Abstract
Vision often requires attending to, and integrating information from, distant parts of the visual field. However, the neural basis for such long-range integration is not clearly understood. Here, we demonstrate a specific neural signature of attentional integration between stimuli in different parts of the visual field. Using functional MRI, we found that a task requiring the integration of information between two attended but spatially separated stimuli actively modulated the degree of functional integration (in terms of effective connectivity) between their retinotopic representations in visual cortical areas V1, V2, and V4. Spatial attention enhanced long-distance coupling between distinct neuronal populations that represented the attended visual stimuli, even at the earliest stages of cortical processing. In contrast, unattended stimulus representations were decoupled both from attended representations and particularly strongly from each other. Furthermore, enhanced functional integration between cortical representations was associated with enhanced behavioral performance. Attention may thus serve to “bind” together cortical loci at multiple levels of the visual hierarchy that are commonly involved in processing attended stimuli, promoting integration between otherwise functionally isolated cortical loci.
Keywords: attention, connectivity, dynamic causal modeling, functional MRI, vision
Vision often requires integration of information from distant locations within the visual field, but how this integration is achieved is currently not well understood. Receptive field properties of visually responsive cortical neurons suggest that, in early visual cortex, information is analyzed largely (although not entirely) locally, with further processing stages combining information from successively larger portions of the visual field (1-3). In such an anatomically convergent pathway, integration of information from distant positions within the visual field might occur entirely in higher visual areas, which have large receptive fields and corresponding possibilities for interactions between distant regions (1-3). However, response properties of cells in early visual areas are also modulated by remote stimuli presented far beyond their classical receptive fields (4-7). Thus, an alternate possibility is that a neural signature reflecting integration of distant information might already be identifiable in very early visual cortex. Possible anatomical substrates for such interactions include long-range horizontal projections within visual areas (8-10), the dense and reciprocal connections between visual areas (7, 11-15), and subcortical pathways via pulvinar and superior colliculus (16).
Here, we used functional MRI (fMRI) in humans to test whether task-dependent interactions between stimulus representations in distant parts of the visual field could be seen in early retinotopic cortex. We placed stimuli in four well separated locations of the visual field and required participants to attend to and integrate information from one pair of distant stimuli while ignoring the others. Our stimulus configuration allowed separate measurements of cortical responses to attended and ignored stimuli in early retinotopic cortex. We could then directly assess interactions between these representations by using established measures of neural connectivity, which have been previously used successfully to study attentional modulation of visual processing (17). We hypothesized that the process of integrating information across distant stimuli would be reflected in enhanced cortico-cortical interactions specific to attended (versus unattended) stimuli. Previous studies of attentional biases on processing of visual stimuli have not directly examined any such distant interactions and therefore cannot address how such integration might be achieved between spatially distant stimulus representations in human vision.
Materials and Methods
We measured participants' brain activity with fMRI while simple visual stimuli (spirals) designed to stimulate the early retinotopic visual areas were presented in the four quadrants (Fig. 1A). On each trial, participants were cued to attend only two of the four quadrants while fixating centrally. To achieve symmetry between conditions, we chose only combinations of immediately adjacent quadrants. Participants were required to perform a demanding discrimination task on just the two attended stimuli. We were able to isolate the multiple representations of each stimulus in different retinotopic areas of visual cortex by using standard techniques (see below). Our stimulus geometry was chosen deliberately to produce significant anatomical separation of these retinotopic representations both within each hemisphere and between the two hemispheres.
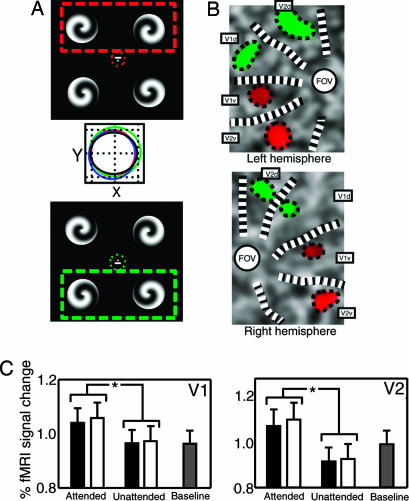
Fig. 1.
Retinotopically specific attentional modulation in V1 and V2. (A) Four high-contrast spirals of random handedness were presented in the four visual quadrants. A small line close to central fixation indicated the two spirals whose handedness should be discriminated. A dashed box (not shown in the experiment) illustrates the spirals to be attended for two representative conditions, attend top (red) and attend bottom (green). Red and green dotted circles around fixation are ellipses plotting eye position during the experiment (mean ± 1 SD during 2-s stimulus presentation). Middle shows eye position under all attention conditions (attend top, red; attend bottom, green; attend left, blue; attend right, black) (grid lines = 0.5 degrees) and reveals no systematic differences. (B) Attentional modulation of cortical responses to the spiral stimuli in V1 and V2 (for representative participant J.G.). Color coding shows stimulus-driven regions with increased responses when either the top two spirals (red) or the bottom two (green) were attended. Attentional enhancement is retinotopically specific. (C) Effect of spatial attention on retinotopic activation averaged across all participants. BOLD responses extracted from the retinotopic representations of individual stimuli are plotted for both V1 (Left) and V2 (Right), averaged across each possible spiral location (separately for attended and unattended) and six participants. BOLD responses (error bar, 1 SD) are shown when the stimulus corresponding to the representation is attended, unattended, or for a fixation baseline. Black bars indicate responses in retinotopic loci when attention was directed to two spirals within either the right or left visual hemifield; white bars indicate corresponding responses when attention is directed to two spirals in different visual hemifields (as shown in A). Significant (P < 0.05) differences are marked with an asterisk. Attention enhanced responses relative to baseline; unattended stimuli evoked activity either at baseline or slightly suppressed. There was little difference in attentional modulation comparing within-field and between-field conditions.
Participants and Experimental Design. Six healthy, right-handed volunteers with normal vision (29-33 years) gave written informed consent to participate; the study was approved by the local ethics committee. Stimuli were presented in blocks of 10 trials during which the attended pair of quadrants remained constant, separated by a rest period of 15 s during which the fixation cross alone was presented. Two seconds before each block, a small cue was presented at the fixation spot that indicated the pair of quadrants to which participants should attend. If no cue appeared, participants were instructed not to attend to any specific stimuli but just to press one of the two response keys on each trial, forming a low-level baseline condition. On each trial, four spiral stimuli of random handedness and phase were presented in the four quadrants for 2 s, followed by a response interval of 1 s. Individual spiral stimuli subtended 5°, had a rotational period of 1 and a slope of 1 period, and were presented on a dark background (luminance of 11 cd/m2) at an eccentricity of 7.5°. Participants indicated by button press whether the two attended spirals were of same or different handedness or, during the low-level baseline condition, simply pressed alternate buttons on successive trials. During each scanning run, 10 such blocks were presented, comprising two pseudorandomized sequences of the five conditions (four pairs of attended quadrants plus the low-level baseline). Before scanning, participants practiced the task to ensure they were able to perform the task and maintain stable fixation. Eye movements were measured during scanning by using long-range infrared eye tracking (Applied Science Laboratories, Bedford, MA).
fMRI Acquisition. An Allegra 3T scanner (Siemens, Erlangen, Germany) was used to acquire between seven and nine runs of 165 fMRI volumes per participant (42 slices; repetition time of 2.73 s; resolution of 3 × 3 × 3 mm). A T1-weighted structural image was also acquired, together with two to three retinotopic mapping runs of 165 volumes each during which participants viewed standard stimuli that mapped the horizontal and vertical meridians.
Data Processing. Data were analyzed by using spm2 (www.fil.ion.ucl.ac.uk/spm). After discarding the first five images of each scanning run to allow for magnetic saturation effects, remaining images were realigned and coregistered to the individual participants' structural scans. Data were modeled voxelwise by using a general linear model that included the five experimental conditions (18) and high-pass filtered at 0.0083 Hz to remove low-frequency signal drifts. To extract activity from individual quadrants in early visual cortex, we used 12 mask volumes, 1 for each region of interest (left and right V1d, V1v, V2d, V2v, V4d, and V4v), using standard methods (19-21).
Functional Connectivity. To test the hypothesis that any integration of information from distant cortical areas would be reflected in specific interactions between cortical loci representing attended stimuli, we examined pairwise relationships between fMRI time courses of activity in areas of visual cortex corresponding to the retinotopic representations of the four visual stimuli presented on each trial. Because our principal experimental hypothesis concerned early visual cortex, we confined our initial investigation to areas V1 and V2. These areas contain neurons with the smallest receptive field sizes in visual cortex and are thus most suitable for retinotopic separation of individual stimuli. We calculated the pairwise correlations between activity in stimulus representations when each pair of cortical loci represented stimuli that were either attended or unattended (Fig. 2B). Such estimates of functional connectivity (correlations between remote neurophysiological events) have the virtue of simplicity but can be confounded by factors unrelated to the experimental manipulation. For example, changes in correlation can result from stimulus-locked or attentional transients evoked by a common input or can reflect stimulus-induced oscillations mediated by synaptic connections (22, 23). To minimize such effects, our analysis of connectivity was based on the residual signals after removing the transient effects of attention and visual stimulation (ref. 24 and Fig. 2 A).
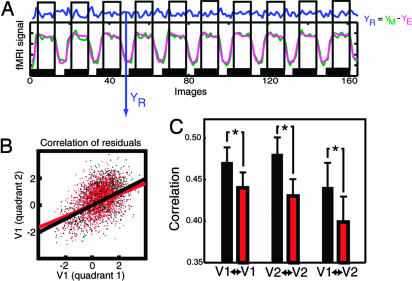
Fig. 2.
Functional connectivity analysis on residual data. (A) Residual data (blue, YR) not influenced by the main effect of stimulation were obtained for each visual quadrant from the fMRI data (green, YM) by subtraction of the data as estimated (modeled) by spm2 (pink, YE). Data were collected selectively for each attention condition and time-shifted by five images to account for the hemodynamic delay (see Materials and Methods). (B) Residual signal in two quadrants of V1 when both were attended (black) or unattended (red; data for one participant). The correlation was decreased in the unattended condition. (C) Average correlation coefficient (across participants) between quadrants that were both attended (black) or unattended (red). V1↔V1 and V2↔V2 refer to within-region correlation, and V1↔V2 refers to correlation between V1 and V2. The correlation between quadrants was significantly decreased when they were both unattended. Note that this change in correlation cannot be a consequence of differences in the main effects, because these differences were removed. Furthermore, the extraction of residuals is time-delayed by five images to avoid contamination by fMRI transients at the onset and offsets of each block. As a result, the residual signals showed no significant difference in variance under attended and unattended conditions (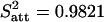 and
and 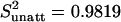 ; T = 1.17; P = 0.15).
; T = 1.17; P = 0.15).
Effective Connectivity. To further investigate cortico-cortical coupling, we next performed an analysis of effective connectivity based on a biologically realistic model of interactions between the retinotopic representations of our visual stimuli (25). Models of effective connectivity are not affected by confounding effects of stimulus- or attention-locked transients. We used an established framework for assessing effective connectivity with fMRI (25) to construct a model that incorporated the retinotopic locations in V1 and V2 where visual stimuli were represented, together with the known direct and indirect anatomical connections between these locations (Fig. 3 Center). In primates, there are topographically precise feed-forward and feedback connections between cortical areas V1 and V2 (11). Horizontal connections within these areas extend up to 10° and preferentially link regions of similar orientation tuning (4, 5, 8, 9). For representations across the vertical meridian, direct callosal connections have not been demonstrated (26), but indirect connections exist in humans by means of the superior colliculus (16) and potentially also the pulvinar, which has a subpopulation of cells with large, bilateral receptive fields (27). We used our fMRI data to estimate how the strength of each connection in the computational model varied with directed spatial attention. We estimated effective connectivity not only for connections within cortical areas V1 and V2 but also independently for the feed-forward and feedback pathways between these areas.
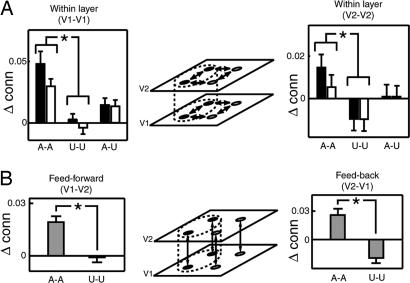
Fig. 3.
Effective connectivity analysis showing modulation of V1 and V2 connectivity by spatial attention. (A) Within-layer connectivity. Center depicts the computational model, with four retinotopic representations in each layer. In this panel, the within-area connections are considered, indicated by the double-headed arrows. Schematically, the dotted line and filled circles indicate the focus of attention. Estimates of effective connectivity parameters (corresponding to rate constants of the modeled neural processes; ref. 25) are plotted in Left (averaged across participants and normalized to the baseline estimate; error bars, SE) between retinotopic loci within V1 for attended-attended, unattended-unattended, and attended-unattended connections and separately for connections within (black) and between (white) hemispheres. In both V1 and V2, there was a significant main effect of attention (V1, F1,5 = 25.29 and P = 0.004; V2, F1,5 = 19.26 and P = 0.007) but no significant main effect of between versus within hemisphere condition (V1, F1,5 = 0.635 and P = 0.462; V2, F1,5 = 4.60 and P = 0.085). Significant (P < 0.05) differences between parameters are marked with an asterisk. Right plots the same estimates of effective connectivity for V2, depicted in the same way. It is apparent that attention enhanced effective connectivity between attended loci (both within and between hemispheres). In contrast, in V2, effective connectivity was decreased between unattended loci. (B) Center is laid out with the same conventions as in A but for between-area connections (i.e., feed-forward and feedback connectivity between V1 and V2). Again, it is apparent that attention strengthened both feed-forward and feedback connectivity but only between pairs of attended retinotopic loci. In contrast, functional integration between unattended loci was reduced for feedback connections from V2 to V1.
The first stage of the effective connectivity model represented the input to, and the interactions between, cortical loci formulated at the level of neural population dynamics (25). Neural activity at each voxel was modeled as the weighted sum of three terms: (i) direct, extrinsic input reflecting stimulus-driven activity; this direct input was only enabled for the four quadrants of V1, (ii) input from other cortical loci by means of task-independent intrinsic connections, including within-layer connections between quadrants (Fig. 3A) and retinotopically specific feed-forward/feedback connections between V1 and V2 (Fig. 3B), and (iii) a bilinear term modeled changes of intrinsic connections that reflected task-dependent effects. This term reflected changes in effective connectivity that depended on which pair of quadrants was attended. The output of this mathematical model of neuronal dynamics was then used as the input to an empirically validated forward model of the hemodynamic response to neural activity (28). This model generated expected blood oxygen level-dependent (BOLD) responses for a neural time series at each voxel for a given set of coupling parameters. A gradient ascent algorithm was then used to find the set of coupling parameters that minimized the difference between predicted and measured BOLD time series. The power and stability of such methods in recovering neural connectivity patterns has been demonstrated previously (25).
This model allowed us to characterize whether any long-range interactions could be identified in early visual areas V1 and V2, where individual neurons have relatively small receptive fields and thus interactions cannot occur within single receptive fields. To compare any findings with those from higher retinotopic areas where neurons with much larger receptive fields could conceivably directly mediate such interaction (1-3), we also constructed a second computational model of connectivity between retinotopic areas V2 and V4. This model incorporated the retinotopic locations in V2 and V4 at which the visual stimuli were represented, together with direct input to V2 and direct (14, 15) and indirect anatomical connections between these loci. We again used the fMRI data to estimate how the strength of each connection in the computational model varied as a function of directed spatial attention.
Results
There was uniformly high task performance (>90% correct for every participant and condition) that did not differ significantly depending on which stimulus pair was attended. Conventional analysis of the fMRI data confirmed that when a stimulus was attended (versus unattended), activity increased in the retinotopic locations corresponding to that stimulus in early visual cortex (see Fig. 1 B and C), replicating previous studies (29-33). Eye tracking data during scanning was available from five of six participants. Fixation was well maintained throughout, and there was no systematic positional bias toward the attended stimuli (Fig. 1 A Middle).
The functional connectivity analysis revealed that directing spatial attention to a pair of visual stimuli significantly changed the correlation between pairs of cortical loci in early visual cortex representing those stimuli (Fig. 2). Specifically, attention led to an enhancement of the correlation between stimulus representations both within V1 and V2 and between V1 and V2. This finding provides preliminary evidence for a neural signature of integration of information across distant cortical locations in early retinotopic cortex, as we hypothesized.
We next undertook a second, more detailed analysis of effective connectivity in early visual areas V1 and V2. Consistent with the functional connectivity analysis, the topography of coupling between distant retinotopic locations representing the stimuli changed in a highly specific and reproducible fashion, according to the direction of spatial attention (Fig. 3). Such changes were evident both for coupling between retinotopic loci within cortical areas V1 and V2 in both hemispheres and for the feed-forward and feedback connections between these cortical areas. There were two specific effects, displayed in Fig. 3 relative to the baseline (fixation task). First, within cortical areas V1 and V2, the effective connectivity increased between each pair of locations representing the attended stimuli (Fig. 3A). In contrast, connectivity decreased between pairs of unattended stimulus representations in cortical area V2. Connectivity between unattended and attended stimulus representations was intermediate (i.e., weaker than the coupling between attended stimulus representations but stronger than that between unattended stimulus representations). Second, between cortical areas V1 and V2 (Fig. 3B), the strength of both feed-forward and feedback connectivity increased between the cortical representations of pairs of attended stimuli. In contrast, feedback connectivity in V2 decreased for the cortical representation of pairs of unattended stimuli. Despite our instructions, it is still possible that participants may have directed some attention to the stimuli in the baseline task. However, the effect of this noncompliance would be to reduce the likelihood of detecting any differences in connectivity between the directed attention conditions and the baseline.
We undertook several different assessments of the validity of our effective connectivity model. First, we compared our full model with a simplified model without modulation of connectivity to validate the presence of modulatory effects. We computed the Bayes factor (34), which is the ratio of model evidence for the two different models. The Bayes factor was 102.5 (corresponding to a probability of P < 0.01), representing strong evidence in favor of the presence of modulatory effects on connectivity. We also verified that parameter estimation for our specific model was stable and unbiased using simulations (25). We generated 49 synthetic data sets from our model (equivalent to the total number of runs across all participants) by fixing parameters to the average values across participants and adding observation noise to achieve an overall signal-to-noise ratio of unity. We then estimated the parameters from the deliberately noised data. Despite the high degree of noise added, the estimated and true parameters were highly correlated (R = 0.97; P < 0.001), confirming that our model allowed for stable and unbiased solutions.
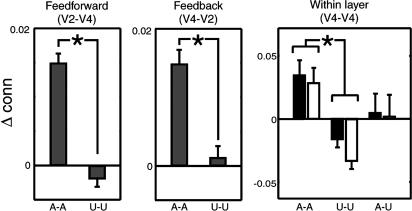
At higher levels of the retinotopic visual system in area V4, our corresponding analysis of effective connectivity confirmed a similar pattern of changes in functional integration between retinotopic stimulus representations as in earlier areas (Fig. 4). In V4, effective connectivity increased between each pair of locations representing the attended stimuli but decreased between pairs of unattended stimulus representations. As in V1 and V2, connectivity between unattended and attended stimulus representations was intermediate. Connectivity between layers V2 and V4 was also enhanced for attended quadrants but not for unattended quadrants (Fig. 4).
Fig. 4.
Effective connectivity analysis of V2 and V4. Left and Center show feed-forward and feedback connectivity between V2 and V4, between both attended and unattended retinotopic locations (as in Fig. 3). Right shows within-layer connectivity in V4 for attended-attended, unattended-unattended, and attended-unattended connections separately for within (black) and between (white) hemispheres (error bars, SE; *, P < 0.05). There was also a significant main effect of attention on stimulus-driven activity in V4 (T47 = 4.3223; P < 0.001).
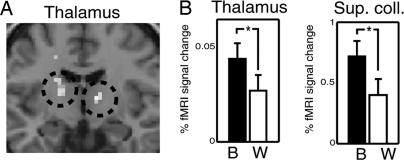
We next investigated the potential neural substrate of integration in conditions that required integrating information across the vertical meridian. When information has to be integrated between the left and right visual fields, the receptive field sizes of neurons in V1 and V2 are too small to permit direct integration of information (in contrast to V4). There are also no callosal connections between the quadrants in V1 and V2 (26). Thus, the behavioral and neural integration we observed must reflect either feedback from higher cortical areas with bilateral receptive fields (35) or indirect subcortical connections (16). To test the latter hypothesis, we examined activity in the superior colliculus and the pulvinar, identified anatomically according to criteria described in ref. 36. We compared activity during between-hemisphere and within-hemisphere attention conditions. Consistent with a subcortical pathway mediating attentional integration, activity in these two subcortical areas was significantly higher when participants were distributing their attention across the vertical meridian (Fig. 5).
Fig. 5.
Subcortical regions with stronger activation for between-hemifield than for within-hemifield attention. (A) Results from a random-effects model computed for normalized images of all participants overlaid on a coronal slice of a T1-weighted canonical structural scan. Two regions of the thalamus showed significantly stronger activation (threshold for display, P < 0.001 uncorrected) when attention was distributed between than within hemispheres. Stereotactic Montreal Neurological Institute (MNI) coordinates of these loci are [12;-11;6] and [-16;-9;10] corresponding to the pulvinar (cf. figure 8 in ref. 36). (B) Activity in thalamic loci depicted in A, plus the superior colliculi (SC) plotted separately for attend between (black bars) and attend within (white bars) hemisphere conditions. Error bars, 1 SE. Activity in both thalamus and SC was significantly enhanced (*, P < 0.05) when participants attended to stimuli distributed across the vertical meridian. Because of the small size of the SC, its position strongly varies between participants in normalized images. To extract activity from the SC, we localized its position on individual participants' anatomical images, where it was clearly visible.
Finally, we investigated whether the neural signature of attentional integration that we observed played any functional role in determining behavior. We measured the correlation between interparticipant variability in task performance and the coupling strengths within and between cortical areas. Remarkably, although participants' performance was close to ceiling, the level of attentional integration (in terms of effective connectivity) predicted how well participants were able to perform the behavioral task. There was a significant correlation between behavioral performance and a global effective connectivity index, which was obtained by averaging connectivity across all attended quadrants of all areas (R = 0.8160; P = 0.047; Fig. 6, which is published as supporting information on the PNAS web site). The correlation between behavioral performance and effective connectivity was positive, indicating that stronger coupling was associated with enhanced performance. In contrast, the overall level of activity within a retinotopic locus was not significantly predictive of performance (R = 0.52; P = 0.15).
Discussion
Integration within a distributed system is normally understood in terms of effective connectivity (25, 37), which reflects the degree to which activity evoked in one neuronal population (here, a retinotopic representation for one quadrant in one cortical area) is influenced by activity in other areas (here, the other retinotopic representations) independently of stimulus-locked transients evoked by sensory stimulation. We found that task-dependent attentional integration of information across distant regions of the visual field was reflected in changes in functional connectivity at multiple levels of processing in early visual cortex. The effect of attentional integration was to “bind” together distinct and spatially separated retinotopic locations across visual cortex that represented the two attended stimuli (Figs. 3 and 4). In contrast, representations of unattended stimuli in V2 showed decreased effective connectivity, reflecting functional decoupling of the associated neuronal populations.
The earliest stage at which interactions between our stimuli could occur within a single receptive field is likely to be V4 (38). Consistent with this, we found enhanced connectivity between retinotopic representations of attended stimuli within this area (Fig. 4). But we also found similarly enhanced connectivity between distant visual field locations at much earlier stages of cortical processing, in V1 and V2. These changes in cortico-cortical coupling occurred for pairs of representations that were separated by considerable anatomical distances (or even located in different hemispheres). Integration in V1 and V2 is unlikely to result monosynaptically from lateral horizontal connections within each area, because the separation of our stimuli was greater than the spread of lateral connections. These connections typically extend only a few millimeters in cortex, or ≈10° of visual angle (4-7, 8-10). The anatomical basis of the functional integration that we observed is therefore likely to involve polysynaptic connections between homologous retinotopic cortical areas, which could involve feedback from higher levels of the visual system such as V4 (14, 15) or even subcortical pathways.
We found enhanced coupling not only when the two attended stimulus representations were located in the same hemisphere but also across the vertical meridian and so between hemispheres. No callosal connectivity has been demonstrated at the quadrantic location of our stimulus representations in V1 and V2 (26). One possibility is that such interhemispheric connectivity is mediated by callosal connections in V4 and then back-propagated to early visual areas. However, our effective connectivity analysis, which allowed independent estimation of feed-forward and feedback coupling, revealed that feedback pathways were not modulated more strongly than feed-forward pathways (Figs. 3 and 4). Thus, the interhemispheric interactions that we observed in V1 and V2 may not be caused by feedback signals alone. An alternate possibility is that anatomical connectivity across the vertical meridian involves interhemispheric connections by means of the superior colliculus (16) or the pulvinar (27). Consistent with such a notion, integration of information across the vertical meridian was associated with significantly enhanced activation of the pulvinar and superior colliculus (Fig. 5). Taken together, our findings therefore suggest that these long-range interactions in V1 and V2 may receive a contribution from subcortical connections through pulvinar and superior colliculus.
Our experimental design allowed us to retinotopically separate individual stimulus representations, permitting direct examination of the degree of interaction between these cortical locations. Previous human neuroimaging studies suggest that attention reduces competitive interactions between stimulus representations but only in higher and not lower retinotopic visual areas (39-41). These findings support the notion that “biased competition” occurs when competing stimuli both fall within the same receptive field, which is more likely to occur in higher visual areas (42-44). Consistent with this, we found enhanced connectivity between retinotopic representations of our stimuli within area V4. But we also found similarly enhanced connectivity between distant retinotopic locations at much earlier stages of cortical processing, in areas V1 and V2, which might reflect a higher sensitivity for our approach of directly studying interactions between retinotopically separable stimulus representations. Previous studies have only examined connectivity indirectly by comparing the effect of attention on the difference in response amplitude between sets of simultaneously and successively presented stimuli in a single visual quadrant (39-41). However, our findings may also suggest that the effect of attention on cortical interactions might not be restricted to cases where stimuli fall into the same receptive field. Consistent with this, top-down signals such as attention and task can modulate the influence of stimuli presented beyond the classical receptive field in monkeys (45, 46). This finding suggests that attention-related signals can dynamically modulate long-range horizontal connections in a task-dependent fashion. The present findings go beyond these earlier observations by showing that, in humans, such modulatory effects of attention on connectivity can even be observed between stimuli presented at much greater distances and even between stimuli presented to different hemispheres.
The changes in coupling that we observed between attended cortical representations complement previous findings that directed attention can modulate the coupling between early visual cortex and higher areas for different features of the same stimulus (17). Conceptually, our findings are close to the notion that spatially distributed representations of the different features of a single object might be “bound” together by attention (47). However, it is important to note that the present findings relate not to two elementary features of a single object but instead to one rather complex feature (“handedness”) of two different objects in distinct spatial locations. It is interesting to speculate whether our findings depend on our specific requirement of integration or comparison of features at two different retinotopic locations. This question could be addressed by comparing in the same experiment our integration task with a simple detection task.
The precise neural mechanisms of such spatial “binding” between representations of attended stimuli are currently unclear, but they could involve long-distance synchronization of activity between different neural populations. This synchronization has been observed at the slow time scale of fMRI signals in the form of coherent variations in gamma-band power (48), plus gamma-band activity has been related to cortical integration of information (49, 50) and can be closely linked to signals measured in BOLD fMRI (51). However, precise characterization of the underlying mechanisms awaits further research. It is important to appreciate that the changes in functional and effective connectivity that we observed between distant retinotopic loci (Fig. 3) were not caused simply by the common main effect of attention on the fMRI signal within these loci (e.g., Fig. 2). Both analyses modeled and removed these main effects of attention (e.g., Fig. 2A). Both empirical data and theoretical investigations (52, 53) have additionally shown that effective connectivity cannot be accounted for by simple correlations between signal amplitude in different areas. Indeed, the independence of our estimates of the main effects of attention within individual areas and those of coupling between areas can be intuitively appreciated by considering that although the pairs of loci representing either attended or unattended stimuli both showed conjoint variation of their main effects, effective connectivity between the pairs changed in opposite directions. Effective connectivity between attended locations increased, but that between unattended locations decreased (Fig. 3). Such dissociations are inconsistent with changes in effective connectivity arising as a simple consequence of conjoint changes in activity comparing attended (versus unattended) epochs or vice versa. We therefore conclude that the functional integration demonstrated here reflects an independent neural signature of attentional integration in the human brain.
Finally, the changes in coupling that we observed between attended cortical representations may play a functional role in generating behavior. The degree of coupling showed a significant correlation with behavioral performance (Fig. 6). Our data are consistent with observations that activity (54) and even connectivity within (45) and between (55, 56) visual areas can be predictive of behavioral performance. Our data go beyond such earlier observations by revealing that the coupling between distinct sectors of early visual cortex can be a better predictor of performance than the activity within a single cortical locus, suggesting a functional role for attentional integration in the generation of behavior.
Supplementary Material
Acknowledgments
We thank Jon Driver, Daniel Glaser, Winrich Freiwald, and Andreas Kreiter for helpful comments; Udo Ernst for spiral stimuli; and Klaas-Enno Stephan and Will Penny for help with dynamic causal modeling and simulations. This work was supported by the Wellcome Trust.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: fMRI, functional MRI; BOLD, blood oxygen level-dependent.
References
- 1.Zeki, S. (1978) J. Physiol. 277, 273-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith, A. T., Singh, K .D., Williams, A. L. & Greenlee, M. W. (2001) Cereb. Cortex 11, 1182-1190. [DOI] [PubMed] [Google Scholar]
- 3.Gattass, R., Sousa, A. P. & Gross, C. G. (1988) J. Neurosci. 8, 1831-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert, D. C., Das, A., Ito, M., Kapadia, M. & Westheimer, G. (1996) Proc. Natl. Acad. Sci. USA 93, 615-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toth, L. J., Rao, S. C., Kim, D. S., Somers, D. & Sur, M. (1996) Proc. Natl. Acad. Sci. USA 93, 9869-9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bringuier, V., Chavane, F., Glaeser, L. & Fregnac, Y. (1999) Science 283, 695-699. [DOI] [PubMed] [Google Scholar]
- 7.Angelucci, A. & Bullier, J. (2003) J. Physiol. (Paris) 97, 141-154. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert, C. D. & Wiesel, T. N. (1983) J. Neurosci. 3, 1116-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockland, K. S. & Lund, J. S. (1983) J. Comp. Neurol. 216, 303-318. [DOI] [PubMed] [Google Scholar]
- 10.Stettler, D. D., Das, A., Bennett, J. & Gilbert, C. (2002) Neuron 36, 739-750. [DOI] [PubMed] [Google Scholar]
- 11.Rockland, K. S. (1994) in Cerebral Cortex: Primary Visual Cortex in Primates, eds. Peters, A. & Rockland, K. S. (Plenum, New York), Vol. 10, pp. 261-300. [Google Scholar]
- 12.Burkhalter, A. & Bernardo, K. L. (1989) Proc. Natl. Acad. Sci. USA 86, 1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelucci, A., Levitt, J. B., Walton, E. J., Hupe, J. M., Bullier, J. & Lund, J. S. (2002) J. Neurosci. 22, 8633-8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gattas, R., Sousa, A. P., Mishkin, M. & Ungerleider, L. G. (1997) Cereb. Cortex 7, 110-129. [DOI] [PubMed] [Google Scholar]
- 15.Rockland, K. S., Saleem, K. S. & Tanaka, K. (1994) Visual Neurosci. 11, 579-600. [DOI] [PubMed] [Google Scholar]
- 16.Tardif, E. & Clarke, S. (2002) Neuroscience 111, 363-372. [DOI] [PubMed] [Google Scholar]
- 17.Friston, K. J. & Büchel, C. (2000) Proc. Natl. Acad. Sci. USA 97, 7591-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friston, K. J., Holmes, A. P., Worsley, K. J., Poline, J. P., Frith, C. D. & Frackowiak, R. S. J. (1995) Hum. Brain Mapp. 2, 189-210. [DOI] [PubMed] [Google Scholar]
- 19.Sereno, M. I., Dale, A. M., Reppas, J. B., Kwong, K. K., Belliveau, J. W., Brady, T. J., Rosen, B. R. & Tootell, R. B. (1995) Science 268, 889-893. [DOI] [PubMed] [Google Scholar]
- 20.Teo, P. C., Sapiro, G. & Wandell, B. A. (1997) IEEE Trans. Med. Imaging 16, 852-863. [DOI] [PubMed] [Google Scholar]
- 21.Wandell, B. A., Chial, S. & Backus, B. T. (2000) J. Cognit. Neurosci. 12, 739-752. [DOI] [PubMed] [Google Scholar]
- 22.Gerstein, G. L. & Perkel, D. H. (1969) Science 164, 828-830. [DOI] [PubMed] [Google Scholar]
- 23.Büchel, C. & Friston, K. (2000) Neural Networks 13, 871-882. [DOI] [PubMed] [Google Scholar]
- 24.Macaluso, E., Frith, C. D. & Driver, J. (2000) Science 289, 1206-1208. [DOI] [PubMed] [Google Scholar]
- 25.Friston, K., Harrison, L. & Penny, W. (2003) NeuroImage 19, 1273-1302. [DOI] [PubMed] [Google Scholar]
- 26.Clarke, S. & Miklossy, J. (1990) J. Comp. Neurol. 298, 188-214. [DOI] [PubMed] [Google Scholar]
- 27.Benevento, L. A. & Miller, J. (1981) J. Neurosci. 1, 1268-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephan, K. E., Harrison, L., Penny, W. D. & Friston, K. J. (2004) Curr. Opin. Neurobiol. 14, 629-635. [DOI] [PubMed] [Google Scholar]
- 29.McMains, S. A. & Somers. D. C. (2004) Neuron 42, 677-686. [DOI] [PubMed] [Google Scholar]
- 30.Tootell, R. B., Hadjikhani, N., Hall, E. K., Marrett, S., Vanduffel, W., Vaughan, J. T. & Dale, A. M. (1998) Neuron 21, 1409-1422. [DOI] [PubMed] [Google Scholar]
- 31.Somers, D. C., Dale, A. M., Seiffert, A. E. & Tootell, R. B. H. (1999) Proc. Natl. Acad. Sci. USA 96, 1663-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gandhi, S. P., Heeger, D. J. & Boynton, G. M. (1999) Proc. Natl. Acad. Sci. USA 96, 3314-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brefczynski, J. A. & Deyoe, E. A. (1999) Nat. Neurosci. 2, 370-374. [DOI] [PubMed] [Google Scholar]
- 34.Penny, W. D., Stephan, K. E., Mechelli, A. & Friston, K. J. (2003) NeuroImage 22, 1157-1172. [DOI] [PubMed] [Google Scholar]
- 35.Tootell, R. B., Mendola, J. D., Hadjikhani, N. K., Liu, A. K. & Dale, A. M. (1998) Proc. Natl. Acad. Sci. USA 95, 818-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kastner, S., O'Connor, D. H., Fukui, M. M., Fehd, H. M., Herwig, U. & Pinsk, M. A. (2004) J. Neurophysiol. 91, 438-448. [DOI] [PubMed] [Google Scholar]
- 37.Friston, K. J., Buechel, C., Fink, G. R., Morris, J., Rolls, E. & Dolan, R. J. (1997) NeuroImage 6, 218-229. [DOI] [PubMed] [Google Scholar]
- 38.Desimone, R., Moran, J., Schein, S. J. & Mishkin, M. (1993). Visual Neurosci. 10, 159-171. [DOI] [PubMed] [Google Scholar]
- 39.Kastner, S., De Weerd, P., Desimone, R. & Ungerleider, L. G. (1998) Science 282, 108-111. [DOI] [PubMed] [Google Scholar]
- 40.Kastner, S. & Ungerleider, L. G. (2000) Ann. Rev. Neurosci. 23, 315-341. [DOI] [PubMed] [Google Scholar]
- 41.Kastner, S. & Ungerleider, L. G. (2001) Neuropsychologia 39, 1263-1276. [DOI] [PubMed] [Google Scholar]
- 42.Desimone, R. & Duncan, J. (1995) Ann. Rev. Neurosci. 18, 193-222. [DOI] [PubMed] [Google Scholar]
- 43.Luck, S. J., Chelazzi, L., Hillyard, S. A. & Desimone, R. (1997) J. Neurophysiol. 77, 24-42. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds, J. H., Chelazzi, L. & Desimone, R. (1999) J. Neurosci. 19, 1736-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito, M. & Gilbert, C. D. (1999) Neuron 22, 593-604. [DOI] [PubMed] [Google Scholar]
- 46.Li, W., Piech, V. & Gilbert, C. D. (2004) Nat. Neurosci. 7, 651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Treisman, A. M. & Gelade, G. (1980) Cognit. Psychol. 12, 97-136. [DOI] [PubMed] [Google Scholar]
- 48.Leopold, D. A., Murayama, Y. & Logothetis, N. K. (2003) Cereb. Cortex 13, 422-433. [DOI] [PubMed] [Google Scholar]
- 49.Gray, C. M., Konig, P., Engel, A. K. & Singer, W. (1989) Nature 338, 334-337. [DOI] [PubMed] [Google Scholar]
- 50.Engel, A. K., Konig, P., Kreiter, A. K., Schillen, T. B. & Singer, W. (1992) Trends Neurosci. 15, 218-226. [DOI] [PubMed] [Google Scholar]
- 51.Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. (2001) Nature 412, 150-157. [DOI] [PubMed] [Google Scholar]
- 52.McIntosh, A. R. & Gonzalez-Lima, F. (1994) Hum. Brain Mapp. 2, 2-22. [Google Scholar]
- 53.Toni, I., Rowe, J., Stephan, K. E. & Passingham, R. E. (2002) Cereb. Cortex 12, 1040-1047. [DOI] [PubMed] [Google Scholar]
- 54.Ress, D. & Heeger, D. J. (2003) Nat. Neurosci. 6, 414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pascual-Leone, A. & Walsh, V. (2001) Science 292, 510-512. [DOI] [PubMed] [Google Scholar]
- 56.Haynes. J. D., Driver, J. & Rees, G. (2005) Neuron 46, 811-821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.