Abstract
Nitric oxide (NO) is a small uncharged free radical that is involved in diverse physiological and pathophysiological mechanisms. NO is generated by three isoforms of NO synthase, endothelial, neuronal, and inducible ones. When generated in vascular endothelial cells, NO plays a key role in vascular tone regulation, in particular. Here, we describe an amplifier-coupled fluorescent indicator for NO to visualize physiological nanomolar dynamics of NO in living cells (detection limit of 0.1 nM). This genetically encoded high-sensitive indicator revealed that ≈1 nM of NO, which is enough to relax blood vessels, is generated in vascular endothelial cells even in the absence of shear stress. The nanomolar range of basal endothelial NO thus revealed appears to be fundamental to vascular homeostasis.
Keywords: fluorescence resonance energy transfer, genetic encoding
Since NO was found to be an endothelium-derived relaxing factor in 1987 (1, 2), it is now well established that NO is a ubiquitous messenger not only for vascular homeostasis but also for neurotransmission and immune systems (3). For the detection of NO, several methods have been devised so far. However, convincing methods are not currently available for visualizing NO dynamics in single living cells that have enough sensitivity (nM) and spatial resolution (sub-μm). Bioimaging of NO has been reported based on chemiluminescence (4) and ESR (5); however, those methods have disadvantages for the functional analysis of NO, such as the use of cytotoxic H2O2 or low spatial resolution. Electrochemical sensing using microelectrodes provides real-time detection of the nanomolar range of NO (6). However, available spatial information is limited to the electrode area and its positioning. Fluorescence methods are generally superior for bioimaging of molecular events in single living cells in terms of their high sensitivity, high spatial resolution (d0 ≅ λ/2), and experimental feasibility (7). Several organic fluorescent indicators have already been developed for bioimaging of NO, such as diamino-fluoresceins (8), diaminorhodamines (9), diamino-boron dipyrromethenes (10), and diamino-cyanines (11). However, these organic dyes covalently react with NO+ but not with NO radical in the presence of dioxygen and are therefore not reversible sensors for NO. The sensitivity of the organic dyes in living cells has not been determined. The organic dyes easily accumulate in subcellular membranes and emit fluorescence signals there in an NO-independent manner. This membrane accumulation of the dyes substantially increases background signals and interferes with the detection of physiologic low concentration of NO in living cells. As to the concentration of cellular NO, the nanomolar range of NO is believed to be physiologically important for exerting its action. To overcome the limitations of previous fluorescent dyes, we have developed a genetically encoded fluorescent indicator for NO that reversibly detects NO with a high sensitivity (detection limit of 0.1 nM) and visualizes the nanomolar dynamics of NO in single living cells.
Materials and Methods
Materials. 8-Bromoguanosine-cGMP (8-Br-cGMP), Nω-nitro-l-arginine methyl ester (l-NAME), 4H-8-bromo-1,2,4-oxadiazolo[3,4-d]benz[1,4]oxazin-1-one (NS 2028), 1,4-dihydro-5-(2-propoxyphenyl)-7H-1,2,3-triazolo(4,5-d)pyrimidin-7-one (zaprinast), and bradykinin were purchased from Sigma. Hanks' balanced salt solution was obtained from Invitrogen. 3-(2-Hydroxy-1-methylethyl-2-nitrosohydrazino)-N-methyl-1-propanamine (NOC-7) was obtained from Wako Pure Chemical (Osaka). Bovine pulmonary artery endothelial cells were obtained from the Japan Collection of Research Bioresources (Osaka).
Plasmid Construction. To construct cDNAs encoding soluble guanylate cyclase (sGC) α-CGY and sGCβ-CGY, each stop codon of sGCα and sGCβ was deleted and a linker sequence, GGEQKLI-SEEDLLESR, was added to each C terminus of sGCα and sGCβ by using PCR. Each cDNA encoding sGCα and sGCβ was connected with cDNA encoding a fluorescent cGMP indicator, CGY. Each cDNA encoding sGCα-CGY and sGCβ-CGY was subcloned at the NheI and NotI sites of a mammalian expression vector, pcDNA3.1(+) (Invitrogen).
Cell Culture and Transfection. CHO-K1 cells were cultured in Ham's F-12 medium supplemented with 10% FCS and 1% penicillin/streptomycin at 37°C in 5% CO2. Vascular endothelial cells were cultured in Eagle's MEM supplemented with 20% FCS, 1% penicillin/streptomycin, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids at 37°C in 5% CO2. Cells were plated onto glass-bottomed dishes, transfected with Lipofectamine 2000 reagent, and left for 24 h at 37°C in 5% CO2.
Preparation of NO Donor, NO, and CO Solutions. NOC-7 is an NO-donating reagent that releases 2 mol of NO per mole. Various concentrations of NOC-7 were separately dissolved in PBS (pH 7.4). To prepare saturated NO solution (2 mM), PBS was bubbled with argon gas for 1 h and then bubbled with NO gas for 1 h. Solutions containing various concentrations of NO were prepared by diluting the saturated NO solution with the deaerated PBS. CO-saturated solution (1 mM) was prepared by bubbling the deaerated PBS with CO gas for 1 h. Solutions containing various concentrations of CO were prepared by diluting the saturated CO solution.
Imaging of Cells. The culture medium was replaced with Hanks' balanced salt solution for fluorescence imaging. As described (12, 13), the cells were imaged at 25°C on a Zeiss Axiovert 200 microscope with a cooled charge-coupled device camera, Cool-SNAP HQ (Roper Scientific, Tucson, AZ), controlled by metafluor (Universal Imaging, West Chester, PA). The exposure time at 440 ± 10-nm excitation was 100 ms. Fluorescence images were obtained through 480 ± 15-nm and 535 ± 12.5-nm filters with a ×40 oil immersion objective (Zeiss). Endothelial cells were exposed to shear stress by applying lateral flow of Hanks' balanced salt solution to the cells with a roller/tube pump.
Results
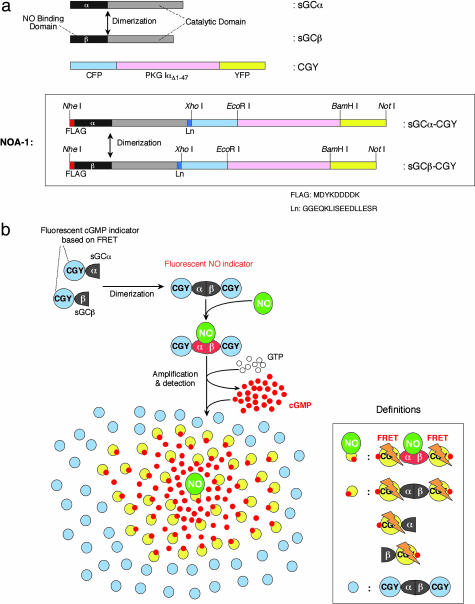
Design of the Present Fluorescent Indicator for NO. NO binds to its receptor protein, sGC, thereby mediating many of effects of NO in its function as a signaling molecule (14, 15). This sGC is a heterodimer consisting of α- and β-subunits (sGCα and sGCβ). sGCβ contains an N-terminal heme-binding region, to which NO reversibly binds. Both sGCα and sGCβ share a C-terminal cyclase domain. After binding of NO to the heme group, the cyclase activity is stimulated up to 400-fold, resulting in the repeated conversion of GTP to cGMP (14, 16). Dimerization of sGCα and sGCβ that spontaneously occurs is required for the NO-dependent stimulation of the cyclase activity. To develop a genetically encoded NO indicator, we designed two chimera proteins (Fig. 1a). One is sGCα connected with a fluorescent cGMP indicator protein that we have previously developed and named CGY (12), and the other is sGCβ connected with CGY (Fig. 1a). This CGY contains a major part of cGMP-dependent protein kinase (PKGIαΔ1–47) having two cGMP-binding sites and thus binds with two molecules of cGMP (Fig. 1a). The CGY bound with two cGMP molecules emits a fluorescence signal based on FRET from cyan fluorescent protein (CFP) to yellow fluorescent protein (YFP), which are, respectively, attached at the N and C terminus of PKGIαΔ1–47 in CGY. When expressed in living cells, sGCα-CGY and sGCβ-CGY are spontaneously associated to form a matured heterodimer as well as sGCα and sGCβ (Fig. 1). This heterodimer of sGCα-CGY and sGCβ-CGY binds with NO, generates cGMP at the rate of 3,000–6,000 molecules per min, and then emits a FRET signal from the CGY domain upon binding with four molecules of the generated cGMP (Fig. 1b). More interestingly, the residual cGMP molecules diffuse and bind to the CGY domain within NO-free heterodimers of sGCα-CGY and sGCβ-CGY surrounding the NO-bound heterodimer. Thus, a single NO molecule provokes FRET signals from a large number of the NO-free heterodimers through the residual cGMP molecules (Fig. 1b). Even if sGCα-CGY and sGCβ-CGY exist as monomers because of their unbalanced expression in living cells, the monomers emit FRET signals upon binding with the residual cGMP as well as the heterodimers of sGCα-CGY and sGCβ-CGY. That is why the two chimera proteins, sGCα-CGY and sGCβ-CGY, actually reported the nanomolar concentration of NO in a reproducible fashion. We named the heterodimer of sGCα-CGY and sGCβ-CGY “NOA-1” (a fluorescent indicator for NO with a signal amplifier) (Fig. 1).
Fig. 1.
An amplifier-coupled fluorescent indicator for visualizing NO in single living cells. (a) Schematic representations of domain structures of sGCα, sGCβ, CGY, sGCα-CGY, and sGCβ-CGY. The amino acid sequence of FLAG tag and linker (Ln) is shown at the bottom. The heterodimer of sGCα-CGY and sGCβ-CGY has been named NOA-1. (b) Principle of the NO indicator NOA-1. sGCα-CGY and sGCβ-CGY are spontaneously associated to form a matured heterodimer, that is, NOA-1. NOA-1 binds with NO and generates cGMP at the rate of 3,000–6,000 molecules per min. Thus generated cGMP binds to the CGY domain in NOA-1 and makes NOA-1 emit a FRET signal. About 99.9% of cGMP molecules thus generated diffusely and bound to NO-free NOA-1. As a result, even a single NO molecule can trigger a large amount of NOA-1 to emit FRET signals. Even if sGCα-CGY and sGCβ-CGY exist as monomers, the monomers also emit FRET signals upon binding with generated cGMP.
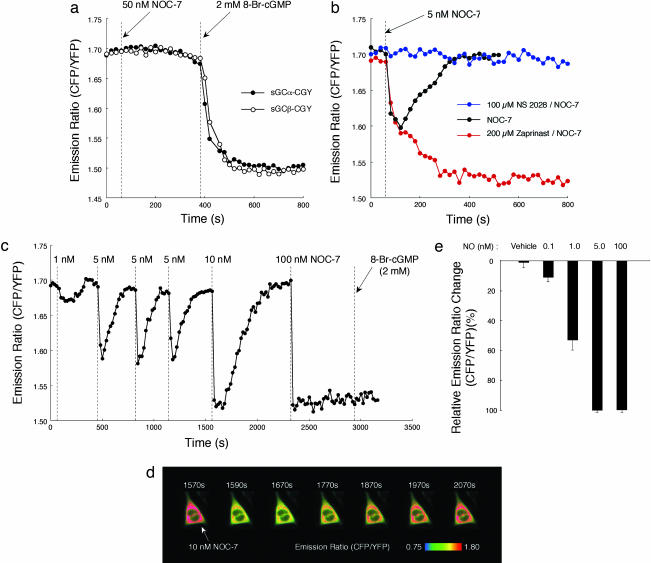
NOA-1 Reversibly Detects the Nanomolar Range of NO. First, we constructed cDNAs encoding sGCα-CGY and sGCβ-CGY and separately expressed them in CHO-K1 cells having a little amount of endogenous sGC. By tethering CGY, the expression of sGCα-CGY and sGCβ-CGY was up to 5-fold compared with recombinant sGCα and sGCβ, respectively (data not shown). When the cells expressed with sGCα-CGY and sGCβ-CGY were separately stimulated with an NO-releasing reagent, 50 nM NOC-7 (17), no significant change was observed in an emission ratio of CFP to YFP by exciting the cells at 440 ± 10 nm (Fig. 2a). This flat fluorescence time course shows no change in the FRET efficiency in the CGY domain. However, subsequent addition of a membrane-permeable analogue of cGMP, 2 mM 8-Br-cGMP, immediately induced FRET in the CGY domain, according to a quick decrease in the CFP/YFP emission ratio (Fig. 2a). This result indicates that sGCα-CGY alone and sGCβ-CGY alone do not have the catalytic cyclase activity to generate cGMP as expected, although the CGY domain works well as a cGMP indicator even when it is connected with sGCα or sGCβ. Next, we coexpressed both sGCα-CGY and sGCβ-CGY in CHO-K1 cells. When these cells were stimulated with 5 nM NOC-7, the CFP/YFP emission ratio immediately decreased but recovered up to the initial level in 300 s (Fig. 2b). The latter recovery of the decreased emission ratio disappeared when the cells were pretreated with an inhibitor of phosphodiesterases (18) that hydrolyses cGMP, 200 μM zaprinast (19) (Fig. 2b). The transient change in the emission ratio by 5 nM NOC-7, however, was completely blocked by pretreating the cells with an inhibitor of sGC, 100 μM 4H-8-bromo-1,2,4-oxadiazolo[3,4-d]benz[1,4]oxazin-1-one (NS 2028) (20) (Fig. 2b). These results indicate that when coexpressed in living cells sGCα-CGY and sGCβ-CGY form a functional heterodimer, namely NOA-1, that generates cGMP upon NO stimulation as expected. Also, cGMP molecules thus generated were shown to be hydrolyzed by endogenous phosphodiesterases in living cells.
Fig. 2.
NOA-1 reversibly sensitizes the nanomolar range of NO in single living cells. (a) Responses of sGCα-CGY (•) and sGCβ-CGY (•) for 50 nM NOC-7 and subsequent 2 mM 8-Br-cGMP stimulation in CHO-K1 cells. (b) Response of the heterodimer of sGCα-CGY and sGCβ-CGY to 5 nM NOC-7 in the absence (black circles) or the presence of 100 μM NS 2028 (blue circles) or 200 μM zaprinast (red circles). (c) Reversible response of NOA-1 for various concentrations of NOC-7. (d) Pseudocolor images of the CFP/YFP emission ratio of NOA-1 before (1,570 s) and after addition of 10 nM NOC-7. (e) Dose–response of NOA-1 for the nanomolar range of NO.
We next examined the FRET response of NOA-1 for various concentrations of NO. All of the imaging experiments were performed in an open system, in which added NO is easily removed from the extracellular media by its volatilization in addition to its oxidation. When the CHO-K1 cell expressed with NOA-1 was stimulated with 1 nM NOC-7, a small, but significant, change in the CFP/YFP emission ratio was transiently observed (Fig. 2c). Subsequent stimulation of the cell with 5 nM NOC-7 elicited an NO-dependent transient change in the emission ratio (Fig. 2c). This transient change in the CFP/YFP emission ratio with 5 nM NOC-7 was observed in a reproducible fashion when the cell was subsequently stimulated two more times with 5 nM NOC-7 (Fig. 2c). Also, upon addition of 10 nM NOC-7, a quick decrease was observed in the CFP/YFP emission ratio (Fig. 2 c and d). The emission ratio then reached a plateau emission ratio, which was sustained for ≈120 s, and recovered up to the basal level in 500 s (Fig. 2 c and d). The CFP/YFP emission ratio reached the plateau as well immediately after the addition of 100 nM NOC-7; however, it did not subsequently show significant recovery for at least 10 min (Fig. 2c). The plateau responses obtained by 100 nM NOC-7 and partly by 10 nM NOC-7 stimulations are caused by the saturation of the CGY domain in NOA-1 by the generated cGMP molecules; judging from that 2 mM 8-Br-cGMP did not further decrease the plateau emission ratio (Fig. 2c). We further confirmed the NO-dependent FRET response of NOA-1 with NO solutions prepared by bubbling NO gas. Fig. 2e shows the averaged peak response of NOA-1 at each concentration of NO in CHO-K1 cells. The NOA-1 was thus confirmed to detect the nanomolar range of NO (detection limit of 0.1 nM). Importantly, NOA-1 senses not only an increase in the NO concentration but also its removal by oxidation and/or volatilization (Fig. 2c). This NO-dependent reversible response of NOA-1 is caused by the reversible binding of NO to the heme group in NOA-1 and endogenous phosphodiesterases that immediately hydrolyze the generated cGMP molecules after the removal of NO.
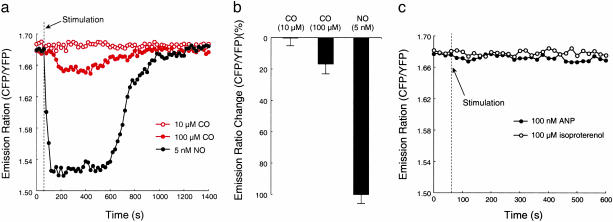
Selectivity of NOA-1. As to the selectivity of NOA-1, several factors such as carbon monoxide (CO) may affect the FRET response of NOA-1. We first examined the response of NOA-1 for CO. When CHO-K1 cells expressed with NOA-1 were stimulated with CO up to 10 μM, we observed no significant change in the CFP/YFP emission ratio (Fig. 3 a and b). At 100 μM CO, a transient but much smaller response of NOA-1 was observed than that for 5 nM NO (Fig. 3 a and b). CO is a sGC activator but has a lower affinity with sGC than NO. In addition, the CO-bound sGC is known to have much lower cyclase activity than NO-bound sGC because of each different coordination to the heme iron (15). Our result on the small response of NOA-1 to CO corresponds with previous biochemical studies. Physiologically generated CO thus appears not to affect the response of NOA-1.
Fig. 3.
Selectivity of NOA-1. (a) Time courses of NOA-1 response for 10 μM CO (red open circle), 100 μM CO (red closed circle), and 5 nM NO (black closed circle) in CHO-K1 cells. (b) Changes in the CFP/YFP emission ratio of NOA-1 upon stimulation with 10 μM CO, 100 μM CO, and 5 nM NO in CHO-K1 cells. (c) Time course of NOA-1 response for 100 nM atrial natriuretic peptide (ANP) or 100 μM isoproterenol in a CHO-K1 cell.
We also examined whether natriuretic peptide stimulation or cAMP generation affects the FRET response of NOA-1. Natriuretic peptides may affect the FRET response of NOA-1 because their receptors also possess the cyclase domains to generate cGMP. However, when several cultured cell types including CHO-K1 cells were stimulated with excess concentration of atrial natriuretic peptide, we observed no significant change in the CFP/YFP emission ratio of NOA-1 (Fig. 3c). The same was observed for cAMP generation. When several cultured cell types, including CHO-K1 cells, were stimulated with excess concentration of isoproterenol to generate cAMP, we observed no significant change in the CFP/YFP emission ratio of NOA-1 (Fig. 3c). These results indicate that the natriuretic peptide stimulation and cAMP generation do not affect the physiologic response of NOA-1 for NO, because the expression of NOA-1 accompanies the overexpression of the NO receptor domain but does not accompany the overexpression of natriuretic peptide receptors. Also, its has previously been shown that the CGY domain in NOA-1 does not to detect the physiologic concentration of cAMP because of its weak affinity with cAMP (12).
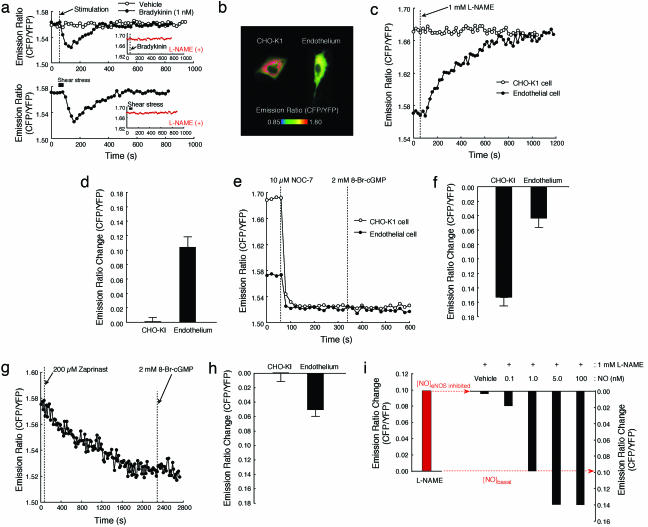
Imaging NO in Vascular Endothelial Cells. We next applied NOA-1 to measure the nanomolar range of NO in vascular endothelial cells. NOA-1 was expressed in endothelial cells from bovine pulmonary artery cultured in a serum-supplemented media. When the endothelial cells were stimulated with a physiologic concentration of vasoactive hormone, 1 nM bradykinin, a transient change in the CFP/YFP emission ratio was observed (Fig. 4a). We also observed a transient change in the emission ratio by applying shear stress that mimics the blood streaming on the endothelial cells (Fig. 4a). In contrast, when the cells were pretreated with an inhibitor for nitric oxide synthase (NOS), 1 mM l-NAME (21), both the response of NOA-1 for bradykinin and that for shear stress disappeared (Fig. 4a). This result confirms that NOA-1 enables the detection of transient generation of NO in vascular endothelial cells upon physiologic stimuli, such as the vasoactive hormone and shear stress.
Fig. 4.
Vascular endothelial cells stably generate the nanomolar range of NO. (a) Response of NOA-1 for NO transiently generated upon 1 nM bradykinin and that upon shear stress in vascular endothelial cells. When the cells were pretreated with 1 mM l-NAME, both the response of NOA-1 for bradykinin and that for shear stress disappeared (Insets). (b) Pseudocolor images of the CFP/YFP emission ratio in CHO-K1 and endothelial cells that are expressed with NOA-1. (c) Time course of NOA-1 response in a CHO-K1 cell and an endothelial cell upon 1 mM l-NAME stimulation. (d) Changes in the CFP/YFP emission ratio of NOA-1 upon 1 mM l-NAME stimulation in CHO-K1 and endothelial cells. (e) Time courses of NOA-1 response in CHO-K1 cell (•) and endothelial cell (•) upon 10 μM NOC-7 and subsequent 2 mM 8-Br-cGMP stimulation. (f) Changes in the CFP/YFP emission ratio of NOA-1 in CHO-K1 (•) and endothelial cells (•) upon 10 μM NOC-7 stimulation. (g) Time course of NOA-1 response upon 200 μM zaprinast and subsequent 2 mM 8-Br-cGMP stimulation in an endothelial cell. (h) Changes in the CFP/YFP emission ratio of NOA-1 upon 200 μM zaprinast stimulation in CHO-K1 and endothelial cells. (i) Basal concentration of NO stably generated in a vascular endothelial cell was measured by in situ calibration of NOA-1 for NO. The endothelial cell was treated in advance with 1 mM l-NAME to remove the basal NO, and various concentrations of NO were subsequently added to the endothelial cell. Solutions containing various concentrations of NO were prepared by diluting the saturated NO solution prepared by bubbling NO gas.
A remarkable observation, however, was obtained by comparing emission profiles of NOA-1 in endothelial cells with those in nonendothelial CHO-K1 cells. The CFP/YFP emission ratio of NOA-1 was found significantly lower in vascular endothelial cells than that in CHO-K1 cells (Fig. 4b). It has not escaped our notice that this difference in the emission ratio may represent the difference in basal NO concentrations between the endothelial and nonendothelial CHO-K1 cells. We first treated both the endothelial and CHO-K1 cells with l-NAME and observed the change in the CFP/YFP emission ratio of NOA-1. The emission ratio showed no significant change in the nonendothelial CHO-K1 cells probably because of the lack of endogenous endothelial NOS (eNOS) (Fig. 4 c and d). In contrast, the emission ratio gradually increased in the endothelial cells and reached nearly the same level as that in the CHO-K1 cells (Fig. 4 c and d). We next compared the response of NOA-1 for excess NOC-7 between these cells. We stimulated the endothelial and CHO-K1 cells with 10 μM NOC-7, which generates enough concentration of NO to immediately saturate the NOA-1 response. Although the basal emission ratios were different between these cells as remarked above, the emission ratios immediately decreased upon stimulation with 10 μM NOC-7 and reached nearly the same plateau level in both of these cells (Fig. 4e). As a result, in endothelial cells, the obtained change in the emission ratio upon the excess NOC-7 stimulation was actually only one-third of that in CHO-K1 cells (Fig. 4f). These results demonstrate that the nanomolar range of basal NO is stably generated in vascular endothelial cells that are cultured in the serum-supplemented media. Also, the results indicate that approximately two-thirds of the expressed NOA-1 is involved in the detection of the basal NO in the endothelial cells.
When endothelial cells expressed with NOA-1 were treated with 200 μM zaprinast, the CFP/YFP emission ratio gradually decreased and reached the saturation of NOA-1 response (Fig. 4g). In contrast, NOA-1 in CHO-K1 showed no significant change in the emission ratio upon treatment with zaprinast, although the CHO-K1 cells endogenously expressed phosphodiesterases as well (Fig. 4h). This result also supports the generation of basal NO in the endothelial cells.
We then measured the basal concentration of NO in each vascular endothelial cell. We first inhibited endogenous eNOS with 1 mM l-NAME to remove the basal NO and cGMP generated by the basal NO. The CFP/YFP emission ratios before and after the inhibition of endogenous eNOS, respectively, represent the basal and zero concentrations of NO in each endothelial cell (Fig. 4i). To quantitate the basal concentration of NO, we subsequently added various concentrations of NO to each endothelial cell, in which eNOS activity was inhibited with l-NAME. The peak response of NOA-1 was plotted at each concentration of NO (Fig. 4i). Based on the obtained dose–response of NOA-1 for various concentrations of NO, we measured ≈1 nM of the basal NO concentration generated in each endothelial cell.
Discussion
In the present study, we have developed a genetically encoded fluorescent indicator, NOA-1, to visualize NO in living cells. Because of its amplifier-coupled mechanism, NOA-1 exhibited a high sensitivity to the nanomolar range of NO (detection limit of 0.1 nM). Because the sensitivities of the organic dyes, diamino-fluoresceins (8), diamino-rhodamines (9), diamino-boron dipyrromethenes (10), and diamino-cyanines (11), have not been determined in living cells, it is impossible to exactly compare the sensitivity of NOA-1 with that of the organic dyes. However, 100 μM of an NO donor, NOC-13, is reported to elicit an approximate half-maximal response of the latest organic dye, diamino-cyanine-P, in rat kidney cells (11). In contrast, we tested that 5 nM NOC-7 provoked an approximate half-maximal response of NOA-1 in living cells (Fig. 2c). Both NOC-13 and NOC-7 generated 2 mole/mole of NO in nearly the same time course. On the basis of these results, NOA-1 appears to have ≈10,000-fold higher sensitivity than the latest organic dye in living cells. Furthermore, in contrast to previous organic dyes, NOA-1 exhibited the reversible response to NO in living cells, because the reversible binding of NO to NOA-1 and endogenous phosphodiesterases that immediately hydrolyze the generated cGMP molecules guarantees this reversible response of NOA-1 to NO in living cells. The reversible response of NOA-1 enabled us to calibrate NOA-1 for various NO concentrations in each living cell after removal of the basal NO. The reversible property thus provided a quantitative basis of NOA-1. In addition, NOA-1 detects biologically important NO radicals; however, previous organic dyes did not detect the NO radical but detected its metabolite, NO+. The superiority of NOA-1 to other organic dyes in terms of sensitivity and reversibility, in particular, has contributed to understanding the biology of the nanomolar range of NO in vascular endothelial cells.
Using NOA-1, we have found that ≈1 nM basal NO is actually generated in vascular endothelial cells cultured in the serum-supplemented media. Basal NO is obviously distinct from the well studied endothelial NO transiently generated upon shear stress. Considering the effective NO concentration required for the relaxation of vascular rings, our measurement of ≈1 nM basal endothelial NO indicates that vascular endothelial cells stably generate enough concentration of NO to relax blood vessels. In addition to the recognized NO generation upon shear stress (22), the basal generation of endothelial NO is thus suggested to play a critical role in the regulation of vascular tone. Endothelial NO also regulates vascular remodeling and angiogenesis and inhibits platelet aggregation, leukocyte activation, and smooth muscle cell proliferation (23). Its decreased bioavailability causes the pathophysiological disorder in vascular wall morphology associated with hypertension and atherosclerosis (24, 25). The failure of physiologic basal NO may represent a major determinant of cardiovascular diseases.
From a methodological viewpoint, it should be noted that the dose–response of NOA-1 is tunable by changing factors related to its FRET response to NO, such as catalytic activity of the cyclase domain and affinity for cGMP of the CGY domain. We have actually succeeded in shifting the dose–response of NOA-1 to that for higher NO by replacing the CGY domain with its lower-affinity variant (see Supporting Text and Fig. 5, which are published as supporting information on the PNAS web site). A lower affinity variant of NOA-1 has escaped from the basal concentration of NO but showed a quick response to NO generated upon stimulation with bradykinin (see Supporting Text and Fig. 5). This tunable property of NOA-1 is advantageous because various cell types such as endothelial cells, neurons, and macrophages generate each different range of NO concentration to be detected.
Supplementary Material
Acknowledgments
We thank Dr. M. Nakane (Abbott Laboratories) for providing cDNAs encoding sGCα and sGCβ and Y. Imai for providing experimental help. This work was supported by Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Agency and grants from the Ministry of Education, Science, and Culture of Japan (to Y.U.), the Takeda Science Foundation (to M.S.), and the Sumitomo Foundation (to M.S.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: 8-Br-cGMP, 8-bromoguanosine-cGMP; l-NAME, Nω-nitro-l-arginine methyl ester; NOC-7, 3-(2-hydroxy-1-methylethyl-2-nitrosohydrazino)-N-methyl-1-propanamine; sGC, soluble guanylate cyclase; CFP, cyan fluorescent protein; YFP, yellow fluorescent protein; NOS, NO synthase; eNOS, endothelial NOS.
References
- 1.Palmer, R. M., Ferrige, A. G. & Moncada, S. (1987) Nature 327, 524-526. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro, L. J., Buga, G. M., Wood, K. S., Byrns, R. E. & Chaudhuri, G. (1987) Proc. Natl. Acad. Sci. USA 84, 9265-9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ignarro, L. J. (2000) Nitric Oxide: Biology and Pathobiology (Academic, New York).
- 4.Leone, A. M., Furst, V. W., Foxwell, N. A., Cellek, S. & Moncada, S. (1996) Biochem. Biophys. Res. Commun. 221, 37-41. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura, T., Yokoyama, H., Fujii, S., Takayama, F., Oikawa, K. & Kamada, H. (1996) Nat. Biotechnol. 14, 992-994. [DOI] [PubMed] [Google Scholar]
- 6.Malinski, T., Mesaros, S. & Tomboulian, P. (1996) Methods Enzymol. 268, 58-69. [DOI] [PubMed] [Google Scholar]
- 7.Nagano, T. & Yoshimura, T. (2002) Chem. Rev. 102, 1235-1269. [DOI] [PubMed] [Google Scholar]
- 8.Kojima, H., Nakatsubo, N., Kikuchi, K., Kawahara, S., Kirino, Y., Nagoshi, H., Hirata, Y. & Nagano, T. (1998) Anal. Chem. 70, 2446-2453. [DOI] [PubMed] [Google Scholar]
- 9.Kojima, H., Hirotani, M., Nakatsubo, N., Kikuchi, K., Urano, Y., Higuchi, T., Hirata, Y. & Nagano, T. (2001) Anal. Chem. 73, 1967-1973. [DOI] [PubMed] [Google Scholar]
- 10.Gabe, Y., Urano, Y., Kikuchi, K., Kojima, H. & Nagano, T. (2004) J. Am. Chem. Soc. 126, 3357-3367. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki, E., Kojima, H., Nishimatsu, H., Urano, Y., Kikuchi, K., Hirata, Y. & Nagano, T. (2005) J. Am. Chem. Soc. 127, 3684-3685. [DOI] [PubMed] [Google Scholar]
- 12.Sato, M., Hida, N., Ozawa, T. & Umezawa, Y. (2000) Anal. Chem. 72, 5918-5924. [DOI] [PubMed] [Google Scholar]
- 13.Sato, M., Ueda, Y., Takagi, T. & Umezawa, Y. (2003) Nat. Cell Biol. 5, 1016-1022. [DOI] [PubMed] [Google Scholar]
- 14.Krumenacker, J. S., Hanafy, K. A. & Murad, F. (2004) Brain Res. Bull. 62, 505-515. [DOI] [PubMed] [Google Scholar]
- 15.Koesling, D., Russwurm, M., Mergia, E., Mullershausen, F. & Friebe, A. (2004) Neurochem. Int. 45, 813-819. [DOI] [PubMed] [Google Scholar]
- 16.Katsuki, S., Arnold, W., Mittal, C. & Murad, F. (1977) J. Cyclic Nucleotide Res. 3, 23-35. [PubMed] [Google Scholar]
- 17.Hrabie, J. A., Klose, J. R., Wink, D. A. & Keefer, L. K. (1993) J. Org. Chem. 58, 1472-1476. [Google Scholar]
- 18.Francis, S. H., Turko, I. V. & Corbin, J. K. (2001) Prog. Nucleic Acid Res. Mol. Biol. 65, 1-52. [DOI] [PubMed] [Google Scholar]
- 19.Gillespie, P. G. & Beavo, J. A. (1989) Mol. Pharmacol. 36, 773-781. [PubMed] [Google Scholar]
- 20.Olesen, S. P., Drejer, J., Axelsson, O., Moldt, P., Bang, L., Nielsen-Kudsk, J. E. & Mulsch, A. (1998) Br. J. Pharmacol. 123, 299-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rees, D., Palmer, R. M., Schulz, R., Hodson, H. F. & Moncada, S. (1990) Br. J. Pharmacol. 101, 746-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boo, Y. C. & Jo, H. (2003) Am. J. Physiol. 285, C499-508. [DOI] [PubMed] [Google Scholar]
- 23.Moncada, S. & Higgs, A. (1993) N. Engl. J. Med. 329, 2002-2012. [DOI] [PubMed] [Google Scholar]
- 24.Zeiher, A. M. (1996) Lancet 348, s10-s12. [DOI] [PubMed] [Google Scholar]
- 25.Napoli, C. & Ignarro, L. J. (2001) Nitric Oxide 5, 88-97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.