR spectrum, corresponding to CO stretching (Figure S7). Reductive amination of 2-Pic with ca. 0.5 equiv of propargylamine in CH2Cl2, followed by the addition of 3 equiv of Na[BH(OAc)3], afforded the neutral amine product 3-Pic as a yellow oil in 90% yield. Similar to its amine-triazole analogue 3-Bn, the picolyl-substituted compound 3-Pic was found to be unstable, converting within several days into intractable mixtures, as evidenced by 1H NMR spectroscopy. Notably, even when spectra were acquired immediately after isolation, the 13C{1H} and 2D 1H–13C HMBC NMR data already revealed impurities and signs of decomposition (Figures S9 and S10). Consequently, both 3-Bn and 3-Pic compounds were immediately reacted with excess propargyl bromide in CH2Cl2 at room temperature, which resulted in white precipitates, characterized as 4-Bn and 4-Pic, in 95% and 70% yields, respectively (pathway (b), Scheme ). The 1H NMR spectra of 4-Bn and 4-Pic in DMSO-d 6 displayed a characteristic singlet resonance at δ 8.57 and 8.63, assignable to trzH, along with an alkynyl 1H peak at δ 4.07 and 4.12 (Figures S11 and S14). In contrast to their neutral amine analogues, the ionic compounds 4-Bn and 4-Pic are thermally robust and air stable.
X-ray quality crystals of 4-Pic were grown from layering Et2O into a concentrated CH3OH solution of 4-Pic to allow slow diffusion at room temperature. As shown in Figure , the crystal structure of 4-Pic features the quaternary ammonium nitrogen (N5) in a distorted tetrahedral geometry, with bond angles ranging from 104.2(2)° to 113.0(2)°. The asymmetric unit cell of the crystal structure indicates significant intermolecular electrostatic interactions of the Br– ion with CCH (2.640 Å) and trzH atoms (2.875 and 2.987 Å). These values were notably shorter than the sum of the van der Waals radii for Br and H atoms (3.05 Å). , In contrast, ionic interactions between the quaternary ammonium center (N5) and the Br– ion appear negligible, with a substantial distance of 6.246 Å observed.
1.
Crystal structures of 4-Pic. C, N, and Br atoms are depicted as thermal ellipsoids at the 30% probability level. (a) The asymmetrical unit of 4-Pic. (b) Packing diagram displaying short H···Br– and H···Ntrz contacts. All H atoms except those involved in short contacts are omitted for clarity.
The dual-substituted, ionic quaternary ammonium species–triazole species containing both benzyl and 2-picolyl substituents, namely, QAS-trzBn 2 Pic 2 , was synthesized via CuAAC between 2-pyCH2N3 and 4-Bn or, alternatively, PhCH2N3 and 4-Pic, as shown in Scheme . The reaction between 4-Pic and 2 equiv of PhCH2N3 in CH3OH catalyzed by CuSO4·5H2O and ascorbic acid at room temperature afforded QAS-trzBn 2 Pic 2 as a dark orange solid in 87% yield. Alternatively, the CuAAC reaction between 4-Bn and 2 equiv of 2-pyCH2N3 in a 3:1 mixture of CH3OH/DMSO solvents under similar conditions afforded the click product in a lower yield of 43%. The reduced yield can be attributed to the limited solubility of 4-Bn in CH3OH, which necessitated the addition of a small amount of DMSO to achieve a homogeneous reaction mixture. However, the remaining DMSO in the product made its isolation challenging, ultimately contributing to the lower yield.
3. Synthesis of QAS-trzBn 2 Pic 2 .

The 1H NMR spectrum of QAS-trzBn 2 Pic 2 in CD3OD displays two singlet resonances at δ 8.87 and 8.77, corresponding to two types of trzH with benzyl and 2-picolyl substituents. In addition, three singlet resonances at δ 5.82, 5.69, and 4.59 can be assigned to trzCH 2-py, trzCH 2-Ph, and +N–CH 2, respectively (Figure S22). Elemental analysis, ESI-MS, and XRF data are consistent with the expected product.
Single crystals of QAS-trzBn 4 and QAS-trzBn 2 Pic 2 , grown from layering Et2O into CH2Cl2 and CH3CN solutions of the respective ionic compounds. Both crystallized in the monoclinic P21/c space group. Figure illustrates both crystal structures, revealing the Lewis acidic nature of +N–CH 2, with significant interactions with pyridine N donors (2.62 Å for QAS-trzBn 2 Pic 2 ) and Br– ions (2.68 and 2.93 Å for QAS-trzBn 4 and QAS-trzBn 2 Pic 2 , respectively).
Catalytic CO2/Epoxide Cycloaddition
The catalytic performance of the ionic QAS-trz compounds as bifunctional, single-component catalysts was evaluated using epichlorohydrin (ECH) as the substrate under 1 atm of CO2 at 100 °C. With 50 mg of QAS-trz catalysts (0.50 mol % Br–), the picolyl-containing derivatives, QAS-trzBn 2 Pic 2 and QAS-trzPic 4 , exhibited high catalytic activity, achieving 98% and 93%, conversion with 95% and 91% selectivity to the cyclic carbonate product after 12 h, respectively (entries 2 and 3, Table ). In contrast, the benzyl analogue QAS-trzBn 4 showed much lower activity, reaching only 39% conversion and 69% selectivity even after 24 h (entry 1). Using TBABr (0.5 mol %) as a control afforded 30% conversion and 73% selectivity at 12 h (entry 4). 1H NMR analysis identified 1-bromo-3-chloropropan-2-ol as the major byproduct, consistent with Br–-promoted epoxide ring opening, followed by protonation (i.e., no CO2 incorporation). Collectively, these results suggest that pyridyl substitution in the QAS-trz framework enhances the catalytic efficiency and favors CO2 incorporation under atmospheric CO2 conditions. Interestingly, in our previous work, the heterogeneous QAS-trz polymer catalyst linked with p-Xylene (QAP-1), which is structurally related to QAS-trzBn 4 , mediated CO2/ECH cycloaddition, achieving 98% conversion and 82% selectivity in 24 h at 1 atm CO2 and 120 °C using 0.59 mol % I– as the nucleophile. The higher apparent catalytic activity of QAP-1 is plausibly due to the stronger nucleophilic iodide and its higher nitrogen content, which enhances the CO2 adsorption at the polymer surface.
2. Catalytic Activity for CO2/ ECH Cycloaddition .
| entry | catalyst | conversion (%) | selectivity (%) |
|---|---|---|---|
| 1 | QAS-trzBn4 | 39 | 69 |
| 2 | QAS-trzBn2Pic2 | 98 | 95 |
| 3 | QAS-trzPic4 | 93 | 91 |
| 4 | TBABr | 30 | 73 |
Conditions: catalyst (0.5 mol % Br–), ECH (1.0 mL, 12.8 mmol), 1 atm CO2, 100 °C, 12 h.
% Conversion and % selectivity were determined using 1H NMR spectroscopy.
Reaction time = 24 h.
20.6 mg of TBABr (0.5 mol % Br–) was used as a catalyst.
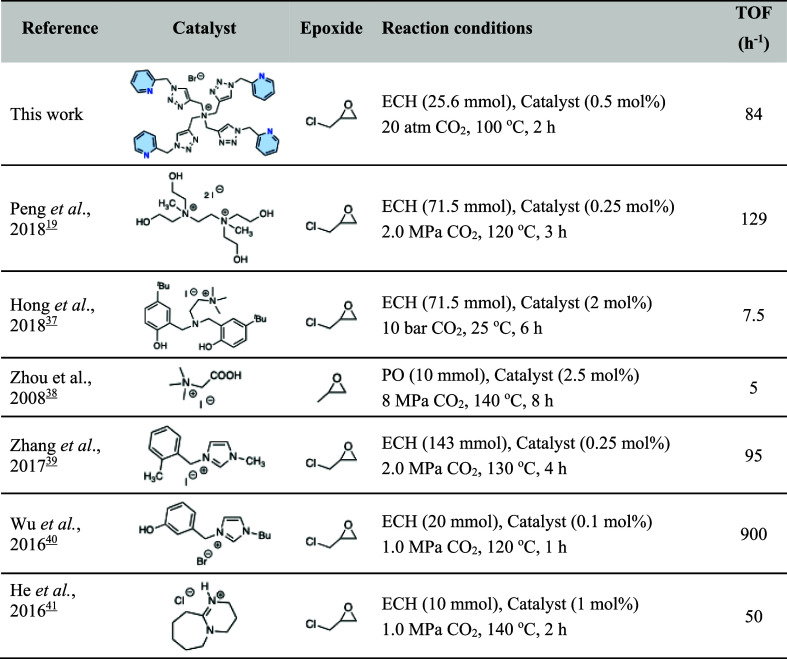
Given its performance and straightforward synthesis, QAS-trzPic 4 was selected for studies at elevated CO2 pressures. At 10 and 20 atm CO2 (0.5 mol % QAS-trzPic 4 ,100 °C), ECH was efficiently converted to the cyclic carbonate, reaching 84% in 2 h and >99% conversions in 6 h, corresponding to TOF = 84 and 33 h–1, respectively (entry 1, Table ). The styrene oxide/CO2 cycloaddition reaction proceeded with exclusive formation of SC, with conversion increasing from 24% at 10 atm of CO2 to 88% at 20 atm of CO2 after 6 h at 100 °C (entry 2). In contrast, the more electron-rich propylene oxide resulted in lower reactivity, affording only 45% conversion to cyclic propylene carbonate under identical conditions at 20 atm of CO2 (entry 3). Both allyl glycidyl ether and ethylene glycol diglycidyl ether underwent cycloaddition with CO2, yielding high conversions of 83% and 97%, respectively, to the corresponding cyclic carbonate products (entries 4 and 5), whereas benzyl glycidyl ether was less reactive (12%, entry 6). Notably, with benzyl glycidyl ether, the QAS-trzPic 4 catalyst remained in the solid phase throughout the reaction, which likely contributes to the low conversion. To test this, the same catalytic reactions (benzyl glycidyl ether, 20 atm of CO2, 100 °C) were run using QAS-trzBn 4 and QAS-trzBn 2 Pic 2 . Both catalysts became completely soluble during the catalysis and gave 60% and 74% conversion, respectively (entry 6). TOF benchmarking against other single-component homogeneous organocatalysts places QAS-trzPic 4 among the more active quaternary ammonium catalyst systems, despite the absence of strong H-bond donor groups such as hydroxyl, phenol, and carboxyl (Table ).
3. Substrate Scope with QAS-trzR 4 .
Conditions: QAS-trzPic 4 (0.5 mol % Br–, 0064 mmol), epoxide (12.8 mmol), 10–20 atm CO2, 100 °C, 2–6 h.
% conversion and % selectivity were determined using 1H NMR spectroscopy.
The QAS-trzPic 4 catalyst remained in the solid phase at the end of the reaction.
Catalyst = QAS-trzBn 4 .
Catalyst = QAS-trzBn 2 Pic 2 .
4. Summary of Catalytic Conditions and TOF of Selected Single-Component Organocatalysts for Cycloaddition between CO2 and Epoxide.
Computational Studies and Proposed Mechanism
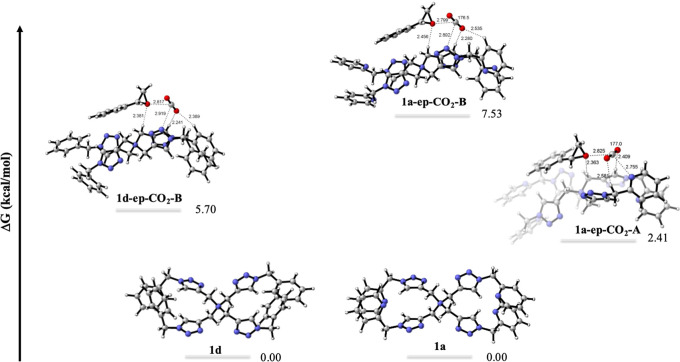
Computational studies were conducted to evaluate the binding of CO2 and styrene oxide (ep) with QAS-trzPic 4 (1a) and QAS-trzBn 4 (1d), as shown in Figure . We found key binding modes that position both epoxide and CO2 in close proximity (∼2.8 Å). This is expected to facilitate the CO2/epoxide cycloaddition, where Oep acts as a nucleophile attacking CO2 (Scheme ). For 1d, the binding mode 1d-ep-CO 2 -B involves interactions of the triazole N atom in 1d with CO2, i.e., Ntrz···CO2, along with +NCH2···Oep, trzH···O2C, and o-ArH···O2C interactions. The binding is endergonic by 5.70 kcal/mol. For picolyl-substituted compound 1a, the corresponding binding mode of 1a-ep-CO 2 -B is also endergonic by 7.53 kcal/mol. However, in the presence of the pyridine groups in 1a, an alternative binding mode was located. In particular, we observe the acid–base interactions between CO2 and the pyridine substituents of 1a, Npy···CO2, along with stabilization from +NCH2···Oep interactions, which yielded a more stabilized complex 1a-ep-CO 2 -A (2.41 kcal/mol). This data suggests that pyridine plays a distinctive role in stabilizing CO2 in 1a-ep-CO 2 -A, making such complexes more thermodynamically favorable than the related complexes with the Ntrz···CO2 interactions. Improved CO2/epoxide stabilization in the presence of pyridine substituents is attributed to the observed higher catalytic activity of QAS-trzPic 4 than QAS-trzBn 4 for CO2/ECH cycloaddition at 1 atm of CO2.
3.
Binding modes and associated relative binding free energies of CO2 and ep (ep = styrene oxide) to QAS-trzPic 4 (1a) and QAS-trzBn 4 (1d). The relative binding free energies (ΔG) are shown in kcal/mol. Selected bond distances are shown in Å.
4. Proposed Mechanism for Catalytic CO2/Epoxide Cycloaddition by QAS-trzPic 4 .
Based on computational results, the proposed mechanism for CO2/epoxide cycloaddition catalyzed by QAS-trzPic 4 is illustrated in Scheme . In the presence of CO2 and epoxide substrates, the acidic H atoms of the triazole ring and the methylene groups surrounded by a quaternary ammonium nitrogen and aromatic triazolyl ring (+NCH 2trz) interact with the O atoms of CO2 and the epoxide (A). Meanwhile, the pyridine N donor, acting as a Lewis base, interacts with the electrophilic carbon of CO2. The reaction proceeds via epoxide ring opening by Br–, followed by the nucleophilic attack of the resulting alkoxide on CO2. The stabilized carbonate intermediate (B) underwent intramolecular ring cyclization to yield the cyclic carbonate product and regenerate the homogeneous catalyst QAS-trzPic 4 .
Conclusion
In this study, a new class of symmetrical and dual-substituted quaternary ammonium bromide featuring triazole functional groups, QAS-trzBn 4 , QAS-trzPic 4 , and QAS-trzBn 2 Pic 2 , were successfully synthesized. Despite the presence of competing anions such as SO4 2– and OH– during the synthesis, XRF and elemental analyses confirmed that Br– was the sole counterion in the final products. X-ray crystallographic analysis of QAS-trzBn 4 and QAS-trzBn 2 Pic 2 revealed strong interactions between Br– and Npy with H atoms of both trzH and +NCH2, suggesting their significant Lewis acidic nature. Catalytic studies showed that picolyl-substituted compounds QAS-trzPic 4 and QAS-trzBn 2 Pic 2 outperform QAS-trzBn 4 as bifunctional, single-component homogeneous catalysts. Both QAS-trzPic 4 and QAS-trzBn 2 Pic 2 efficiently promoted the CO2/ECH cycloaddition, affording the cyclic carbonate product at 1 atm of CO2, 100 °C. In addition, QAS-trzPic 4 remains highly efficient across a range of epoxides under elevated CO2 pressures. Computational studies further indicate that pyridine substituents play a crucial role in stabilizing the QAS-trzPic 4 -CO 2 -epoxide complex, likely contributing to its enhanced catalytic efficiency. Based on these insights, a catalytic mechanism was proposed, highlighting trzH and +NCH2 as key Lewis acidic sites responsible for the binding of CO2 and epoxide activation. This study demonstrates the significant impact of triazolyl substituents on improving the catalytic performance in CO2/epoxide cycloaddition. Notably, pyridyl-substituted triazole functional groups enable CO2 stabilization, while the +NCH2 Lewis acid sites facilitate epoxide activation, as supported by computational and single-crystal analyses.
Supplementary Material
Acknowledgments
This research project has been supported by Mahidol University (Fundamental Fund: fiscal year 2025 by the National Science Research and Innovation Fund (NSRF), Grant No. FF-077/2568). The authors also appreciate support from the Faculty of Science, Mahidol University, under the National Research Universities Initiatives; Center for Excellence for Innovation; as well as the CIF Grant.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.5c04751.
1H, 13C{1H} NMR spectra, HRMS, and FT-IR spectra of ligand substrates and quaternary ammonium-triazole salts (QAS-trzBn 4 , QAS-trzBn 2 Pic 2 , and QAS-trzPic 4 ); elemental analysis and XRF results of QAS-trzBn 4 , QAS-trzBn 2 Pic 2 , and QAS-trzPic 4 ; 1H NMR spectra of the cycloaddition reactions under 1 and 10 atm CO2 pressures and various epoxide substrates; and computational details including methodology and parameters used in DFT calculations (PDF)
DpPyBr-150K 2 (CIF)
QAS-trzBn4 (CIF)
QAS-trzBn2Pic2 (CIF)
Declaration of generative AI and AI-assisted technologies in the writing process During the preparation of this work, the author(s) used ChatGPT-4o in order to improve the readability of the article. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
The authors declare no competing financial interest.
References
- Pescarmona P. P.. Cyclic carbonates synthesised from CO2: Applications, challenges and recent research trends. Curr. Opin. Green Sustainable Chem. 2021;29:100457. doi: 10.1016/j.cogsc.2021.100457. [DOI] [Google Scholar]
- Kamphuis A. J., Picchioni F., Pescarmona P. P.. CO2-fixation into cyclic and polymeric carbonates: principles and applications. Green Chem. 2019;21(3):406–448. doi: 10.1039/C8GC03086C. [DOI] [Google Scholar]
- Usman M., Rehman A., Saleem F., Abbas A., Eze V. C., Harvey A.. Synthesis of cyclic carbonates from CO2 cycloaddition to bio-based epoxides and glycerol: an overview of recent development. RSC Adv. 2023;13(33):22717–22743. doi: 10.1039/D3RA03028H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Monica F., Capacchione C.. Recent Advancements in Metal-catalysts Design for CO2/Epoxide Reactions. Asian J. Org. Chem. 2022;11(8):e202200300. doi: 10.1002/ajoc.202200300. [DOI] [Google Scholar]
- Cokoja M., Wilhelm M. E., Anthofer M. H., Herrmann W. A., Kühn F. E.. Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide by Using Organocatalysts. ChemSusChem. 2015;8(15):2436–2454. doi: 10.1002/cssc.201500161. [DOI] [PubMed] [Google Scholar]
- Rehman A., Resul M. G., Eze V. C., Harvey A.. A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate. Green Process. Synth. 2019;8(1):719–729. doi: 10.1515/gps-2019-0042. [DOI] [Google Scholar]
- Wang J.-Q., Dong K., Cheng W.-G., Sun J., Zhang S.-J.. Insights into quaternary ammonium salts-catalyzed fixation carbon dioxide with epoxides. Catal. Sci. Technol. 2012;2(7):1480–1484. doi: 10.1039/c2cy20103h. [DOI] [Google Scholar]
- Guo L., Lamb K. J., North M.. Recent Developments in Organocatalysed Transformation of Epoxides and Carbon Dioxide into Cyclic Carbonates. Green Chem. 2021;23:77–118. doi: 10.1039/D0GC03465G. [DOI] [Google Scholar]
- Kumatabara Y., Okada M., Shirakawa S.. Triethylamine Hydroiodide as a Simple Yet Effective Bifunctional Catalyst for CO2 Fixation Reactions with Epoxides under Mild Conditions. ACS Sustainable Chem. Eng. 2017;5(8):7295–7301. doi: 10.1021/acssuschemeng.7b01535. [DOI] [Google Scholar]
- Meng X., Ju Z., Zhang S., Liang X., von Solms N., Zhang X., Zhang X.. Efficient transformation of CO2 to cyclic carbonates using bifunctional protic ionic liquids under mild conditions. Green Chem. 2019;21(12):3456–3463. doi: 10.1039/C9GC01165J. [DOI] [Google Scholar]
- Aoyagi N., Furusho Y., Endo T.. Efficient Catalysts of Acyclic Guanidinium Iodide for the Synthesis of Cyclic Carbonates from Carbon Dioxide and Epoxides under Mild Conditions. Synthesis. 2020;52(01):150–158. doi: 10.1055/s-0037-1610735. [DOI] [Google Scholar]
- Emelyanov M. A., Stoletova N. V., Lisov A. A., Medvedev M. G., Smol’yakov A. F., Maleev V. I., Larionov V. A.. An octahedral cobalt(iii) complex based on cheap 1,2-phenylenediamine as a bifunctional metal-templated hydrogen bond donor catalyst for fixation of CO2 with epoxides under ambient conditions. Inorg. Chem. Front. 2021;8(16):3871–3884. doi: 10.1039/D1QI00464F. [DOI] [Google Scholar]
- Emelyanov M. A., Lisov A. A., Medvedev M. G., Maleev V. I., Larionov V. A.. Cobalt(III) Complexes as Bifunctional Hydrogen-Bond Donor Catalysts Featuring Halide Anions for Cyclic Carbonate Synthesis at Ambient Temperature and Pressure: A Mechanistic Insight. Asian J. Org. Chem. 2022;11(5):e202100811. doi: 10.1002/ajoc.202100811. [DOI] [Google Scholar]
- Calabrese C., Giacalone F., Aprile C.. Hybrid Catalysts for CO2 Conversion into Cyclic Carbonates. Catalysts. 2019;9(4):325. doi: 10.3390/catal9040325. [DOI] [Google Scholar]
- D’Elia V., Kleij A. W.. Surface science approach to the heterogeneous cycloaddition of CO2 to epoxides catalyzed by site-isolated metal complexes and single atoms: a review. Green Chem. Eng. 2022;3(3):210–227. doi: 10.1016/j.gce.2022.01.005. [DOI] [Google Scholar]
- Caló V., Nacci A., Monopoli A., Fanizzi A.. Cyclic carbonate formation from carbon dioxide and oxiranes in tetrabutylammonium halides as solvents and catalysts. Org. Lett. 2002;4(15):2561–2563. doi: 10.1021/ol026189w. [DOI] [PubMed] [Google Scholar]
- Sun J., Zhang S., Cheng W., Ren J.. Hydroxyl-functionalized ionic liquid: a novel efficient catalyst for chemical fixation of CO2 to cyclic carbonate. Tetrahedron Lett. 2008;49(22):3588–3591. doi: 10.1016/j.tetlet.2008.04.022. [DOI] [Google Scholar]
- Arruda da Mata Á. F., Glanzmann N., Fazza Stroppa P. H., Terra Martins F., das Chagas R. P., Da Silva A. D., Milani J. L. S.. Single-component, metal-free, solvent-free HO-functionalized 1, 2, 3-triazole-based ionic liquid catalysts for efficient CO 2 conversion. New J. Chem. 2022;46(25):12237–12243. doi: 10.1039/d2nj02052a. [DOI] [Google Scholar]
- Peng J., Wang S., Yang H.-J., Ban B., Wei Z., Wang L., Lei B.. Highly efficient fixation of carbon dioxide to cyclic carbonates with new multi-hydroxyl bis-(quaternary ammonium) ionic liquids as metal-free catalysts under mild conditions. Fuel. 2018;224:481–488. doi: 10.1016/j.fuel.2018.03.119. [DOI] [Google Scholar]
- Suleman S., Younus H. A., Khattak Z. A., Ullah H., Elkadi M., Verpoort F.. Co-catalyst and solvent free nitrogen rich triazole based organocatalysts for cycloaddition of CO2 into epoxide. Mol. Catal. 2020;493:111071. doi: 10.1016/j.mcat.2020.111071. [DOI] [Google Scholar]
- Chen Y., Chen C., Li X., Feng N., Wang L., Wan H., Guan G.. Hydroxyl-ionic liquid functionalized metalloporphyrin as an efficient heterogeneous catalyst for cooperative cycloaddition of CO2 with epoxides. J. CO2 Util. 2022;62:102107. doi: 10.1016/j.jcou.2022.102107. [DOI] [Google Scholar]
- Promchan P., Srifa A., Rungtaweevoranit B., Pananusorn P., Phomphrai K., Sangtrirutnugul P.. Ionic quaternary ammonium-triazole polymers as efficient single-component catalysts for CO2 conversion. J. CO2 Util. 2024;90:102989. doi: 10.1016/j.jcou.2024.102989. [DOI] [Google Scholar]
- Abdinejad M., Irtem E., Farzi A., Sassenburg M., Subramanian S., Iglesias van Montfort H.-P., Ripepi D., Li M., Middelkoop J., Seifitokaldani A.. et al. CO2 electrolysis via surface-engineering electrografted pyridines on silver catalysts. ACS Catal. 2022;12(13):7862–7876. doi: 10.1021/acscatal.2c01654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z., Saito T., Jiang D.-e.. Ab initio screening of CO2-philic groups. J. Phys. Chem. A. 2015;119(16):3848–3852. doi: 10.1021/acs.jpca.5b01892. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Das M., Manna A., Krishna R., Das S.. Newly designed 1, 2, 3-triazole functionalized covalent triazine frameworks with exceptionally high uptake capacity for both CO 2 and H 2. J. Mater. Chem. A. 2019;7(3):1055–1068. doi: 10.1039/C8TA08185A. [DOI] [Google Scholar]
- Somsri S., Gopalakrishnan M., Ratvijitvech T., Worakul T., Surawatanawong P., Kuwamura N., Konno T., Rungtaweevoranit B., Sangtrirutnugul P.. Nitrogen-rich, click-based porous organic polymers featuring flexible amine cores for catalytic CO2/epoxide cycloaddition. React. Funct. Polym. 2023;191:105690. doi: 10.1016/j.reactfunctpolym.2023.105690. [DOI] [Google Scholar]
- Kögel J. F., Abacılar N. C., Weber F., Oelkers B., Harms K., Kovačević B., Sundermeyer J.. Constrained-Geometry Bisphosphazides Derived from 1, 8-Diazidonaphthalene: Synthesis, Spectroscopic Characteristics, Structural Features, and Theoretical Investigations. Chem. - Eur. J. 2014;20(20):5994–6009. doi: 10.1002/chem.201304498. [DOI] [PubMed] [Google Scholar]
- Mamidyala S. K., Cooper M. A.. Probing the reactivity of o-phthalaldehydic acid/methyl ester: synthesis of N-isoindolinones and 3-arylaminophthalides. Chem. Commun. 2013;49(75):8407–8409. doi: 10.1039/c3cc43838d. [DOI] [PubMed] [Google Scholar]
- Chen H., Hou S., Tan Y.. An ‘in-water’halogen-ion compatible “click” catalyst for cucurbituril guest ligation. Supramol. Chem. 2016;28(9–10):801–809. doi: 10.1080/10610278.2016.1142089. [DOI] [Google Scholar]
- Díaz Velázquez H., Ruiz García Y., Vandichel M., Madder A., Verpoort F.. Water-soluble NHC-Cu catalysts: applications in click chemistry, bioconjugation and mechanistic analysis. Org. Biomol. Chem. 2014;12(46):9350–9356. doi: 10.1039/c4ob01350f. [DOI] [PubMed] [Google Scholar]
- Brotherton W. S., Michaels H. A., Simmons J. T., Clark R. J., Dalal N. S., Zhu L.. Apparent copper (II)-accelerated azide– alkyne cycloaddition. Org. Lett. 2009;11(21):4954–4957. doi: 10.1021/ol9021113. [DOI] [PubMed] [Google Scholar]
- Song H., Rogers N. J., Brabec V., Clarkson G. J., Coverdale J. P., Kostrhunova H., Phillips R. M., Postings M., Shepherd S. L., Scott P.. Triazole-based, optically-pure metallosupramolecules; highly potent and selective anticancer compounds. Chem. Commun. 2020;56(47):6392–6395. doi: 10.1039/D0CC02429E. [DOI] [PubMed] [Google Scholar]
- Nakarajouyphon V., Bunchuay T., Yoshinari N., Konno T., Sangtrirutnugul P.. Unsymmetrical PEG-substituted tris (triazolyl) amines as bi-functional surfactants for copper-catalyzed aerobic oxidation of alcohols in water. New J. Chem. 2022;46(13):6009–6017. doi: 10.1039/D1NJ04812K. [DOI] [Google Scholar]
- Somepalli, G. ; Goldblum, M. ; Schwarzschild, A. ; Bruss, C. B. ; Goldstein, T. . SAINT: Improved neural networks for tabular data via row attention and contrastive pre-training. 2021, arXiv preprint arXiv:2106.01342. [Google Scholar]
- Batsanov S. S.. Van der Waals radii of elements. Inorg. Mater. 2001;37(9):871–885. doi: 10.1023/A:1011625728803. [DOI] [Google Scholar]
- Mantina M., Chamberlin A. C., Valero R., Cramer C. J., Truhlar D. G.. Consistent van der Waals radii for the whole main group. J. Phys. Chem. A. 2009;113(19):5806–5812. doi: 10.1021/jp8111556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M., Kim Y., Kim H., Cho H. J., Baik M.-H., Kim Y.. Scorpionate Catalysts for Coupling CO2 and Epoxides to Cyclic Carbonates: A Rational Design Approach for Organocatalysts. J. Org. Chem. 2018;83(16):9370–9380. doi: 10.1021/acs.joc.8b00722. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Hu S., Ma X., Liang S., Jiang T., Han B.. Synthesis of cyclic carbonates from carbon dioxide and epoxides over betaine-based catalysts. J. Mol. Catal. A: Chem. 2008;284(1):52–57. doi: 10.1016/j.molcata.2008.01.010. [DOI] [Google Scholar]
- Wang T., Zheng D., Ma Y., Guo J., He Z., Ma B., Liu L., Ren T., Wang L., Zhang J.. Benzyl substituted imidazolium ionic liquids as efficient solvent-free catalysts for the cycloaddition of CO2 with epoxides: Experimental and Theoretic study. J. CO2 Util. 2017;22:44–52. doi: 10.1016/j.jcou.2017.09.009. [DOI] [Google Scholar]
- Wu S., Wang B., Zhang Y., Elageed E. H. M., Wu H., Gao G.. Phenolic hydroxyl-functionalized imidazolium ionic liquids: Highly efficient catalysts for the fixation of CO2 to cyclic carbonates. J. Mol. Catal. A: Chem. 2016;418–419:1–8. doi: 10.1016/j.molcata.2016.03.002. [DOI] [Google Scholar]
- Yang Z.-Z., He L.-N., Miao C.-X., Chanfreau S.. Lewis Basic Ionic Liquids-Catalyzed Conversion of Carbon Dioxide to Cyclic Carbonates. Adv. Synth. Catal. 2010;352(13):2233–2240. doi: 10.1002/adsc.201000239. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.