Abstract
In yeast, environmental conditions control the transcription factor Msn2, the nuclear accumulation and function of which serve as a sensitive indicator of nutrient availablity and environmental stress load. We show here that the nuclear localization signal (NLS) of Msn2 is a direct target of cAMP-dependent protein kinase (cAPK). Genetic analysis suggests that Msn2-NLS function is inhibited by phosphorylation and activated by dephosphorylation. Msn2-NLS function is unaffected by many stress conditions that normally induce nuclear accumulation of full-length Msn2. The Msn2-NLS phosphorylation status is, however, highly sensitive to carbohydrate fluctuations during fermentative growth. Dephosphorylation occurs in >2 min after glucose withdrawal but the effect is reversed rapidly by refeeding with glucose. This response to glucose depletion is due to changes in cAPK activity rather than an increase in protein phosphatase activity. Surprisingly, the classical glucose-sensing systems are not connected to this rapid response system. Our results further imply that generic stress signals do not cause short-term depressions in cAPK activity. They operate on Msn2 by affecting an Msn5-dependent nuclear export and/or retention mechanism.
Keywords: cAMP/nuclear localization/Saccharomyces cerevisiae/stress/TOR
Introduction
The combined influences of nutrients and stress factors determine whether and at what rate a microorganism such as the budding yeast Saccharomyces cerevisiae will grow and divide. Glucose is the preferred carbon source of this yeast, and it is therefore not surprising that pathways involved in glucose sensing and signalling are crucial regulators of cellular physiology. Transduction of glucose signals involves two main regulatory systems that respond in opposite ways. The protein kinase Snf1, a relative of mammalian AMP-dependent protein kinases, is activated by glucose limitation, thereby orchestrating the induction of glucose-repressed genes (Gancedo, 1998; Carlson, 1999). In contrast, the cAMP-dependent protein kinase (cAPK) functions as a positive, Snf1-independent mediator of high glucose levels. cAPK is also a key regulator of cellular growth, cell cycle progression and the metabolic reprogramming at the diauxic growth transition. The kinase further influences the extent of transcriptional responses to environmental stress signals and nutrient availability (Thompson-Jaeger et al., 1991; Thevelein, 1994). Mutants lacking Bcy1 (the negative regulatory cAPK subunit) exhibit cAMP-independent, high constitutive cAPK activity. They are sensitive to stress and nutrient limitation, fail to accumulate glycogen and are severely impeded in their ability to grow on carbon sources other than glucose. Mutants with low cAPK display the opposite phenotype, including high resistance to stress (Iida, 1988; Norbeck and Blomberg, 2000). The second messenger cAMP is synthesized by adenylate cyclase which, in the case of budding yeast, is stimulated either by Ras proteins (possibly by reacting to intracellular acidification) or by glucose via the Gpr1–Gpa2 G-protein system (Broach and Deschenes, 1990; Thevelein and de Winde, 1999; Rolland et al., 2001). Addition of glucose to carbohydrate-starved cells has been shown temporarily to activate cAMP production and cAPK. Large differences in cAPK activity also seem to exist between cells that are logarithmically growing on glucose and cells that have passed through the diauxic shift and are adapted to non-fermenting growth. Recent evidence shows that yeast cells will also react quickly to a sudden drop in carbohydrate levels. They respond to such an insult within minutes by rapidly reorganizing the intracellular localization of Gal83, a regulatory factor of the Snf1 kinase (Vincent et al., 2001), by activation of the Yak1 protein kinase (Moriya et al., 2001) and by changes in the translational apparatus (Ashe et al., 2000). They also respond by activating a transcriptional programme dedicated to general stress protection (C.Schüller, unpublished). Despite these observations, surprisingly little information is available on cAMP levels and cAPK activity upon acute glucose depletion. Moreover, the question of whether cAPK activities are regulated by generic stress conditions, either through cAMP levels or by cAMP-independent mechanisms, has never been clarified.
Msn2 and Msn4 are C2H2 zinc finger proteins that recognize the so-called stress response elements (STREs) (Ruis and Schüller, 1995; Martinez-Pastor et al., 1996). The transcription of ∼180 genes is up-regulated by these two factors in response to environmental stresses (Gasch et al., 2000). Regulation is thought to be achieved through controlled binding of Msn2 to respective target promoters. Furthermore, the intracellular localization of Msn2 and Msn4 is highly sensitive to environmental stress conditions such as weak organic acids, osmotic stress, heat stress and nutrient starvation (Görner et al., 1998). During logarithmic growth, both proteins are predominantly cytoplasmic. However, in stressed cells or when glucose is suddenly withdrawn, they rapidly concentrate in the nucleus. Moreover, the intracellular distribution of Msn2 and Msn4 is closely linked to the status of cAPK activity. Low cAPK activity leads to nuclear accumulation of Msn2 and Msn4, whereas high activity causes nuclear Msn2 and Msn4 to be redistributed to the cytoplasm. High cAPK activity may counteract stress-induced nuclear import, accelerate nuclear export or change the interaction with localized retention factors (Görner et al., 1998, 1999). The connection between the cAPK regulatory system and the transcriptional component of the stress response is physiologically highly relevant. Down-regulation of both Msn2 and Msn4 and consequently STRE-regulated genes, by cAPK is a key requirement for yeast growth and division; yeast cells lacking cAPK are inviable, but cells deficient in Msn2/Msn4 or in the STRE-regulated gene YAK1 will grow without cAPK (Smith et al., 1998).
The TOR signal pathway is another prominent player dedicated to the regulation of cellular growth (Barbet et al., 1996; Di Como and Arndt, 1996). Yeast have two TOR kinases, which are both inhibited by complexes consisting of the drug rapamycin and FK506-binding protein Fpr1 (Heitman et al., 1991). Cells treated with rapamycin arrest in G1, down-regulate translation and exhibit the physiological characteristics of nutrient starvation (G0 arrest). Recently, the TOR pathway has been implicated in the regulation of Msn2 and Msn4 localization (Beck and Hall, 1999). Both transcription factors were found to interact with the 14-3-3 protein Bmh2 in vivo. In the presence of rapamycin, the interaction was weakened, coinciding with nuclear accumulation of Msn2. Rapamycin did not affect Msn2 localization in the drug-resistant TOR1-1 mutant background (Beck and Hall, 1999; for a review see Schmelzle and Hall, 2000), suggesting that inactivation of TOR kinase activity is a prerequisite for Msn2 nuclear accumulation during nitrogen starvation.
The mechanism by which glucose and different stress regimens converge on Msn2 has remained an interesting problem serving as a model for how different signals are integrated by one transcription factor. In this study, we focused on the question of how regulated nuclear import of Msn2 could contribute to the overall localization of Msn2. Interestingly, most changes in external conditions did not affect the functional state of the main nuclear localization signal (NLS) of Msn2. However, a large effect was noted after withdrawal of glucose. Subsequently, we could demonstrate that the NLS-containing domain is directly modified by cAPK in vitro and in vivo. We propose that phosphorylation will decrease its activity and thereby influence the overall intracellular distribution of Msn2. During glucose starvation, dephosphorylation-mediated activation of the Msn2-NLS can be surprisingly rapid and among the first easily recognizable events. On the whole, it is dependent on the rapid down-regulation of cAPK activity via a Bcy1-dependent mechanism. Measuring Msn2-NLS phosphorylation thus provides a novel and effective assay for monitoring in vivo fluctuations in cAPK activity. Such measurements allowed us to propose that the cAPK system is dedicated to the signalling of glucose sufficiency to Msn2. Stress-related signals do not touch the regulation of the nuclear import system but are focused on a region conferring nuclear export.
Results
The Msn2-NLS responds to glucose starvation
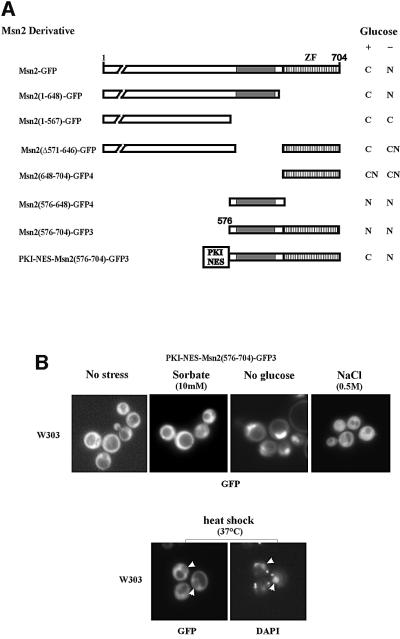
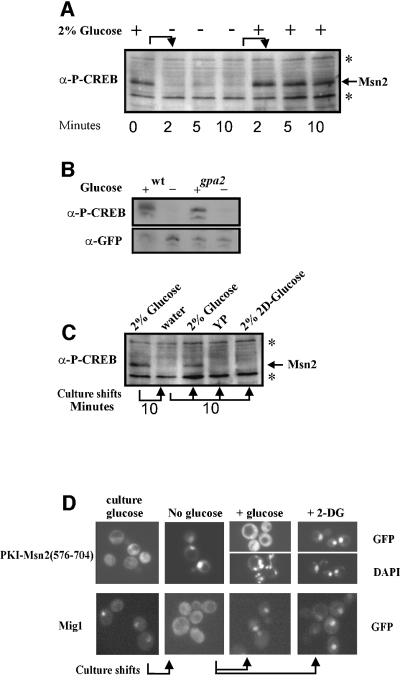
Environmental stresses and low cAPK activity trigger nuclear accumulation of Msn2, which apparently serves as an integration point for these signals. To identify the molecular mechanisms for this integration, we analysed the domain responsible for nuclear import of Msn2. A region containing such function had been mapped previously near the C-terminal DNA-binding domain (Görner et al., 1998). Further deletion analysis, as summarized in Figure 1A, showed that Msn2 nuclear import is mediated by a region between amino acids 576 and 648. A second region, but with much weaker nuclear import activity, is apparently overlapping the Msn2 zinc finger domain (amino acids 648–704). Both domains were able to impose predominantly nuclear localization on a 3× green fluorescent protein (GFP) fusion protein. To compensate for the absence of nuclear export, the fragments were fused N-terminally to the heterologous protein kinase A inhibitor nuclear export signal (PKI-NES) (Wen et al., 1994), an export signal unresponsive to the stress regimens used in this study (data not shown). On their own, neither of the putative Msn2-NLS domains was able to over come the activity provided by the constitutive PKI-NES (not shown). A PKI-NES–Msn2(576–704)–GFP3 fusion protein containing both NLS domains was also cytoplasmic under optimal growth conditions but nuclear upon glucose starvation stress (Figure 1). Unexpectedly, any other types of stress (sorbate, NaCl, heat shock; Figure 1B; rapamycin, see Figure 4C) did not trigger an increased nuclear signal. In the case of heat shock, even partial nuclear exclusion of the construct was observed. This large discrepancy in the effects suggested that most stress signals must differ fundamentally from those generated by acute glucose depletion. Furthermore, the C-terminus of Msn2 could be viewed as an exclusive target of signals induced by acute glucose starvation.
Fig. 1. Definition of the nuclear localization sequence of Msn2 by localization studies on Msn2–green fluorescent protein (GFP) derivatives. (A) Msn2–GFP derivatives were expressed in strain W303 msn2msn4 and grown to logarithmic phase. The subcellular localization of Msn2–GFP derivatives was determined before and 5 min after glucose withdrawal [indicated intracellular localization: (N) nuclear, (C) cytoplasmic, (CN) slight nuclear staining]. The constitutive nuclear export signal of protein kinase A inhibitor (PKI) is indicated by PKI-NES. The striped bars represent the zinc finger domain, grey bars the parts of the Msn2 nuclear import domain mediating regulation. (B) Intracellular localization of PKI-NES–Msn2(576–704)–GFP3 fusion protein under non-stress and stress conditions. Logarithmic cells were treated for 10 min with the indicated stresses. Micrographs of GFP and DAPI fluorescence from living cells are shown. Arrows (heat shock experiment) indicate areas of nuclear exclusion of the GFP construct.
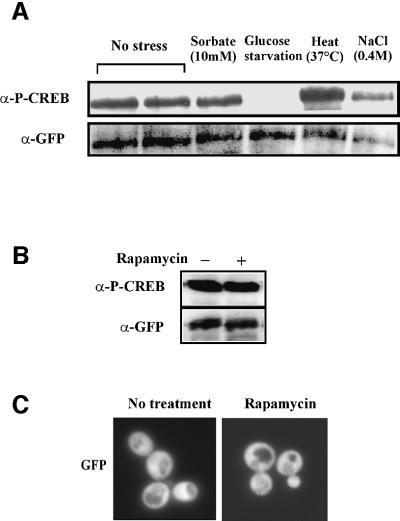
Fig. 4. Phosphorylation state of the Msn2-NLS under different stress conditions. (A) Extracts from W303 cells expressing full-length Msn2–GFP fusion protein before or after 10 min exposure to stress. Phosphorylation of the Msn2-NLS was assayed by α-P-CREB (S133) antibody. (B) Rapamycin treatment of W303 cells expressing the PKI-NES–Msn2(576–704)–GFP fusion protein. Rapamycin was added to 200 ng/ml for 10 min. (C) Intracellular localization of the PKI-NES–Msn2(576–704)–GFP. Conditions are as described in (B).
cAPK directly phosphorylates the Msn2-NLS
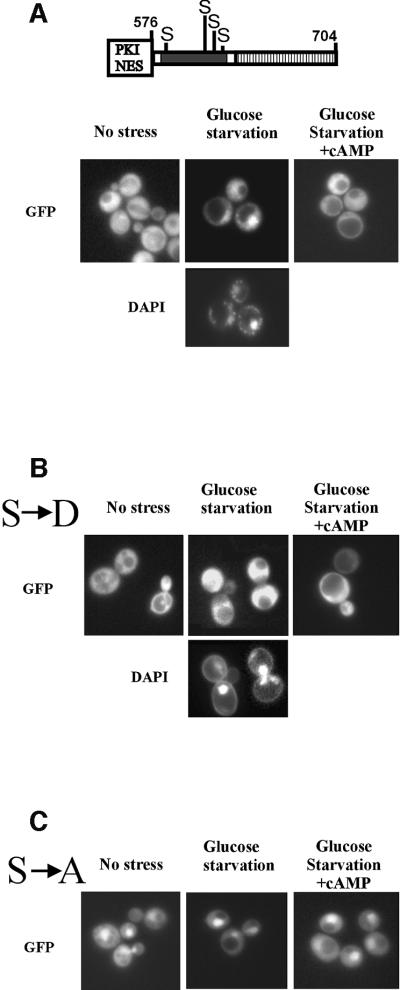
The Msn2-NLS, as defined above, contains a cluster of four cAPK consensus sites (RRXS). It was therefore reasonable to suspect a regulatory role for cAPK for the activity of the Msn2-NLS. If this was the case, high cAPK activity should attenuate or even prevent the nuclear import of the PKI–Msn2(576–704)–GFP3 fusion protein. Indeed, nuclear accumulation induced by glucose starvation was reversed by activation of cAPK by supplying exogenous cAMP in a pde2 strain. In contrast, when nuclear import was blocked by high cAPK activity, the fusion protein accumulated in the cytoplasm (Figure 2A). To assay whether the putative cAPK consensus sites of the Msn2-NLS have a regulatory role, aspartate and alanine substitutions of the respective serine residues were generated. Aspartate mutations mimicking permanent phosphorylation of serines prevented the Msn2-NLS mutant form from accumulating in the nucleus when glucose was withdrawn (Figure 2B). In contrast, alanine mutations led to permanent nuclear localization, even when cAPK activity was induced by exogenous cAMP (Figure 2C). Alanine substitutions at individual sites had no dramatic effects, suggesting their functional redundancy and equivalence for the response. Combinations of mutations of fewer than four sites had partial phenotypes whose severity depended more on their number than their exact position (see below and data not shown). The functional redundancy becomes apparent, for example, by evaluating the basal expression of an Msn2-dependent lacZ reporter gene in the different substitution mutants (Figure 3C); increases in expression level only seem to correlate with the number of alanine substitutions but are independent of their position. At the same time, these results indicate that different states of Msn2 modification might not only have functional consequences but also physiological consequences for Msn2-NLS activity.
Fig. 2. Putative cAPK sites within the Msn2-NLS control nuclear import activity. Subcellular localization of PKI-NES– Msn2(576–704)–GFP3 in logarithmic cells (no stress), under glucose starvation stress and after addition of cAMP (20 mM) to stressed cells for 10 min. Serine residues of consensus cAPK sites (RRXS) are indicated. (A) Wild-type allele. (B) Effect of serine to aspartate mutations (S→D). (C) Serine to alanine mutations (S→A). Three tandem GFP fusions were used to rule out diffusion effects.
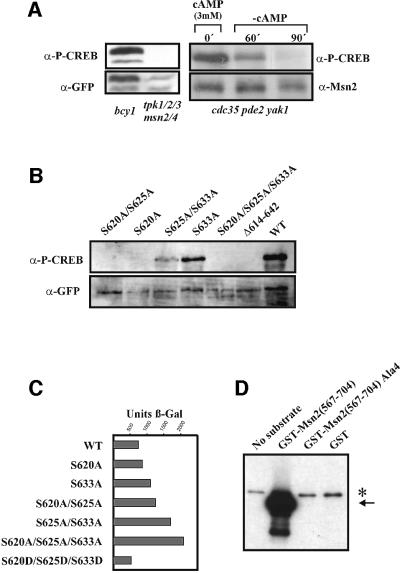
Fig. 3. cAPK modifies the Msn2-NLS by phosphorylation. (A) PKI-NES–Msn2(576–704)–GFP was expressed in W303 bcy1 (unregulated PKA activity), W303 tpk123msn2msn4 (no cAPK activity) and W303 cdc35pde2yak1 (adjustable cAPK activity) mutant strains. The phosphorylation state of Msn2-NLS Ser620 was assayed with anti-phospho-CREB (S133) antibody (α-P-CREB). The amounts of Msn2–GFP fusion proteins and Msn2p were monitored using anti-GFP antibody (α-GFP) and a polyclonal Msn2 antiserum (α-Msn2). A conditional CUP1-MSN2 gene was used to transiently increase the level of Msn2p in the cdc35pde2yak1 background. (B) Recognition of α-P-CREB antibody requires intact cAPK consensus sites within the Msn2-NLS. Plasmids harbouring Msn2–GFP derivatives with combinations of S→A substitutins were expressed in strain W303 bcy1. Anti-GFP antibody was used for the loading control. (C) Transcriptional activity of MSN2 NLS substitution mutants: Msn2 variants with the indicated modifications were assayed in W303 msn2msn4 harbouring a 7× STRE-lacZ reporter. β-galactosidase activity was determined in extracts from unstressed cells. (D) Autoradiograph of an in vitro phosphorylation assay using cAPK purified from yeast. Escherichia coli-derived GST fusions of the Msn2-NLS were incubated with cAPK and [γ-32P]ATP. Msn2-NLS with serines in cAPK consensus sites exchanged for alanine residues and GST alone were used as negative controls. Arrows indicate phosphorylation-dependent bands of the Msn2-NLS. The asterisk indicates a substrate-independent band.
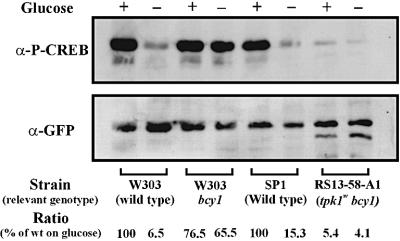
Consequently, we investigated whether the cAPK consensus sites of the Msn2-NLS are indeed phosphorylated and whether this phosphorylation is responsive to cAPK activity and stress. Amino acids 614–625 of the Msn2-NLS are identical to the region around Ser133 of the mammalian transcription factor cAMP response element-binding protein (CREB). Therefore, we tested whether a commercial, phosphorylation-specific antibody against this motif could recognize an Msn2-NLS–GFP fusion protein. A bcy1 strain provided the source for extracts with high level cAPK activity, whereas a tpk1tpk2tpk3msn2msn4 strain was used as a control strain lacking all cAPK activity. Yeast strains with defects in all three TPK genes are unable to grow in the presence of Msn2. Therefore, this experiment was carried out in a strain lacking Msn2 and Msn4 (Smith et al., 1998) using a transcriptionally inactive Msn2-NLS construct. The phosphorylation patterns of the NLS of the full-length Msn2 and of the truncated Msn2-NLS protein as assayed with the phospho-CREB antibody were identical in all experiments carried out in this study. In the bcy1 strain, a strong signal was observed in western blots with phospho-CREB antibody. This signal was absent in extracts from a cAPK-deficient strain. GFP tags detected with anti-GFP antibody were used as an internal reference for the level of protein (Figure 3A). Using a strain that allows complete manipulation of cAPK activity by exogenous sources (cdc35pde2yak1), we could also establish a direct correlation between the extent of phosphorylation and extracellular cAMP concentration (Figure 3A).
Antibody reactivity was highly dependent on the integrity of one of the CREB-related motifs found in the Msn2-NLS. Alanine substitution at S620 abolished recognition of the full-length Msn2–GFP fusion, whereas such substitutions at S625 and S633 caused only a minor or no reduction (Figure 3B). Protein kinase assays with yeast cAPK purified as a complex with TAP-tagged Bcy1 (Rigaut et al., 1999; J.Norbeck, in preparation) confirmed that yeast cAPK phosphorylates the Msn2-NLS in vitro as the quadruple alanine substitution protein remained unphosphorylated under the same conditions (Figure 3D). From the results summarized in Figure 3, we infer that Msn2-S620 is directly phosphorylated by cAPK and that the signal detected by the phospho-CREB antibody faithfully reflects cAPK activity towards the Msn2-NLS.
Msn2-NLS phosphorylation is insensitive towards stress and TOR pathway activity
Accepting that the modification-specific antibody provides a reliable reagent for monitoring the phosphorylation state of the Msn2-NLS, we tested whether any changes in phosphorylation occurred after stress treatment. W303 wild-type cells were grown to logarithmic phase and then treated with different stress conditions for 5 min. It should be noted that any of the employed stress regimens induces rapid nuclear accumulation of the full-length Msn2–GFP fusion (Görner et al., 1998). Extracts were prepared from unwashed cell pellets and compared on western blots after phospho-CREB and anti-GFP antibody detection (Figure 4A). As expected, the Msn2-NLS is heavily phosphorylated under non-stress conditions. Stress treatment with sorbate (10 mM), osmotic stress (0.4 M NaCl; Figure 4A) and rapamycin (200 ng/ml; Figure 4B) did not cause any changes in the phospho-CREB signal. Surprisingly, heat shock even seemed to increase the levels of phosphorylation, an effect that was found to depend on the presence of cAPK (W.Görner, unpublished observation). Removal of external glucose, however, was followed by a significant loss of the phospho-CREB signal (Figure 4A). Thus, with regard to the initiating events, the phosphorylation data show exquisite correlation with the cytological measurements for nuclear import activity. Together, both data sets point towards a fundamental separation of carbohydrate starvation and stress signals.
The TOR pathway is an important system connecting nitrogen availability to cell growth. Rapamycin treatment is thought to mimic nitrogen depletion due to its inhibition of the TOR kinase pathway. Interestingly, Msn2 accumulates in the nucleus of cells exposed to rapamycin (Beck and Hall, 1999). We confirmed this observation in living cells. After addition of rapamycin to the medium of logarithmically growing cells, we could observe a shift of full-length Msn2–GFP to the nucleus within 5 min (not shown). However, the Msn2-NLS was not dephosphorylated by this treatment (Figure 4B). Indeed, a PKI-NES–Msn2-NLS fusion protein remained cytoplasmic (Figure 4C), despite the fact that the same rapamycin concentrations efficiently inhibited growth of the yeast strains under study (not shown). By inference from independent biochemical and cell biological observations, Beck and Hall (1999) proposed that the 14-3-3 protein Bim2 might function as a rapamycin-sensitive cytoplasmic anchor, perhaps by controlling access of Msn2 to its nuclear transporters. We therefore looked at the distribution of the PKI-NES–Msn2-NLS–GFP fusion in bmh1 bmh2 and bmh2 cells. We found that such cells do not exhibit stronger basal nuclear GFP signals than wild-type cells, even if the appropriate nucleo/cytoplasmic redistribution pattern can be induced easily in the mutant cells through glucose starvation and refeeding (data not shown). Thus, it is unlikely that a putative Bmh2-dependent retention mechanism operates through the NLS domain. Moreover, since a downshift in TOR pathway activity per se does not impart on the Msn2 nuclear import signals, we infer that, in the short term, nitrogen depletion signals do not spill into the cAPK pathway.
Dephosphorylation of the Msn2-NLS requires Bcy1-dependent down-regulation of cAPK
As shown above, glucose withdrawal is closely followed by rapid dephosphorylation of the Msn2-NLS. Principally, this event could either be due to down-regulation of cAPK activity or due to up-regulation of an NLS-specific protein phosphatase. To differentiate between these two possibilities, Msn2-NLS phosphorylation levels were assayed in the presence of glucose and after glucose withdrawal in the presence and absence of the Bcy1 regulatory subunit. The experiment was carried out with strains expressing wild-type cAPK and catalytically attenuated cAPK (Cameron et al., 1988). On glucose, the bcy1 strains and the corresponding wild-type strains exhibited quite similar Msn2-NLS phosphorylation levels (Figure 5A). However, while glucose starvation triggered dephosphorylation in the wild-type strains (a 7- to 15-fold decrease), dephosphorylation was almost negligible in the absence of Bcy1 (1.2- to 1.3-fold difference). Dephosphorylation seemd to be insignificant in bcy1 mutants regardless of their level of cAPK activity (TPK wild-type or tpk1w strains). This observation indicated that acute glucose starvation must mainly affect the phosphorylation status of the Msn2-NLS by down-regulation of cAPK via Bcy1, and not by up-regulation of an NLS-specific protein phosphatase.
Fig. 5. Dephosphorylation of the Msn2-NLS in different cAPK mutant backgrounds after glucose starvation. PKI-NES–Msn2(576– 704)–GFP fusion protein was expressed in strains W303 bcy1, RS13-58-A1 (tpk1w tpk2tpk3bcy1) and in the corresponding wild-type strains. Extracts were prepared from cells before and after 10 min of glucose starvation. Phosphorylation of the Msn2-NLS was detected using α-P-CREB (S133) antibody. α-GFP antibody was used as loading control. Phosphorylation was quantified as described in Materials and methods.
Acute Msn2-NLS dephosphorylation may require loss of phosphoglucose metabolites
Glucose is sensed by yeast cells through a variety of systems, including a G-protein-coupled membrane sensor, hexose transporters, glucose phosphate sensors and metabolic changes. We therefore tried to gain some understanding about which of these systems might couple glucose starvation to Msn2 localization. We tested gpa2 mutants for their behaviour under sudden glucose deprivation (Figure 6B); however, they showed no difference from wild-type cells. We further determined how quickly cells would recover after refeeding. Figure 6A shows that logarithmic cells quickly recover high levels of Msn2-NLS modification upon readdition of glucose. Thus, we asked whether addition of unmetabolizable 2-deoxyglucose could also cause such a reversal. Unlike glucose, 2-deoxyglucose was unable to readjust Msn2-NLS modification (Figure 6C) and GFP localization (Figure 6D). This behaviour of the Msn2-NLS is quite different from that observed for the Snf1 system monitored here via the localization of the Snf1-regulated repressor Mig1. Mig1–GFP quickly accumulates in the cytoplasm upon glucose depletion but its return to the nucleus can be triggered by glucose as well as by 2-deoxyglucose (Figure 6D). Since 2-deoxyglucose can be imported into the cell and converted to 2-deoxyglucose-6-phosphate, we assume that sensing systems at or above this level are not sufficient for cAPK reactivation.
Fig. 6. Dynamic phosphorylation of the Msn2-NLS in response to glucose availability. (A) Rapid and reversible phosphorylation of endogenous Msn2. Logarithmic W303 cells were starved for glucose and incubated with readded glucose for the times indicated. Phosphorylation of endogenous Msn2 was monitored using α-P-CREB (S133) antibody. (B) Phosphorylation of the Msn2-NLS does not require Gpa2. PKI-NES–Msn2(576–704)–GFP fusion protein was expressed in a GPA2 deletion mutant and the respective wild type. Phosphorylation of the Msn2-NLS was detected by α-P-CREB (S133) antibody. (C) Rephosphorylation of the Msn2-NLS requires metabolizable glucose. Logarithmic W303 cells were starved for glucose and resuspended in YP containing either none, 2% glucose or 2% 2-deoxyglucose. (D) Localization of PKI-NES–Msn2(576– 704)–GFP and Mig1–GFP fusion proteins under the conditions described in (C).
The Msn2-NLS dephosphorylation response is maximal during early logarithmic growth
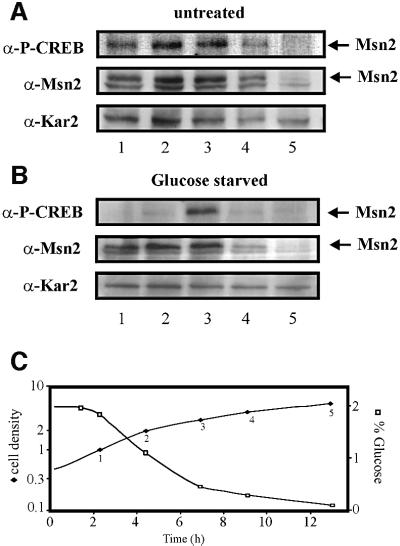
cAMP levels drop when cells exhaust glucose stores and switch from fermentation to respiration, a growth transition also called the diauxic shift. Considering the close connection between cAPK activity and Msn2-NLS modification, we studied the behaviour of NLS-phosphorylation during the diauxic shift. Throughout logarithmic growth, we could indeed observe high constant levels of Msn2-NLS phosphorylation (Figure 7A). As predicted, the relative strength of the phospho-CREB signal became perceptibly weaker as glucose levels in the medium dropped (∼OD600 = 4). Cells from these growth phases were also tested for their reaction to glucose depletion (Figure 7B). A sharp drop in Msn2 modification as a physiological reaction was readily detectable up to culture densities of 2 (OD600), a point where the cells appeared to enter the diauxic shift. We noticed that the Msn2-NLS became refractory to dephosphorylation by complete carbon source removal at a density of OD600 = 3 (when sugar levels have dropped significantly but are not yet completely exhausted; see Figure 7C). Either the cAPK system becomes uncoupled from the relevant glucose-sensing system during this growth transition or the equilibrium of phosphorylation is changed due to the inhibition of the underlying protein phosphatase system.
Fig. 7. Changes of the Msn2-NLS phosphorylation and glucose starvation response in a growing culture. Msn2 phosphorylation was investigated at the time points listed in (C). Samples were split; one half was left untreated before further processing (A) and the other was starved 10 min for glucose (B). Levels and phosphorylation of endogenous Msn2 were monitored using α-Msn2 and α-P-CREB antibodies, respectively. α-Kar2 antibody was used for the loading control. (C) Glucose concentrations in dependency of cell growth. The first sample was taken 2 h after dilution of a logarithmically growing culture into fresh growth medium (time 0). Cell density is given in 107 cells/ml.
Stress and nitrogen starvation modulate Msn2 localization through a region containing the nuclear export signal
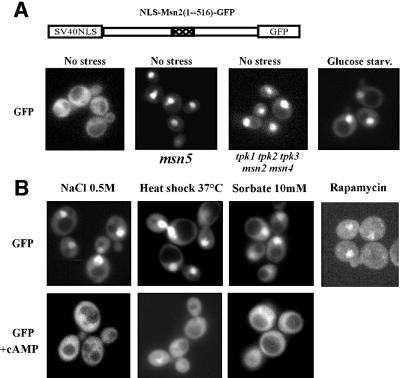
Our observation that the nuclear import sequence was unresponsive to generic stresses suggested that such conditions must control the localization of native Msn2 through either modulating nuclear export or retention factors. To test this proposition, we analysed a region of Msn2 that contains two blocks of homology (HDI/HDII) with the functionally redundant zinc finger protein Msn4. Connecting this region with GFP and the stress-insensitive nuclear import signal from SV40 (not shown) results in a polypeptide with mostly cytosolic localization in unstressed wild-type cells but nuclear localization in msn5 mutants (Figure 8A) that are lacking a nuclear export factor (Görner et al., 1999; Chi et al., 2001). The same GFP fusion protein exhibits fast nuclear accumulation after stress induction and inhibition of the TOR pathway (Figure 8B). Thus, as expected, stress-induced nuclear concentration of this construct is the consequence of diminished nuclear export. Moreover, as previously suggested, the Msn2-HDI/HDII nuclear export activity was also responding to cAMP levels (Görner et al., 1998). Further analysis, as presented here, showed that this domain (Msn2-HDI/HDII) responds to cAPK activity, glucose depletion and glucose refeeding in much the same way as the nuclear import signal. In pde2 cells, the addition of cAMP also antagonized and reversed any stress-related nuclear accumulation of the Msn2-HDI/HDII product (Figure 8B). In this case, however, the target(s) of the kinase has yet to be determined. Taking also into account our Msn2-NLS data, we postulate that cAPK antagonizes stress signals converging on the Msn2-HDI/HDII domain rather than the other way round; thus, stress signals block neither cAPK activity nor its access to Msn2 (see also Figure 9).
Fig. 8. Nuclear export of Msn2 is mediated by exportin Msn5 and regulated by stresses and cAMP/cAPK. (A) Micrographs of the SV40-NLS-Msn2(1–516)–GFP fusion protein in the msn5 mutant and the respective wild type. (B) Intracellular localization of the SV40-NLS-Msn2(1–516)–GFP fusion protein in the pde2 strain before and after treatment with the indicated stresses for 10 min. Rapamycin was added to a final concentration of 200 ng/ml. For relocalization experiments, stressed cells were incubated with 10 mM cAMP for 15 min. Pictures were recorded from living cells.
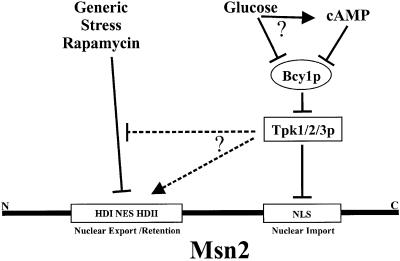
Fig. 9. A model for the integration of different environmental signals through distinct functional domains of Msn2. Glucose signals could take two possible routes, namely cAMP-dependent and -independent routes, to regulate cAPK via Bcy1. cAPK antagonizes nuclear accumulation of Msn2 by targeting two domains. One of the targets, the NLS, is directly modified and provides an exclusive regulatory element for cAPK. In contrast, the region responsible for Msn2 nuclear export and/or retention is reacting promiscuously to signals generated by generic stress, signals from the TOR pathway and cAPK.
Discussion
Msn2-NLS modification is a readout for cAPK activity
Many unrelated external conditions such as nutrient starvation and different forms of environmental stresses increase the nuclear concentration of the transcription factor Msn2. This means that all these diverse signals must somehow be integrated at the level of Msn2 or above. Previous evidence suggested that cAPK could function as a major integration point because it behaves like a master regulator for Msn2 localization; an increase in cAMP concentration is able to antagonize nuclear accumulation independently of the inducing agent; a decrease in cAMP alone is sufficient to trigger nuclear concentration of Msn2 (Görner et al., 1998; this study). One extreme interpretation of these observations is that many, if not all, fluctuations in environmental parameters influence cAPK activity, which then modulates both the import and export signals of Msn2. Such a model is attractive but is clearly invalidated by the results presented here. Instead, it has become apparent, at least for short-term responses, that stress signals and nitrogen starvation signals must operate independently of cAPK function.
At the core of all our arguments is the finding that Msn2 harbours an NLS that is functionally affected by the manipulation of cAPK levels and by glucose starvation, but not by any common stress regimen (see below). Several lines of evidence support the notion that the element must be a direct target of cAPK and that phosphorylation catalysed by it has a measurable functional consequence. First, the protein is a substrate of cAPK in vitro. Secondly, a modification-specific antibody can detect phosphorylation of one of the putative cAPK motifs in the NLS and the stength of the signal can be directly correlated with in vivo cAPK activity. Amino acid substitutions at the candidate modification site will cause loss of detection by the antibody. Finally, a genetic analysis of multiple substitution mutants is fully consistent with the functional importance of the modification events. Serine to alanine replacements at all four predicted cAPK sites in the NLS not only prevent NLS phosphorylation, but result in constitutive nuclear import, whereas serine to aspartate replacements result in permanent cytoplasmic localization of an Msn2-NLS–GFP reporter construct. Exogenous cAMP has no effect on the aspartate and alanine mutant proteins. In our view, these findings constitute convincing evidence that cAPK-driven phosphorylation occurs at the Msn2-NLS and that its phosphorylation status is reflected by nuclear import activity.
Two factors could have been responsible for the switch in NLS phosphorylation: a rapid increase in dephosphorylation activity or, in the case of a high permanent phosphate turnover, a loss in phosphorylating activity. Bcy1, the cAMP-binding, negative regulatory subunit of cAPK, is supposed to connect cAMP levels to cAPK activity. Hence, the loss of NLS regulation in bcy1 mutants allows us to conclude that the fast and transient changes in the level of NLS modification are determined mostly through Bcy1-modulated cAPK activity. In short-term regulatory situations, one might safely interpret the behaviour of the Msn2 nuclear import domain as a direct reflection of cAPK activity. Most importantly, under this assumption, one should be able to obtain direct information on cAPK activity in vivo, in real time and without extrapolation from cAMP measurements.
Msn2-NLS dephosphorylation is a response specific to carbohydrate starvation
Out of all the environmental insults, only glucose withdrawal was followed rapidly by dephosphorylation and activation of the Msn2-NLS. Until recently, not many investigations have dealt with the physiological effects of acute carbohydrate starvation during growth. Most studies have concentrated rather on events taking place during refeeding of starvation-adapted cells with glucose. The classical studies on glucose derepression, on the other hand, were based largely on long-term growth transitions, for example comparing logarithmically growing cells with early stationary cells that had passed the diauxic shift. Such conditions were also predominant in the identification of glucose-sensing mechanisms. A reluctance to analyse cells after acute glucose depletion might have come from the assumption that such cells could quickly encounter an energy-deficient state. The fact that acutely starved cells still induce transcription of stress-protecting genes (not shown) and still regulate their nuclear trafficking might alleviate such concerns. In this respect, one may also note that bcy1 cells maintain a highly phosphorylated state of Msn2 even after glucose deprivation, indicating that general kinase activities are not compromised by this treatment.
Overall, glucose surveillance has turned out to be a complex issue involving hexose transport, hexose phosphorylation, a G-protein-coupled membrane sensor and perhaps intracellular acidification as putative cues for the status of glucose availability. Our results indicate that some of these sensing systems may not make crucial contributions during acute glucose deprivation. Novel glucose signalling mechanisms recently have been proposed to act in the case of sudden carbohydrate depletion (Moriya et al., 2001; Vincent et al., 2001). For example, an analysis of the glucose-repressed protein kinase Snf1 indicated that a kinase complex accumulates in the nucleus under glucose deprivation (Vincent et al., 2001). Similar observations were made for Yak1 kinase (Moriya et al., 2001). In both cases, the addition of 2-deoxyglucose could override the starvation response, leading to rapid relocalization of nuclear Gal83 and Yak1 to the cytoplasm. Even if the sensory system responding to changes in activated glucose is unknown, one could have speculated that it might also feed into the cAPK regulatory system through an early branching of the pathways. Since Yak1 contains putative cAPK modification sites, the proposal that Msn2-NLS function and Yak1 activation are coordinately regulated by the same sensory and signal transduction system would have been quite attractive. This contention is not supported by our data, since 2-deoxyglucose is insufficient for the rephosphorylation of the Msn2-NLS. We rather conclude that metabolic steps beyond glucose phosphorylation influence the signals required for activation of cAPK. In this respect, the sensing mechanism could be very similar if not identical to one of the signal branches proposed for the polysomal response to acute glucose deprivation (Ashe et al., 2000).
Using the phosphorylation pattern of the Msn2-NLS as an indicator, we found that environmental stress and rapamycin do not perturb cAPK activity and possibly the upstream parts of the cAMP pathway. Thus, at least in the short term, these environmental conditions should not down-regulate the cAMP generation systems nor should they intersect with other modulators of cAPK such as the proposed FGM pathway (Thevelein, 1994; Ashe et al., 2000). This conclusion is consistent with earlier findings showing that bcy1 mutants exhibit a repressed level of STRE-dependent transcription while still displaying stress regulation, albeit at low levels (Marchler et al., 1993). Also, mutants with reduced cAPK activity exhibit high basal rates of Msn2-dependent transcription but still allow some degree of stress induction (Marchler et al., 1993; Norbeck and Blomberg, 2000). Consequently, cAPK cannot function as an effector of stress signals, but rather must act by pre-setting a maximal level for Msn2 activation.
It is now evident that stress and rapamycin must affect Msn2 localization by mechanisms independent of nuclear import. Indeed, as shown here, a large domain of high similarity between Msn2 and Msn4 (HDI/HDII) is solely responsible for stress-related relocalizations and must therefore provide the core of the signal integrator. This region incorporates the Msn2 NES. Regulation of Msn2 nuclear export might be achieved through direct control of the export domain and/or regulated nuclear retention. Interestingly, export activity of Msn2-HDI/HDII is inactivated by both generic stress and glucose depletion, an effect antagonized by increased cAMP levels and, consequently, enhanced cAPK activity. This effect might be caused by inactivation of a stress-sensing mechanism located in Msn2-HDI/HDII by increased cAPK activity. Extrapolating from the Msn2-NLS regulation, however, we may exclude that stress controls the Msn2-HDI/HDII region indirectly through modulation of cAPK.
A simplified diagram of how signals converge on Msn2 according to our results is presented in Figure 9. In summary, one has to conclude that variations in glucose levels and environmental stresses target Msn2 on different functional domains, through mechanistically distinct processes even though the outcome is essentially the same, namely a high nuclear concentration of Msn2.
Materials and methods
Yeast strains, media and growth conditions
Standard methods were used as described by Görner et al. (1998). For an overview on yeast strains, see Table I. Unless otherwise indicated, strains are derivatives of W303. Isogenic crossing of W303 tpk2tpk3, W303 msn2msn4 and W303 tpk1tpk2 generated W303 tpk123msn2msn4. TPK gene deletions in W303 are according to Toda et al. (1987) and were a gift from M.Rep (Leuven). The BCY1 gene in W303 was disrupted with URA3 (Cannon and Tatchell, 1987). The cdc35::kanMX disuption cassette was recovered from the systematic deletion set (Euroscarf).
Table I. Saccharomyces cerevisiae strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| W303-1A | a leu2 ura3 his3 trp1 ade2 can1 | K.Nasmyth (Vienna) |
| W303 msn2msn4 | a msn2::HIS3 msn4::TRP1 | Görner et al. (1998) |
| W303 tpk2tpk3 | α tpk2::HIS3 tpk3::TRP1 | M.Rep (Leuven) |
| W303 tpk1tpk2 | α tpk1::URA3 tpk2::HIS3 | M.Rep (Leuven) |
| W303 tpk123msn2msn4 | a tpk1::URA3 tpk2::HIS3 tpk3::TRP1 msn2::HIS3 msn4::TRP1 | this study |
| W303 msn5 | a msn5::HIS3 | this study |
| W303 bcy1 | a bcy1::URA3 | this study |
| W303 pde2 | a pde2::TRP1 | Görner et al. (1998) |
| SP1 | a leu2 his3 trp1 ade8 can1 ura3 | Cameron et al. (1988) |
| RS13-58A-1 | a tpk1w1 tpk2::HIS3 tpk3::TRP1 bcy1::LEU2 | Cameron et al. (1988) |
| W303 cdc35pde2yak1 | a cdc35::KanMX pde2::TRP1 yak1::LEU2 | this study |
Yeast cells were grown on YPD or synthetic medium to an OD600 of 1–1.5 and either exposed to stress (10 mM sorbate, 7% ethanol or heat shock) for the times indicated or used immediately. For heat stress treatment, cultures were grown for at least four generations at 26°C before the temperature was raised to 37°C. Rapamycin was applied as described by Beck and Hall (1999). Copper induction of Cup1–Msn2p was performed by adding 10 µM Cu2SO4 for 20 min to the medium.
Plasmids
Deletions and mutations in the MSN2 gene are derivatives of pAdh1-Msn2–GFP (Görner et al., 1998). For sequences of oligonucleotides used, see Table II. SalI–NcoI-cut PCR fragments were inserted into SalI–NcoI-cut pAdh1-Msn2–GFP, resulting in formation of pMsn2(1–648)–GFP O1/O2, pMsn2(1–567)–GFP O4/O1, pMsn2(567–704)–GFP O9/O6 and pMsn2(567–648)–GFP O9/O2. To obtain pMsn2Δ(571–649)–GFP, the NcoI-cut PCR fragment obtained with oligonucleotides O6/O7 was inserted in NcoI-cut pMsn2(1–567)–GFP. pAdh1-Msn2–GFP was digested by XhoI–SalI and religated to generate pMsn2(648–704)–GFP. Multiple GFP moieties were generated by isolating multiple integrants of the NotI–NcoI–EGFP–NotI cassette (Görner et al., 1998). To construct pPKI-NES–Msn2(567–704)–GFP, the annealed oligonucleotides PKI-W and PKI-C were inserted into SalI-cut pMsn2(567–704)–GFP andSalI-cut pMsn2(567–648)–GFP. The PKI-NES–Msn2–GFP derivatives were constructed as described by Görner et al. (1998). Mutant allele S582,620,625,633D was generated by ligating the PCR fragment obtained with the oligonucleotides O20/O6 [using plasmid pAMG7 as template; described in Görner et al. (1998)] into SalI–NcoI-cut Msn2(567–704)–GFP. All PCRs were performed with Vent polymerase (NEB). GST fusion proteins were constructed by cloning SalI–NcoI fragments of Msn2(576–704) and Msn2(576–704) (S582,620,625,633A) into vector pGEX3-1 (Pharmacia). pCup1–Msn2 was generated by replacing the MSN2 promoter with a 500 bp KspI–SalI PCR fragment of CUP1 in the vector YCplac111.
Table II. Oligonucleotides used in this study.
| Name | Sequence |
|---|---|
| O1 | 5′-CTAAAATGACGGTCGACCATG-3′ |
| O2 | 5′-CCATGGGACAGTGGAACGGTTTCTCCTCGAG-3′ |
| O4 | 5′-AGCCATGGTATGGTGACCCTGCTGTGGG-3′ |
| O5 | 5′-TCTCGAGCGTAACCCCAGCA CCATTG-3′ |
| O6 | 5′-TGGCCGTTTACGGTCGACCATG-3′ |
| O7 | 5′-AAAGGATCCGCCATGGCACCGTTCCACTGTCACATTTGTCC-3′ |
| O9 | 5′-GTCGACCATACCATGAACTCTAAAATCGG-3′ |
| O20 | 5′-AAGTCGACTCCCTTCGGAGGCGGAAGGATGCTGTGCCTTTGATGGG-3′ |
| PKI-W | 5′-TCGAGAATGAATTAGCCTTGAAATTAGCAGGTCTTGATATCAACAAGACAG-3′ |
| PKI-C | 5′-TCGACTGTCTTGTTGATATCAAGACCTGCTAATTTCAAGGCTAATTCATTC-3′ |
Protein analysis, enzyme activities
β-galactosidase activity of extracts prepared by breakage with glass beads was assayed after high speed clearing using o-nitrophenyl-β-d-galactopyranoside as substrate. For western blots, cells were grown to OD600 = 1, harvested and suspended in cold extraction buffer [50 mM Tris pH 7.5, 0.4 M (NH4)2SO4, 1 mM EDTA, 5% glycerol/NaF/o-NaVO4, β-glycerophosphate/Na-pyrophosphate and complete protease inhibitor cocktail (Boehringer Mannheim)]. Cells were disrupted with glass beads, and the lysate was clarified and boiled with 4× SDS sample buffer (Laemmli, 1970). Alternatively, equal amounts of cells were resuspended in 2× SDS sample buffer and glass beads were added. Samples were incubated for 5 min at 95°C, vortexed (5 min) and incubated again at 95°C (5 min). Proteins were separated by 10 or 8% SDS–PAGE. Blots were probed with GFP polyclonal antibody (Clontech) and α-P-CREB (S133) antibody (Cell Signalling). α-Msn2 antibody was kindly provided by F.Estruch (Valencia). α-Kar2 antibody was from A.Kal (University of Amsterdam). Bound antibodies were detected using ECL (Amersham). To quantify western blots, short exposures of three independent experiments were scanned and quantified using Image Quant software.
Yeast cAPK in vitro phosphorylation assays
Substrate proteins were expressed and purified as GST fusions in Escherichia coli according to the procedure described (Ausubel et al., 1999). Yeast cAPK was purified using its interaction with TAP-tagged Bcy1 (J.Norbeck, in preparation). cAPK catalytic kinase subunits were eluted from immobilized Bcy1 with cAMP in cAPK buffer (10 mM Tris pH 8, 150 mM NaCl, 0.1% Triton X-100). [γ-32P]ATP (10 µCi, 3000 Ci/mmol) was added to each reaction, and addition of equal amounts of substrates was verified by Coomassie stain (not shown).
GFP fluorescence microscopy
Unfixed living cells were used for fluorescence microscopy. Cellular DNA was stained for 10 min by addition of 2 µg/ml 4′,6 diamidino-2-phenylindole (DAPI) to the cell suspension in SD medium. Stress conditions were applied as specified for individual experiments. Images were recorded on a Zeiss Axioplan 2 fluorescence microscope with a Quantix CCD camera using IP-LAB or Lightview software and processed in Adobe Photoshop 5.
Acknowledgments
Acknowledgements
We are grateful to M.Rep and F.Estruch for materials. We thank all the members of the Ruis, Ammerer and Kuchler laboratories, but especially W.Reiter and J.Norbeck for fruitful discussions and material help, M.Matz and M.Galova for technical support, and C.Brocard for critically reading the manuscript. This work was supported by grants P11303, P12015 and P13493 from the Fonds zur Förderung der wissenschaftlichen Forschung, Vienna, Austria.
References
- Ashe M.P., De Long,S.K. and Sachs,A.B. (2000) Glucose depletion rapidly inhibits translation initiation in yeast. Mol. Biol. Cell, 11, 833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel F., Brent,R., Kingston,R., Moore,D., Seidman,J. and Struhl,K. (1999) Current Protocols in Molecular Biology. John Wiley & Sons, Inc., New York, NY.
- Barbet N.C., Schneider,U., Helliwell,S.B., Stansfield,I., Tuite,M.F. and Hall,M.N. (1996) TOR controls translation initiation and early G1 progression in yeast. Mol. Biol. Cell, 7, 25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck T. and Hall,M.N. (1999) The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature, 402, 689–692. [DOI] [PubMed] [Google Scholar]
- Broach J.R. and Deschenes,R.J. (1990) The function of ras genes in Saccharomyces cerevisiae. Adv. Cancer Res., 54, 79–139. [DOI] [PubMed] [Google Scholar]
- Cameron S., Levin,L., Zoller,M. and Wigler,M. (1988) cAMP-independent control of sporulation, glycogen metabolism and heat shock resistance in S.cerevisiae. Cell, 53, 555–566. [DOI] [PubMed] [Google Scholar]
- Cannon J.F. and Tatchell,K. (1987) Characterization of Saccharomyces cerevisiae genes encoding subunits of cyclic AMP-dependent protein kinase. Mol. Cell. Biol., 7, 2653–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M. (1999) Glucose repression in yeast. Curr. Opin. Microbiol., 2, 202–207. [DOI] [PubMed] [Google Scholar]
- Chi Y., Huddleston,M.J., Zhang,X., Young,R.A., Annan,R.S., Carr,S.A. and Deshaies,R.J. (2001) Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev., 15, 1078–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Como C.J. and Arndt,K.T. (1996) Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes Dev., 10, 1904–1916. [DOI] [PubMed] [Google Scholar]
- Gancedo J.M. (1998) Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev., 62, 334–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch A.P., Spellman,P.T., Kao,C.M., Carmel-Harel,O., Eisen,M.B., Storz,G., Botstein,D. and Brown,P.O. (2000) Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell, 11, 4241–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görner W., Durchschlag,E., Martinez-Pastor,M.T., Estruch,F., Ammerer,G., Hamilton,B., Ruis,H. and Schüller,C. (1998) Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev., 12, 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görner W., Schüller,C. and Ruis,H. (1999) Being at the right place at the right time: the role of nuclear transport in dynamic transcriptional regulation in yeast. Biol. Chem., 380, 147–150. [DOI] [PubMed] [Google Scholar]
- Heitman J., Movva,N.R. and Hall,M.N. (1991) Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science, 253, 905–909. [DOI] [PubMed] [Google Scholar]
- Iida, H. (1988) Multistress resistance of Saccharomyces cerevisiae is generated by insertion of retrotransposon Ty into the 5′ coding region of the adenylate cyclase gene. Mol. Cell. Biol., 8, 5555–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Marchler G., Schuller,C., Adam,G. and Ruis,H. (1993) A Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J., 12, 1997–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pastor M.T., Marchler,G., Schüller,C., Marchler-Bauer,A., Ruis,H. and Estruch,F. (1996) The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). EMBO J., 15, 2227–2235. [PMC free article] [PubMed] [Google Scholar]
- Moriya H., Shimizu-Yoshida,Y., Omori,A., Iwashita,S., Katoh,M. and Sakai,A. (2001) Yak1p, a DYRK family kinase, translocates to the nucleus and phosphorylates yeast Pop2p in response to a glucose signal. Genes Dev., 15, 1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbeck J. and Blomberg,A. (2000) The level of cAMP-dependent protein kinase A activity strongly affects osmotolerance and osmo-instigated gene expression changes in Saccharomyces cerevisiae. Yeast, 16, 121–137. [DOI] [PubMed] [Google Scholar]
- Rigaut G., Shevchenko,A., Rutz,B., Wilm,M., Mann,M. and Seraphin,B. (1999) A generic protein purification method for protein complex characterization and proteome exploration. Nature Biotechnol., 17, 1030–1032. [DOI] [PubMed] [Google Scholar]
- Rolland F., Winderickx,J. and Thevelein,J.M. (2001) Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem. Sci., 26, 310–317 [DOI] [PubMed] [Google Scholar]
- Ruis H. and Schüller,C. (1995) Stress signaling in yeast. BioEssays, 17, 959–965. [DOI] [PubMed] [Google Scholar]
- Schmelzle T. and Hall,M.N. (2000) TOR, a central controller of cell growth. Cell, 103, 253–262. [DOI] [PubMed] [Google Scholar]
- Smith A., Ward,M.P. and Garrett,S. (1998) Yeast PKA represses Msn2p/Msn4p-dependent gene expression to regulate growth, stress response and glycogen accumulation. EMBO J., 17, 3556–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J.M. (1994) Signal transduction in yeast. Yeast, 10, 1753–1790. [DOI] [PubMed] [Google Scholar]
- Thevelein J.M. and de Winde,J.H. (1999) Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol., 33, 904–918. [DOI] [PubMed] [Google Scholar]
- Thompson-Jaeger S. Francois,J, Gaughran,J.P. and Tatchell,K. (1991) Deletion of SNF1 affects the nutrient response of yeast and resembles mutations which activate the adenylate cyclase pathway. Genetics, 129, 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Cameron,S., Sass,P., Zoller,M., Scott,J.D., McMullen,B., Hurwitz,M., Krebs,E.G. and Wigler,M. (1987) Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol. Cell. Biol., 7, 1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent O., Townley,R., Kuchin,S. and Carlson,M. (2001) Subcellular localization of the Snf1 kinase is regulated by specific β subunits and a novel glucose signaling mechanism. Genes Dev., 15, 1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W., Harootunian,A.T., Adams,S.R., Feramisco,J., Tsien,R.Y., Meinkoth,J.L. and Taylor,S.S. (1994) Heat-stable inhibitors of cAMP-dependent protein kinase carry a nuclear export signal. J. Biol. Chem., 269, 32214–32220. [PubMed] [Google Scholar]