Abstract
Background
Lipopolysaccharide (endotoxin) from the cell wall of Gram-negative bacteria is a potent trigger for the release of host-derived inflammatory mediators. The relationship between endotoxaemia, Gram-negative infection and the clinical syndrome of sepsis has been difficult to establish, in part because of the limitations of available endotoxin assays.
Methods
We performed an observational cohort study in critically ill patients in the medical/surgical intensive care unit (ICU) of a tertiary care hospital. Whole blood endotoxin levels on the day of ICU admission were measured using a novel chemiluminescent assay – the endotoxin activity assay (EAA) – and the chromogenic modification of the limulus amoebocyte lysate (LAL) assay.
Results
We studied 74 consecutive admissions. Endotoxin levels were higher in patients with a diagnosis of sepsis (470 ± 57 pg/ml) than in patients admitted with a diagnosis other than sepsis (157 ± 140 pg/ml; P < 0.001). Endotoxaemia was significantly associated with Gram-negative infection (P < 0.05); no patient with a Gram-negative infection had an endotoxin level below 50 pg/ml. White blood cell counts of patients with EAA-detected endotoxaemia were significantly higher (15.7 ± 9.1 × 109 cells/l for endotoxaemic patients versus 10.8 ± 6.2 × 109 cells/l for patients without endotoxaemia; P < 0.05).
Conclusion
Endotoxaemia is associated with Gram-negative infection from any source, and with a diagnosis of sepsis and leukocytosis. These correlations were not apparent using the LAL method. The EAA may be a useful diagnostic tool for the investigation of invasive Gram-negative infection and incipient sepsis.
Keywords: critical illness, endotoxaemia, endotoxin assay, infection, sepsis
Introduction
Lipopolysaccharide (endotoxin) from the cell wall of Gram-negative bacteria is a potent trigger for the release of host-derived inflammatory mediators of sepsis, including proteins, free radicals and bioactive lipids [1,2,3]. A single intravenous bolus of Escherichia coli endotoxin given to healthy humans evokes many of the same responses (fever, tachycardia, tachypnoea and leukocytosis) that characterize Gram-negative infection [4].
Endotoxaemia has been detected in a variety of disorders, including sepsis [5,6,7], liver disease [8,9] and vascular disease [10], and in patients who have sustained trauma [11] or who have undergone cardiopulmonary bypass [12]. Even within aetiologically homogeneous populations of patients with sepsis, there is considerable variability in the prevalence of endotoxaemia and its association with important clinical outcomes [5,6,7]. Inability to detect endotoxaemia reliably in the clinical setting has impeded assessment of its precise role in inflammatory responses in critically ill patients [13].
A minority of patients with clinical sepsis are bacteraemic by conventional blood culture methods [6,14,15], and only 30–60% of bacteraemic patients develop fever and evidence of organ dysfunction [16]. Moreover, the association of endo-toxaemia with Gram-negative bacteraemia is poor, perhaps in part because of the low sensitivity and specificity of blood cultures. Antibiotic administration may further confound culture results by triggering endotoxin release from dying organisms [17].
Measurement of endotoxin in biological fluids is notoriously difficult [18]. The most widely used method – the chromogenic modification of the limulus amoebocyte assay (LAL) [19,20] – permits detection of endotoxin in picogram quantities and has become the standard assay system used to detect endotoxin contamination in pharmaceutical manufacturing. Plasma proteins can interfere with the assay, making it less reliable in biological fluids. Moreover, the limulus coagulation cascade can be triggered by fungal products, rendering the assay relatively nonspecific. A recent report [21] documented an association between the chromogenic LAL and fungaemia, but found a negative correlation with Gram-negative bacteraemia.
We have developed an alternative technique for detecting endotoxin in whole blood based on the detection of enhanced respiratory burst activity in neutrophils following their priming by complexes of endotoxin and a specific anti-endotoxin antibody [22]. The EAA shows excellent performance characteristics in recovering endotoxin from spiked samples and can be performed within 30 min, using less than 100 μl whole blood. We therefore undertook the present study to compare the LAL assay with the EAA, to determine the prevalence of endotoxaemia in a population of patients admitted to an ICU, to define its association with invasive infection, and to evaluate the association of endotoxaemia at ICU admission with clinical outcomes.
Patients and methods
We studied an inception cohort of 74 consecutive patients admitted to the medical-surgical ICU of the Toronto Hospital – a 900-bed tertiary care referral center. The study protocol was reviewed and approved by the Committee for Research on Human Subjects (The Toronto Hospital), and need for informed consent was waived. Excluded patients included those who were admitted on the weekend, who had no arterial line, or who had a terminal prognosis.
Data collection and study definitions
Comprehensive clinical and laboratory data were collected on the day of ICU admission. Survival status was assessed at 30 days, and at ICU and hospital discharge. Admission multiple organ dysfunction score [23] and Acute Physiology and Chronic Health Evaluation II score [24] were calculated. Systemic inflammatory response syndrome (SIRS) was defined in accordance with the criteria of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference [25]. Sepsis was defined as SIRS plus infection. Infection was diagnosed by the presence of microorganisms invading an otherwise sterile body fluid or site. Coagulase-negative staphylococci grown in one of two bottles from a blood culture was considered a contaminant. Blood for endotoxin assay was collected within 12 hours of admission in endotoxin-free tubes, with EDTA as an anticoagulant. Aliquots of platelet-poor plasma were stored at -70°C for subsequent LAL assay in pyrogen-free tubes. Endotoxin assays were compared with cultures drawn within 8 hours of the endotoxin sample.
Hypotension was defined as a systolic blood pressure below 90 mmHg for at least 1 hour, despite adequate filling pressures (pulmonary artery wedge pressure >12 mmHg), or as administration of an intravenous fluid bolus greater than 500 ml or the use of inotropic support (dopamine >5 g/kg per min, or phenylephrine, epinephrine or norepinephrine at any dose to maintain systolic blood pressure >90 mmHg).
Assay of endotoxin by endotoxin activity assay
The EAA assay is described in detail elsewhere [22]. Briefly, a 2-ml sample of whole blood was drawn through an indwelling arterial line into an endotoxin-free K3EDTA blood collection tube (Vacutainer systems; Becton Dickinson, Franklin Lakes, NJ, USA). Samples were immediately transported to a laboratory adjacent to the ICU for assay. Blood samples were maintained at room temperature and all samples were assayed within 30 min of collection.
To assay levels of endotoxin, a 10 l aliquot of whole blood was placed in each of three tubes containing luminol buffer (300 μl/tube). The control tube contained blood and buffer only, whereas a positive control contained a maximum stimu-latory concentration of endotoxin (2 ng/ml); the final tube contained the test sample. All three tubes were incubated at 37°C for 5 min and assayed in triplicate. Chemiluminescence was initiated by the addition of 20 l/tube human complement opsonized zymosan. Continuous measurements were made of light emissions at 30-s intervals over a total period of 20 min in a reciprocating tube luminometer (Autolumat LB 953; E. G. & G. Berthold, Wildbad, Germany).
Quantitation of endotoxin in whole blood was based on a standard curve of averaged light emission versus endotoxin concentration [22]. For each patient, a normalized response factor was calculated by subtracting the averaged 20-min light integral of the control from the assay tube and the maximally stimulated tube. The response factor was the difference light integral of the test sample divided by the difference integral of the maximally stimulated tube. The endotoxin concentration was interpolated from the dose–response curve of response factor versus endotoxin concentration.
Chromogenic limulus amoebocyte lysate assay
Determination of endotoxin using the LAL technique was conducted with the Associates of Cape Cod LAL assay (Pyrochrome Assay Kit; Associates of Cape Cod, Falmouth, MA, USA), with the sample pretreatment protocol of Tamura [26]. The assay was performed according to the manufacturer's instructions, using a 96-well microplate format (Pyrochrome 96-well plates; Associates of Cape Cod). All dilutions of standards and reagents were performed in glass vessels that had been depyrogenated by heating to 220°C for a minimum of 4 hours. All pipette tips were endotoxin free (Diamed Laboratory Supplies Inc., Mississauga, Ontario, Canada). Solutions were prepared with endotoxin-free water, as verified by LAL assay.
In order to perform the assay, 100 l plasma was added to 200 μl 1.32 N nitric acid and 200 l 0.5% Triton X-100, vortexed for 30 s, and incubated at 37°C for 5 min. The tubes were centrifuged at 1500 g for 5 min at room temperature. An aliquot of 200 l supernatant was pipetted into a tube containing 200 l 0.55 N sodium hydroxide for assay. Twofold serial dilutions were performed on all samples using endotoxin-free water. Endotoxin values were calculated from the highest dilutions that yielded plateau endotoxin concentrations. To achieve maximum sensitivity, assays were performed using diazo coupling to N-(1-naphthyl)-ethelenediamine, with absorbance detection at 550 nm. Most samples displayed dilution enhancement.
Statistical analyses
Continuous variables are described as means and standard deviations, unless otherwise specified. Normality was ensured using normal probability plots, and continuous biological variables were compared using Student's t-test. Dichotomous variables were compared using a χ2 test or Fisher's exact test, where appropriate. By convention, an α level of P < 0.05 was considered to be statistically significant.
Results
During the 7-week study period, 126 patients were admitted to the medical/surgical ICU. Seventy-four patients met the inclusion criteria for the study. Patients were excluded for the following reasons: lack of an in-dwelling arterial line (five patients), predicted ICU stay less than 24 h (19 patients), admission from another ICU (three patients) or readmission (three patients), organ donors (three patients), or admission on the weekend (19 patients). Demographic data for the patients included in the study are summarized in Table 1.
Table 1.
Demographic characteristics of the study population
| Parameter | Mean ± standard deviation (range) |
| Age (years) | 59 ± 17 (18–87) |
| Gender (% female) | 42 |
| APACHE II Score | 15.8 ± 8.0 |
| MOD score | 5.0 ± 3.0 |
| Mortality (%) | |
| ICU | 20 |
| Hospital | 28 |
| Duration of stay (days) | |
| ICU | 6.2 ± 15.0 (1–114) |
| Hospital | 25.6 ± 30.0 (1–155) |
APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; MOD, multiple organ dysfunction.
Endotoxin activity assay reliably detects endotoxaemia in critically ill patients
Lipopolysaccharide was added to whole blood of four ICU patients with no detectable endotoxin levels, in graded doses of 100, 400 and 800 pg/ml. The resulting dose–response curve was indistinguishable from one constructed from the blood of nonhospitalized ambulatory normal control individuals [22]. In a parallel series of studies (Fig. 1), patient samples with elevated endotoxin levels were treated with polymyxin B to chelate lipopolysaccharide (300 g/ml blood for 20 min at 37°C) and reassayed using the EAA. This treatment resulted in complete abrogation of the endotoxin-dependent signal in the majority of samples.
Figure 1.
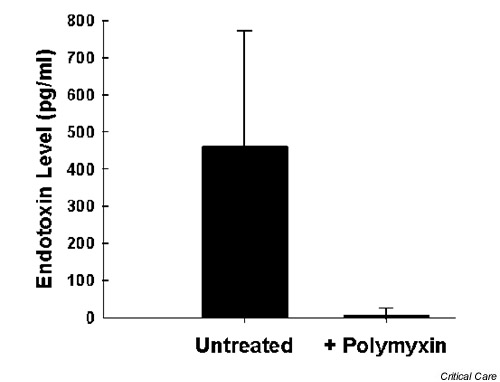
Effect of polymyxin-sulphate preincubation on endotoxin recovery in the endotoxin activity assay. Samples from 15 intensive care unit patients with endotoxaemia ranging from 50 to more than 800 pg/ml were pretreated with polymyxin-sulphate at a concentration of 300 g/ml for 20 min at 37°C. Endotoxin concentrations are expressed as means ± standard deviation.
Prevalence of endotoxaemia
Using the EAA, 43 out of 74 patients (58%) had endotoxin levels greater than 50 pg/ml at the time of ICU admission. The association of endotoxin levels with reason for admission is shown in Table 2. Endotoxin levels by EAA were highest in patients with an admission diagnosis of sepsis, and endotoxin levels greater than 50 pg/ml significantly correlated with an admission diagnosis of sepsis (P < 0.01). This association was not evident when blood samples were assayed using the LAL.
Table 2.
Endotoxin levels as a function of reason for intensive care unit admission
| Mean ± standard error of the mean (% >50 pg/ml) | |||
| Reason for admission | n (%) | EAA assay | LAL assay |
| Elective surgery | 21 (28) | 180 ± 58 (53) | 165 ± 16 (68) |
| Transplant | 11 (14) | 240 ± 110 (50) | 260 ± 48 (91) |
| Renal failure | 5 (7) | 320 ± 196 (40) | 390 ± 170 (80) |
| Sepsis | 19 (25) | 470 ± 57 (85) | 130 ± 37 (60) |
| Hepatic failure | 3 (4) | 210 ± 180 (33) | 200 ± 45 (100) |
| Respiratory failure | 6 (8) | 190 ± 60 (50) | 240 ± 70 (100) |
| Post-CPR | 6 (8) | 160 ± 120 (33) | 190 ± 40 (83) |
| Other | 3 (4) | 450 ± 160 (100) | 85 ± 35 (67) |
Transplants performed were liver, lung, and pancreas. Post-CPR refers to in-hospital cardiopulmonary resuscitation (CPR) cases. Others: acetaminophen overdose, pre-eclampsia and deep vein thrombosis. EAA, endotoxin activity assay; LAL, limulus amoebocyte lysate.
Endotoxaemia is associated with Gram-negative infection
Culture-proven infection was present in 19 (26%) patients at the time of ICU admission: eight patients had pneumonia, eight had positive blood cultures, two had positive cultures from an intra-abdominal source, and one had a positive urine culture. Ten patients had culture-proven Gram-negative infections (five pneumonias, two bacteraemias, two intra-abdominal infections and one urinary tract infection). All patients with Gram-negative infections had endotoxin levels greater than 50 pg/ml using the EAA (Tables 3,4,5). As shown in Fig. 2, patients with Gram-negative infection had higher mean endotoxin levels than did those without Gram-negative infection when measured using the EAA (P < 0.05); no difference in endotoxin levels was observed when the same samples were assayed using the whole-blood LAL method.
Table 3.
Endotoxin levels in patients with bacteraemia
| Patient number | Gram positive | Gram negative | EAA (pg/ml) | LAL (pg/ml) |
| 005 | Group A streptococci | 140 | 0 | |
| 015 | Klebsiella pneumoniae | 380 | 50 | |
| 022 | Coagulase-negative staphylococci | 35 | 100 | |
| 031 | Corynebacterium afermentans | 800 | 160 | |
| 039 | Unspecified | 240 | 150 | |
| 040 | Clostridium difficile | 800 | 160 | |
| 059 | Acinetobacter baumannii | 800 | 900 | |
| 074 | Coagulase-negative staphylococci | 10 | 315 |
EAA, endotoxin activity assay; LAL, limulus amoebocyte lysate.
Table 4.
Endotoxin levels in patients with pneumonia
| Patient number | Gram positive | Gram negative | EAA (pg/ml) | LAL (pg/ml) |
| 007 | Staphylococcus aureus | 380 | 330 | |
| 010* | Lactobacillus spp. | Enterobacter cloacae | 330 | 60 |
| 020 | Klebsiella pneumoniae | 140 | 80 | |
| 025 | S. aureus | 800 | 39 | |
| 039† | K. pneumoniae | 240 | 150 | |
| 042 | Stenotrophomonas maltophilia | 200 | 645 | |
| 061 | S. aureus | 45 | 45 | |
| 068 | Sten. maltophilia | 800 | 225 |
*Patient also had yeast growing in the culture. †Patient also had an intra-abdominal infection. EAA, endotoxin activity assay; LAL, limulus amoebocyte lysate.
Table 5.
Endotoxin levels in patients with infections other than bacteraemia or pneumonia
| Patient number | Site of infection | Gram positive | Gram negative | EAA (pg/ml) | LAL (pg/ml) |
| 002 | Intra-abdominal abcess drain | GNB (nonspecified) | 800 | 90 | |
| 007 | Urine | Citrobacter freundii | 380 | 160 | |
| 039 | Intra-abdominal abcess drain | Pseudomonas aeruginosa | 240 | 150 | |
| 074 | Rectal swab | VRE | 10 | 315 |
EAA, endotoxin activity assay; GNB, Gram-negative bacilli; LAL, limulus amoebocyte lysate; VRE, vancomycin-resistant enterococcus.
Figure 2.
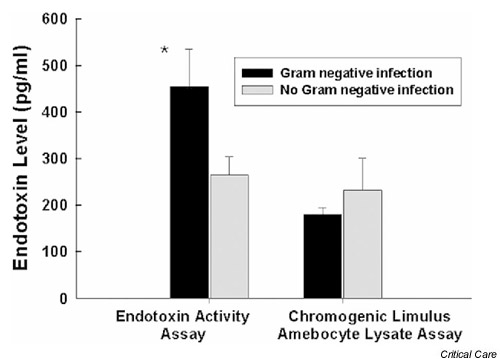
Endotoxin levels in patients with Gram-negative infection. Endotoxin levels were significantly higher by endotoxin activity assay (P < 0.05, n = 10). Endotoxin concentrations are expressed as means ± standard deviation.
Endotoxaemia does not correlate with mortality or illness severity
There were no statistically significant associations between an ICU admission endotoxin level greater than 50 pg/ml and ICU or hospital mortality (Table 6). Patients who had endotoxin levels below 50 pg/ml, as measured using the EAA method, had a trend toward a shorter duration of ICU stay (3.5 ± 6.2 days) than did patients with levels greater than 50 pg/ml (8.1 ± 18.8 days; P = 0.082, by Mann–Whitney U test). Acute Physiology and Chronic Health Evaluation II scores were not significantly different between patients who were endotoxaemic and those who were not.
Table 6.
Endotoxin levels and intensive care unit outcomes
| Endotoxin level | ||
| Characteristic | <50 pg/ml | ≥ 50 pg/ml |
| APACHE II score ± SD | 13.7 ± 7.8 | 15.8 ± 8.6 |
| MOD score ± SD | 5.6 ± 2.8 | 4.5 ± 3.3 |
| ICU mortality (%) | 26 | 30 |
| ICU duration of stay (days) ± SD | 3.5 ± 6.2 | 8.1 ± 18.8 |
APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; MOD, multiple organ dysfunction; SD, standard deviation.
Endotoxaemia is associated with leukocytosis but not with systemic inflammatory response syndrome
SIRS, as defined by the presence of two or more criteria [25], was present in 60 out of 74 patients (80%) at the time of admission to the ICU; however, its presence was not associated with endotoxaemia. Mean white blood cell counts for patients with endotoxaemia were significantly higher (15.7 ± 9.1 × 109 cells/l for endotoxaemic patients versus 10.8 ± 6.2 × 109 cells/l for patients without endotoxaemia; P = 0.014). Using the EAA method, patients who were hypotensive on admission had a mean endotoxin level of 440 ± 350 pg/ml, versus 320 ± 330 pg/ml for normotensive patients; however, the difference was not statistically significant (P = 0.18).
Discussion
We compared results obtained with a new, rapid, near-patient assay for endotoxin with those obtained using the chromogenic LAL assay in a group of patients admitted to an ICU. In this heterogeneous patient population, 58% of patients had endotoxin levels greater than 50 pg/ml at the time of ICU admission. Endotoxaemia was associated with Gram-negative infection from any source, a diagnosis of sepsis and an elevated white blood cell count, but only when samples were assayed using the EAA; no such correlations were found when samples were assayed using the LAL method.
To date the only widely available method for measuring endotoxin has been the LAL assay; however, its use to detect and quantify endotoxin in plasma or whole blood has been problematic. Circulating inhibitors of the limulus reaction have been described [18,27], and published reports show considerable variability in the prevalence of endotoxaemia or its association with Gram-negative infection [6,16,21].
The EAA detects endotoxin as the priming of the patient's own neutrophils by complexes of endotoxin and a specific antiendotoxin antibody; it is thus sensitive and more specific than the LAL assay [22]. It can be performed using as little as 100 μl whole blood and results are available within 30 min. We previously showed the assay to be specific for Gram-negative bacterial endotoxin [22]. Similar performance characteristics are reported here for recovery studies using blood from critically ill patients. In a subset of 15 patients with elevated endotoxin levels, as assayed using the EAA, polymyxin B – a specific endotoxin-binding agent – eliminated or markedly attenuated the chemiluminescent signal (Fig. 1).
Hurley [26] conducted a meta-analysis of 11 studies including a total of 738 patients, in whom the correlation between endotoxaemia, Gram-negative bacteremia and infection was evaluated. Of the patients studied 18% had endotoxaemia and Gram-negative bacteraemia, 12% had Gram-negative bacteraemia alone and 19% had endotoxaemia alone; in 51% of patients with suspected sepsis, neither endotoxin or bacteraemia was detected. The concomitant detection of endotoxin and Gram-negative bacteraemia was strongly associated with a fatal outcome.
Endotoxaemia has been reported to be common in patients with sepsis (79%) [16], chronic liver disease with cirrhosis (46%) [9], and leukaemia or lymphoma (81%) [28], as well as in patients undergoing cardiopulmonary bypass (100%) [12]. Endotoxaemia correlates with mortality in patients with meningococcaemia [29], severe pancreatitis [30] and extensive burn injury [31]. The data presented here do not suggest an association with increased risk for mortality in an heterogeneous population of critically ill patients.
Conflicting findings have been reported regarding the association of endotoxaemia with Gram-negative bacteraemia [5,6,7,13,16,17]. Endotoxaemia in the absence of viable Gram-negative microorganisms may reflect translocation of lipopolysaccharide from either a haematogenous or extravas-cular site of Gram-negative bacterial invasion [32]. Antibiotics can accelerate endotoxin release [33] and may result in falsely negative blood cultures. Endotoxin may also appear in the blood stream following hypotensive episodes that result in reduced splanchnic blood flow [10]. Animal studies have similarly documented systemic translocation of endotoxin from the lungs [34]. Several patients in the present study with Gram-positive infections also demonstrated high levels of endotoxin (Tables 3,4,5). This phenomenon has been described by others [5,35] and may represent concomitant Gram-negative infection or translocation of endotoxin through gut or lung tissue.
Clarification of the pathogenic role of endotoxin in Gram-negative sepsis is further complicated by the failure of clinical trials evaluating antiendotoxin therapies. Although preliminary studies of antiendotoxin strategies suggested promise, larger phase III trials have yielded disappointing results [15]. Inability to identify appropriate populations for study on the basis of clinical criteria alone may partly account for the lack of apparent benefit. Wortel and coworkers [7] used the LAL assay to measure plasma endotoxin levels in a subset of 82 patients enrolled in a phase III antiendotoxin trial. Those investigators found a 58% decrease in mortality for patients with documented endotoxaemia who received the antiendotoxin antibody as compared with endotoxaemic patients who received placebo (P = 0.034).
More studies using the EAA are required to establish the role of endotoxaemia in the pathophysiology of critical illness. However, the ability to detect endotoxaemia reliably and rapidly may expedite the investigation of suspected infection in critical illness, and may help to identify appropriate high-risk populations for intervention with conventional antibiotic or surgical strategies, or emerging therapies that target endotoxin per se.
Key messages
· Circulating endotoxin can be rapidly detected in critically ill patients using the technique of whole blood chemiluminescence.
· Significant endotoxemia is a common finding in critical illness.
· Endotoxin levels are most elevated in patients with clinical sepsis.
Competing interests
Dr Paul Walker is President and Chief Executive Officer of Spectral Diagnostics and Sepsis Inc. Dr Alex Romaschin is Vice President of Sepsis Inc. Both have financial interests in the chemiluminescent assay.
Debra Foster, Anastasia Derszko, David Harris, Melanie Ribeiro and Jeffrey Paice are all employees of Sepsis Inc.
Abbreviations
EAA = endotoxin activity assay; ICU = intensive care unit; LAL = limulus amoebocyte lysate; SIRS = systemic inflammatory response syndrome.
See related Commentary: http://ccforum.com/content/6/4/289
References
- Morrison DC, Ulevitch RJ. The effects of bacterial endotoxin on host mediation systems. Am J Pathol. 1978;93:527–601. [PMC free article] [PubMed] [Google Scholar]
- Parillo JE. Pathogenic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- Watson RWG, Redmond HP, Bouchier-Hayes D. Role of endotoxin in mononuclear phagocyte-mediated inflammatory responses. J Leukoc Biol. 1994;56:95–103. doi: 10.1002/jlb.56.1.95. [DOI] [PubMed] [Google Scholar]
- Santos AA, Wilmore DW. The systemic inflammatory response: perspective of human endotoxemia. Shock. 1996;6(suppl 1):S50–S56. [PubMed] [Google Scholar]
- Danner RL, Elin RJ, Hosseini JM, Wesley RA, Reilly JM, Parilllo JE. Endotoxemia in human septic shock. Chest. 1991;99:169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- Wortel CH, von der Mohlen MA, van Deventer SJ, Sprung CL, Jastremski M, Lubbers MJ, Smith CR, Allen IE, ten Cate JW. Effectiveness of a human monoclonal anti-endotoxin antibody (HA-1A) in Gram negative sepsis: relationship to endotoxin and cytokine levels. J Infect Dis. 1992;166:1367–1374. doi: 10.1093/infdis/166.6.1367. [DOI] [PubMed] [Google Scholar]
- Bion JF, Badger I, Crosby HA, Hutchings P, Kong KL, Baker J, Hutton P, McMaster P, Buckels JA, Elliott TS. Selective decontamination of the digestive tract reduces Gram-negative pulmonary colonization but not systemic endotoxemia in patients undergoing elective liver transplantation. Crit Care Med. 1994;22:40–49. doi: 10.1097/00003246-199401000-00011. [DOI] [PubMed] [Google Scholar]
- Gaeta GB, Perna P, Adinolfi LE, Utili R, Ruggiero G. Endotoxemia in a series of 104 patients with chronic liver diseases: prevalence and significance. Digestion. 1982;23:239–244. doi: 10.1159/000198756. [DOI] [PubMed] [Google Scholar]
- Roumen RMH, Frieling JTM, van Tits HWHJ, van der Vliet JA, Goris RJ. Endotoxemia after major vascular operations. J Vasc Surg. 1993;18:853–857. [PubMed] [Google Scholar]
- Schlag G, Redl H, Dinges HP, Davies J. Sources of endotoxin in the posttraumatic setting. In: Sturk A, van Deventer SJH, ten Cate JW, Buller HR, Thijs LG, Levin J, editor. In Bacterial Endotoxins: Cytokine Mediators and New Therapies for Sepsis. Amsterdam: Wiley-Liss Inc.; 1991. pp. 121–134. [Google Scholar]
- Jansen PG, Te Velthuis H, Oudemans-Van Straaten HM, Bulder ER, Van Deventer SJ, Sturk A, Eijsman L, Wildevuur CR. Perfusion-related factors of endotoxin release during cardiopulmonary bypass. Eur J Cardiothorac Surg. 1994;8:125–129. doi: 10.1016/1010-7940(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Natanson C, Hoffman WD, Suffredini AF, Eichacker PQ, Danner RL. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenisis. Ann Intern Med. 1994;120:771–783. doi: 10.7326/0003-4819-120-9-199405010-00009. [DOI] [PubMed] [Google Scholar]
- Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;272:117–123. [PubMed] [Google Scholar]
- Ziegler EJ, Fisher CJ, Jr, Sprung CL, Straube RC, Sadoff JC, Foulke GE, Wortel CH, Fink MP, Dellinger RP, Teng NN, et al. Treatment of Gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. N Engl J Med. 1991;324:429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- van Deventer SJH, Buller HR, ten Cate JW, Sturk A, Pauw W. Endotoxemia: an early predictor of septicemia in febrile patients. Lancet. 1988;1:605–608. doi: 10.1016/s0140-6736(88)91412-2. [DOI] [PubMed] [Google Scholar]
- Hurley JC. Antibiotic-induced release of endotoxin: a reappraisal. Clin Infect Dis. 1992;15:840–854. doi: 10.1093/clind/15.5.840. [DOI] [PubMed] [Google Scholar]
- Elin RJ, Robinson RA, Levine AS, Wolff SM. Lack of clinical usefulness of the limulus test in the diagnosis of endotoxemia. N Engl J Med. 1975;293:521–524. doi: 10.1056/NEJM197509112931102. [DOI] [PubMed] [Google Scholar]
- Levin J, Bang FB. Clottable protein in limulus: its localization and kinetics of its coagulation by endotoxin. Thromb Diath Haemorrh. 1968;19:186–197. [PubMed] [Google Scholar]
- Tamura H, Tanaka S, Obayashi T, Yoshida M, Kawai T. A new sensitive method for determining endotoxin in whole blood. Clin Chim Acta. 1991;200:35–42. doi: 10.1016/0009-8981(91)90331-6. [DOI] [PubMed] [Google Scholar]
- Bates DW, Parsonnet J, Ketchum PA, Novitsky TJ, Sands K, Hibberd PL, Graman PS, Lanken PN. Limulus amebocyte lysate assay for detection of endotoxin in patients with sepsis syndrome. AMCC Sepsis Project Working Group. Clin Infect Dis. 1998;27:582–591. doi: 10.1086/514713. [DOI] [PubMed] [Google Scholar]
- Romaschin AD, Harris DM, Ribeiro MB, Paice J, Foster DM, Walker PM, Marshall JC. A rapid assay of endotoxin in whole blood using autologous neutrophil dependent chemiluminescence. J Immunol Methods. 1998;212:169–185. doi: 10.1016/s0022-1759(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- Members of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Definitions of sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;23:376–393. [PubMed] [Google Scholar]
- Hurley JC. Re-appraisal with meta analysis of bacteremia, endotoxemia and mortality in Gram negative sepsis. J Clin Microbiol. 1995;33:1278–1282. doi: 10.1128/jcm.33.5.1278-1282.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Baek L, Bertelen T, Koch C. Quantification of the endotoxin-neutralizing capacity of serum and plasma. APMIS. 1995;103:712–730. doi: 10.1111/j.1699-0463.1995.tb01429.x. [DOI] [PubMed] [Google Scholar]
- Behre G, Schedel I, Nentwig B, Wormann B, Essink M, Hiddemann W. Endotoxin concentration in neutropenic patients with suspected Gram-negative sepsis: correlation with clinical outcome and determination of anti-endotoxin core antibodies during therapy with polyclonal immunoglobulin M-enriched immunoglobulins. Antimicrob Agents Chemother. 1992;36:2139–2146. doi: 10.1128/aac.36.10.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P, Kierulf P, Gaustad P, Skulberg A, Bruun JN, Halvorsen S, Sorensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- Exley AR, Leese T, Holliday MP, Swann RA, Cohen J. Endotoxemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut. 1992;33:1126–1128. doi: 10.1136/gut.33.8.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YM, Sheng ZY, Tian HM, Wang YP, Guo ZR, Gao WY. The association of circulating endotoxemia with the development of multiple organ failure in burned patients. Burns. 1995;21:255–258. doi: 10.1016/0305-4179(95)93867-j. [DOI] [PubMed] [Google Scholar]
- Go LL, Healey PJ, Watkins SC, Simmons RL, ROWE MI. The effect of endotoxin on intestinal mucosal permeability to bacteria in vitro. Arch Surg. 1995;130:53–58. doi: 10.1001/archsurg.1995.01430010055011. [DOI] [PubMed] [Google Scholar]
- Shenep JL, Flynn PM, Barrett FF, Stidham GL, Westenkirchner DF. Serial quantitation of endotoxemia and bacteremia during therapy for Gram-negative bacterial sepsis. J Infect Dis. 1988;157:565–568. doi: 10.1093/infdis/157.3.565. [DOI] [PubMed] [Google Scholar]
- Nahum A, Hoyt J, Schmitz L, Moody J, Shapiro R, Marini JJ. Effect of mechanical ventilation strategy on dissemination of intra-tracheally instilled Escherichia coli in dogs. Crit Care Med. 1997;25:1733–1743. doi: 10.1097/00003246-199710000-00026. [DOI] [PubMed] [Google Scholar]
- Opal SM, Scannon PJ, Vincent JL, White M, Carroll SF, Palardy JE, Parejo NA, Pribble JP, Lemke JH. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999;180:1584–1589. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]


