Abstract
Smac/DIABLO is a mitochondrial protein that potentiates some forms of apoptosis, possibly by neutralizing one or more members of the IAP family of apoptosis inhibitory proteins. Smac has been shown to exit mitochondria and enter the cytosol during apoptosis triggered by UV- or γ-irradiation. Here, we report that Smac/DIABLO export from mitochondria into the cytosol is provoked by cytotoxic drugs and DNA damage, as well as by ligation of the CD95 death receptor. Mitochondrial efflux of Smac/DIABLO, in response to a variety of pro-apoptotic agents, was profoundly inhibited in Bcl-2-overexpressing cells. Thus, in addition to modulating apoptosis-associated mitochondrial cytochrome c release, Bcl-2 also regulates Smac release, suggesting that both molecules may escape via the same route. However, whereas cell stress-associated mitochondrial cytochrome c release was largely caspase independent, release of Smac/DIABLO in response to the same stimuli was blocked by a broad-spectrum caspase inhibitor. This suggests that apoptosis-associated cytochrome c and Smac/DIABLO release from mitochondria do not occur via the same mechanism. Rather, Smac/DIABLO efflux from mitochondria is a caspase-catalysed event that occurs downstream of cytochrome c release.
Keywords: apoptosis/caspases/cytochrome c/DIABLO/Smac
Introduction
Apoptosis is a cell-autonomous programmed cell death mechanism that is utilized extensively during development, tissue homeostasis and maintenance of the immune system in adult organisms (Jacobson et al., 1997). The morphological changes typical of apoptosis, characterized by cell shrinkage, plasma membrane blebbing, chromatin compaction and nuclear fragmentation, are orchestrated by the activity of a family of cysteine proteases called caspases (Kerr et al., 1972; Martin and Green, 1995; Earnshaw et al., 1999).
One important route to caspase activation involves the translocation of cytochrome c from the mitochondrial intermembrane space into the cytosol (Liu et al., 1996). Following release from mitochondria, cytochrome c promotes the assembly of a protein complex called the apoptosome, which includes caspase-9 bound to the CED-4 homologue Apaf-1 (Li et al., 1997; Zou et al., 1997; Adrain et al., 1999; Rodriguez and Lazebnick, 1999; Cain et al., 2000; Adrain and Martin, 2001). Upon activation, caspase-9 instigates a proteolytic cascade involving multiple caspases, which culminates in the cleavage of numerous substrate proteins and, ultimately, in macrophage engulfment of apoptotic corpses (Srinivasula et al., 1998; Earnshaw et al., 1999; Nicholson, 1999; Slee et al., 1999a,b, 2001; Savill and Fadok, 2000).
Precisely how the efflux of pro-apoptotic molecules from the mitochondrial intermembrane space occurs is not clear at present (for recent reviews, see Desagher and Martinou, 2000; Adrain and Martin, 2001; Martinou and Green, 2001; Zamzami and Kroemer, 2001). However, accumulating evidence suggests a model whereby the death-promoting ‘BH-3-only’ members of the Bcl-2 family, such as caspase-cleaved Bid (tBid), induce the homo-oligomerization of death promoting molecules Bax and/or Bak and their insertion into the outer mitochondrial membrane (Li et al., 1998; Gross et al., 1999; Desagher and Martinou, 2000; Wei et al., 2000; Antonsson et al., 2001; Martinou and Green, 2001; Wei et al., 2001). Several lines of evidence suggest that Bax or Bak oligomers may form cytochrome c-conducting channels within the mitochondrial outer membrane (Antonsson et al., 2000; Saito et al., 2000). Bcl-2, a key inhibitor of apoptosis, blocks cytochrome c release, possibly by preventing Bax/Bak oligomerization and consequent pore formation (Kluck et al., 1997; Yang et al., 1997; Mikhailov et al., 2001).
Recently, a novel mitochondria-derived pro-apoptotic molecule called Smac/DIABLO was identified (Du et al., 2000; Verhagen et al., 2000). Based largely upon in vitro studies, Smac/DIABLO appears to function by neutralizing the caspase-inhibitory properties of the IAP family of proteins, particularly XIAP (Roy et al., 1997; Deveraux et al., 1998; Du et al., 2000; Verhagen et al., 2000). Current data suggest a model whereby the ability of XIAP to repress active caspase-9 within the apoptosome complex is overcome by displacement of XIAP from caspase-9 by Smac/DIABLO (Ekert et al., 2001; Srinivasula et al., 2001).
Similar to cytochrome c, Smac/DIABLO is encoded by a nuclear gene and is subsequently imported into mitochondria (Stuart and Neupert, 1990; Du et al., 2000; Verhagen et al., 2000). During mitochondrial import, the N-terminus of Smac/DIABLO is removed by limited proteolysis to generate the mature form of the molecule (Chai et al., 2000; Du et al., 2000). Interestingly, mature Smac/DIABLO exists as a dimer, mediated by a hydrophobic interface within the N-termini of individual Smac/DIABLO molecules (Chai et al., 2000). Mutations that disrupt Smac/DIABLO dimer formation abrogate the XIAP-neutralizing ability of this molecule, suggesting that Smac/DIABLO dimerization is essential for its pro-apoptotic activity (Chai et al., 2000). Because the Smac/DIABLO dimer behaves as an ∼100 kDa molecule, whereas cytochrome c has a mol. wt of ∼12 kDa, an important question that remains unresolved is whether the channel responsible for mediating cytochrome c release is large enough to accommodate Smac/DIABLO (Chai et al., 2000; Du et al., 2000; Martinou and Green, 2001).
In this study, we generated a Smac/DIABLO-specific antibody to examine the efflux of endogenous Smac/DIABLO from mitochondria in response to diverse pro-apoptotic stimuli. Using a range of cytotoxic drugs/DNA-damaging stimuli, we show that mitochondrial Smac/DIABLO release is a general feature of apoptosis, and that Bcl-2 regulates this event. However, in sharp contrast to the behaviour of cytochrome c during stress-associated apoptosis, we find that Smac/DIABLO release from mitochondria is largely attenuated by caspase inhibition. Thus, Smac/DIABLO efflux from mitochondria is a caspase-catalysed event that occurs downstream of cytochrome c translocation.
Results
Generation and characterization of Smac/DIABLO-specific antisera
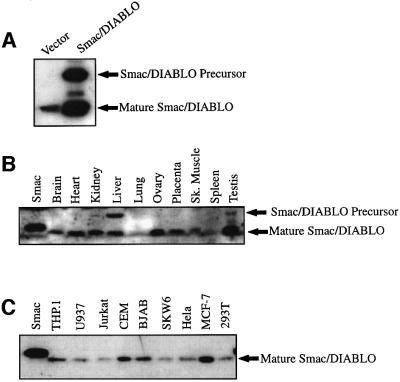
To explore Smac/DIABLO redistribution from mitochondria to the cytosol during apoptosis, we generated polyclonal antibodies against this protein by immunizing rabbits with bacterially expressed polyhistidine-tagged human Smac/DIABLO (Du et al., 2000). Following affinity purification, the specificity of the antiserum was confirmed by the detection of endogenous and overexpressed Smac/DIABLO in HEK 293T whole-cell lysates (Figure 1A).
Fig. 1. Tissue distribution of Smac/DIABLO. (A) Characterization of Smac/DIABLO antibody. Whole-cell extracts from 293T cells transfected with vector plasmid, or an expression plasmid encoding full-length human Smac/DIABLO were immunoblotted with purified anti-Smac/DIABLO antibodies. (B) One hundred micrograms of the indicated human tissue lysates were subjected to SDS–PAGE, followed by immunoblotting with Smac/DIABLO antibodies. The left-hand lane contains 25 ng of bacterially expressed Smac/DIABLO as a control. (C) Fifty micrograms of the indicated tumour cell lysates were subjected to SDS–PAGE and immunoblotted with Smac/DIABLO antibodies. The left-hand lane contains 25 ng of bacterially expressed Smac/DIABLO as a control.
We also examined Smac/DIABLO expression in a panel of adult human tissues and in tumour cell lysates (Figure 1B and C). Consistent with data relating to Smac/DIABLO mRNA distribution in adult human tissues (Du et al., 2000), mature Smac/DIABLO protein was detected in the majority of tissues examined (Figure 1B). Smac/DIABLO was also ubiquitously expressed in a panel of tumour cell lines, including those derived from monocytic, T lymphocytic and B lymphocytic lineages, and in epitheloid-like cells (Figure 1C).
Smac/DIABLO release from mitochondria is a general feature of apoptosis
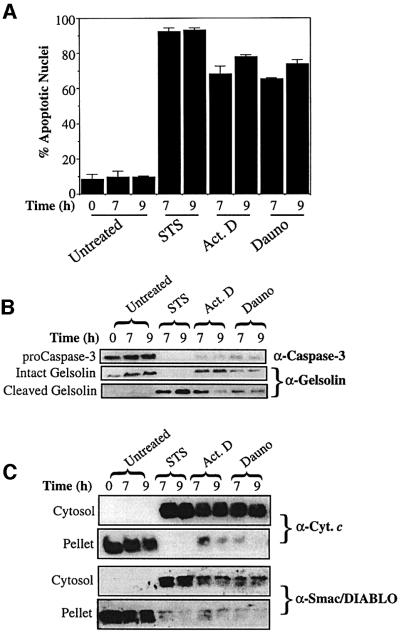
Previous studies have reported the translocation of Smac/DIABLO from mitochondria to the cytosol in cells stimulated to undergo apoptosis by UVB-irradiation, γ-irradiation or glucocorticoids (Du et al., 2000; Verhagen et al., 2000; Chauhan et al., 2001; Ekert et al., 2001). However, it is unclear whether Smac/DIABLO release from mitochondria, like that of cytochrome c, is a general feature of apoptosis (Liu et al., 1996; Kluck et al., 1997; Yang et al., 1997; Bossy-Wetzel et al., 1998; Li et al., 1998; Luo et al., 1998; Goldstein et al., 2000; Wei et al., 2000). To explore this, we treated Jurkat cells with a panel of pro-apoptotic agents established to provoke cytochrome c release and apoptosis (Figure 2). Cells treated with staurosporine, actinomycin D and daunorubicin readily exhibited features typical of apoptosis, including nuclear condensation/fragmentation, activation of caspase-3 and cleavage of the caspase-3 substrate gelsolin (Figure 2A and B).
Fig. 2. Smac/DIABLO release from mitochondria is a general feature of apoptosis. Jurkat cells, either untreated or treated with 250 nM staurosporine (STS), 10 µM actinomycin D (Act. D) or 10 µM daunorubicin (Dauno), were harvested at the indicated timepoints. (A) The percentage of cells exhibiting apoptotic features was determined as described in Materials and methods. (B) Cells (107) were subjected to a digitonin-based subcellular fractionation procedure as described in Materials and methods. Cytosolic fractions (∼50 µg per lane) were subjected to SDS–PAGE, followed by immunoblotting with the indicated antibodies. (C) Cytosolic and pellet fractions prepared from the same cells were subjected to SDS–PAGE, followed by probing with cytochrome c and Smac/DIABLO-specific antibodies, as indicated.
Using a digitonin-based subcellular fractionation procedure, we analysed cytosolic and mitochondrial fractions obtained from these cells for the presence of cytochrome c and Smac/DIABLO, respectively (Figure 2C). Whereas untreated cells exhibited negligible redistribution of cytochrome c from mitochondria into the cytosol, cytochrome c was present in the cytosols of dying cells, as expected (Figure 2C). Smac/DIABLO similarly accumulated in the cytosols of dying cells and was lost from the mitochondrial pellet fractions (Figure 2C). Thus, taken together with previous reports on Smac/DIABLO release in the context of UV- and γ-irradiation, redistribution of Smac/DIABLO appears to be a general feature of apoptosis.
Cytochrome c and Smac/DIABLO release occur with similar kinetics in death receptor-induced apoptosis
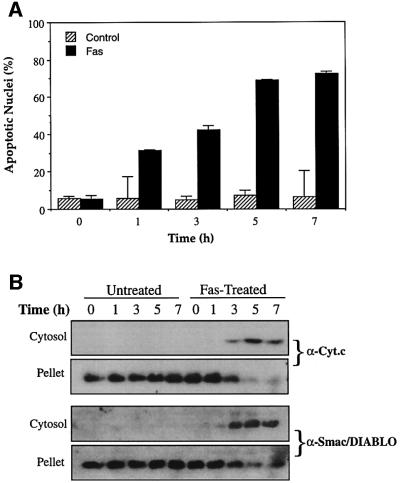
In certain cells such as hepatocytes, apoptosis initiated via the CD95 (Fas/APO-1) death receptor requires engagement of the mitochondrial pathway in order to complete the apoptotic programme (Scaffidi et al., 1998). In this context, cytochrome c release is achieved via caspase-8-mediated cleavage of Bid and the subsequent translocation of the Bid C-terminus to mitochondria (Li et al., 1998; Luo et al., 1998; Gross et al., 1999). To explore whether death receptor-initiated apoptosis was also associated with Smac/DIABLO redistribution to the cytosol, Jurkat cells were treated with agonistic anti-Fas antibody, followed by assessment of cytochrome c and Smac/DIABLO release from mitochondria. Figure 3 illustrates that ligation of the CD95 receptor provoked extensive apoptosis, which was accompanied by the accumulation of cytochrome c in the cytosol and its loss from mitochondrial pellets. Smac/DIABLO was also detected in cytosolic fractions at all timepoints where cytochrome c was observed, beginning 3 h after treatment with anti-Fas antibodies (Figure 3B). These data demonstrate that cytochrome c and Smac/DIABLO are both released from mitochondria in response to death receptor ligation, with similar kinetics.
Fig. 3. Timecourse analysis of Fas-induced cytochrome c and Smac/DIABLO release. Jurkat cells, treated with 100 ng/ml of the agonistic anti-Fas antibody CH-11, were harvested at the indicated timepoints. (A) The percentage of cells exhibiting apoptotic features was determined as described in Materials and methods. (B) Subcellular fractions were subjected to SDS–PAGE, followed by immunoblotting with anti-cytochrome c and anti-Smac/DIABLO antibodies, as indicated.
Bcl-2 blocks Smac/DIABLO release provoked by diverse pro-apoptotic agents
Given their common location within the mitochondrial intermembrane space and their mutual efflux in response to divergent pro-apoptotic stimuli (Figures 2 and 3), it is possible that the pore which enables cytochrome c efflux also permits Smac/DIABLO release. However, whereas cytochrome c has a mol. wt of ∼12 kDa, Smac/DIABLO has been reported to form ∼100 kDa dimers in solution (Du et al., 2000). In addition, although hypotonic buffers are generally sufficient to enable cytochrome c release from mitochondria, Smac/DIABLO release from similar organelle fractions has been reported to require the use of detergents (Du et al., 2000). Moreover, a recent study suggests that glucocorticoid-induced Smac/DIABLO release from mitochondria proceeds in the absence of detectable cytochrome c export (Chauhan et al., 2001). Thus, it remains unclear whether the channel involved in modulating cytochrome c release also regulates Smac/DIABLO efflux.
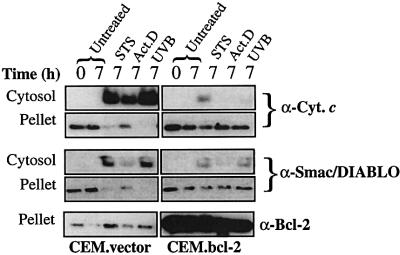
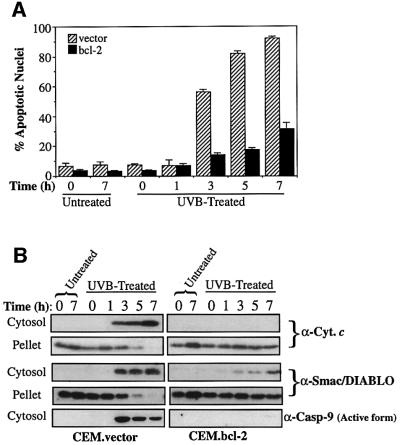
It is well established that Bcl-2 plays a critical role in regulating apoptosis by blocking efflux of cytochrome c from the mitochondrial intermembrane space (Kluck et al., 1997; Yang et al., 1997). To explore whether mitochondrial Smac/DIABLO release during apoptosis was similarly Bcl-2 sensitive, we used a well-characterized CEM cell line stably transfected with a Bcl-2 expression plasmid (CEM.Bcl-2), versus empty vector-transfected control cells (CEM.vector). These cell lines were compared in terms of their response to a panel of pro-apoptotic agents (Figure 4). Exposure of CEM.vector cells to a variety of stimuli, including staurosporine, actinomycin D and UVB-irradiation, readily provoked morphological changes indicative of apoptosis in these cells (data not shown). CEM.Bcl-2 cells were largely protected from apoptosis under the same conditions, exhibiting a morphology similar to untreated cells (data not shown). In response to these pro-apoptotic agents, CEM.vector cells released substantial amounts of cytochrome c and Smac/DIABLO into the cytosol. In contrast, CEM.Bcl-2 cells exhibited minimal accumulation of cytosolic cytochrome c or Smac/DIABLO under the same conditions (Figure 4). Com parison of the corresponding pellet fractions confirmed that loss of mitochondrial cytochrome c, or Smac/DIABLO, was substantially attenuated in CEM.Bcl-2 cells (Figure 4).
Fig. 4. Bcl-2 blocks Smac/DIABLO translocation. CEM cells stably transfected with either vector (left panels) or a Bcl-2 expression plasmid (right panels) were treated with 250 nM staurosporine (STS), 10 µM actinomycin D (Act. D) or UVB-irradiated for 90 s on a transilluminator (UVB). Cells were harvested after 7 h and subjected to subcellular fractionation as described in Materials and methods. Cytosolic and pellet fractions were subjected to SDS–PAGE, followed by immunoblotting with the indicated antibodies. Pellet fractions were probed with a Bcl-2-specific antibody to indicate the relative levels of endogenous versus overexpressed Bcl-2.
Bcl-2-regulated cytochrome c and Smac/DIABLO efflux occur with similar kinetics
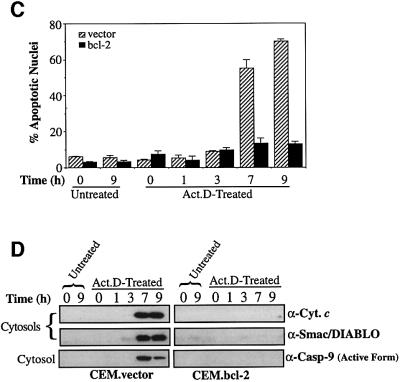
To investigate whether Smac/DIABLO and cytochrome c release generally occur within the same timeframe, we treated cells with UVB radiation or actinomycin D, and compared the kinetics of cytochrome c and Smac/DIABLO export in control versus Bcl-2-overexpressing cells (Figure 5).
Fig. 5. Timecourse analysis of Bcl-2-regulated Smac/DIABLO release. CEM.vector and CEM.Bcl-2 cells were UVB-irradiated for 90 s (A and B) or treated with 10 µM actinomycin D (C and D) and harvested at the indicated timepoints. (A) The percentage of cells exhibiting apoptotic features was determined as described in Materials and methods. (B) Cytosolic and pellet subcellular fractions were subjected to SDS–PAGE and immunoblotted with the indicated antibodies. Cytosolic fractions were also probed with an antibody that recognizes only the active form of caspase-9. (C) The percentage of cells exhibiting apoptotic features was determined as described in Materials and methods. (D) Cytosolic fractions from the same cells were subjected to SDS–PAGE, followed by immunoblotting with anti-cytochrome c and Smac/DIABLO-specific antibodies, as indicated. Cytosols were also probed for active caspase-9 as described in (B).
As illustrated in Figure 5B, cytosolic fractions prepared from UVB-treated CEM.vector cells had accumulated substantial amounts of cytochrome c within 3 h of treat ment. Similarly, cytosolic fractions from actinomycin D-treated vector transfectants contained substantial levels of cytochrome c within 7 h (Figure 5D). However, in response to both agents, the release of cytochrome c was potently blocked in the Bcl-2 transfectants (Figure 5B and D). Probing the same fractions for Smac/DIABLO revealed that the latter accumulated in the CEM.vector cytosols over a similar timescale to cytochrome c and that its release was similarly blocked by Bcl-2 (Figure 5B and D). Cells treated with the DNA-damaging agent daunorubicin also exhibited similar kinetics of cytosolic cytochrome c and Smac/DIABLO accumulation (data not shown).
Death receptor-initiated mitochondrial release of cytochrome c and Smac/DIABLO is caspase dependent
As discussed above, several lines of evidence suggest that the pore involved in mediating Smac/DIABLO release from mitochondria may be distinct from the cytochrome c-releasing channel (Du et al., 2000; Chauhan et al., 2001; Martinou and Green, 2001). However, thus far, we have observed that Smac/DIABLO and cytochrome c efflux occur with similar kinetics, and that both events are repressed by Bcl-2. This suggests that release of the two molecules may be regulated by the same mitochondrial pore (Figures 4 and 5).
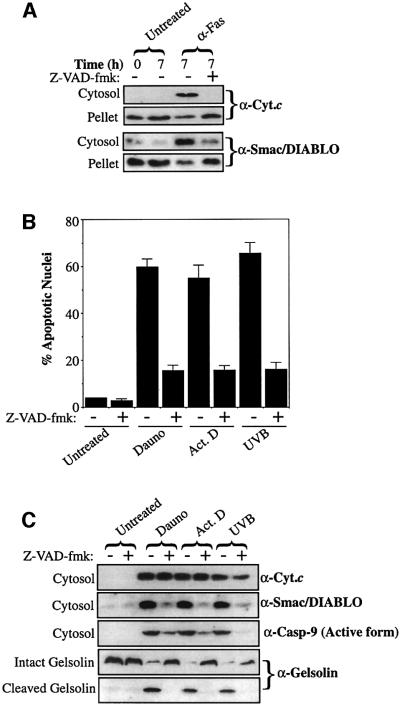
With the important exception of caspase-8-catalysed Bid cleavage associated with death receptor engagement, mitochondrial cytochrome c release has been reported to be caspase independent in most cases (Bossy-Wetzel et al., 1998; Li et al., 1998; Luo et al., 1998; Scaffidi et al., 1998; Zhuang and Cohen, 1998; Gross et al., 1999; Goldstein et al., 2000; Waterhouse et al., 2001). To explore whether the channel responsible for regulating Smac/DIABLO release was similarly caspase independent, we pre-treated Jurkat cells with the pan-caspase inhibitor, z-VAD-fmk, before stimulating them with a panel of pro-apoptotic agents (Figure 6).
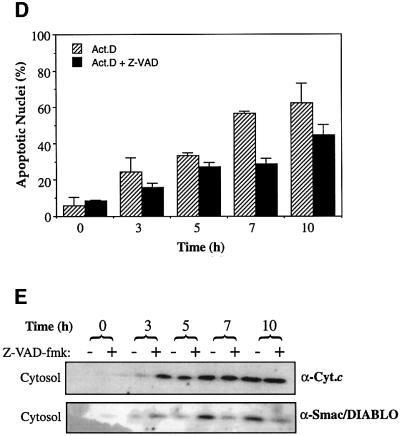
Fig. 6. Smac/DIABLO release requires active caspases. (A) Jurkat cells, pre-incubated in medium containing z-VAD-fmk (50 µM) where indicated, were induced to undergo apoptosis by treatment with 100 ng/ml anti-Fas (CH-11). Cytosolic and pellet fractions were prepared and subjected to SDS–PAGE, followed by immunoblotting with the indicated antibodies. (B) Jurkat cells were induced to undergo apoptosis (in the presence or absence of 50 µM z-VAD-fmk) by treatment with 10 µM daunorubicin (Dauno), 10 µM actinomycin D (Act. D) or UVB-irradiation (90 s) (UVB) for 7 h. The percentage of cells exhibiting apoptotic features was determined as described in Materials and methods. (C) Cytosolic fractions from the same cells were subjected to SDS–PAGE, followed by immunoblotting with the indicated antibodies. (D) Jurkat cells, pre-treated with 50 µM z-VAD-fmk where indicated, were induced to undergo apoptosis by the addition of 20 µM actinomycin D to culture media and harvested at the indicated timepoints. The percentage of cells exhibiting apoptotic features was determined as described in Materials and methods. (E) Cytosolic and pellet fractions from the same cells were subjected to SDS–PAGE, followed by immunoblotting with the indicated antibodies.
Initially, we examined Smac/DIABLO release in anti-Fas-treated cells, because it has been established that cytochrome c release is impaired by caspase inhibition in this cell death model (Bossy-Wetzel and Green, 1999). As expected, anti-Fas-treated cells readily underwent apoptosis, which was substantially attenuated by pre-incubation with z-VAD-fmk (data not shown). Analysis of subcellular fractions of these cells revealed that cytochrome c accumulation within the cytosol (and its loss from mitochondria) was potently repressed by z-VAD-fmk (Figure 6A). This is probably due to the requirement for caspase-mediated cleavage of Bid (tBid), or a similar factor, to modulate cytochrome c release in this context (Li et al., 1998; Luo et al., 1998; Gross et al., 1999). Fas-mediated Smac/DIABLO release from mitochondria was also substantially attenuated by pre-treatment of the cells with z-VAD-fmk, suggesting a similar requirement for a caspase-cleaved factor for Smac/DIABLO release in Fas-induced death (Figure 6A). Thus, during death receptor-induced apoptosis, active caspases are required to fuel Smac/DIABLO release.
Cell stress-associated Smac/DIABLO efflux from mitochondria requires active caspases
We examined the ability of z-VAD-fmk to modulate Smac/DIABLO release in response to types of stimuli established to provoke caspase-independent cytochrome c translocation (Kluck et al., 1997; Bossy-Wetzel et al., 1998; Zhuang and Cohen, 1998; Chen et al., 2000). As anticipated, cells pre-treated with z-VAD-fmk were protected from apoptosis induced by cytotoxic drugs and UVB-irradiation, as judged by the paucity of apoptotic nuclei in these samples (Figure 6B), the absence of active caspase-9 within the cytosols of the same cells or by the cleavage of the caspase-3 substrate gelsolin (Figure 6C). As previously reported, the accumulation of cytochrome c within the cytosols of cells undergoing apoptosis in response to cytotoxic drugs and UV-irradiation was largely insensitive to caspase inhibition (Figure 6C; Kluck et al., 1997; Bossy-Wetzel et al., 1998; Zhuang and Cohen, 1998; Chen et al., 2000). However, in sharp contrast, the accumulation of Smac/DIABLO within the same cytosolic fractions was potently repressed in z-VAD-fmk-treated cells, strongly suggesting that caspases were required to mediate Smac/DIABLO release in response to these pro-apoptotic stimuli (Figure 6C).
To examine further the role of caspases in modulating Smac/DIABLO release from mitochondria, we also performed timecourse experiments (Figure 6D and E). Jurkat cells, pre-incubated with z-VAD-fmk, were treated with actinomycin D and subcellular fractions were generated at the indicated timepoints (Figure 6E). In agreement with the endpoint experiment (Figure 6B and C), whereas cytosolic cytochrome c accumulation was largely uninhibited by caspase inhibition, z-VAD-fmk pre-treated cells exhibited reduced levels of cytosolic Smac/DIABLO at all timepoints examined (Figure 6E). Similar results were obtained for cells induced to undergo apoptosis in response to UV-irradiation (data not shown).
Immunolocalization of Smac/DIABLO and cytochrome c reveals divergent release patterns upon caspase inhibition
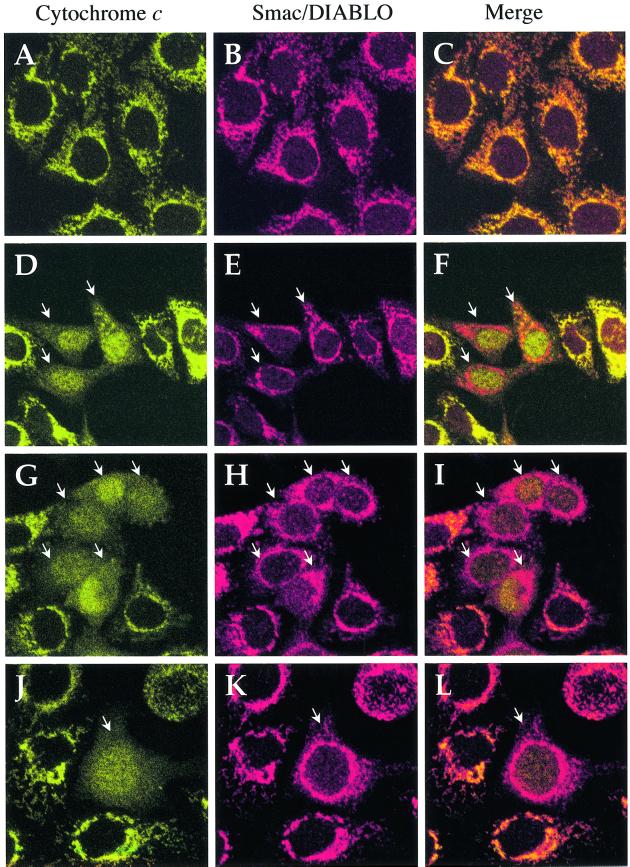
To confirm that caspase inhibition could uncouple cytochrome c and Smac/DIABLO release from mitochondria, we also performed immunolocalization experiments in HeLa cells. Figure 7A–C illustrates that cytochrome c and Smac/DIABLO co-localized to mitochondria in viable (non-apoptotic) cells, as expected. However, when cells were pre-treated with z-VAD-fmk and induced to undergo apoptosis by exposure to UV radiation or actinomycin D, numerous cells could be found containing mitochondria that had clearly released cytochrome c but not Smac/DIABLO (Figure 7). In the latter cells, cytochrome c had assumed a diffuse staining pattern within the cell and could also be seen within the nucleus (Figure 7D–L). In sharp contrast, Smac/DIABLO was clearly retained within mitochondria and exhibited a punctate distribution similar to that seen in surrounding cells where cytochrome c release had not yet occurred (Figure 7D–L). Where z-VAD-fmk was not added to the culture medium, cells that had released cytochrome c from mitochondria also released Smac/DIABLO and rapidly progressed to a rounded and blebbed apoptotic phenotype. In the latter situation, both proteins exhibited a diffuse extramitochondrial distribution within the cell (Figure 8).
Fig. 7. Immunolocalization of Smac/DIABLO and cytochrome c in HeLa cells. (A–C) Viable (non-apoptotic) HeLa cells were stained with antibodies to cytochrome c (green) and Smac/DIABLO (red) as indicated. Note that both proteins clearly co-localize within punctate, mitochondrial structures. (D–F) HeLa cells, pre-incubated with z-VAD-fmk (100 µM), were UV-irradiated followed by further incubation for 8 h. Note the cells where cytochrome c has exited mitochondria and can be seen in a diffuse pattern throughout the cell, including the nucleus (white arrows). In the latter case, note the retention of Smac/DIABLO in punctate mitochondrial structures within the cytoplasm. (G–I) HeLa cells, pre-incubated with z-VAD-fmk (100 µM), were then treated with 10 µM actinomycin D for 8 h. Once again note the retention of Smac/DIABLO in mitochondrial structures. (J–L) HeLa cells were treated as described in (G–I). A close-up of a single cell that has undergone mitochondrial cytochrome c release, but where Smac/DIABLO is retained within mitochondria, is shown.
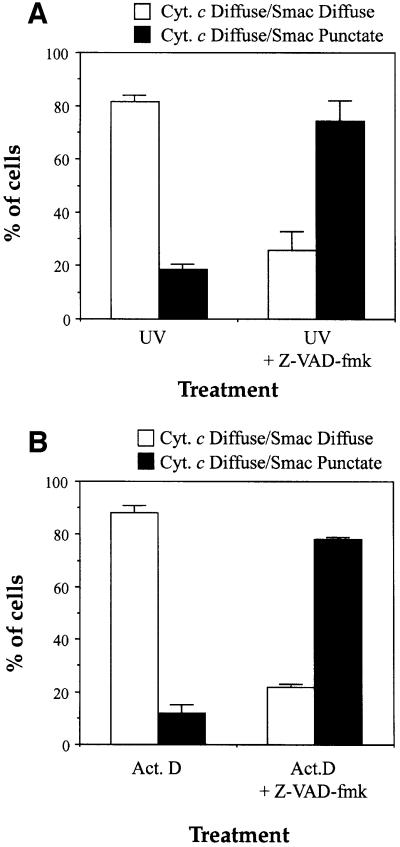
Fig. 8. Quantitative analysis of cytochrome c versus Smac/DIABLO release in apoptotic cells. HeLa cells, pre-incubated where indicated in the presence of 100 µM z-VAD-fmk, were either UV-irradiated (A), or treated with 10 µM actinomycin D (B). Upon appearance of apoptotic cells in the cultures (8 h after UV-irradiation or actinomycin D treatment) cells were immunostained with anti-cytochrome c and anti-Smac/DIABLO antibodies as described in Materials and methods. Cells exhibiting a diffuse (extra-mitochondrial) cytochrome c staining pattern were evaluated for their pattern of Smac/DIABLO staining (diffuse or punctate). Open bars represent the proportion of cells that exhibited doubly diffuse cytochrome c and Smac/DIABLO staining patterns. Filled bars indicate the percentage of cells that exhibited diffuse cytochrome c staining but retained punctate (mitochondrial) Smac/DIABLO staining patterns. Error bars denote the standard error of the mean of three fields of at least 100 cells within a representative experiment.
Thus, caspase inhibition attenuates the cytosolic accumulation of Smac/DIABLO but not cytochrome c. Taken together, these data suggest that whereas the pore responsible for mediating cytochrome c export is largely caspase-independent, active caspases are required to enable Smac/DIABLO efflux from mitochondria provoked by the same stimuli.
Discussion
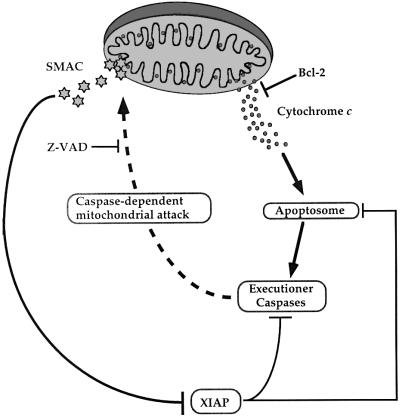
Here we report that Smac/DIABLO release from mitochondria is a general feature of apoptosis. In cells stimulated to die in response to diverse pro-apoptotic agents, including death receptor ligation, cytotoxic drugs and DNA-damaging agents, Smac/DIABLO accumulation within the cytosol was readily detected (Figures 2 and 3). This coincided with a concomitant loss of Smac/DIABLO from mitochondria. Using a cell line that constitutively overexpresses Bcl-2, we have also demonstrated that Bcl-2 blocks apoptosis-associated release of Smac/DIABLO from mitochondria (Figures 4 and 5). These data expand the already established anti-apoptotic roles of Bcl-2. Whereas cytochrome c release lies upstream of caspase activation in many forms of stress-induced apoptosis, data presented herein suggest that active caspases are required to enable Smac/DIABLO release (Figures 6, 7 and 8). This would suggest a model whereby the ability of Bcl-2 to inhibit Smac/DIABLO release from mitochondria may not relate to its ability to regulate a Smac/DIABLO-exporting channel directly (Figure 9). Rather, by regulating cytochrome c translocation into the cytosol and preventing apoptosome assembly, it is conceivable that Bcl-2 indirectly regulates Smac/DIABLO release by blocking caspase activation via the apoptosome (Figure 9).
Fig. 9. Hypothetical model of Bcl-2- and caspase-regulated Smac/DIABLO release from mitochondria. The capacity of Bcl-2 to regulate Smac/DIABLO release from mitochondria may derive from its ability to block cytochrome c efflux. Once released from mitochondria, cytochrome c promotes the assembly of the apoptosome, which results in caspase-9 activation and the propagation of a caspase cascade. Under these conditions, XIAP may attenuate caspase activity by integration into the apoptosome complex, or repress the activity of active executioner caspases-3 and -7. As a counter-measure, caspase-mediated attack of a (unidentified) mitochondrial component may result in the opening of a Smac/DIABLO-conducting pore. Once released into the cytosol, Smac/DIABLO may enhance caspase activity by binding to IAPs, including XIAP, thereby neutralizing their caspase-inhibitory properties.
Clearly, one implication of this observation is that the nature of the Smac/DIABLO-escape channel may differ from that of the cytochrome c-releasing pore. Given the significant difference in size between cytochrome c (∼12 kDa) and of Smac/DIABLO dimers (∼100 kDa), one possibility is that the latter molecule is simply too large to escape from the cytochrome c-conducting channel (Martinou and Green, 2001). This is consistent with the observation that extraction of Smac/DIABLO from mitochondria requires the use of detergents, whereas cytochrome c extraction does not (Du et al., 2000). Our data further support the possibility that the mechanisms governing cytochrome c and Smac/DIABLO release differ. In contrast to cytochrome c, Smac/DIABLO release from mitochondria appears to require caspase-catalysed attack of a mitochondrial component (Figure 9).
Within this context, it is interesting to note data concerning the role of caspases in promoting the loss of mitochondrial transmembrane potential (Bossy-Wetzel et al., 1998; Marzo et al., 1998; Waterhouse et al., 2001). Apoptosis-associated cytochrome c release appears to involve limited permeabilization of the outer mitochondrial membrane and maintenance of transmembrane potential. However, cytochrome c/apoptosome-mediated caspase activation appears to result in swelling of the mitochondrial matrix and rupture of the inner and outer mitochondrial membranes (Martinou and Green, 2001; Zamzami and Kroemer, 2001). Interestingly, it has been established that the megapore spanning the inner and outer mitochondrial membranes created by permeability transition pore opening enables the efflux of molecules larger than cytochrome c (Marzo et al., 1998; Kroemer, 1999; Patterson et al., 2000). Thus, caspase-dependent mitochondrial attack, which may result in permeability transition pore opening or generalized mitochondrial destruction, could facilitate Smac/DIABLO release from mitochondria downstream of cytochrome c efflux (Figure 9). Clearly, further studies will be required to evaluate this hypothesis.
Materials and methods
Reagents
Anti-cytochrome c, anti Bcl-2, anti-caspase-3 and anti-gelsolin antibodies were purchased from BD PharMingen (UK). Antibodies selectively recognizing active caspase-9 (generated by cleavage at Asp330) were purchased from New England Biolabs (UK). Anti-actin antibodies were from ICN (Aurora, OH). z-VAD-fmk was purchased from Bachem (UK). Human multi-tissue lysates were purchased from Clontech (UK). The anti-Fas agonistic antibody (clone CH-11) was obtained from Kamiya Biomedical Co. Unless otherwise indicated, all other reagents were purchased from Sigma (Ireland) Ltd.
Generation and affinity purification of Smac/DIABLO-specific antisera
BL21 bacteria, harbouring a plasmid encoding the mature form of human Smac/DIABLO, which lacks the mitochondrial targeting sequence (a kind gift of Dr Xiaodong Wang, University of Texas Medical Center), were induced to express recombinant His6-Smac by the addition of 1 mM IPTG to culture media. The pellets from 1 l of bacterial culture were lysed by sonication in lysis buffer [20 mM Tris–Cl pH 7.9, 500 mM NaCl, 5 mM imidazole, 0.1% NP-40, 100 µM phenymethylsulfonyl fluoride (PMSF), 10 µg/ml leupeptin, 2 µg/ml aprotinin]. Recombinant polyhistidine-tagged Smac was captured from the clarified lysate by incubation with Ni-agarose beads (Qiagen, UK) followed by extensive washing in wash buffer (20 mM Tris–HCl pH 7.9, 500 mM NaCl, 60 mM imidazole). Affinity-purified Smac/DIABLO was eluted by incubation in wash buffer supplemented with 500 mM imidazole and the resultant soluble protein was washed extensively in phosphate-buffered saline (PBS) pH 7.2 in a microfiltration unit (Vivascience Ltd, UK). A rabbit polyclonal anti-Smac/DIABLO antibody was generated by immunizing rabbits with purified recombinant Smac/DIABLO protein. To purify the antibody, the crude antiserum was pre-cleared with Ni-agarose beads for 6 h at 4°C. Smac/DIABLO-specific antibodies were subsequently captured for 12 h using bead-immobilized recombinant Smac/DIABLO protein as an affinity matrix. Bead-captured antibodies were washed extensively in PBS pH 7.2, then acid eluted into 100 mM glycine pH 3.0, followed by neutralization with one-tenth of a volume of 1 M Tris, pH 8.0. Prior to use, purified antibodies were washed extensively in PBS and concentrated ∼8-fold in a microfiltration unit.
Determination of Smac/DIABLO expression patterns
To determine the tissue distribution of Smac/DIABLO, a panel of human tissue lysates (Clontech) were subjected to standard 12% SDS–PAGE, followed by immunoblotting with Smac/DIABLO antibodies. To assess Smac/DIABLO expression levels in human tumour cells, a variety of cell lines were lysed at a density of 107 cells/ml in standard SDS–PAGE loading buffer. Samples (50 µg) were subjected to 12% SDS–PAGE and immunoblotted with anti-Smac/DIABLO antibodies.
Induction and assessment of apoptosis
To induce apoptosis, cells were seeded at a final density of 2 × 106/ml in the presence of pro-apoptotic agents at concentrations indicated in the Figure legends. Alternatively, cells were exposed to UVB-irradiation for 90 s on a Bio-Rad UV transilluminator 2000 (Bio-Rad, UK). Where indicated, cells were pre-incubated in medium containing 50 µM z-VAD-fmk for 2 h prior to the induction of apoptosis.
To assess the extent of apoptosis, cells (106) were harvested by centrifugation at 800 g, for 10 min. Cell pellets were resuspended in 100 µl of ice-cold fixative solution (4% paraformaldehyde in PBS pH 7.2) supplemented with 10 µM Hoechst. The percentage of cells exhibiting apoptotic nuclei, as judged by chromatin condensation and nuclear fragmentation, was then determined by fluorescence microscopy.
Subcellular fractionation
Following induction of apoptosis, cytosolic and pellet (mitochondrial) fractions were generated using a digitonin-based subcellular fractionation technique, essentially as described previously (Ekert et al., 2001; Waterhouse et al., 2001). Briefly, 107 cells were harvested by centrifugation at 800 g, washed in PBS pH 7.2, and re-pelletted. Cells were digitonin-permeabilized for 5 min on ice at a density of 3 × 107/ml in cytosolic extraction buffer (250 mM sucrose, 70 mM KCl, 137 mM NaCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4 pH 7.2, 100 µM PMSF, 10 µg/ml leupeptin, 2 µg/ml aprotinin, containing 200 µg/ml digitonin). Plasma membrane permeabilization of cells was confirmed by staining in a 0.2% trypan blue solution. Cells were then centrifuged at 1000 g for 5 min at 4°C. The supernatants (cytosolic fractions) were saved and the pellets solubilized in the same volume of mitochondrial lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 0.2 % Triton X-100, 0.3% NP-40, 100 µM PMSF, 10 µg/ml leupeptin, 2 µg/ml aprotinin), followed by pelletting at 10 000 g for 10 min at 4°C. For the detection of Smac/DIABLO or cytochrome c, equal volumes (28 µl) of cytosolic and pellet fractions were supplemented with 5× SDS–PAGE loading buffer, subjected to standard 12% SDS–PAGE and transferred to nitrocellulose membranes (Schleicher and Schuell, UK). Blots were probed with anti-cytochrome c or anti-Smac/DIABLO antibodies and detected with horseradish peroxidase-coupled secondary antisera using the Supersignal West Pico or Supersignal West Dura chemiluminescence reagents (Pierce, UK).
Immunostaining of cytochrome c and Smac/DIABLO
HeLa cells were seeded on coverslips at a density of 5 × 104 cells/well in 6-well tissue culture plates 48 h prior to treatment. Prior to induction of apoptosis, cells were pre-incubated in control media or in the presence of 100 µM z-VAD-fmk for 2 h. Cells were then UVB-irradiated for 7 min, or treated with 10 µM actinomycin D, to induce apoptotic changes. Eight hours later, cells were washed three times in PBS pH 7.2, followed by fixation in 3% paraformaldehyde/PBS for 10 min. Fixed cells were then washed three times in PBS and permeabilized in 0.15% Triton X-100 in PBS for 15 min. Cells were blocked for 30 min in 2% bovine serum albumin (BSA)/PBS and probed with a 1:100 dilution of anti-cytochrome c (clone 6H2.B4, BD PharMingen) and anti-Smac/DIABLO antibodies (this study) for 1 h at room temperature. After incubation with primary antibodies, cells were washed three times (10 min per wash) in 2% BSA/PBS with gentle rocking, followed by probing for 45 min with a 1:250 dilution of fluorescein-conjugated anti-mouse and rhodamine-conjugated anti-rabbit antibodies (Molecular Probes, The Netherlands). Immuno stained cells were then washed three times in PBS and mounted in Slowfade Light Antifade mounting medium (Molecular Probes). Confocal images were obtained using a Bio-Rad confocal microscope.
Acknowledgments
Acknowledgements
We thank Dr Xiaodong Wang for the provision of the Smac bacterial expression plasmid. E.M.C. was supported by a Health Research Board of Ireland Post-Doctoral Fellowship. This work was funded by a Wellcome Trust Senior Fellowship Award in Biomedical Science (047580) and EU grant (QLG1-1999-00739) to S.J.M. We also thank Science Foundation Ireland for support of ongoing work in our laboratory.
References
- Adrain C. and Martin,S.J. (2001) The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem. Sci., 26, 390–397. [DOI] [PubMed] [Google Scholar]
- Adrain C., Slee,E.A., Harte,M.T. and Martin,S.J. (1999) Regulation of apoptotic protease activating factor-1 oligomerization and apoptosis by the WD-40 repeat region. J. Biol. Chem., 274, 20855–20860. [DOI] [PubMed] [Google Scholar]
- Antonsson B., Montessuit,S., Lauper,S., Eskes,R. and Martinou,J.C. (2000) Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J., 345, 271–278. [PMC free article] [PubMed] [Google Scholar]
- Antonsson B., Montessuit,S., Sanchez,B. and Martinou,J.C. (2001) Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem., 276, 11615–11623. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E. and Green,D.R. (1999) Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J. Biol. Chem., 274, 17484–17490. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E., Newmeyer,D.D. and Green,D.R. (1998) Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J., 17, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain K., Bratton,S.B., Langlais,C., Walker,G., Brown,D.G., Sun,X.M. and Cohen,G.M. (2000) Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J. Biol. Chem., 275, 6067–6070. [DOI] [PubMed] [Google Scholar]
- Chai J., Du,C., Wu,J.W., Kyin,S., Wang,X. and Shi,Y. (2000) Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature, 406, 855–862. [DOI] [PubMed] [Google Scholar]
- Chauhan D., Hideshima,T., Rosen,S., Reed,J.C., Kharbanda,S. and Anderson,K.C. (2001) Apaf-1/cytochrome c-independent and Smac-dependent induction of apoptosis in multiple myeloma cells. J. Biol. Chem., 276, 24453–24456. [DOI] [PubMed] [Google Scholar]
- Chen Q., Gong,B. and Almasan,A. (2000) Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induce apoptosis. Cell Death Differ., 7, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S. and Martinou,J.C. (2000) Mitochondria as the central control point of apoptosis. Trends Cell Biol., 10, 369–377. [DOI] [PubMed] [Google Scholar]
- Deveraux Q.L., Roy,N., Stennicke,H.R., Van Arsdale,T., Zhou,Q., Srinivasula,S.M., Alnemri,E.S., Salvesen,G.S. and Reed,J.C. (1998) IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J., 17, 2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C., Fang,M., Li,Y., Li,L. and Wang,X. (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell, 102, 33–42. [DOI] [PubMed] [Google Scholar]
- Earnshaw W.C., Martins,L.M. and Kaufmann,S.H. (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem., 68, 383–424. [DOI] [PubMed] [Google Scholar]
- Ekert P.G., Silke,J., Hawkins,C.J., Verhagen,A.M. and Vaux,D.L. (2001) DIABLO promotes apoptosis by removing MIHA/XIAP from processed caspase 9. J. Cell Biol., 152, 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.C., Waterhouse,N.J., Juin,P., Evan,G.I. and Green,D.R. (2000) The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nature Cell. Biol., 2, 156–162. [DOI] [PubMed] [Google Scholar]
- Gross A., Yin,X.M., Wang,K., Wei,M.C., Jockel,J., Milliman,C., Erdjument-Bromage,H., Tempst,P. and Korsmeyer,S.J. (1999) Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem., 274, 1156–1163. [DOI] [PubMed] [Google Scholar]
- Jacobson M.D., Weil,M. and Raff,M.C. (1997) Programmed cell death in animal development. Cell, 88, 347–354. [DOI] [PubMed] [Google Scholar]
- Kerr J.F., Wyllie,A.H. and Currie,A.R. (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer, 26, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck R.M., Bossy-Wetzel,E., Green,D.R. and Newmeyer,D.D. (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science, 275, 1132–1136. [DOI] [PubMed] [Google Scholar]
- Kroemer G. (1999) Mitochondrial control of apoptosis: an overview. Biochem. Soc. Symp., 66, 1–15. [DOI] [PubMed] [Google Scholar]
- Li H., Zhu,H., Xu,C.J. and Yuan,J. (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell, 94, 491–501. [DOI] [PubMed] [Google Scholar]
- Li P., Nijhawan,D., Budihardjo,I., Srinivasula,S.M., Ahmad,M., Alnemri,E.S. and Wang,X. (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell, 91, 479–489. [DOI] [PubMed] [Google Scholar]
- Liu X., Kim,C.N., Yang,J., Jemmerson,R. and Wang,X. (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell, 86, 147–157. [DOI] [PubMed] [Google Scholar]
- Luo X., Budihardjo,I., Zou,H., Slaughter,C. and Wang,X. (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell, 94, 481–490. [DOI] [PubMed] [Google Scholar]
- Martin S.J. and Green,D.R. (1995) Protease activation during apoptosis: death by a thousand cuts? Cell, 82, 349–352. [DOI] [PubMed] [Google Scholar]
- Martinou J.C. and Green,D.R. (2001) Breaking the mitochondrial barrier. Nature Rev. Mol. Cell. Biol., 2, 63–67. [DOI] [PubMed] [Google Scholar]
- Marzo I., Susin,S.A., Petit,P.X., Ravagnan,L., Brenner,C., Larochette,N., Zamzami,N. and Kroemer,G. (1998) Caspases disrupt mitochondrial membrane barrier function. FEBS Lett., 427, 198–202. [DOI] [PubMed] [Google Scholar]
- Mikhailov V., Mikhailova,M., Pulkrabek,D.J., Dong,Z., Venkatachalam,M.A. and Saikumar,P. (2001) Bcl-2 prevents bax oligomerization in the mitochondrial outer membrane. J. Biol. Chem., 276, 18361–18374. [DOI] [PubMed] [Google Scholar]
- Nicholson D.W. (1999) Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ., 6, 1028–1042. [DOI] [PubMed] [Google Scholar]
- Patterson S.D., Spahr,C.S., Daugas,E., Susin,S.A., Irinopoulou,T., Koehler,C. and Kroemer,G. (2000) Mass spectrometric identification of proteins released from mitochondria undergoing permeability transition. Cell Death Differ., 7, 137–144. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. and Lazebnik,Y. (1999) Caspase-9 and APAF-1 form an active holoenzyme. Genes Dev., 13, 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N., Deveraux,Q.L., Takahashi,R., Salvesen,G.S. and Reed,J.C. (1997) The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J., 16, 6914–6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M., Korsmeyer,S.J. and Schlesinger,P.H. (2000) BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nature Cell Biol., 2, 553–555. [DOI] [PubMed] [Google Scholar]
- Savill J. and Fadok,V. (2000) Corpse clearance defines the meaning of cell death. Nature, 407, 784–788. [DOI] [PubMed] [Google Scholar]
- Scaffidi C., Fulda,S., Srinivasan,A., Friesen,C., Li,F., Tomaselli,K.J., Debatin,K.M., Krammer,P.H. and Peter,M.E. (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J., 17, 1675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee E.A. et al. (1999a) Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol., 144, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee E.A., Adrain,C. and Martin,S.J. (1999b) Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ., 6, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Slee E.A., Adrain,C. and Martin,S.J. (2001) Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem., 276, 7320–7326. [DOI] [PubMed] [Google Scholar]
- Srinivasula S.M., Ahmad,M., Fernandes-Alnemri,T. and Alnemri,E.S. (1998) Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol. Cell, 1, 949–957. [DOI] [PubMed] [Google Scholar]
- Srinivasula S.M. et al. (2001) A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature, 410, 112–116. [DOI] [PubMed] [Google Scholar]
- Stuart R.A. and Neupert,W. (1990) Apocytochrome c: an exceptional mitochondrial precursor protein using an exceptional import pathway. Biochimie, 72, 115–121. [DOI] [PubMed] [Google Scholar]
- Verhagen A.M., Ekert,P.G., Pakusch,M., Silke,J., Connolly,L.M., Reid,G.E., Moritz,R.L., Simpson,R.J. and Vaux,D.L. (2000) Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell, 102, 43–53. [DOI] [PubMed] [Google Scholar]
- Waterhouse N.J., Goldstein,J.C., von Ahsen,O., Schuler,M., Newmeyer,D.D. and Green,D.R. (2001) Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J. Cell Biol., 153, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M.C., Lindsten,T., Mootha,V.K., Weiler,S., Gross,A., Ashiya,M., Thompson,C.B. and Korsmeyer,S.J. (2000) tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev., 14, 2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei M.C. et al. (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science, 292, 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu,X., Bhalla,K., Kim,C.N., Ibrado,A.M., Cai,J., Peng,T.I., Jones,D.P. and Wang,X. (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science, 275, 1129–1132. [DOI] [PubMed] [Google Scholar]
- Zamzami N. and Kroemer,G. (2001) The mitochondrion in apoptosis: how Pandora’s box opens. Nature Rev. Mol. Cell Biol., 2, 67–71. [DOI] [PubMed] [Google Scholar]
- Zhuang J. and Cohen,G.M. (1998) Release of mitochondrial cytochrome c is upstream of caspase activation in chemical-induced apoptosis in human monocytic tumour cells. Toxicol. Lett., 102–103, 121–129. [DOI] [PubMed] [Google Scholar]
- Zou H., Henzel,W.J., Liu,X., Lutschg,A. and Wang,X. (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell, 90, 405–413. [DOI] [PubMed] [Google Scholar]