Abstract
Cell migration plays important roles in embryonic development and inflammation, and this process is highly regulated to ensure tissue homeostasis. A number of barriers exist to prevent the inappropriate migration of leukocytes into healthy peripheral tissues, including retention of these cells in the inactive state and maintenance of the integrity and charge of the vascular endothelium. However, active signals also are likely to exist that can repulse cells or abolish existing cell migration. One such paradigm exists in the developing nervous system, where neuronal migration is mediated by a balance between chemoattractive and chemorepulsive signals. The ability of the guidance molecule netrin-1 to repulse or abolish attraction of neuronal cells expressing the UNC5b receptor makes it an attractive candidate for the regulation of inflammatory cell migration. Here, we show that netrin-1 is expressed on vascular endothelium, where it is regulated by infection and inflammatory cytokines. The netrin-1 receptor UNC5b is strongly expressed by leukocytes, upon which netrin-1 acts as a potent inhibitor of migration to different chemotactic stimuli both in vivo and in vitro. These data suggest that endothelial expression of netrin-1 may inhibit basal cell migration into tissues and that its down-regulation with the onset of sepsis/inflammation may facilitate leukocyte recruitment.
Keywords: chemotaxis, inflammation, guidance cue
Cell migration to specific sites of inflammation or infection is a critical immune response to eliminate foreign or infectious agents. However, inappropriate migration of these cells into organs can result in tissue destruction. Positive guidance cues, such as chemokines, have been well characterized in recent years (1). These secreted molecules play a critical role in regulating leukocyte extravasation and accumulation in tissues during response to infection or inflammation. Because the inappropriate migration of leukocytes into peripheral tissues can have serious consequences, barriers are likely to exist at a number of levels to regulate leukocyte migration. The circulation of the leukocyte in an inactivated form, the integrity of the vascular endothelial barrier, and the charge of the leukocyte and endothelium are known impediments to aberrant infiltration. However, negative guidance cues also are likely to exist to control inappropriate leukocyte migration or to quench an existing migration, and to date, these have been less well described. Negative guidance cues have been identified in the developing nervous system, where neurons and axons are precisely guided to their final location by a balance of chemoattractive and chemorepulsive signals to establish elaborate neuronal circuitry (2, 3). Several families of such conserved neuronal guidance cues have been identified, including the slits, netrins, ephrins, and semaphorins, and migration is achieved by a complex integration of these signals. One of these guidance molecules, slit-2, has recently been reported to be expressed in peripheral tissues, where it can regulate the migration of granulocytes, lymphocytes, monocytes, and dendritic cells (4-7). These findings suggest that neuronal guidance cues may have additional roles outside the CNS.
Netrin-1 is a laminin-related molecule that is secreted at the spinal cord midline, where it plays a defined role in guiding vertebrate commissural axons (8, 9). Interestingly, this molecule can act as both a chemoattractant and a chemorepulsive force. This versatility is achieved by modulation of receptor expression by target cells to mediate context-dependent responses. For example, neurons expressing the deleted in colon cancer (DCC) receptor are attracted by netrin-1 secreted at the midline (2, 10). Once these neurons reach the midline, they do not linger but rather proceed across the midline as if repelled. This sudden shift in perception of the netrin-1 signal is caused by the expression of an additional netrin-1 receptor, UNC5 (10, 11). Coexpression of UNC5 homologue b (UNC5b, previously known as UNC5H2) and DCC converts netrin-1 attraction to repulsion, thus directing axons away from the midline and netrin-1. Recently, a number of studies have proposed additional guidance roles for netrin-1, including directing the retinal nerve entering the optical nerve head in Xenopus (12) and functioning as an instructional molecule for the building of complex nonneuronal structures in organs, such as the inner ear and the mammary gland (13, 14). Furthermore, we have shown that UNC5b is expressed in immune tissues, suggesting that netrin-1 may play a role in modulating immune cell function (15).
We investigated whether netrin-1 expression is regulated in a model of acute pulmonary inflammation and whether it plays a role in modulating leukocyte recruitment. We report that netrin-1 is highly expressed by the vascular endothelium, particularly in postcapillary venules, and that its expression is down-regulated during Staphylococcus aureus infection in vivo. Furthermore, netrin-1 is a potent inhibitor of monocyte, lymphocyte, and granulocyte migration in vitro and in vivo. Netrin-1 dose-dependently inhibited chemokine-mediated migration of these cells and blocked leukocyte accumulation in a mouse model of peritonitis. Blocking of the UNC5b receptor abolished the inhibitory effects of netrin-1 on cell migration. Together, these data suggest that netrin-1 performs a function in modulating leukocyte migration similar to what it does in guiding neuronal cells. Normal tissues are leukocyte-poor, but with infection, there is a rapid influx of inflammatory cells. We suggest that modulation of endothelial expression of netrin-1 in part regulates leukocyte infiltration of tissues.
Materials and Methods
Leukocyte Isolation. Lymphocytes were isolated from peripheral blood according to the manufacturer's protocol by using Ficoll-Paque PLUS (Amersham Pharmacia Biosciences), and monocytes and granulocytes were isolated by using Histopaque (Sigma) centrifugation. Cells were resuspended in RPMI medium 1640 containing 1% BSA or 10% FCS at a cell density of 1-5 × 106 cells per ml. Cells were kept at 4°C until use; cells were used within 5 h after isolation.
Cell Culture. Human umbilical vein endothelial cells (Cambrex, Walkersville, MD) were trypsinized and plated in a six-well plate. The following day, the cells were treated with medium alone (unstimulated control) or medium containing 10 ng/ml TNF-α or 10 ng/ml IFN-γ for 6 h. Cells were then washed in ice-cold PBS and lysed for RNA extraction and protein measurement.
Human 293-EBNA T cells were maintained in DMEM containing 10% FCS, penicillin (100 units/ml), and streptomycin (100 μg/ml). Cells were transiently transfected with the human DCC or UNC5b cDNA in the pCDNA3.1 vector by using Lipofectamine 2000 (Invitrogen) as suggested by the manufacturer. DCC and UNC5b expressions were verified after 48 h. The DCC and UNC5b plasmids were provided by Marc T. Tessier-Lavigne (Genentech).
Migration Assays. Chemotaxis of monocytes, lymphocytes, and granulocytes was measured by using transfilter migration assays. Cell migration was measured in the absence and presence of chemotactic agents, including stromal cell-derived factor 1α (10 ng/ml), IL-8 (50 ng/ml), and 10 nM formylmethionylleucylphenylalanine (fMLP), and with these chemotactic agents plus recombinant chicken netrin-1 (500 ng/ml, R & D Systems). Chemotactic agents and/or netrin-1 (in a total of 28 μl) were placed in the lower wells of a 48-well Boyden chamber (Neuroprobe, Cabin John, MD). Cell suspensions (50 μl) with or without netrin-1 (500 ng/ml, ≈7.2 nM) were added to the upper wells. The upper and lower wells were separated with a polyvinylpyrrolidone-free polycarbonate filter (Neuroprobe), which was precoated with type IV collagen (Sigma) and had a 3-μm (granulocytes) or 5-μm pore size (monocytes and lymphocytes). After incubation at 37°C with 5% CO2 (from 30 min to 1.5 h), cells on the top surface of the filter were removed. Cells that had migrated through to the undersurface of the filter were fixed, stained, and counted. In addition, migration assays were performed by using a 96-well Boyden chamber (Neuroprobe), where cells migrating into the lower chamber were counted. Migration was expressed as chemotactic index per high-power field, which was calculated by dividing the number of migrating cells in the treated groups by the number of migrating cells in the lowest control well. The results of chemotaxis assays are representative of at least three independent experiments performed on triplicate samples.
In experiments where the UNC5b receptor was blocked, cells were isolated and incubated at 37°C for 15 min in RPMI medium 1640 and 0.1% BSA with either goat Ig or anti-UNC5b antibody (R & D Systems) at the stated concentrations before the chemotaxis assay.
Quantification of Superoxide Radical Production. Superoxide radical production by granulocytes was determined by measuring the superoxide dismutase-inhibitable reduction of 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-1) (Roche Applied Science) in accordance with the manufacturer's guidelines. Cells (1.2 × 105) were plated in a 96-well plate in 150 μl of media. WST-1 (final concentration 500 μM) plus catalase (final concentration 20 μg/ml), with or without superoxide dismutase (final concentration 20 μg/ml), was added. The granulocytes were stimulated with 1 μM fMLP in the presence or absence of netrin (500 ng/ml) at 37°C. After 30 min, superoxide production was measured spectrophotometrically (optical density at 450 nm). Superoxide dismutase-inhibitable absorbance is reported.
Western Blotting and Quantitative Real-Time RT-PCR. Western blotting and quantitative real-time RT-PCR were performed as described in refs. 16 and 17. The primers used for netrin-1 were 5′-AAGCCTATCACCCACCGGAAG-3′ and 5′-GCATCAAGATTCCTGTGGCG-3′; for DCC, 5′-CCTCTTCACAGGATTGGAGAAAGG-3′and 5′-CATCATGAGTTGGACACCTCC-3′; and for UNC5b, 5′-TGGGTCTTGAGCTGAAAGATCCA-3′and 5′-TGGATCTTTCAGCTCAAAGACCCA-3′.
Immunohistochemistry. 293-T cells transfected with UNC5b or DCC plasmids were grown in chamber slides, and isolated leukocytes from peripheral blood were spun onto slides by using a Cytospin cytocentrifuge (Shandon, Pittsburgh). The slides were air-dried, then fixed in methanol and subsequently in 4% paraformaldehyde. Lung tissues were placed in Cryomolds (Miles), which were then filled with tissue-freezing medium (Triangle Biomedical Sciences, Durham, NC) and frozen at -70°C. Six-micrometer-thick sections were placed on slides, air-dried, and fixed in 4% paraformaldehyde. Air-dried lung sections and cells were permeabilized with 0.2% Triton X-100 in PBS, washed three times in PBS, and blocked with 5% donkey serum (Jackson ImmunoResearch). Primary antibodies included netrin-1 rabbit anti-mouse (Oncogene Science); UNC5b rabbit anti-human, generated in our lab against an extracellular domain, SQAGTDSGSEVLPDS; DCC goat anti-mouse (Santa Cruz Biotechnology); PECAM-1 goat anti-mouse (Santa Cruz Biotechnology); and CD45 (mouse anti-human, Abcam, Cambridge, MA). Experimental negative controls were incubated in PBS/2% BSA with rabbit IgG generated in our lab, goat IgG (Santa Cruz Biotechnology), and mouse IgG (Santa Cruz Biotechnology). Secondary antibodies include FITC-conjugated donkey anti-rabbit for UNC5b, FITC donkey anti-goat for DCC and PECAM-1, Cy3-conjugated donkey anti-rabbit for netrin-1, and Cy3-conjugated donkey anti-mouse for CD45 (Jackson ImmunoResearch). Hoechst 33342 stain (blue) (Invitrogen) was used for nuclear staining.
Immunostaining of the intestine also was performed by using the avidin-biotin complex immunoperoxidase technique, with diaminobenzidine as substrate using conditions similar to those used for immunofluorescent staining described above. Slides were counter-stained with hematoxylin.
Mouse Sepsis Model. Adult (8- to 12-week-old) female C57BL/6 mice were injected with S. aureus CP5 (Reynolds capsular serotype 5) at 5 × 107 colony-forming units per mouse in 0.2 ml of saline through the lateral tail vein. At the indicated time points, mice were killed and their organs were processed immediately for RNA isolation.
Peritonitis Model. Mice (8- to 12-week-old female C57BL/6 mice, n = 6 per group) were injected i.p. with 10 nM fMLP either alone or in combination with recombinant chicken netrin-1 (500 ng/ml, R & D Systems) in a total volume of 1 ml of PBS containing 1% BSA. Recruited leukocytes were harvested 4 h later by peritoneal lavage with calcium- and magnesium-free ice-cold Hanks' balanced salt solution containing 1 μM EDTA and analyzed as described in ref. 18. Collected cells were washed, resuspended in 2 ml of Hanks' balanced salt solution, and counted. Cytospin samples were prepared, and cells were stained with Diff-Quick (American Hospital Supply, McGraw Park, IL) to differentiate leukocyte subpopulations. All reagents used were endotoxin-free.
Results
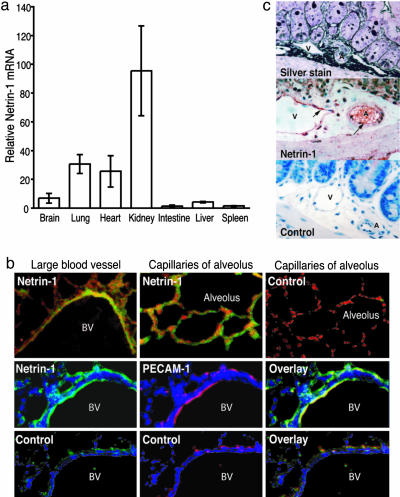
We, as well as others, have demonstrated that netrin-1 is highly expressed in the lung and brain (17, 19). Furthermore, we found that its receptor UNC5b was highly expressed in immune tissues, as well as the heart, kidney, and lung. We reasoned that, if netrin-1 plays a role in the regulation of leukocyte migration, it should have a broad distribution, with particularly strong expression in tissues with large vascular beds and blood supply. To explore further the distribution of netrin-1, we examined netrin-1 mRNA expression in tissues by quantitative real-time RT-PCR. In addition to its expression in the brain, netrin-1 was detected in the lung, heart, kidney, and, to a lesser extent, the intestine, liver, and spleen (Fig. 1a). To define more precisely the localization of netrin-1 expression in the lung, we performed immunohistochemistry on paraformaldehyde-fixed tissues (Fig. 1b). We found that netrin-1 was highly expressed in the lung, with abundant staining detected in the vascular endothelium of large and small blood vessels. This endothelial expression was confirmed by colocalization of netrin-1 with the endothelial cell marker PECAM-1. Expression of netrin-1 in this location is consistent with a role in the regulation of inflammatory cell migration.
Fig. 1.
Netrin-1 tissue expression and cellular localization. (a) Netrin-1 mRNA expression in mouse tissues was quantified by quantitative real-time RT-PCR. Each sample was analyzed in triplicate, and the amount of netrin-1 was calculated from a standard curve of known template. Results represent the average of three samples, normalized to GAPDH. (b) Immunofluorescent staining of the lung demonstrates high netrin-1 (green) expression in this tissue, particularly in the endothelium of large blood vessels and capillaries. Colocalization of netrin-1 (green, Center) with the endothelial cell marker PECAM-1 (red, Center) is seen in the merged image (yellow). Primary antibodies were omitted in control staining. (c) Immunoperoxidase staining for netrin-1 in postcapillary venules of the small intestine. Serial sections of the small intestine were stained with silver stain to show venules (V) and arterioles (A) (Top), anti-netrin-1 antibody (Middle), and an isotype control antibody (Bottom). Netrin-1 (red, arrows) appeared to be expressed on the luminal surface of postcapillary venules and, to a lesser extent, by arteriole endothelial cells. No staining was detected when netrin-1 was replaced with an isotype control antibody (Bottom).
Because we postulate a role for netrin-1 in the regulation of chemotaxis, we would expect it to be expressed on postcapillary venules and on the luminal surface of endothelial cells. Postcapillary venules are easily identified in the gastrointestinal tract and in the skin. We used immunoperoxidase staining to detect netrin-1 expression in the intestine, because this technique is superior to immunofluorescence for protein quantification and localization. Arterioles and venules were identified in the sections of the jejunum by silver staining (Fig. 1c). Netrin-1 was found to be highly expressed in postcapillary venules and appeared to be associated with the luminal surface of endothelial cells. Lesser netrin-1 staining was detected on arterioles (Fig. 1c). These data confirm the localization of netrin-1 to endothelial cells where it can engage circulating leukocytes.
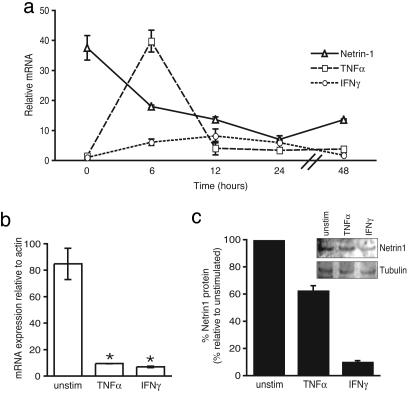
If netrin-1 were to regulate leukocyte migration at the endothelium, we hypothesized that its expression would be modulated during acute inflammation due to infection. To test this hypothesis, we used a mouse model of S. aureus infection in which the lung is a site of abscess formation. We chose to evaluate expression of netrin-1 in the lung, because we had previously characterized its expression in this tissue during development (17). We found that, in mice injected with S. aureus, netrin-1 expression was rapidly down-regulated in the lung (Fig. 2a). At 6 h after injection with S. aureus, there was a 2-fold reduction in netrin-1 mRNA in the lung as determined by quantitative real-time RT-PCR. Expression of the inflammatory cytokines TNF-α and IFN-γ corresponds with the accumulation of leukocytes in the lung. We found that the reduction in netrin-1 coincided with a 6-fold increase in IFN-γ and a 27-fold increase in TNF-α mRNA (Fig. 2a), indicating that netrin-1 is inversely correlated with inflammatory cytokine expression and leukocyte invasion of this tissue. Netrin-1 levels continued to decline until 24 h after infection (25% of baseline), and this decline was followed by a trend upward by 48 h. We did not observe gross destruction of the lung on histology, and thus, this decline in netrin-1 levels is unlikely to be related to tissue destruction, particularly as the levels of netrin-1 are normalized to a house-keeping gene. These data suggest that, in this model of acute inflammation of the lung, netrin-1 expression is rapidly down-regulated during the initial phase of inflammation.
Fig. 2.
Modulation of netrin-1 expression in the lung during infection. (a) Expression of netrin-1, IFN-γ, and TNF-α mRNA in the lung was measured by quantitative real-time RT-PCR in mice infected with S. aureus. Netrin-1 is rapidly down-regulated. Regulation of netrin-1 mRNA showed an inverse relationship to the expression of the inflammatory cytokines IFN-γ and TNF-α. (b-c) Treatment of human umbilical vein epithelial cells with 10 ng/ml TNF-α or IFN-γ for 6 h reduced netrin-1 expression. (b) Netrin-1 mRNA was measured by quantitative RT-PCR and normalized to β-actin. (c) Netrin-1 protein was detected by Western blotting and normalized to levels of tubulin.
Cytokines such as TNF-α and IFN-γ are major mediators of acute inflammation and were found to have an expression pattern inverse to that of netrin-1 in the lung during S. aureus infection. To test whether these proinflammatory cytokines could modulate netrin-1 expression in vascular endothelium, human umbilical vein epithelial cells were stimulated with TNF-α and IFN-γ. Both of these cytokines significantly reduced netrin-1 expression, resulting in a 90% decrease in mRNA levels 6 h after stimulation (Fig. 2b). Western blotting confirmed a 40% and 90% decrease in netrin-1 protein in response to TNF-α and IFN-γ, respectively, whereas no change was observed in tubulin, which was used as an internal control (Fig. 2c). These data suggest a role for cytokines in the down-regulation of netrin-1 expression by endothelium during inflammation.
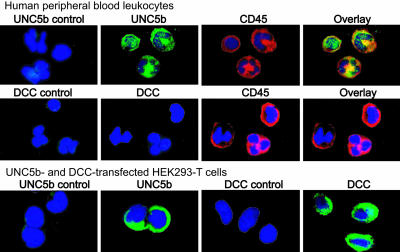
Understanding the cellular distribution of netrin-1 receptors is likely to provide insight into the function of this guidance molecule. Four vertebrate homologues of UNC5 have been described (20). UNC5a is predominantly expressed in the ventral spinal cord; UNC5c in migrating neural crest cells, lung, kidney, and cartilage; and UNC5d in the brain, inner ear, forming limb, and glands (20, 21). We demonstrated in ref. 15 that UNC5b is strongly expressed in the lung and brain, as well as in hematopoietic and immune tissues. To characterize further the expression of netrin-1 receptors that mediate both chemoattractive and chemorepulsive responses to this molecule, we performed immunohistochemical staining for DCC and UNC5b, respectively, in human leukocyte populations. Expression of the netrin-1 chemoattractive receptor DCC was not detected in human granulocytes, monocytes, or lymphocytes by immunohistochemistry (Fig. 3) or by quantitative RT-PCR (not shown). However, DCC was readily detected by immunohistochemical methods in transfected 293-T cells, indicating that leukocytes do not express this receptor (Fig. 3). In contrast, UNC5b was abundantly expressed on the surface of monocytes, granulocytes, and lymphocytes, and this expression colocalized with the cell surface marker CD45 (Fig. 3).
Fig. 3.
Leukocytes express the netrin-1 receptor UNC5b but not DCC. Immunohistochemical staining demonstrates robust expression of UNC5b (green, Top) on the cell surface of monocytes, lymphocytes, and granulocytes that colocalizes with the leukocyte cell-surface marker CD45 (red). By contrast, DCC (green, Middle) expression is not detected on CD45-positive leukocytes. UNC5b- and DCC-transfected 293-T cells served as positive controls and show high expression of these receptors (Bottom). Negative controls included replacing primary antibodies with rabbit (UNC5b) or goat (DCC) IgG. Nuclei were stained with Hoechst 33342 stain (blue).
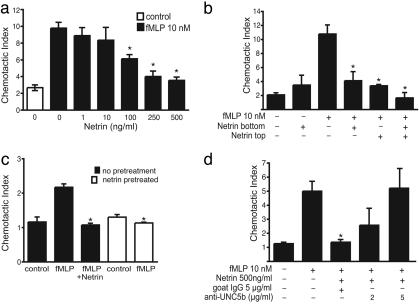
Because netrin-1 abolishes attraction and repulses neurons expressing UNC5 homologues, we hypothesized that it also might negatively regulate leukocyte migration. To test the effect of netrin-1 on the migration of human monocytes, we used Boyden chamber-based migration assays. We found that netrin-1 strongly inhibited fMLP-induced chemotaxis of monocytes in a dose-dependent manner (Fig. 4a). Initial experiments were performed by measuring the migration of cells through the 5-μm pores of polyvinylpyrrolidone-free polycarbonate filter and counting cells that migrated onto the undersurface. These assays showed reduced monocyte migration to the filter undersurface in response to fMLP when netrin-1 was present. However, because this result could be explained by altered adhesion of netrin-1-treated monocytes, the experiment was repeated by using a 96-well assay system where migration of cells into the lower chamber media was measured. Identical results were seen with both techniques. The inhibition of monocyte migration by netrin-1 was robust and not affected by serum concentrations in the media.
Fig. 4.
Netrin-1 inhibits active migration of monocytes, granulocytes, and lymphocytes in vitro. (a) Netrin-1 dose-dependently inhibits monocyte migration in response to 10 nM fMLP. Control represents monocyte migration in the absence of fMLP and netrin-1 (*, P < 0.05). (b) Netrin-1 inhibits fMLP-induced monocyte migration when present in either the lower or upper chamber, or both, in a Boyden migration plate (*, P < 0.05). Monocytes were placed in the top chamber, and migration to 10 nM fMLP in the lower chamber was measured in the presence and absence of netrin-1 (500 ng/ml) in the lower chamber or both. Netrin-1 alone did not alter baseline cell migration. (c) Netrin-1 pretreatment inhibits monocyte migration to fMLP. Monocytes were untreated (filled bars) or pretreated with netrin-1 (500 ng/ml) for 90 min and washed extensively (open bars), and migration to 10 nM fMLP was measured. Both netrin-1 pretreatment of cells and the presence of netrin-1 in the lower chamber inhibited monocyte migration to fMLP to a similar extent (*, P = 0.002). (d) Treatment of monocytes with anti-UNC5b antibody, but not control antibody, dose-dependently reverses the inhibitory effect of netrin-1 on monocyte migration (*, P < 0.05).
Netrin-1 alone did not significantly alter baseline cellular migration, even when placed in the upper chamber with the monocytes, where it could exert repulsive forces (Fig. 4b). In the presence of fMLP, netrin-1 added to either the top or bottom chamber reduced monocyte chemotaxis down to baseline levels (Fig. 4b). These results suggest that netrin-1 is an inactivator of chemotaxis. Although this effect is likely to be mediated through UNC5b on leukocytes, it also could be due to a direct interaction of netrin-1 with fMLP. To test this possibility, the cells were pretreated with netrin-1 for 90 min and washed extensively with PBS before exposure to fMLP. Netrin-1 pretreatment of monocytes also abolished fMLP-induced chemotaxis, excluding the possibility of a direct interaction between netrin-1 and fMLP (Fig. 4c). To determine whether these inhibitory effects of netrin-1 were mediated through the UNC5b receptor, monocytes were preincubated with antibodies that bind to the extracellular domain of this receptor. These antibodies blocked the inhibitory effect of netrin-1 on monocyte migration to fMLP in a dose-dependent manner (Fig. 4d). By contrast, control antibodies had no effect on the ability of netrin-1 to inhibit cell migration, suggesting that UNC5b is specifically required for this effect (Fig. 4d). Similar results also were obtained by using a second anti-UNC5b antibody (data not shown).
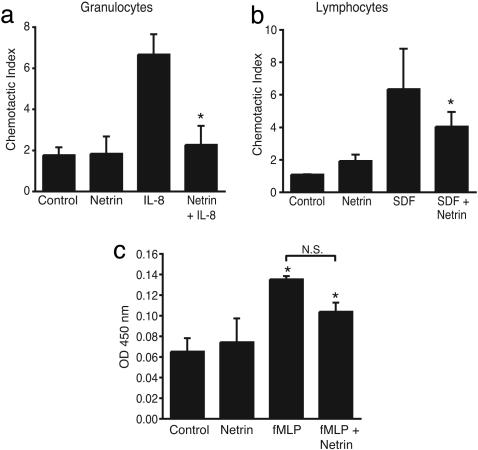
This profound effect on leukocyte migration was not specific to either this chemotactic stimulus or cell type, because we found that netrin-1 also potently inhibited granulocyte migration to IL-8 (Fig. 5a) and lymphocyte migration to stromal cell-derived factor 1α (Fig. 5b). As seen with monocytes, treatment of granulocytes with netrin-1 reduced chemokine-mediated migration to baseline levels, whereas treatment of lymphocytes with netrin-1 resulted in a 50% decrease in migration. Together, these data demonstrate that netrin-1 abolished chemotactic responses of all leukocyte subpopulations and chemokines tested, suggesting that it may play a general role in inhibiting leukocyte migration. However, because chemokines also regulate other leukocyte functions, including superoxide production, we tested the effect of netrin-1 on superoxide production by granulocytes. fMLP robustly induced superoxide production by granulocytes, and this induction was only modestly attenuated by netrin-1 (≈20%) (Fig. 5c). Although this trend was seen in five experiments, the difference was not statistically significant. No effect of netrin-1 alone on superoxide production was detected. Thus, netrin-1 seems to specifically regulate leukocyte migration.
Fig. 5.
Netrin-1 is a broad inhibitor of leukocyte chemotaxis. (a and b) Netrin-1 inhibits both IL-8 (50 ng/ml)-induced granulocyte migration and stromal cell-derived factor 1α (10 ng/ml)-induced lymphocyte migration when placed in the lower well of a Boyden chamber (*, P ≤ 0.005). (c) Netrin-1 does not significantly affect superoxide production by neutrophils treated with 10 nM fMLP (*, P < 0.05 significantly different from control).
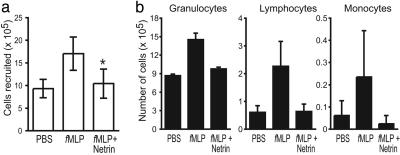
Our finding that expression of netrin-1 is modulated during acute infection with S. aureus in vivo and is down-regulated in endothelial cells by inflammatory cytokines in vitro suggests that this guidance molecule may regulate leukocyte movement into tissues. We used a peritonitis model to assess the effect of netrin-1 on cell recruitment in vivo. Mice were injected i.p. with fMLP, netrin-1, or both, and recruited cells were collected by peritoneal lavage after 4 h, counted, and stained with Giemsa stain as described in ref. 18. We found that fMLP caused a rapid recruitment of leukocytes to the peritoneum, compared with vehicle alone (PBS/1% BSA) (Fig. 6a). Leukocytes recruited by fMLP were predominantly granulocytes (92%), with few monocytes (2%) or lymphocytes (6%) (Fig. 6b). In the additional presence of netrin-1, the total number of leukocytes recruited to the peritoneum was reduced by 45% (P < 0.005) (Fig. 6a). Netrin-1 did not alter the distribution of leukocyte subpopulations recruited by fMLP, but rather reduced the migration of all leukocytes into the peritoneum. Injection of netrin-1 alone did not induce leukocyte recruitment into the peritoneum and was similar to injection of PBS/1% BSA alone (data not shown). The baseline granulocyte count in the peritoneal washes of the control mice was high. We concluded that this finding was explained by the use of 1% BSA in PBS as a vehicle. When this vehicle was used, we saw similar results. Similar results were obtained with an IL-8 peritonitis model (data not shown), where netrin-1 inhibited i.p. recruitment of leukocytes by 48%. Thus, similar to its effect on cell migration in vitro, netrin-1 is a potent inhibitor of leukocyte chemoattraction in vivo.
Fig. 6.
Netrin-1 inhibits leukocyte recruitment in vivo in a model of mouse peritonitis. (a) Mice were injected i.p. with 1 ml of PBS/1% BSA, fMLP (10 nM), netrin-1 (500 ng), or both fMLP plus netrin-1, and recruited leukocytes were harvested. fMLP induced a 2-fold increase in leukocytes in the peritoneum that was abrogated in the presence of netrin-1 (*, P < 0.005). Injection of netrin-1 alone was similar to that of the PBS control (data not shown). (b) Analysis of the leukocyte subpopulations in the peritoneal lavage revealed that 4 h after fMLP injection, a predominance of granulocytes was recruited. Netrin-1 inhibited the recruitment of all leukocyte subpopulations.
Discussion
Migration of inflammatory cells to the site of infection is a critical host response to limit the infectious agent and mount an immune response. However, inappropriate migration of these cells into organs also can result in tissue destruction, and this abnormal influx is thought to be the mechanism involved in many autoimmune diseases. Known barriers to aberrant cell migration include an intact vascular endothelium and the circulation of leukocytes in the inactive state. However, additional regulators of cell migration that prevent the unnecessary influx of cells into healthy organs are likely to exist. We have identified a previously uncharacterized function for the neuronal guidance cue netrin-1 outside the CNS, as a regulator of leukocyte migration during inflammation. In the lung, netrin-1 is highly expressed in the vascular endothelium under basal conditions, and during acute S. aureus infection, expression of this secreted molecule is down-regulated coincident with an influx of leukocytes into this tissue and inflammatory cytokine expression. This decrease in netrin-1 expression could be reproduced by treating vascular endothelial cells with TNF-α and IFN-γ, two cytokines that we found were highly induced with S. aureus infection. Netrin-1 potently inhibited chemokine-induced migration of leukocytes, consistent with the idea that, under steady-state conditions, netrin-1 may act as a barrier to prevent inflammatory cell penetration of the vascular endothelium, but at the times of infection, this barrier would be lowered to allow influx of leukocytes into affected tissues. As netrin-1 returns to baseline, excess leukocyte influx is kept in check, thus preventing excessive tissue destruction. Our identification of netrin-1 in tissues that are highly vascularized and sensitive to infection, such as the lungs, heart, and kidneys, is consistent with this proposed protective effect of netrin-1.
Netrin-1 was found to be a potent inhibitor of migration in all leukocyte subpopulations tested and was capable of inhibiting multiple chemotactic stimuli. This broad inhibitory effect was not due to a direct interaction of netrin-1 with the chemoattractants, because pretreatment of cells with netrin-1 rendered them refractory to subsequent stimuli. Consistent with its role in other tissues and species, netrin-1 appeared to predominantly affect migration of leukocytes. We postulate that netrin-1 may inhibit the effects of multiple chemokines by interrupting signaling through G protein-coupled receptors. We have previously shown that UNC5b, the netrin receptor that abolishes chemoattraction, binds to the Gαi subunit (15). By interrupting this pathway, netrin-1 would be expected to have a broad inhibitory effect on multiple chemoattractants and leukocyte subpopulations. Recently, a member of an unrelated family of neuronal guidance molecules, slit-2, also was shown to inhibit migration of granulocytes, lymphocytes (4), monocytes (5, 6), dendritic cells (7), and breast cancer cells (22), providing further evidence that neuronal guidance cues may play roles outside the CNS. These studies suggest that these important developmental signals may have previously unappreciated roles in regulating immune cell responses by directing cell migration.
The highly conserved attraction/repulsion paradigm is central to establishing neuronal patterning, and it is interesting to speculate on the potential roles of such a guidance system in the regulation of immune cells. In addition to acting as a barrier molecule, netrin-1 could potentially fine-tune the position of leukocytes by mediating similar context-dependent signaling through various receptors. Our finding that netrin-1 receptors are expressed in these tissues suggests that further exploration of the function of this neuronal guidance cue in regulating leukocyte trafficking pathways is likely to yield exciting new roles for these molecules (15).
The identification of endogenous factors that limit infection and inflammation has important medical implications. Leukocyte recruitment is a critical immune response to infection and injury, and likewise, pathways that prevent inflammatory cell migration into normal tissues in the absence of these conditions play an important role in preventing autoimmune tissue damage. Indeed, many human diseases are characterized by excessive inflammatory reactions. Pharmacologic anti-inflammatory agents (corticosteroids, nonsteroidal anti-inflammatory drugs, and cytokine antagonists) are widely used in clinical practice to treat a broad array of inflammatory conditions. The use of these anti-inflammatory agents is, unfortunately, frequently complicated by adverse events, which limits their clinical efficacy. Thus, the identification of an endogenous pathway that down-regulates inflammation would provide important new targets for pharmaceutical intervention.
Acknowledgments
We thank Lei Shi and Kazue Takahashi for their assistance with the S. aureus infection model and Marc T. Tessier-Lavigne for technical advice. This work was supported by National Institutes of Health Grants 501 HL 58819-04 (to T.B.K.) and AG20255 (to K.J.M.) and the Ellison Medical Foundation (to K.J.M.).
Conflict of interest statement: Massachusetts General Hospital has filed for a patent on netrin-1's anti-inflammatory actions in Drs. Kinane, Ly, and Komatsuzaki's name. This patent has not been issued. This potential patent has been licensed.
Abbreviations: DCC, deleted in colon cancer; UNC5b, UNC5 homologue B; fMLP, formylmethionylleucylphenylalanine.
References
- 1.Baggiolini, M. (2001) J. Intern. Med. 250, 91-104. [DOI] [PubMed] [Google Scholar]
- 2.Tessier-Lavigne, M. & Goodman, C. S. (1996) Science 274, 1123-1133. [DOI] [PubMed] [Google Scholar]
- 3.Yu, T. W. & Bargmann, C. I. (2001) Nat. Neurosci. 4, Suppl., S1169-S1176. [DOI] [PubMed] [Google Scholar]
- 4.Wu, J. Y., Feng, L., Park, H. T., Havlioglu, N., Wen, L., Tang, H., Bacon, K. B., Jiang, Z., Zhang, X. & Rao, Y. (2001) Nature 410, 948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, B., Blair, D. G., Plisov, S., Vasiliev, G., Perantoni, A. O., Chen, Q., Athanasiou, M., Wu, J. Y., Oppenheim, J. J. & Yang, D. (2004) J. Immunol. 173, 5914-5917. [DOI] [PubMed] [Google Scholar]
- 6.Kanellis, J., Garcia, G. E., Li, P., Parra, G., Wilson, C. B., Rao, Y., Han, S., Smith, C. W., Johnson, R. J., Wu, J. Y. & Feng, L. (2004) Am. J. Pathol. 165, 341-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan, H., Zu, G., Xie, Y., Tang, H., Johnson, M., Xu, X., Kevil, C., Xiong, W. C., Elmets, C., Rao, Y., et al. (2003) J. Immunol. 171, 6519-6526. [DOI] [PubMed] [Google Scholar]
- 8.Serafini, T., Kennedy, T. E., Galko, M. J., Mirzayan, C., Jessell, T. M. & Tessier-Lavigne, M. (1994) Cell 78, 409-424. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy, T. E., Serafini, T., de la Torre, J. R. & Tessier-Lavigne, M. (1994) Cell 78, 425-435. [DOI] [PubMed] [Google Scholar]
- 10.Hong, K., Hinck, L., Nishiyama, M., Poo, M. M., Tessier-Lavigne, M. & Stein, E. (1999) Cell 97, 927-941. [DOI] [PubMed] [Google Scholar]
- 11.Keleman, K. & Dickson, B. J. (2001) Neuron 32, 605-617. [DOI] [PubMed] [Google Scholar]
- 12.Hopker, V. H., Shewan, D., Tessier-Lavigne, M., Poo, M. & Holt, C. (1999) Nature 401, 69-73. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan, K., Strickland, P., Valdes, A., Shin, G. C. & Hinck, L. (2003) Dev. Cell 4, 371-382. [DOI] [PubMed] [Google Scholar]
- 14.Salminen, M., Meyer, B. I., Bober, E. & Gruss, P. (2000) Development (Cambridge, U.K.) 127, 13-22. [DOI] [PubMed] [Google Scholar]
- 15.Komatsuzaki, K., Dalvin, S. & Kinane, T. B. (2002) Biochem. Biophys. Res. Commun. 297, 898-905. [DOI] [PubMed] [Google Scholar]
- 16.Dalvin, S., Komatsuzaki, K., Anselmo, M. A., Kling, D. E., Schnitzer, J. J. & Kinane, T. B. (2004) Dev. Growth Differ. 46, 275-282. [DOI] [PubMed] [Google Scholar]
- 17.Dalvin, S., Anselmo, M. A., Prodhan, P., Komatsuzaki, K., Schnitzer, J. J. & Kinane, T. B. (2003) Gene Expr. Patterns 3, 279-283. [DOI] [PubMed] [Google Scholar]
- 18.El Khoury, J. B., Moore, K. J., Means, T. K., Leung, J., Terada, K., Toft, M., Freeman, M. W. & Luster, A. D. (2003) J. Exp. Med. 197, 1657-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, Y., Stein, E., Oliver, T., Li, Y., Brunken, W. J., Koch, M., Tessier-Lavigne, M. & Hogan, B. L. (2004) Curr. Biol. 14, 897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonardo, E. D., Hinck, L., Masu, M., Keino-Masu, K., Ackerman, S. L. & Tessier-Lavigne, M. (1997) Nature 386, 833-838. [DOI] [PubMed] [Google Scholar]
- 21.Przyborski, S. A., Knowles, B. B. & Ackerman, S. L. (1998) Development (Cambridge, U.K.) 125, 41-50. [DOI] [PubMed] [Google Scholar]
- 22.Prasad, A., Fernandis, A. Z., Rao, Y. & Ganju, R. K. (2004) J. Biol. Chem. 279, 9115-9124. [DOI] [PubMed] [Google Scholar]