Abstract
Rearrangements of the RET receptor tyrosine kinase gene generating RET/PTC oncogenes are specific to papillary thyroid carcinoma (PTC), the most frequent thyroid tumor. Here, we show that the RET/PTC1 oncogene, when exogenously expressed in primary normal human thyrocytes, induces the expression of a large set of genes involved in inflammation and tumor invasion, including those encoding chemokines (CCL2, CCL20, CXCL8, and CXCL12), chemokine receptors (CXCR4), cytokines (IL1B, CSF-1, GM-CSF, and G-CSF), matrix-degrading enzymes (metalloproteases and urokinase-type plasminogen activator and its receptor), and adhesion molecules (L-selectin). This effect is strictly dependent on the presence of the RET/PTC1 Tyr-451 (corresponding to RET Tyr-1062 multidocking site). Selected relevant genes (CCL20, CCL2, CXCL8, CXCR4, L-selectin, GM-CSF, IL1B, MMP9, UPA, and SPP1/OPN) were found up-regulated also in clinical samples of PTC, particularly those characterized by RET/PTC activation, local extrathyroid spread, and lymph node metastases, when compared with normal thyroid tissue or follicular thyroid carcinoma. These results, demonstrating that the RET/PTC1 oncogene activates a proinflammatory program, provide a direct link between a transforming human oncogene, inflammation, and malignant behavior.
Keywords: chemokines, inflammation, papillary thyroid carcinoma, gene expression
Several lines of evidence suggest a strong association between chronic inflammation and increased susceptibility to neo-plastic transformation and cancer development (1-4).
A number of studies in murine tumor models have demonstrated the protumoral role of inflammatory mediators at distinct phases of malignant progression (5-8). Conversely, inhibition of selected proinflammatory cytokines (e.g., TNF, IL1) or of inflammation-related transcription factors (e.g., NF-κB) has resulted in reduced susceptibility to carcinogenesis and slower tumor development in experimental tumor models (7-9).
The persistent release of inflammatory molecules may affect tumor progression in a variety of ways, for instance by increasing tumor cell proliferation and resistance to apoptosis, by promoting angiogenesis and stroma remodeling, and by inhibiting the establishment of a protective antitumor immunity (4). Tumor-associated cells of the innate and adaptive immunity play a pivotal role in this context, as they release a whole array of proinflammatory mediators (10).
Indeed, it has been estimated that up to 20% of all tumors arise from conditions of persistent inflammation mainly because of chronic infections or autoimmune diseases (1). For example, the autoimmune inflammatory disorders, chronic Hashimoto's thyroiditis and Graves' disease, are both associated with an increased incidence of papillary thyroid carcinoma (PTC) (11). On the other hand, tumor cells themselves may support their own growth and invasive phenotype through direct expression of inflammation-related molecules (2).
PTC is the most frequent thyroid malignancy in humans. Rearrangements of the RET receptor tyrosine kinase (RTK) gene, caused by chromosomal inversions or translocations, is a frequent genetic event (≈30%) in PTC (12). These rearrangements mediate fusion of the tyrosine kinase-encoding domain of RET with heterologous genes, leading to the generation of chimeric RET/PTC oncogenes. All RET/PTCs, differing for the 5′ donor gene involved, display ligand-independent constitutive dimerization and activation. RET/PTC1, the H4-RET fusion (13), is one of the prevalent variants. The multidocking site Tyr-1062 (Tyr-451 in RET/PTC1) in the C-terminal region of RET, interacting with a number of transduction molecules and involved in almost all downstream pathways activated by RET (14), was demonstrated necessary for the transforming activity of RET/PTC oncogenes (15). Alternative pathogenetic events have been found in PTC and include chromosomic rearrangement involving TRKA (12) or BRAF point mutations or rearrangement (16-18).
PTCs are unique among epithelial tumors in that they appear to develop into a full-fledged malignancy in one step without any apparent benign preinvasive counterpart. It is well established that the translocation generating RET/PTCs is an early event playing a causative role in the pathogenesis of a significant fraction of PTC. In fact, RET/PTC expression has been found in ≈40% of occult PTCs (19). In PC-Cl3 rat thyroid epithelial cells, RET/PTCs induce morphological transformation (20) and lead to the development of thyroid tumors resembling human PTC in transgenic mice (21). Furthermore, RET/PTC1 expression is sufficient to cause, in primary cultures of normal human thyrocytes, changes in the nuclear envelope and chromatin diagnostic for PTC (22).
These observations prompted us to use primary human thyrocytes as an in vitro model to study the RET oncogene-dependent molecular mechanisms driving follicular thyroid cells to malignant transformation and to challenge the concept of its direct role in triggering an inflammatory program.
In this study, we provide evidence that the RET/PTC1 transforming oncogene, exogenously expressed in primary normal human thyrocytes, regulates through its multidocking site, Tyr-451, the expression of a distinct set of genes involved in inflammation and tumor invasion. The in vivo relevance of in vitro identified profiles was validated by analysis of PTC specimens. Overall, these results demonstrate a direct connection between a transforming human oncogene and a distinct inflammatory program in primary human cells.
Materials and Methods
Cell Cultures and Retroviral Infections. Primary cultures of normal human thyroid cells were infected with retroviral vectors containing RET/PTC1 or RET/PTC1-Y451F short isoforms as detailed in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Cell Growth Assay. Cell growth was evaluated by sulforhodamine B colorimetric assay as detailed in Supporting Materials and Methods.
Protein Extraction and Western Blotting. Mass populations of G418-selected thyrocytes stably expressing RET/PTC1 or RET/PTC1-Y451F as well as parental thyrocytes were processed for Western blot analysis as described in Supporting Materials and Methods.
RNA Extraction and Microarray Analysis. RNA extraction and purification and cDNA amplification were performed as detailed in Supporting Materials and Methods. Human genome set U133 GeneChips in duplicate (Affymetrix, Santa Clara, CA) were used. The data were analyzed with Affymetrix microarray suite version 5 (MASv5), and statistical analyses were performed by using software designed in our institute (Institute of Molecular Oncology Foundation, Milan) (23). All of the microarray data is deposited in the ArrayExpress database (accession no. E-MEXP-429).
Real-Time Quantitative PCR (Q-PCR). Total RNA used for microarray experiments and from 24 thyroid specimens was used to analyze the expression of a total of 12 genes: CCL2, CCL20, CXCL8, CXCL12, CXCR4, SELL, GM-CSF, IL1B, MMP9, TIMP3, UPA, and SPP1 (labeled with 6-carboxyfluorescein). H18S (labeled with VIC, Applied Biosystems) was assayed as a representative housekeeping gene. For details, see Supporting Materials and Methods.
Detection of Chemokines by ELISAs and Gelatinase Activity Assay. The supernatants of parental and RET/PTC1-expressing thyrocytes, maintained in serum-free medium for 24 h, were collected and analyzed. The amounts of CCL2, CCL20, CXCL8, and CXCL12 released in the supernatant were quantified by ELISA with a commercial kit by R & D Systems. MMP2/MMP9 activity was quantified by the gelatinase assay kit (Chemicon).
Migration Assay. Cell migration of parental and RET/PTC1- and RET/PTC1-Y451F-expressing thyrocytes was evaluated in the presence/absence of CXCL12 and of the CXCR4 inhibitor AMD3100, as detailed in Supporting Materials and Methods.
Tissue Samples. Thyroid samples, 3 normal and 21 tumor specimens [17 PTC and 4 follicular thyroid carcinomas (FTC)], were selected for this study and characterized as detailed in Supporting Materials and Methods.
Results
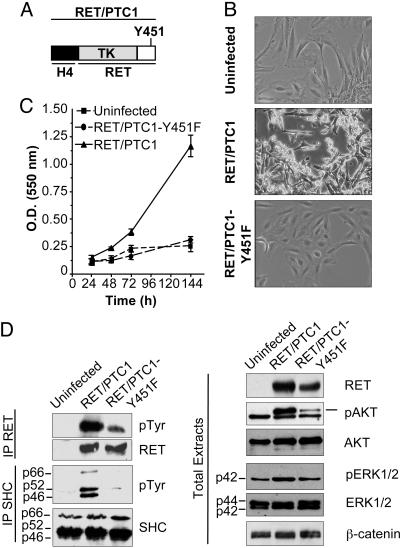
Biological and Biochemical Characterization of Normal Human Thyroid Follicular Cells Ectopically Expressing RET/PTC1 Oncogene. To study the molecular mechanisms triggered by RET/PTC oncogenes to transform thyroid cells, we exogenously expressed RET/PTC1 (13) oncogene (Fig. 1A) in normal human thyroid cells. Cultured primary thyrocytes from a healthy thyroid were infected with pLNCX retroviral vector expressing RET/PTC1 or, as control, RET/PTC1-Y451F mutant. Tyr-451 of RET/PTC1 corresponds to RET-Y1062 multidocking site, previously demonstrated necessary for the transforming activity of RET/PTC oncogenes in NIH/3T3 cells (15). Mass populations of thyrocytes stably expressing RET/PTC1 or RET/PTC1-Y451F and parental thyrocytes were used for all this study.
Fig. 1.
Characterization of normal human thyroid follicular cells ectopically expressing RET/PTC1 oncogene. (A) Schematic representation of RET/PTC1 oncogene short isoform. A portion of H4 donor gene is juxtaposed to RET tyrosine kinase (TK) intracellular portion. The Y451 docking site is marked. (B) Phase-contrast micrographs of human thyroid cells either uninfected or infected with RET/PTC1 or RET/PTC1-Y451F mutant. (Magnification ×100.) (C) Proliferative capacity of parental and RET/PTC1- and RET/PTC1-Y451F-expressing thyrocytes. Each point represents the mean of eight independent replicates ± SD. (D) Biochemical analysis of parental and RET/PTC1-infected thyrocytes. Cell extracts were analyzed with the indicated antisera. IP, immunoprecipitate. β-Catenin is shown as a control for protein loading.
Thyrocytes expressing RET/PTC1-Y451F mutant and parental thyrocytes showed a similar morphology that differed markedly from that of thyrocytes expressing RET/PTC1 (Fig. 1B). In fact, whereas parental thyrocytes, as well as those expressing RET/PTC1-Y451F, are flat and growth contact inhibited, the thyrocytes expressing the fully functional oncogene showed a “transformed phenotype,” being more refractile and growing without contact inhibition. In addition, they showed the characteristic nuclear envelope irregularity and chromatin clearing of human PTCs as described in ref. 22. The nuclear morphology of human thyrocytes expressing RET/PTC1-Y451F was identical to that of the uninfected ones (A.F., M.G.B., and A.G., unpublished results).
Uninfected and RET/PTC1-infected thyrocytes were monitored for cell growth. The thyrocytes expressing RET/PTC1-Y451F and the parental cells showed similar low proliferative capacity, whereas those expressing fully functional RET/PTC1 oncoprotein grew rapidly with a doubling time of 36 h (Fig. 1C). When RET/PTC1-expressing thyrocytes were treated with the RET kinase inhibitor RPI-1 (24), their proliferative capacity became similar to that of parental cells (data not shown). These results confirm the growth-promoting effect of RET/PTC1 and the relevance of Tyr-451 in the transmission of proliferative signals in our human thyrocyte model.
To biochemically characterize cells infected with the two RET/PTC1 oncogenes, the expression and tyrosine phosphorylation status of RET/PTC1 as well as the activation of known RET downstream effectors were analyzed by Western blot, as shown in Fig. 1D. The expression levels of RET/PTC1 and RET/PTC1-Y451F proteins in infected cells were comparable, and both proteins were tyrosine-phosphorylated. As expected, SHC proteins were constitutively tyrosine phosphorylated in RET/PTC1-expressing cells but not in cells expressing RET/PTC1 lacking Tyr-451, the SHC docking site, and in parental thyrocytes. SHC is known to play a crucial role in the transforming activity induced by oncogenic RET/PTC proteins in different cell environments (15). AKT was not activated in uninfected thyrocytes, whereas strong AKT activation was achieved in thyrocytes expressing RET/PTC1 and, to a lesser extent, in those expressing RET/PTC1-Y451F. ERK1 phosphorylation was slightly increased in RET/PTC1-expressing cells compared with the other cells. Phospholipase Cγ (PLCγ) protein was Tyr-phosphorylated in both RET/PTC1- and RET/PTC1-Y451F-expressing cells, as expected, but not in parental thyrocytes, whereas p38 phosphorylation was not induced by either of the RET/PTC proteins (data not shown). Overall, the biochemical analysis confirmed that the analyzed thyrocyte populations expressed comparable amounts of WT and mutant RET/PTC1 and that the RET/PTC1 oncogene activates transforming intracellular pathways involving SHC, PLCγ, ERK1, and AKT.
RET/PTC1 Expression Induces Inflammatory Molecules in Thyrocytes. The gene expression profiles of infected cells compared with parental thyrocytes was analyzed on human genome set U133 GeneChips to detect genes induced in human thyroid cells on RET/PTC1-mediated transformation. Systematic comparison of genes present in RET/PTC1-expressing cells vs. parental thyrocytes or RET/PTC1-Y451F-expressing cells was performed.
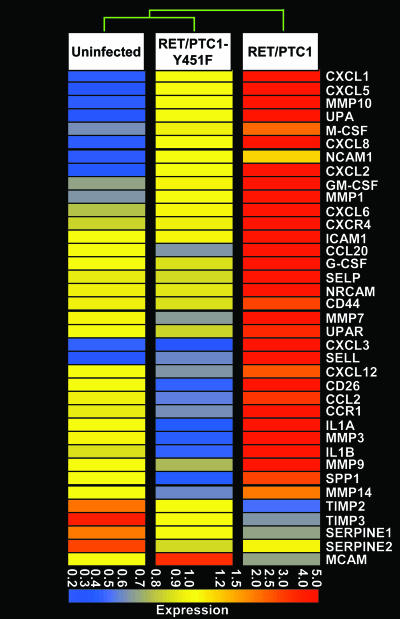
Using a cutoff of 2-fold, we identified 1,258 and 612 regulated genes in RET/PTC1- and RET/PTC1-Y451F-expressing cells, respectively, compared with uninfected thyrocytes. Comparison of the RET/PTC1 with RET/PTC1-Y451F mutant expressing cells showed 1,161 regulated genes, thus suggesting that the multidocking site is responsible for the majority of RET/PTC transcriptional effects. We found 320 genes, i.e., ≈1/4th of those regulated by RET/PTC1-WT, regulated also by RET/PTC1-Y451F, although to a lesser extent. Among the changes induced by RET/PTC1, we observed a prominent up-regulation of genes related to inflammation and tumor invasion. As listed in Table 1, which is published as supporting information on the PNAS web site, and shown in Fig. 2, nine chemokines and three chemokine receptors were induced. In addition, stimulating factors for macrophages or granulocytes, M-CSF, GM-CSF, and G-CSF were all up-regulated. Other molecules involved in angiogenesis and invasion, such as six different metalloproteinases (MMPs), the transmembrane protease dipeptidyl peptidase IV (DPPIV), and the urokinase-type plasminogen activator (UPA) and its receptor (UPAR), were all up-regulated, whereas the tissue inhibitors of MMP2 and MMP3 (TIMP2 and TIMP3) were down-regulated. Moreover, the adhesion molecules L-selectin (SELL), P-selectin (SELP), intracellular adhesion molecule 1 (ICAM1), and neural and neuronal cell adhesion molecules (NCAM and NRCAM, respectively) were induced. In some cases, both the ligand and the corresponding receptor were induced, suggesting an autocrine loop similar to the chemokine receptor 4 (CXCR4) and its ligand chemokine ligand 12 (CXCL12) and osteopontin/secreted phosphoprotein 1 (OPN/SPP1) and one of its receptors (CD44), UPA and UPAR. Almost all of the genes up-regulated in RET/PTC1-cells compared with uninfected thyrocytes were also up-regulated when compared with RET/PTC1-Y451F-cells, as shown in Fig. 2.
Fig. 2.
Expression plot of the inflammation/invasion-related genes regulated by RET/PTC1 oncogene. Shown is unsupervised hierarchical clustering analysis of genes related to inflammation/invasion in uninfected thyrocytes or in thyrocytes expressing RET/PTC1 or RET/PTC1-Y451F. Each row represents one probe set corresponding to one gene. The dendrogram illustrates the degree of similarity between the expression profiles of the three cell types. Different colors in the rectangles represent different levels of MAS 5-derived signals after per chip and per gene normalization (Gene Spring, Agilent Technologies, Palo Alto, CA). The color bar beneath the dendrogram represents the average expression values for this subset of genes.
This finding is supported by the dendrogram at the top of Fig. 2, which highlights the similarity of uninfected thyrocytes with RET mutant-infected cells.
Real-time Q-PCR (TaqMan gene expression assay) was performed to confirm expression of representative molecules of the different classes of induced genes. In particular, the gene expression levels of CXCL12, CCL2, SPP1, TIMP3, GM-CSF, MMP9, UPA, CXCL8, CCL20, CXCR4, IL1B, and SELL were analyzed (Fig. 6, which is published as supporting information on the PNAS web site). Real-time Q-PCR results confirmed the microarray data. To avoid the possibility of an effect specific to one patient's sample of cells, we have performed real-time Q-PCR analyses also on thyroid cultures from a distinct donor infected with the same RET/PTC1 or RET/PTC1-Y451F-expressing retroviruses. The RET/PTC1-expressing thyrocytes from the two donors showed comparable results for all genes except TIMP3 (data not shown).
To confirm gene expression at the protein level, supernatants of the three cell cultures were measured by ELISA for the chemokines CCL2, CCL20, CXCL8, and CXCL12. In line with the gene expression analysis, cells infected with RET/PTC1 actively produced a large amount of inflammatory chemokines, as shown in Fig. 3A. CXCL8 protein was strongly induced by RET/PTC1, being increased ≈70-fold, whereas both CCL2 and CCL20 chemokines were increased ≈10-fold. The levels of CXCL12 showed lower but reproducible increase.
Fig. 3.
Expression of inflammation/invasion-related molecules. (A) Analysis of chemokine production by ELISA and of MMP activity. One representative experiment of three independent experiments is shown. (B) Spontaneous and CXCL12-induced migration of uninfected and RET/PTC1- or RETPTC-Y451F-infected thyrocytes. Results are expressed as the mean number of migrated cells counted in 10 microscope high-power fields. (C) Dose-response migration of RET/PTC1-infected thyrocytes to CXCL12. (D) Effect of the CXCR4 inhibitor AMD3100 (1 μg/ml) on CXCL12-induced (100 ng/ml) chemotaxis. (E) Phase-contrast micrographs of RET/PTC1-infected thyroid cells, treated with CXCL12 (100 ng/ml) or medium for 3 h.
Of the several MMPs whose expression increased after RET/PTC1 infection (listed in Table 1), we tested the functional activity of MMP2 and MMP9. Fig. 3A shows that, in the supernatants of RET/PTC1-infected cells, gelatinase activity is much higher compared with the other cell cultures.
The strong up-regulation of CXCR4 in RET/PTC1-expressing cells prompted us to analyze the functional activity of this chemokine receptor in chemotaxis assays. RET/PTC1-expressing thyrocytes significantly migrated in response to the specific ligand CXCL12 in a dose-dependent manner, whereas thyroid cells lacking Tyr-451 and parental thyrocytes were completely unresponsive to the chemokine (Fig. 3 B and C). In line with these results, RET/PTC1-cells exposed to CXCL12 showed a polarized morphology (Fig. 3E). To test the specificity of the CXCL12-induced chemotaxis in RET/PTC1-expressing thyrocytes, AMD3100, a specific antagonist to CXCR4, was used. Cell treatment with AMD3100 almost completely inhibited migration in response to CXCL12 (Fig. 3D).
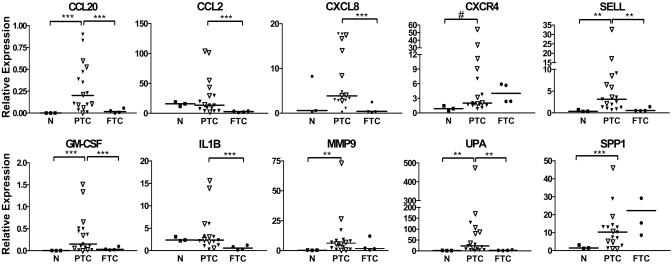
Expression Analysis of RET/PTC1-Induced Genes in PTC and FTC Specimens. The expression of a set of genes induced by RET/PTC1 in the in vitro experimental model was tested in a panel of thyroid carcinomas and normal thyroid samples. The genes were selected among those most differentially expressed and for being representative of different functional classes. RNAs from 17 PTCs, 4 FTCs, and 3 normal thyroid samples were analyzed.
The expression of genes encoding CCL20, CXCR4, SELL, GM-CSF, MMP9, UPA, and SPP1 was significantly higher (P < 0.05 or P < 0.01) in PTC specimens compared with normal thyroid samples, thus confirming the presence “in vivo” of molecules identified by the “in vitro” model (Fig. 4). The median of CCL2 and CXCL8 expression tended to be higher in PTC compared with normal thyroid; however, because of the high dispersion of expression levels in PTC specimens and the small number of normal thyroid samples, the differences are not statistically significant (Fig. 4).
Fig. 4.
Real-time Q-PCR analysis of selected genes in normal and tumor specimens. The results are given as relative expression (mRNA expression normalized for 18S rRNA levels). Medians and P values are indicated. As determined by using the Student's t test with unequal variance, **, P < 0.05; ***, P < 0.01; #, P = 0.05. N, normal thyroid; ▿, PTC from patients characterized by RTK (RET/PTC1, RET/PTC3, and TRK) rearrangements and pT4N1 presentation.
Moreover, the median expression levels of CCL20, CCL2, CXCL8, SELL, GM-CSF, IL1B, and UPA genes were significantly higher (P < 0.05 or P < 0.01) in PTC specimens also when compared with FTCs. PTC and FTC have an identical origin, but molecular and clinical features are different.
To explain differences in expression levels among the clinical PTC cases (see Fig. 4), we correlated them with clinical and molecular parameters (Table 2, which is published as supporting information on the PNAS web site). Seven patients displayed RET/PTC rearrangements including RET/PTC1 (three cases) and RET/PTC3 (four cases). TRKA rearrangement was present in one case, and BRAF mutations were present in six cases. Three cases did not have RET/TRKA rearrangements or mutations in BRAF (17). Clinicopathological parameters {sex, age, PTC subtype (25), tumor side, and TNM [description of primary tumor (T), the extent of the regional lymph node metastasis (N), and distant metastasis (M)] classification (26)} for each patient are indicated (Table 2). Tumor samples displaying RTK rearrangements (RET/PTCs and TRK) and extrathyroid spread and metastasis to regional lymph nodes (pT4N1) tended to express higher levels of CCL2, CXCR4, SELL, GM-CSF, MMP9, and UPA genes (Table 2 and Fig. 4), suggesting a role for these genes in locoregional spread. Further studies in larger case lists are needed to validate this (these) inflammatory signature(s) as a correlate of molecular pathogenesis and clinical aggressiveness.
Discussion
In this study, we show that the RET/PTC1 oncogene, exogenously expressed in normal primary human thyrocytes, directly induces the up-modulation of several inflammatory genes, including chemokines and their receptors, cytokines, matrix-degrading enzymes, and adhesion molecules. As schematically depicted in Fig. 5, these molecules may affect tumor progression and invasion of surrounding tissues.
Fig. 5.
Inflammation/invasion molecules with specified functions triggered by RET/PTC1 oncogene in primary human thyrocyte.
There is accumulating evidence that the complex chemokine network present in human tumors influences both extent and phenotype of immune cell infiltration as well as several tumor properties. Here, we show that RET/PTC1 induces the expression of both CXCR4 and CXCL12, thus suggesting a possible autocrine loop. The CXCR4/CXCL12 axis may be involved in tumor cell proliferation and survival, as well as in the metastatic capacity of tumor cells (27). Indeed, we demonstrated that CXCR4-positive thyrocytes migrate in response to CXCL12.
Interestingly, all of the components of ELR+ CXC chemokine family (CXCL1, -2, -3, -5, -6, and -8), with proangiogenic activity, were strongly up-regulated in RET/PTC1-infected thyrocytes. Because the appropriate chemokine receptor (CXCR2) was not expressed (data not shown), a paracrine effect for these chemokines could be envisaged. Accordingly, in a mouse model of lung cancer, the protumor effect of ELR+ CXC chemokines was demonstrated to be mainly because of their action on nontumor cells (28). ELR+ chemokines could facilitate tumor development through the stimulation of blood vessel growth and recruitment of inflammatory cells, as recently demonstrated in RAS-transformed HeLa cells (29).
RNA and protein levels of the chemokines CCL2 and CCL20 were also up-regulated by RET/PTC1. CCL20 expression has been reported to be associated with the presence of immature dendritic cells in PTC, where, in general, they promote tumor progression (30). CCL2 is a major tumor-derived chemoattractant for blood monocytes (1), and established data demonstrate that CCL2 levels correlate with the macrophage contents of several tumor types (10). In addition, RET/PTC1 also induces the expression of myeloid CSFs, which not only recruit myeloid cells but also sustain their differentiation and survival (31). As documented by correlative studies, the presence of high numbers of macrophages at the tumor site is more frequently associated with poor prognosis (32).
Overall, the de novo expression of a large number of chemokines is a feature of RET/PTC-infected human thyrocytes. This finding is in line with the work of Melillo et al. (33) describing the up-regulation of some chemokines (CXCL1, CXCL10, and CCL2) and of CXCR4 in a rat thyroid cell line expressing RET/PTC (33-35) and suggesting a chemokine role in proliferation and invasion.
The induction of high levels of the serine protease UPA and UPAR, known to be overexpressed in 80% of PTC, is another putative autocrine loop triggered by RET/PTC1 expression. A number of findings suggest that the UPA system is causally involved at multiple steps in cancer progression, metastasis, and shortened survival in cancer patients. UPAR has been suggested to be induced by HGFR/MET, a protein overexpressed in PTC (36). In this paper, we found that RET/PTC oncogenes induce MET expression in thyrocytes, as reported in ref. 36, but that the induction is independent from RET-Y1062 multidocking site (data not shown) instead necessary for inducing UPA and UPAR.
Interestingly, at variance with the induction of the MET gene, the up-regulation of the majority of inflammatory molecules was strictly dependent on the presence of Tyr-451 (the Tyr-1062 multidocking site in proto-RET). Tyr-1062 has been demonstrated as necessary for the transforming activity of RET/PTC oncogenes (15), for the activation of NF-κB, a key player in inflammation (14), and for induction of CCL2 and GM-CSF by RET/PTC3 oncoprotein (35).
Another putative autocrine loop triggered by RET/PTC1 expression in our study is SPP1, a multifunctional secreted phosphoprotein, whose overexpression is associated with cell transformation, and one of its receptors, CD44. The expression of SPP1/CD44 has been shown to be triggered by RET/PTC oncogenes in rat thyroid cells and to sustain tumor cell proliferation and invasion (37).
Several MMPs and the dipeptidyl peptidase IV (DPPIV) were induced by RET/PTC1. The proposed pathogenic role of MMPs in cancer is tissue remodeling by degrading extracellular matrix (ECM) during invasive tumor growth and neoangiogenesis and, more recently, in processing molecules such as chemokines, chemokine receptors, and cytokines (38). Similarly, the transmembrane protease DPPIV has been implicated in regulating the activity of chemotactic peptides and appears to have a critical role in angiogenesis (39, 40). It is worth mentioning that DPPIV was recently suggested (41) as a molecular marker for differentiated thyroid carcinoma.
Finally, RET/PTC1 induced the adhesion molecule L-selectin. L-selectin plays an important role in homing of lymphocytes to lymphoid tissues, and it has also been shown to facilitate cancer metastasis (42). Thus, induction of L-selectin and CXCR4 by RET/PTC may arm PTC with molecular tools to disseminate to lymph nodes. The fact that many RET/PTC-induced molecules are known to interact with each other is in agreement with the scenario that different components of an inflammatory/invasive program are coordinately induced by this oncogene.
Selected molecules induced by RET/PTC1 in our in vitro model of PTC were also present in tumor specimens of PTC. In particular, expression levels of CCL20, CXCR4, SELL, GM-CSF, MMP9, UPA, and SPP1 were significantly higher in PTC specimens compared with normal thyroid tissues. CCL20, CCL2, CXCL8, SELL, GM-CSF, IL1B, and UPA expression levels were significantly higher in PTC also when compared with FTC specimens. Furthermore, among different PTC specimens, enhanced expression of inflammatory genes was more frequently seen in tumors positive for RTK rearrangement (RET/PTC1, RET/PTC3, and TRK), rather than in BRAF-positive cases. Profiling in PTC tumors carrying RTK rearrangements or BRAF mutations already showed a different set of gene expression in these tumors (17). Finally, tumors with more aggressive clinical behavior (pT4N1 presentation) tended to express higher levels of selected inflammatory molecules CCL2, CXCR4, SELL, GM-CSF, MMP9, and UPA, suggesting a role for these molecules in locoregional spread, a pathological characteristic of PTC. However, further studies in larger case lists are needed to validate this inflammatory signature as a correlate of molecular pathogenesis and clinical aggressiveness.
In conclusion, the RET/PTC1 oncogene activates in normal human primary thyrocytes a transcriptional program related to inflammation. RET/PTC1-induced genes were also expressed in specimens of PTC, particularly those with rearranged RTK and pT4N1 presentation, thus validating the in vivo relevance of the in vitro identified profile. These results establish a direct connection between a transforming oncogene involved in the pathogenesis of a human tumor and the activation of a proinflammatory program in the primary human cell type originating that tumor. Of note, PTC is associated, in ≈30% of cases, with chronic inflammatory thyroiditis (43). The set of inflammation-related genes activated by RET/PTC1 in human thyrocytes is therefore likely to contribute to tumor progression and locoregional metastasis, characteristic of this type of thyroid cancer.
Supplementary Material
Acknowledgments
We thank Loris De Cecco, James F. Reid, and Simone Minardi for their contribution to gene profiling. We also thank Mrs. Maria Grazia Rizzetti for technical assistance and Mrs. Cristina Mazzadi for secretarial help. This work was supported by the Associazione Italiana per la Ricerca sul Cancro.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PTC, papillary thyroid carcinoma; RTK, receptor tyrosine kinase; FTC, follicular thyroid carcinomas; UPA, urokinase-type plasminogen activator; UPAR, UPA receptor; MMPs, metalloproteases; Q-PCR, quantitative PCR.
Data deposition: The gene expression data have been deposited at the ArrayExpress database (accession no. E-MEXP-429).
References
- 1.Balkwill, F. & Mantovani, A. (2001) Lancet 357, 539-545. [DOI] [PubMed] [Google Scholar]
- 2.Coussens, L. M. & Werb, Z. (2002) Nature 420, 860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal, B. B. (2004) Cancer Cell 6, 203-208. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill, F., Charles, K. A. & Mantovani, A. (2005) Cancer Cell 3, 211-217. [DOI] [PubMed] [Google Scholar]
- 5.Voronov, E., Shouval, D. S., Krelin, Y., Cagnano, E., Benharroch, D., Iwakura, Y., Dinarello, C. A. & Apte, R. N. (2003) Proc. Natl. Acad. Sci. USA 100, 2645-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vidal-Vanaclocha, F., Alvarez, A., Asumendi, A., Urcelay, B., Tonino, P. & Dinarello, C. A. (1996) J. Natl. Cancer Inst. 88, 198-205. [DOI] [PubMed] [Google Scholar]
- 7.Greten, F. R., Eckmann, L., Greten, T. F., Park, J. M., Li, Z. W., Egan, L. J., Kagnoff, M. F. & Karin, M. (2004) Cell 118, 285-296. [DOI] [PubMed] [Google Scholar]
- 8.Pikarsky, E., Porat, R. M., Stein, I., Abramovitch, R., Amit, S., Kasem, S., Gutkovich-Pyest, E., Urieli-Shoval, S., Galun, E. & Ben Neriah, Y. (2004) Nature 431, 461-466. [DOI] [PubMed] [Google Scholar]
- 9.Moore, R. J., Owens, D. M., Stamp, G., Arnott, C., Burke, F., East, N., Holdsworth, H., Turner, L., Rollins, B., Pasparakis, M., et al. (1999) Nat. Med. 5, 828-831. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. (2002) Trends Immunol. 23, 549-555. [DOI] [PubMed] [Google Scholar]
- 11.From, G., Mellemgaard, A., Knudsen, N., Jorgensen, T. & Perrild, H. (2000) Thyroid 10, 697-700. [DOI] [PubMed] [Google Scholar]
- 12.Alberti, L., Carniti, C., Miranda, C., Roccato, E. & Pierotti, M. A. (2003) J. Cell. Physiol. 195, 168-186. [DOI] [PubMed] [Google Scholar]
- 13.Fusco, A., Grieco, M., Santoro, M., Berlingieri, M. T., Pilotti, S., Pierotti, M. A., Della Porta, G. & Vecchio, G. (1987) Nature 328, 170-172. [DOI] [PubMed] [Google Scholar]
- 14.Arighi, E., Borrello, M. G. & Sariola, H. (2005) Cytokine Growth Factor Rev. 16, 441-467. [DOI] [PubMed] [Google Scholar]
- 15.Mercalli, E., Ghizzoni, S., Arighi, E., Alberti, L., Sangregorio, R., Radice, M. T., Gishizky, M. L., Pierotti, M. A. & Borrello, M. G. (2001) Oncogene 20, 3475-3485. [DOI] [PubMed] [Google Scholar]
- 16.Kimura, E. T., Nikiforova, M. N., Zhu, Z., Knauf, J. A., Nikiforov, Y. E. & Fagin, J. A. (2003) Cancer Res. 63, 1454-1457. [PubMed] [Google Scholar]
- 17.Frattini, M., Ferrario, C., Bressan, P., Balestra, D., De Cecco, L., Mondellini, P., Bongarzone, I., Collini, P., Gariboldi, M., Pilotti, S., et al. (2004) Oncogene 23, 7436-7440. [DOI] [PubMed] [Google Scholar]
- 18.Ciampi, R., Knauf, J. A., Kerler, R., Gandhi, M., Zhu, Z., Nikiforova, M. N., Rabes, H. M., Fagin, J. A. & Nikiforov, Y. E. (2005) J. Clin. Invest. 115, 94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viglietto, G., Chiappetta, G., Martinez-Tello, F. J., Fukunaga, F. H., Tallini, G., Rigopoulou, D., Visconti, R., Mastro, A., Santoro, M. & Fusco, A. (1995) Oncogene 11, 1207-1210. [PubMed] [Google Scholar]
- 20.Santoro, M., Melillo, R. M., Grieco, M., Berlingieri, M. T., Vecchio, G. & Fusco, A. (1993) Cell Growth Differ. 4, 77-84. [PubMed] [Google Scholar]
- 21.Jhiang, S. M., Sagartz, J. E., Tong, Q., Parker-Thornburg, J., Capen, C. C., Cho, J. Y., Xing, S. & Ledent, C. (1996) Endocrinology 137, 375-378. [DOI] [PubMed] [Google Scholar]
- 22.Fischer, A. H., Bond, J. A., Taysavang, P., Battles, O. E. & Wynford-Thomas, D. (1998) Am. J. Pathol. 153, 1443-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finocchiaro, G., Parise, P., Minardi, S. P., Alcalay, M. & Muller, H. (2004) Bioinformatics 20, 3670-3672. [DOI] [PubMed] [Google Scholar]
- 24.Cuccuru, G., Lanzi, C., Cassinelli, G., Pratesi, G., Tortoreto, M., Petrangolini, G., Seregni, E., Martinetti, A., Laccabue, D., Zanchi, C., et al. (2004) J. Natl. Cancer Inst. 96, 1006-1014. [DOI] [PubMed] [Google Scholar]
- 25.DeLellis, R. A., Lloyd, R. V. & Heitz, P. U. (2004) in Pathology and Genetics of Tumor Endocrine Organs, World Health Organization Classification of Tumors, eds. DeLellis, R. A., Lloyd, R. V., Heitz, P. U. & Eng, C. (IARC, Oxford), Vol. 8, pp. 57-66. [Google Scholar]
- 26.Sobin, L. H. & Fleming, I. D. (1997) Cancer 80, 1803-1804. [DOI] [PubMed] [Google Scholar]
- 27.Balkwill, F. (2004) Nat. Rev. Cancer 4, 540-550. [DOI] [PubMed] [Google Scholar]
- 28.Keane, M. P., Belperio, J. A., Xue, Y. Y., Burdick, M. D. & Strieter, R. M. (2004) J. Immunol. 172, 2853-2860. [DOI] [PubMed] [Google Scholar]
- 29.Sparmann, A. & Bar-Sagi, D. (2004) Cancer Cell 6, 447-458. [DOI] [PubMed] [Google Scholar]
- 30.Scarpino, S., Stoppacciaro, A., Ballerini, F., Marchesi, M., Prat, M., Stella, M. C., Sozzani, S., Allavena, P., Mantovani, A. & Ruco, L. P. (2000) Am. J. Pathol. 156, 831-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollard, J. W. (2004) Nat. Rev. Cancer 4, 71-78. [DOI] [PubMed] [Google Scholar]
- 32.Bingle, L., Brown, N. J. & Lewis, C. E. (2002) J. Pathol. 196, 254-265. [DOI] [PubMed] [Google Scholar]
- 33.Melillo, R. M., Castellone, M. D., Guarino, V., De Falco, V., Cirafici, A. M., Salvatore, G., Caiazzo, F., Basolo, F., Giannini, R., Kruhoffer, M., et al. (2005) J. Clin. Invest. 115, 1068-1081. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Castellone, M. D., Guarino, V., De Falco, V., Carlomagno, F., Basolo, F., Faviana, P., Kruhoffer, M., Orntoft, T., Russell, J. P., Rothstein, J. L., et al. (2004) Oncogene 23, 5958-5967. [DOI] [PubMed] [Google Scholar]
- 35.Russell, J. P., Shinohara, S., Melillo, R. M., Castellone, M. D., Santoro, M. & Rothstein, J. L. (2003) Oncogene 22, 4569-4577. [DOI] [PubMed] [Google Scholar]
- 36.Ruco, L. P., Stoppacciaro, A., Ballarini, F., Prat, M. & Scarpino, S. (2001) J. Pathol. 194, 4-8. [DOI] [PubMed] [Google Scholar]
- 37.Castellone, M. D., Celetti, A., Guarino, V., Cirafici, A. M., Basolo, F., Giannini, R., Medico, E., Kruhoffer, M., Orntoft, T. F., Curcio, F., et al. (2004) Oncogene 23, 2188-2196. [DOI] [PubMed] [Google Scholar]
- 38.Stamenkovic, I. (2003) J. Pathol. 200, 448-464. [DOI] [PubMed] [Google Scholar]
- 39.Bauvois, B. (2004) Oncogene 23, 317-329. [DOI] [PubMed] [Google Scholar]
- 40.Van Damme, J., Struyf, S., Wuyts, A., Van Coillie, E., Menten, P., Schols, D., Sozzani, S., De Meester, I. & Proost, P. (1999) Chem. Immunol. 72, 42-56. [PubMed] [Google Scholar]
- 41.Huang, Y., Prasad, M., Lemon, W. J., Hampel, H., Wright, F. A., Kornacker, K., LiVolsi, V., Frankel, W., Kloos, R. T., Eng, C., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 15044-15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian, F., Hanahan, D. & Weissman, I. L. (2001) Proc. Natl. Acad. Sci. USA 98, 3976-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosai, J., Carcangiu, M. L. & DeLellis, R. A. (1992) in Tumors of the Thyroid Gland, Atlas of Tumor Pathology (Armed Forces Institute of Pathology, Washington, DC), 3rd Ed., pp. 161-182.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.