Abstract
For more than a century, the process of stabilization has been a central issue in the research of learning and memory. Namely, after a skill or memory is acquired, it must be consolidated before it becomes resistant to disruption by subsequent learning. Although it is clear that there are many cases in which learning can be disrupted, it is unclear when learning something new disrupts what has already been learned. Herein, we provide two answers to this question with the demonstration that perceptual learning of a visual stimulus disrupts or interferes with the consolidation of a previously learned visual stimulus. In this study, we trained subjects on two different hyperacuity tasks and determined whether learning of the second task disrupted that of the first. We first show that disruption of learning occurs between visual stimuli presented at the same orientation in the same retinotopic location but not for the same stimuli presented at retinotopically disparate locations or different orientations at the same location. Second, we show that disruption from stimuli in the same retinotopic location is ameliorated if the subjects wait for 1 h before training on the second task. These results indicate that disruption, at least in visual learning, is specific to features of the tasks and that a temporal delay of 1 h can stabilize visual learning. This research shows that visual learning is susceptible to disruption and elucidates the processes by which the brain can consolidate learning and thus protect what is learned from being overwritten.
Keywords: plasticity, psychophysics, vision, consolidation, hyperacuity
Living organisms are constantly learning new skills and improving their abilities. When does the learning of something new disrupt that which has already been learned? For more than a century, the process of stabilization has been a central issue in the research of learning and memory (1, 2). Namely, after a skill or memory is acquired, it must undergo a period of consolidation before it becomes resistant to disruption by subsequent learning of a similar task (3). Although it is clear that there are many cases in which learning can be disrupted, the process of disruption and its specificity are unclear.
Disruption of learning has been best detailed in studies of motor learning. These studies show that skill acquisition from a task (A) is disrupted or interfered with by subsequent practice of a second task (B). For instance, subjects in a hand-reaching task can learn to adapt to a force field, which alters their movements. This learning persists on subsequent days of testing. If subjects first adapt to force field A and then adapt to force field B, they no longer show improvements for force field A when tested on the following day (3-6). Similar disruption has been found in tasks of visual-motor sequence learning (7). Disruption has also been found in visual-motor reaching tasks, but only between tasks involving similar visual-motor transformations (8). Some motor studies show that the disruption of A does not occur if there is a waiting period of a few hours before the training of B (3, 6), but recent research brings these results into question by showing that disruption occurs even for delays of 24 h between adaptation to force field A and subsequent adaptation to force field B (4).
Although studies of the disruption of visual-motor learning have provided important insights into the processes of learning, they are, in some cases, difficult to interpret because many brain systems, including perceptual, cognitive, and motor processing, are involved in the learning of a given task. This diversity poses a complexity in interpreting findings of disruption because interference between tasks can presumably occur in any of these processes, and the contribution of each stage may differ significantly between tasks and even individual subjects. An alternative model by which to study the disruption of learning is that of perceptual learning, which takes place in the early sensory systems.
Perceptual learning is here defined as a performance increase on a task involving primitive sensory features and which involves plasticity in one's sensory brain areas. This type of learning is thought to help us to better perceive the visual environment. The advantage of perceptual learning for studies of learning disruption is that, in many cases, perceptual learning is highly specific to features being trained; for example, visual detection or discrimination thresholds can be reduced and show a high degree of specificity for the orientation (9-11), motion direction (12-15), retinotopic location (9, 10, 16), and sometimes ocularity (9) of the trained visual stimuli. These results are consistent with plasticity of early visual areas and are supported by neurophysiological evidence of neuronal plasticity in early visual areas (17-22). Learning of features in other modalities such as audition (23-25), somatosensation (26, 27), and motor functions (28, 29) also implicates neural changes in the primary cortical areas for these modalities. Through techniques used to study perceptual learning, we can potentially restrict learning, and thus disruption of learning, to take place within a very limited set of brain regions.
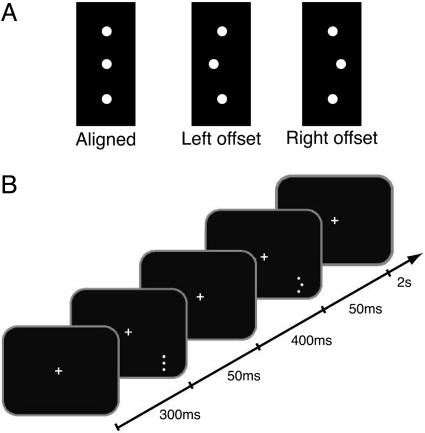
Herein, we report the finding that the disruption of learning occurs in perceptual learning. We do this by training subjects on a three-dot hyperacuity task (Fig. 1A). The advantage of using this hyperacuity task is that the learning improvements from this task are highly specific to the location in retinotopic space and the orientation of the trained stimuli (9). Separate A and B conditions, of different offset directions (left or right), were presented in sequential blocks or interleaved, depending on the experiment. When subjects were trained for 5 days with sequential A-then-B (AB) sessions, the learning of A was disrupted by the training of B. This disruption was retinotopically specific in that subjects trained with sequential AB sessions at retinotopically different locations showed no disruption of the learning of A by the training of B. Additionally, disruption was orientation-specific in that subjects trained with A and B conditions of different stimulus orientation (vertical or horizontal) showed no disruption.
Fig. 1.
Stimulus and task. (A) A white vertical three-dot hyperacuity stimulus was presented on a black background on a computer screen. Either the three dots were aligned or the middle dot was offset to the left or right. (B) For the experimental procedure, two stimuli (one offset and one aligned stimulus) were presented in succession in the lower right visual field. After a fixation period of 300 ms, each stimulus was presented for 50 ms, separated by an interval of 400 ms. Subjects fixated a central cross, which flashed to indicate the presentation of the peripheral stimulus. After the stimulus presentation, subjects had 2 s to indicate with a key-press whether the first or the second stimulus was offset. The color of the fixation cross indicated whether the response was correct (green) or incorrect (red).
We also show evidence for a consolidation period for perceptual learning on this task. A delay between training of A and B of 1 h ameliorates the disruption of A by B. This finding suggests that, compared with other brain areas that take 6 h (3, 6) or exhibit no consolidation with 24 h or longer (4, 5), consolidation can occur relatively quickly in perceptual learning, which itself can lasts for months (13).
Methods
Subjects. Five different groups of 10 subjects participated in each of five conditions. Subject ages ranged from 20 to 35 years old. Data from 49 of 50 subjects are reported because a single subject in the task testing for orientation specificity (the 2ORI condition) was unable to perform beyond chance level.
Stimuli. A white vertical three-dot hyperacuity stimulus (82 cd/m2) was presented on a black background (1.1 cd/m2) on a computer screen. The distance between the centers of the top and the bottom dots was 20′ arc, and each dot had a radius of 2′ arc. Either the three dots were either aligned or the middle dot was offset to the left or right (Fig. 1A). Five different difficulties of offsets (0.9′, 1.8′, 2.7′, 3.6′, and 4.5′ arc) were used.
Procedure. Each group of subjects was trained on the three-dot hyperacuity task: unless otherwise noted, two stimuli (one offset and one aligned stimulus) were presented in succession in the lower right visual field (7.5° arc in the periphery). After a fixation period of 300 ms, each stimulus was presented for 50 ms, separated by an interval of 400 ms. Subjects fixated a central cross, which flashed to indicate the presentation of the peripheral stimulus. After the stimulus presentation, subjects had 2 s to indicate with a key-press whether the central dot was offset in the first or the second stimulus. The color of the fixation cross indicated whether the response was correct (green) or incorrect (red). Subject's heads were restrained with a stereotaxic chin rest.
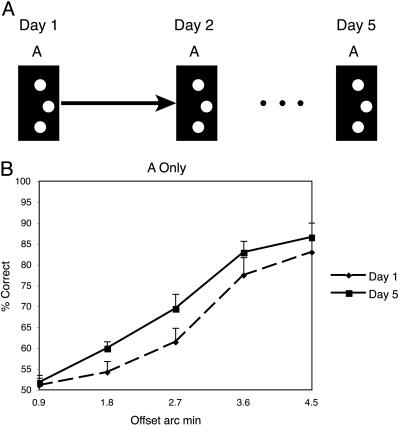
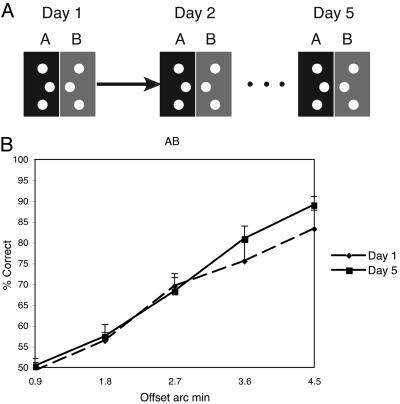
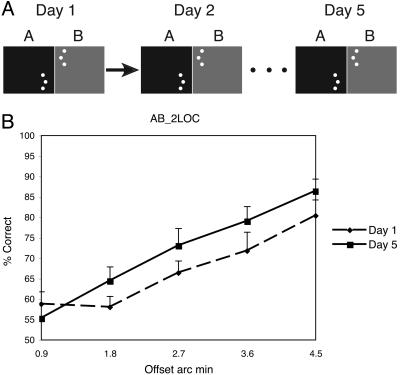
A different task condition was performed by each of the five groups of subjects. Each group conducted five sets of training sessions per task, with each set of training sessions conducted on a separate day. Training sessions consisted of 400 trials (80 trials per offset size), divided up into blocks of 20 trials of the same offset, after which subjects could rest their eyes. The order of presentations of the five offsets was randomly determined in each session. Learning was evaluated for each of the five different groups of subjects: For the control group, the offset was to one side throughout the experiment (Fig. 2A). Group AB performed an additional session (B) each day immediately after performing session A. Session B was the identical to session A, except that the offset was to the opposite side (Fig. 3A). Group 2LOC also performed sequential AB sessions with opposite offsets, but for this group, the hyperacuity stimuli in the B session were presented in the upper left visual field (Fig. 4A). Group 2ORI had interleaved A and B conditions and received training in two locations (upper left and lower right), also interleaved. At location 1, the A and B stimuli were both oriented vertically (similar to group AB), but at location 2, the A stimulus was oriented vertically and the B stimulus was oriented horizontally (Fig. 5A). Group AB-1 h was identical to group AB, but the B session was conducted 1 h after the end of session A (Fig. 6A). For all groups, the offset side used for the A and the B conditions was counterbalanced across subjects. Note that because session A occurred before session B for Groups AB, AB-1 h, and 2LOC, the condition under which the A session was conducted on day 1 was identical across all groups, with the exception of group 2ORI, which performed interleaved trials of different conditions.
Fig. 2.
Control condition. (A) The offset of the stimulus was to one side throughout the experiment and subjects performed one session per day. (B) Performance for each offset magnitude on day 1 (dashed line) and day 5 (solid line). (Error bars reflect standard error.)
Fig. 3.
Condition AB. (A) On each day, subjects performed two sequential sessions. In the first session, the offset was to one side, and in the second session, the offset was to the other side. (B) Performance from the A session for each offset magnitude on day 1 (dashed line) and day 5 (solid line). (Error bars reflect standard error.)
Fig. 4.
Condition 2LOC. (A) On each day, subjects performed two sequential sessions. In the first session, the offset was to one side and in the lower right visual quadrant, and in the second session, the offset was to the other side in the upper left visual quadrant. (B) Performance from the A session for each offset magnitude on day 1 (dashed line) and day 5 (solid line). (Error bars reflect standard error.)
Fig. 5.
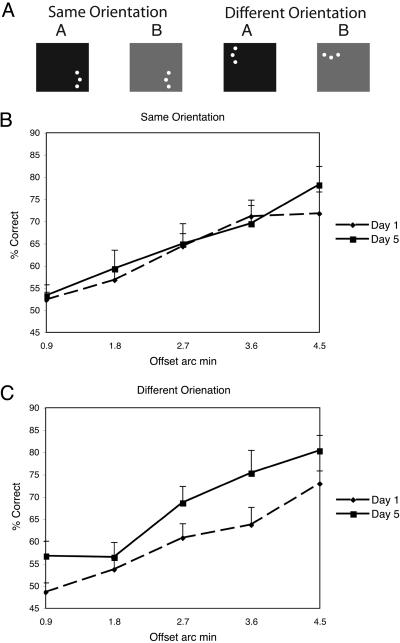
Condition 2ORI. (A) On each day, subjects performed a session with interleaved trials of two stimulus orientations and two stimulus locations. In one location, stimuli were always presented vertically and the offset was either to the left or right. In the other location, stimuli were presented either vertically or horizontally but with a single offset side. (B) Performance from the same orientation trials for each offset magnitude on day 1 (dashed line) and day 5 (solid line). (C) Performance from the vertical condition in the different orientation trials for each offset magnitude on day 1 (dashed line) and day 5 (solid line). (Error bars reflect standard error.)
Fig. 6.
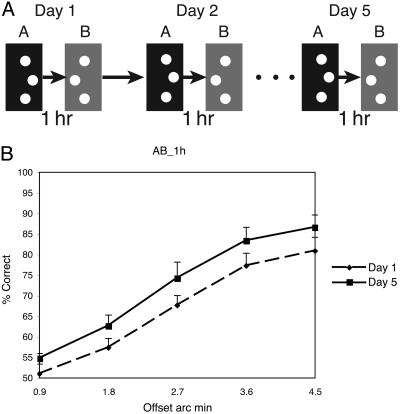
Condition AB-1 h. (A) On each day, subjects performed two sessions with a 1-h break between sessions. In the first session, the offset was to one side, and in the second session, the offset was to the other side. (B) Performance from the A session for each offset magnitude on day 1 (dashed line) and day 5 (solid line). (Error bars reflect standard error.)
Results
To demonstrate that learning can be disrupted, we must first verify that learning occurs for subjects trained only on a single offset side (control). In Fig. 2B, the across-subject average is shown for the first day (dashed line) and last day (solid line) of the 5-day training. To quantify learning, we ran a within-subject, two-way ANOVA, which showed a significant effect across days [f(1,9) = 6.4, mean square error (MSE) = 0.055, P < 0.05] as well as a significant effect of the offset size [f(4,9) = 55.2, MSE = 0.42, P < 0.0001].
Next, we examined subjects (group AB) who performed sequential sessions in which they were first trained on one offset side (A), and then immediately afterward were trained on the other offset side (B). For this group, no significant learning was found for offset side A [f(1,9) = 0.87, MSE = 0.014, P = 0.37; see Fig. 3B], indicating that learning for these subjects was disrupted by their practice of offset side B, although there was some improvement for the easiest two offsets (see Discussion). In addition, there was a significant difference in the degree of improvement between the control group and the AB group for the three most difficult conditions [f(1,9) = 5.4, MSE = 0.06, P < 0.05], but not for the easiest two conditions [f(1,9) = 0.1, MSE = 0.005, P = 0.76]. On the other hand, significant learning [f(1,9) = 4.7, MSE = 0.042, P < 0.05] was found for these subjects on offset B (data not shown), indicating that these subjects were capable of learning this task. Day 1 performance for the A and B offset sides were not different [f(1,9) = 0.1, MSE = 0.004, P = 0.76], suggesting that the lack of learning for A was not due to a plateau in performance being reached in the first session.
Although we have shown that the learning of A is disrupted through the training of B, it is unclear what aspect of the B training is responsible for this disruption. For instance, it is possible that disruption of A will occur from the immediate performance of any subsequent demanding task, irrespective of the features of that task. To test whether this hypothesis was the case, we ran a new set of subjects on a third experiment, which was identical to the AB condition with the exception that the B session was performed with the stimulus in a different visual quadrant than that of the A session (group 2LOC; see Fig. 4A). If it is merely the performance of any subsequent task that results in disruption, then no learning of A should be expected, because the demands of the B session for the 2LOC group are the same as in that for the B session of the AB group. On the other hand, if no disruption occurs, it would imply that disruption is specific to the visual feature, in this case, the retinotopic location of the stimulus, being trained.
The results of the 2LOC experiment confirm that disruption is specific to the retinotopic location of the stimulus. This effect can be seen in Fig. 4B, which plots the average subject's performance on offset side A for the first day (dashed line) and last day (solid line) of the 5-day training. There is a clear improvement across days, and this improvement is statistically significant [f(1,9) = 11.8, MSE = 0.052, P < 0.01; see Fig. 4B].
Next, we examined whether this disruption of learning is specific to the orientation of the trained stimuli. In this experiment, we took advantage of the finding that disruption was spatially specific and, therefore, used a within-subject design in which each subject was trained with A and B stimuli of the same (vertical) orientation, but different offset sides, in one location and in another location with A and B stimuli of different (vertical or horizontal) orientations. In addition, we interleaved all four conditions (A and B for same and different orientations) to ensure that our results of interference were not specific to our blocking procedure. Our hypothesis was that interference would occur between stimuli of the same orientation, as shown with group AB, but that interference would not be found for stimuli of different orientation. A result of this type would be in accord with perceptual learning studies showing that learning of hyperacuity stimuli is specific to the stimulus orientation (9, 30).
The results of the 2ORI experiment confirm that disruption is specific to the orientation of the stimulus. This effect can be seen in Fig. 5 B and C, which plots the average subject's performance for the same orientation and different orientation conditions, respectively, for the first day (dashed line) and last day (solid line) of the 5-day training. Although there was no learning in the location of training with the same orientation [f(1,8) = 0.34, MSE = 0.007, P = 0.57; see Fig. 5B], there was significant improvement across days for the location of training with different orientations [f(1,8) = 6.5, MSE = 0.13, P < 0.01; see Fig. 5C]. The lack of learning for the same orientation conditions shows that interleaving the A and B conditions results in interference and confirms the finding of disruption in group AB.
Although we have shown that the disruption of learning can occur between visual tasks and that this disruption is specific to the location and orientation of the trained visual feature, one may ask what mechanisms exist by which to protect learning from disruption. For instance, does there exist a consolidation process that stabilizes the learning of A after some recess from training? To address this question, we ran an additional experiment with a new group of subjects, which was identical to that of group AB, with the exception that session B was run 1 h after the completion of session B (group AB-1 h; Fig. 5A).
The results of this experiment indicate that a delay of 1 h is sufficient to ameliorate the disruptive effects of the training of offset side B on the learning of offset side A. This can be seen in the performance differences on offset side A between the first and last experimental sessions of the AB-1 h condition (see Fig. 6b). These differences are significant [f(1,9) = 13.2, MSE = 0.076, P < 0.01]. These results indicate that a consolidation period as short as 1 h after the completion of session A abolishes the disruptive effects of the training of offset side B on the learning of offset side A.
Discussion
Our studies have three important findings. First, that perceptual learning can be disrupted by the performance of a subsequent task or between learning of interleaved conditions. Second, that this disruption is specific to the visual features that are common between the two tasks, in this case, the retinotopic location and orientation of the trained stimuli. Third, that the learning from the first task is protected from disruption by performance of subsequent tasks if the temporal interval between the tasks is 1 h or longer.
A number of studies have shown that perceptual learning is highly specific to particular stimulus features. In the case of the three-dot hyperacuity stimulus as used here, learning effects are specific to the orientation and retinotopic location of the stimuli (9, 30). Given this featural specificity and the fact that the discriminations involved are of 1′ arc, psychophysicists have postulated that the learning involves primary visual cortex. This hypothesis has been supported by physiological experiments in monkeys that indicate neuronal plasticity in primary visual cortex associated with a similar hyperacuity task (31, 32).
Models of perceptual learning suggest that a neuron or cluster of neurons form a template that is optimized to process the features of the task. For instance, a classic feed-forward model by Poggio et al. (30) suggests that the hyperacuity task can be performed by forming a template for each offset side based on a few exemplars of each stimulus. In this model, Gaussian input filters are processed by a set of radial-basis functions to determine a binary output. Later models have shown how more neurobiologically plausible networks involving recurrent connections and/or oriented receptive fields can perform similar tasks (33-37).
Our results show that the disruption of this learning is specific to the orientation, spatial location, and the offset side of the stimulus. Because the perceptual judgment occurs over just a few minutes of arc, in the AB condition, the same visual neurons may subserve both the A and the B tasks. This specificity suggests that the cause of disruption is that the learning of the second task “overwrites” the templates of the first.
The fact that a 1-h delay between the sessions was sufficient to eliminate the disruption suggests the existence of a mechanism that prevents the learning of A from being overwritten by the learning of B. One suggestion is that higher-level brain areas can help solve this problem by learning to distinguish between the different task conditions. This idea is supported by physiological and psychophysical lines of evidence. For instance, primary visual cortex neurons respond differently to identical hyperacuity stimuli, depending on the task that the animal is performing (19). Behavioral performance curves change differently for the same stimuli trained with different tasks (38). Likewise, modeling and imaging studies of the consolidation of motor learning suggest that higher-level motor areas serve to help the lower-level motor centers learn distinct motor plans and thus overcome interference of motor learning (39-41). Also, feedback processes have been shown to be important to maintain the stability of auditory learning (42, 43).
Although we have demonstrated that consolidation occurs, it is unclear how to relate its time course to that found in other studies. There is a great deal of controversy regarding when consolidation occurs and what circumstances allow for learning to be disrupted. For instance, whereas some studies of motor learning show that 6 h are required for consolidation (3, 6), our study found that only 1 h is sufficient for visual learning. Further, early studies by Müller and Pilzecker (1, 2) of the learning of word lists found a consolidation period of only 10 min. To add to this confusion, a recent thorough study of motor learning found that, in many instances, consolidation does not occur and that a delay of 24 h was not sufficient to prevent disruption (4). A possible reconciliation of this controversy was provided by Walker et al. (7). In this study, consolidation was observed, but practice with only a few probe sessions of the A condition was enough to make A again liable to disruption of B. Thus, although it is clear that consolidation exists, it seems that small differences in conditions are important.
To date, consolidation in perceptual learning has been studied only in relation to sleep (44, 45). Such studies demonstrate that sleep is necessary for the manifestation of performance enhancements after learning. In particular, these studies show that performance actually deteriorates when the subject is tested multiple times on the same task without an intervening period of sleep. These studies employ very different methods to assay consolidation are not in conflict with our results. In particular, they compare performance across sessions within a day, whereas we compare performance across session performed on separate days. Walker et al. (7) reconcile these methods and demonstrate that consolidation in motor learning involves multiple dissasociable processes. Their study found that the consolidation of motor sequences takes 6 h, but that the manifestation of performance enhancements requires sleep. Thus, studies of consolidation in visual learning also likely confound what may be two separate processes; one process of stabilization, which we have identified in this study, and a second process of enhancement, which requires sleep.
An interesting trend in our data is that in the AB and 2ORI conditions, subjects showed some improvement for the easiest offset difficulties. This pattern fits well within what is known about visual learning. It has been shown that the degree of location and orientation specificity of perceptual learning depends on the difficulty of the training condition (32, 46). This finding has led to the suggestion that the more difficult discriminations are learned in low-level visual areas, whereas easier trials are learned in higher-level areas (46). In our study, interference was found only for stimuli with small offsets, which require the most difficult and finest-grained spatial discriminations. For the easier trials, which are thought to mainly involve learning in higher cortical areas, task attributes that generalize across the A and B conditions might have been learned.
One concern is that the results of these studies could result from some form of facilitation between the A and B conditions. In this framework, training with the B condition would cause learning to reach a plateau by the end of day 1 and thus no learning would occur on subsequent days. For group AB, this explanation can be ruled out because for day 1, the A condition was identical to that of the control group and there was no significant difference in performance between these groups [f(1,9) = 0.43, MSE = 0.005, P = 0.51]. For the 2ORI condition, facilitation can be ruled out because there was no significant difference between the same orientation vs. the different orientation conditions on day 1[f(1,8) = 0.65, MSE = 0.03, P = 0.44], and there was a significant interaction between these conditions and the day of training [f(1,8) = 6.7, MSE = 0.037, P < 0.05]. Thus, although there may still be some degree of facilitation between the A and B conditions, it does not explain the interference found in our study.
Disruption of the learning on one task by subsequent training on a second task is an important technique by which to study consolidation of learning. Nevertheless, disruption has not been directly studied for learning in the visual system, whose physiological and computational mechanisms are better known than most of other brain systems. The present study shows that visual perceptual learning for a stimulus can be disrupted by subsequent training on a different visual stimulus. We also found that this disruption is specific to the stimulus orientation and occurs only within a limited spatial and temporal window. The limited spatial window suggests that interference overwrites the template of new learning on an existing template. The temporal window suggests that the overwriting is effective for a short time in perceptual learning, perhaps because of the quick stabilization of learning. Perceptual learning is an appropriate technique to study consolidation because it occurs in the early sensory areas and the learning effects are highly specific to the trained stimuli.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 EY015980-01, Human Frontiers Grant RGP18/2004 (to T.W.), and a grant from the National Institute of Information and Communications Technology (to M.K.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AB, A-then-B; MSE, mean square error.
References
- 1.Müller, G. E. & Pilzecker, A. (1900) Z. Psychol. Ergänzungsband 1, 1-300. [Google Scholar]
- 2.Lechner, H. A., Squire, L. R. & Byrne, J. H. (1999) Learn. Mem. 6, 77-87. [PubMed] [Google Scholar]
- 3.Brashers-Krug, T., Shadmehr, R. & Bizzi, E. (1996) Nature 382, 252-255. [DOI] [PubMed] [Google Scholar]
- 4.Caithness, G., Osu, R., Bays, P., Chase, H., Klassen, J., Kawato, M., Wolpert, D. M. & Flanagan, J. R. (2004) J. Neurosci. 24, 8662-8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osu, R., Hirai, S., Yoshioka, T. & Kawato, M. (2004) Nat. Neurosci. 7, 111-112. [DOI] [PubMed] [Google Scholar]
- 6.Shadmehr, R. & Brashers-Krug, T. (1997) J. Neurosci. 17, 409-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker, M. P., Brakefield, T., Hobson, J. A. & Stickgold, R. (2003) Nature 425, 616-620. [DOI] [PubMed] [Google Scholar]
- 8.Krakauer, J. W., Ghilardi, M. F. & Ghez, C. (1999) Nat. Neurosci. 2, 1026-1031. [DOI] [PubMed] [Google Scholar]
- 9.Fahle, M., Edelman, S. & Poggio, T. (1995) Vision Res. 35, 3003-3013. [DOI] [PubMed] [Google Scholar]
- 10.Fiorentini, A. & Berardi, N. (1980) Nature 287, 43-44. [DOI] [PubMed] [Google Scholar]
- 11.Schoups, A. A., Vogels, R. & Orban, G. A. (1995) J. Physiol. (London) 483, 797-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe, T., Nanez, J. E. & Sasaki, Y. (2001) Nature 413, 844-848. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe, T., Nanez, J. E., Sr., Koyama, S., Mukai, I., Liederman, J. & Sasaki, Y. (2002) Nat. Neurosci. 5, 1003-1009. [DOI] [PubMed] [Google Scholar]
- 14.Seitz, A. R. & Watanabe, T. (2003) Nature 422, 36. [DOI] [PubMed] [Google Scholar]
- 15.Ball, K. & Sekuler, R. (1981) J. Exp. Psychol. Hum. Percept. Perform. 7, 780-794. [DOI] [PubMed] [Google Scholar]
- 16.Dill, M. & Fahle, M. (1997) Proc. R. Soc. London Ser. B 264, 1031-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zohary, E., Celebrini, S., Britten, K. H. & Newsome, W. T. (1994) Science 263, 1289-1292. [DOI] [PubMed] [Google Scholar]
- 18.Yang, T. & Maunsell, J. H. (2004) J. Neurosci. 24, 1617-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, W., Piech, V. & Gilbert, C. D. (2004) Nat. Neurosci 7, 651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoups, A., Vogels, R., Qian, N. & Orban, G. (2001) Nature 412, 549-553. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert, C. D., Sigman, M. & Crist, R. E. (2001) Neuron 31, 681-697. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz, S., Maquet, P. & Frith, C. (2002) Proc. Natl. Acad. Sci. USA 99, 17137-17142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilgard, M. P. & Merzenich, M. M. (1998) Science 279, 1714-1718. [DOI] [PubMed] [Google Scholar]
- 24.Bao, S., Chan, V. T. & Merzenich, M. M. (2001) Nature 412, 79-83. [DOI] [PubMed] [Google Scholar]
- 25.Bao, S., Chang, E. F., Woods, J. & Merzenich, M. M. (2004) Nat. Neurosci. 7, 974-981. [DOI] [PubMed] [Google Scholar]
- 26.Dinse, H. R., Ragert, P., Pleger, B., Schwenkreis, P. & Tegenthoff, M. (2003) Science 301, 91-94. [DOI] [PubMed] [Google Scholar]
- 27.Kaas, J. H., Merzenich, M. M. & Killackey, H. P. (1983) Annu. Rev. Neurosci. 6, 325-356. [DOI] [PubMed] [Google Scholar]
- 28.Li, C. S., Padoa-Schioppa, C. & Bizzi, E. (2001) Neuron 30, 593-607. [DOI] [PubMed] [Google Scholar]
- 29.Pascual-Leone, A., Tarazona, F., Keenan, J., Tormos, J. M., Hamilton, R. & Catala, M. D. (1999) Neuropsychologia 37, 207-217. [DOI] [PubMed] [Google Scholar]
- 30.Poggio, T., Fahle, M. & Edelman, S. (1992) Science 256, 1018-1021. [DOI] [PubMed] [Google Scholar]
- 31.Crist, R. E., Kapadia, M. K., Westheimer, G. & Gilbert, C. D. (1997) J. Neurophysiol. 78, 2889-2894. [DOI] [PubMed] [Google Scholar]
- 32.Liu, Z. (1999) Proc. Natl. Acad. Sci. USA 96, 14085-14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adini, Y., Sagi, D. & Tsodyks, M. (2002) Nature 415, 790-793. [DOI] [PubMed] [Google Scholar]
- 34.Dosher, B. A., Liu, S. H., Blair, N. & Lu, Z. L. (2004) Vision Res. 44, 1257-1271. [DOI] [PubMed] [Google Scholar]
- 35.Dosher, B. A. & Lu, Z. L. (1998) Proc. Natl. Acad. Sci. USA 95, 13988-13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dosher, B. A. & Lu, Z. L. (1999) Vision Res. 39, 3197-3221. [DOI] [PubMed] [Google Scholar]
- 37.Zhaoping, L., Herzog, M. H. & Dayan, P. (2003) Network 14, 233-247. [PubMed] [Google Scholar]
- 38.Koyama, S., Harner, A. & Watanabe, T. (2004) Perception 33, 1139-1147. [DOI] [PubMed] [Google Scholar]
- 39.Imamizu, H., Kuroda, T., Miyauchi, S., Yoshioka, T. & Kawato, M. (2003) Proc. Natl. Acad. Sci. USA 100, 5461-5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imamizu, H., Kuroda, T., Yoshioka, T. & Kawato, M. (2004) J. Neurosci. 24, 1173-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawato, M. (1999) Curr. Opin. Neurobiol. 9, 718-727. [DOI] [PubMed] [Google Scholar]
- 42.Konishi, M. (2004) Ann. N.Y. Acad. Sci. 1016, 463-475. [DOI] [PubMed] [Google Scholar]
- 43.Leonardo, A. & Konishi, M. (1999) Nature 399, 466-470. [DOI] [PubMed] [Google Scholar]
- 44.Karni, A., Tanne, D., Rubenstein, B. S., Askenasy, J. J. & Sagi, D. (1994) Science 265, 679-682. [DOI] [PubMed] [Google Scholar]
- 45.Mednick, S., Nakayama, K. & Stickgold, R. (2003) Nat. Neurosci. 6, 697-698. [DOI] [PubMed] [Google Scholar]
- 46.Ahissar, M. & Hochstein, S. (1997) Nature 387, 401-406. [DOI] [PubMed] [Google Scholar]