Abstract
Background
Acupressure and qigong are Chinese medicine-based modalities that can work in synergy to improve chemotherapy-induced peripheral neuropathy (CIPN). This study examines the effect of a combined qigong and acupressure intervention on CIPN among cancer patients.
Patients and methods
This randomized controlled trial included cancer patients experiencing CIPN. Participants were randomly allocated to intervention and waitlist control groups (1 : 1). The intervention group received a 16-week combined qigong and self-administered acupressure intervention. The primary outcome was self-reported CIPN severity (Functional Assessment of Cancer Therapy/Gynecologic Oncology Group—Neurotoxicity). Other outcomes included objectively measured CIPN severity (DPNCheck), handgrip strength (dynamometer), physical performance (Short Physical Performance Battery), fall incidence, and health-related quality of life (HRQoL, Functional Assessment of Cancer Therapy—General). Data were collected at baseline, 16 weeks, and 28 weeks. Intention-to-treat analysis was carried out.
Results
The study randomly assigned 110 patients [mean (standard deviation) age 61.86 years (9.31 years); 49 (89.1%) female], among whom 103 completed the study. After intervention, the reduction in self-reported CIPN severity in the intervention group was significantly larger than in the control group (mean difference = −3.86, 95% confidence interval CI −6.14 to −1.58), P = 0.001). The intervention showed significant benefit in reducing self-reported CIPN severity (group × time P = 0.004), physical performance (group × time P = 0.025), and HRQoL (group × time P < 0.001). The effect on objectively measured CIPN severity, handgrip strength, and fall incidence did not reach statistical significance.
Conclusions
A combined qigong and acupressure intervention versus waitlist control significantly improved self-reported CIPN among cancer patients. Incorporating the combined intervention into standard practice is recommended.
Key words: randomized controlled trial, cancer, chemotherapy-induced peripheral neuropathy, qigong, acupressure
Highlights
-
•
110 cancer patients with CIPN were randomly assigned to the intervention and the control group.
-
•
The intervention group received a 16-week combined qigong and acupressure intervention.
-
•
The combined intervention showed significant benefits in reducing self-reported CIPN severity.
-
•
Future research should explore longer term effects and mechanisms of action of this combined intervention.
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a prevalent and clinically relevant side-effect of neurotoxic chemotherapy. The American Society of Clinical Oncology’s 2020 guideline update identified no effective approach for treating CIPN except limited benefits from duloxetine.1 Considering the adverse effects of pharmacological agents,2,3 exploring nonpharmacological options is essential. However, conclusive evidence for first-line nonpharmacological treatments for CIPN remains lacking.1 Although recent reviews and meta-analyses suggest potential benefits from nonpharmacological interventions such as acupuncture4, 5, 6 and physical activity,7, 8, 9 the evidence is limited by the small number and varied quality of studies. Furthermore, barriers such as low availability of acupuncturists, needle phobia, and functional limitations may deter cancer patients with CIPN from receiving acupuncture and conventional exercise interventions. Consequently, there is a critical need for more accessible, appealing, and mechanistically driven nonpharmacological self-care modalities.
Acupressure is a noninvasive variant of acupuncture, with both adopting the meridian theory of traditional Chinese medicine (TCM) that stimulating acupoints across meridians facilitates the flow of qi (energy) and blood, thereby restoring health and treating disease. Qigong is a mind–body exercise also rooted in the meridian theory of TCM. Through a combination of movement, breath control, and meditation, meridians can be opened, and the flow of qi and blood stimulated, to restore health. Both acupressure and qigong are effective in treating cancer-related symptoms10, 11, 12, 13, 14, 15, 16 and are acceptable to symptomatic cancer patients due to their gentle and noninvasive nature. In the context of CIPN, preliminary studies of acupressure and qigong alone showed a significant benefit in reducing CIPN but did not indicate clinical meaningfulness.17, 18, 19
The potential mechanisms of acupuncture/acupressure and qigong for improving CIPN may complement each other and enhance their effectiveness. While acupressure may enhance the circulation of blood,20, 21, 22 modulating nerve growth factors23 and ion channels24,25 and/or reducing inflammation,25,26 qigong may act on the central nervous system, stimulating endorphin production,27 reducing pro-inflammatory cytokines,28,29 influencing neurotrophic proteins,30 and/or encouraging mindfulness.31 The combination is likely to elicit complementary physiologic adaptations on mechanisms involving both the peripheral and central nervous systems, thereby inducing a larger and potentially clinically meaningful improvement in CIPN. TCM meridian theory, the foundation of both modalities, also suggests their potential compatibility in practice.
This study evaluates the effects of a combined qigong and acupressure intervention for reducing self-reported CIPN severity (primary) and objective CIPN severity, improving handgrip strength and physical performance, decreasing fall incidence, and improving health-related quality of life (HRQoL) (secondary), relative to a waitlist control group.
Materials and methods
Study design
This study was a two-arm, assessor-blinded randomized controlled trial (RCT; ClinicalTrials.gov, NCT05764447) conducted from 10 March 2023 to 31 March 2025. Eligible participants were randomly assigned to the intervention or the waitlist control group (1 : 1). The study was approved by the ethics committees of the study sites. All participants provided written informed consent for participation at baseline.
Participants and setting
The inclusion criteria were age ≥18 years, diagnosis of cancer, experiencing CIPN (tingling, numbness, or pain in the extremities in the past week on the basis of a score ≥4 on an 11-point numerical rating scale, due to receiving neurotoxic chemotherapy), Eastern Cooperative Oncology Group performance status between 0 and 2, able to communicate in Cantonese or Putonghua, and having completed neurotoxic chemotherapy (taxanes, platinum derivatives, or vinca alkaloids) at least 1 month before enrollment. Participants on a stable dosage regimen of antineuropathy medications for the past 3 months were also eligible. Exclusion criteria were psychiatric disorders or conditions precluding intervention practice; regular engagement in qigong or acupressure (more than once per week) in the previous 6 months; current acupuncture treatment; pregnancy or lactation; infection, injury, or ulcers around acupoints; or peripheral neuropathy before chemotherapy.
The sample size was computed using GPower, based on self-reported CIPN severity. The minimal important difference (MID) was defined as a 3.3-4.4 point change in the 11-item Functional Assessment of Cancer Therapy/Gynecologic Oncology Group—Neurotoxicity (FACT/GOG-Ntx).32 The change in intervention group patients’ score was expected to be −4 with a standard deviation (SD) of 4.75,33 whereas that in the usual care group was expected to be −1.35 (SD 2.95).33 Assuming a type I error rate of 5%, 72 patients were needed to achieve a power of 80%. Assuming an attrition rate of 30%, a sample size of 104 (52 per arm) was needed.
Procedure
Participants were recruited from the oncology department of a major public hospital and nongovernment organizations serving cancer patients in Hong Kong. Attending patients were first offered an information leaflet, and those interested were screened for eligibility. They then provided informed consent for participation and completed a baseline assessment. Participants were randomly assigned to the intervention or control groups using block randomization. An independent statistician generated the randomization list using a computer, with the use of permuted blocks of varying sizes with an allocation weight of 1 : 1. The group assignment was sealed in sequential opaque envelopes, which were opened by a research assistant whenever a participant was recruited. The researcher performing data analysis and the outcome assessors were blinded.
Intervention
The combined intervention consisted of qigong and self-administered acupressure. For the qigong component, Baduanjin, which comprises eight simple standardized movements combined with breath control and mindfulness, was used. For the acupressure component, six acupoints were chosen based on previous reviews:33, 34, 35 LI4 (Hegu), LI11 (Quchi), ST36 (Zusanli), SP6 (Sanyinjiao), CV6 (Qihai), and LV3 (Taichong).
The intervention comprised 16 weeks of twice-weekly supervised group sessions (Supplementary Material S1). The first 8 weeks focused on training (90 min each) led by a qigong master and a TCM practitioner, in which participants learned qigong and acupressure separately for 3 weeks, started practicing the combined intervention at week 4, and received feedback from both trainers. From week 9 onward, group sessions were reduced to 60 min twice weekly, led by the qigong master (who was also trained in acupressure), to reinforce practice and provide remedial training.
Participants also received a self-practice prescription of three times per week on non-group-session days (30 min each time, 90 min/week in total). Each self-practice session included 20 min of Baduanjin (each movement repeated three times; eight movements total) and 10 min of acupressure (1 min per point). This sequence exploits the complementary properties of qigong and acupressure, as qigong first opens the meridians, with acupressure further stimulating the flow of qi.
Intervention fidelity
The TCM practitioner and the qigong master were certified by an experienced TCM practitioner researcher (Yeung). The principal investigator conducted spot checks of classes every 2 months to ensure fidelity of intervention implementation. Participants recorded their self-practice time and duration in logbooks, which were reviewed weekly by the trainer. A booklet and videos were also provided to aid home practice. At the end of week 8, participants’ competency was assessed by the trainers using a predesigned checklist (score range 16-104) (Supplementary Material S2).
Control condition
The waitlist control group received usual care alone during the study period and were offered a free modality of their choice after the last follow-up.
Outcome measures
Data collection took place at baseline, after intervention (week 16), and 12 weeks after the intervention ended (Week 28).
Primary outcome
Self-reported CIPN severity was measured by the 11-item FACT/GOG-Ntx subscale (Version 4).36 It has four domains: sensory, motor, hearing, and dysfunction. Items are rated on a scale of 0 (not at all) to 4 (very much), yielding a total score between 0 and 44, with higher scores indicating more severe CIPN. The Chinese version of FACT/GOG-Ntx has satisfactory reliability and validity.37 A 3.3- to 4.4-point change on the scale indicates an MID.32
Secondary outcomes
Objective CIPN severity was quantified by measuring sensory nerve action potential amplitude (SNAP) and sensory nerve conduction velocity (SNCV), conducted by trained research assistants using DPNCheck, a hand-held device that has been validated for diabetic peripheral neuropathy38 and has previously quantified CIPN severity.39 Lower SNAP and SNCV values indicate more severe CIPN.
Handgrip strength was measured twice using a dynamometer to obtain the maximum reading in a standing position with full elbow extension using the dominant hand.40
Physical performance was measured using the Short Physical Performance Battery (SPPB), which consists of three functional tasks.41 Scores range from 0 to 12, with higher scores indicating higher levels of physical functioning.
Fall incidence since last follow-up was assessed by self-reports at week 16 and 28. A fall is defined as unintentionally coming to rest on the ground or at some other lower level.
HRQoL was measured using the 27-item Functional Assessment of Cancer Therapy—General (FACT-G). It has four subscales: physical, emotional, social/family, and functional well-being. The score range is 0-108, with higher scores representing better HRQoL. FACT-G has been validated as reliable in Chinese populations.42
Background characteristics on demographic and disease information were assessed using an investigator-designed form. Adverse events were recorded by the research personnel. Treatment credibility and expectations of change were rated by intervention group participants at the end of the first session, using the six-item Credibility/Expectancy Questionnaire.43
Acceptability was examined using a three-item investigator-designed survey to assess satisfaction with supervised sessions, self-practice, and the combined intervention among the intervention group. Also, qualitative interviews were conducted and transcribed by a trained staff member to explore participants’ experience, using a semistructured interview guide (Supplementary Material S3).
Statistical analysis
All analyses were conducted using SPSS (Version 28.0) by a researcher blinded to group allocation, with a two-sided significance level of 0.05 for all tests. Student’s t-tests and chi-square tests were carried out to identify baseline differences between groups. Participant characteristics, adherence, and satisfaction were summarized using descriptive statistics.
Primary and secondary outcomes were analyzed based on the intention-to-treat principle using a mixed-effects model to handle missing data naturally. Differences in change from baseline between the intervention and control groups at post-intervention (primary analysis) and follow-up were examined. Group allocation and time were fixed factors and intercept was a random factor. The interaction between group and time (group × time) was included to examine changes in between-group differences. Effect size was computed via Cohen’s d accompanied by 95% confidence interval (CI). For the data on fall incidence, multiple imputation was carried out (with five imputations and an automatic imputation method selection). Logistic regression was conducted to report the adjusted odds ratio (AOR). Participant characteristics such as cancer type, age, and sex were adjusted in mixed-effects models and regressions.
Furthermore, per-protocol analysis was carried out to include only participants who had completed the study and had good adherence to the study protocol (>70% attendance and self-practice prescription). Qualitative feedback was analyzed by two researchers using thematic analysis.
Results
Participants
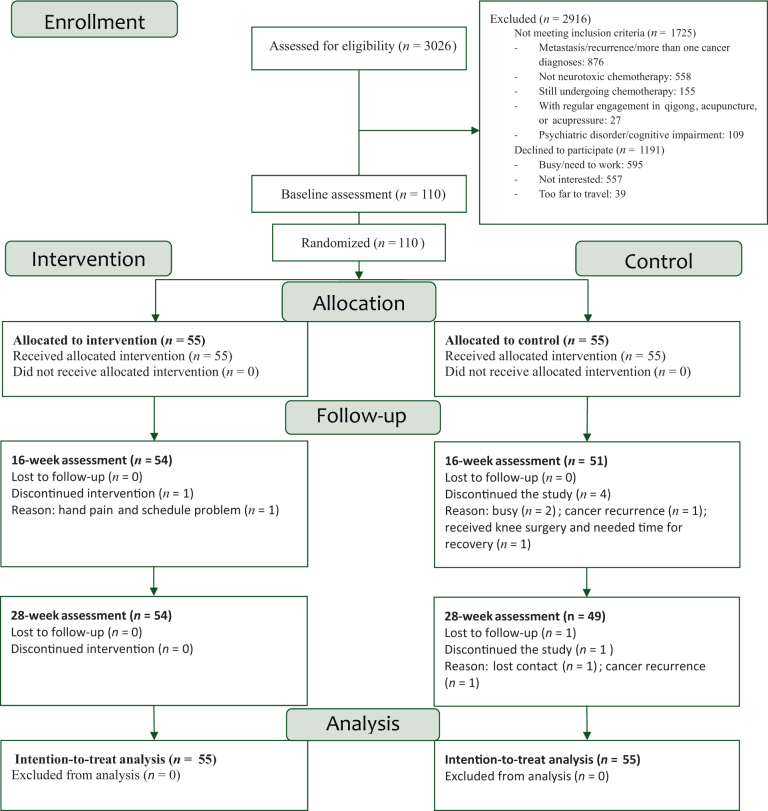
Overall, 110 participants were randomly assigned (Figure 1). The study was completed by 98.1% (54 of 55) of the participants in the intervention group and 89% (49 of 55) in the control group. The reasons for withdrawal were mainly busy schedules and loss of interest. At baseline, there was no significant difference in background characteristics between the groups (Table 1).
Figure 1.
CONSORT flow diagram.
Table 1.
Baseline characteristics
| Background characteristic | Overall, n (%) | Group, n (%) |
P value | |
|---|---|---|---|---|
| Intervention group (n = 55) | Control group (n = 55) | |||
| Age, mean ± SD, years | 61.86 ± 9.31 | 61.16 ± 9.05 | 62.56 ± 9.59 | 0.433 |
| Sex | 0.567 | |||
| Male | 14 (12.7) | 6 (10.9) | 8 (14.5) | |
| Female | 96 (87.3) | 49 (89.1) | 47 (85.5) | |
| Marital status | 0.432 | |||
| Single/divorced | 42 (38.2) | 23 (41.8) | 19 (34.5) | |
| Married | 68 (61.8) | 32 (58.2) | 36 (65.5) | |
| Education | 0.241 | |||
| Junior secondary or below | 23 (20.9) | 14 (25.5) | 9 (16.4) | |
| Senior secondary or above | 87 (79.1) | 41 (74.5) | 46 (83.6) | |
| Regular exercise habit | 0.589 | |||
| Yes | 94 (85.5) | 46 (83.6) | 48 (87.3) | |
| No | 16 (14.5) | 9 (16.4) | 7 (12.7) | |
| Type of cancer | 0.539 | |||
| Breast | 75 (68.2) | 39 (70.9) | 36 (65.5) | |
| Othera | 35 (31.8) | 16 (29.1) | 19 (34.5) | |
| Stage of cancer | 0.699 | |||
| Early stage | 64 (58.2) | 33 (60.0) | 31 (56.4) | |
| Stage ≥3 | 46 (41.8) | 22 (40.0) | 24 (43.6) | |
| Neurotoxic chemotherapy received | ||||
| Taxanes | 50 (45.5) | 26 (47.3) | 24 (43.6) | 0.884 |
| Platinum derivatives | 36 (32.7) | 18 (32.7) | 18 (32.7) | |
| Vinca alkaloids | 24 (21.8) | 11 (20) | 13 (23.6) | |
| Receiving any cancer treatment now (other than chemotherapy) | 0.684 | |||
| Yes | 36 (32.7) | 19 (34.5) | 17 (30.9) | |
| No | 74 (67.3) | 36 (65.5) | 38 (69.1) | |
| Time since cancer diagnosis, mean ± SD, months | 32.79 ± 23.64 | 31.00 ± 22.96 | 34.58 ± 24.38 | 0.429 |
| Time since neurotoxic chemotherapy completion, mean ± SD, months | 37.62 ± 56.09 | 34.55 ± 55.77 | 40.46 ± 56.93 | 0.64 |
| Any prescribed medical for CIPN? | 0.449 | |||
| Yes | 19 (17.3) | 11 (20.0) | 8 (14.5) | |
| No | 91 (82.7) | 44 (80.0) | 47 (85.5) | |
| Any financial difficulties? | 0.634 | |||
| Yes | 22 (20.0) | 12 (21.8) | 10 (18.2) | |
| No | 88 (80.0) | 43 (78.2) | 45 (81.8) | |
CIPN, chemotherapy-induced peripheral neuropathy; SD, standard deviation.
Other cancers include colorectal, lung, prostate, liver, stomach, thyroid, laryngeal, cervical, lymphoma, esophageal, bladder, and kidney.
Of the completers in the intervention group, 68.5% attended >70% of supervised sessions and 81.5% self-practiced >70% of the prescribed amount (Table 2). The average competency score was 72.03 ± 9.57 out of 104. The credibility/expectancy score was 38.10 ± 6.41 out of 54. No adverse events occurred.
Table 2.
Fidelity and satisfaction parameters among completers in the intervention group (N = 54)
| Fidelity parameters | Mean ± standard deviation/n (%) |
|---|---|
| Combined intervention competency score (score range 16-104) | 72.03 ± 9.57 |
| Credibility/expectancy total scores (score range 6-54) | 38.10 ± 6.41 |
| Credibility subscale (score range 3-27) | 9.65 ± 3.63 |
| Expectancy subscale (score range 3-27) | 18.44 ± 3.36 |
| Number of supervised classes attended (maximum = 32) | 24.5 ± 5.58 |
| Attended >70% of supervised classes | 37 (68.5) |
| Self-practice (min/week) (prescribed = 90 min/week) | 117.71 ± 6.3 |
| Self-practiced >70% of prescribed amount | 44 (81.5) |
| Satisfaction parameters | |
| Satisfaction with the supervised sessions (score range 1-5) | 4.87 ± 0.39 |
| Satisfaction with the self-practice (score range 1-5) | 3.93 ± 0.84 |
| Satisfaction with the combined intervention (score range 1-5) | 4.74 ± 0.52 |
Primary outcome
For self-reported CIPN severity, the intervention group reported a significant decrease in FACT/GOG-Ntx score at 16 weeks [mean difference (md) −4.47, 95% CI –6.42 to –2.51), P < 0.001] and at 28 weeks (md −4.39, 95% CI –6.31 to –2.48, P < 0.001), whereas the control group decreased nonsignificantly at 16 weeks (md −0.61, 95% CI −2.61 to 1.39, P = 1.000) and significantly at 28 weeks (md −2.79, 95% CI –4.77 to –0.82, P = 0.003) (Table 3). Overall, the intervention group had a more significant improvement over time (group × time P = 0.004), with the intervention group showing a significantly greater decrease than the control group at 16 weeks (Δ change −3.86, 95% CI –6.14 to –1.58, P = 0.001; effect size d = –0.64].
Table 3.
Change in outcomes based on the intention-to-treat analysis
| Time point | Intervention group (n = 55) |
Control group (n = 55) |
Intervention group change from baseline |
Control group change from baseline |
Between-group difference in change (intervention–control) |
Time P value | Group P value | Group × time interaction P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P value | Mean (95% CI) | P value | Mean (95% CI) | P value | Effect size (Cohen’s d) | ||||
| Functional Assessment of Cancer Therapy/Gynecologic Oncology Group—Neurotoxicity (FACT/GOG-Ntx) | ||||||||||||
| Baseline | 12.42 (8.48-16.36) | 12.77 (9.03-16.52) | – | – | – | – | – | – | – | ∗<0.001 | ∗0.028 | ∗0.004 |
| 16 weeks | 7.95 (3.90-12.01) | 12.16 (8.29-16.04) | –4.47 (–6.42 to –2.51) | ∗<0.001 | –0.61 (–2.61 to 1.39) | 1.000 | –3.86 (–6.14 to –1.58) | ∗0.001 | –0.64 (–1.02 to –0.26) | – | – | – |
| 28 weeks | 8.02 (4.08-11.97) | 9.98 (6.23-13.73) | –4.39 (–6.31 to –2.48) | ∗<0.001 | –2.79 (–4.77 to –0.82) | ∗0.003 | –1.60 (–3.84 to 0.64) | 0.160 | –0.27 (–0.65 to 0.11) | – | – | – |
| Handgrip strength | ||||||||||||
| Baseline | 21.02 (18.19-23.85) | 20.51 (17.82-23.20) | – | – | – | – | – | – | – | ∗0.002 | 0.117 | 0.256 |
| 16 weeks | 22.44 (19.55-25.32) | 21.04 (18.27-23.81) | 1.41 (0.44-2.39) | ∗0.002 | 0.53 (–0.52 to 1.58) | 0.663 | 0.88 (–0.29 to 2.05) | 0.137 | 0.29 (–0.09 to 0.66) | – | – | – |
| 28 weeks | 22.29 (19.43-25.15) | 20.98 (18.23-23.72) | 1.27 (0.31-2.22) | ∗0.005 | 0.47 (–0.53 to 1.47) | 0.775 | 0.80 (–0.33 to 1.92) | 0.163 | 0.27 (–0.11 to 0.64) | – | – | – |
| Short Physical Performance Battery (SPPB) | ||||||||||||
| Baseline | 7.92 (6.79-9.05) | 8.22 (7.15-9.30) | – | – | – | – | – | – | – | ∗<0.001 | 0.543 | ∗0.025 |
| 16 weeks | 8.84 (7.70-9.97) | 8.63 (7.52-9.73) | 0.91 (0.39-1.44) | ∗<0.001 | 0.40 (–1.15 to 0.95) | 0.229 | 0.51 (–0.10 to 1.13) | 0.103 | 0.31 (–0.06 to 0.69) | – | – | – |
| 28 weeks | 9.32 (8.18-10.45) | 8.78 (7.68-9.89) | 1.40 (0.88-1.91) | ∗<0.001 | 0.56 (0.03-1.10) | ∗0.037 | 0.83 (0.23-1.44) | ∗0.007 | 0.52 (0.14-0.90) | – | – | – |
| Functional Assessment of Cancer Therapy—General (FACT-G) | ||||||||||||
| Baseline | 76.60 (67.02-86.18) | 79.71 (70.61-88.80) | – | – | – | – | – | – | – | ∗0.002 | 0.558 | ∗<0.001 |
| 16 weeks | 84.18 (74.50-93.86) | 78.28 (69.06-87.50) | 7.58 (3.76-11.40) | ∗<0.001 | –1.43 (–5.38 to 2.53) | 1.000 | 9.01 (4.53-13.49) | ∗<0.001 | 0.76 (0.37-1.15) | – | – | – |
| 28 weeks | 83.36 (73.67-93.05) | 82.05 (72.82-91.29) | 6.76 (2.49-11.03) | ∗<0.001 | 2.35 (–2.09 to 6.78) | 0.602 | 4.41 (–0.61 to 9.43) | 0.084 | 0.33 (–0.04 to 0.71) | – | – | – |
| Sensory nerve action potential amplitude (SNAP) | ||||||||||||
| Baseline | 2.06 (–3.22 to 7.35) | 4.87 (–0.09 to 9.83) | – | – | – | – | – | – | – | 0.240 | 0.235 | 0.318 |
| 16 weeks | 4.49 (–0.40 to 9.39) | 4.64 (–0.20 to 9.48) | 2.43 (–0.64 to 5.50) | 0.169 | –0.23 (–3.75 to 3.30) | 1.000 | 2.66 (–1.15 to 6.46) | 0.168 | 0.27 (–0.11 to 0.64) | |||
| 28 weeks | 5.05 (0.16-9.93) | 4.92 (0.06-9.77) | 2.98 (–0.12 to 6.09) | 0.063 | 0.05 (–3.51 to 3.61) | 1.000 | 2.93 (–0.91 to 6.77) | 0.132 | 0.29 (–0.09 to 0.67) | |||
| Sensory nerve conduction velocity (SNCV) | ||||||||||||
| Baseline | 43.64 (39.42-47.89) | 44.68 (40.75-48.61) | – | – | – | – | – | – | – | 0.935 | 0.627 | 0.467 |
| 16 weeks | 43.47 (39.11-47.84) | 44.30 (40.10-48.50) | –0.17 (–2.84 to 2.49) | 1.000 | –0.39 (–3.39 to 2.62) | 1.000 | 0.21 (–3.05 to 3.48) | 0.896 | 0.03 (–0.35 to 0.40) | |||
| 28 weeks | 44.23 (39.97-48.48) | 43.95 (39.88-48.02) | 0.58 (–1.46 to 2.62) | 1.000 | –0.74 (–3.14 to 1.67) | 1.000 | 1.32 (–1.25 to 3.88) | 0.309 | 0.20 (–0.18 to 0.57) | |||
CI, confidence interval.
Secondary outcomes
For handgrip strength, the intervention group reported a significant increase at 16 weeks (md 1.41, 95% CI 0.44-2.39, P = 0.002) and at 28 weeks (md 1.27, 95% CI 0.31-2.22, P = 0.005), whereas the control group did not report significant changes at either time point. Overall, there was no significant difference in between-group change over time (group × time P = 0.256).
For physical performance, the intervention group reported a significant increase in SPPB score at 16 weeks (md 0.91, 95% CI 0.39-1.44, P < 0.001) and at 28 weeks (md 1.4, 95% CI 0.88-1.91, P < 0.001) respectively, whereas those in the control group increased nonsignificantly at 16 weeks (md 0.4, 95% CI –1.15 to 0.95, P = 0.229) and significantly at 28 weeks (md 0.56, 95% CI 0.03-1.10, P = 0.037) (Table 3). Overall, the intervention group had a more significant improvement over time (group × time P = 0.025), with the intervention group showing a significantly greater change than the control group at 28 weeks (Δ change = 0.83, 95% CI 0.23-1.44, P = 0.007; d = 0.52).
For HRQoL, the intervention group reported a significant increase in FACT-G score at 16 weeks (md 7.58, 95% CI 3.76-11.40, P < 0.001) and at 28 weeks (md 6.76, 95% CI 2.49- 11.03, P < 0.001), whereas the control group remained similar at both time points. Overall, the intervention group had a more significant improvement over time (group × time P < 0.001), with the intervention group showing a significantly greater change than the control group at 16 weeks (Δ change = 9.01, 95% CI 4.53-13.49) P < 0.001; d = 0.76).
For objectively measured CIPN parameters, i.e. SNAP and SNCV, there was no significant change in either group at either time point. Fall incidence also did not differ significantly between the two groups at either time point (Supplementary Material S4).
Per-protocol analysis
The results of the per-protocol analysis (n = 83) are in Supplementary Materials S4 and S5. There were no major differences from the intention-to-treat analysis.
Participant acceptability
Participants expressed strong satisfaction with the combined intervention (Table 2). Five themes emerged from qualitative feedback, namely bothersome CIPN symptoms after chemotherapy, experience of changes since joining the study, potential reasons for the improvement in CIPN symptoms, reflection of the learning process, and room for improvement (Supplementary Material S6). Overall, most participants experienced relief in CIPN and related symptoms, while some attributed the improvement to increased flow of blood and qi within the body based on the feeling of warmth in the feet and hands after practicing the intervention. Participants also highlighted slight rebounds of symptoms after the intervention ended due to decreased self-practice. Other changes since joining the study included positive emotions, and strengthened peer support and social network. Areas for improvements included a preference for indoor venues for classes and a need for lengthened class time for acupressure training.
Discussion
In this RCT, a 16-week combined qigong and acupressure intervention led to significant improvement in self-reported CIPN severity, physical performance, and QoL among cancer patients compared with the waitlist controls. Improvements in these outcomes were sustained 12 weeks after intervention. However, the intervention did not show a significant effect on handgrip strength, fall incidence, or objectively measured CIPN severity at either time point.
We found a medium effect size of therapeutic benefit of combined qigong and acupressure for reducing FACT/GOG-Ntx score (Cohen’s d = –0.64; mean difference in CIPN score = –3.86), which exceeded the MID.32 This effect size is comparable to or larger than the pooled effect sizes reported in previous meta-analyses of conventional exercise (standardized mean difference = 0.45-0.68)7 and acupuncture (mean difference in FACT/GOG-Ntx score = 2.09).6 In cancer patients experiencing CIPN, symptom burden and accessibility to acupuncturists may limit the uptake of conventional exercise and acupuncture interventions. Qigong and acupressure are both self-care strategies that are flexible, low cost, and safe.44,45 They can be practiced even by frail and symptomatic patients and may thus have a wider reach than conventional exercise and acupuncture interventions. Our study demonstrates not only the effects of a combination approach on CIPN, but also its acceptability and feasibility among patients experiencing CIPN.
Notably, most previous nonpharmacological studies on CIPN were limited by the lack of post-intervention follow-ups, leaving the long-term effect of the interventions inconclusive.46, 47, 48 In our study, the effect size at follow-up, although it remained significant, became small (0.31), indicating that the sustainability of the intervention decreased over time. Strategies to maintain the intervention’s effects are needed, or such effects may wear off over time.
There is a lack of consensus on the optimal clinical method of CIPN assessment. Patient-reported outcome measures are valuable tools to assess CIPN, with FACT/GOG-Ntx recommended with the most supporting evidence.49, 50, 51, 52 For objective measurement, traditional nerve conduction studies have limited value in discriminating clinically relevant CIPN and might cause discomfort to patients; therefore, they are not recommended as outcome measurements in CIPN symptom management trials.53 This study adopted the DPNCheck, an easy-to-use device that elicited minimal discmfort.39 Although SNAP and SNCV showed improvement from baseline toward the follow-up, the change was not significant, probably due to a lack of power. Further research could examine optimal objective measurements that have high diagnostic accuracy, cause no discomfort, and are sensitive to changes in CIPN.
Limitations
Firstly, our sample is from a Chinese population, who may be more culturally open to receiving TCM-based interventions. Its acceptability in and generalizability to other cultural groups should be assessed. Secondly, although the combined intervention is based on the rationale that acupressure and qigong complement each other, the inability of the present study to examine the underlying mechanisms and the individual effects of acupressure and qigong may limit our understanding of the full picture of the intervention effect. Thirdly, the intervention’s effect on specific domains of CIPN symptoms was not assessed due to unclear factor structure of the Chinese version of the FACT/GOG-Ntx.54 Fourthly, the study was not powered to detect changes in objective measures. Fifthly, the effect of intervention may have been overestimated due to the lack of treatment in the waitlist control group.
Implications
This study provides evidence on a combined qigong and acupressure intervention to relieve CIPN. The intervention is standardized, low cost, and safe, making it ideal for patients experiencing CIPN. Health care providers can launch the intervention based on the detailed regimen provided, making it readily adaptable to clinical practice and patients’ daily routines. Further research is needed to examine the underlying mechanisms of the intervention by including physiologic markers. Adequately powered trials can be conducted to detect changes in both self-reported and objective measures. Strategies to prolong the sustainability of the intervention effect are also warranted.
Conclusions
Compared with waitlist controls, a combined qigong and acupressure intervention resulted in improved self-reported CIPN, physical performance, and HRQoL among cancer patients. Incorporating the intervention into standard oncology care is recommended.
Acknowledgements
We thank the participants for participating in the study, research assistants for assisting with project implementation, trainers for delivering the intervention, and nongovernment organizations including Cancer Fund and Maggie’s Cancer Caring Centre for referring participants to us. We also wish to honor the memory of Professor Yeh Chao Hsing, whose invaluable contributions in study conceptualization greatly enriched this work.
Funding
This study was supported by the General Research Fund of the Research Grants Council, Hong Kong SAR Government [grant number 17611922], which was not involved in the design, conduct, analysis, and reporting of the trial.
Disclosure
The authors have declared no conflicts of interest.
Data Sharing
Data can be accessed from the corresponding author upon reasonable request.
Supplementary data
References
- 1.Loprinzi C.L., Lacchetti C., Bleeker J., et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO Guideline Update. J Clin Oncol. 2020;38(28):3325–3348. doi: 10.1200/JCO.20.01399. [DOI] [PubMed] [Google Scholar]
- 2.Mezzanotte J.N., Grimm M., Shinde N.V., et al. Updates in the treatment of chemotherapy-induced peripheral neuropathy. Curr Treat Options Oncol. 2022;23(1):29–42. doi: 10.1007/s11864-021-00926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C., Chen S., Jiang W. Treatment for chemotherapy-induced peripheral neuropathy: a systematic review of randomized control trials. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1080888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh M.L., Hsu C.C., Lin M., Lin C.J., Lin J.G. Effects of acupuncture-related intervention on chemotherapy-induced peripheral neuropathy and quality of life: an umbrella review. Complement Ther Med. 2025;89 doi: 10.1016/j.ctim.2025.103131. [DOI] [PubMed] [Google Scholar]
- 5.Yeh M.L., Liao R.W., Yeh P.H., Lin C.J., Wang Y.J. Acupuncture-related interventions improve chemotherapy-induced peripheral neuropathy: a systematic review and network meta-analysis. BMC Complement Med Ther. 2024;24(1):310. doi: 10.1186/s12906-024-04603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L., Huang Y., An C., et al. Acupuncture in the treatment of chemotherapy-induced peripheral neuropathy: a meta-analysis and data mining. Front Neurol. 2024;15 doi: 10.3389/fneur.2024.1442841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y., Tan T., Liu L., et al. Exercise for reducing chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis of randomized controlled trials. Front Neurol. 2024;14 doi: 10.3389/fneur.2023.1252259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu N., Cao H., Du S., et al. Effect of exercise intervention on chemotherapy-induced peripheral neuropathy symptoms in cancer patients: a meta-analysis. Cancer Nurs. 2024 doi: 10.1097/NCC.0000000000001435. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa N., Yamamoto S., Hanai A., Oiwa A., Arao H. Exercise intervention for the management of chemotherapy-induced peripheral neuropathy: a systematic review and network meta-analysis. Front Neurol. 2024;15 doi: 10.3389/fneur.2024.1346099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S., Li Y., Liang Z., et al. Comparing the effects of different non-pharmacological traditional Chinese medicine therapies on cancer survivors: a Bayesian Network Meta-analysis. Complement Ther Med. 2025;90 doi: 10.1016/j.ctim.2025.103164. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh S.H., Wu C.R., Romadlon D.S., Hasan F., Chen P.Y., Chiu H.Y. The effect of acupressure on relieving cancer-related fatigue: a systematic review and meta-analysis of randomized controlled trials. Cancer Nurs. 2021;44(6):E578–E588. doi: 10.1097/NCC.0000000000000997. [DOI] [PubMed] [Google Scholar]
- 12.Yang C., Huang Y., Ling W., Cheung D.S.T., Lee J.J. The effectiveness of acupressure on sleep quality in cancer patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Nurs. 2025 doi: 10.1111/jocn.17707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung D.S.T., Xu X., Smith R., et al. Invasive or noninvasive? A systematic review and network meta-analysis of acupuncture and acupressure to treat sleep disturbance in cancer patients. Worldviews Evid Based Nurs. 2023;20(3):202–211. doi: 10.1111/wvn.12617. [DOI] [PubMed] [Google Scholar]
- 14.Wayne P.M., Lee M.S., Novakowski J., et al. Tai Chi and Qigong for cancer-related symptoms and quality of life: a systematic review and meta-analysis. J Cancer Surviv. 2018;12(2):256–267. doi: 10.1007/s11764-017-0665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung D.S.T., Takemura N., Smith R., et al. Effect of qigong for sleep disturbance-related symptom clusters in cancer: a systematic review and meta-analysis. Sleep Med. 2021;85:108–122. doi: 10.1016/j.sleep.2021.06.036. [DOI] [PubMed] [Google Scholar]
- 16.Takemura N., Cheung D.S.T., Smith R., et al. Effectiveness of aerobic exercise and mind-body exercise in cancer patients with poor sleep quality: a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2020;53 doi: 10.1016/j.smrv.2020.101334. [DOI] [PubMed] [Google Scholar]
- 17.Kim S.Y., Park J.S. The effect of self-acupressure on peripheral neuropathy, disturbance in daily activity, and quality of life in breast cancer patients undergoing chemotherapy. Asian Oncol Nurs. 2021;21(3):129. [Google Scholar]
- 18.Yeh C.H., Lukkahatai N., Campbell C., et al. Preliminary effectiveness of auricular point acupressure on chemotherapy-induced neuropathy: part 1 self-reported outcomes. Pain Manag Nurs. 2019;20(6):614–622. doi: 10.1016/j.pmn.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Oh B., Butow P.N., Boyle F., et al. Effects of qigong on quality of life, fatigue, stress, neuropathy, and sexual function in women with metastatic breast cancer: a feasibility study. J Clin Oncol. 2014;32(suppl 15) [Google Scholar]
- 20.Litscher G., Wang L., Huber E., Nilsson G. Changed skin blood perfusion in the fingertip following acupuncture needle introduction as evaluated by laser doppler perfusion imaging. Lasers Med Sci. 2002;17(1):19–25. doi: 10.1007/s10103-002-8262-9. [DOI] [PubMed] [Google Scholar]
- 21.Mehta P., Dhapte V., Kadam S., Dhapte V. Contemporary acupressure therapy: adroit cure for painless recovery of therapeutic ailments. J Tradit Complement Med. 2016;7(2):251–263. doi: 10.1016/j.jtcme.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X., Hirokawa M., Inoue Y., Sugano N., Qian S., Iwai T. Effects of acupressure on lower limb blood flow for the treatment of peripheral arterial occlusive diseases. Surg Today. 2007;37(2):103–108. doi: 10.1007/s00595-006-3347-x. [DOI] [PubMed] [Google Scholar]
- 23.Jiang S., Chen W., Zhang Y., et al. Acupuncture induces the proliferation and differentiation of endogenous neural stem cells in rats with traumatic brain injury. Evid Based Complement Alternat Med. 2016;2016(1) doi: 10.1155/2016/2047412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao C., Zeng X., Zhang S., et al. Acupoint massage: a comprehensive descriptive review of its forms, applications, and underlying mechanisms. Chin Med. 2025;20(1):54. doi: 10.1186/s13020-025-01105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y.X., Yu X.C., Gao J.H., Yao M.J., Zhu B. Acupuncture for paclitaxel-induced peripheral neuropathy: a review of clinical and basic studies. J Pain Res. 2021;14:993–1005. doi: 10.2147/JPR.S296150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen A., Li M., Ning H., et al. The promising application of acupressure for management of cancer-related lymphedema: a scoping review. Asia-Pac J Oncol Nurs. 2025;12 doi: 10.1016/j.apjon.2025.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryu H., Lee H.S., Shin Y.S., et al. Acute effect of qigong training on stress hormonal levels in man. Am J Chin Med. 1996;24(2):193–198. doi: 10.1142/S0192415X96000256. [DOI] [PubMed] [Google Scholar]
- 28.Bower J.E., Irwin M.R. Mind–body therapies and control of inflammatory biology: a descriptive review. Brain Behav Immun. 2016;51:1–11. doi: 10.1016/j.bbi.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan C.L.W., Wang C.W., Ho R.T.H., et al. A systematic review of the effectiveness of qigong exercise in supportive cancer care. Support Care Cancer. 2012;20(6):1121–1133. doi: 10.1007/s00520-011-1378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You T., Ogawa E.F. Effects of meditation and mind-body exercise on brain-derived neurotrophic factor: a literature review of human experimental studies. Sports Med Health Sci. 2020;2(1):7–9. doi: 10.1016/j.smhs.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilton L., Hempel S., Ewing B.A., et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51(2):199–213. doi: 10.1007/s12160-016-9844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yost K.J., Eton D.T. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof. 2005;28(2):172–191. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 33.Chien T.J., Liu C.Y., Fang C.J., Kuo C.Y. The efficacy of acupuncture in chemotherapy-induced peripheral neuropathy: systematic review and meta-analysis. Integr Cancer Ther. 2019;18 doi: 10.1177/1534735419886662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin Y., Wang Y., Zhang J., Xiao X., Zhang Q. Efficacy and safety of acupuncture against chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020(1) doi: 10.1155/2020/8875433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang M.S., Lee H.Y., Choi T.Y., et al. A systematic review and meta-analysis of the efficacy of acupuncture and electroacupuncture against chemotherapy-induced peripheral neuropathy. Medicine (Baltimore) 2020;99(17) doi: 10.1097/MD.0000000000019837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang H.Q., Brady M.F., Cella D., Fleming G. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer. 2007;17(2):387–393. doi: 10.1111/j.1525-1438.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 37.Cheng H.L., Molassiotis A. longitudinal validation and comparison of the Chinese version of the European Organization for Research and Treatment of Cancer Quality of Life—Chemotherapy-Induced Peripheral Neuropathy Questionnaire (EORTC QLQ-CIPN20) and the Functional Assessment of Cancer—Gynecologic Oncology Group—Neurotoxicity subscale (FACT/GOG-Ntx) Asia Pac J Clin Oncol. 2019;15(1):56–62. doi: 10.1111/ajco.13000. [DOI] [PubMed] [Google Scholar]
- 38.Shibata Y., Himeno T., Kamiya T., et al. Validity and reliability of a point-of-care nerve conduction device in diabetes patients. J Diabetes Investig. 2019;10(5):1291–1298. doi: 10.1111/jdi.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuoka A., Mitsuma A., Maeda O., et al. Quantitative assessment of chemotherapy-induced peripheral neurotoxicity using a point-of-care nerve conduction device. Cancer Sci. 2016;107(10):1453–1457. doi: 10.1111/cas.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts H.C., Denison H.J., Martin H.J., et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 41.Welch S.A., Ward R.E., Beauchamp M.K., Leveille S.G., Travison T., Bean J.F. The short physical performance battery (SPPB): a quick and useful tool for fall risk stratification among older primary care patients. J Am Med Dir Assoc. 2021;22(8):1646–1651. doi: 10.1016/j.jamda.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu C.L., Fielding R., Chan C.L., et al. Measuring quality of life of Chinese cancer patients: a validation of the Chinese version of the Functional Assessment of Cancer Therapy—General (FACT-G) scale. Cancer. 2000;88(7):1715–1727. [PubMed] [Google Scholar]
- 43.Devilly G.J., Borkovec T.D. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 44.Mazzocco K., Milani A., Ciccarelli C., Marzorati C., Pravettoni G. Evidence for choosing qigong as an integrated intervention in cancer care: an umbrella review. Cancers. 2023;15(4):1176. doi: 10.3390/cancers15041176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng H.L., Yeung W.F., Wong H.F., Lo H.T., Molassiotis A. Self-acupressure for symptom management in cancer patients: a systematic review. J Pain Symptom Manage. 2023;66(1):e109–e128. doi: 10.1016/j.jpainsymman.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Ronconi G., Gatto D.M., Codazza S., et al. Conservative non-pharmacological treatments for chemotherapy-induced peripheral neuropathies in women treated for breast cancer: a systematic review. Eur J Phys Rehabil Med. 2024;60(3):505–513. doi: 10.23736/S1973-9087.24.08197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang M., Pei Z., Molassiotis A. Recent advances in managing chemotherapy-induced peripheral neuropathy: a systematic review. Eur J Oncol Nurs. 2022;58 doi: 10.1016/j.ejon.2022.102134. [DOI] [PubMed] [Google Scholar]
- 48.Tanay M.A.L., Armes J., Moss-Morris R., Rafferty A.M., Robert G. A systematic review of behavioural and exercise interventions for the prevention and management of chemotherapy-induced peripheral neuropathy symptoms. J Cancer Surviv. 2023;17(1):254–277. doi: 10.1007/s11764-021-00997-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haryani H., Fetzer S.J., Wu C.L., Hsu Y.Y. Chemotherapy-induced peripheral neuropathy assessment tools: a systematic review. Oncol Nurs Forum. 2017;44(3):E111–E123. doi: 10.1188/17.ONF.E111-E123. [DOI] [PubMed] [Google Scholar]
- 50.Li T., Park S.B., Battaglini E., et al. Assessing chemotherapy-induced peripheral neuropathy with patient reported outcome measures: a systematic review of measurement properties and considerations for future use. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2022;31(11):3091–3107. doi: 10.1007/s11136-022-03154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffith K.A., Merkies I.S.J., Hill E.E., Cornblath D.R. Measures of chemotherapy-induced peripheral neuropathy: a systematic review of psychometric properties. J Peripher Nerv Syst. 2010;15(4):314–325. doi: 10.1111/j.1529-8027.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 52.Curcio K. Instruments for assessing chemotherapy-induced peripheral neuropathy: a review of the literature. Clin J Oncol Nurs. 2016;20(2):144–151. doi: 10.1188/16.CJON.20-01AP. [DOI] [PubMed] [Google Scholar]
- 53.Wang M., Bandla A., Sundar R., Molassiotis A. The phenotype and value of nerve conduction studies in measuring chemotherapy-induced peripheral neuropathy: a secondary analysis of pooled data. Eur J Oncol Nurs. 2022;60 doi: 10.1016/j.ejon.2022.102196. [DOI] [PubMed] [Google Scholar]
- 54.Cheng H.L., Lopez V., Lam S.C., et al. Psychometric testing of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-Ntx) subscale in a longitudinal study of cancer patients treated with chemotherapy. Health Qual Life Outcomes. 2020;18(1):246. doi: 10.1186/s12955-020-01493-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.