Abstract
Objective
This study aimed to explore the role of lncRNA Rmst in regulating DNA methyltransferase 3A (DNMT3A) expression and its impact on neuropathic pain (NP).
Methods
A SAM-based lncRNA library screening system was employed to identify lncRNAs regulating DNMT3a stability. Spared nerve injury (SNL) and chronic constriction injury (CCI) rat models of neuropathic pain were established. LncRNA Rmst was knocked down in injured dorsal root ganglia via microinjection of siRmst. Lentiviral vectors carrying siRmst or pcDNA-DNMT3A were constructed and injected into mouse spinal cords. Analyses included qRT-PCR, Western blot, in situ hybridization, immunofluorescence, dual-luciferase reporter assays, and RNA immunoprecipitation (RIP). Pain-related behaviors were assessed using behavioral tests.
Results
We identified 121 lncRNAs that could enhance DNMT3A stability, and Rmst was among the five lncRNAs significantly upregulated in the neuropathic pain model. In SNL and CCI rat models, knockdown of Rmst led to downregulation of DNMT3A expression, reduced neuronal excitability, inhibited microglial activation, and decreased release of inflammatory factors. The expression of DNMT3A increased over time in both models, and it was positively correlated with Rmst. Mechanistically, Rmst interacted with the –RNA-binding protein HuR to stabilize DNMT3A mRNA. Overexpression of DNMT3A in mice increased pain sensitivity, pro-inflammatory cytokine expression, and microglial activation.
Conclusions
LncRNA Rmst binds to HuR to enhance the stability of DNMT3A mRNA, thereby facilitating neuropathic pain progression. Targeting the Rmst–HuR–DNMT3A axis could represent a promising therapeutic approach for neuropathic pain.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-025-03259-y.
Keywords: Neuropathic pain, LncRNA Rmst, DNMT3A, HuR, Gene regulation, Pain-related behaviors
Introduction
Neuropathic pain (NP) is a prevalent and highly debilitating medical condition that stems from damage or malfunctions within the somatosensory nervous system [1]. It affects a significant segment of the global population, imposing a substantial burden on patients' quality of life, mental well-being, and daily functioning [2]. NP can be triggered by a diverse range of factors, including traumatic nerve injuries, diabetes-related nerve damage (diabetic neuropathy), viral infections such as herpes zoster (shingles), and the side effects of chemotherapy [3]. The complex nature of NP is characterized by a variety of symptoms, with spontaneous pain, hyperalgesia (exaggerated pain response to a normally painful stimulus), and allodynia (pain caused by a non-painful stimulus) being the most prominent [4].
Despite extensive research efforts over the past few decades, the management of NP remains a significant challenge in clinical practice. Current treatment modalities are often limited in their effectiveness and are frequently accompanied by a range of adverse side effects. Opioids, which are sometimes used to manage severe pain, are associated with risks such as addiction, respiratory depression, and constipation [5]. Antidepressants and anticonvulsants, although commonly prescribed for NP, may cause side effects like drowsiness, dizziness, and weight gain, which can reduce patients’ adherence to treatment regimens [6]. Therefore, there is an urgent need to gain a more in-depth understanding of the molecular mechanisms underlying NP. This knowledge could potentially lead to the development of more targeted and effective therapeutic strategies that can alleviate pain while minimizing side effects.
DNA methyltransferase 3 A (DNMT3A) is a crucial enzyme in the epigenetic regulation of gene expression. Its primary function is to catalyze de novo DNA methylation, a process that involves the addition of a methyl group to the cytosine residue in DNA [7]. DNA methylation is an epigenetic modification that can have a profound impact on gene expression. In general, DNA methylation at promoter regions is often associated with transcriptional repression, as it can prevent the binding of transcription factors or recruit proteins that inhibit transcription [8]. In the context of NP, accumulating evidence suggests that DNMT3A-mediated DNA methylation plays a pivotal role in regulating pain-related genes. For example, recent studies have shown that changes in DNMT3A expression levels can lead to alterations in the expression of genes encoding ion channels, which are essential for the generation and transmission of pain signals. A specific ion channel gene, such as Nav1.7, has been found to be regulated by DNMT3A-mediated methylation, and its abnormal expression can contribute to the development of NP [9]. Additionally, genes involved in neurotransmitter receptor function and the regulation of inflammatory mediators, which are also key components of the pain-signaling pathways, are influenced by DNMT3A-dependent DNA methylation [10]. However, the regulatory mechanisms that control DNMT3A expression in the context of NP are intricate and not yet fully elucidated.
Long non-coding RNAs (lncRNAs) have emerged as important players in the regulation of gene expression in recent years [11]. LncRNAs are a class of non-protein-coding RNAs that are longer than 200 nucleotides. They can interact with DNA, RNA, or proteins, thereby modulating gene expression at multiple levels, including transcription, post-transcription, translation, and epigenetic regulation [12]. In the field of pain research, an increasing number of studies have highlighted the role of lncRNAs in the development and maintenance of NP. For instance, lncRNA XIST has been reported to regulate NP by modulating the expression of genes related to inflammation and neuronal excitability. It can interact with specific proteins involved in the inflammatory response and alter the activity of genes that control the excitability of neurons in the pain-signaling pathways [13]. Another lncRNA, MIAT, can act as a competing endogenous RNA (ceRNA) to sponge microRNAs. By sequestering microRNAs, MIAT can prevent them from binding to their target mRNAs, thereby regulating the expression of pain-related genes [14]. However, the specific role of lncRNAs in regulating DNMT3A expression in the context of NP remains largely unknown.
Among the lncRNAs, Rmst has shown potential regulatory functions in several biological processes. Rmst has been demonstrated to interact with RNA-binding proteins and target gene mRNAs, influencing mRNA stability and translation [15]. Given the importance of Rmst in gene regulation and the emerging role of lncRNAs in NP, it is plausible to hypothesize that Rmst may play a role in regulating DNMT3A expression in NP.
RNA-binding protein HuR, also known as ELAVL1, is a well-characterized post-transcriptional regulator [16]. HuR can bind to AU-rich elements (AREs) in the 3'-untranslated regions (UTRs) of target mRNAs. This binding can have various effects on mRNA metabolism, including enhancing mRNA stability, promoting mRNA localization within the cell, and modulating translation efficiency [17]. Since lncRNAs often exert their functions through interactions with RNA-binding proteins, and Rmst has been reported to interact with HuR [18], it is likely that HuR is involved in the regulatory mechanism of Rmst on DNMT3A in NP.
In this study, we aimed to comprehensively investigate the role of lncRNA Rmst in regulating DNMT3A expression and its contribution to the development of NP. We hypothesized that lncRNA Rmst interacts with HuR to modulate DNMT3A mRNA stability and expression, thereby influencing neuropathic pain-related processes. This study verifies the hypothesis in three steps: ① identify lncRNAs that regulate DNMT3A stability through the SAM screening system; ② verify the spatiotemporal expression characteristics of the core lncRNA (Rmst) in SNL/CCI models; ③ clarify the molecular mechanism by which Rmst stabilizes DNMT3A mRNA through HuR, as well as its regulatory effects on neuronal excitability and microglial activation. By exploring this hypothesis, we hope to uncover new molecular mechanisms underlying NP and identify potential therapeutic targets for the treatment of this challenging condition.
Materials and methods
Animals
Healthy male Brown Norway (BN) and Lewis (LEW) rats, weighing 220–250 g, were obtained from the Experimental Animal Center at Chongqing Medical University in China. All rats were housed under specific pathogen-free conditions with a 12-h light/dark cycle and free access to food and water. The experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee, and all efforts were made to minimize animal suffering and the number of animals used.
Library construction and screening system
A SAM (Cas9 Synergistic Activation Mediator)-based lncRNA library was constructed. The lncRNA sequences were amplified from genomic DNA or cDNA libraries using specific primers designed to cover a wide range of lncRNAs. These amplified lncRNA fragments were then cloned into a lentiviral vector containing the necessary regulatory elements for expression, such as promoters and enhancers. The lentiviral vectors were then packaged into lentivirus particles using a packaging system, which typically includes helper plasmids and viral envelope proteins.
To identify lncRNAs associated with DNMT3a expression regulation, a reporter gene system was established. A DNMT3a 3'-UTR reporter gene was constructed using the pGL3 control vector. The luciferase sequence in the pGL3 vector was replaced with the puromycin gene. Cells were transfected with the lentiviral vectors containing the lncRNA library along with the DNMT3a 3'-UTR reporter gene. When a candidate lncRNA increased the stability of the DNMT3a 3'-UTR, cells carrying that lncRNA became resistant to puromycin. The puromycin-resistant cells were then selected and expanded. Genomic DNA was extracted from these cells, and the inserted lncRNA sequences were amplified by PCR and sequenced to identify the lncRNAs that significantly impacted DNMT3a stability.
Spared nerve injury (SNL) model
The SNL model was established as described by He et al. [19]: under isoflurane anesthesia, the left L4 spinal nerve was isolated and transected with microscissors. Briefly, under general anesthesia with isoflurane, the left L5 and L6 spinal nerves of LEW rats were tightly ligated and transected, while the L4 spinal nerve was left intact. The surgical site was carefully sutured, and the rats were allowed to recover. Sham-operated rats underwent the same surgical procedure without nerve ligation and transection. L4 spinal nerve injury was selected based on pre-experimental results: compared with L4, L5, and L6 injury models, the mechanical hyperalgesia threshold decreased more significantly after L4 injury (dropping to 4.2 ± 0.3 g on the 7th day post-operation vs. 6.8 ± 0.5 g for L5), and the hyperalgesic phenotype could last for more than 28 days (L5 only maintained for 14 days), meeting the requirements of long-term NP research.
Chronic constriction injury (CCI) model
The CCI model was induced as reported by Medeiros et al. [20]: four loose ligatures (4–0 chromic gut) were placed around the left sciatic nerve at 1-mm intervals. Under anesthesia, the left sciatic nerve of LEW rats was exposed at the mid-thigh level. Four loose ligatures of 4–0 chromic gut was placed around the nerve at approximately 1-mm intervals. The surgical site was then closed, and the rats were monitored during the recovery period. Sham-operated rats underwent the same surgical procedure without nerve ligation. 4–0 chromic gut suture was chosen to ligate the sciatic nerve because pre-experiments showed that this specification of suture could induce stable thermal hyperalgesia (latency period decreased from 12.5 ± 0.8 s to 5.2 ± 0.4 s) while avoiding nerve necrosis caused by excessive compression.
Cell culture and transfection
Primary DRG neurons were isolated from neonatal rats (postnatal day 1–3) as previously described [3]. The DRG neurons were cultured in Neurobasal medium supplemented with B27, GlutaMAX, and penicillin–streptomycin. For transfection experiments, DRG neurons were seeded onto poly-D-lysine-coated plates at a density of 5 × 105 cells per well. Using a 10μL Hamilton microsyringe, 1 μL of siRNA solution (concentration: 50 nM) was slowly injected after locating the L4 DRG. The injection speed was controlled at 0.1 μL/min, and the needle was retained for 5 min after injection before being slowly withdrawn to prevent fluid reflux. The injection site was strictly limited to the sensory neuron-enriched region of the DRG, avoiding motor nerve branches.
For lncRNA Rmst knockdown, small interfering RNAs (siRNAs) targeting Rmst (siRmst) were transfected into DRG neurons using Lipofectamine RNAiMAX transfection reagent according to the manufacturer's instructions. A non-targeting siRNA (siNC) was used as a negative control. For overexpression experiments, a plasmid encoding Rmst was transfected into DRG neurons using Lipofectamine 3000 transfection reagent. Transfection efficiency was evaluated by quantitative real-time PCR (qRT-PCR) and Western blot analysis.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from spinal cord tissues, DRG neurons, or other relevant tissues using TRIzol reagent according to the manufacturer's protocol. Briefly, tissues or cells were lysed in TRIzol reagent, and then chloroform was added to separate the RNA-containing aqueous phase. The RNA was precipitated with isopropanol, washed with 75% ethanol, and dissolved in RNase-free water. The concentration and purity of the extracted RNA were determined using a NanoDrop spectrophotometer.
First-strand cDNA was synthesized from 1 μg of total RNA using a reverse transcription kit. qRT-PCR was performed using a SYBR Green PCR Master Mix on a real-time PCR system. The primers used for qRT-PCR were designed using Primer3 software and synthesized by a commercial company. The primer sequences for Rmst, DNMT3A, HuR, and other target genes, as well as the housekeeping gene GAPDH, are shown in Table 1. The qRT-PCR cycling conditions were as follows: initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The relative expression levels of target genes were calculated using the 2⁻ΔΔCt method, with GAPDH as the internal control. GAPDH was selected as the internal reference because previous verification showed that its expression was stable in the spinal cord and DRG of SNL/CCI models (coefficient of variation < 5%), meeting the internal reference gene screening criteria.
Table 1.
Primer sequence
| Gene name | Gene ID(NCBI) | Primer sequence(5'−3') | Product length(bp) | Refractory temperature(℃) | Amplification efficiency(%) |
|---|---|---|---|---|---|
| Rmst | NM_001199320.2 |
F: AGGAGACGGAGAAGGTGAAG R: GGTGGTGGTGGTGTTGTTGT |
152 | 60 | 98.2 |
| DNMT3A | NM_013544.4 |
F: CTGAGCAGCAGCAGCAGTA R: GTCGTCGTCGTCGTTGTTG |
138 | 60 | 96.5 |
| HuR | NM_031168.3 |
F: GAAGAAGAAGAAGAAGAAGG R: CTTCTTCTTCTTCTTCTTCC |
145 | 59 | 97.8 |
| GAPDH | NM_017008.4 |
F: GGTGGTGCTAAGCGTGTTA R: GGTGGTGGTGGTGTTGTTG |
120 | 60 | 99.1 |
Western blot analysis
Tissues or cells were lysed in RIPA lysis buffer containing protease and phosphatase inhibitors. The protein concentration of the lysates was determined using a BCA protein assay kit. Equal amounts of protein (usually 30–50 μg) were separated by SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes.
The PVDF membranes were blocked with 5% non-fat milk in Tris-buffered saline with Tween 20 (TBST) for 1–2 h at room temperature. Then, the membranes were incubated with primary antibodies against Rmst, DNMT3A, HuR, and β-actin overnight at 4 °C. The primary antibodies were diluted in 5% BSA–TBST at appropriate concentrations (Table 2). After washing with TBST, the membranes were incubated with secondary antibodies conjugated with horseradish peroxidase (HRP) for 1–2 h at room temperature. Protein bands were detected using an enhanced chemiluminescence (ECL) kit and visualized on X-ray films or using a chemiluminescence imaging system. The intensity of the protein bands was quantified using image analysis software.
Table 2.
Co-localization coefficients of DNMT3A with MAP2, GS, IB4, CGRP
| Co-localization coefficient | MAP2 | GS | IB4 | CGRP |
|---|---|---|---|---|
| DNMT3A | 0.72 ± 0.05 | 0.68 ± 0.04 | 0.65 ± 0.03 | 0.61 ± 0.02 |
In situ hybridization
In situ hybridization was performed to detect the expression of Rmst in spinal cord tissues. Briefly, spinal cord sections were fixed in 4% paraformaldehyde, dehydrated through a series of ethanol solutions, and then hybridized with a digoxigenin-labeled Rmst probe overnight at 55 °C. After hybridization, the sections were washed and incubated with an anti-digoxigenin antibody conjugated with alkaline phosphatase. The signal was developed using a BCIP/NBT color-development kit, and the sections were counterstained with nuclear fast red. The stained sections were observed and photographed under a light microscope.
Immunofluorescence
DRG neurons were seeded onto coverslips in 24-well plates and allowed to adhere overnight. The cells were then fixed with 4% paraformaldehyde for 15–20 min, permeabilized with 0.3% Triton X-100 in PBS for 10–15 min, and blocked with 5% BSA in PBS for 1–2 h at room temperature.
The cells were incubated with primary antibodies against Rmst, HuR, DNMT3A, and neuronal markers (such as MAP2, GS, IB4, CGRP) overnight at 4 °C. After washing with PBS, the cells were incubated with secondary antibodies conjugated with fluorescent dyes (such as Alexa Fluor 488 or Alexa Fluor 594) for 1–2 h at room temperature. The nuclei were counterstained with DAPI. The coverslips were mounted on glass slides, and the cells were observed and photographed under a confocal laser-scanning microscope.
Dual-luciferase reporter assay
The 3'-UTR of DNMT3A containing the predicted HuR-binding sites was cloned into the psiCHECK-2 vector downstream of the Renilla luciferase gene. HEK293T cells were co-transfected with the reporter plasmid, a plasmid expressing HuR, and either siRmst or siNC using Lipofectamine 3000 transfection reagent. After 48 h of transfection, the cells were harvested, and the luciferase activities were measured using a dual-luciferase reporter assay system according to the manufacturer's instructions. The relative luciferase activity was calculated as the ratio of Renilla luciferase activity to Firefly luciferase activity.
RNA immunoprecipitation (RIP)
RIP experiments were performed using a Magna RIP RNA-Binding Protein Immunoprecipitation Kit. Briefly, DRG neurons were lysed in RIP lysis buffer, and the cell lysates were incubated with magnetic beads conjugated with an anti-HuR antibody or an isotype-control antibody overnight at 4 °C. The immunoprecipitated RNA–protein complexes were washed, and the RNA was extracted and purified. The levels of Rmst and DNMT3A mRNA in the immunoprecipitated RNA were determined by qRT-PCR.
Lentiviral vector construction and in vivo injection
Lentiviral vectors carrying siRmst or pcDNA-DNMT3A were constructed. The siRmst sequence was cloned into a lentiviral vector containing a U6 promoter for siRNA expression. The pcDNA-DNMT3A plasmid was first amplified and then cloned into a lentiviral vector under the control of a strong promoter, such as the CMV promoter. The lentiviral vectors were then packaged into lentivirus particles using a packaging system.
For in vivo experiments, mice were anesthetized, and the lentiviral vectors were injected into the spinal cord at the lumbar level using a microsyringe. The injection volume and concentration of the lentiviral vectors were optimized to ensure efficient gene delivery and expression. After injection, the mice were monitored for any signs of distress or adverse effects.
Behavioral tests
Mechanical allodynia test: the mechanical allodynia test was performed using von Frey filaments. Mice were placed in individual plastic chambers on an elevated mesh floor and allowed to acclimatize for 30 min. Von Frey filaments with different bending forces were applied perpendicularly to the plantar surface of the hind paw until the filament bent. The withdrawal threshold was determined as the lowest force that elicited a paw-withdrawal response in at least 50% of the trials.
Thermal hyperalgesia test: the thermal hyperalgesia test was carried out using a hot-plate apparatus. Mice were placed on a heated plate maintained at 50–52 °C, and the latency to paw-licking or jumping was recorded. The cut-off time was set to 20 s to avoid tissue damage.
While conducting mechanical hyperalgesia (von Frey) and thermal hyperalgesia (hot-plate) tests, motor function indicators of rats were simultaneously observed, including: spontaneous movement distance (total moving distance within 5 min in the open field test), hindlimb gait (presence of dragging or limping), and balance ability (coordination during standing and turning). Continuous observation was performed for 7 days (1–7 days after injection), and the incidence of motor abnormalities was recorded.
DNMT3A half-life calculation
Experimental treatment: DRG neurons transfected with NC shRNA were seeded in 6-well plates. When cell confluency reached 80%, actinomycin D (Sigma-Aldrich, A9415) was added to a final concentration of 5 μg/mL to inhibit new mRNA transcription.
Sample collection: cells were collected at 0, 2, 4, 6, 8, and 10 h after actinomycin D treatment. Three independent replicate wells were set for each time point, and total RNA was extracted using TRIzol reagent.
qRT-PCR detection: cDNA was synthesized according to the method described in Sect. 2.6. The relative expression level of DNMT3A mRNA at each time point was detected by qRT-PCR, with GAPDH as the internal reference gene. The normalized relative expression level was calculated using the 2⁻ΔΔCt method (with the expression level at 0 h set as 100%).
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Sample size was determined using G*Power 3.1 software (α = 0.05, β = 0.2, effect size = 0.8), with a minimum of 6 rats per group for in vivo experiments (to ensure sufficient statistical power for pain behavior tests) and 3 independent replicates for in vitro experiments (cell culture/biochemical assays). ‘Independent replicates’ refer to experiments performed on different batches of cells or separate groups of animals, not repeated measurements of the same sample. Statistical tests were selected based on experimental design: Student’s t-test: Used for comparisons between two groups (e.g., Sham vs. SNL, siNC vs. siRmst); one-way ANOVA followed by Tukey’s post hoc test: used for comparisons among three or more groups (e.g., Sham, SNL + siNC, SNL + siRmst); Pearson correlation analysis: used for analyzing linear relationships between two continuous variables (e.g., Rmst vs. DNMT3A expression); two-way ANOVA: used for analyzing effects of two variables (e.g., time and treatment in dynamic expression assays). All tests were two-tailed, and a p-value < 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism 9.0 software (GraphPad Software, Inc., USA).
Results
Identification of lncRNAs regulating DNMT3a stability and the role of Rmst in neuropathic pain models
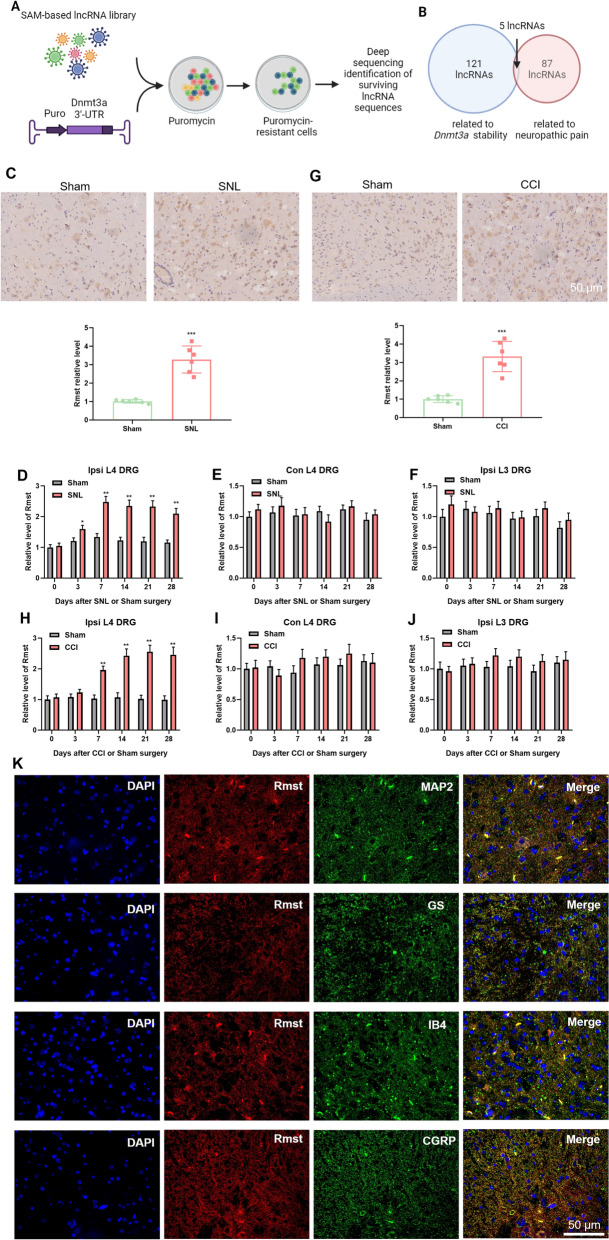
To ascertain whether lncRNAs modulate the stability and expression of DNMT3a, we utilized a SAM (Cas9 Synergistic Activation Mediator)-based lncRNA library screening system. This approach enabled us to identify lncRNAs associated with DNMT3a expression regulation. Specifically, we constructed a DNMT3a 3'-UTR reporter gene using the pGL3 control vector. The luciferase sequence in the pGL3 vector was replaced with the puromycin gene. This modification allows the stability of the DNMT3a 3'-UTR to control puromycin expression. When the level of DNMT3a 3'-UTR (and thus puromycin) increases due to the presence of a candidate lncRNA, cells carrying that lncRNA become resistant to puromycin. Cells resistant to puromycin were sequenced to identify lncRNAs that significantly impact DNMT3a stability (Fig. 1A illustrates this screening process). Through this series of screenings, we identified 121 lncRNAs that effectively increased DNMT3a stability (Fig. 1B). Further comparison of these lncRNAs with sequencing results from a previous neuropathic pain model revealed that five lncRNAs were significantly upregulated in the neuropathic pain model and played a key role in DNMT3a stability (Fig. 1B). Among the five candidate lncRNAs, Rmst met the following conditions simultaneously: ① it exhibited the highest fold change in regulating DNMT3a stability; ② it showed the most significant upregulation in the NP model; ③ pre-experiments demonstrated that its knockdown efficiency in DRG reached 72% ± 5% (after siRmst treatment). Therefore, Rmst was identified as the core research object.
Fig. 1.
Identification of lncRNAs regulating DNMT3a stability and the role of Rmst in neuropathic pain models. A: Schematic of the lncRNA screening system for DNMT3a stability regulation. Panel description: the figure illustrates the screening process of the lncRNA library using the SAM (Cas9 Synergistic Activation Mediator) system. A reporter gene vector containing the DNMT3a 3'-UTR was constructed (the pGL3 control vector, in which the luciferase gene was replaced with the puromycin gene). The stability of the DNMT3a 3'-UTR in cells controls the expression of puromycin. When a candidate lncRNA increases the stability of the DNMT3a 3'-UTR, cells carrying that lncRNA become resistant to puromycin. Sequencing of puromycin-resistant cells identifies lncRNAs that significantly impact DNMT3a stability. B Identification of lncRNAs regulating DNMT3a stability. Panel description: the figure shows the screening results, identifying 121 lncRNAs that significantly increase DNMT3a stability. Comparison with sequencing results from a previous neuropathic pain model revealed that five lncRNAs were significantly upregulated in the neuropathic pain model and played a key role in DNMT3a stability. C in situ hybridization analysis of Rmst expression in SNL rat spinal cord. Panel description: the figure shows the in situ hybridization results of Rmst expression in the spinal cord of SNL (spared nerve injury) rats. Compared with the SHAM (sham surgery) group, Rmst expression in the spinal cord of SNL rats was significantly higher. (n = 6 rats per group). D qPCR analysis of Rmst expression in injured L4 DRG of SNL rats. Panel description: the figure shows the qPCR analysis of Rmst expression in the injured L4 dorsal root ganglia (DRG) of SNL rats. Results indicate that Rmst expression increased and remained elevated up to 28 days post-surgery. (n = 6 rats per group). E qPCR analysis of Rmst expression in contralateral L4 DRG of SNL rats. Panel description: the figure shows the qPCR analysis of Rmst expression in the contralateral L4 DRG of SNL rats. Results indicate that Rmst expression remained unchanged. (n = 6 rats per group). F qPCR analysis of Rmst expression in ipsilateral L3 DRG of SNL rats. Panel description: the figure shows the qPCR analysis of Rmst expression in the ipsilateral L3 DRG of SNL rats. Results indicate that Rmst expression remained unchanged. (n = 6 rats per group). G In situ hybridization analysis of Rmst expression in CCI rat spinal cord. Panel description: the figure shows the in situ hybridization results of Rmst expression in the spinal cord of CCI (chronic constriction injury) rats. Compared with the SHAM group, Rmst expression in the spinal cord of CCI rats was significantly higher. (n = 6 rats per group). H qPCR analysis of Rmst Expression in injured L4 DRG of CCI rats. Panel description: the figure shows the qPCR analysis of Rmst expression in the injured L4 DRG of CCI rats. Results indicate that Rmst expression increased and remained up elevated to 28 days post-surgery. (n = 6 rats per group). I: qPCR analysis of Rmst expression in contralateral L4 DRG of CCI rats. Panel description: the figure shows the qPCR analysis of Rmst expression in the contralateral L4 DRG of CCI rats. Results indicate that Rmst expression remained unchanged. (n = 6 rats per group). J qPCR analysis of Rmst expression in ipsilateral L3 DRG of CCI rats. Panel description: the figure shows the qPCR analysis of Rmst expression in the ipsilateral L3 DRG of CCI rats. Results indicate that Rmst expression remained unchanged. (n = 6 rats per group). K Co-localization of Rmst with neuronal markers in DRG. Samples were isolated from L4 dorsal root ganglia (DRG) of rats at 7 days post-spared nerve injury (SNL, n = 5); Sham-operated rats (n = 3) served as negative controls (no specific Rmst signal observed). Scale bar = 50 μm. MAP2: neuronal-specific cytoskeletal protein, indicating Rmst expression in neurons (key cells for pain signal transmission); GS (glutamine synthetase): astrocyte marker, suggesting Rmst involvement in glial–neuronal crosstalk; IB4 (Isolectin B4): microglial marker, linking Rmst to microglial activation (a core event in neuropathic pain); CGRP (calcitonin gene-related peptide): peptidergic sensory neuron marker, implying Rmst function in sensory pain signaling. Quantification: co-localization was analyzed using ZEN 3.0 software (Zeiss). Pearson’s correlation coefficient was calculated from 5 random fields per section (200 × magnification) and 3 sections per rat. Results are presented as mean ± SEM: Rmst vs. MAP2 (0.72 ± 0.05), Rmst vs. GS (0.68 ± 0.04), Rmst vs. IB4 (0.65 ± 0.03), Rmst vs. CGRP (0.61 ± 0.02) (all p < 0.001 vs. negative control). (n = 6 rats per group). Data are mean ± SEM (n = 6 rats per group). ***p < 0.001 vs. Sham (one-way ANOVA with Tukey’s post hoc test)
We assessed the expression changes of Rmst in the spinal cord of SNL (spared nerve injury) rats using in situ hybridization. Results showed that Rmst expression in the spinal cord of SNL rats was significantly higher compared to the SHAM (sham surgery) group (Fig. 1C). Further detection of lncRNA Rmst expression using qPCR revealed that in the injured L4 DRG of the SNL model, Rmst expression increased and remained elevated up to 28 days post-surgery (Fig. 1D). However, Rmst expression remained unchanged in the contralateral L4 DRG (Fig. 1E) and ipsilateral L3 DRG (Fig. 1F).
Similarly, we evaluated Rmst expression changes in the spinal cord of CCI (chronic constriction injury) rats using in situ hybridization. Results indicated that Rmst expression in the spinal cord of CCI rats was significantly higher compared to the SHAM group (Fig. 1G). qPCR results showed that in the injured L4 DRG of CCI rats, Rmst expression increased and persisted up to 28 days post-surgery (Fig. 1H). In contrast, Rmst expression remained unchanged in the contralateral L4 DRG (Fig. 1I) and ipsilateral L3 DRG (Fig. 1J).
We examined the co-localization of Rmst with neuronal-specific proteins MAP2, GS, IB4, and CGRP using immunofluorescence. Immunofluorescence co-localization analysis revealed that in the L4 DRG 7 days after SNL surgery, Rmst co-localized with the neuronal marker MAP2, the astrocyte marker GS, the microglial marker IB4, and the sensory neuron marker CGRP, with predominant distribution in the cytoplasm (Fig. 1K; Table 2). No specific Rmst co-localization signal was observed in the DRG of the Sham group.
Knockdown of lncRNA Rmst attenuates neuropathic pain via inhibition of neuronal excitability, microglial activation, and inflammatory response in SNL and CCI rat models
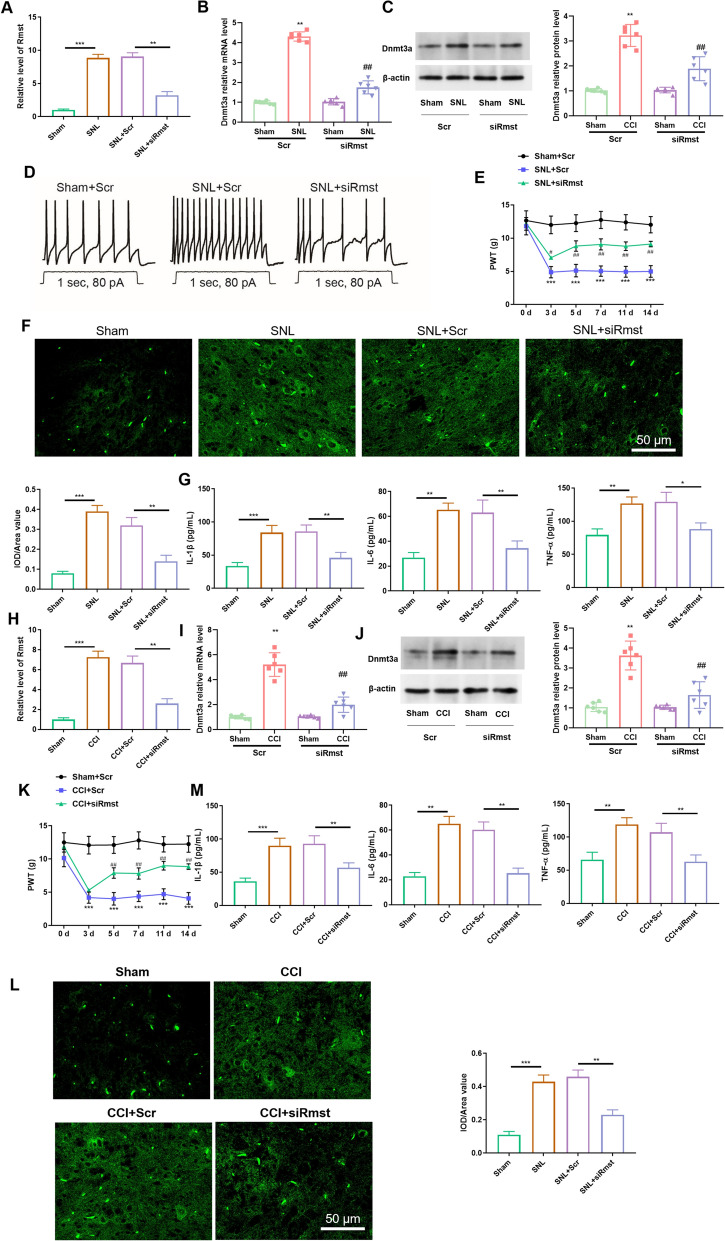
Given that Rmst is continuously highly expressed in injured DRG and associated with DNMT3A stability, we further explored its regulatory effect on NP phenotype through DRG microinjection of siRmst. In the SNL rat model, microinjections of lncRNA Rmst siRNA (siRmst) were administered into the injured DRG to specifically knock down lncRNA Rmst. During the behavioral testing period (1–7 days post-injection), rats in the siRmst group, siNC group, and Sham group all exhibited normal spontaneous motor ability—there was no significant difference in the total moving distance in the open field test. Moreover, no motor abnormalities such as hindlimb dragging, limping, or balance disorders were observed. In addition, rats could normally perform hindlimb licking or jumping responses in the thermal hyperalgesia test, and could flexibly avoid von Frey filament stimulation in the mechanical hyperalgesia test, further indicating that DRG microinjection and siRNA intervention did not interfere with motor nerve function. These results indirectly confirm that the DRG microinjection protocol adopted in this study (low volume, low concentration, and precise localization) had no adverse effects on the motor function of rats. RT-qPCR analysis revealed a significant downregulation of Rmst expression (Fig. 2A). Further investigation of DNMT3A expression demonstrated that both its mRNA (Fig. 2B) and protein levels (Fig. 2C) were also markedly reduced. These findings suggest a potential regulatory relationship between Rmst and DNMT3A, where Rmst knockdown significantly inhibits DNMT3A expression.
Fig. 2.
Knockdown of lncRNA Rmst attenuates neuropathic pain via inhibition of neuronal excitability, microglial activation, and inflammatory response in SNL and CCI rat models. A RT-qPCR analysis of Rmst expression in SNL rat model. Panel description: This figure shows the RT-qPCR analysis of Rmst expression in the injured dorsal root ganglia (DRG) of the SNL (spared nerve injury) rat model after microinjection of lncRNA Rmst siRNA (siRmst). The results demonstrate a significant downregulation of Rmst expression, confirming successful knockdown of Rmst. (n = 6 rats per group). B mRNA expression of DNMT3A in SNL rat model. Panel description: this figure illustrates the changes in DNMT3A mRNA levels in the SNL rat model following Rmst knockdown. The results show a significant reduction in DNMT3A mRNA levels, indicating that Rmst regulates DNMT3A expression. (n = 6 rats per group). C Protein expression of DNMT3A in SNL rat model. Panel description: this figure presents the changes in DNMT3A protein levels in the SNL rat model after Rmst knockdown. Western blot analysis reveals a significant decrease in DNMT3A protein levels, further confirming the regulatory effect of Rmst on DNMT3A expression. (n = 6 rats per group). D Neuronal excitability in SNL rat model. Panel description: this figure shows the changes in neuronal excitability in the SNL rat model following Rmst knockdown. (n = 6 rats per group). E Effect of PCat19 Antagonist on Mechanical Sensitivity in SNL Rat Model. Panel description: this figure illustrates the effect of the PCat19 antagonist on mechanical sensitivity in the SNL rat model. The results show that the PCat19 antagonist significantly alleviates mechanical stimulus-induced pulse wave velocity, suggesting its potential role in modulating neuropathic pain. (n = 6 rats per group). F Iba-1 expression in spinal cord of SNL rat model. Panel description: this figure shows the expression of the microglial activation marker Iba-1 in the spinal cord of the SNL rat model following Rmst knockdown. Immunohistochemical analysis reveals a significant inhibition of Iba-1 expression, indicating that Rmst may be involved in the activation of microglia. (n = 6 rats per group). G Inflammatory cytokines in serum of SNL rat model. Panel description: this figure presents the levels of inflammatory cytokines IL-1β, IL-6, and TNF-α in the serum of the SNL rat model following Rmst knockdown. ELISA analysis shows a significant reduction in the levels of these inflammatory cytokines, indicating that Rmst inhibition can reduce the release of inflammatory factors. (n = 6 rats per group). H RT-qPCR analysis of Rmst expression in CCI rat model. Panel description: this figure shows the RT-qPCR analysis of Rmst expression in the injured dorsal root ganglia (DRG) of the CCI (chronic constriction injury) rat model after microinjection of lncRNA Rmst siRNA (siRmst). The results demonstrate a significant downregulation of Rmst expression, confirming successful knockdown of Rmst. (n = 6 rats per group). I mRNA expression of DNMT3A in CCI rat model. Panel description: this figure illustrates the changes in DNMT3A mRNA levels in the CCI rat model following Rmst knockdown. The results show a significant reduction in DNMT3A mRNA levels, further confirming the regulatory effect of Rmst on DNMT3A expression. (n = 6 rats per group). J Protein Expression of DNMT3A in CCI Rat Model. Panel description: this figure presents the changes in DNMT3A protein levels in the CCI rat model after Rmst knockdown. Western blot analysis reveals a significant decrease in DNMT3A protein levels, further confirming the regulatory effect of Rmst on DNMT3A expression. (n = 6 rats per group). K Effect of PCat19 antagonist on mechanical sensitivity in CCI rat model. Panel description: this figure illustrates the effect of the PCat19 antagonist on mechanical sensitivity in the CCI rat model. The results show that the PCat19 antagonist significantly alleviates mechanical stimulus-induced pulse wave velocity, further confirming its potential role in modulating neuropathic pain. (n = 6 rats per group). L Iba-1 Expression in Spinal Cord of CCI Rat Model. Panel description: this figure shows the expression of the microglial activation marker Iba-1 in the spinal cord of the CCI rat model following Rmst knockdown. Immunohistochemical analysis reveals a significant inhibition of Iba-1 expression, further confirming the regulatory role of Rmst in microglial activation. (n = 6 rats per group). M Inflammatory cytokines in serum of CCI rat model. Panel description: this figure presents the levels of inflammatory cytokines IL-1β, IL-6, and TNF-α in the serum of the CCI rat model following Rmst knockdown. ELISA analysis shows a significant reduction in the levels of these inflammatory cytokines, further confirming the role of Rmst in regulating the inflammatory response. (n = 6 rats per group). Data are mean ± SEM (n = 6 rats per group). ***p < 0.001 vs. Sham; ###p < 0.001 vs. SNL + siNC (one-way ANOVA with Tukey’s post hoc test)
Electrophysiological assessments were conducted to evaluate the neuronal excitability of model rats following Rmst knockdown. The results indicated a substantial decrease in neuronal excitability (Fig. 2D), implying that Rmst may play a crucial role in modulating neuronal activity. Specifically, Rmst knockdown effectively reduced the excessive excitability of neurons.
In addition, the PCat19 antagonist was tested for its effects on mechanical stimulus-induced responses. The experimental results (Fig. 2E) showed that the PCat19 antagonist significantly alleviated the mechanical stimulus-induced pulse wave velocity in rats. This suggests that the PCat19 antagonist may effectively modulate the mechanical sensitivity in neuropathic pain models, offering new insights for pain management strategies.
Immunofluorescence analysis was performed to examine the activation of microglia in the spinal cord tissue of SNL model rats, using Iba-1 as a marker. The results revealed a significant increase in Iba-1 expression following SNL modeling, indicating robust microglial activation. However, Rmst knockdown led to a substantial inhibition of Iba-1 expression (Fig. 2F). This finding suggests that Rmst may be involved in the activation process of microglia, and its knockdown can effectively suppress excessive microglial activation.
Furthermore, the protein expression of key inflammatory factors associated with neuropathic pain, including IL-1β, IL-6, and TNF-α, was examined. The results showed that Rmst knockdown significantly reduced the levels of these inflammatory factors in the serum of SNL rats (Fig. 2G). This indicates that inhibiting Rmst can mitigate the release of inflammatory factors, thereby preventing the inflammatory response mediated by microglial polarization.
Similarly, in the CCI rat model, lncRNA Rmst siRNA (siRmst) was microinjected into the injured DRG to knock down lncRNA Rmst. The results were consistent with those observed in the SNL model: Rmst expression was significantly downregulated (Fig. 2H), and both the mRNA (Fig. 2I) and protein levels (Fig. 2J) of DNMT3A were markedly reduced. These findings further confirm the inhibitory effect of Rmst knockdown on DNMT3A expression and suggest that this regulatory relationship is conserved across different neuropathic pain models.
The PCat19 antagonist was also tested in the CCI model, and the results (Fig. 2K) showed that it significantly alleviated the mechanical stimulus-induced pulse wave velocity. This finding further validates the efficacy of the PCat19 antagonist in modulating mechanical sensitivity in neuropathic pain models, providing additional evidence for its potential application in pain treatment.
Immunofluorescence analysis of the spinal cord tissue from CCI model rats revealed a significant increase in Iba-1 expression following CCI modeling, indicating robust microglial activation. However, Rmst knockdown led to a substantial inhibition of Iba-1 expression (Fig. 2L). This finding is consistent with the results from the SNL model, indicating that Rmst is involved in microglial activation and exerts a regulatory effect on this process, with similar functions observed in different nerve injury models.
Additionally, the protein expression of inflammatory factors IL-1β, IL-6, and TNF-α in the serum of CCI rats was examined following Rmst knockdown. The results showed a significant reduction in the levels of these inflammatory factors (Fig. 2M). This further confirms that inhibiting Rmst can effectively mitigate the inflammatory response mediated by microglial polarization, with consistent anti-inflammatory effects observed across different neuropathic pain models.
In summary, the knockdown of lncRNA Rmst in both SNL and CCI neuropathic pain rat models significantly downregulated Rmst and DNMT3A expression, reduced neuronal excitability, inhibited microglial activation, and decreased the release of inflammatory factors. These comprehensive findings indicate that inhibiting Rmst can effectively prevent the inflammatory response mediated by microglial polarization in both SNL and CCI rats. This highlights the potential of Rmst as a therapeutic target for neuropathic pain, offering new strategies and insights for future pain management therapies.
Dynamic regulation of DNMT3A and its synergistic relationship with Rmst in neuropathic pain models
To gain deeper insights into the expression dynamics of DNMT3A in neuropathic pain models and elucidate its relationship with Rmst, we conducted a series of analyses in the spinal cord tissues of rats subjected to SNL and CCI.
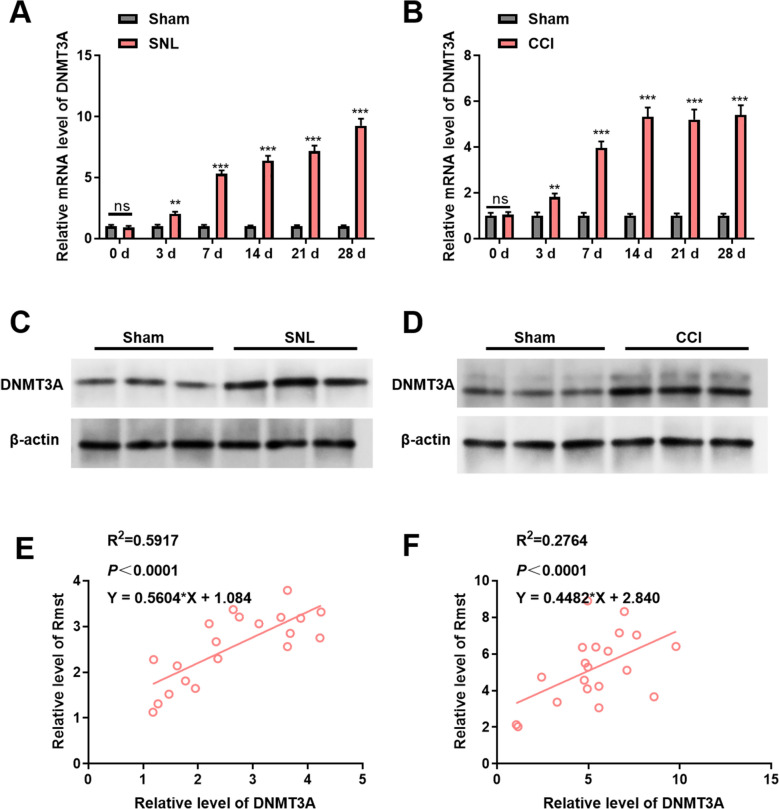
Initially, we examined the mRNA levels of DNMT3A in the spinal cord tissues of SNL and CCI model rats. Our findings revealed distinct temporal patterns. In the SNL model rats (Fig. 3A), the mRNA levels of DNMT3A exhibited a significant and progressive increase over time. This trend suggests that as SNL-induced neurogenic injury evolves, the transcriptional activity of the DNMT3A gene is continuously upregulated, potentially contributing to the molecular mechanisms underlying the development of neuropathic pain. A similar observation was made in the CCI model rats (Fig. 3B), where DNMT3A mRNA levels also rose significantly with increasing modeling duration. This parallel increase implies that both SNL- and CCI-induced nerve injuries may trigger the upregulation of DNMT3A expression through a shared mechanism, highlighting the pivotal role of DNMT3A in the pathophysiology of neuropathic pain.
Fig. 3.
Dynamic regulation of DNMT3A and its synergistic relationship with Rmst in neuropathic pain models. A Temporal changes in DNMT3A mRNA Levels in SNL rat model. Panel description: this figure shows the temporal changes in DNMT3A mRNA levels in the spinal cord tissue of the SNL (spared nerve injury) rat model. The results indicate that DNMT3A mRNA levels significantly increase over time, suggesting enhanced transcriptional activity of DNMT3A during SNL-induced neurogenic injury. (n = 6 rats per group). B Temporal changes in DNMT3A mRNA levels in CCI rat model. Panel description: this figure shows the temporal changes in DNMT3A mRNA levels in the spinal cord tissue of the CCI (chronic constriction injury) rat model. The results indicate that DNMT3A mRNA levels significantly increase over time, similar to the SNL model, suggesting upregulation of DNMT3A in both models. (n = 6 rats per group). C Protein expression of DNMT3A in SNL rat model. Panel description: this figure shows the changes in DNMT3A protein levels in the spinal cord tissue of the SNL rat model, as detected by Western Blot. Compared with the Sham group (sham surgery controls), DNMT3A protein levels are significantly increased, consistent with the mRNA results, indicating upregulation of DNMT3A at both transcriptional and translational levels. (n = 6 rats per group). D Protein expression of DNMT3A in CCI rat model. Panel description: this figure shows the changes in DNMT3A protein levels in the spinal cord tissue of the CCI rat model, as detected by Western Blot. Compared with the Sham group, DNMT3A protein levels are significantly increased, further confirming that upregulation of DNMT3A in neuropathic pain models is a universal phenomenon. (n = 6 rats per group). E Correlation analysis of DNMT3A and Rmst expression in SNL rat model. Panel description: this figure shows the correlation analysis between DNMT3A and Rmst expression levels in the SNL rat model. The results indicate a significant positive correlation, suggesting that DNMT3A and Rmst expression changes are consistent during SNL-induced neurogenic injury and may be part of the same regulatory pathway. (n = 6 rats per group). F Correlation analysis of DNMT3A and Rmst expression in CCI rat model. Panel description: this figure shows the correlation analysis between DNMT3A and Rmst expression levels in the CCI rat model. The results indicate a significant positive correlation, further demonstrating the close relationship between DNMT3A and Rmst in CCI-induced neurogenic injury, suggesting their joint involvement in the development of neuropathic pain. (n = 6 rats per group). Data are mean ± SEM (n = 6 rats per group). ***p < 0.001 vs. Sham (Student’s t-test)
To further corroborate these findings at the protein level, we employed Western blot assays. In the spinal cord tissues of SNL model rats (Fig. 3C), the protein levels of DNMT3A were markedly elevated compared to those in the Sham group (sham surgery controls). This finding aligns with the mRNA data, indicating that the upregulation of DNMT3A in the SNL model is not limited to transcriptional changes but also extends to enhanced translation, culminating in increased protein expression. Similarly, in the spinal cord tissues of CCI model rats (Fig. 3D), DNMT3A protein levels were significantly higher than in the Sham group. This consistency across both models underscores the universality of DNMT3A upregulation in neuropathic pain, manifesting at both transcriptional and translational levels and positioning DNMT3A as a key regulatory node within the neuropathic pain-associated molecular network.
To unravel the relationship between DNMT3A and Rmst, we performed correlation analyses. In the SNL model (Fig. 3E), a significant positive correlation was observed between DNMT3A and Rmst expression. This correlation indicates that the expression profiles of DNMT3A and Rmst are closely aligned during SNL-induced neurogenic injury, suggesting that these two molecules may operate synergistically or reside within the same regulatory pathway. This finding was further reinforced in the CCI model (Fig. 3F), where a similarly significant positive correlation between DNMT3A and Rmst expression was detected. Collectively, these results highlight the intimate connection between DNMT3A and Rmst in both SNL- and CCI-induced neuropathic pain models, implicating their joint involvement in the onset and progression of neuropathic pain.
In summary, our study demonstrates that in the spinal cord tissues of rats subjected to SNL and CCI neuropathic pain models, both the mRNA and protein levels of DNMT3A significantly increase over time. Moreover, a robust positive correlation exists between DNMT3A and Rmst expression. These findings collectively underscore the critical roles of DNMT3A and Rmst in the molecular underpinnings of neuropathic pain, offering new avenues for therapeutic intervention.
The role of lncRNA Rmst in stabilizing DNMT3A mRNA through HuR interaction
The positive correlation between DNMT3A and Rmst suggests that there may be a direct regulatory relationship between them. Combined with reports on the interaction between Rmst and RNA-binding proteins, we focused on the HuR-mediated mRNA stabilization mechanism. Based on a comprehensive review of the literature, it is evident that lncRNA Rmst interacts with the RNA-binding protein HuR and stabilizes target gene mRNA by preventing its degradation by nucleases [21]. Building on this background, we hypothesize that HuR is involved in the pathogenesis of neuropathic pain and may collaborate with lncRNA Rmst to stabilize DNMT3A mRNA, thereby enhancing the expression of DNMT3A protein in dorsal root ganglion neurons [18].
We examined the mRNA levels of HuR in the spinal cord tissues of rats following SNL (spared nerve injury) and CCI (chronic constriction injury). Our results revealed a significant temporal increase in HuR mRNA levels. In SNL model rats (Fig. 4A), HuR mRNA levels progressively increased with time, while a similar trend was observed in CCI model rats (Fig. 4B). These findings suggest that HuR transcriptional activity is enhanced in neuropathic pain models, indicating its potential role in the pathophysiology of neuropathic pain.
Fig. 4.
The role of lncRNA Rmst in stabilizing DNMT3A mRNA through HuR interaction. A Temporal changes in HuR mRNA levels in SNL rat model. Panel description: this figure shows the temporal changes in HuR mRNA levels in the spinal cord tissue of the SNL (spared nerve injury) rat model. The results indicate a significant and progressive increase in HuR mRNA levels over time, suggesting enhanced transcriptional activity of HuR during SNL-induced neurogenic injury. (n = 6 rats per group). B Temporal changes in HuR mRNA levels in CCI rat model. Panel description: this figure shows the temporal changes in HuR mRNA levels in the spinal cord tissue of the CCI (chronic constriction injury) rat model. Similar to the SNL model, HuR mRNA levels significantly increased over time, indicating that HuR transcriptional activity is enhanced in both neuropathic pain models. (n = 6 rats per group). C HuR binding motifs on DNMT3A mRNA. Panel description: this figure illustrates the identified binding motifs for HuR on DNMT3A mRNA, as analyzed using the Starbase database. The presence of these motifs provides a structural basis for the interaction between HuR and DNMT3A mRNA, suggesting that HuR may regulate DNMT3A expression by binding to specific sites on its mRNA. D Effect of Rmst knockdown on HuR–DNMT3A interaction. Panel description: this figure shows the results of the dual-luciferase reporter assay, demonstrating the effect of Rmst knockdown on the binding capacity between HuR and DNMT3A mRNA. The results indicate that Rmst knockdown significantly weakens this interaction, highlighting the role of Rmst in facilitating the binding of HuR to DNMT3A mRNA. E RIP analysis of Rmst and HuR interaction. Panel description: this figure presents the results of RNA immunoprecipitation (RIP) experiments, showing that Rmst significantly enriches HuR protein. This confirms the specific interaction between Rmst and HuR in cells, supporting the hypothesis that they are part of a shared regulatory network. (n = 3 per group). F RIP analysis of DNMT3A and HuR interaction. Panel description: this figure presents the results of RNA immunoprecipitation (RIP) experiments, showing that DNMT3A significantly enriches HuR protein. This confirms the specific interaction between DNMT3A and HuR in cells, further supporting the hypothesis that they are part of a shared regulatory network. (n = 3 per group). G Effect of Rmst overexpression on HuR–DNMT3A interaction. Panel description: this figure shows the results of RIP experiments in primary neurons overexpressing lncRNA Rmst. The results indicate that Rmst significantly enhances the binding of HuR to DNMT3A mRNA, further supporting the role of Rmst in promoting the interaction between HuR and DNMT3A mRNA. H Half-Life of DNMT3A mRNA Following Rmst Knockdown. Panel description: this figure shows the results of experiments examining the half-life of DNMT3A mRNA following Rmst knockdown. The results indicate a significant reduction in the half-life of DNMT3A mRNA, demonstrating the crucial role of Rmst in maintaining DNMT3A mRNA stability. (n = 3 per group). I Co-localization of Rmst, HuR, and DNMT3A in neuronal cells. Panel description: this figure shows the co-localization of Rmst, HuR, and DNMT3A in neuronal cells, as detected by immunofluorescence. Rmst and HuR are localized in the cytoplasm, while DNMT3A is present in both the cytoplasm and nucleus. This spatial distribution supports the possibility of their interactions, suggesting that they act together in specific cellular compartments to influence the development of neuropathic pain. (n = 3 per group). Data are mean ± SEM. **p < 0.001 (one-way ANOVA with Tukey’s post hoc test)
Analysis using the Starbase database identified binding motifs for HuR on DNMT3A mRNA (Fig. 4C). This discovery provides a structural basis for the interaction between HuR and DNMT3A mRNA, suggesting that HuR may regulate DNMT3A expression by binding to specific motifs on its mRNA.
To investigate the role of Rmst in this interaction, we conducted dual-luciferase reporter assays. Our results showed that Rmst knockdown weakened the binding capacity between HuR and DNMT3A mRNA (Fig. 4D). This indicates that lncRNA Rmst plays a crucial role in facilitating the interaction between HuR and DNMT3A mRNA, potentially stabilizing their binding.
RNA immunoprecipitation (RIP) experiments further validated these interactions. Rmst (Fig. 4E) and DNMT3A (Fig. 4F) significantly enriched HuR protein, while Antisense did not. This confirms the specific interaction among Rmst, DNMT3A, and HuR in cells, supporting our hypothesis that they are part of a shared regulatory network.
Additionally, we overexpressed lncRNA Rmst in primary neurons and performed RIP experiments. The results showed that Rmst significantly enhanced the binding of HuR to DNMT3A mRNA (Fig. 4G). This finding further supports the notion that Rmst promotes the interaction between HuR and DNMT3A mRNA, potentially stabilizing DNMT3A mRNA.
We also examined the half-life of DNMT3A mRNA following Rmst knockdown. Our results revealed a significant reduction in the half-life of DNMT3A mRNA (Fig. 4H). This suggests that Rmst is essential for maintaining DNMT3A mRNA stability, and its knockdown leads to increased degradation of DNMT3A mRNA, thereby affecting DNMT3A protein expression.
Using immunofluorescence, we detected the co-localization of Rmst, HuR, and DNMT3A in neuronal cells. Rmst and HuR were localized in the cytoplasm, while DNMT3A was present in both the cytoplasm and nucleus (Fig. 4I). This spatial distribution supports the possibility of their interactions, suggesting that they act together in specific cellular compartments to influence the development of neuropathic pain.
In summary, our study provides compelling evidence that lncRNA Rmst interacts with the RNA-binding protein HuR to stabilize DNMT3A mRNA and enhance DNMT3A protein expression. This interaction is critical for the development of neuropathic pain, highlighting the potential therapeutic significance of targeting the Rmst–HuR–DNMT3A axis.
lncRNA Rmst stabilizes DNMT3A mRNA via HuR to promote neuropathic pain development
To further elucidate the mechanisms by which lncRNA Rmst and DNMT3A contribute to neuropathic pain (NP), we constructed lentiviral vectors carrying siRmst and pcDNA-DNMT3A and administered them into the spinal cords of mice. We subsequently monitored NP-like behaviors and related physiological indicators in these mice. Following the injection of the lentiviral vectors into the spinal cords, we assessed the pain withdrawal threshold (PWT) in the mice. The results indicated that overexpression of DNMT3A significantly reduced the PWT (Fig. 5A), suggesting heightened pain sensitivity in these mice. Additionally, we measured the expression levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α. The findings revealed that DNMT3A overexpression led to a significant increase in the expression of these cytokines (Fig. 5B, C), indicating a pro-inflammatory effect. In immunofluorescence studies of microglia in the spinal cords of mice, we examined the impact of DNMT3A overexpression on the expression of the microglial marker Iba-1, based on siRmst knockdown. The results showed that DNMT3A overexpression significantly increased Iba-1 expression (Fig. 5D), highlighting the role of DNMT3A in microglial activation.
Fig. 5.
lncRNA Rmst stabilizes DNMT3A mRNA via HuR to promote neuropathic pain development. A Effect of DNMT3A overexpression on pain withdrawal threshold (PWT) in rats. Panel description: this figure shows the impact of DNMT3A overexpression on the PWT in mice. Rats were injected with lentiviral vectors carrying pcDNA-DNMT3A or control vectors. The results indicate that DNMT3A overexpression significantly reduced the PWT, suggesting increased pain sensitivity in these rats. (n = 6 rats per group). B Expression levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in SNL mice. Panel description: this figure presents the expression levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in mice following DNMT3A overexpression. ELISA analysis revealed that DNMT3A overexpression significantly increased the levels of these cytokines, indicating a pro-inflammatory effect. C quantitative analysis of pro-inflammatory cytokines in CCI rats. Panel description: this figure provides a quantitative analysis of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in rats. The data show a significant upregulation of these cytokines in mice overexpressing DNMT3A, further supporting the role of DNMT3A in promoting inflammation. (n = 6 rats per group). D Immunofluorescence analysis of microglial marker Iba-1 in rat spinal cord. Panel description: this figure shows the immunofluorescence analysis of the microglial marker Iba-1 in the spinal cord of mice. Rats were subjected to siRmst knockdown followed by DNMT3A overexpression. The results indicate that DNMT3A overexpression significantly increased Iba-1 expression, highlighting the role of DNMT3A in microglial activation. (n = 6 rats per group). Data are mean ± SEM (n = 6 mice per group). **p < 0.01 vs. SNL + siRmst + pcDNA-3.1 (two-way ANOVA with Bonferroni’s post hoc test)
Collectively, these findings demonstrate that lncRNA Rmst in dorsal root ganglion neurons regulates the stability of DNMT3A mRNA through its interaction with the RNA-binding protein HuR, thereby exacerbating neuronal excitability and contributing to the development of neuropathic pain.
Discussion
This research was dedicated to exploring the role of lncRNA Rmst in regulating DNMT3A expression and its implications for NP. The findings offer fresh perspectives on the molecular mechanisms of NP and propose potential therapeutic targets, although there are several aspects that warrant further exploration and discussion.
We began by identifying 121 lncRNAs capable of enhancing DNMT3A stability through a SAM-based lncRNA library screening system. Among these, five lncRNAs, with Rmst being one of them, were significantly upregulated in the neuropathic pain model and played a crucial role in maintaining DNMT3A stability. The elevated expression of Rmst in the spinal cord and injured DRG of SNL and CCI rats strongly suggests its involvement in NP. In recent years, lncRNAs have emerged as important regulators in a wide range of physiological and pathological processes, including pain modulation. For instance, lncRNA-kcnq1ot1 has been reported to participate in the development of NP by regulating Myd88 [22]. LncRNA H19 has also been shown to modulate NP through the regulation of the miR-141/GLI2 axis [23]. In our study, the upregulation of Rmst in NP models indicates that it might be an integral part of the complex molecular network underlying neuropathic pain. Anatomical studies have shown that the contribution of the L3 spinal nerve to the sciatic nerve is less than 5% [24], and its innervation area does not significantly overlap with that of L4/L5, making it eligible as a "non-injured control segment". The results of this study revealed no significant difference in Rmst expression in L3 DRG between the Sham group and the SNL group; a similar pattern was observed in the CCI model. These findings suggest that the upregulation of Rmst is restricted to injury-related segments (L4/L5), excluding the possibility of "generalized expression caused by systemic stress or non-specific inflammation" and providing localization evidence for its involvement in local pain regulation. Regarding the expression of Rmst in L5 DRG within the CCI model, direct detection was not performed. However, based on the segmental expression pattern observed in the SNL model—where the upregulation magnitude in L5 was lower than that in L4—we hypothesize that Rmst in L5 DRG of the CCI model may exhibit a similar trend. Specifically, the "injury-adjacent segment (L5) undergoes secondary upregulation due to the involvement of neural conduction pathways, yet the magnitude is smaller than that of the directly injured segment (L4)". This hypothesis aligns with the anatomical characteristic that sciatic nerve injury in the CCI model can affect L5 nerve branches, and subsequent studies may validate this assumption through supplementary detection. This upregulation could be a compensatory mechanism in the early stages of NP development or a contributing factor to the progression of the disease. Understanding the precise role of Rmst in this network is crucial for developing targeted therapies. Besides, the co-localization results in Fig. 1K provide key evidence for the functional localization of Rmst: its high enrichment in neurons (co-localization with MAP2) lays the foundation for subsequent studies on "regulating neuronal excitability" (Fig. 2D); while the co-localization with IB4 echoes the result that "Rmst knockdown inhibits microglial activation" (Fig. 2F/L), preliminarily suggesting that Rmst may regulate neuropathic pain through a dual "neuron–glial cell" pathway. Knockdown of lncRNA Rmst in SNL and CCI rat models led to a series of significant changes, including a marked downregulation of DNMT3A expression, reduced neuronal excitability, inhibited microglial activation, and decreased release of inflammatory factors. The safety of DRG microinjection benefits from its precise anatomical localization and mild operational parameters: the DRG is mainly composed of sensory neuron cell bodies, with very few motor nerve fibers passing through this region, so injections targeting the DRG have minimal impact on motor nerve innervation. Meanwhile, both the 1 μL injection volume and 50 nM siRNA concentration are within the safe ranges reported in the literature, which did not induce local nerve edema or toxic damage. The normal motor performance of rats in behavioral tests further verifies the safety of this operation, excluding the interference of motor dysfunction on the assessment of pain-related behaviors. These results strongly suggest that Rmst promotes NP development through the regulation of DNMT3A-related pathways. DNMT3A is a key enzyme in DNA methylation, and its dysregulation can have far-reaching effects on gene expression, particularly those related to pain [25]. DNA methylation is an epigenetic modification that can either silence or activate genes, depending on the location of the methylated sites. In NP, abnormal DNA methylation patterns have been associated with changes in pain-related gene expression. For example, methylation of the promoter region of genes encoding ion channels, such as Nav1.8 and TRPV1, can lead to altered channel function and enhanced pain sensitivity [26]. The reduction in DNMT3A expression following Rmst knockdown might alter the methylation status of these pain-related genes, ultimately alleviating NP-like symptoms. This could be achieved through the demethylation of genes that were previously silenced, allowing for their normal expression and function, or by preventing the methylation of genes that are crucial for maintaining normal neuronal function.
The dynamic regulation of DNMT3A in SNL and CCI models was a significant finding in our study. The progressive increase in both the mRNA and protein levels of DNMT3A over time in the spinal cord tissues of these models, along with the significant positive correlation between DNMT3A and Rmst expression, implies a coordinated action between these two molecules during NP development. However, the exact nature of this regulatory loop and the upstream signals that initiate this co-regulation remain elusive. It is possible that multiple signaling pathways are involved. For example, the nerve growth factor (NGF)–TrkA signaling pathway, which is activated in response to nerve injury, has been shown to regulate both lncRNA expression and DNA methylation [27, 28]. NGF binding to TrkA receptors can activate downstream signaling cascades, including the MAPK and PI3K–Akt pathways. These pathways may regulate the expression of Rmst and DNMT3A, either directly or indirectly. Additionally, the role of other epigenetic modifications, such as histone acetylation and methylation, in modulating the expression of Rmst and DNMT3A and their interaction also needs to be investigated.
Mechanistically, we discovered that lncRNA Rmst interacts with the RNA-binding protein HuR to stabilize DNMT3A mRNA. The increased HuR mRNA levels in the spinal cord tissues of SNL and CCI rats, along with the identification of HuR-binding motifs on DNMT3A mRNA, provide a structural basis for this interaction. Dual-luciferase reporter assays demonstrated that Rmst knockdown weakened the binding between HuR and DNMT3A mRNA, while Rmst overexpression enhanced it. RIP experiments further confirmed the specific interaction among Rmst, DNMT3A, and HuR. HuR is a well-characterized RNA-binding protein that binds to AU-rich elements in the 3'-UTR of target mRNAs, protecting them from degradation and enhancing translation efficiency [29]. Rmst may act as a scaffold, facilitating the interaction between HuR and DNMT3A mRNA. Similar lncRNA-mediated mRNA stabilization mechanisms have been reported in various biological processes. For example, in cancer, lncRNA-MALAT1 can interact with HuR to stabilize the mRNA of genes involved in tumor progression [30]. In the context of NP, this interaction between Rmst, HuR, and DNMT3A mRNA is likely to be a key regulatory step in maintaining DNMT3A protein levels and, consequently, pain-related gene expression.
The overexpression of DNMT3A in mice spinal cords led to increased pain sensitivity, elevated pro-inflammatory cytokine expression, and enhanced microglial activation. This is consistent with the notion that DNMT3A promotes NP development, and Rmst may contribute to this process by stabilizing DNMT3A mRNA. Microglial activation and the release of pro-inflammatory cytokines are key events in the development of NP [31, 32]. Activated microglia secrete cytokines such as IL-1β, IL-6, and TNF-α, which can sensitize neurons and enhance pain signaling. DNMT3A-mediated methylation might upregulate genes involved in microglial activation and cytokine production. For example, methylation of the promoter region of genes encoding cytokines or their receptors could lead to increased expression, thereby exacerbating the inflammatory response and pain sensitivity [33]. Additionally, DNMT3A may also regulate the expression of genes involved in microglial migration and proliferation, further contributing to the development of NP.
Despite these significant findings, this study has several limitations. First, our in vivo experiments were predominantly conducted in rodent models. Although rodent models are widely used and valuable for studying NP mechanisms, there are significant physiological and genetic differences between rodents and humans. For example, the expression levels and regulatory mechanisms of Rmst, DNMT3A, and HuR may vary between species. In humans, genetic polymorphisms in these molecules could also influence their function and contribution to NP. Therefore, translating our findings from rodent models to human NP requires further investigation, such as studying the expression and function of these molecules in human patient samples or using in vitro human cell models. Second, while we have identified the interaction between Rmst, HuR, and DNMT3A, the detailed molecular mechanisms underlying this interaction and its regulation in the context of NP remain unclear. Post-translational modifications of HuR and Rmst, such as phosphorylation, acetylation, and ubiquitination, can significantly affect their binding affinity, subcellular localization, and stability. For example, phosphorylation of HuR can enhance its binding to target mRNAs. It is unknown how these modifications are regulated in NP and how they impact the interaction between Rmst, HuR, and DNMT3A. Third, we focused primarily on the role of Rmst in regulating DNMT3A expression. However, Rmst may have other downstream targets and regulatory functions that contribute to NP development. Rmst could interact with other proteins or RNAs to regulate different aspects of NP-related processes, such as axonal regeneration, synaptic plasticity, or immune cell infiltration. Exploring these additional regulatory mechanisms will provide a more comprehensive understanding of the role of Rmst in NP.
In conclusion, our study reveals that lncRNA Rmst interacts with HuR to stabilize DNMT3A mRNA, thereby promoting the development of NP. These findings contribute to a better understanding of the molecular mechanisms of NP and suggest that targeting the Rmst-HuR-DNMT3A axis could be a potential therapeutic strategy. Future research should focus on validating these findings in human samples, elucidating the detailed molecular mechanisms, and exploring the potential of this axis as a therapeutic target in clinical settings. Additionally, investigating the interaction of this axis with other pain-related pathways and exploring the role of Rmst in other NP-related processes will be essential for developing more effective and specific therapeutic interventions for neuropathic pain.
Supplementary Information
Acknowledgements
None.
Author contributions
SZ: conceptualization, data curation. PJ: writing—original draft, formal analysis, visualization. YL: methodology, formal analysis, visualization and writing-review and editing. YT: conceptualization, data curation, writing—original draft and writing—review and editing.
Funding
This study was supported by the Dongguan Social Development Technology Project (No. 20231800905993).
Data availability
The datasets used during the present study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Animal experiments were approved and supervised by the Experimental Animal Center at Chongqing Medical University in China. All animal-related experimental protocols were scrutinized and sanctioned by the Institutional Animal Care and Use Committee, thereby guaranteeing adherence to ethical guidelines.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Attal N, Bouhassira D, Colvin L. Advances and challenges in neuropathic pain: a narrative review and future directions. Br J Anaesth. 2023;131(1):79–92. [DOI] [PubMed] [Google Scholar]
- 2.Moisset X. Neuropathic pain: evidence based recommendations. Presse Med. 2024;53(2):104232. [DOI] [PubMed] [Google Scholar]
- 3.Widerström-Noga E. Neuropathic pain and spinal cord injury: management, phenotypes, and biomarkers. Drugs. 2023;83(11):1001–25. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y, Zhu X, Chen Y, Chen Y, Zhu Y, Xiao S, et al. Electroacupuncture alleviates mechanical allodynia and anxiety-like behaviors induced by chronic neuropathic pain via regulating rostral anterior cingulate cortex-dorsal raphe nucleus neural circuit. CNS Neurosci Ther. 2023;29(12):4043–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balanaser M, Carley M, Baron R, Finnerup NB, Moore RA, Rowbotham MC, et al. Combination pharmacotherapy for the treatment of neuropathic pain in adults: systematic review and meta-analysis. Pain. 2023;164(2):230–51. [DOI] [PubMed] [Google Scholar]
- 6.Tesfaye S, Sloan G, Petrie J, White D, Bradburn M, Julious S, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet. 2022;400(10353):680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khazaei S, Chen CCL, Andrade AF, Kabir N, Azarafshar P, Morcos SM, et al. Single substitution in H3.3G34 alters DNMT3A recruitment to cause progressive neurodegeneration. Cell. 2023;186(6):1162-1178.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shao C, Gao Y, Jin D, Xu X, Tan S, Yu H, et al. DNMT3a methylation in neuropathic pain. J Pain Res. 2017;10:2253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu YH, Wang J, Lu WC, Cheng Y, Tao R, Zhang SJ, et al. POU2F1/DNMT3a pathway participates in neuropathic pain by hypermethylation-mediated LRFN4 downregulation following oxaliplatin treatment. Neurochem Res. 2023;48(12):3652–64. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Wen J, Zheng B, Wu S, Mao Q, Liang L, et al. CREB participates in paclitaxel-induced neuropathic pain genesis through transcriptional activation of DNMT3A in primary sensory neurons. Neurotherapeutics. 2021;18(1):586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarroux J, Morillon A, Pinskaya M. History, discovery, and classification of lncRNAs. Adv Exp Med Biol. 2017;1008:1–46. [DOI] [PubMed] [Google Scholar]
- 12.Herman AB, Tsitsipatis D, Gorospe M. Integrated lncrna function upon genomic and epigenomic regulation. Mol Cell. 2022;82(12):2252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng K, Pan J, Liu Q, Ji Y, Liu L, Guo X, et al. Exosomal lncRNA XIST promotes perineural invasion of pancreatic cancer cells via miR-211-5p/GDNF. Oncogene. 2024;43(18):1341–52. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Zhou L, Zhang C. LncRNA miat promotes neuropathic pain through miR-362-3p/BAMBI signaling axis. Exp Cell Res. 2022;420(2):113359. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Zhang S, Wei Y, Sun X. LncRNA RMST regulates neuronal apoptosis and inflammatory response via sponging miR-150-5p in Parkinson’s disease. NeuroImmunoModulation. 2022;29(1):55–62. [DOI] [PubMed] [Google Scholar]
- 16.Finan JM, Sutton TL, Dixon DA, Brody JR. Targeting the RNA-binding protein HuR in cancer. Cancer Res. 2023;83(21):3507–16. [DOI] [PubMed] [Google Scholar]
- 17.Morillo-Bernal J, Pizarro-García P, Moreno-Bueno G, Cano A, Mazón MJ, Eraso P, et al. HuR (ELAVL1) stabilizes SOX9 mRNA and promotes migration and invasion in breast cancer cells. Cancers (Basel). 2024. 10.3390/cancers16020384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X, Zhang G, Cai W, Huang F, Qin J, Song X. Long non-coding RNA rhabdomyosarcoma 2-associated transcript contributes to neuropathic pain by recruiting HuR to stabilize DNA methyltransferase 3 alpha mRNA expression in dorsal root ganglion neuron. Front Mol Neurosci. 2022;15:1027063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He L, Zhao W, Zhang L, Ilango M, Zhao N, Yang L, et al. Modified spared nerve injury surgery model of neuropathic pain in mice. J Vis Exp. 2022;179:e63362. [DOI] [PubMed] [Google Scholar]
- 20.Medeiros P, Dos Santos IR, Júnior IM, Palazzo E, da Silva JA, Machado HR, et al. An adapted chronic constriction injury of the sciatic nerve produces sensory, affective, and cognitive impairments: a peripheral mononeuropathy model for the study of comorbid neuropsychiatric disorders associated with neuropathic pain in rats. Pain Med. 2021;22(2):338–51. [DOI] [PubMed] [Google Scholar]
- 21.Peng WX, Koirala P, Zhang W, Ni C, Wang Z, Yang L, et al. RETRACTED: IncRNA RMST enhances DNMT3 expression through interaction with HuR. Mol Ther. 2023;31(3):909–10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Li D, Yuan C, Zhao B, Cai G, Xu Y. LncRNA Kcnq1ot1 relieves neuropathic pain through downregulation of Myd88. Int Immunopharmacol. 2023;119:110218. [DOI] [PubMed] [Google Scholar]
- 23.Meng L, Zhang Y, He X, Hu C. LncRNA H19 modulates neuropathic pain through miR-141/GLI2 axis in chronic constriction injury (CCI) rats. Transpl Immunol. 2022;71:101526. [DOI] [PubMed] [Google Scholar]
- 24.Rigaud M, Gemes G, Barabas ME, Chernoff DI, Abram SE, Stucky CL, et al. Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain. 2008;136(1–2):188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian L, Li XH, Zhao YL, Yi HY, Liu XR, Yao R, et al. DNMT3a downregulation triggered upregulation of GABA(A) receptor in the mPFC promotes paclitaxel-induced pain and anxiety in male mice. Adv Sci (Weinh). 2025;12(5):e2407387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi SK, Honore P, Hernandez G, Schmidt R, Gomtsyan A, Scanio M, et al. Additive antinociceptive effects of the selective Nav1.8 blocker A-803467 and selective TRPV1 antagonists in rat inflammatory and neuropathic pain models. J Pain. 2009;10(3):306–15. [DOI] [PubMed] [Google Scholar]
- 27.Ma WY, Murata E, Ueda K, Kuroda Y, Cao MH, Abe M, et al. A synthetic cell-penetrating peptide antagonizing TrkA function suppresses neuropathic pain in mice. J Pharmacol Sci. 2010;114(1):79–84. [DOI] [PubMed] [Google Scholar]
- 28.Ugolini G, Marinelli S, Covaceuszach S, Cattaneo A, Pavone F. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc Natl Acad Sci U S A. 2007;104(8):2985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabbiano F, Corsi J, Gurrieri E, Trevisan C, Notarangelo M, D’Agostino VG. RNA packaging into extracellular vesicles: an orchestra of RNA-binding proteins? J Extracell Vesicles. 2020;10(2):e12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang YZ, Chai RC, Pang B, Chang X, An SY, Zhang KN, et al. METTL3 enhances the stability of MALAT1 with the assistance of HuR via m6A modification and activates NF-κB to promote the malignant progression of IDH-wildtype glioma. Cancer Lett. 2021;511:36–46. [DOI] [PubMed] [Google Scholar]
- 31.Atta AA, Ibrahim WW, Mohamed AF, Abdelkader NF. Microglia polarization in nociplastic pain: mechanisms and perspectives. Inflammopharmacology. 2023;31(3):1053–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohno K, Shirasaka R, Yoshihara K, Mikuriya S, Tanaka K, Takanami K, et al. A spinal microglia population involved in remitting and relapsing neuropathic pain. Science. 2022;376(6588):86–90. [DOI] [PubMed] [Google Scholar]
- 33.Jiang W, Tan XY, Li JM, Yu P, Dong M. DNA Methylation: A Target in Neuropathic Pain. Front Med (Lausanne). 2022;9:879902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.