Abstract
Saffron (Crocus sativus L.) is one of the most valuable medicinal plants, but its production is highly constrained by drought stress and excessive dependence on chemical fertilizers, which adversely affect both yield and quality while raising environmental concerns. The aim of this study was to evaluate the impact of endophytic fungi (Penicillium chrysogenum and Serendipita indica) and silicon concentrations under different irrigation regimes on growth, yield and nutritional quality of saffron (Crocus sativus L.) with an intention to reduce reliance on chemical fertilizers utilizing sustainable agricultural practices. This experiment was conducted as a factorial experiment in a completely randomized block design with 27 treatment combinations of Penicillium chrysogenum, Serendipita indica, silicon, and different irrigation levels with three replications during the 2022–2023. Application of Penicillium chrysogenum, and Serendipita indica fungi, solely or in combination with silicon significantly enhanced the stigma fresh weight, stigma dry weight, saffron yield, stigma length, daughter corm number, daughter corm weight, leaf length and number, leaf dry weight, root length, root fresh and dry weight, picrocrocin and safranal. The treatment of Serendipita indica + 200 ppm silicon was the most effectiveness, so application of Serendipita indica + 200 ppm silicon caused increases by 419.1, 29.2, 279.4, 286.5, 284.5, 55.4, 371.2, 316.9, 120.8, 163.9, 312.4, 177.6, 116.5, 116.5, 40.0, and 157.8%, respectively in the values of stigma fresh weight, stigma dry weight, saffron yield, stigma length, daughter corm number, daughter corm weight, leaf length, leaf number per plant, leaf dry weight, root length, root fresh weight, root dry weigh, picrocrocin and safranal. We recommend the application of Serendipita indica + 200 ppm silicon in order to enhancement growth and flowering, saffron yield, and saffron nutritional quality in non-stress and drought stress condition. In conclusion, this study provides a promising strategy for sustainable saffron production, and future research should validate these findings under diverse agro-climatic conditions and explore their long-term economic feasibility.
Keywords: Biostimulants, Drought stress, Picrocrocin, Plant growth regulators, Saffron, Sustainable agriculture
Introduction
Crocus sativus L. (Saffron) from the Iridaceae family, as a valuable plant is well known for the color, flavor, and extensive medicinal properties of its dried stigma. Crocus sativus is a male-sterile, triploid flower crop [1]. This plant is well adapted to cool to cold winters with autumn–winter–spring precipitation and warm summers with very little rainfall [2]. the global saffron market was valued at approximately USD 602.2 million. It is expected to grow at a robust compound annual growth rate (CAGR) of 7.1% from 2024 to 2030, potentially exceeding a market value of USD 1 billion by the end of the decade [3]. Saffron product, which is processed from the stigma of C. sativus, is one of the most valuable, attractive, expensive, and widely used spices in the world due to its pleasant aroma and taste and distinct medicinal properties [4–6]. Three key bioactive components (safranal, crocin, and picrocrocin) are mainly liable for its flavor and pharmacological properties [7] [8]. Crocins, a major group of glycosylated apocarotenoids, are significant potential for diverse applications across the food, pharmaceutical, and cosmetic industries [9]. saffron (Crocus sativus L.) exhibits allelopathic properties, particularly through its corms, which have been shown to inhibit seed germination in various crops. For instance, a study by [10] demonstrated that saffron corms possess allelopathic effects that can suppress seed germination in several important crops.
Drought stress affects the quality and quantity of food, which endangers human health in some regions of the world. Agricultural scientists are working responsively to diminish plant water consumption, especially in drought-prone countries [11]. They use various agronomic techniques, including biotechnology, nanotechnology, and agronomic improvement to achieve this goal. The application of plant growth-promoting microorganisms (PGPM) is an effective, economical and environmentally friendly approach to enhancement plant drought resilience [11], resulting supplementation yield and nutritional content of agricultural products to increment human health and food security [12, 13]. As well as, causes reduction in photosynthesis, and ultimately reduction in plant growth and development [14, 15].
PGPM include fungi and bacteria can improve nutrient absorption as well as the plants yield under stress conditions [16–18]. They play a vital role in encouraging sustainable agriculture by reducing the use of chemical fertilizers and water consumption [19]. Plant-growth-promoting fungi (PGPF), a group of non-pathogenic soil-borne fungi can enhance plant growth through producing phytohormones, fixing nitrogen, and inducing systemic resistance [20–22]. These microorganisms provide an integrated management opportunity for nutrient gaining, local and systemic resistance to disease and stress tolerance in plants [12, 23–27]. Endophytic fungi including Penicilliums and Serendipita are known to be naturally occurring beneficial partners of plants which resides as good symbiont and proven a confident role in plant growth advancement and improved resistance against stresses [28, 29].
Penicilliums as an antibiotic agent, can dissolve insoluble phosphate and improve its uptake by plant roots [18, 30, 31]. Penicillium chrysogenum, an endophyte species, can definitely be used as a plant growth promoter inducing resistance to abiotic and biotic stresses. It achieves this through various mechanisms, including the production of plant growth-promoting hormones and bioactive compounds and solubilization of minerals [32]. Serendipita indica (formerly named Piriformospora indica), belonging to the family Thelephoraceae, a root endophytic fungus, colonizes the roots of a wide range of hosts including various members of gymnosperms and angiosperms [28, 33]. S. indica can initiate a signaling cascade involving the activation of 3-phosphoinositide-dependent protein kinase 1, oxidative signal inducible 1, and mitogen-activated protein kinases 3/6 to promote the growth of host plants [34]. S. indicca display important role in nutrient uptake, especially nitrogen and phosphate [35]. The Pht1 subfamily belongs to the high-affinity P transporter proteins located in the cell membrane, which are induced by P deficiency stress and are involved in the transport of phosphate from the rhizosphere soil to the root inside [36, 37]. S. indica increased leaf CoPht1;1, CoPht1;2, and CoPht1;3 expression, suggests that this fungus might be providing sufficient phosphate to the plant [25, 26, 38].
The application of trace elements biostimulants is an eco-friendly crop management tool [39, 40]. In recent years, application of these biostimulants gained attention to improve plant tolerance to environmental stress [41–43]. Silicon (Si) a non-essential element, will enhance crop yield by promoting several desirable plant physiological processes including resistance to biotic and abiotic stresses, promoting nutrient uptake, and strengthening plant structure [44–46]. The adequate Si levels appear to be essential to mitigating environmental stresses, increase yield, and sustain plant production in drought conditions [47]. Recent studies have stated a multiple role of silicon in modifying abiotic stress through regulating various Physio-biochemical, and molecular manners [48, 49]. Overall, silicon regulates root water uptake and transpiration, resulting improves plant–water relations, boosts nutrient uptake, increases photosynthesis pigments and activity, enhances antioxidant activity, and regulates the biosynthesis of phytohormones [50–53].
However, despite extensive studies on saffron physiology and the individual role of plant growth-promoting microorganisms or silicon in improving plant stress tolerance, little information is available on their combined effects on saffron, particularly under drought stress. Previous reports have mainly focused on other crops or on either PGPM or silicon alone [54, 55], while comprehensive studies addressing their synergistic role in enhancing saffron growth, yield, and bioactive compounds are scarce, in addition to, Given the growing demand for environmentally friendly and resource-efficient agricultural practices, sustainable strategies for saffron production have become essential. This gap highlights the need for systematic investigation into the integrated application of endophytic fungi and silicon in saffron under drought conditions. It was hypothesized that the application of endophytic fungi (Penicillium chrysogenum and Serendipita indica), either alone or in combination with silicon, would enhance vegetative and reproductive growth, yield, and nutritional quality of saffron under both normal and drought stress conditions. Furthermore, the synergistic effect of Serendipita indica with 200 ppm silicon was expected to provide the greatest improvement. In current study, the multifaceted effects of plant growth stimulants; Penicillium chrysogenum, Serendipita indica, and silicon on the morph-physiological, phytochemical, and yield characteristics of saffron were investigated in order to increase saffron quality and quantity and the drought tolerance of this plant.
Materials and methods
Experimental site and design
The experiment was conducted during the 2022–2023 growing season in the research greenhouse of Basrah University, Iraq. Uniform, healthy corms of saffron (Crocus sativus L.) with an average weight of 8–10 g was used and cultivated in pots. This experiment was conducted as a factorial experiment in a completely randomized block design with 27 treatment combinations. Saffron (Crocus sativus L.) corms used in this study were obtained from the mother field of a traditional saffron farm located in Asfij District, Bahabad County, Yazd Province, Iran. The plant material was not collected from the wild. Instead, the corms were sourced from cultivated stocks commonly propagated and planted by hundreds of local farmers in the region. The taxonomic identification of the plant material (genus and species) was formally confirmed by Dr. Heidar Meftaḥizadeh, (Associate Professor in the Department of Horticultural science, Medicinal and Aromatic Plants, Ardakan University, Yazd, Iran. Hmeftahizade@ardakan.ac.ir), based on morphological characteristics, based on morphological characteristics. The treatments consisted of three irrigation levels [100% Field Capacity (FC) = Non-Drought Stress (NDS), 70% FC = Medium Drought Stress (MDS), and 40% FC = High Drought Stress (HDS)], two endophytic fungi (Penicillium chrysogenum and Serendipita indica), and three concentrations of silicon (0,100 and 200 ppm) as follows:
T1: 100% FC irrigation: Non Drought Stress (NDS), T2: 70% FC irrigation: Medium Drought Stress (MDS), T3: 40% FC irrigation: High Drought Stress (HDS), T4: 100 ppm silicon (Si), T5: Si 100 ppm + MDS, T6: Si 100 ppm + HDS, T7: Si 200 ppm, T8: Si 200 ppm + MDS, T9: Si 200 ppm + HDS, T10: Penicillium chrysogenum (PC) + NDS, T11: PC + MDS, T12: PC + HDS, T13: PC + Si 100 ppm, T14: PC + Si 100 ppm + MDS, T15: PC + Si 100 ppm + HDS, T16: PC + Si 200 ppm, T17: PC + Si 200 ppm + MDS, T18: PC + Si 200 ppm + HDS, T19: Serendipita indica (SI), T20: SI + MDS, T21: SI + HDS, T22: SI + Si 100 ppm, T23: SI + Si 100 ppm + MDS, T24: SI + Si 100 ppm + HDS, T25: Si + S 200 ppm, T26: SI + Si 200 ppm + MDS, T27: SI + Si 200 ppm + HDS.
Fungal inoculation and silicon application
Endophytic fungi were cultured on potato dextrose agar (PDA) at 28 °C for 7 days. The fungal inoculum (1 × 10⁷ spores/mL) was applied to the corms by soaking them for 2 h prior to planting. Silicon (potassium silicate Merck company) was applied as a foliar spray using sodium silicate solution at concentrations of 100 or 200 ppm, applied at 15-day intervals throughout the growing period (September to March).
Investigated parameters
Number of flowers
The number of flowers in each plot was counted and recorded from the beginning to the end of the flowering period.
Fresh and dry yield of the stigma
The flower stigmas were carefully separated from other floral parts. To determine the fresh weight, the total fresh weight of the stigmas collected during the flowering period was measured for each plot [56]. using a digital analytical balance (Sartorius, CPA224S, Germany) with 0.001 g precision. For dry weight determination, the stigmas were dried in an oven at 40 °C. The samples were weighed at 12-hour intervals until no significant change in weight was observed between consecutive measurements, indicating that constant dry weight had been achieved [57]. After drying, the total number of harvested stigmas per plot was recorded, and the dry saffron yield per square meter was calculated.
Measurement of root and shoot length
After harvesting the samples, the root length from the collar area to the tip of the longest root and the leaf length from the collar area to the tip of the longest leaf were measured using a ruler.
Measurement of root and shoot weight
The roots and aerial parts of the plants were separated and thoroughly washed with water. After removing surface moisture, their fresh weights were recorded using a digital balance with 0.001 g precision (Sartorius, CPA224S, Germany). Subsequently, half of the samples were placed in an oven at 70 °C for 72 h to achieve constant weight. After the drying period, the dry weights of the samples were measured. Also, daughter corm number and daughter corm weight were measured at harvesting time [56].
Measurement of Relative Water Content (RWC)
At the end of the experimental period, the relative water content (RWC) of leaves in all treatments was measured. For this purpose, 0.2 g of a fully expanded leaf was sampled from each treatment. The leaf segments were placed in lidded Petri dishes containing distilled water and kept at 4 °C in darkness for 16 h to attain full turgidity. After removal from the water, excess surface moisture was blotted using two layers of filter paper, and the turgid weight (TW) was recorded. The samples were then dried in an oven at 70 °C for 48 h to determine their dry weight (DW). RWC was then calculated according to [58] using the Eq. 1.
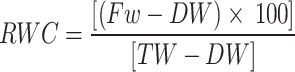 |
1 |
 |
Stigma length
In order to calculate the stigma length, the length of 30 stigmas (from the top to the orange part) was measured daily using a ruler in centimeters, and the average length of the stigmas during the flowering period was considered as the average stigma length for each plot [59].
Measurement of Picrocrocin and Safranal
50 mg of the sample was weighed with a balance with an accuracy of 0.0001 g in a watch glass and transferred to a 50 ml volumetric flask. Then 45 ml of distilled water was added to it. The contents of the volumetric flask were placed on a magnetic stirrer at a speed of 1000 rpm for 30 min. Then the volumetric flask was made up to the mark with distilled water to a volume of 50 ml, closed and mixed well until a uniform solution was obtained. One ml of this solution was removed with a pipette and brought to a volume of 10 ml and stirred until a uniform solution was obtained. After centrifugation, one ml of the supernatant was removed and distilled water was used as a control and regulator. The amount of picrocrocin and safranal in the stigma was determined at wavelengths of 257 and 330 nm, respectively. Then the percentage of chemical compounds in saffron stigma was calculated according to the Eq. 2.
 |
2 |
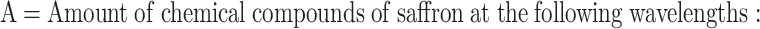 |
Picrocrocin
Absorption at a wavelength of 257 nm, Safranal: absorption at a wavelength of 330 nm, M = Mass of saffron sample (grams), H = Amount of moisture and volatile substances in the sample [59].
Statistical analysis
Data were analyzed using IBM-SPSS (Ver. 27) software. Means compared by Duncan’s Multiple Range Test at a 5% significance level. Principal Component Analysis (PCA), Regression Analysis, and Person’s Correlation Estimation were done using Minitab Statistical Software (Ver. 22).
Results
Means comparison
ANOVA analysis designated significant differences at the level of 1% between endophyte fungi (EF) for all studied variable except stigma length. Also, between silicon (Si) treatments observed significant differences for all studied variables, except stigma length, daughter corm weight, and RWC, at the level of 1%. Drought stress (DS) treatments showed significant differences only for daughter corm number and root length at the level of 1%. The interaction between EF × Si was significant for all variables except stigma length, daughter corm number, leaf length, root fresh weight, root dry weight, and RWC. Also, the interaction effect of EF×DS and Si×DS only were significant for root length. Furthermore, the interaction effect of EF × Si × DS was significant for daughter corm number and root length too.
The amounts of all studied traits were not significantly influenced by drought, application of silicon, and drought × silicon interaction (T2-T9), as the values of these variables significantly had not changed compared to control under effect of T2-T9. However, the application of endophyte fungi (Penicillium chrysogenum (PC) or Serendipita indica (SI), solely or in combination with silicon increased significantly the values of phytochemicals, morpho-physiological, yield and yield components of saffron under none or drought stress conditions compared to control. In addition, results demonstrated that application of SI endophyte is more effective than PC endophyte, as the highest values of all studied variable were observed in the plants treated with SI endophyte with combination of 200 ppm silicon, or in some case with combination of 100 ppm silicon.
As exhibited in Fig. 1a, the flower number exponentially has increased with application of treatments from control to T 27. The extreme flower number, 156.7, 147.0, and 142.0, respectively was recorded in plants treated with SI + 100 ppm silicon (T22), SI + 200 ppm silicon + medium drought stress (T26), and SI + 200 ppm silicon under non drought stress (T25).
Fig. 1.

Number of flower (a) and stigma fresh weight (b) (Duncan test, α = 0.05). T1: 100% FC irrigation: Non Drought Stress (NDS), T2: 70% FC irrigation: Medium Drought Stress (MDS), T3: 40% FC irrigation: High Drought Stress (HDS), T4: 100 ppm silicon (Si), T5: Si 100 ppm + MDS, T6: Si 100 ppm + HDS, T7: Si 200 ppm, T8: Si 200 ppm + MDS, T9: Si 200 ppm + HDS, T10: Penicillium chrysogenum (PC) + NDS, T11: PC + MDS, T12: PC + HDS, T13: PC + Si 100 ppm, T14: PC + Si 100 ppm + MDS, T15: PC + Si 100 ppm + HDS, T16: PC + Si 200 ppm, T17: PC + Si 200 ppm + MDS, T18: PC + Si 200 ppm + HDS, T19: Serendipita indica (SI), T20: SI + MDS, T21: SI + HDS, T22: SI + Si 100 ppm, T23: SI + Si 100 ppm + MDS, T24: SI + Si 100 ppm + HDS, T25: Si + S 200 ppm, T26: SI + Si 200 ppm + MDS, T27: SI + Si 200 ppm + HDS
The increase trend of stigma fresh weight showed a low changes and slope across application of T1 to T27 (Fig. 1b). The highest amounts of this variable including 27.67, 27.00, 26.00, and 25.00 gr/m2, respectively were obtained with application of SI + 200 ppm silicon under non drought stress (T25), SI + 200 ppm silicon in response to high drought stress (T27), SI + 100 ppm silicon under high drought stress (T24), and SI + 200 ppm silicon in response to medium drought stress (T26).
The maximum stigma dry weights (Fig. 2a) were found as follow: 5.67 g/m2 in the plants treated with SI + 200 ppm silicon under non drought stress (T25), 5.4 at SI + 200 ppm silicon under high drought stress (T27), 5.2 under application of SI + 100 ppm silicon under high drought stress (T24), 5.17 using SI + 200 ppm silicon under medium drought stress (T26) 4.6 in the plants treated with SI + 100 ppm silicon + MDS (T23), and finally, 4 g/m2 under effect of SI + 100 ppm silicon (T22).
Fig. 2.
Stigma dry weight (a) and saffron yield (b), (Duncan test, α = 0.05). T1: 100% FC irrigation: Non Drought Stress (NDS), T2: 70% FC irrigation: Medium Drought Stress (MDS), T3: 40% FC irrigation: High Drought Stress (HDS), T4: 100 ppm silicon (Si), T5: Si 100 ppm + MDS, T6: Si 100 ppm + HDS, T7: Si 200 ppm, T8: Si 200 ppm + MDS, T9: Si 200 ppm + HDS, T10: Penicillium chrysogenum (PC) + NDS, T11: PC + MDS, T12: PC + HDS, T13: PC + Si 100 ppm, T14: PC + Si 100 ppm + MDS, T15: PC + Si 100 ppm + HDS, T16: PC + Si 200 ppm, T17: PC + Si 200 ppm + MDS, T18: PC + Si 200 ppm + HDS, T19: Serendipita indica (SI), T20: SI + MDS, T21: SI + HDS, T22: SI + Si 100 ppm, T23: SI + Si 100 ppm + MDS, T24: SI + Si 100 ppm + HDS, T25: Si + S 200 ppm, T26: SI + Si 200 ppm + MDS, T27: SI + Si 200 ppm + HDS
Although, the saffron yield has increased significantly with application of two endophytic fungi in response to all drought treatment and silicon levels compared to control and non-endophyte fungi-treated plants, the peak of saffron yield (Fig. 2b) were gotten with application of SI endophyte fungus, as 7.90 kg ha−1 saffron were gained with application of SI endophyte + 200 ppm silicon (T25) under non-stress condition, which wasn’t significantly differ to its quantities in the plants treated with SI + 200 ppm silicon under high drought condition (T27), SI + 100 ppm silicon under high drought stress (T24), and SI + 200 ppm silicon under medium drought stress (T26), respectively 7.61, 7.28, and 7.09 kg ha−1 (Fig. 2b).
As showed in the Fig. 3a stigma length significantly was improved with application of endophytic fungi, solely or in combination with silicon under non and stress conditions. The largest stigma was observed, respectively in plants treated with SI + 200 ppm silicon under medium drought condition (3.7 cm, T26), and 3.63 cm in the plants treated with SI + 200 ppm silicon under non stress condition (T25), and SI + 100 ppm silicon under high drought stress (10.6 cm, T24).
Fig. 3.

Stigma length (a) and daughter corm number (b) (Duncan test, α = 0.05). T1: 100% FC irrigation: Non Drought Stress (NDS), T2: 70% FC irrigation: Medium Drought Stress (MDS), T3: 40% FC irrigation: High Drought Stress (HDS), T4: 100 ppm silicon (Si), T5: Si 100 ppm + MDS, T6: Si 100 ppm + HDS, T7: Si 200 ppm, T8: Si 200 ppm + MDS, T9: Si 200 ppm + HDS, T10: Penicillium chrysogenum (PC) + NDS, T11: PC + MDS, T12: PC + HDS, T13: PC + Si 100 ppm, T14: PC + Si 100 ppm + MDS, T15: PC + Si 100 ppm + HDS, T16: PC + Si 200 ppm, T17: PC + Si 200 ppm + MDS, T18: PC + Si 200 ppm + HDS, T19: Serendipita indica (SI), T20: SI + MDS, T21: SI + HDS, T22: SI + Si 100 ppm, T23: SI + Si 100 ppm + MDS, T24: SI + Si 100 ppm + HDS, T25: Si + S 200 ppm, T26: SI + Si 200 ppm + MDS, T27: SI + Si 200 ppm + HDS
Application of SI endophytic fungus, and in the next time, PC endophytic fungus caused notable increases in the number of daughter corm solely or in combination with silicon in the drought control plants; and plants under medium and high stress. Figure 3b exhibited that the highest daughter corm number (14.0 n) was found under effect of high drought stress and application of SI endophyte + 200 ppm silicon (T27). Furthermore, in the next steps, 13.37 and 10.6 numbers of daughter corm, respectively were obtained in plants treated with SI + 100 ppm silicon under non stress condition (T22) and SI + 100 ppm silicon under high drought stress (T24).
As previously we explained abought enhancing effects of applied endophyte treatments on daughter corm number, these treatments displayed expected effects on daughter corn weight (Fig. 4a), so the highest daughter corm weights (7.70, 7.42, 7.08, 6.89, 6.37, and 5.40 g m2) respectively, were measured in the saffron plants treated with T25, T27, T24, T26, T23, and T22; combination treatments of SI endophyte, different silicon levels, and different drought conditions). The largest leaf length (Fig. 4b) was measured, respectively in plants treated with SI endophyte + 200 ppm silicon under medium drought condition (T26) (14.1 cm), SI + 200 ppm silicon in the non-stress condition (T25) (13.0 cm), SI + 200 ppm silicon under high drought condition (T27) (12.9 cm), and SI + 100 ppm silicon under high drought stress (T24) (12.7 cm).
Fig. 4.
Daughter corm weight (a) and leaf length (b) (Duncan test, α = 0.05). T1: 100% FC irrigation: Non Drought Stress (NDS), T2: 70% FC irrigation: Medium Drought Stress (MDS), T3: 40% FC irrigation: High Drought Stress (HDS), T4: 100 ppm silicon (Si), T5: Si 100 ppm + MDS, T6: Si 100 ppm + HDS, T7: Si 200 ppm, T8: Si 200 ppm + MDS, T9: Si 200 ppm + HDS, T10: Penicillium chrysogenum (PC) + NDS, T11: PC + MDS, T12: PC + HDS, T13: PC + Si 100 ppm, T14: PC + Si 100 ppm + MDS, T15: PC + Si 100 ppm + HDS, T16: PC + Si 200 ppm, T17: PC + Si 200 ppm + MDS, T18: PC + Si 200 ppm + HDS, T19: Serendipita indica (SI), T20: SI + MDS, T21: SI + HDS, T22: SI + Si 100 ppm, T23: SI + Si 100 ppm + MDS, T24: SI + Si 100 ppm + HDS, T25: Si + S 200 ppm, T26: SI + Si 200 ppm + MDS, T27: SI + Si 200 ppm + HDS
The uppermost numbers of leaf per plant including 10.67, 10.67, 10.33, and 10.33 number, respectively were counted in plants treated with T26, T27, T21, and T25, demonstrated positive effects of SI fungus × silicon levels on vegetative growth of saffron under non and drought stress condition (Fig. 5a), in addition the longest roots: 15.33, 14.07, 13.67, and 12.50 cm, respectively were observed in saffron plants treated with SI endophyte + 200 ppm silicon in the high drought stress condition (T27), SI endophyte + 200 ppm silicon in the medium drought stress condition (T26), SI + 100 ppm silicon and moderate drought stress (T23), and finally, SI + 100 ppm silicon (T22), which is illustrated that drought stress caused meaningfully enlargement in the root growth and synergetic effects of silicon and SI fungus on root development (Fig. 5a). The highest leaf dry weight 10.90 g was measured in plants treated with SI + 100 ppm silicon under high drought stress (T24), which not significantly vary with its values observed in the plants treated with SI endophyte fungus ± silicon under stressful or non-stress conditions (T19-T23 and T25-T27), illustrating the fundamental role of SI endophyte in vegetative growth expansion and water status improvement (Fig. 5a).
Fig. 5.
Leaf no per plant, leaf dry weight, and root length (a); root fresh and dry weight (b), relative water content (c); and picrocrocin and safranal content of saffron (d) (Duncan test, α = 0.05). T1: 100% FC irrigation: Non Drought Stress (NDS), T2: 70% FC irrigation: Medium Drought Stress (MDS), T3: 40% FC irrigation: High Drought Stress (HDS), T4: 100 ppm silicon (Si), T5: Si 100 ppm + MDS, T6: Si 100 ppm + HDS, T7: Si 200 ppm, T8: Si 200 ppm + MDS, T9: Si 200 ppm + HDS, T10: Penicillium chrysogenum (PC) + NDS, T11: PC + MDS, T12: PC + HDS, T13: PC + Si 100 ppm, T14: PC + Si 100 ppm + MDS, T15: PC + Si 100 ppm + HDS, T16: PC + Si 200 ppm, T17: PC + Si 200 ppm + MDS, T18: PC + Si 200 ppm + HDS, T19: Serendipita indica (SI), T20: SI + MDS, T21: SI + HDS, T22: SI + Si 100 ppm, T23: SI + Si 100 ppm + MDS, T24: SI + Si 100 ppm + HDS, T25: Si + S 200 ppm, T26: SI + Si 200 ppm + MDS, T27: SI + Si 200 ppm + HDS
The highest root fresh weight including 14.57, 14.33, 13.53, and 13.23 g and the highest root dry weight 2.91, 2.87, 2.71, and 2.65 g, respectively were observed in response to SI endophyte + 200 ppm silicon under medium drought condition (T26), SI + 200 ppm silicon under high drought condition (T27), SI + 200 ppm silicon in the non-stress condition (T25), and SI + 100 ppm silicon under high drought stress (T24), which demonstrated the positive role of drought stress, silicon, and endophytic fungus (SI) in the root development (Fig. 5b).
In order to relative water content, our observations designated that the RWC values were increased significantly with application of SI endophyte, regardless silicon treatments and drought condition, as these values in plants treated with T19-T27 classified in a one group (a) with rare change from 64.00 to 67.00 values (Fig. 5C), while these values significantly differ from those which observed under effect of other treatment, i.e. in plants treated with PC fungus. These observations confirmed the prominent modulator effect of SI fungus on plant water status too.
The chemical compounds of saffron including picrocrocin and safranal content (%) effectively were increased with application of SI fungus, so the highest amounts of these chemical were measured under application of SI + 100 ppm silicon + high drought stress, SI + 200 ppm silicon + non- drought stress, SI + 200 ppm silicon + medium drought stress, and finally SI + 200 ppm silicon + high drought stress. These peak values ranged from 12.37 to 12.83% for picrocrocin and 3.06–3.31% for safranal (Fig. 5d).
Overall, findings demonstrated that drought treatments had no significant influence on most growth variable, yield and chemicals of saffron plants. PC fungus, in some cases, effectively increased morpho-physiological, chemical, and performance attributes of saffron, however these improvements were less than those induced with application of SI endophytic fungus. Silicon levels in combination with endophytic fungi have notably increased the studied traits.
The general pattern of effects of different treatments, regardless of irrigation levels, on different traits (in terms of percentage increase or decrease compared to the control) is presented in Fig. 6. Diagram shows that, although the values of all studied traits significantly were enhanced by application of each two endophyte fungi, solely or in combination with silicon, nevertheless, SI fungus + 200 ppm silicon was the most effectiveness treatments, so that application of SI + 200 ppm silicon caused increases by 419.1, 29.2, 279.4, 286.5, 284.5, 55.4, 371.2, 316.9, 120.8, 163.9, 312.4, 177.6, 116.5, 116.5, 40.0, and 157.8%, respectively in the values of stigma fresh weight, stigma dry weight, saffron yield, stigma length, daughter corm number, daughter corm weight, leaf length, leaf number per plant, leaf dry weight, root length, root fresh weight, root dry weigh, picrocrocin and safranal (Fig. 6).
Fig. 6.
The general trend of changes (%) in the different variables caused by application of the treatments compared to control, regardless drought stress condition
Principal component analysis
Principal component analysis discovered 0.92 of variance between variables; the first component (Eigenvalue = 14.22) reveled 0.89 of variance, and the second component 0.03 of variance (Fig. 7). These results indicated the major positive correlation between first PC and variables. The all morpho-physiological, yield, physiological, and biochemical attributes showed the same trend and exhibited highly relationship to treatments contain SI endophyte fungus; subsequently the extreme values of all variables were obtained in the plants treated with SI fungus (T19-T27), regardless silicon treatments and drought condition. Of course, should be mentioned that, all studied variable exhibited significantly increases, in the plants treated with PC endophyte fungus (T10-T18) too, regardless application of silicon or drought conditions. Of course, this progresses less than those which treated with application of SI endophyte. Over-all, based on PCA results, the combination of SI + 100 ppm silicon + medium drought stress (T23), was the most effective treatment on morpho-physiological variables. However, the supreme values of yield component and picrocrocin were obtained in the T24-T27. In addition, the quality and quantity attributes exhibited a modest relationship to silicon application solely, while the application of silicon in combined with SI fungi had fairly significant effects on growth enhancement. The treatments T1-T9 (without endophyte fungi) displayed the inverse relationship with studied traits, so they had the largest distance with all variables, therefore the least values of all studied attributes were measured in plants treated with these treatments.
Fig. 7.
PCA graph of 2 first components for morpho-physiological, yield components, and biochemical variables of saffron plants under different endophyte fungi × silicon × drought stress treatments; T1: 100% FC irrigation: Non Drought Stress (NDS), T2: 70% FC irrigation: Medium Drought Stress (MDS), T3: 40% FC irrigation: High Drought Stress (HDS), T4: 100 ppm silicon (Si), T5: Si 100 ppm + MDS, T6: Si 100 ppm + HDS, T7: Si 200 ppm, T8: Si 200 ppm + MDS, T9: Si 200 ppm + HDS, T10: Penicillium chrysogenum (PC) + NDS, T11: PC + MDS, T12: PC + HDS, T13: PC + Si 100 ppm, T14: PC + Si 100 ppm + MDS, T15: PC + Si 100 ppm + HDS, T16: PC + Si 200 ppm, T17: PC + Si 200 ppm + MDS, T18: PC + Si 200 ppm + HDS, T19: Serendipita indica (SI), T20: SI + MDS, T21: SI + HDS, T22: SI + Si 100 ppm, T23: SI + Si 100 ppm + MDS, T24: SI + Si 100 ppm + HDS, T25: Si + S 200 ppm, T26: SI + Si 200 ppm + MDS, T27: SI + Si 200 ppm + HDS. NF: Number of Flower (n/m2), SFW: Stigma Fresh Weight (g), SDW: Stigma Dry Weight (g), SAFY: Saffron Yield (kg/ha), SL: Stigma Length (cm), DCN: Daughter Corm Number (n), DCW: Daughter Corm Weight (g), LF: Leaf length (cm), LN/p: Leaf No/plant (n), LDW: Leaf Dry Weight(g), RL: Root Length (cm), RFW: Root Fresh Weight (g), RDW: Root Dry Weight (g), RWC: Relative water Content, PIC: Picrocrocin (%), and SAF: Safranal (%)
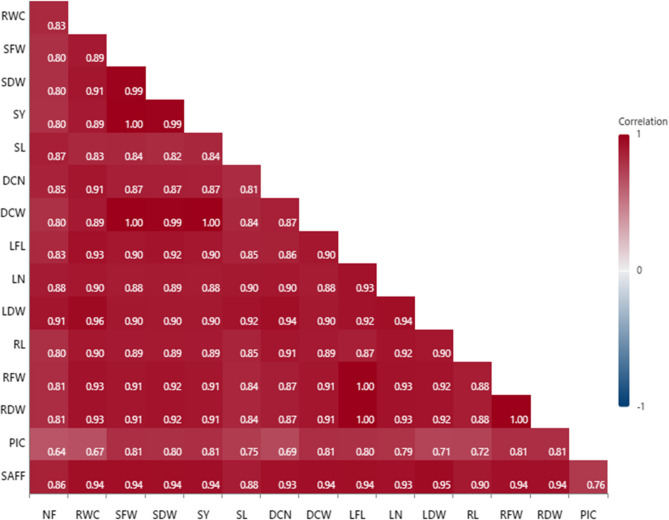
Person’s correlation Estimation
The person’s correlation estimation (Fig. 8) discovered strong positive correlations between saffron yield, yield components, morpho-physiological attributes, and safranal content (r ≥ 0.80**), additionally, significant positive correlations between picrocrocin and other studied traits (0.64*≤ r ≤ 0.81**). These strong correlations help breeders to enhancement quality and quantity of stigma yield.
Fig. 8.
correlogram of Pearson’s correlation for different studied traits of saffron plants under effects of endophyte fungi × silicon × drought stress. NF: Number of Flower (n/m2), SFW: Stigma Fresh Weight (g), SDW: Stigma Dry Weight (g), SAFY: Saffron Yield (kg/ha), SL: Stigma Length (cm), DCN: Daughter Corm Number (n), DCW: Daughter Corm Weight (g), LFL: Leaf Length (cm), LN/p: Leaf No/plant (n), LDW: Leaf Dry Weight(g), RL: Root Length (cm), RFW: Root Fresh Weight (g), RDW: Root Dry Weight (g), RWC: Relative water Content, PIC: picrocrocin, SAFF: safranal
Result of regression
The results of the stepwise regression analysis discovered positive significant regressions between saffron yield as dependent variable and number of flowers, saffron fresh weight, stigma length, leaf length, leaf dry weight, and root dry weight at the level of 1% (Table 1). Subsequently, linear regression analysis was performed between these traits and saffron yield and the corresponding regression plots were generated (Fig. 9). The linear regression analysis between numbers of flowers (predictor) versus saffron yield indicated a positive linear relationship. The adjusted coefficient of determination (R² adj) for this regression was 64.8%, and the fitted linear equation was: Y = 1.098 + 0.0.034 X (Fig. 9a). As showed in Fig. 9b, the linear regression between saffron fresh weight (predictor) and saffron yield was positive, indicating that increasing saffron fresh weight directly leads to increasing saffron yield. The adjusted R² for this model was 100%, and the fitted linear equation was Y = −0.0412 + 0.2842 X. According to Fig. 9c, there was a positive linear relationship between the stigma length as independent variable and saffron yield as dependent variable, indicating that longer stigma production caused higher saffron yield. The adjusted R² for this regression was 0.68%, and the fitted linear equation was: Y = −5.082 + 3.121 X. The fitted linear equation for positive regression between leaf length (independent variable) and saffron yield was Y = −2.50 + 0.72 X (Fig. 9d). The adjusted coefficient of determination for this regression was 80%. Figure 9e presented a positive regression between saffron yield and leaf dry weight (R2 = 0.79.8%, Y = 0.25 + 0.57 X), demonstrating with improvement in leaf fresh weight, saffron yield was increased. Finally, the linear regression between root dry weight and saffron yield (Fig. 9f) showed a direct positive relationship, suggesting that higher root dry weight is associated with higher saffron yield (R² = 81.5% and Y = −2.73 + 3.53 X).
Table 1.
Results of ANOVA for regression between saffron yields (independent variable) and yield components (predictors) under effects of endophyte fungi × silicon × drought stress
| Source | df | Saffron yield - number of flowers | Saffron yield - saffron fresh weight | Saffron yield - stigma length |
|---|---|---|---|---|
| MS | MS | MS | ||
| Regression | 1 | 63.38** | 100.10** | 69.89** |
| Error | 25 | 1.47 | 0.001 | 1.24 |
| Source | Saffron yield - leaf length | Saffron yield - leaf dry weight | Saffron yield - root dry weight | |
| Source | df | MS | MS | MS |
| Regression | 1 | 81.58** | 80.72** | 82.31** |
| Error | 14 | 0.75 | 0.78 | 0.71 |
**Significant at the 1% level
Fig. 9.
linear regression model between saffron yield as independent variable and predictors: number of flowers (a), saffron fresh weight (b), stigma length (c), leaf length (d), leaf dry weight (e), and root dry weight (f) in response to endophyte fungi × silicon × drought stress
Discussion
Saffron is a drought resistance plant with low water requirement. We found that the most morpho-physiological, biochemical, and yield attributes did not influence by drought. However, drought caused significant enhancements in the root length and root dry weight. In a study, the role of drought stress in significantly increasing the activity of saffron fibrous roots has been proven [60]. In line with our results, an increase has observed in root growth (length) in response to drought stress. Mzabri et al. (2017) fund that drought stress even at severe level (40% FC irrigation) non-significantly decrease the chlorophyll content, proline content, soluble sugars, total phenols, RWC, Malondialdehyde (MAD), and stigmas yield [61]. Some authors have reported different results. For example: In a study, the saffron secondary metabolites (crocin, picrocrocin and safranal) significantly has increased under irrigation of 50% field capacity, and conversely, the dry weight of daughter corms decreased [62]. In research conducted by Gusain and Joshi (2025), growth and leaf water content were decreased under drought stress condition [63]. Polyethylene glycol 30%, w/v has reduced various growth parameters including shoot fresh and dry weight, shoot height, stigma yield, and relative leaf water content [64]. Sevindik (2024) has reported significant reduction in plant dry weight, plant fresh weight, corm diameter, daughter corm number, and flower number in saffron plants treated PEG 6000 [65].
In a research, saffron petal dry weights have significantly decreased under 50% FC irrigation compared to 70% FC yield [66], however the amounts of picrocrocin improved by 5.6% with decreasing irrigation water from 70% to 50% FC [66]. These findings are not consistent with our results. The reason for these different and sometimes contradictory results may be different ecological conditions of the experiments, including soil type and texture, regional temperature, altitude above sea level, etc., other than irrigation.
Data analysis showed that application of PC or especially SI endophytes, solely or in combination with silicon caused notable enhancement in the all studied variable (Fig. 6) including number of flowers per m2, stigma fresh weight, stigma dry weight, saffron yield, stigma length, the number of daughter corm, daughter corm weight, leaf length, leaf number per plant, leaf dry weight, root length, root fresh weight, root dry weight, relative water content, picrocrocin content, and safranal percent. So far, in order to Crocus sativus, application of endophyte fungi did not study, however the beneficial effect of other plant growth promotor such rhizobacteria and plant hormones to improvement growth in saffron plants has provand. For example: The application of rhizobacteria Rahnella aquatilis and Variovorax paradoxus isolated from the saffron rhizosphere affects positively different saffron plant parameters, such as the number of leaves and chlorophyll content. In addition, these PGRs has significantly improved daughter corms production and also the concentrations of crocin, picrocrocin, and safaranal [67]. In a previous study, Heidari et al. (2022) illustrated that application of GA3 500 µM upsurges flower fresh weight and stigma dry weight of Crocus sativus, while SA increases numbers of daughter corms, leaf dry weight, and leaf area index [68]. Priming of saffron corm with IAA has increased petal and stigma dry weights, dry weight of daughter corms, and saffron yield under 70% FC irrigation [66]. Application of plant growth regulators, including gibberellic acid (GA3) and γ-aminobutyric acid (GABA) under different light recipes has improved saffron quality and quantity, so that exogenous GABA application and combined red and blue LED lights enhanced the number of flowers as well as the fresh and dry weight of flowers and stigmas in the indoor-cultivated saffron. Furthermore, GABA increased safranal content up to 5.03% and picrocrocin up to 15.8%, while GA3 induced crocin up to 25.1% [69].
The beneficial effects of endophytic fungi on increasing growth and development, resistance to adverse environmental conditions, and promotion of physiological and biochemical processes have been proven in many plants [18, 19, 30, 33, 70]. S. indica improves plant health through adjustment of soil pH, releasing acid phosphatase and nutrient solubilization, increase uptake and transport of nutrients such N, P, Mg and S [12, 71–73]. was involved in the acquisition of root P rather than the subsequent transfer of P from the roots to leaves [74]. The results of a study showed that inoculation with S. indica suppressed root CoPht1;1, CoPht1;2, CoPht1;3, and CoPht1;4 expression and increased leaf CoPht1;1, CoPht1;2, and CoPht1;3 expression [26].
In oilseed crop (Camellia oleifera) under field conditions, Inoculation with S. indica significantly increased the plant height (46.81%), net photosynthetic rate (69.16%), nitrogen balance index (14.44%), chlorophyll index (21.08%), and mineral content of leaf [38]. S. indica colonization stimulates nutrient uptake and efficiency, leading to amended plant quality and yield [75]. This fungus endorses the plant growth and development i.e., height, leaf number, shoot and root growth, fresh weight, and dry weight [23, 27]. In addition, it significantly has increased yield and yield components of wheat plants under light deficiency [76]. In wheat plants under arsenic stress, application of S. indica has developed the leaf palisade and spongy tissues, resulting improved CO2 uptake and endorsing photosynthesis [70]. It improved morpho-physiological characteristics as well as photosynthetic pigment and phytohormone synthesis in crops, such Sesamum indicum, zea mize, Juglans regia L., and rice [77–80].
In addition to improving the growth and production of crops, S. indica has improved the quality of the products. This impact may be attributable to increased root growth and root hair formation in plants treated with S. indica, which results in improved mineral nutrition acquisition and water uptake. Inoculation with S. indica significantly increased the net photosynthetic rate, Nbi, and Chl in the leaves of Camellia oleifera plants by 69.16%, 14.44%, and 21.08%, respectively, while significantly decreasing the intercellular CO2 concentration by 17.39% compared with the control [38]. In the Carthamus tinctorius L. (safflower) cv. Sina cultivated in soil contaminated with different levels of Pb and cadmium, application of S. indica caused greater growth parameters, photosynthetic pigments, chlorophyll fluorescence, and antioxidant enzymes activities of CAT, APX and SOD [81, 82].
Our results and the evidence from other authors mentioned above confirmed the extraordinary role of this endophytic fungus in promoting plant biological processes, increasing plant production, and plant tolerance to environmental stress conditions.
Using silicon in agricultural systems helps to achieve optimal crop harvest while reducing water consumption [83]. Application of silicon can improve plant growth under a water deficit [84] and alleviate drought stress of plants by regulating physiological and biochemical processes [52]. Silicon aids maintain higher relative water content, upsurges total phenolics, boosts antioxidant activity, and improves potassium and nitrogen uptake, while dipping sodium accumulation in both roots and leaves. This leads to better cell membrane stability and overall plant health under abiotic stress [85–87]. As evidences for this claim, in soybean plants treated with silicon, the activity of the enzymes nitrate reductase (NR) and glutamine synthetase (GS), two enzymes involved in nitrogen metabolism, increased by 25 to 30% [88]. Additionally, superoxide dismutase activity has increased by 15% in plants treated with n-Sio2 [88]. Applying Si to the leaf surface of Glycyrrhiza uralensis and Glycyrrhiza inflata under salt stress conditions has promoted water transportation, enhanced the relative leaf water content, and reduced the decomposition of photosynthetic pigments [89].
The main effect of silicon and its interaction with endophytes fungi significantly improved the most morpho-physiological phenomenon, biochemical and yield characteristics of saffron. Previous studies proven silicon improves plant–water relations, regulates nutrient and root water uptake, and increases photosynthesis and antioxidant activity, through phytohormones biosynthesis and activity [50–53]. There are a lot of evidences for the confident role of silicon in increasing plant growth and development, some of which are mentioned below. Application of Si in Capsicum annuum L. plants developed physiological and biochemical process, and significantly improved the root length (17% and 24%), root biomass (23% and 27%), and carotenoids (16% and 19%) compared to control [90]. Maize seedlings, treated with combined Si and putrescine foliar spray, showed a significant increase of 22.70% in ear length, 12.77% in number of grains per ear, 18.30% in 100-grain weight, and 10.29% in grain yield under drought stress conditions compared to the control [91]. Application of Si-based biostimulants enhanced above-ground biomass and tuber weight in Potato compared to control. The total tuber yield was higher, on average, by 10% to 13% and the marketable tuber yield by 11% to 15% [52]. Furthermore, 3.0 mM silicon significantly has increased grain yield of Eragrostis tef (Zucc.) Trotter by 100% and aboveground biomass yield by 45% [92]. Finally, we clarified the extremely positive correlations and positive regressions between saffron yield and morpho-physiological traits, which provide a strong basis for improvement the quantity and quality of saffron yield using agronomic and breeding techniques.
Conclusion
Regard of highlight findings in this study, we recommend the application of Serendipita indica (SI) and Penicillium chrysogenum (PC) as effective plant growth promoters, to enhancing growth and yield of saffron under non or drought stress in combination with silicon or without. Especially, we endorse a combination of Serendipita indica + 200 ppm silicon as an eco-friendly fertilization strategy, in order to enhancement saffron quantity and quality under different irrigation strategy. this study supplies a strategy for sustainable saffron production, and future research should validate these findings under diverse agro-climatic conditions and explore their long-term economic feasibility. In conclusion, we suggest that further studies are needed in the future to optimize the levels of SI fungus in combination with chemical fertilizers and/or different bio-fertilizers to achieving the further crop quantity and quality in saffron cultivation.
Acknowledgments
Authorship contribution statement
The authors conducted the experiments in collaboration and wrote the manuscript.
Authors’ contributions
SaharAA. Malik Al-Saadi, Conceptualization, Data curation, administration affairs. KarzanOmer Qader, Supervision, administration, writing original draft and Heidar Meftahizade, Conceptualization, Data curation, Formal analysis, Investigation and writing manuscript.
Funding
No specific financial credit was used in this experiment.
Data availability
All data generated during this study are included in the article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kazemi-Shahandashti S-S et al. Ancient artworks and crocus genetics both support saffron’s origin in early Greece. Front Plant Sci. 2022;13. 10.3389/fpls.2022.834416. [DOI] [PMC free article] [PubMed]
- 2.Saxena RB. Botany, taxonomy and cytology of crocus sativus series. Ayu. 2010;31(3):374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grand View Research. Saffron market size, share, trends & growth report. https://www.grandviewresearch.com/industry-analysis/saffron-market. 2024. (Accessed 25 April 2025), 2030.
- 4.Leone S, et al. Phytotherapic use of the crocus sativus L. (Saffron) and its potential applications: A brief overview. Phytother Res. 2018;32(12):2364–75. [DOI] [PubMed] [Google Scholar]
- 5.Siddique HR, Fatma H, Khan MA. Medicinal Properties of Saffron With Special Reference to Cancer—A Review of Preclinical Studies. 2020. 10.1016/B978-0-12-818462-2.00018-8.
- 6.Lachkar A, Amari K, Ben I, Atia. Phytochemical properties of local and introduced apricot cultivars grown under organic cultivation system in Tunisia. J Hortic Postharvest Res. 2022;5(Issue 4):349–62. [Google Scholar]
- 7.Yousefi M, Shafaghi K. Saffron in Persian traditional medicine. 2020;393–404. 10.1016/B978-0-12-818638-1.00025-3.
- 8.Khorasany AR, Hosseinzadeh H. Therapeutic effects of saffron (Crocus sativus L.) in digestive disorders: a review. Iran J Basic Med Sci. 2016;19(5):455–69. [PMC free article] [PubMed] [Google Scholar]
- 9.Lachguer K, et al. Saffron (Crocus sativus L.) cultivation and properties: A review. Int J Hortic Sci Technol. 2025;12(2):627–46. [Google Scholar]
- 10.Mahdieh Kheirabadi M, Azizi, Seyedeh Faezeh Taghizadeh and Yoshiharu Fujii. Recent advances in saffron soil remediation: Activated carbon and zeolites effects on allelopathic potential. 2020;9:1714. 10.3390/plants9121714 [DOI] [PMC free article] [PubMed]
- 11.Khan AA et al. Mechanistic insights and future perspectives of drought stress management in staple crops. 2025;16. 10.3389/fpls.2025.1547452. [DOI] [PMC free article] [PubMed]
- 12.Shekhawat PK, et al. Serendipita indica: Harnessing its versatile potential for food and nutritional security. Physiol Mol Plant Pathol. 2021;116:101708. [Google Scholar]
- 13.Kumar S, Sindhu SS. Drought stress mitigation through bioengineering of microbes and crop varieties for sustainable agriculture and food security. Curr Res Microb Sci. 2024;7:100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali S et al. Exploring physiological and molecular dynamics of drought stress responses in plants: challenges and future directions. 2025;16. 10.3389/fpls.2025.1565635. [DOI] [PMC free article] [PubMed]
- 15.Dirie KA, Maamor S, Alam MM. Impacts of climate change in post-conflict somalia: is the 2030 agenda for SDGs endangered? World Development Perspectives. 2024. 10.1016/j.wdp.2024.100598.
- 16.Shahrajabian MH, et al. Sustainable agriculture systems in vegetable production using Chitin and Chitosan as plant biostimulants. Biomolecules. 2021;11(6):819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahrajabian MH, et al. Biostimulants application: A low input cropping management tool for sustainable farming of vegetables. Biomolecules. 2021;11(5):698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahrajabian MH, Cheng Q, Sun W. Using bacteria and fungi as plant biostimulants for sustainable agricultural production systems. Recent Patents Biotechnol. 2023;17(3):206–44. [DOI] [PubMed] [Google Scholar]
- 19.Sun W, Shahrajabian MH, Guan L. The Biocontrol and Growth-Promoting Potential of Penicillium spp. and Trichoderma spp. in Sustainable Agriculture. 2025;14(13). 10.3390/plants14132007. [DOI] [PMC free article] [PubMed]
- 20.Sun W, Shahrajabian MH. The effectiveness of rhizobium bacteria on soil fertility and sustainable crop production under cover and catch crops management and green manuring. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2022;50(2):12560. 10.15835/nbha50212560.
- 21.Chabbi N et al. Plant-Growth-Promoting rhizobacteria improve seeds germination and growth of Argania spinosa. Plants (Basel). 2024;13(15). [DOI] [PMC free article] [PubMed]
- 22.Al Raish SM, Sourani OM, Abu-Elsaoud AM. Plant Growth-Promoting microorganisms as biocontrol agents: Mechanisms, Challenges, and future prospects. Appl Microbiol. 2025;5. 10.3390/applmicrobiol5020044.
- 23.Li L, et al. Phytoremediation effect of medicago sativa colonized by Piriformospora indica in the phenanthrene and cadmium co-contaminated soil. BMC Biotechnol. 2020;20(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouzouina M, Kouadria R, Lotmani B. Fungal endophytes alleviate salt stress in wheat in terms of growth, ion homeostasis and osmoregulation. J Appl Microbiol. 2021;130(3):913–25. [DOI] [PubMed] [Google Scholar]
- 25.Bandyopadhyay P et al. Piriformospora indica and azotobacter Chroococcum consortium facilitates higher acquisition of N, P with improved carbon allocation and enhanced plant growth in Oryza sativa. J Fungi (Basel). 2022;8(5). 10.3390/jof8050453. [DOI] [PMC free article] [PubMed]
- 26.Cao M-A, et al. Symbiotic fungi alter the acquisition of phosphorus in camellia Oleifera through regulating root Architecture, plant phosphate transporter gene expressions and soil phosphatase activities. J Fungi. 2022;8. 10.3390/jof8080800. [DOI] [PMC free article] [PubMed]
- 27.Saleem S, Sekara A, Pokluda R. Serendipita indica-A review from agricultural point of view. Plants (Basel). 2022;11(24). 10.3390/plants11243417. [DOI] [PMC free article] [PubMed]
- 28.Varma A, et al. Piriformospora indica: A novel plant Growth-Promoting mycorrhizal fungus. Agricultural Res. 2012;1(2):117–31. [Google Scholar]
- 29.Sharma N et al. Interaction studies of serendipita indica and Zhihengliuella sp. ISTPL4 and their synergistic role in growth promotion in rice. Front Plant Sci. 2023;14. 10.3389/fpls.2023.1155715. [DOI] [PMC free article] [PubMed]
- 30.Embacher J et al. Serpula lacrymans reacts with a general, unspecialized chemical response during interaction with mycoparasitic trichoderma spp. And bacteria. 2023;63:101230. 10.1016/j.funeco.2023.101230.
- 31.Shahrajabian MH, Cheng Q, Sun W. The effects of amino acids, phenols and protein hydrolysates as biostimulants on sustainable crop production and alleviated stress. Recent Patents Biotechnol. 2022;16(4):319–28. [DOI] [PubMed] [Google Scholar]
- 32.Mattos Silva Galeano R, et al. Penicillium chrysogenum strain 34-P promotes plant growth and improves initial development of maize under saline conditions. Rhizosphere. 2023;26:100710. [Google Scholar]
- 33.Xu G, et al. Colonization of Piriformospora indica enhances rice resistance against the brown planthopper Nilaparvata lugens. Pest Manag Sci. 2024;80(9):4386–98. [DOI] [PubMed] [Google Scholar]
- 34.Kundu A, Vadassery J. Molecular mechanisms of Piriformospora indica mediated growth promotion in plants. Plant Signal Behav. 2022;17(1):2096785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu W-J, et al. Rhizoglomus intraradices is more prominent in improving soil aggregate distribution and stability than in improving plant physiological activities. Agronomy. 2023;13. 10.3390/agronomy13051427.
- 36.Cao Y, et al. Functional analysis of the phosphate transporter gene MtPT6 from medicago truncatula. Front Plant Sci. 2020;11:620377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balasubramaniam T, et al. Plants’ response mechanisms to salinity stress. Plants. 2023;12(12):2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu W-L, et al. Serendipita indica: A promising biostimulant for improving Growth, nutrient Uptake, and sugar accumulation in camellia Oleifera. Horticulturae. 2024;10. 10.3390/horticulturae10090936.
- 39.Zulfiqar F, et al. Biostimulants: A sufficiently effective tool for sustainable agriculture in the era of climate change? Plant Physiol Biochem. 2024;211:108699. [DOI] [PubMed] [Google Scholar]
- 40.Mittal U, et al. Role of beneficial elements in developing resilience to abiotic and biotic stresses in plants: present status and future prospects. J Plant Growth Regul. 2022;42:1–25. [Google Scholar]
- 41.Nunes da Silva M, et al. Non-Essential elements and their role in sustainable agriculture. Agronomy. 2022;12. 10.3390/agronomy12040888.
- 42.Sarraf M et al. Understanding the role of beneficial elements in developing plant stress resilience: Signalling and crosstalk with phytohormones and microbes. Plant Stress. 2023: p. 100224. 10.1016/j.stress.2023.100224.
- 43.Singhal R et al. Beneficial elements: new players in improving nutrient use efficiency and abiotic stress tolerance. Plant Growth Regul, 2022. 100. 10.1007/s10725-022-00843-8.
- 44.Zargar SM, et al. Role of silicon in plant stress tolerance: opportunities to achieve a sustainable cropping system. 3 Biotech. 2019;9(3):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hassan KM, et al. Silicon: A powerful aid for medicinal and aromatic plants against abiotic and biotic stresses for sustainable agriculture. Horticulturae. 2024;10. 10.3390/horticulturae10080806.
- 46.Pereira S, et al. Silicon, an emergent strategy to lighten the effects of (A)Biotic stresses on crops: A review. J Agron Crop Sci. 2024;210(6):e12762. [Google Scholar]
- 47.Thakral V, et al. Silicon, a quasi-essential element: availability in soil, fertilizer regime, optimum dosage, and uptake in plants. Plant Physiol Biochem. 2024;208:108459. [DOI] [PubMed] [Google Scholar]
- 48.Wang M, et al. Functions of silicon in plant drought stress responses. Hortic Res. 2021;8(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siddiqi KS, et al. Harnessing silicon nanoparticles and various forms of silicon for enhanced plant growth performance under salinity stress: application and mechanism. Discover Nano. 2025;20(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahire ML, et al. Multifaceted roles of silicon in mitigating environmental stresses in plants. Plant Physiol Biochem. 2021;169:291–310. [DOI] [PubMed] [Google Scholar]
- 51.Kovács S, Kutasy E, Csajbók J. The multiple role of silicon nutrition in alleviating environmental stresses in sustainable crop production. Plants. 2022;11. 10.3390/plants11091223. [DOI] [PMC free article] [PubMed]
- 52.Wadas W, Kondraciuk T. The role of Foliar-Applied silicon in improving the growth and productivity of early potatoes. Agriculture. 2025;15. 10.3390/agriculture15050556.
- 53.Abdullah MM, et al. Improving soybean drought tolerance via silicon-induced changes in growth, physiological, biochemical, and root characteristics. Plant Signal Behav. 2025;20(1):2465232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fahimi Jamila Bouzoubaâ, Zakia A, Fouad S, Nabil MR. Effect of silicon application on talioune crocus sativus L. cultivation under salt stress. Int J Res -GRANTHAALAYAH. 2018;6(9):291–300. [Google Scholar]
- 55.Huertas V, Jiménez A, Diánez F, Chelhaoui R, Santos M. Importance of dark septate endophytes in agriculture in the face of climate change. J Fungi. 2024;10:329. 10.3390/jof10050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mollafilabi A. Experimental findings of production and echophysiological aspects of saffron (Crocus sativus L). Acta Hort. 2004;650:195–200. [Google Scholar]
- 57. Saeidirad, Mohammad Hossein, Parvin Sharayei, and Saeed Zarifneshat. "Effect of Drying Temperature, Air Velocity and Flower Types on Dried Saffron Flower Quality." Agricultural Engineering International: CIGR Journal. 2014;16(4):251–254.
- 58.Turner NC. Techniques and experimental approaches for the measurement of plant water status. Plant Soil. 1981;58(1):339–66. [Google Scholar]
- 59.Ahmad Ahmadian Y, Esmaeilian A, Tavassoli. Jesús Fernández-Gálvez, Andrés Caballero-Calvo,Application of a superabsorbent hydrogel for improving water productivity and quality of saffron (Crocus sativus L.) under water deficit conditions. Sci Hort. 2024;336:113411. [Google Scholar]
- 60.Nasiri M, et al. Pivotal role of fibrous roots in drought tolerance of saffron (Crocus sativus L.) and mitigation of oxidative stress by penconazole. Sci Rep. 2025;15(1):19324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mzabri B et al. Effect of drought stress on the growth and development of saffron (Crocus Sativus. L) in Eastern Morocco. Atlas J Biology, 2017: pp. 364–70. 10.5147/ajb.2017.0150.
- 62.Ziaei SM, et al. Reduction of drought stress effects on saffron (Crocus sativus L.) using phytohormones. J Med Plants By-products. 2025;14(1):54–62. [Google Scholar]
- 63.Gusain S, Joshi R. Morphological, Physiological, and transcriptional changes in crocus sativus L. Under in vitro polyethylene Glycol-Induced water stress. Biology (Basel). 2025;14(1). 10.3390/biology14010078. [DOI] [PMC free article] [PubMed]
- 64.Gavyar PHH, et al. Exogenous application of serotonin, with the modulation of redox homeostasis and photosynthetic characteristics, enhances the drought resistance of the saffron plant. Sci Rep. 2024;14(1):23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sevindik B. Elucidation of volatile and morphological properties of saffron (Crocus sativus) flower as affected by controlled drought stress induced by polyethylene glycol. Flavour Fragr J. 2024;40. 10.1002/ffj.3830.
- 66.Ziaei SM, et al. Yield and quality of saffron (Crocus sativus L.) in response to priming treatments and water deficit. Italian J Agron. 2024;19(3):100020. [Google Scholar]
- 67.Chamkhi I, Sbabou L, Aurag J. Improved growth and quality of saffron (Crocus sativus L.) in the field conditions through inoculation with selected native plant growth-promoting rhizobacteria (PGPR). Ind Crops Prod. 2023;197:116606. [Google Scholar]
- 68.Heidari F, et al. Comparative effects of four plant growth regulators on yield and field performance of crocus sativus L. Horticulturae. 2022;8(9):799. [Google Scholar]
- 69.Eftekhari M, et al. Alteration of flower yield and phytochemical compounds of saffron (Crocus sativus L.) by application of different light qualities and growth regulators. Horticulturae. 2023;9(2):169.https://doi.org/10.3390/horticulturae9020169 [Google Scholar]
- 70.Abdolmaleki AK et al. Endophytic fungi improve growth and yield of wheat (Triticum aestivum L.) under limited light conditions. 2023;75(5):1517–29. 10.1007/s10343-022-00816-x.
- 71.Liang S-M, et al. Root-associated symbiotic fungi enhance waterlogging tolerance of Peach seedlings by increasing flavonoids and activities and gene expression of antioxidant enzymes. Chemical and Biological Technologies in Agriculture. 2023;10(1):124.
- 72.Rahman SU, et al. Piriformospora indica alter root-associated Microbiome structure to enhance Artemisia annua L. tolerance to arsenic. J Hazard Mater. 2023;457:131752. [DOI] [PubMed] [Google Scholar]
- 73.Singh M, Sharma J, Giri B. Augmentative role of arbuscular mycorrhizal Fungi, Piriformospora indica, and plant Growth-Promoting bacteria in mitigating salinity stress in maize (Zea Mays L). J Plant Growth Regul. 2023;43:1–21. [Google Scholar]
- 74.Yang L, et al. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci Hort. 2021;277:109815. [Google Scholar]
- 75.Su ZZ, et al. Piriformospora indica promotes growth, seed yield and quality of brassica Napus L. Microbiol Res. 2017;199:29–39. [DOI] [PubMed] [Google Scholar]
- 76.Karimi A, et al. Endophytic fungi improve growth and yield of wheat (Triticum aestivum L.) under limited light conditions. Gesunde Pflanzen. 2022;75:1–13. [Google Scholar]
- 77.Zhang W et al. Eendophytic fungus Piriformospora indica promotes growth and confers drought tolerance in Sesame (Sesamum indicum L). Chin J Oil Crop Sci. 2014;1(11). 10.7505/j.issn.1007-9084.2014.01.011.
- 78.Xu L, et al. Piriformospora indica confers drought tolerance on Zea Mays L. through enhancement of antioxidant activity and expression of drought-related genes. Crop J. 2017;5(3):251–8. [Google Scholar]
- 79.Liu B, et al. Serendipita indica alleviates drought stress responses in walnut (Juglans regia L.) seedlings by stimulating osmotic adjustment and antioxidant defense system. Appl Microbiol Biotechnol. 2021;105(23):8951–68. [DOI] [PubMed] [Google Scholar]
- 80.Tsai H-J, et al. Piriformospora indica symbiosis improves water stress tolerance of rice through regulating stomata behavior and ROS scavenging systems. Plant Signal Behav. 2020;15(2):1722447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shahabivand S, Tayebnia V, Aliloo AA. Impact of endophyte fungus serendipita indica on fungus-assisted phyto-stabilization and performance of Carthamus tinctorius in a lead polluted soil. J Plant Res (Iranian J Biology). 2019;31(4):838–51. [Google Scholar]
- 82.Shahabivand S, Parvaneh A, Aliloo AA. Root endophytic fungus Piriformospora indica affected growth, cadmium partitioning and chlorophyll fluorescence of sunflower under cadmium toxicity. Ecotoxicol Environ Saf. 2017;145:496–502. [DOI] [PubMed] [Google Scholar]
- 83.Verma KK et al. Unlocking the role of silicon against biotic stress in plants. Front Plant Sci. 2024;15. 10.3389/fpls.2024.1430804. [DOI] [PMC free article] [PubMed]
- 84.Wadas W. Potato (Solanum tuberosum L.) growth in response to foliar silicon application. Agronomy. 2021;11(12):2423. [Google Scholar]
- 85.Jamila A, et al. EFfect of silicon application ontaliouine crocus sativus (l) cultivation undersalt stresS. International Journal of Research -Granthaalayah. 2018. 10.5281/zenodo.1443451.
- 86.Fahimi J et al. Effect of silicon on improving salinity tolerance of Taliouine Crocus sativus L. Silicon. 2017:219–228. 10.17660/ActaHortic.2017.1184.31.
- 87.Khoshpeyk S, Haghighi S, Ahmadian A. The effect of irrigation water quality and application of Silicon, nano Silicon, and super absorbent polymer on some physiological responses of leaves and saffron yield. Silicon. 2022;15:2953–61. [Google Scholar]
- 88.Wei J et al. Silicon Nano-Fertilizer-Enhanced soybean resilience and yield under drought stress. Plants (Basel), 2025. 14(5). 10.3390/plants14050751. [DOI] [PMC free article] [PubMed]
- 89.Shen Z, et al. Effects of silicon application on leaf structure And physiological characteristics of glycyrrhiza uralensis Fisch. And glycyrrhiza inflata Bat. Under salt treatment. BMC Plant Biol. 2022;22(1):390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ansari WA et al. Silicon (Si) foliar treatment modulates capsicum annuum L. (green chilli) growth and stress responses under cadmium and lead stress. Frontires Plant Sci. 2025;16. 10.3389/fpls.2025.1590148. [DOI] [PMC free article] [PubMed]
- 91.El-Beltagi SH, et al. Sole and combined foliar application of silicon and Putrescine alleviates the negative effects of drought stress in maize by modulating the morpho-physiological and antioxidant defence mechanisms. Plant Soil Environ. 2024;70(1):26–39. [Google Scholar]
- 92.Ligaba-Osena A, et al. Silicon enhances biomass and grain yield in an ancient crop tef [Eragrostis tef (Zucc.) Trotter]. Front Plant Sci. 2020;11:608503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated during this study are included in the article.