Abstract
The glucocorticoid receptor (GR) acts both as a transcription factor itself on genes carrying GR response elements (GREs) and as a modulator of other transcription factors. Using mice with a mutation in the GR, which cannot activate GRE promoters, we examine whether the important anti-inflammatory and immune suppressive functions of glucocorticoids (GCs) can be established in this in vivo animal model. We find that most actions are indeed exerted in the absence of the DNA-binding ability of the GR: inhibition of the inflammatory response of locally irritated skin and of the systemic response to lipopolysaccharides. GCs repress the expression and release of numerous cytokines both in vivo and in isolated primary macrophages, thymocytes and CD4+ splenocytes. A transgenic reporter gene controlled by NF-κB exclusively is also repressed, suggesting that protein– protein interaction with other transcription factors such as NF-κB forms the basis of the anti-inflammatory activity of GR. The only defect of immune suppression detected so far concerns the induced apoptosis of thymocytes and T lymphocytes.
Keywords: anti-inflammation/cytokines/innate immune system/lymphocytes/NF-κB
Introduction
Mammalian organisms have acquired a large repertoire of responses to external attacks (stress responses, immune and inflammatory responses, acute phase response, specialized mobilization of energy stores, repair and wound healing reactions, etc.). Each of these responses needs to be regulated and restricted in magnitude such that homeostasis can be re-established and healthy survival is guaranteed. To give an example, the specific expansion of antigen-triggered lymphocytes cannot be allowed to proceed towards ‘leukemic’ levels. As an even more drastic example, an uncontrolled response to the invasion of bacteria leads to septic shock. A particularly important organismic regulatory loop is formed by the release of glucocorticoids (GCs) (Wilckens and De Rijk, 1997). GCs turn off cytokine synthesis and thus protect the organism from undue proliferation and from septic shock. With regard to this function, GCs are in widespread medical use for the treatment of inflammatory disorders such as rheumatoid arthritis, asthma and dermatitis, and autoimmune diseases such as Crohn’s disease (reviewed in Karin, 1998).
The effects of GCs are exerted through the glucocorticoid receptor (GR), a ligand-induced transcription factor, which belongs to the nuclear receptor superfamily (Evans, 1988; Beato et al., 1995). GR controls transcription by two major modes of action. One involves binding of GR homodimers to glucocorticoid response elements (GREs) in regulatory sequences of GR target genes. In cell culture experiments, a second mode of action has been identified: GR modulates the activity of other transcription factors such as AP-1, NF-κB and Stat5, independently of direct DNA contact, a process designated as cross-talk (Jonat et al., 1990; Stöcklin et al., 1996; reviewed in Beato et al., 1995; Herrlich, 2001). The interference with the activities of these other transcription factors appears to occur at a late stage of transcriptional initiation, after formation of the pre-initiation complex (Nissen and Yamamoto, 2000; Herrlich, 2001). The GR itself does not need to bind to DNA for this second mode of action. In fact, GR mutants in the transactivation domain or in the dimerization domain (D-loop), which cannot activate GRE promoters, are perfectly proficient in cross-talk (Caldenhoven et al., 1995; Heck et al., 1994, 1997).
We have recently established a mouse model which permits exclusive study of the cross-talk function in the absence of GR-dependent gene transcription (Reichardt et al., 1998). By gene targeting, the point mutation A458T was introduced into the GR D-loop (homozygotic GRdim mice). In these mice, AP-1-mediated gene expression is repressed efficiently by GCs in the absence of transcriptional activation of classical GRE-regulated genes (Reichardt et al., 1998; Tuckermann et al., 1999). Here, we have exploited GRdim mice to supply the first in vivo proof that local and systemic inflammatory responses are repressed potently by GR in the absence of DNA binding. The basis for the anti-inflammatory activity of the DNA binding-defective GR appears to be its normal ability to repress inflammation-relevant genes in various cell types, predominantly by negative interference with NF-κB activity.
Results and discussion
Inflammatory responses are repressed efficiently by glucocorticoids in mice carrying a DNA binding-defective GR
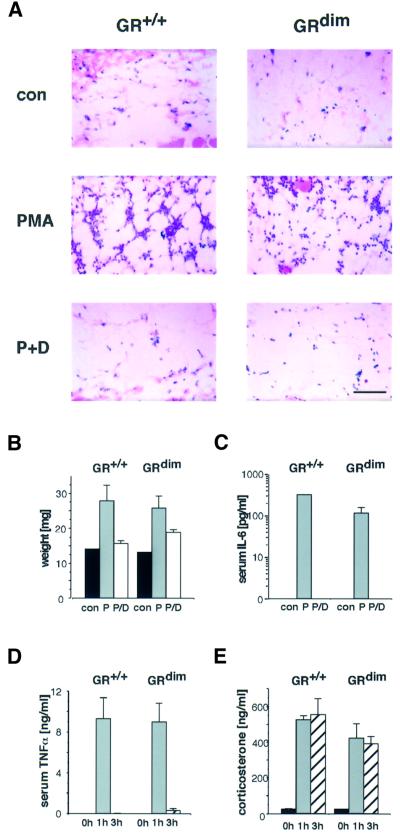
A frequently used model of acute inflammation is phorbol ester (phorbol 12-myristate 13-acetate; PMA)-induced oedema formation (Gschwendt et al., 1984; Lloret and Moreno, 1995). Application of PMA to the skin causes swelling associated with increased vascular permeability and rapid influx into the skin of neutrophilic granulocytes and mononuclear cells. This inflammatory response can be inhibited efficiently by topical application of GCs. To address the question of whether this repressive effect is retained in mice with a DNA binding-defective GR, we analysed the response of PMA-irritated skin to GC treatment. Topical application of PMA to the dorsal skin of either wild-type or GRdim mice induced rapid infiltration of inflammatory cells into the fat tissue underlying the dermis, exhibiting the features of panniculitis (Figure 1A). Simultaneous treatment with the synthetic GC dexamethasone almost completely inhibited the panniculitic phenotype in mice of both genotypes.
Fig. 1. GCs potently suppress local and systemic inflammatory responses in GRdim mice. (A) Hematoxylin–eosin-stained sections of subdermal fat tissue derived from the back skin of wild-type (GR+/+) and GRdim mice treated with either vehicle acetone (con), 10 nmol PMA (P) or PMA plus 50 µg dexamethasone (P+D) for 6 h. Scale bar: 100 µm. (B) Vehicle acetone (con), 1 nmol PMA (P) or PMA plus 5 µg dexamethasone (P/D) were applied ectopically to the ears of the mice and the swelling measured after 6 h. (C) IL-6 levels in serum of mice treated as described in (A) were determined by ELISA. (D and E) Wild-type (GR+/+) or GRdim mice were injected with 100 µg of LPS per mouse and killed at the time points indicated. TNF-α serum levels were measured by ELISA (D) and corticosterone serum levels by RIA (E).
To quantitate the inhibition of swelling, PMA was also applied to the ears. This topical treatment led to a substantial increase in weight due to massive oedema formation, in both wild-type and GRdim mice (Figure 1B). In the presence of GC, this increase was strongly reduced, although slightly less efficiently in GRdim mice than in wild-type mice.
PMA-induced local inflammation of the skin also led to a marked systemic response, as demonstrated by an elevation of the interleukin-6 (IL-6) serum levels (Figure 1C). The secretion of IL-6 into the blood was completely prevented by GC. In accordance with these data, the synthesis of IL-6 mRNA and the expression of the NF-κB-dependent E-selectin gene, which facilitates rolling of immune cells (Brostjan et al., 1997), was inhibited efficiently in the skin of wild-type and GRdim mice (data not shown). Taken together, these findings demonstrate that the topical anti-inflammatory activity of GCs as employed in the treatment of numerous skin disorders, as well as inhibition of the release of acute phase mediators, are established predominantly by the DNA binding-independent function of GR.
To mimic a bacterial infection with subsequent systemic inflammatory response, we injected lipopolysaccharides (LPS) into wild-type and GRdim mice. The response elicited, typical for endotoxic shock, can be monitored by the release of cytokines into the blood. Physiologically the response is then terminated by the action of endogenous GCs, which are released after activation of the hypothalamus–pituitary–adrenal (HPA) axis, and therefore abolished in adrenalectomized or hypophysectomized animals due to their inability to execute a corticosterone response (Zuckerman et al., 1989). To study the response, we followed secretion of the cytokine tumour necrosis factor-α (TNF-α) over time and found that TNF-α was massively induced at 1 h after LPS injection in both wild-type and mutant mice. The levels returned to basal values after 3 h (Figure 1D). In parallel, the serum concentrations of corticosterone were up-regulated (Figure 1E). The magnitude and kinetics of both TNF-α and corticosterone serum levels were similar in mice of both genotypes. This clearly indicates that DNA binding by GR is not required for terminating a systemic inflammatory response as induced by LPS injection.
The GRdim receptor represses cytokine gene expression in primary immune cells
Complex inflammatory responses such as the phorbol ester-induced oedema of the skin or endotoxic shock after LPS injection involve multiple cell types and the production of a variety of inflammatory mediators (Barnes, 1998). For instance, macrophages and T lymphocytes release cytokines. The sum of these reactions forms the pre-conditions for the development of innate and adaptive immunity. Inhibition by GCs occurs on several levels: removal of cells by apoptosis which could result from the reduction of intracellular components required for survival or from direct induction of a proapoptotic gene product; and inhibition of effector functions, e.g. the synthesis of cell surface proteins, of cytokines or proteins involved in invasion of immune cells into tissues (Barnes, 1998). We tested several of these parameters in our mutant mice.
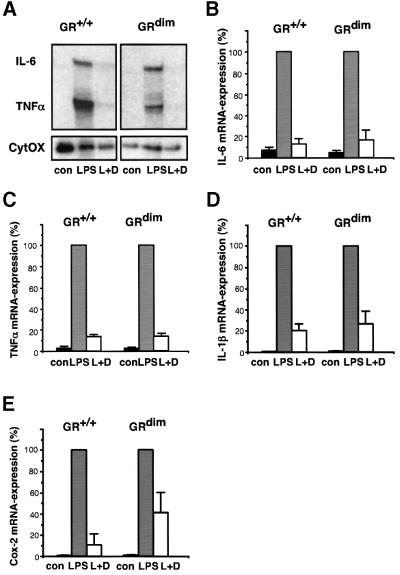
The potency of the DNA binding-defective GR to repress cytokine production in the living animal was explored by measuring the expression of cytokines in primary macrophages and lymphocytes. Peritoneal macrophages were isolated after thioglycolate treatment and cultured for 24 h prior to stimulation by LPS. mRNAs transcribed from the cytokine genes TNF-α, IL-6 and IL-1β were found to be up-regulated after 2 h of LPS treatment (Figure 2A–D). Regardless of the genotype, this induction was strongly inhibited when cells had been pre-treated with dexamethasone. In addition to cytokines, the release of prostaglandins is indicative of an inflammatory process. mRNA induction of the rate-limiting cyclooxygenase-2 gene (Cox-2) by LPS could be blocked efficiently by dexamethasone in macrophages of GRdim and wild-type mice (Figure 2E). Similarly, effective repression was observed for PMA-induced transcription in mouse embryonic fibroblasts (MEFs; data not shown).
Fig. 2. Repression of cytokine genes by GC in peritoneal macrophages does not require the DNA-binding function of GR. (A) TNF-α and IL-6 mRNA levels were determined by RNase protection assay in peritoneal macrophages of either wild-type (GR+/+) or GRdim mice cultured under the following conditions: mock-treated (con), treated with 100 ng/ml LPS for 2 h (LPS) and LPS reatment in the presence of 10–6 M dexamethasone (L+D). Dexamethasone was added 1 h prior to LPS. mRNA levels for CytOx (cytochrome oxidase) were used for normalization. (B and C) Quantitative evaluation of the data in (A). The levels after induction by LPS were taken as 100%. mRNA levels for IL-1β (D) and cyclooxygenase-2 (Cox-2) (E) were determined by quantitative PCR using HPRT for normalization.
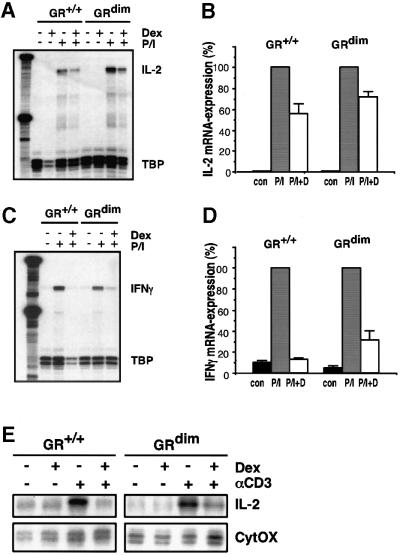
GCs inhibited the expression of T-cell cytokines. To this end, primary thymocytes from wild-type and GRdim mice were treated with PMA/ionomycin (P/I) as a model for antigen-induced activation of T cells. P/I application led to strong induction of IL-2 and interferon-γ (IFN-γ) mRNA expression, whereas simultaneous treatment with dexamethasone suppressed the P/I-induced levels in both types of mice to a similar extent (Figure 3A–D). Finally, CD4+ splenocytes were analysed as an example of mature T lymphocytes. T-cell receptor ligation by αCD3 caused induction of IL-2 mRNA expression, which was completely prevented by dexamethasone treatment (Figure 3E). No difference in the ability to suppress T-cell activity was found between wild-type and GRdim splenocytes.
Fig. 3. Repression of cytokine genes by GCs in T lymphocytes does not require the DNA-binding function of GR. Primary thymocytes of either wild-type (GR+/+) or GRdim genotype were cultured in the absence (con) or presence of 10–6 M dexamethasone and 10 µg/ml PMA/0.5 µg/ml ionomycin (P/I) for 6 h. (A and C) IL-2 and IFN-γ mRNA levels were determined by RNase protection analysis and normalized to TATA box-binding protein (TBP). (B and D) Quantitative evaluation of the data shown in (A) and (C). The level after induction by P/I was taken as 100%. (E) IL-2 analysis of primary CD4+ splenocytes by RNase protection using CytOX RNA for normalization. Cells were cultured on αCD3-coated culture dishes in the absence or presence of 10–6 M dexamethasone for 4 h.
Cycloheximide experiments indicated that the effects of GCs were direct (not shown), which is compatible with the properties of the mutant GR. DNA binding by GR is obviously dispensable for the repression of Cox-2 transcription and that of various cytokine genes in all primary cells tested.
Glucocorticoids repress pro-inflammatory NF-κB activity in the absence of GR DNA binding
The expression of most pro-inflammatory genes, including those investigated here, depends on the activation of NF-κB (Baeuerle and Baltimore, 1996). NF-κB most probably represents the major target of GR in the inhibition of an inflammatory response. Two modes of repression of NF-κB activity have been described: (i) inhibition occurs by protein–protein interaction between promoter-bound NF-κB and GR (Caldenhoven et al., 1995; Heck et al., 1997; Nissen and Yamamoto, 2000); and (ii) GCs induce the transcription of IκBα resulting in sequestering nuclear NF-κB into the cytoplasm, thereby abolishing transcription (Auphan et al., 1995; Scheinman et al., 1995). In the mutant mice experiments described here, a direct GRE-dependent transcription of IκBα does not seem to play a major role as the mutant GR cannot activate genes and yet the inflammatory response is inhibited. One could, however, imagine the existence of a mode of IκBα induction not requiring direct GR–DNA binding. Such GR action has been found for the synergy with Jun homodimers (Diamond et al., 1990; Teurich and Angel, 1995). We therefore investigated the fate of IκBα and NF-κB activity in GRdim mice.4
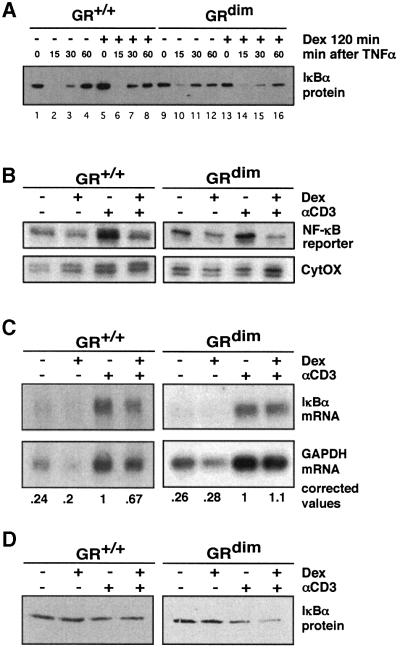
Fig. 4. Induction of IκBα expression is not required for repression of NF-κB activity in MEFs and CD4+ splenocytes. (A) GR+/+ and GRdim MEFs were treated with 10–7 M dexamethasone for 2 h followed by treatment with 50 ng/ml TNF-α. IκBα protein content was measured by immunoblotting. (B) CD4+ splenocytes from NF-κB transgenic reporter mice expressing either wild-type (GR+/+) or DNA binding-defective GR (GRdim) were cultured on αCD3-coated culture dishes in the absence or presence of 10–6 M dexamethasone for 4 h. Expression of the reporter gene (B) was determined by RNase protection analysis and normalized to CytOX. Relative IκBα mRNA levels (C) were determined by northern blot analysis and IκBα protein (D) was detected by western blot analysis.
The expression of IκBα was measured in MEFs and splenic CD4+ cells. Primary MEFs isolated from both wild-type and GRdim mice responded to treatment with TNF-α with disappearance and then delayed resynthesis of IκBα protein. In wild-type cells, but not cells from GRdim mice, GC treatment caused >2-fold increases of IκBα mRNA (data not shown) and protein beyond basal levels (Figure 4A, compare lanes 1 and 5 for wild-type and lanes 9 and 13 for GRdim), also reflected in earlier resynthesis (Figure 4A, compare lanes 3 and 7). IκBα transcription thus appears to depend on proper GR–GRE binding or on an unknown reaction requiring GR dimer formation. In electrophoretic mobility shift assay (EMSA) experiments, the same cells of both genotypes showed identical kinetics for the formation and stability of NF-κB–DNA complexes after stimulation with TNF-α, both with and without treatment with GCs (not shown), compatible with the idea that inhibition occurs after binding of NF-κB to DNA.
Splenic CD4+ cells were isolated from both wild-type and GRdim mice into both of which we had crossed a transgene reporter carrying three NF-κB-binding sites as the only promoter elements in front of the β-globin TATA box (Lernbecher et al., 1993). This setting permits analysis of IL-2 transcription, IκBα and exclusive NF-κB activity in the same primary cells. Stimulation of the cells with αCD3 led to a marked increase of mRNA expression of the reporter (Figure 4B) and of IL-2 (not shown), indicating induction of NF-κB activity. Concomitant addition of GC repressed reporter expression (as well as IL-2) equally well in both genotypes (Figure 4B), demonstrating that NF-κB activity is fully inhibited by GR in the absence of its DNA-binding function. Interestingly, in CD4+ cells IκBα mRNA and protein were not induced by GC alone (Figure 4C and D), perhaps suggesting a cell type-specific contribution to its regulation. The levels achieved upon αCD3 treatment were somewhat inhibited by the presence of dexamethasone. These data further confirm that the inhibition of NF-κB does not depend on elevated IκBα.
Taken together, the results obtained in MEFs and CD4+ splenocytes demonstrate that efficient repression of NF-κB activity occurs in the absence of DNA binding by GR and that induction of IκBα by GC does not play a significant role in conferring GC-dependent repression of NF-κB activity under the conditions tested here. These results are in agreement with the finding that GCs do not interfere with the formation of a pre-initiation complex at an NF-κB-dependent promoter but rather trigger a subsequent step of transcriptional initiation (Nissen and Yamamoto, 2000).
The reduction of cytokine release upon GC treatment may well suffice to bring dependent cells into apoptosis. The ability of GCs to induce apoptosis is indeed used for the treatment of leukemia. In cell culture, a transactivation-defective GR with non-disturbed inhibitory activity on AP-1 could indeed block the proliferation of appropriately designed Jurkat cells (Helmberg et al., 1995). However, peripheral T lymphocytes, thymocytes (Reichardt et al., 1998) and thymocytes in fetal thymus organ culture (not shown) were resistant, indicating that a GR dimer-specific gene programme was needed to bring these cells into apoptosis. The difference from leukemic cells may be that the survival of these primary cells has not yet become cytokine dependent. For the anti-inflammatory action of GCs in vivo, induced apoptosis thus does not seem necessary or limiting.
Conclusions
GCs are among the most widely employed anti-inflammatory drugs, although long-term application is accompanied by massive side effects. Thus, it is conceivable that the search for improved drugs is a major challenge for pharmacological research. The finding that GR mediates the effects of GCs by two different modes of action has suggested that interfering with one of these may provide a tool to develop new anti-inflammatory compounds. Since DNA binding is abrogated in GRdim mice, these animals are the ideal model to determine the contribution of protein–protein interaction of GR to the anti-inflammatory action of GCs in vivo. In this study, we have shown that inflammatory responses in the living animal are repressed efficiently in the absence of DNA binding. These results strongly suggest that this repression is due, at least in part, to the unimpaired ability of the GRdim receptor to inhibit pro-inflammatory protein production by macrophages and T lymphocytes, through negative interference with NF-κB and possibly other pro-inflammatory transcription factors. The reduction of cytokines may also explain why B cells of GRdim mice enter apoptosis (our unpublished data). A separate pro-apoptotic programme requiring GR DNA binding exists in thymocytes and mature T cells. Our findings suggest that ligands selectively acting via the DNA binding-independent function of GR should suffice for the treatment of inflammatory diseases. Such ligands would possibly help to avoid the adverse side effects of long-term treatment, such as GC-induced osteoporosis, growth retardation, redistribution of fat, muscle degeneration and, when topically applied, atrophy of the skin.
Materials and methods
RNA analysis
Total RNA was isolated after guanidinium isothiocyanate extraction according to standard procedures. RNase protection assays and cDNA synthesis were done as described previously (Reichardt et al., 1998). RNase protection assays were quantified using a phosphoimager. Quantitative PCR was performed using a Lightcycler System according to the manufacturer’s instructions (Roche Diagnostics, Mannheim, Germany) using the following primers: 5′-CATTATGCCGAGGATTTG and 5′-TGGGGCTGTACTGCTTA for the amplification of a 389 bp fragment of hprt, 5′-GCAAACGCTTCTCCCTGAAG and 5′-CGCTTGCATTGATGGTGGCTG for the amplification of a 389 bp fragment of cox-2, and 5′-TCCTGAACTCAACTGTGA and 5′-CCAGCAGGTTATCATCAT for amplification of a 469 bp fragment of IL-1β.
Protein analysis
For western blotting analysis, proteins were extracted from cells in 50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 10 mM NaF, 0.5 mM sodium vanadate, leupeptin (10 µg/ml), 1% (v/v) NP-40 and 10% (v/v) glycerol, and separated on a 12% SDS– polyacrylamide gel. Proteins were transferred to a polyvinylidene difluoride membrane, hybridized with a rabbit IκBα antiserum (kindly provided by Dr L.Schmitz, Heidelberg, Germany) and immunoreactive bands visualized by enhanced chemiluminescence.
Isolation and cultivation of primary cells
MEFs were isolated from day 14.5 embryos as described previously (Reichardt et al., 1998). The CD4+ subpopulation of splenic lymphocytes was isolated by magnetic bead selection and cultured on anti-CD3-coated culture dishes (mAb145-2C11, 1 µg/cm2). Primary thymocytes were isolated as described previously (Reichardt et al., 1998) and cultured at a concentration of 106 cells/ml. To isolate macrophages, mice were injected intraperitoneally with 1.5 ml of sterile thioglycollate medium 4 days prior to the experiment. Macrophages were harvested by peritoneal lavage with 2 ml of sterile phosphate-buffered saline, centrifuged and resuspended in RPMI medium supplemented with 10% fetal calf serum (FCS). The cells were seeded at a concentration of 106 cells/ml in 24-well plates, incubated for 24 h at 37°C and washed to remove non-adherent cells. During the experiment, FCS was omitted.
Hormone determination
Serum was obtained by centrifugation of freshly isolated EDTA-blood at 5000 r.p.m. for 10 min. The concentration of TNF-α and IL-6 was determined by enzyme-linked immunosorbent assay (ELISA) and the level of corticosterone by radioimmunoassay (RIA) using commercially available kits (Endogen, Woburn, USA and ICN Biomedicals, Meckenheim, Germany).
Animal experimentation
To study systemic inflammation, mice were injected intraperitoneally with 100 µg of LPS, killed by CO2 and the blood collected for cytokine and hormone determination.
To induce local inflammation in the dorsal skin, mice were shaved 4 days before experimentation and treated topically with 10 nmol PMA or in combination with 50 µg of dexamethasone dissolved in 200 µl of acetone as described (Tuckermann et al., 1999). The animals were killed 6 h after application and both skin biopsies and the blood were collected for histological analysis and cytokine measurement. The ear oedema formation assay (Gschwendt et al., 1984) was performed by ectopic application of the vehicle acetone, 1 nmol PMA or PMA plus 5 µg of dexamethasone to the ear of the mice. After 6 h, a defined area of the ear was excised and the weight determined.
Acknowledgments
Acknowledgements
We are grateful to Nadine Sold, Heike Alter and Margarethe Litfin for technical assistance, Thomas Wirth for providing the NF-κB reporter mice, Lienhard Schmitz for providing IκBα antibodies and Kathrin Michelsen and Jens Eberlein for their help with the inflammation experiments. This work was supported by the TMR and Biomed-2 programs of the European Community (contracts CT-960044 and CT-963505) and the Boehringer Ingelheim Fonds.
References
- Auphan N., DiDonato,J.A., Rosette,C., Helmberg,A. and Karin,M. (1995) Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science, 270, 286–290. [DOI] [PubMed] [Google Scholar]
- Baeuerle P.A. and Baltimore,D. (1996) NF-κB: ten years after. Cell, 87, 13–20. [DOI] [PubMed] [Google Scholar]
- Barnes P.J. (1998) Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci., 94, 557–572. [DOI] [PubMed] [Google Scholar]
- Beato M., Herrlich,P. and Schütz,G. (1995) Steroid hormone receptors: many actors in search of a plot. Cell, 83, 851–857. [DOI] [PubMed] [Google Scholar]
- Brostjan C., Anrather,J., Csizmadia,V., Natarajan,G. and Winkler,H. (1997) Glucocorticoids inhibit E-selectin expression by targeting NF-κB and not ATF/c-Jun. J. Immunol., 158, 3836–3844. [PubMed] [Google Scholar]
- Caldenhoven E., Liden,J., Wissink,S., Van de Stolpe,A., Raaijmakers,J., Koenderman,L., Okret,S., Gustafsson,J.A. and Van der Saag,P.T. (1995) Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids. Mol. Endocrinol., 9, 401–412. [DOI] [PubMed] [Google Scholar]
- Diamond M.I., Miner,J.N., Yoshinaga,S.K. and Yamamoto,K.R. (1990) Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science, 249, 1266–1272. [DOI] [PubMed] [Google Scholar]
- Evans R.M. (1988) The steroid and thyroid hormone receptor superfamily. Science, 240, 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwendt M., Kittstein,W., Furstenberger,G. and Marks,F. (1984) The mouse ear edema: a quantitatively evaluable assay for tumor promoting compounds and for inhibitors of tumor promotion. Cancer Lett., 25, 177–185. [DOI] [PubMed] [Google Scholar]
- Heck S., Kullmann,M., Gast,A., Ponta,H., Rahmsdorf,H.J., Herrlich,P. and Cato,A.C. (1994) A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J., 13, 4087–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck S., Bender,K., Kullmann,M., Gottlicher,M., Herrlich,P. and Cato,A.C. (1997) IκBα-independent downregulation of NF-κB activity by glucocorticoid receptor. EMBO J., 16, 4698–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmberg A., Auphan,N., Caelles,C. and Karin,M. (1995) Gluco corticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J., 14, 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P. (2001) Cross-talk between glucocorticoid receptor and AP-1. Oncogene, 20, 2465–2475. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf,H.J., Park,K.K., Cato,A.C., Gebel,S., Ponta,H. and Herrlich,P. (1990) Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell, 62, 1189–1204. [DOI] [PubMed] [Google Scholar]
- Karin M. (1998) New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell, 93, 487–490. [DOI] [PubMed] [Google Scholar]
- Lernbecher T., Muller,U. and Wirth,T. (1993) Distinct NF-κB/Rel transcription factors are responsible for tissue-specific and inducible gene activation. Nature, 365, 767–770. [DOI] [PubMed] [Google Scholar]
- Lloret S. and Moreno,J.J. (1995) Effects of an anti-inflammatory peptide (antiflammin 2) on cell influx, eicosanoid biosynthesis and oedema formation by arachidonic acid and tetradecanoyl phorbol dermal application. Biochem. Pharmacol., 50, 347–353. [DOI] [PubMed] [Google Scholar]
- Nissen R.M. and Yamamoto,K.R. (2000) The glucocorticoid receptor inhibits NFκB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev., 14, 2314–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt H.M. et al. (1998) DNA binding of the glucocorticoid receptor is not essential for survival. Cell, 93, 531–541. [DOI] [PubMed] [Google Scholar]
- Scheinman R.I., Cogswell,P.C., Lofquist,A.K. and Baldwin,A.S.,Jr (1995) Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science, 270, 283–286. [DOI] [PubMed] [Google Scholar]
- Stöcklin E., Wissler,M., Gouilleux,F. and Groner,B. (1996) Functional interactions between Stat5 and the glucocorticoid receptor. Nature, 383, 726–728. [DOI] [PubMed] [Google Scholar]
- Teurich S. and Angel,P. (1995) The glucocorticoid receptor synergizes with Jun homodimers to activate AP-1-regulated promoters lacking GR binding sites. Chem. Senses, 20, 251–255. [DOI] [PubMed] [Google Scholar]
- Tuckermann J.P., Reichardt,H.M., Arribas,R., Richter,K.H., Schütz,G. and Angel,P. (1999) The DNA binding-independent function of the glucocorticoid receptor mediates repression of AP-1-dependent genes in skin. J. Cell Biol., 147, 1365–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens T. and De Rijk,R. (1997) Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol. Today, 18, 418–424. [DOI] [PubMed] [Google Scholar]
- Zuckerman S.H., Shellhaas,J. and Butler,L.D. (1989) Differential regulation of lipopolysaccharide-induced interleukin 1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticoids and the role of the pituitary–adrenal axis. Eur. J. Immunol., 19, 301–305. [DOI] [PubMed] [Google Scholar]