Abstract
The radiation-induced bystander effect is defined as “the induction of biological effects in cells that are not directly traversed by a charged particle but are in close proximity to cells that are.” Although these bystander effects have been demonstrated with a variety of biological endpoints in both human and rodent cell lines (as well as in 3D tissue samples), the mechanism of the phenomenon is not known. Although gap junction communication and the presence of soluble mediator(s) are both known to play important roles in the bystander response, the precise signaling molecules have yet to be identified. By using the Columbia University charged particle beam in conjunction with a strip dish design, we show here that the cyclooxygenase-2 (COX-2, also known as prostaglandin endoperoxide synthase-2) signaling cascade plays an essential role in the bystander process. Treatment of bystander cells with NS-398, which suppresses COX-2 activity, significantly reduced the bystander effect. Because the critical event of the COX-2 signaling is the activation of the mitogen-activated protein kinase pathways, our finding that inhibition of the extracellular signal-related kinase phosphorylation suppressed bystander response further confirmed the important role of mitogen-activated protein kinase signaling cascade in the bystander process. These results provide evidence that the COX-2-related pathway, which is essential in mediating cellular inflammatory response, is the critical signaling link for the bystander phenomenon.
Ionizing radiation is a well established human carcinogen. Established dogma has relied on the assumption that DNA of the nucleus is the main target for radiation-induced genotoxicity and carcinogenesis. At doses above 50 millisievert, the radiation-induced cancer risk can be estimated based on the cancer incidence among the Japanese atomic bomb survivors (1, 2). At lower doses, because fewer cells are likely to be directly damaged, the deleterious effect of radiation is expected to decline proportionally as well. However, evidence accumulated over the past decade has indicated that both extranuclear targets and extracellular events may play an important role in determining the biological responses to ionizing radiation (3–6). A major paradigm shift in radiation biology in the last decade has resulted from work involving the bystander effect (see refs. 7 and 8 for a review), which could have an important impact on our thinking as well as immediate application in radiation protection.
Radiation-induced bystander effect is defined as the induction of biological effects in cells that are not directly traversed by a charged particle but are in close proximity to cells that are, or have received signals from, these irradiated cells. There is evidence that very low doses of α-particles induced clastogenic responses (principally sister chromatid exchanges) in both Chinese hamster ovary (CHO) and human fibroblast cultures at levels significantly higher than expected based on the number of cellular nuclei that had been traversed by a particle (3, 5, 9). In CHO cells irradiated with low doses of α particles where <1% of the nuclei were estimated to have been traversed by a particle, an increase in sister chromatid exchanges was observed in >30% of the cells (3). In other words, either cytoplasmic damages or signals received from an extracellular component may have modulated the observed genotoxic response. Although circumstantial evidence in support of a bystander effect appears to be consistent, direct proof of such extranuclear and extracellular effects is most convincingly demonstrated by using charged particle microbeams (4, 10–12).
Results from experiments using microbeam provide evidence that the bystander effects can be demonstrated by using a variety of endpoints of biological damage, including micronucleus induction, cell lethality, gene expression, mutation, and oncogenic transformation (10–15) in various human and rodent cell lines. Many of these bystander effects resembled those phenotypes seen in cells that are directly irradiated, albeit in the absence of any dose–response relationship. It has been shown, for example, that irradiation of 10% of a confluent human hamster hybrid (AL) cell population with a single α particle per nucleus results in a mutant yield similar to that observed when all of the cells in the population are irradiated (4). A similar observation has also been made by using primary human bronchial epithelial cells and incorporating G2 phase premature chromosome condensation as an endpoint (16). Furthermore, the use of AL cells that are dominant negative for connexin 43 and lack gap junction formation demonstrated an attenuated bystander mutagenic effect (4).
There is also evidence that media from irradiated culture (upon transferal to nonirradiated cells) can induce biological effects in the latter. Mothersill and Seymour first demonstrated a highly significant reduction in cloning efficiency in both nonirradiated normal as well as malignant epithelial cell lines that had received media from 60Co-γ-ray-irradiated cultures (17). These results suggested that irradiated cells secreted a cytotoxic factor into the culture medium, which was capable of killing nonirradiated cells. Furthermore, transferring media from low linear energy transfer-irradiated cultures to nonirradiated cells lead to increased levels of various bystander effects, such as cell killing (18–20), neoplastic transformation (21), and genomic instability (22). In contrast, in feeder layer culture, metabolic cooperation between cells of similar or different types increases the clonogenic survival of the nonirradiated cells by providing growth factors and matrix support and is considered a positive aspect of the bystander phenomenon.
Although the bystander effects have been well described over the past decade, the mechanisms of the process remain unclear. In subconfluent cultures, there is evidence that reactive oxygen species, nitric oxide, and cytokines such as TGFβ are involved in mediating the process (19, 23, 24). On the other hand, gap junction-mediated cell–cell communications have been shown to be critical in mediating bystander effects in confluent cultures of either human (25) or rodent cells (4, 10). It is likely that a combination of pathways involving both primary and secondary signaling processes is involved in producing a bystander process. In the present study, we used a strip dish method to demonstrate the induction of bystander hypoxanthine-guanine phosphoribosyltransferase (HPRT-) mutations among the nonirradiated primary human lung fibroblasts. Microarray analyses of differentially expressed genes among the bystander cells showed a 3-fold overexpression of cyclooxygenase-2 (COX-2). Furthermore, suppression of COX-2 activity in bystander cells by using the chemical inhibitor NS-398 significantly reduced the bystander effect. Because the critical event of the COX-2 signaling pathway is activation of the mitogen-activated protein kinase (MAPK) pathways, such as extracellular signal-related kinase (ERK), c-Jun N-terminal kinase, and p38 kinase, we found that inhibition of ERK phosphorylation similarly suppressed the bystander response. These results provide evidence that the COX-2 signaling pathway, which is essential in mediating a cellular inflammatory response, may be a critical signaling event for producing a bystander effect.
Materials and Methods
Cell Culture. Normal human lung fibroblasts (NHLF) in frozen vials were purchased from Cambrex BioScience (Walkersville, MD). The NHLF cells were thawed and maintained in a fibroblast basal medium supplemented with 2% FBS and other growth additives, including bovine insulin, human fibroblast growth factor-β, gentamycin sulfate, and amphotercin-B, as supplied by the manufacturer. Cultures were maintained at 37°C in a humidified 5% CO2 incubator. The medium was changed every 3 days, and the cells were passaged at confluence.
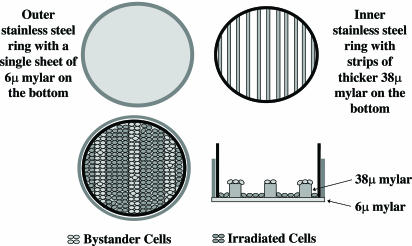
Irradiation Procedure. The newly designed strip mylar dishes were used in the present studies. Briefly, two concentric stainless steel rings were fitted with mylar bottoms with the outer and inner rings covered by a 6- and 38-μm-thick mylar sheet, respectively. The thicker mylar sheet of the inner ring was sliced into strips as shown in Fig. 1. The dishes were sterilized by soaking in 70% pure ethanol bath for 1 h, air-dried, and then rinsed in the medium once before use. Exponentially growing NHLF cells were plated in the concentric strip dishes 3 days before irradiation to ensure a confluent state. Because the fibroblasts seeded on the 38-μm-thick mylar strips would not be irradiated due to the short penetrating distance of the α particles, these cells would effectively become the bystander cells, being seeded right next to the cells plated on the 6-μm mylar dishes that were directly irradiated (Fig. 1). A 50-cGy dose of 4He ions (120 keV/μm) was delivered to the NHLF cells by using the 4-MV van de Graff accelerator at the Radiological Research Accelerator Facilities of Columbia University, as described (4, 10, 13–15). After irradiation, at selected time points, the inner and outer mylar dishes were separated, and the cells from each growth surface were trypsinized and individually pooled for endpoint analysis.
Fig. 1.
A schematic diagram of the stripped dish used in the present study. A6-μm-thick mylar sheet was epoxied to the outer stainless ring to provide a bottom for the dish. The mylar of the inner rings (38 μm thick) was cut as strips. Exponentially growing NHLF cells were plated into the concentric rings 2 days before irradiation at a density to ensure a confluent state. (See Materials and Methods for further details.)
Survival of Irradiated and Nonirradiated Bystander Cells. Nonirradiated bystander, irradiated, and control cells were collected after irradiation at different time points. Cultures were trypsinized, counted with a Coulter counter, and aliquots of the cells were replated into 100-mm-diameter dishes for colony formation. Representative aliquots of cells were further incubated for mutation assay, cDNA expression array analysis, and Western blot. Cultures for clonogenic survival assays were incubated for 12 days, at which time they were fixed with formaldehyde and stained with Giemsa. The number of colonies was counted to determine the surviving fraction as described (10, 13).
Quantification of Mutations at the HPRT- Locus. To determine the mutant fraction, after a 7-day expression period, 2 × 105 cells per dish were plated into 25–30 100-mm dishes in a total of 12 ml of the growth medium, with 100 μM 6-thioguanine (6-TG, Sigma). The cultures were further incubated for 12 days, at which time the cells were fixed and stained and the number of HPRT- mutant colonies scored. Meanwhile, aliquots of 500 cells were plated in dishes containing growth medium without 6-TG for determination of the plating efficiency. The cultures derived from each group were tested for mutant yield for 2 consecutive weeks to ensure full expression of the mutations. The mutant fraction was calculated as the number of surviving colonies divided by the total number of cells plated after correction for the plating efficiency.
cDNA Microarray. The signal transduction pathway finder array (SuperArray, Frederick, MD), which includes 96 genes involved in 18 different signal transduction pathways, was used in the experiments. RNA from control, bystander, and irradiated cells were extracted by using TRIzol reagent (Invitrogen), and the same amount of total RNA was used for each array according the protocol provided by the manufacturer (26, 27).
RT-PCR. The Advantage RT-PCR Kit (BD Biosciences, Franklin Lakes, NJ) was used for RT-PCR analysis. Briefly, cDNA was synthesized from 0.8 μg of total RNA based on the protocol provided. Five microliters of the diluted cDNA was used for each 50-μl PCR reaction. The efficiency of cDNA synthesis was estimated by using GAPDH as a positive internal control. Primers for COX-2 were: sense, GCTTCCATTGCCAGAGCAGGCA; antisense, GAGCTCTGGATCTGGAACACTG; and for insulin growth factor (IGF)-binding protein-3 (IGFBP-3), sense, ACTTCTCCTCCGAGTCCAAGCG; antisense, CGCTTCGACCAACATGTGGTGAGC.
Western Blotting. Protein was extracted from either control or bystander cells by lysis in extracting buffer (50 mM Tris·HCl (pH 8)/150 mM NaCl/1% Nonidet P-40/0.1% SDS/1 mM phenylmethylsulfonyl fluoride, and mixture of protease inhibitors (Roche Applied Sciences, Indianapolis, IN). The concentration of the protein was determined by Bio-Rad Protein Assay (Bio-Rad). Equivalent amounts of protein (100 μg) were fractionated on 10% SDS polyacrylamide gel overnight, and proteins were transferred to nitrocellulose membranes under semidry conditions. For the COX-2 study, the primary antibodies used were goat polyclonal anti-human COX-2 (Santa Cruz Biotechnology). COX-2 protein was detected by using horseradish peroxidase (HRP)-conjugated donkey anti-goat IgG diluted 1:10,000 in 5% skim milk in TBS-Tween for 60 min at room temperature. For MAPK pathways, the primary antibodies used were polyclonal anti-phospho p44/42 MAPK (Thr-202/Tyr-204), anti-p44/42 MAPK, anti-phospho-p38 MAPK (Thr-180/Tyr-182), anti-p38 MAPK (Cell Signaling Technology, Beverly, MA) and monoclonal anti-β-actin (Sigma). The secondary antibodies (anti-rabbit, anti-mouse, or anti-goat) were conjugated to HRP (dilution 1:5,000–1:10,000). After incubation, the band intensities were evaluated by phosphorimaging and normalized to the β-actin expression level.
Statistical Analysis. Data were calculated as means and standard deviations. Comparisons of surviving fractions and induced mutant fractions between treated and control groups were made by the students' t tests. A P value of ≤0.05 between groups was considered significant.
Results
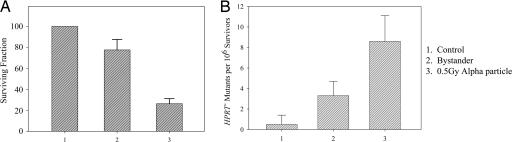
Bystander Cytotoxicity and Mutagenesis in NHLF Induced by α Particles by Using Double Mylar Dishes. In our quest to identify the signaling pathways involved in radiation induced bystander effect, we first focused on the genes that are differentially expressed among the bystander vs. control cells. Because the microbeam can irradiate only one cell at a time, and a large number of cells are needed for gene array analyses, we used the double mylar dish approach (described above) to define the bystander response. Fig. 2 shows the cytotoxicity and mutagenesis at the HPRT- locus for primary human lung fibroblasts that either are directly irradiated (seeded on the 6-μm mylar) or are bystander cells (seeded in the 38-μm mylar). Consistent with our previous findings, NHLF irradiated with a 0.5-Gy dose of α particles resulted in a survival level of 0.21 ± 0.07 (Fig. 2 A) and a mutant fraction of 8.4 ± 2.2 mutants per 106 survivors (Fig. 2B). In contrast, the nonirradiated bystander cells showed a reduced surviving fraction of 0.76 ± 0.11, a level that was significantly differed from the nontreated controls (P < 0.05). Similarly, nonirradiated bystander NHLF showed increased HPRT- mutagenesis (3.2 ± 1.4 mutants per 106 survivors) at a level significantly higher than the background mutant fraction of 0.4 ± 1.1 (P < 0.05).
Fig. 2.
Survival fraction and HPRT- mutations in bystander and directly irradiated cells. Cultures were irradiated with a 0.5-Gy dose of α particle radiation by using the stripped dishes. Data are pooled from six to eight independent experiments. Bar represents ± SD.
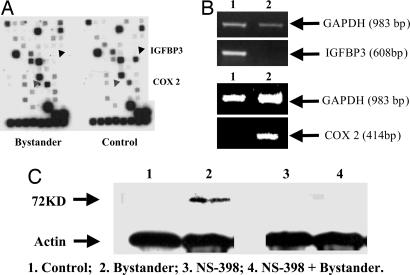
Use of Gene Array to Identify Differentially Expressed Genes Among Bystander vs. Control Cells. By using a signal transduction pathway-specific SuperArray, we compared the differentially expressed genes among the nonirradiated control NHLF and bystander cells (Fig. 3A). Among the 96 genes represented on the platform, transcription level of one gene, COX-2, was found to be consistently up-regulated by >3-fold, whereas the RNA level of IGFBP-3 was found to be consistently lower by >7-fold in multiple analyses of multiple bystander samples. Semiquantitative RT-PCR was used to confirm the expression levels of these two genes by using expression level of the GAPDH gene as internal control (Fig. 3B). The expression of the COX-2 protein in the nonirradiated bystander cells was further confirmed by Western blotting (Fig. 3C). Addition of the COX-2 inhibitor NS-398 (50 μM) suppressed COX-2 level in NHLF cells. After 24 h, the COX-2 protein in bystander cells was reduced to a nondetectable level (lane 2 vs. lane 4). These results indicated that expression of COX-2 is associated with the bystander effect. Because the COX-2 gene plays an important role in arachidonic acid metabolism, the discovery is consistent with the finding that TGFβ may be linked to the bystander signaling cascade (23).
Fig. 3.
Identification of differentially expressed genes in radiation-induced bystander cells. (A) Differentially expressed signaling genes in bystander and control normal human lung fibroblasts using the cDNA signal transduction pathway finder array (SuperArray). (B) RT-PCR analysis and confirmation of the array data showing down-regulated IGFBP-3 and up-regulated COX-2.(C) Western blotting of COX-2 protein expression (72 kD) in bystander and control cells with or without treatment with NS 398 (50 μM), a specific COX-2 inhibitor.
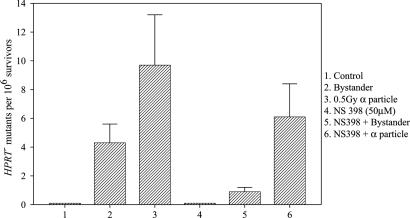
Effects of COX-2 Inhibitor on the Bystander Effect. If the COX-2 gene is causally linked to the bystander signaling pathways, it should be possible to modulate the bystander response by using a specific inhibitor of the COX-2 enzymatic activity. Fig. 4 shows the effect of a noncytotoxic and nonmutagenic dose of NS-398, a specific inhibitor of COX-2 activity, on bystander mutagenesis at the HPRT- locus in NHLF cells irradiated with a 0.5-Gy dose of α particles by using the track segment beam. NHLF cells showed a bystander mutagenic yield of ≈4.2 ± 1.2 mutants per 106 survivors. In contrast, in cultures cotreated with NS-398 (50 μM) that did not increase the spontaneous mutant yield by itself, the bystander mutant fraction was reduced by >6-fold to a level of ≈0.7 ± 0.2 mutants per 106 survivors. Although NS-398 treatment was able to reduce the HPRT- mutant fraction in the directly irradiated population as well, the magnitude of suppression, from 9.2 ± 3.5 to 5.9 ± 2.2 mutants per 106 survivors was only 36%. Similar findings were also obtained by using cytotoxicity as an endpoint (data not shown).
Fig. 4.
Effect of NS 398 treatment (50 μM, 24 h before and maintained overnight after irradiation), a specific COX-2 inhibitor, on HPRT- mutant fractions of NHLFs. Data are from three to four independent experiments. Bar represents ± SD.
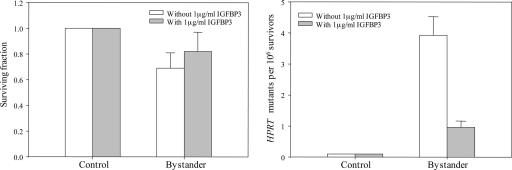
Effects of IGFBP-3 on the Bystander Effect. IGFBP-3 inhibits IGF signaling activity at the cellular level by competitively binding IGFs and, as a result, decreases their ability to bind to the cell surface receptor. Because bystander cells show a 7-fold decrease in the levels of IGFBP-3 RNA, we hypothesize that IGF may play an important role in mediating the bystander effect. As a result, the addition of exogenous IGFBP-3 may suppress the bystander effect. Fig. 5 shows the effects of exogenously applied IGFBP-3 (1 μg/ml) on both bystander survival as well as HPRT- mutant fraction in NHLF cells. Addition of IGFBP-3 increased the bystander survival from 0.68 ± 0.11 to 0.81 ± 0.13, although the result was not statistically significant (P > 0.05). In contrast, addition of IGFBP-3 reduced the mutant yield by ≈4-fold from 3.9 to 0.9 mutants per 106 survivors. It should be noted that the bystander mutant fraction obtained in this set of experiments was comparable to that of the COX-2 studies described above.
Fig. 5.
Effect of IGFBP-3 treatment (1 μg/ml, 24 h before and maintained overnight after irradiation) on survival fraction (Left) and HPRT- mutation fraction (Right) in control and bystander NHLF cells in the vicinity of cells irradiated with 0.5-Gy α particle plated in strip dishes. Data are pooled from three independent experiments. Bar represents ± SD.
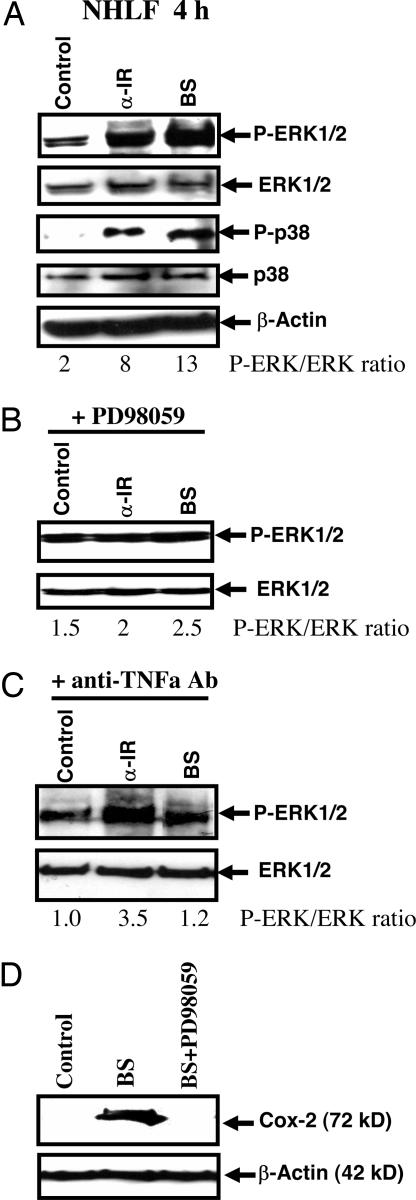
Activation of MAPK Signaling Pathways in Bystander Cells. IGF and other cytokines activate MAPK signaling cascade, and activation of ERK by phosphorylation is a critical upstream event preceding COX-2 expression. Determination of ERK activity by Western blot analysis demonstrated strong up-regulation of phospho-ERK levels in both α irradiated and bystander NHLF 4 h after treatment (Fig. 6A). Increased levels of phospho-ERK could even be detected 16 h after treatment, indicating a persistent response to the bystander signaling (data not shown). In contrast, activity of MAPK p38 kinase was found to be increased 4 h after treatment (Fig. 6A) and was not detectable 16 h after irradiation (data not shown). It should be noted that, when compared with the controls, the ratio of phosphorylated ERK over native ERK increased from 2 to 13 among the bystander cells. To further confirm the activation of ERK in bystander cells, we used PD 98059 (50 μM), a specific inhibitor of MAPK kinase (MEK)-ERK, which had been added to cell cultures immediately after irradiation for a period of 4 h. In the presence of PD 98059, the phosphorylated form of ERK and its activation were suppressed in both α particle-irradiated and bystander cells (Fig. 6B). Because NHLFs respond to different forms of stress, including radiation, by up-regulation of the production of various cytokines, we used inhibitory anti-TNF-α monoclonal antibody to determine the probable role of TNF-α in bystander activation. This mAb (2 μg/ml), which had been added immediately after irradiation, significantly blocked ERK activation in bystander cells, whereas effects on irradiated cells were very mild (Fig. 6C). Down-regulation of ERK activity in bystander cells in the presence of PD98059 (50 μM) completely suppressed COX-2 expression (Fig. 6D).
Fig. 6.
Activation of the MAPK signaling pathways is involved in the radiation-induced bystander effects. Western blot analysis of phospho-ERK1/2, total ERK1/2, phospho-p38, and total p38 expression in control, α-irradiated, and bystander NHLF cells 4 hr after irradiation with a 0.5-Gy dose of α particle plated in stripped dishes (A). β-actin was used as a loading control. Phospho-ERK/ERK ratio is indicated. PD 98059 (50 μM), an inhibitor of MEK-ERK, was added to the cultures immediately after irradiation (B). Anti-TNF-α mAb (2 μg/ml) was added to the cultures immediately after irradiation (C), PD98059 (50 μM) suppressed expression of COX-2 in bystander cells (D).
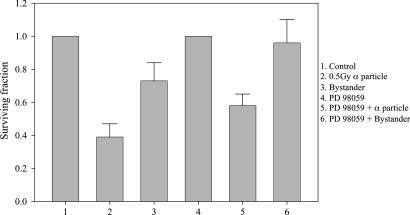
Inhibition of ERK Signaling Suppresses the Bystander Response. If activation of the MAPK signaling cascade and ERK phosphorylation are essential in mediating the bystander effect, it should be possible to mitigate the later response by using a specific inhibitor of the MEK-ERK signaling cascade. Fig. 7 shows the bystander toxicity in the presence or absence of a noncytotoxic dose of PD 98059 (50 μM) when added to cultures immediately after irradiation for a period of 4 h, as described above. The surviving fraction of the bystander cells was 0.72 ± 0.1, and treatment with PD 98059 increased survival significantly to almost control level, 0.95 ± 0.12 (P < 0.05).
Fig. 7.
Effect of PD 98059 treatment (50 μM, immediately after and maintained overnight after irradiation), an inhibitor of MEK-ERK, on surviving fractions of control, directly irradiated, and bystander NHLF cells. Data are from three independent experiments. Bar represents ± SD.
Conclusion
Radiation-induced bystander effects have been extensively studied in the past decade. The plethora of data now available concerning this effect falls into two categories: (i) in confluent cultures where physical contacts between irradiated and nonirradiated cells are made and where gap junctional communications have been shown to be essential for the process, and (ii) in sparsely populated cultures where bystander effects may be mediated by damage signals released into the culture medium by the irradiated cells. As a result, incubation of nonirradiated cells with conditioned medium from irradiated cultures may lead to induction of biological effects. In the present study, spatially distinct confluent monolayers of NHLF were used. It is not clear whether the signaling molecules involved in the two bystander processes are mutually exclusive. In fact, it is likely that some common initiating or intermediate steps are involved.
The mechanism of the radiation-induced bystander effect, whether involving cell–cell contact or mediated by soluble factors, is not clear, is likely to be complex, and involves multiple pathways. It is, however, clear that p53 gene function is not necessary for the effect, because cells without normal p53 function (such as Chinese hamster ovary cells) show a large bystander response in either bystander pathway. It is likely that multiple signaling cascades involving both an initiating event and downstream signaling steps are necessary to mediate the bystander process. In the present study, we have used a systematic approach to first identify the signaling genes that are differentially altered among the bystander cells, followed by functional assays to identify and assess the biological significance of the signaling molecules.
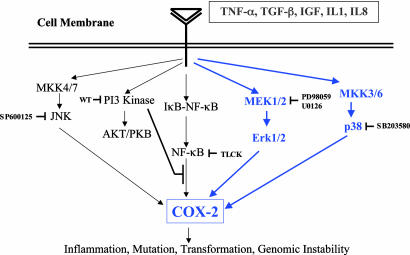
By using a microarray specific for cell signaling genes, we identified the transcription of two genes, COX-2 and IGFBP-3, that were consistently altered in the bystander cells. As proposed in our working model (Fig. 8), the functions of these two genes might work in a coordinated manner to mediate the bystander effect. COX-2 is a member of the COX family of genes that have important functions in mediating the cellular immune response. There are two isoforms of COX: COX-1 and COX-2, which differ in many respects. For example, COX-1 is constitutively expressed in normal tissues, but COX-2 is inducible after treatment with different growth factors and cytokines, such as TGFβ, TNF-α, IL1β, and different stress factors (28, 29). All these stimuli induce Ras-Raf-MEK-ERK-AP1 and inhibitor nuclear factor κB kinase (IKK)-NF-κB pathways and finally target COX-2 gene transcription. COX-2 is the initial and rate-limiting enzymatic step in the metabolism of arachidonic acid into a complex group of signaling lipid mediators called prostaglandins (30), which play important roles in modulating cellular inflammation, carcinogenesis and genomic instability.
Fig. 8.
Working model of the signaling pathways involved in the radiation-induced bystander effect. Binding of TNF-α, TGFβ, IGF, IL-1, and IL-8 ligands to the corresponding receptors activate signaling pathways including MEK1/2; mitogen-activated protein kinase kinase (MKK3/6), and p38 kinase (all in blue lines), which lead to COX-2 gene expression. Specific inhibitors of the various signaling pathways, such as Wortmannin (wt) for phosphatidylinositol 3-kinase and N-(α)-tosyl-l-lysine chloromethylketone hydrochloride for the NF-κB can be used to delineate specific signaling routes involved in the bystander phenomenon.
Because the abundance of COX-2 RNA is increased by 3-fold in the bystander cells, it is necessary to neutralize the COX-2 signaling pathways to establish its function in the bystander effect. We used NS-398, a specific inhibitor of COX-2, to achieve this goal. The IC50 values of NS-398 for the human recombinant form of COX-1 and COX-2 have been established to be 75 and 1.8 μM, respectively (31). In the presence of NS-398, the bystander mutagenic effect was reduced by >6-fold in NHLF cells, thereby establishing the functional link of COX-2 to the bystander cascade of events. However, because both directly irradiated and nonirradiated bystander NHLF cells were maintained in the same medium with NS-398, we cannot rule out the possible effects of NS-398 on the directly irradiated cells in promoting radiation-induced bystander effects.
Another gene found to be differentially expressed in the bystander NHLF cells is IGFBP-3. In humans, the majority of the circulating IGFs are bound to IGFBP-3, which is produced mainly in the liver (32). IGFBP-3 inhibits IGF activity at the cellular level by binding competitively to IGFs and, thereby, preventing their binding to the IGF receptors in the cell surface. By preventing IGFs from binding to the IGF receptors, IGFBP-3 effectively inhibits IGF-mediated signaling pathways (33). The observation that there was a 7-fold decrease in levels of IGFBP-3 among the bystander cells suggests that the IGF signaling process is active and could potentially play a functional role in the bystander process. Our findings that exogenously applied IGFBP-3 can eliminate both bystander survival and mutagenic effects provide strong support for an important role of the IGF activating loop. This finding is consistent with our observations that pretreatment with anti-human TNF-α monoclonal antibody (5 μg/ml), which does not affect ERK phosphorylation in irradiated fibroblasts, completely abolished ERK activation in the bystander cells.
As mentioned above, one of the consequences of the binding of TNF-α, IGF, and other leukotrienes to the surface receptor sites is activation of the MAPK cellular signaling pathways. The three classes of MAPK include ERK, c-Jun N-terminal kinase (JNK), and p38 kinases (34). ERKs are believed to be strongly activated and to play an important role in intracellular signaling induced by various growth factors, such as epidermal growth factor and tumor promoters (e.g., phorbol esters) (35). On the other hand, JNK and p38 kinases are activated by multiple stresses including UV, ionizing radiation, cytotoxic chemicals, and reactive oxygen species (36). Although the role of p38 kinase signaling in the cellular response to external stimuli is diverse, responses of ERK and JNK are fairly reliable. This is consistent with our present finding, where the activation of ERK is robust and persistent.
Furthermore, there is evidence that TGFβ may play an important role in mediating bystander effects in medium-transfer experiments (23). Similarly, Lehnert et al. (37, 38) have demonstrated the contribution of reactive oxygen species (ROS) to the induction of sister chromatid exchanges among nonirradiated cultures exposed to irradiated culture media. Because both TGFβ and ROS can trigger the MAPK signaling cascade, these findings are consistent with the present observation of the role of COX-2 in the bystander effect. Likewise, the role of nitric oxide in medium-mediated bystander response has been described (24). The observations that (i) nitric oxide is known to regulate the expression of IL-8 in some human cells (39) and (ii) nitric oxide synthase, which is critical to the biosynthesis of peroxynitrite anions, has been shown to be involved in the regulation of COX-2 expression (40) provide a functional link for the role of nitric oxide and COX-2 in mediating the bystander effect. It is likely that some common initiating or intermediate steps are involved in the two processes.
Last, a better understanding of the cellular and molecular mechanisms of the bystander phenomenon, together with evidence of their occurrence in vivo, will allow us to formulate a more accurate model in assessing the health effects of low doses of ionizing radiation.
Acknowledgments
This research was supported by National Institutes of Health Grants CA 49062 and ES 12888, Environmental Center Grant ES10349, National Cancer Institute Research Resource Grant RR 11623, National Institute of General Medical Sciences Grant GM 52493, and Department of Energy Grant DE-FG02-03ER63441.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: COX-2, cyclooxygenase-2; ERK, extracellular signal-related kinase; HPRT-, hypoxanthine-guanine phosphoribosyltransferase; IGF, insulin growth factor; IGFBP-3, IGF-binding protein 3; MAPK, mitogen-activated protein kinase; MEK, MAPK kinase; NHLF, normal human lung fibroblast.
References
- 1.International Commission on Radiological Protection (1991) Recommendations (Pergamon, New York), Report No. 60.
- 2.National Council on Radiation Protection and Measurements (1993) Report 116 (National Council on Radiation Protection and Measurements, Bethesda, MD).
- 3.Nagasawa, H. & Little, J. B. (1992) Cancer Res. 52, 6394-6396. [PubMed] [Google Scholar]
- 4.Zhou, H., Suzuki, M., Randers-Pehrson, G., Vannais, D., Chen, G., Trosko, J. E., Waldren, C. A. & Hei, T. K. (2001) Proc. Natl. Acad. Sci. USA, 98, 14410-14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzam, E. I., De Toledo, S. M., Spitz, D. R. & Little, J. B. (2002) Cancer Res. 62, 5436-5442. [PubMed] [Google Scholar]
- 6.Hickman, A. W., Jaramillo, R. J., Lechner, J. F. & Johnson, N. F. (1994) Cancer Res. 54, 5797-5800. [PubMed] [Google Scholar]
- 7.Morgan, W. F. (2003) Radiat. Res. 159, 567-580. [DOI] [PubMed] [Google Scholar]
- 8.Little, J. B. (2003) Oncogene 22, 6978-6987. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande, A., Goodwin, E. H., Bailey, S. M., Marrone, B. L. & Lehnert, B. E. (1996) Radiat. Res. 145, 260-267. [PubMed] [Google Scholar]
- 10.Zhou, H., Randers-Pehrson, G., Waldren, C. A., Vannais, D., Hall, E. J. & Hei, T. K. (2000) Proc. Natl. Acad. Sci. USA 97, 2099-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prise, K. M., Belyakov, O. V., Folkard, M. & Michael, B. D. (1998) Int. J. Radiat. Biol. 74, 793-798. [DOI] [PubMed] [Google Scholar]
- 12.Prise, K. M., Belyakov, O. V., Folkard, M., Ozols, A., Schettino, G., Vojnovic, B. & Michael, B. D. (2002) Adv. Space Res. 30, 871-876. [DOI] [PubMed] [Google Scholar]
- 13.Ponnaiya, B., Jenkins-Baker, G., Bigelow, A., Marino, S. & Geard, C. R. (2004) Mutat. Res. 568, 41-48. [DOI] [PubMed] [Google Scholar]
- 14.Sawant, S. G., Randers-Pehrson, G., Geard, C. R., Brenner, D. J. & Hall, E. J. (2001) Radiat. Res. 155, 397-401. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell S. A., Randers-Pehrson, G., Brenner, D. J. & Hall, E. J. (2004) Radiat. Res. 161, 397-401. [DOI] [PubMed] [Google Scholar]
- 16.Hei, T. K., Persaud, R., Zhou, H. & Suzuki, M. (2004) Mutat Res. 568, 111-120. [DOI] [PubMed] [Google Scholar]
- 17.Mothersill, C. & Seymour, C. (1997) Int. J. Radiat. Biol. 71, 421-427. [DOI] [PubMed] [Google Scholar]
- 18.Nagar, A., Smith, L. E. & Morgan, W. F. (2003) Cancer Res. 63, 324-328. [PubMed] [Google Scholar]
- 19.Mothersill, C. & Seymour, C. B. (1998) Radiat. Res. 149, 256-262. [PubMed] [Google Scholar]
- 20.Lyng, F. M., Seymour, C. B. & Mothersill, C. (2000) Br. J. Cancer. 83, 1223-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis, D. A., Mayhugh, B. M., Qin, Y., Trott, K. & Mendonca, M. S. (2001) Radiat. Res. 156, 251-258. [DOI] [PubMed] [Google Scholar]
- 22.Seymour, C. B. & Mothersill, C. (1997) Radiat. Oncol. Invest. 5, 106-110. [DOI] [PubMed] [Google Scholar]
- 23.Iyer, R., Lehnert. B. E. & Svensson, R. (2000) Cancer Res. 60, 1290-1298. [PubMed] [Google Scholar]
- 24.Matsumoto, H., Hayashi, S., Hatashita, M., Ohnishi, K., Shioura, H., Ohtsubo, T., Kitai, R., Ohnishi, T. & Kano, E. (2001) Radiat. Res. 155, 387-396. [DOI] [PubMed] [Google Scholar]
- 25.Azzam, E. I., de Toledo, S. M. & Little, J. B. (2001) Proc. Natl. Acad. Sci. USA. 98, 473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy, D., Calaf, C. & Hei, T. K. (2001) Mol. Carcinogen. 192, 203. [DOI] [PubMed] [Google Scholar]
- 27.Zhao, Y. L., Piao, C. Q. & Hei, T. K. (2002) Br. J. Cancer 86, 1923-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picot, D., Loll, P. J. & Garavito, R. M. (1994) Nature 367, 243-245. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell, J. A., Akarasereenont, P., Thiemermann, C., Flower, R. J. & Vane, J. R. (1993) Proc. Natl. Acad. Sci. USA 90, 11693-11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marnett, L. J. (1992) Cancer Res. 52, 5575-5589. [PubMed] [Google Scholar]
- 31.Barnett, J., Chow, J., Ives, D., Chiou, M., Mackenzie, R., Osen, E., Nguyen, B., Tsing, S., Bach, C. & Freire, J. (2004) Biochem. Biophys. Acta 1209, 130-139. [DOI] [PubMed] [Google Scholar]
- 32.Chin, E., Zhou, J., Dai, J., Baxter, R. C. & Bondy, C. A. (1994) Endocrinology 134, 2498-2504. [DOI] [PubMed] [Google Scholar]
- 33.Grimberg, A. (2000) Mol. Genet. Metab. 70, 85-98. [DOI] [PubMed] [Google Scholar]
- 34.Boulton, T. G., Nye, S. H., Robbins, D. J., Ip, N. Y., Radziejewska, E., Morgenbesser, S. D., DePinho, R. A., Panayotatos, N., Cobb, M. H. & Yancopoulos, G. D. (1991) Cell 65, 663-675. [DOI] [PubMed] [Google Scholar]
- 35.Minden, A., Lin, A., McMahon, M., Lange-Carter, C., Derijard, B., Davis, R. J., Johnson, G. L. & Karin, M. (1994) Science 266, 1719-1723. [DOI] [PubMed] [Google Scholar]
- 36.Dent, P., Yacoub, A., Contessa, J., Caron, R., Amorino, G., Valerie, K., Hagan, M. P., Grant, S. & Schmidt-Ullrich, R. (2003) Radiat. Res. 159, 283-300. [DOI] [PubMed] [Google Scholar]
- 37.Narayanan, P. K., Goodwin, E. H. & Lehnert, B. E. (1997) Cancer Res. 57, 3963-3971. [PubMed] [Google Scholar]
- 38.Lehnert, B. E., Goodwin, E. H. & Deshpande, A. (1997) Cancer Res. 57, 2164-2171. [PubMed] [Google Scholar]
- 39.Xiong, Q., Shi, Q., Le, X., Wang, B. & Xie, K. (2001) J. Interferon Cytokine Res. 21, 529-537. [DOI] [PubMed] [Google Scholar]
- 40.Landino, L. M., Crews, B. C., Timmons, M. D., Morrow, J. D. & Marnett, L. J. (1996) Proc. Natl. Acad. Sci. USA 93, 15069-15074. [DOI] [PMC free article] [PubMed] [Google Scholar]