Abstract
Anorexia nervosa (AN) is a complex psychiatric disorder characterized by severe caloric restriction and distorted body image, leading to significant psychological and physiological complications. Brain-derived neurotrophic factor (BDNF) plays a critical role in cognitive function and metabolic regulation. A mutation in the BDNF gene is associated with anorexia nervosa. This study examines the effects of food restriction, refeeding and short-term refeeding on the expression of Bdnf and its receptor (tropomyosin receptor kinase B TrkB/Ntrk2) in key brain regions involved in reward and cognitive function. We assessed BDNF mRNA levels in the dorsal striatum (DS), nucleus accumbens, ventral tegmental area, and prefrontal cortex (PFC) of AN-like mice subjected to different feeding regimes combined with or without physical activity. Cognitive flexibility was assessed using the Y-maze test. Whole RNA sequencing was also performed to analyse gene expression changes. Food restriction induced a transient decrease in cognitive flexibility and significantly decreased Bdnf expression in the DS and PFC. Progressive refeeding restored Bdnf in the DS but not the PFC. Short refeeding restored Bdnf levels to baseline. TrkB expression is increased by restriction only in the PFC. The presence of a running wheel cancelled these effects, suggesting an interaction between physical activity and diet. Pathway analysis of dysregulated genes revealed enrichment in immune regulation and cell-cell communication pathways. These findings highlight the complex relationship between diet, exercise, and brain function in AN-like mouse model and suggest avenues for further research into the clinical relevance of BDNF and TrkB as biomarkers of eating disorders.
Subject terms: Physiology, Molecular neuroscience
Introduction
Anorexia nervosa (AN) is a complex and potentially life-threatening eating disorder characterized by self-imposed dietary restriction and usually excessive physical exercise [1]. Individuals with AN experience severe weight loss due to caloric restriction, leading to a number of somatic complications such as hormonal and metabolic changes, and loss of bone density [2, 3]. Metabolic changes are often associated with psychiatric symptoms across various mental diseases and more specifically in AN where recent genomic analysis encourage for a reconceptualization of AN as to be a metabo-psychiatric disorder [4, 5]. These include high levels of anxiety and depression associated with intense fear of weight gain, distorted body image, obsessive behaviors related to food and body shape and impaired cognitive flexibility and decision-making, which further complicate the clinical presentation and treatment approach [6–8]. The disorder predominantly affects adolescents and young adults, with a higher prevalence in women, accounting for up to 90% of cases [9]. Epidemiological studies estimate that the prevalence of AN to be around 1% in young adult females [10]. Despite various therapeutic approaches, there is currently no cure for AN, and many individuals experience recurrent relapses. It is estimated that 9–52% of patients relapse after treatment [11], underlining the urgent need to understand the factors that contribute to relapse. Indeed, the impact of different refeeding protocols on brain function remains poorly understood, which is an important gap, as these neural changes may play a critical secondary role in influencing relapse vulnerability. Furthermore, given that AN has the highest mortality rate of any psychiatric disorder [12–14], there is an urgent need to improve treatment outcomes and reduce the high risk of mortality associated with this disorder [13]. Genome-wide association studies (GWAS) and case-controlled studies have identified several genetic risk factors associated with the disorder [4, 15–17]. Among these, a specific allele of the brain-derived neurotrophic factor (BDNF) gene, specifically the Val66Met polymorphism (also known as rs6265), has received considerable attention [18], and is now under intense scrutiny [19, 20].
Known for its role in neuronal development, neurogenesis, and synaptic plasticity [21, 22], BDNF has recently emerged as a key player in metabolic regulation, influencing processes both centrally in the brain and peripherally. Centrally, BDNF influences hypothalamic circuits that regulate energy balance, appetite, and satiety, contributing to its anorexic effects [23]. Peripherally, BDNF can directly affect metabolic processes, including increasing lipid oxidation and energy expenditure, ultimately leading to weight loss and increased physical activity levels [24], highlighting a comprehensive effect of BDNF on metabolic health and physical activity. The Val66Met mutation in the BDNF gene, has been implicated in several metabolic diseases and psychiatric disorders, particularly in AN, affecting both neuroplasticity and metabolic regulation [19, 20]. In individuals with AN, Met variant carriers show altered reward function, as evidenced by increased reward circuit activity in response to images of thinness in the ventral striatum, a key region for reward processing [25, 26].
Finding animal models that mimic the full spectrum of AN symptoms is challenging due to their specificity to humans. A widely used AN-like rodent model is the activity-based anorexia (ABA) model, which combines time-limited access to food with free and continuous access to a running wheel [27]. This paradigm leads to a paradoxical pattern of behavior in which animals voluntarily increase their physical activity levels while simultaneously reducing food intake, resulting in severe weight loss and physiological, behavioral and cognition changes reminiscent of human AN [27].
The expression of BDNF in brain regions associated with the neurobiological basis of AN has been studied, partly using the ABA paradigm. For instance, it has been observed that rodents exposed to the ABA typically exhibit reduced BDNF expression in the medial prefrontal cortex (PFC) and amygdala [28, 29]. The medial PFC is involved in decision-making and executive function, both of which may be impaired in individuals with AN. Meanwhile, the amygdala plays a key role in emotional responses and fear, both of which are heightened in individuals with AN [30]. Conversely, increased BDNF levels in the hippocampus, a region involved in memory and stress responses, suggest adaptive or maladaptive responses to food restriction and stress [28]. Levels remain relatively unchanged in the nucleus accumbens, a region central to reward processing. This suggests that some reward-related behaviors in anorexia nervosa may not be directly related to changes in BDNF in this region [31].
However, the relatively short duration of the ABA protocol may not fully capture the chronicity and complexity of human AN, limiting its translational relevance. This highlights the need for further refinement of this model to better understand the long-term neurobiological effects of AN and to develop more effective treatments. In this study, we used a modified version of the ABA protocol, known as the Food Restriction and Wheel (FRW) model, which incorporates the chronic aspect of AN, a critical feature that closely aligns with the prolonged course of the disorder [32, 33]. The FRW model has been well validated, particularly in terms of its metabolic relevance, and effectively mimics the metabolic and endocrine changes observed in AN [32–34].
This study aimed to assess the levels of gene expression of brain-derived neurotrophic factor (BDNF), its high-affinity receptor tropomyosin receptor kinase B (TrkB) [35] and the BDNF regulatory enzyme N-acetyltransferase 8-like (Nat8l), in key brain regions implicated in AN, the dorsal striatum, the nucleus accumbens, the ventral tegmental area and the prefrontal cortex in the FRW model of anorexia nervosa. The primary hypothesis was that alterations in the expression of Nat8l, a regulator of BDNF in the dorsal striatum (DS) [36], modulate BDNF/TrkB signaling in a region-specific manner, thereby contributing to the behavioral and neurophysiological abnormalities characteristic of anorexia nervosa. Gene expression profiles were assessed in three nutritional states: chronic food restriction, progressive refeeding, and short-term refeeding. Progressive refeeding models the clinical phase of structured nutritional rehabilitation during inpatient treatment for AN, characterized by a controlled and gradual caloric increase aimed at physiological recovery and body mass index normalization. In contrast, short-term refeeding simulates the spontaneous, unstructured refeeding episodes observed in some patients, involving abrupt and excessive calorie intake, which may affect neural recovery processes. The final aim of this study was thus to determine whether changes in Nat8l, BDNF, and TrkB expression across these conditions provide mechanistic insight into the neural adaptations associated with the onset, maintenance, and treatment response of anorexia nervosa.
Materials and methods
Animals
Given that anorexia nervosa predominantly affects young women (with a sex ratio of approximately 9:1), our study will focus exclusively on female C57BL/6 mice (n = 10 per group, aged 7 weeks) (Charles River Laboratories, L’Arbresle, France) weighing 18.3 ± 0.1 g were housed in pairs in standard Plexiglas cages, to reduce external stressors such as isolation and hypothermia while ensuring adequate monitoring of food intake and physical activity. For one week, mice had access to food to calculate ad libitum food intake of standard chow (3% fat, 16% protein, 60% carbohydrate, 4% fibre, 2.79 kcal/g; Safe A04, Germany). Cages were maintained in a specific pathogen-free environment at a temperature range of 19 to 21 °C with a 12-h light-dark cycle (with lights on from 06:00 am to 06:00 pm). Baseline body weight and ad libitum food intake were recorded in each cage during the first week acclimatization period and throughout the protocol. All experimental procedures were in accordance with the guidelines of the European Communities Council Directives (86/609/EEC). In addition, the study protocol was approved by the Regional Ethics Committee (CEEA.34) of the University Paris Cité, France.
Food restriction and refeeding protocols
We used the food restriction and wheel protocols [32, 34, 37]. During the acclimatization week, daily food intake was measured during the last five days of ad libitum access to standard chow, to determine mean food consumption. Animals were then equally distributed into four groups based on initial body weight (mean 16.19 ± 0.20 g) at the end of the acclimatization period to homogenise the groups. Groups were designated as ad libitum (AL), ad libitum with wheel (ALW), food restriction (FR) and food restriction with wheel (FRW). FR and FRW groups experienced 30% food restriction for three days followed by 50% for 15 days, based on the calculation of the mean food consumption during the ad libitum phase. To prevent competition between littermates, food was provided directly in the home cages at 5:00 pm daily. ALW and FRW groups had access to a running wheel (diameter: 230 mm; width: 50 mm; 1 revolution = 0.72 m), whose activity was continuously monitored and analyzed during the periprandial period (1:00 pm–6:00 pm) (ActiWheel software; Intellibio, Seichamps, France. The recorded running wheel activity reflects the combined activity of both mice housed within a single cage. Body weight was monitored daily and blood glucose levels were measured three times per week (FreeStyle Optium Neo, Abbott, Netherlands).
Three food restriction protocols were performed (Fig. 1). In the first experiment, mice (n = 6 per group) were subjected to a 15-day food restriction protocol (Fig. 1A). In experiment 2, mice (n = 8 per group) started with a 15-day food restriction protocol followed by a progressive refeeding regimen in which the amount of food was gradually increased every two days for a total period of 10 days (60, 70, 80, 90, and 100%; Fig. 1B). In experiment 3, mice (n = 6 per group) were briefly refed under ad libitum conditions for 24 h, after the 15-day restriction protocol (Fig. 1C).
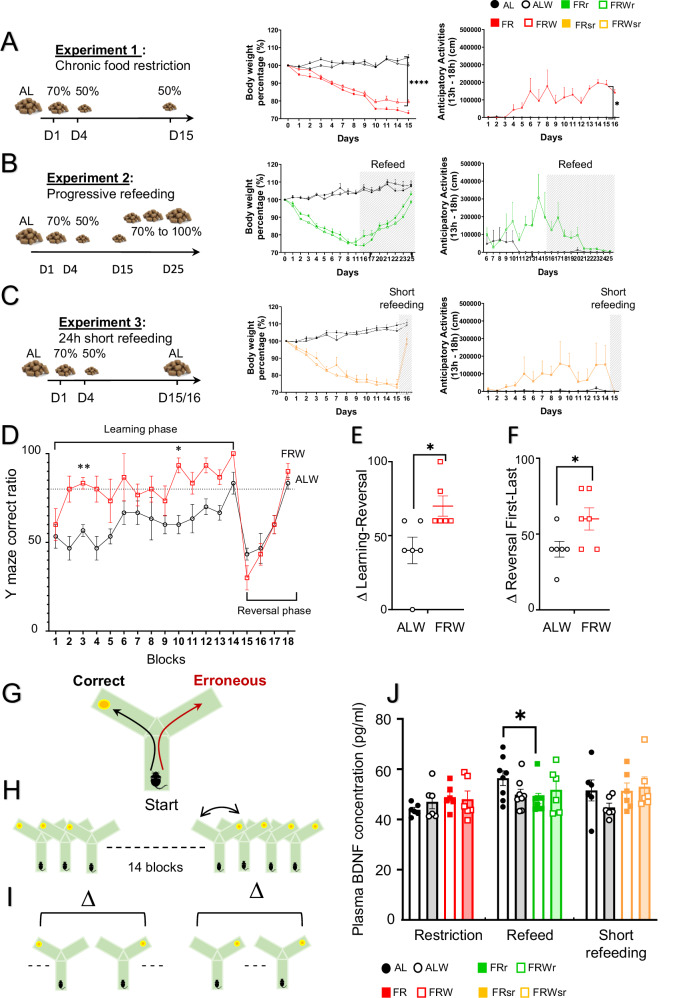
Fig. 1. Effects of chronic food restriction and refeeding protocols on body weight, physical activity, cognitive flexibility and plasma BDNF levels.
A–C Different feeding protocols (chronic food restriction, progressive refeeding and 24 h short refeeding) with the evolution of the body weight and physical activity (for ALW and FRW mice). D Y-maze reversal learning test showing the ratio of correct arm choice across learning and reversal phases in ad libitum with wheel (ALW) and food restriction with wheel (FRW) groups. E Delta correct ratio between the ad libitum learning and reversal phases. F Delta correct ratio between the first and last blocks of the reversal phase. G–I Description of the reversal learning test with the delta of (H) the performance obtained between the last trial of the learning phase and the first trial of the reversal phase and (I) the performance obtained in the first trial of the reversal phase with the last trial. The orange dot corresponds to a piece of Miel Pop cereal (Kellogg’s) as a reward. J Plasma BDNF protein levels measured by ELISA across dietary protocols. Group names are as follows: ad libitum (AL, black), ad libitum with wheel (ALW, black), food restriction (FR, red), food restriction with wheel (FRW, red), progressive refeeding (FRpr, green), progressive refeeding with wheel (FRWpr, green), short refeeding (FRsr, yellow), and short refeeding with wheel (FRWsr, yellow). Data are presented as mean ± SEM for each experimental group. Significant differences are indicated as *p < 0.05, **p < 0.01, ****p < 0.0001. Statistical significance was determined using multiple t-test, unpaired t-test and one-way ANOVA followed by post-hoc Tukey’s test.
Tissue collection
At the end of each experiment, mice were sacrificed using an anaesthetic injection (ketamine/xylazine, ketamine 100 mg/kg and xylazine 10 mg/kg, intraperitoneally), followed by cardiac puncture for blood collection. Blood was centrifuged at 1000 rpm for 10 min at −4 °C to separate plasma, which was then frozen on dry ice. Ethylenediaminetetraacetic acid (EDTA, 2 mg/mL of blood) was added to the blood to prevent clotting, and polyhexamethylene biguanide (PHMB, 0.4 mM final concentration) was added to the plasma to inhibit protease activity. Brain tissues, including the prefrontal cortex (PFC), dorsal striatum (DS), nucleus accumbens, and ventral tegmental area were microdissected (Fig supplementary 1), frozen in liquid nitrogen, and stored at −80 °C.
Y maze reversal learning protocol
In a separate study, 6 mice per group were subjected to the food restriction protocol for up to 25 days including the reversal learning test. A Y-maze reversal test was performed to assess cognitive flexibility under food restriction and ad libitum conditions. The Y-maze consisted of two arms made of black Plexiglass, each measuring 35 cm (Fig. 1G). The test consisted of two phases: habituation (day 13–15 of food restriction) and testing which was divided into a learning phase (from day 16) and a reversal learning phase (from day 23). During habituation, the mice were habituated to the Y-maze during a 15-min session and allowed to explore all arms freely with no food reward on days 13 and 14 and with the reward on day 15. To familiarize the mice with the reward, they were given half a Miel Pop cereal (Kellogg’s) at feeding time in their home cages on days 13 and 14. During the testing phase, a small piece of Miel Pop was randomly placed in one of the arms to assess learning. Mice were placed at the intersection of the maze and the time to choose an arm and the probability of choosing the correct arm (with the reward) were recorded. After entering in an arm, the chosen arm was closed and the mouse was removed from the maze after 20 s. If the correct arm was chosen, the mouse consumed the reward; otherwise, no reward was given. Each mouse underwent 10 sessions per day until an 80% correct choice rate was achieved, which occurred after 7 days of testing, indicating successful learning. A reversal learning phase was then performed, in which the reward location was switched to the opposite arm. Testing continued until one group reached 80% correct choice (Fig. 1G-I).
RNA extraction and cDNA preparation
For RNA extraction, tissues were homogenized in TRIzol reagent, followed by phase separation and RNA precipitation. The extracted RNA was washed with ethanol (75%) and resuspended in RNase-free water. The concentration and purity of the RNA was determined spectrophotometrically. Subsequently, cDNA synthesis was performed using the Maxima cDNA synthesis kit (Thermo Fisher scientific, Waltham, MA USA).
Quantitative polymerase chain reaction (qPCR) and RNA sequencing
Real-time quantitative PCR (qPCR) was performed using the SYBR Green qPCR Master Mix (2X, Roche Diagnostics, Meylan, France) on a 96-well plate (Roche Diagnostics, Meylan, France). The gene PPIA (cyclophilin A) was used as the housekeeping gene for normalization. The purity of the PCR products was assessed using dissociation curves. Data analysis was performed using the comparative threshold (Ct) method with gene expression normalised to internal controls, and expressed using the 2−ΔΔCt method. PCR primers are described in Supplementary Table 1.
RNA sequencing was performed by the GENOM’IC core of the Cochin institute on the prepared samples. RNA sequencing was performed by the GENOM’IC core of the Cochin institute on the prepared samples. Libraries were prepared from 3 μg of extracted total RNA. The captured, purified and clonally amplified libraries were then sequenced on a Novaseq instrument (Illumina) according to the manufacturer’s recommendations. Sequence reads were aligned to the mouse genome (mm10) using BWA software. Downstream processing was carried out using the Genome Analysis Toolkit (GATK), SAMtools and Picard Tools (http://picard.sourceforge.net). Fastq files were then aligned using the STAR algorithm (version 2.7.6a), against the Ensembl release 101 reference (GRCm38). Reads were then counted using RSEM (v1.3.1) and the statistical analyses on the read counts were performed using R (version 3.6.3) and the DESeq2 package (DESeq2_1.26.0) to determine the proportion of differentially expressed genes between two conditions. We used the standard DESeq2 normalization method (DESeq2’s median of ratios with the DESeq function), with a pre-filter of reads and genes (reads uniquely mapped on the genome, or up to 10 different loci with a count adjustment, and genes with at least 10 reads in at least 3 different samples). Following the package recommendations, we used the Wald test with the contrast function and the Benjamini-Hochberg FDR control procedure to identify the differentially expressed genes. R scripts and parameters are available on the platform, https://github.com/GENOM-IC-Cochin/RNA-Seq_analysis.
Enzyme-linked immunosorbent assay
To quantify the total brain-derived neurotrophic factor (BDNF) in plasma, we used the BDNF ELISA kit (R&D Systems, Minneapolis, MN, USA) following the manufacturer’s protocol. Plasma samples were collected and stored at −80 °C until assayed. Plasma samples were diluted 1:10 and the assay was performed in duplicate. The precision of the BDNF ELISA assay was assessed by intra- and inter-assay variability. Intra-assay precision was assessed by testing three samples of known concentration 20 times on a single plate, yielding CVs of 3.2, 2.4, and 3%. Inter-assay precision was assessed by 20 separate assays performed by at least three technicians on two batches of components, yielding CVs of 7.2, 4.3, and 4.7%.
Statistical analysis
Statistical analyses were performed using GraphPad Prism and R. Data were first tested for normality using the Shapiro-Wilk test to determine the appropriate statistical tests. Outliers were identified and removed using z-score analysis, where data points with a z-score greater than ± 3 were considered outliers. For normally distributed data, parametric tests such as one- or two-way analysis of variance (ANOVA) were used to compare group means, followed by post hoc analysis with Tukey’s test when appropriate.
The unpaired Student’s t-test test was used for comparisons between two groups. The Y-maze results were analysed using repeated measures ANOVA to account for the within-subject variability across sessions. Correlations between variables were assessed using Pearson’s correlation coefficients depending on the data distribution.
All data are presented as mean ± standard error of the mean (SEM) and a p-value of less than 0.05 was considered statistically significant.
Results
Body weight and anticipatory activity responses to food restriction and refeeding in in AN-like mice
To validate our AN-like mouse model by food restriction, we evaluated body weight and food anticipatory activity (FAA), defined as increased physical activity prior to scheduled feeding, under different feeding protocols (Fig. 1A-C). Mice in the FR and FRW groups exhibited significant body weight loss (~25%) by the end of the fasting period (F(3,10) = 135.7, p < 0.001, Fig. 1A), with no differences between FR and FRW, indicating no effect of wheel access on weight loss. FRW mice showed a significant increase in FAA compared to the ALW group (F(1,4) = 15.02, p = 0.018, Fig. 1A).
During progressive refeeding, mice recovered their baseline body weight over 10 days (Fig. 1B), with a concomitant reduction in FAA (Fig. 1B). In the short refeeding group, weight was recovered within one day (Fig. 1C), accompanied by an immediate cessation of FAA (Fig. 1C).
Behavioral responses in the Y-maze test
We tested if food restriction alters cognitive abilities. Cognitive function was assessed using the Y-maze reversal learning test. During the learning phase, FRW mice acquired the task faster than ALW mice (t = 5.657, p = 0.004 for day 3 and t = 5.000, p = 0.011 for day 10, Fig. 1D). However, there were no significant differences between groups in the reversal learning phase (Fig. 1D). FRW mice initially showed an impaired response during the first day of reversal learning, as indicated by a more significant reduction in the percentage of correct responses compared to the last day of learning (F(5, 5) = 1.714, p = 0.024, Fig. 1E). However, no permanent deficits were observed as FRW mice quickly relearned the task (F(5, 5) = 2, p = 0.050, Fig. 1F), indicating that they eventually adapt quickly to novel rules.
Expression of BDNF, TrkB, and Nat8l in different brain regions
We compared the peripheral BDNF levels and the Bdnf gene expression in different brain regions between different groups of animals. Plasma BDNF protein concentrations were not different during the restriction period, whereas progressive refeeding resulted in a significant decrease only in FR mice (AL vs FR t = 2.231, p = 0.035), with no significant effect observed in the short-term refeeding group (Fig. 1J).
We then quantified the mRNA expression levels of Bdnf, TrkB, and Nat8l (Fig. 2). In the DS, food restriction significantly reduced Bdnf expression (AL vs FR t = 2.679, p = 0.014, Fig. 2A), with no effect of wheel access. Progressive refeeding and short refeeding fully restored Bdnf levels (Fig. 2A). TrkB and Natl8l expression was not significantly altered by dietary restriction (Fig. 2B, C). In the PFC, Bdnf expression decreased with dietary restriction (AL vs FR t = 2.193, p = 0.040; ALW vs FRW t = 2.209, p = 0.039, Fig. 3A), but in contrast to DS, progressive refeeding did not restore these levels (ALW vs FRW t = 3.734, p = 0.001, Fig. 3A). However, short refeeding with wheel access reversed the reduction (AL vs FR t = 2.359, p = 0.029, Fig. 3A). TrkB expression in the PFC followed different trend than BDNF, with an increase during restriction and normalization by progressive refeeding (AL vs FR t = 3.052, p = 0.007, Fig. 3B). Bdnf, TrkB, and Nat8l expression in the nucleus accumbens and ventral tegmental area was not affected by dietary protocols or wheel activity (figs S2, S3).
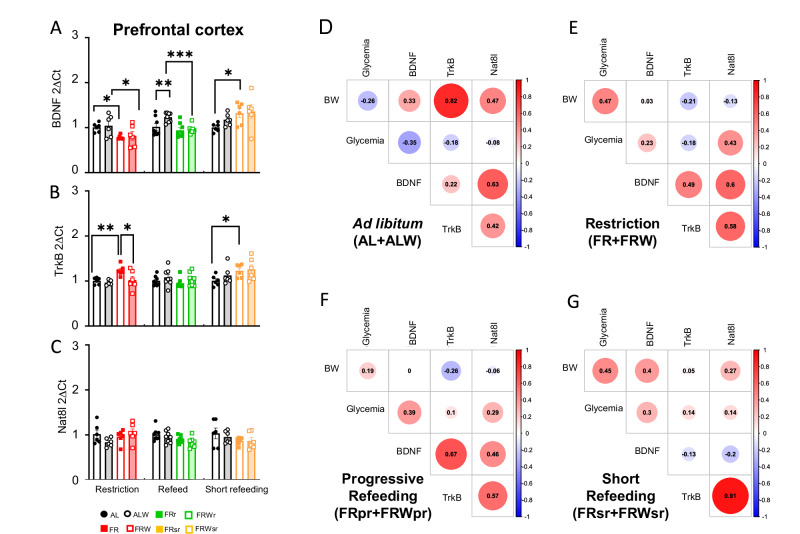
Fig. 2. Effects of chronic food restriction, progressive refeeding, and short refeeding on Bdnf, TrkB and Nat8l expression in the dorsal striatum and prefrontal cortex. Correlation between gene expression and metabolic parameters.
mRNA expression levels of (A) Bdnf, (B) TrkB and (C) Nat8l in the dorsal striatum under different feeding protocols. D–G Correlation heatmaps showing the relationship between gene expression and body weight (BW), glycemia (before feeding time) and circulating BDNF levels in different experimental groups. D ad libitum (AL + ALW), (E) Food restriction (FR + FRW), (F) Refeeding (FRpr + FRWpr), and (G) short refeeding (FRsr + FRWsr). The intensity of the color of the circles represents the strength of the correlation, with red indicating a positive correlation and blue indicating a negative correlation. The numbers represent correlation coefficients (r), and the size of the circles corresponds to the significance of the correlation (the larger the circle, the smaller the p-value). Group names are as follows: ad libitum (AL, black line), ad libitum with wheel (ALW, black filled), food restriction (FR, red line), food restriction with wheel (FRW, red filled), progressive refeeding (FRpr, green line), progressive refeeding with wheel (FRWpr, green filled), short refeeding (FRsr, yellow line), and short refeeding with wheel (FRWsr, yellow filled). Data are expressed as mean ± SEM. Significant differences are indicaed as *p < 0.05. Statistical significance was determined by one-way ANOVA with post-hoc Tukey’s test.
Fig. 3. Effects of chronic food restriction, progressive refeeding, and short refeeding on Bdnf, TrkB and Nat8l expression in the prefrontal cortex. Correlation between gene expression and metabolic parameters.
mRNA expression levels of (A) Bdnf, (B) TrkB and (C) Nat8l in the prefrontal cortex under different feeding protocols. D–G Correlation heatmaps showing the relationship between gene expression and body weight (BW), glycemia (before feeding time) and circulating BDNF levels in different experimental groups. D ad libitum (AL + ALW), (E) Food restriction (FR + FRW), (F) Refeeding (FRpr + FRWpr), and (G) short refeeding (FRsr + FRWsr). The intensity of the color of the circles represents the strength of the correlation, with red indicating a positive correlation and blue indicating a negative correlation. The numbers represent correlation coefficients (r), and the size of the circles corresponds to the significance of the correlation (the larger the circle, the smaller the p-value). Group names are as follows: ad libitum (AL, black line), ad libitum with wheel (ALW, black filled), food restriction (FR, red line), food restriction with wheel (FRW, red filled), progressive refeeding (FRpr, green line), progressive refeeding with wheel (FRWpr, green filled), short refeeding (FRsr, yellow line), and short refeeding with wheel (FRWsr, yellow filled). Data are expressed as mean ± SEM. Significant differences are indicated as *p < 0.05, **p < 0.01, ***p < 0.001. Statistical significance was determined by one-way ANOVA with post-hoc Tukey’s test.
Two-way ANOVA showed that food restriction significantly decreased Bdnf expression in both the DS and PFC (F(1, 20) = 10.56, p = 0.004 for DS; F(1, 20) = 9.619, p = 0.006 for PFC, fig S4A,J), while no effect was observed on TrkB expression (Fig S4D,M). In contrast, Nat8l showed a significant interaction effect of restriction combined with running wheel exclusively in the DS (F(1, 20) = 10.17, p = 0.005, Fig S4G,P). However, no significant effect of wheel access alone was observed (Figs S4, 5). No significant changes were noted in the nucleus accumbens and the ventral tegmental area.
In addition, in the food restriction condition, Bdnf expression was positively correlated with body weight in the DS and nucleus accumbens and with fasting blood glucose levels in the DS, PFC and nucleus accumbens (Figs. 2E, 3E, S2E). This suggests region-specific responses to metabolic changes under chronic food restriction.
Whole RNA sequencing in the dorsal striatum and prefrontal cortex
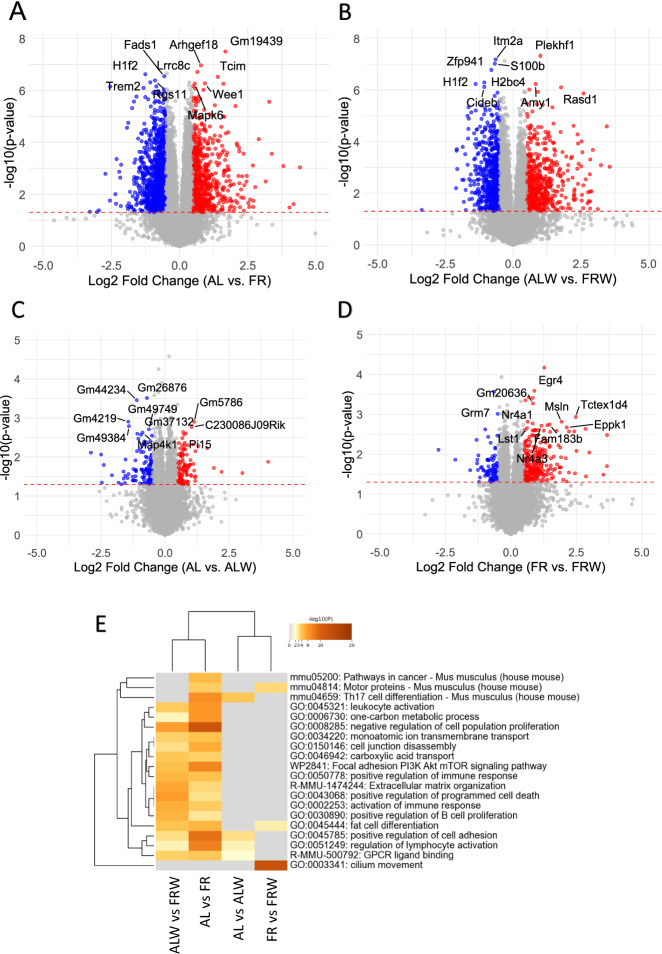
Then, to replicate the Bdnf expression and identify biological pathways, we investigated the entire gene expression profile in the brain regions. RNA sequencing in the DS also revealed the decrease of Bdnf expression under chronic restriction and identified significant gene expression changes between AL and FR groups, as well as and between wheel access groups (ALW vs FRW) (Fig. 4A-D). Food restriction resulted in significant up- and down-regulation of genes (Fig. 4A, Table 1), while wheel access under ad libitum conditions also altered gene expression (Fig. 4B). However, wheel access generally had a minimal effect on gene expression in both ad libitum and restricted conditions (Fig. 4C, D).
Fig. 4. RNA sequencing analysis reveals differentially expressed genes in the dorsal striatum across feeding and physical activity conditions.
A–D Volcano plots show gene expression changes between different comparison groups: ad libitum (AL) vs food restriction (FR) (A), ad libitum with wheel (ALW) vs food restriction with wheel (FRW) (B), ad libitum (AL) vs ad libitum with wheel (ALW) (C), and food restriction (FR) vs food restriction with wheel (FRW) (D). Significantly downregulated genes (p < 0.05, log2FC < −0.5) are highlighted in blue, and significantly upregulated genes (p < 0.05, log2FC > 0.5) are in red. E Gene Ontology (GO) analysis of enriched pathways using Metascape. The heatmap represents the most significantly enriched biological processes across experimental conditions, including pathways related to cancer, cell proliferation, MAPK signalling, PI3K-Akt signalling, and immune regulation. Statistical significance was determined using Student’s t-test.
Table 1.
Modulation of mRNA expression levels in various brain structures by chronic restriction or wheel running.
| Tissue | *Down-regulated | *Up-regulated | Top 10 significant | |
|---|---|---|---|---|
| DS | Restriction | 1049 | 671 | H1f2, Gap43, Fads1, Rgs11, Trem2, Arhgef18, Lrrc8c, Golph3, Tcim, Gm19439 |
| Wheel | 115 | 106 | Gm26876, Gm4219, Gm44294, Gm49749, Map4k1, Gm5786, Gm37132, nPi19, C230086J09Rik | |
| PFC | Restriction | 632 | 460 | Rskr, Igfn1, Panx2, Akt2, Colq, 4930447C04Rik, Gm29674, Tmem74b |
| Wheel | 75 | 49 | Gm37728, Gm9320, 2900076G11Rik, Rps27l, Pomc, A930035D04Rik, Kif11, Rps15a-ps6, Gm4117 Pm20d1 | |
*Genes with an adjusted p-value < 0.05 and a Log2Fold Change < -0.5 or > 0.5 were selected for inclusion.
DS dorsal striatum, PFC prefrontal cortex.
Pathway enrichment analysis revealed significant changes in key pathways, including “MAPK signalling”, “PI3K-Akt signalling” and “Apoptosis”, reflecting the broad effects of dietary status on metabolic, neuronal and immune functions. Similar effects were observed in the restriction and wheel-running groups (Fig. 4E, Supplementary Tables 2 and 3).
Cross-comparison of the transcriptomic data with the BDNF protein-protein interaction (PPI) network revealed that most of the differentially expressed genes were involved in metabolism, neuronal development, and immune regulation (Table 2, Supplementary Tables 4 to 8).
Table 2.
Modulation of mRNA expression levels of genes in protein-protein interaction (PPI) with BDNF in various brain structures by chronic food restriction or wheel running.
| Tissue | Effect of Restriction | Effect of Wheel Running | |
|---|---|---|---|
| DS | up-regulated | Mme, Atp7a, Fgf22, Ppargc1a, Ror1, Calca, Jak2, Pik3r1, Slc2a1, Musk, Ehd1, Nt5e, Stat3, Htr1b, Nrtn, Gfap, Htr2a, Dnmt3b, Fkbp5, Mc4r, Pkp2, Scn10a | Atp7a, Calca |
| down-regulated | Tph2, Itgam, Pkp1, Ngfr, Fgf3, Trem2, Nr4a2, Bche, Fgf17, Rasal3, S100b, Cx3cr1, Sec16b, mt-Nd2, Aif1, Tph1, Cck, Mog, Grin3b, Mmp2, Cadps2, Ptn, Rtn4r, Fgf10, Ntsr1, Artn, Stmn2, Esr1, Angpt1, Nfia, Npas4, Fos | Grap2 | |
| PFC | up-regulated | Grm8, Tacr1, Ddc, Mmp9, Ppp1r1b, Pkp2, Dnmt3b, Fkbp5, Mc4r, Scn10a | Pm20d1, Bmp4, Pomc |
| down-regulated | Itgam, Neurog2, Npas4, Trem2, Fgf17, Cx3cr1, Nptx2, Tek, Artn, Bche, Grm3, mt-Nd2 | Fgf18, Dynlt1c | |
Protein-protein interactions were analyzed using string.com. Genes with an adjusted p-value < 0.05 and a Log2Fold Change < -0.5 or > 0.5 were selected for inclusion.
DS dorsal striatum, PFC prefrontal cortex.
Gene expression in the PFC followed a similar pattern to that in the DS, with minimal effects of wheel activity (Fig S6A-D, Table 1). Wheel running reversed gene expression patterns under restriction (Fig S7A, B, Table S2), with differences in the specific genes affected between the DS and PFC (Table S2). Pathway enrichment in the PFC highlighted immune regulation and cell-cell communication as major processes (Fig S6E).
Discussion
Our study shows that, in our AN-like mouse model, chronic food restriction, progressive refeeding and short-term refeeding modulate the expression of Bdnf, TrkB and Nat8l in brain regions involved in cognitive and reward functions, resulting in region-specific responses. Interestingly, refeeding failed to restore Bdnf levels in the prefrontal cortex (PFC), whereas short-term refeeding induced an increase in Bdnf levels in the same region. This suggests that the type of refeeding elicits differential responses. Furthermore, TrkB patterns mirrored those of Bdnf in the DS, whereas in the PFC, Bdnf and TrkB were regulated in opposite directions. These region-specific modifications in the expression of Bdnf and TrkB genes likely have consequences for signal transduction and the ability of cells, especially neurons, to undergo neuroprotection and neuroplasticity. These findings suggest that nutritional status, as observed in anorexia nervosa, profoundly affects neuroplasticity and reward circuit dynamics, but in a region-specific manner. Chronic food restriction was found to impair reversal learning on the first day as evidence in the Y-maze test, however this effect appears to be reversible as no long-term deficits were observed.
Female individuals with AN often struggle with cognitive flexibility, particularly in task switching [8]. The DS is critical for this function [38], with BDNF in the DS playing a key role [39], in addition to BDNF in the PFC [40]. While no long-term cognitive inflexibility was observed in our study with the AN-like mouse model, we did find short-term impairments, likely related to reduced BDNF in the DS and PFC due to chronic food restriction. This suggests that reduced BDNF in these regions may contribute to transient cognitive challenges during dietary stress.
We observed that mice experiencing food restriction performed better during the learning phase than ad libitum fed mice and had more “correct” trials in the learning phase to consolidate. This would possibly explain the initial perseveration on the first day of reversal learning when the baited arm was reversed.
Our results on plasma Bdnf are consistent with previous studies showing that patients with AN exhibit reversible decreased plasma BDNF levels with weight recovery [41–43], supporting not only the relevance of our model in mimicking key physiological aspects of AN but also the potential role of BDNF as a biomarker for the disease. However, no previous study has investigated BDNF mRNA or protein levels in the DS under chronic food restriction, making our study the first to do so. Previous studies have found no significant changes in the PFC, NAc or VTA in both male and female rats under similar conditions [44]. Our results are consistent with these findings in the mesolimbic regions. However, in our study we observed a decrease in Bdnf expression in the PFC that may be specific to the specie of mice that we used or specific to females.
The function of BDNF is not consistent across brain regions, with contrasting effects observed between the hippocampus/PFC and the reward circuits [45]. In areas such as the PFC, higher levels of BDNF are often associated with improved cognitive outcomes and protection against stress-related mood disorders [46, 47]. However, in the mesolimbic pathway (NAc-VTA) and in the DS, BDNF appears to play a more complex and, in some cases, pro-depressive role [36, 48]. Our findings contribute to the growing body of evidence suggesting that patients with AN may engage in food restrictive behaviors to modulate mood, possibly through reward pathways [49].
Nat8l, a positive regulator of BDNF expression in the DS, is thought to play a key role in modulating these mood-related effects [36]. Our results show that food restriction combined with wheel running, significantly reduced Nat8l expression in the DS, which may further reduce Bdnf levels and contribute to stress susceptibility. Nat8l has been implicated in epigenetic modulation that influences brain resilience to chronic stress [36]. The observed decrease in both Nat8l and Bdnf under food restriction conditions suggests that the DS may become more susceptible to stress or fail to properly regulate reward mechanisms that are often disrupted in AN [5]. This may contribute to the persistence of reduced feeding in AN as a maladaptive response to stress [50, 51]. Further studies are needed to determine whether these changes are part of a protective mechanism or a driver of maladaptive behaviors in AN, and how they might influence potential therapeutic targets aimed at restoring neuroplasticity in these regions.
The RNA sequencing results revealed significant gene expression changes in the DS and PFC under chronic food restriction. Key genes such as Fasn, Gap43, and Trem2 were upregulated in the DS, indicating an adaptive response to metabolic stress, likely related to synaptic plasticity and cellular signalling as shown by GO analysis. In the PFC, genes such as Egr4 and Golph3 were altered, with GO analysis suggesting effects on immune regulation and metabolic processes. These findings suggest that food restriction-induced molecular changes in brain regions involved in cognitive flexibility and reward processing may contribute to the behavioral outcomes observed in AN.
Physical activity increases Bdnf in the PFC and DS as shown in rodent models. In our study, although physical activity had a limited overall effect on gene expression, it reversed the effects of food restriction on specific genes in the DS and PFC, such as Gap43, Trem2, and Arhgef18. In the DS, genes such as Egr4 and Fasn were reversed by wheel access, suggesting that exercise may play a role in regulating metabolic pathways under chronic restriction. Similarly, Tceal5 and Atat1 in the PFC were modulated by exercise. These results highlight the interaction between nutritional status and physical activity in modulating brain function under metabolic stress.
Finally, although BDNF protein can cross the blood-brain barrier [52], our study suggests that plasma BDNF levels may not accurately reflect region-specific brain changes due to the heterogeneous regulation of BDNF in different brain regions and the lack of consistency with the expression pattern of a single brain region. Future work should focus on proteomic analyses to fully understand how BDNF and TrkB signalling contribute to the neurobiological basis of AN and explore whether other peripheral markers may more accurately reflect brain changes.
A limitation of this study is the use of the Y-maze reversal learning task, which may not have been sufficiently challenging enough to fully assess potential deficits in cognitive flexibility. Future studies should consider the use of more challenging tasks, such as the water maze. Food restriction may also induce olfactory sensitisation [53], which may lead to a bias of the test we have used. In addition, although we measured Bdnf mRNA expression in key brain regions, we did not quantify protein in these regions. Direct measurement of BDNF protein levels in the brain would provide a more complete understanding of their relationship with plasma BDNF levels and allow a more accurate cross-comparison between peripheral and central changes under restrictive conditions. The design of our study, which relied mainly on dissection and molecular tools, we were unable to investigate the level of neuronal circuitry required to gain a clear view of the interactions between DS and its subregions, the PFC, and the infralimbic, prelimbic and anterior cingulate regions.
In conclusion, chronic food restriction and subsequent refeeding significantly affects brain plasticity in the DS and PFC, regions critical for cognitive function, with implications for the development of more tailored treatment strategies in AN. Understanding these complex interactions between diet, brain plasticity, and physical activity is critical for improving treatment outcomes in this population.
Supplementary information
Supplementary figures and tables legends
Figure S1. Schematic illustration of the brain regions of interest dissected
Figure S2. Effects of chronic food restriction, progressive refeeding, and short refeeding on Bdnf, TrkB and Nat8l expression in the nucleus accumbens. Correlation between gene expression and metaboli
Figure S3. Effects of chronic food restriction, progressive refeeding, and short refeeding on Bdnf, TrkB and Nat8l expression in the ventral tegmental area
Figure S4. Bdnf, TrkB and Nat8l mRNA expression in the dorsal striatum and the prefrontal cortex regions under different feeding protocols and physical activity conditions, highlighting the interactio
Figure S5. Bdnf, TrkB and Nat8l mRNA expression in the nucleus accumbens and the ventral tegmental area under different feeding protocols and physical activity conditions, highlighting the interaction
Figure S6. RNA sequencing analysis reveals differentially expressed genes in the prefrontal cortex across feeding and physical activity conditions
Figure S7. Cross-comparison of gene expression changes in response to food restriction and wheel running across the dorsal striatum and prefrontal cortex
Supplementary Table 1. Primers used for qPCR experiments
Supplementary Table 2. Functional Annotation of Differentially Expressed Genes in the dorsal striatum from Table 1
Supplementary Table 3. Functional Annotation of Differentially Expressed Genes in the prefrontal cortex from Table 1
Supplementary Table 4. Functional Annotation of Differentially Expressed Genes in the dorsal striatum from Table 2
Supplementary Table 5. Functional Annotation of Differentially Expressed Genes in the prefrontal cortex from Table 2
Supplementary Table 6. Top gene expression in interaction between chronic food restriction and wheel running. CR: caloric restriction; DS: dorsal striatum; PFC: Prefrontal cortex
Supplementary Table 7. Functional Annotation of Differentially Expressed Genes in the dorsal striatum from Supplementary Table 6
Supplementary Table 8. Functional Annotation of Differentially Expressed Genes in the prefrontal cortex from Supplementary Table 6
Acknowledgements
This work was supported by the Fédération pour la Recherche sur le Cerveau (FRC to NR), the Fondation de France – Maladies Psychiatriques (FdF to PG), the Université Paris Cité and the Institut National de la Santé et de la Recherche Médicale (INSERM). JC is a PhD student of a doctoral fellow of the University Paris Cité, ED562 BioSPC. CT is a recipient of a PhD fellowship (FDM202006011161) funded by the Fondation pour la Recherche Médicale (FRM). The authors would like to thank Ludivine Therreau and Gwenaëlle Le Pen of the IPNP PhenoBrain core facility and the staff of the animal facility, for their invaluable help in the handling and managing the animals used in this study.
Author contributions
Odile Viltart, Nicolas Ramoz, Jingxian Cao and Virginie Tolle designed the experiments. Jingxian Cao, Nicolas Lebrun, Shiou-ping Chen, Chloé Tezenas du Montcel, Céline Cruciani-Guglielmacci and Odile Viltart performed the experiments. Jingxian Cao, Philip Gorwood, Nicolas Ramoz, and Odile Viltart carried out the analysis. The manuscript was drafted by Jingxian Cao, Nicolas Ramoz, and Odile Viltart. All authors discussed results, made figures, and edited the manuscript.
Data availability
Data, protocols and detailed materials are available on request from the corresponding author (nicolas.ramoz@inserm.fr).
Competing interests
PG received during the last 5 years fees for presentations at congresses or participation in scientific boards from Biogen, Janssen, Lundbeck, Merk, Otsuka, Richter and Viatris. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nicolas Ramoz, Odile Viltart.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-025-03618-7.
References
- 1.Micali N, Hagberg KW, Petersen I, Treasure JL. The incidence of eating disorders in the UK in 2000-2009: findings from the general practice research database. BMJ Open. 2013;3:e002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mainz V, Schulte-Rüther M, Fink GR, Herpertz-Dahlmann B, Konrad K. Structural brain abnormalities in adolescent anorexia nervosa before and after weight recovery and associated hormonal changes. Psychosom Med. 2012;74:574–82. [DOI] [PubMed] [Google Scholar]
- 3.Rosen E, Bakshi N, Watters A, Rosen HR, Mehler PS. Hepatic complications of anorexia nervosa. Dig Dis Sci. 2017;62:2977–81. [DOI] [PubMed] [Google Scholar]
- 4.Watson HJ, Yilmaz Z, Thornton LM, Hübel C, Coleman JR, Gaspar HA, et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet. 2019;51:1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duriez P, Ramoz N, Gorwood P, Viltart O, Tolle V. A metabolic perspective on reward abnormalities in anorexia nervosa. Trends Endocrinol Metab. 2019;30:915–28. [DOI] [PubMed] [Google Scholar]
- 6.Gaudio Santino, Quattrocchi CC. Neural basis of a multidimensional model of body image distortion in anorexia nervosa. Neurosci Biobehav Rev. 2012;36:1839–47. [DOI] [PubMed] [Google Scholar]
- 7.Hagman J, Gardner RM, Brown DL, Gralla J, Fier JM, Frank GK. Body size overestimation and its association with body mass index, body dissatisfaction, and drive for thinness in anorexia nervosa. Eat Weight Disord. 2015;20:449–55. [DOI] [PubMed] [Google Scholar]
- 8.Duriez P, Kaya Lefèvre H, Di Lodovico L, Viltart O, Gorwood P. Increased cognitive flexibility mediates the improvement of eating disorders symptoms, depressive symptoms and level of daily life functioning in patients with anorexia nervosa treated in specialised centres. Euro Eat Disord Rev. 2021;29:600–10. [DOI] [PubMed] [Google Scholar]
- 9.Treasure J, Zipfel S, Micali N, Wade T, Stice E, Claudino A, et al. Anorexia nervosa. Nat Rev Dis Prim. 2015;1:15074. [DOI] [PubMed] [Google Scholar]
- 10.Smink FRE, van Hoeken D, Hoek HW. Epidemiology, course, and outcome of eating disorders. Curr Opin Psychiatry. 2013;26:543–8. [DOI] [PubMed] [Google Scholar]
- 11.Khalsa SS, Portnoff LC, McCurdy-McKinnon D, Feusner JD. What happens after treatment? A systematic review of relapse, remission, and recovery in anorexia nervosa. J Eat Disord. 2017;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68:724–31. [DOI] [PubMed] [Google Scholar]
- 13.Duriez P, Goueslard K, Treasure J, Quantin C, Jollant F. Risk of non-fatal self-harm and premature mortality in the three years following hospitalization in adolescents and young adults with an eating disorder: A nationwide population-based study. Intl J Eat Disord. 2023;56:1534–43. [DOI] [PubMed] [Google Scholar]
- 14.Di Lodovico L, Al Tabchi A, Clarke J, Mancusi RL, Messeca D, Duriez P, et al. Trajectories and predictive factors of weight recovery in patients with anorexia nervosa completing treatment. a latent class mixed model approach. Euro Eat Disord Rev. 2024;32:758–70. [DOI] [PubMed] [Google Scholar]
- 15.Abou Al Hassan S, Cutinha D, Mattar L. The impact of COMT, BDNF and 5-HTT brain-genes on the development of anorexia nervosa: a systematic review. Eat Weight Disord. 2021;26:1323–44. [DOI] [PubMed] [Google Scholar]
- 16.Baker JH, Schaumberg K, Munn-Chernoff MA. Genetics of anorexia nervosa. Curr Psychiatry Rep. 2017;19:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Versini A, Ramoz N, Le Strat Y, Scherag S, Ehrlich S, Boni C, et al. Estrogen receptor 1 gene (ESR1) is associated with restrictive anorexia nervosa. Neuropsychopharmacol. 2010;35:1818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribases M, Gratacos M, Fernandez-Aranda F, Bellodi L, Boni C, Anderluh M, et al. Association of BDNF with anorexia, bulimia and age of onset of weight loss in six European populations. Hum Mol Genet. 2004;13:1205–12. [DOI] [PubMed] [Google Scholar]
- 19.Cao J, Gorwood P, Ramoz N, Viltart O. The role of central and peripheral brain-derived neurotrophic factor (bdnf) as a biomarker of anorexia nervosa reconceptualized as a metabo-psychiatric disorder. Nutrients. 2024;16:2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramoz N, Versini A, Gorwood P. Eating disorders: an overview of treatment responses and the potential impact of vulnerability genes and endophenotypes. Expert Opin Pharmacother. 2007;13:2029–44. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci. 2005;8:164–72. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Christian K, Lu B. BDNF: a key regulator for protein-synthesis dependent LTP and long-term memory?. Neurobiol Learn Mem. 2008;89:312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S-W, Xu B. Rapid and lasting effects of activating BDNF-expressing PVH neurons on energy balance. eNeuro. 2022;9:ENEURO.0009-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iu ECY, Chan CB. Is brain-derived neurotrophic factor a metabolic hormone in peripheral tissues?. Biology. 2022;11:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke J, Ramoz N, Fladung A-K, Gorwood P. Higher reward value of starvation imagery in anorexia nervosa and association with the Val66Met BDNF polymorphism. Transl Psychiatry. 2016;6:e829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fladung A-K, Grön G, Grammer K, Hernberger B, Schilly E, Grasteit S, et al. A neural signature of anorexia nervosa in the ventral striatal reward system. AJP. 2010;167:206–12. [DOI] [PubMed] [Google Scholar]
- 27.Chowdhury TG, Chen Y-W, Aoki C. Using the activity-based anorexia rodent model to study the neurobiological basis of anorexia nervosa. JoVE. 2015;22:52927. 10.3791/52927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelegen C, van den Heuvel J, Collier DA, Campbell IC, Oppelaar H, Hessel E, et al. Dopaminergic and brain-derived neurotrophic factor signalling in inbred mice exposed to a restricted feeding schedule. Genes Brain Behav. 2008;7:552–9. [DOI] [PubMed] [Google Scholar]
- 29.Mottarlini F, Rizzi B, Targa G, Fumagalli F, Caffino L. Long-lasting BDNF signaling alterations in the amygdala of adolescent female rats exposed to the activity-based anorexia model. Front Behav Neurosci. 2022;16:1087075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennerley SW, Walton ME. Decision making and reward in frontal cortex: complementary evidence from neurophysiological and neuropsychological studies. Behav Neurosci. 2011;125:297–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho EV, Klenotich SJ, McMurray MS, Dulawa SC. Activity-based anorexia alters the expression of BDNF transcripts in the mesocorticolimbic reward circuit. PLoS ONE. 2016;11:e0166756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Méquinion M, Caron E, Zgheib S, Stievenard A, Zizzari P, Tolle V, et al. Physical activity: benefit or weakness in metabolic adaptations in a mouse model of chronic food restriction?. Am J Physiol -Endocrinol Metab. 2015;308:E241–E255. [DOI] [PubMed] [Google Scholar]
- 33.Duriez P, Nilsson IAK, Le Thuc O, Alexandre D, Chartrel N, Rovere C, et al. Exploring the mechanisms of recovery in anorexia nervosa through a translational approach: from original ecological measurements in human to brain tissue analyses in mice. Nutrients. 2021;13:2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tezenas du Montcel C, Duriez P, Cao J, Lebrun N, Ramoz N, Viltart O, et al. The role of dysregulated ghrelin/LEAP-2 balance in anorexia nervosa. iScience. 2023;26:107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein R, Parada LF, Coulier F, Barbacid M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 1989;8:3701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyanishi H, Muramatsu S, Nitta A. Striatal Shati/Nat8l–BDNF pathways determine the sensitivity to social defeat stress in mice through epigenetic regulation. Neuropsychopharmacol. 2021;46:1594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tezenas du Montcel C, Cao J, Mattioni J, Hamelin H, Lebrun N, Ramoz N, et al. Chronic food restriction in mice and increased systemic ghrelin induce preference for running wheel activity. Psychoneuroendocrinology. 2023;155:1–10. [DOI] [PubMed] [Google Scholar]
- 38.Darvas M, Palmiter RD. Contributions of striatal dopamine signaling to the modulation of cognitive flexibility. Biol Psychiatry. 2011;69:704–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh V, Naughton SX, Yegla B, Guzman DM. Impact of partial dopamine depletion on cognitive flexibility in BDNF heterozygous mice. Psychopharmacology. 2016;233:1361–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houlton J, Zhou LYY, Barwick D, Gowing EK, Clarkson AN. Stroke Induces a BDNF-dependent improvement in cognitive flexibility in aged mice. Neural Plasticity. 2019;2019:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keeler JL, Patsalos O, Chung R, Schmidt U, Breen G, Treasure J, et al. Short communication: Serum levels of brain-derived neurotrophic factor and association with pro-inflammatory cytokines in acute and recovered anorexia nervosa. J Psychiatr Res. 2022;150:34–39. [DOI] [PubMed] [Google Scholar]
- 42.Keeler JL, Bahnsen K, Wronski M-L, Bernardoni F, Tam F, Arold D, et al. Longitudinal changes in brain-derived neurotrophic factor (BDNF) but not cytokines contribute to hippocampal recovery in anorexia nervosa above increases in body mass index. Psychol Med. 2024;54:2242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trinh S, Keller L, Herpertz-Dahlmann B, Seitz J. The role of the brain-derived neurotrophic factor (BDNF) in anorexia nervosa. Psychoneuroendocrinology. 2023;151:106069. [DOI] [PubMed] [Google Scholar]
- 44.Pan Y, Chau L, Liu S, Avshalumov MV, Rice ME, Carr KD. A food restriction protocol that increases drug reward decreases tropomyosin receptor kinase B in the ventral tegmental area, with no effect on brain-derived neurotrophic factor or tropomyosin receptor kinase B protein levels in dopaminergic forebrain regions. Neuroscience. 2011;197:330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyanishi H, Nitta A. A role of BDNF in the depression pathogenesis and a potential target as antidepressant: the modulator of stress sensitivity “Shati/Nat8l-BDNF System” in the dorsal striatum. Pharmaceuticals. 2021;14:889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang B, Zhang J-C, Han M, Yao W, Yang C, Ren Q, et al. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology. 2016;233:3647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shirayama Y, Chen AC-H, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. [DOI] [PubMed] [Google Scholar]
- 49.Wayda-Zalewska M, Grzegorzewski P, Kot E, Skimina E, Santangelo PS, Kucharska K. Emotion dynamics and emotion regulation in anorexia nervosa: a systematic review of ecological momentary assessment studies. IJERPH. 2022;19:13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller SP, Erickson SJ, Branom C, Steiner H. Habitual response to stress in recovering adolescent anorexic patients. Child Psychiatry Hum Dev. 2009;40:43–54. [DOI] [PubMed] [Google Scholar]
- 51.Guarda AS, Schreyer CC, Boersma GJ, Tamashiro KL, Moran TH. Anorexia nervosa as a motivated behavior: relevance of anxiety, stress, fear and learning. Physiol Behav. 2015;152:466–72. [DOI] [PubMed] [Google Scholar]
- 52.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology. 1998;37:1553–61. [DOI] [PubMed] [Google Scholar]
- 53.Apelbaum AF. Rats habituated to chronic feeding restriction show a smaller increase in olfactory bulb reactivity compared to newly fasted rats. Chem Senses. 2003;28:389–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables legends
Figure S1. Schematic illustration of the brain regions of interest dissected
Figure S2. Effects of chronic food restriction, progressive refeeding, and short refeeding on Bdnf, TrkB and Nat8l expression in the nucleus accumbens. Correlation between gene expression and metaboli
Figure S3. Effects of chronic food restriction, progressive refeeding, and short refeeding on Bdnf, TrkB and Nat8l expression in the ventral tegmental area
Figure S4. Bdnf, TrkB and Nat8l mRNA expression in the dorsal striatum and the prefrontal cortex regions under different feeding protocols and physical activity conditions, highlighting the interactio
Figure S5. Bdnf, TrkB and Nat8l mRNA expression in the nucleus accumbens and the ventral tegmental area under different feeding protocols and physical activity conditions, highlighting the interaction
Figure S6. RNA sequencing analysis reveals differentially expressed genes in the prefrontal cortex across feeding and physical activity conditions
Figure S7. Cross-comparison of gene expression changes in response to food restriction and wheel running across the dorsal striatum and prefrontal cortex
Supplementary Table 1. Primers used for qPCR experiments
Supplementary Table 2. Functional Annotation of Differentially Expressed Genes in the dorsal striatum from Table 1
Supplementary Table 3. Functional Annotation of Differentially Expressed Genes in the prefrontal cortex from Table 1
Supplementary Table 4. Functional Annotation of Differentially Expressed Genes in the dorsal striatum from Table 2
Supplementary Table 5. Functional Annotation of Differentially Expressed Genes in the prefrontal cortex from Table 2
Supplementary Table 6. Top gene expression in interaction between chronic food restriction and wheel running. CR: caloric restriction; DS: dorsal striatum; PFC: Prefrontal cortex
Supplementary Table 7. Functional Annotation of Differentially Expressed Genes in the dorsal striatum from Supplementary Table 6
Supplementary Table 8. Functional Annotation of Differentially Expressed Genes in the prefrontal cortex from Supplementary Table 6
Data Availability Statement
Data, protocols and detailed materials are available on request from the corresponding author (nicolas.ramoz@inserm.fr).