Abstract
The mechanism by which hypoxia [low partial pressure of O2 (pO2)] elicits signaling to regulate pulmonary arterial pressure is incompletely understood. We considered the possibility that, in addition to its effects on smooth muscle, hypoxia may influence pulmonary vascular tone through an effect on RBCs. We report that exposure of native RBCs to sustained hypoxia is accompanied by a buildup of heme iron-nitrosyl (FeNO) species that are deficient in pO2-governed intramolecular transfer of NO to cysteine thiol, yielding a deficiency in the vasodilator S-nitrosohemoglobin (SNO-Hb). S-nitrosothiol (SNO)-deficient RBCs produce impaired vasodilator responses in vitro and exaggerated pulmonary vasoconstrictor responses in vivo and are defective in oxygenating the blood. RBCs from hypoxemic patients with elevated pulmonary arterial pressure (PAP) exhibit a similar FeNO/SNO imbalance and are thus deficient in pO2-coupled vasoregulation. Chemical restoration of SNO-Hb levels in both animals and patients restores the vasodilator activity of RBCs, and this activity is associated with improved oxygenation and lower PAPs.
Keywords: hemoglobin, red blood cell vasodilation, S-nitrosylation
In the systemic microcirculation, blood flow is regulated by physiological O2 gradients that couple the O2 content of blood to regulated vasodilation and vasoconstriction (1-3). Blood flow is thereby matched to tissue O2 demand. An analogous mechanism operates in the lungs, where O2 uptake (ventilation) is optimized through regulated vasodilation and vasoconstriction (perfusion). Blood flow is thereby matched to alveolar ventilation (2). Because it is Hb O2 saturation, not the partial pressure of O2 (pO2), that is coupled to blood flow in vivo (1, 3) it has been deduced that RBCs may serve as O2 sensors within the integrated vascular system. In support of this idea, it has been shown recently that RBCs can act as O2-responsive transducers of vasodilator and vasoconstrictor activity (4-10), at least partly by modulating the availability of NO (6-8, 10, 11). According to these studies, RBCs release NO bioactivity under hypoxia and sequester it at hyperoxia. The release of NO bioactivity would facilitate hypoxic vasodilation in peripheral tissues and oppose hypoxic pulmonary vasoconstriction (HPV) in the lungs.
The mechanism by which NO bioactivity escapes from RBCs is incompletely understood. It is generally accepted that the rapid reaction of NO with the hemes of Hb produces a heme-iron nitrosyl adduct (Hb[FeNO]) that exhibits no vasodilator activity (4, 7, 12). Hb also sustains S-nitrosylation at two cysteine residues conserved in all mammals and birds. Biochemical and mutational analyses (β93Cys→Ala) indicate that S-nitrosohemoglobin (SNO-Hb) is formed upon oxygenation of Hb[FeNO] by means of heme-to-Cys NO transfer (13-15) and by transnitrosylative transfer from low-mass S-nitrosothiols (SNOs) (16, 17). SNO-Hb is very stable in the oxygenated (or R) structure and thus cannot effectively dilate blood vessels (5, 10, 18). However, upon deoxygenation [or with change in the spin state of the hemes (3)], the vasodilator potency of SNO-Hb is markedly potentiated (5, 16, 18). Crystal structures and molecular models show that the β-Cys NO gains solvent access in the deoxygenated (or T) state (3, 19). Solvent-exposed NO can exchange with acceptor thiols within the N-terminal cytoplasmic domain of the RBC membrane anion exchange protein (AE1; band 3) (4, 15). Transnitrosylation of AE1 by SNO-Hb involves a direct protein-protein interaction. The steps by which the NO group is subsequently transferred to the vessel wall are not yet established, although recent studies suggest that membrane SNO becomes accessible to plasma reactants, including glutathione (10, 20).
Hb is continuously cycling in vivo between oxygenated and deoxygenated states that influence the propensity for binding vs. release of NO groups (5, 16, 18, 21). In support of this proposition, SNO-Hb levels have been found by several independent groups and methods (3) to be higher in oxygenated blood than in deoxygenated blood of adults (6, 8, 10) and newborns (22) and to vary as a function of tissue oxygen saturations (6, 8, 10, 23). Collectively, these studies raise the idea that NO bioactivity in vivo is dispensed to dilate blood vessels in proportion to the degree of hypoxia. Whereas the focus to date has been on the potential role of this mechanism across the systemic arteriolar O2 gradient (regulating tissue O2 delivery), the hypoxic influence on RBCs is exerted throughout the microcirculation, venous system, and pulmonary arterial circuit, and the amounts of SNO-Hb entering the lungs of humans are believed to be quite substantial (6, 8, 10). RBCs are therefore potentially poised to regulate pulmonary vascular resistance (PVR) and ventilation-perfusion (V/Q) matching at basal conditions.
Methods
Measurement of HbNO in RBCs. SNO-Hb, Hb[FeNO], and total HbNO were measured by using photolysis-chemiluminescence and EPR as described in ref. 6 (see also Supporting Text, which is published as supporting information on the PNAS web site). To avoid a point of confusion in the literature, we note that nitrite (<1-2% yield) and nitrate (in the presence or absence of thiol; <0.01% yield) are not detected by photolysis-chemiluminescence (and samples are also desalted before analysis).
EPR. EPR was performed as described in ref. 24; see also Supporting Text.
Vascular Bioassay. RBC vasoactivity was studied as described in ref. 6; see Supporting Text.
Isolated Perfused Lung. Isolated rabbit lungs were perfused with buffer; see Supporting Text.
Open-Chest Pig Preparation and RBC Infusion. Anesthetized (with isoflurane) and paralyzed (with pancuronium) pigs were mechanically ventilated. See Supporting Text.
Patient Characteristics, Hemodynamic and Laboratory Measurements, Ethyl Nitrite (ENO) Synthesis and Administration, and Statistical Analysis. See Supporting Text. The protocol was approved by Duke University's Institutional Review Board.
Results
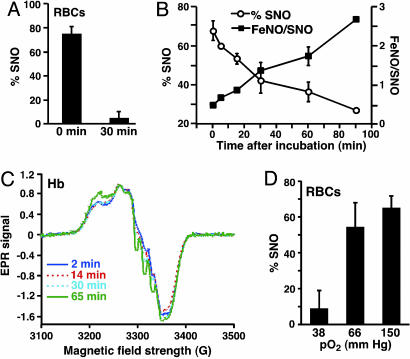
O2 Concentration Dependence and Time Dependence of SNO Yield in Native RBCs. Exposure of human venous blood RBCs [pO2 ≈ 40 mmHg (1 mmHg = 133 Pa)] to room air (pO2 ≈ 150 mmHg) immediately after phlebotomy triggers the formation of SNO-Hb from Hb[FeNO] (6), recapitulating the oxygenation-induced production of SNO-Hb across the lungs (6, 8, 13). We hypothesized that the depressed Hb O2 saturation (i.e., sustained hypoxia) that accompanies elevated pulmonary arterial pressure (PAP) might limit the ability to form SNO-Hb and thereby exacerbate pulmonary vasoconstriction. As a first step in testing this idea, we compared the amount of SNO-Hb produced in human RBCs exposed to air immediately (within 5 min) after venipuncture vs. after a 30-min delay, during which the RBCs were maintained at a physiological venous pO2 (38 mmHg) (Fig. 1A). Whereas SNO-Hb was abundant in RBCs that had been aerated rapidly, it was undetectable in RBCs after more prolonged (30 min) exposure to mild hypoxia (Fig. 1 A). Total amounts of NO bound by Hb (heme plus thiol) did not differ between the two conditions (data not shown). Thus, prolonged exposure to low physiological O2 saturation impairs the O2-induced exchange of NO between heme and cysteine thiol.
Fig. 1.
Sustained hypoxia impairs production of SNO-Hb. (A) SNO-Hb yield in venous RBCs exposed to room air either immediately (0 min) or 30 min after acquisition. (B) SNO-Hb yield from NO/deoxyHb mixtures (Hb[FeNO]) (pO2 < 1 mm Hg; 1 μM NO) aerated at varying times after incubation. (C) EPR spectra of Hb[FeNO] mixtures held at low pO2 (HbO2 saturation ≈ 45%) for varying periods. (D) SNO yield in normal RBCs oxygenated at pO2 = 38 mmHg, pO2 = 66 mmHg, or pO2 = 150 mmHg (room air).
O2 Concentration Dependence and Time Dependence of SNO Yield in Isolated Hb. Transfer of NO from the hemes to thiols of Hb takes place within the β-subunit (3). The binding of NO to the β-subunit is favored in oxygenated (R-state) molecules and, conversely, disfavored in deoxgenated (T-state) molecules (3), where it ultimately lodges on the α-hemes (25). A peculiarity of NO binding to the α-chain is its tendency to generate a so-called five-coordinate (high-affinity) heme-NO complex. NO bound as five-coordinate α-NO cannot migrate readily to the β-chains upon oxygenation (25). Thus, the predicted order of efficiency with which NO can migrate from hemes to thiols is β > six-coordinate α > five-coordinate α. Because the disposition of NO bound to Hb is allosterically linked with blood oxygenation (6), we hypothesized that the depressed Hb O2 saturation accompanying lung disease might limit the ability to form SNO-Hb.
To investigate this idea, we analyzed the efficiency of SNO-Hb formation in deoxyHb/NO preparations (Hb[FeNO][FeII]3) that were oxygenated after NO had been added (to enhance the EPR signal), immediately or with increasing intervals of sustained hypoxia. SNO-Hb yield was greatest when the aeration of Hb[FeNO] was immediate and fell progressively as a function of the duration of hypoxic incubation (Fig. 1B). EPR showed that the initial NO-heme product was evenly distributed between six-coordinate α-NO and β-NO (Fig. 1C). However, features of both six- and five-coordinate α-heme-NO complexes strengthened over time. In other words, NO had migrated from the β- to the α-heme subunits and, within the α-subunit, had partly induced the five-coordinate α-heme NO state (25) (Fig. 1C). It should be noted that whereas five-coordinate α-NO is the major NO species produced at high NO saturations of Hb (26), it was a relatively minor product at these more physiological concentrations of NO. In addition, we noted that SNO-Hb was not detected under anaerobic conditions and, predictably, that the amounts of SNO-Hb that formed upon oxygenation were dependent on the pO2 of the oxygenating solution (Fig. 1D). Thus, the yield of SNO is a function of both the extent and duration of hypoxia.
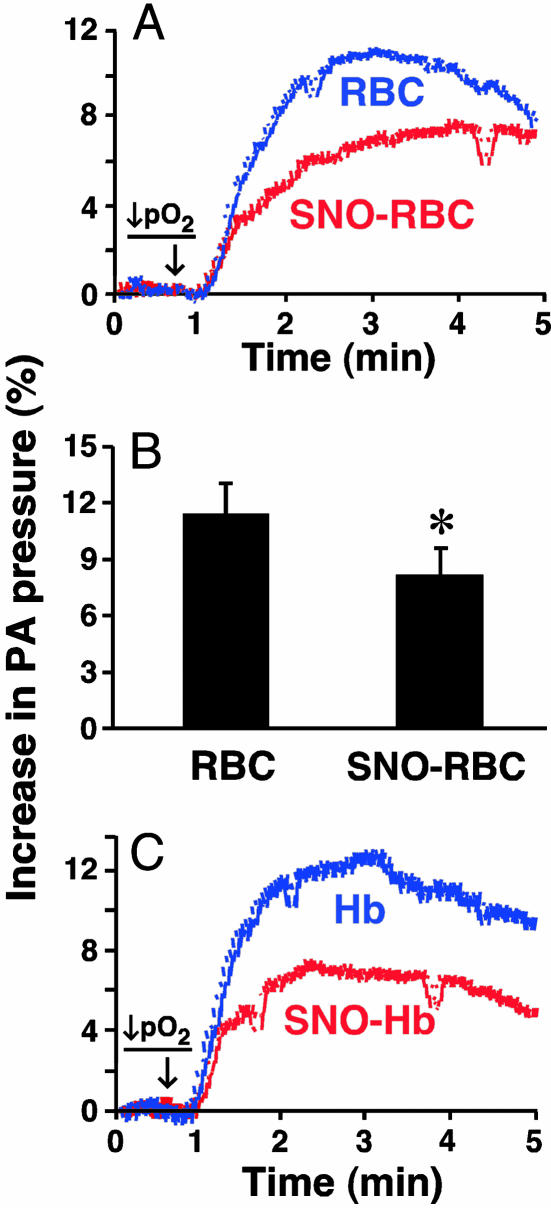
Influence of RBC SNO on PAP ex Vivo. Hypoxia raises PAP [HPV (6)], and HPV may strengthen as a function of Hb concentration (27). In contrast, it has recently been reported that RBCs containing SNO-Hb dilate pulmonary arteries under hypoxia (10). We reasoned that the net effect of RBCs might reflect their SNO content. Indeed, in the isolated perfused rabbit lung, HPV was blunted by SNO-replete RBCs relative to the response with RBCs in which SNOs were depleted to ≈85% of basal levels by sustained deoxygenation (see Methods and Fig. 1) and then reoxygenated without adding NO (Fig. 2 A and B). HPV was also weaker in the presence of isolated SNO-Hb (1 μM) vs. unmodified Hb (which augmented HPV) (Fig. 2C). Thus, RBC-SNO may protect against excessive increases in PVR induced by hypoxia.
Fig. 2.
Influence of SNO-Hb depletion on RBC-dependent hypoxic pressor responses (HPV) in isolated perfused lungs. (A and B) HPV in the presence of RBCs either depleted of SNO-Hb or replete in SNO-Hb (≈10 nM final concentration). Representative tracings are depicted in A, and mean (±SEM) of n = 5 experiments is shown in B. (C) Influence of Hb or SNO-Hb (1 μM) HPV (pO2 = 25 mmHg) in isolated perfused lungs.
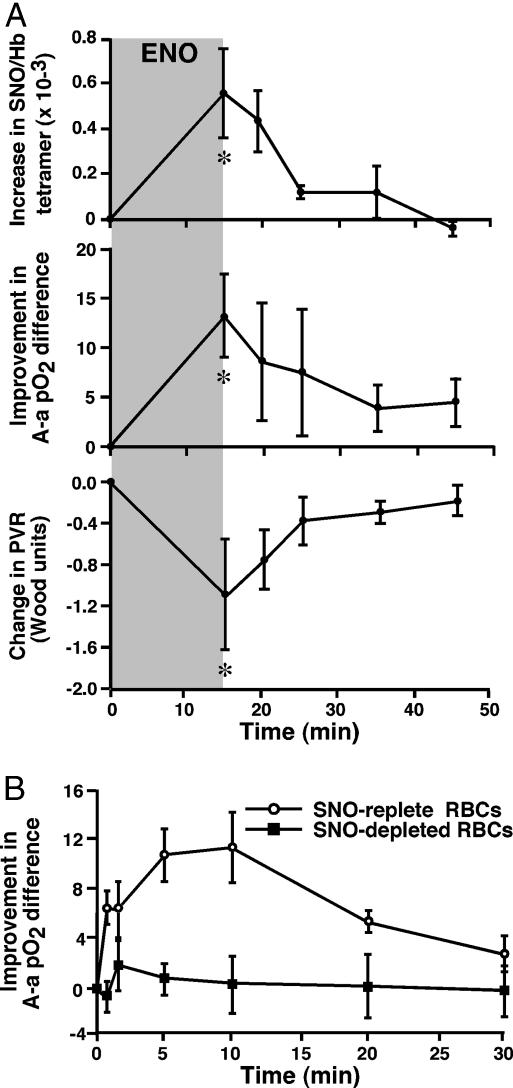
Effects of RBC-SNO on Hemodynamics and Gas Exchange in Vivo. In the intact animal, HPV serves a physiological role in matching Q to V. Impairment in oxygenation (V/Q mismatch) arises if HPV is either excessive or insufficient. To establish that RBC-SNO can affect PVR in vivo and to assess its effect on oxygenation, we performed two complementary experiments in an adult porcine model. In the first experiment, RBC-SNO levels were raised in vivo by ventilating pigs for 15 min with 100 ppm ENO, a SNO-generating prodrug (28, 29), after which the gas was stopped and SNO-Hb levels were correlated with hemodynamic response and gas exchange for an additional 30 min. In the second experiment, porcine RBCs (100 ml) containing or depleted of SNO (through hypoxic exposures; see Methods) were infused directly into the main pulmonary artery, and the effects on arterial blood gases were compared over 30 min. Fig. 3A shows that PVR and arterial blood oxygenation rise and fall commensurate with changes in SNO-Hb levels, and Fig. 3B shows that infusion of SNO-containing RBCs recapitulates these effects: RBC-SNO induced profound improvements in blood oxygenation and a significant fall in PVR (8 ± 10% decrease at 5 min; n = 6; mean ± SD) relative to SNO-deficient RBCs (3 ± 8% increase in PVR; n = 18; P = 0.035). These data strongly suggest a cause-and-effect relationship between SNO-Hb levels and improvements in pulmonary parameters.
Fig. 3.
Influence of RBC-SNO repletion in vivo or ex vivo on hemodynamics and gas exchange in pigs. (A) Temporal correlation of changes in SNO-Hb level (Top), alveolar-arterial (A-a) pO2 difference (Middle), and mean PVR (Bottom) in pigs inhaling the SNO-generating gas ENO (100 ppm, 15 min). SNO/Hb, mol of SNO per mol of Hb (tetramer). Baseline mean (±SEM) pO2 and pCO2 were 91 (±3) and 38 (±2) mm Hg, respectively. (B) RBC-mediated improvement in A-a pO2 difference in pigs after i.v. infusion of either SNO-depleted or SNO-replete porcine RBCs (P < 0.05). Baseline mean (±SEM) pO2 and pCO2 were 97 (±5) and 38 (±1) mm Hg in the group receiving SNO-depleted RBCs, and 99 (±5) and 38 (±1) mm Hg in those receiving SNO-replete RBCs.
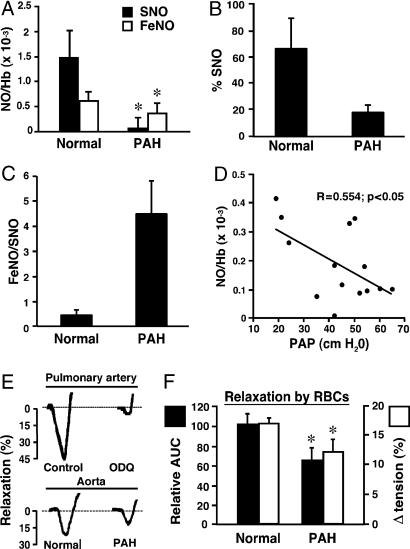
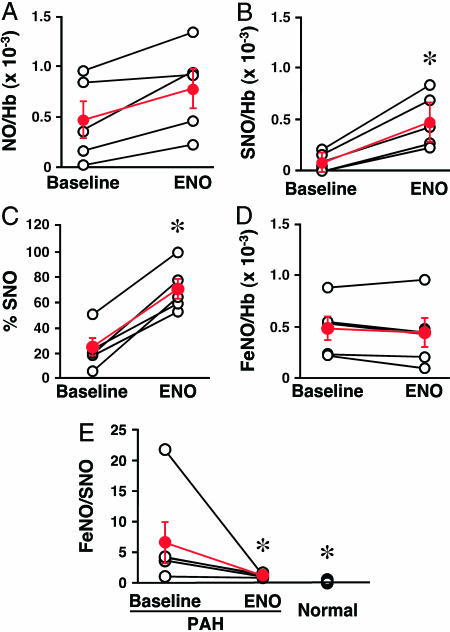
SNO-Hb and Hb[FeNO] Levels in Pulmonary Arterial Hypertension (PAH). To place our findings in pathophysiological context, we measured the levels of Hb[FeNO] and SNO-Hb in RBCs from five hypoxemic patients with moderate to severe PAH (see Table 1, which is published as supporting information on the PNAS web site) and compared them with healthy human controls (n = 8). Total levels of NO bound to Hb in normal patients were approximately five times higher than in PAH patients (Fig. 4A; P < 0.01). SNO-Hb, the bioactive component (4, 7, 10), was virtually absent in all PAH subjects (Fig. 4 A and B; P < 0.01 vs. normal patients), whereas levels of Hb[FeNO] did not differ significantly between the two groups. Thus, the decrease in HbNO was associated with a disproportionate loss in SNO-Hb (Fig. 4C). In other words, hypoxemic PAH patients show a buildup of heme iron-nitrosyl species that are evidently deficient in the pO2-governed exchange of NO with cysteine thiol, emulating the defect created by sustained hypoxia in vitro (Fig. 1). To test for a relationship between the deficiency of SNO-Hb and elevated PAP, we measured Hb-NO levels in subjects undergoing right heart catheterization. An inverse correlation was found between mean PAP and venous Hb-NO levels (n = 14; P < 0.05; R = 0.55; Fig. 4D). In many cases, SNO-Hb was too low to be plotted accurately as a function of PAP.
Fig. 4.
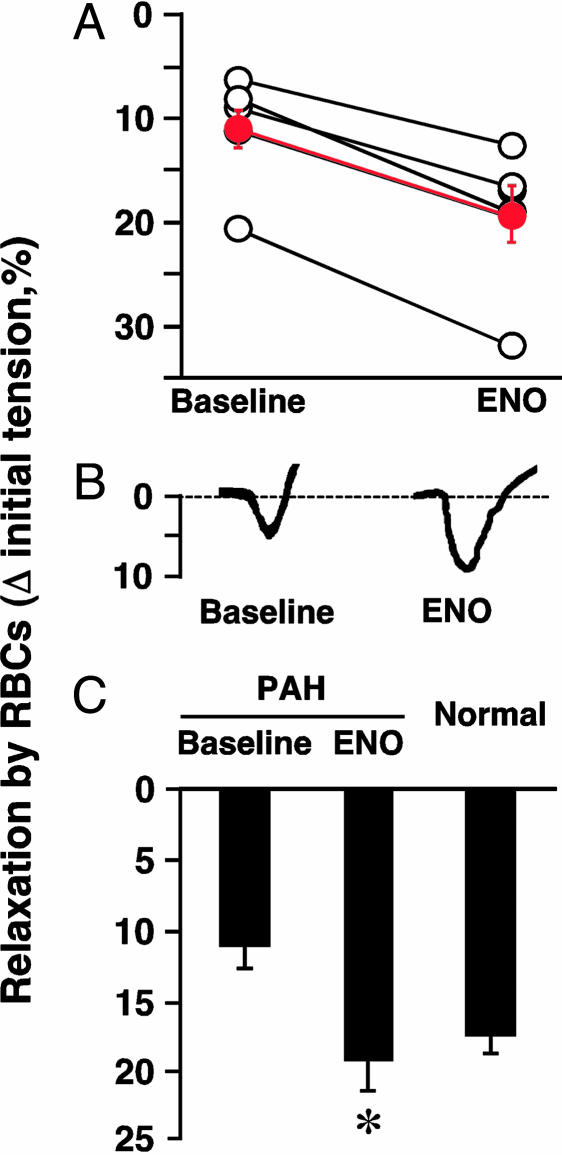
RBC-NO levels, RBC function, and PAP in patients. (A) Levels of SNO-Hb and Hb[FeNO] in arterial blood of normal human subjects and PAH patients. NO/Hb, mol of NO per mol of Hb (tetramer). *, P < 0.05 vs. normal patients; n = 5-8. (B) SNO content of blood presented as the percentage of total NO bound to Hb. (C) Increased FeNO/SNO signature of PAH. (D) Inverse correlation between RBC-NO levels and PAP. Hb-NO levels (expressed as mol of NO per mol of Hb tetramer) and baseline PAP in 14 patients. (E and F) Vasorelaxation (percent change in initial tension) by RBCs from normal subjects and from PAH patients. (E) Actual tracings of the hypoxia-mediated vasodilator response by RBCs of deendothelialized pulmonary artery [in the presence and absence of the guanylate cyclase inhibitor ODQ [1H(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one] (1 μM)] and intact aortic rings. (F) Mean aortic ring results (±SEM) expressed as the relative area under the time-tension curve (AUC) or as the peak decrease in tension; data are from seven individuals in each group. Hypoxia-mediated vasodilation by RBCs is impaired in PAH. *, P < 0.05 vs. normal subjects.
RBC Bioactivity in Normal Patients vs. PAH Patients. Native RBCs elicit graded vasodilator responses that are inversely proportional to pO2s (3, 6, 10). As shown in Fig. 4E, hypoxic vasorelaxations by RBCs from healthy subjects are (i) qualitatively similar in pulmonary artery and aorta, (ii) largely preserved in deendothelialized vessels, and (iii) largely blocked by the guanylate cyclase inhibitor ODQ [1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one] (1 μM; Fig. 4E). Moreover, RBCs from normal subjects produced greater vasorelaxation than those of PAH patients (Fig. 4 E and F, P < 0.05). (To address a point of potential confusion, we emphasize that we do not add NO or nitrite to RBCs, and find no effect of added nitrite under these conditions). Thus deficiency of SNO-Hb is associated with impaired vasodilation by RBCs.
In Vivo Repletion of RBC-SNO in Patients. We reasoned that repletion of SNO-Hb in patients with PAH should normalize RBC vasodilation and that, to the extent that the impairment in vasodilation by RBCs contributes to pulmonary hypertension or V/Q mismatching, SNO repletion of RBCs with ENO should demonstrate salutary effects (for protocols, see Supporting Text). SNO-Hb levels. Inhalation of ENO significantly increased levels of RBC-NO compared with baseline values (P < 0.05; Fig. 5A). This increase was attributable primarily to an increase in SNO-Hb (Fig. 5 B and C), whereas no significant change in Hb[FeNO] was detected (Fig. 5D). Levels of SNO-Hb in ENO-treated patients were not statistically different from those of controls (Fig. 5E).
Fig. 5.
In vivo RBC-SNO repletion by ENO inhalation in PAH patients. (A-C) Increases in total Hb-bound NO (A) and SNO-Hb (B and C) were seen in every patient treated with ENO inhalation (70 ppm, 10 min). (D) Hb[FeNO] did not change during ENO inhalation. (E) ENO normalized the FeNO/SNO ratio. *, significant difference vs. baseline by paired t test (A and B).
RBC vasodilation. RBCs obtained from patients during inhalation of ENO elicited greater relaxation than did RBCs obtained from patients at baseline (Fig. 6 A and B). The extent of vasorelaxation by RBCs from patients who had breathed ENO did not differ from that produced by RBCs from normal subjects (Figs. 4C and 6C). Thus, ENO normalized the hypoxic vasodilator activity of RBCs of patients with PAH. [At high pO2, RBCs sampled before or during ENO treatment produced comparable degrees of vasoconstriction (not shown)].
Fig. 6.
In vivo restoration of RBC bioactivity by ENO inhalation in PAH patients. RBC relaxation in PAH patients before and after ENO (A), and individual (B) and mean data from eight patients (C) are shown. RBC-induced vasorelaxation was enhanced by ENO inhalation (70 ppm, 10 min). *, P < 0.05 relative to baseline, which did not differ significantly from normal RBCs.
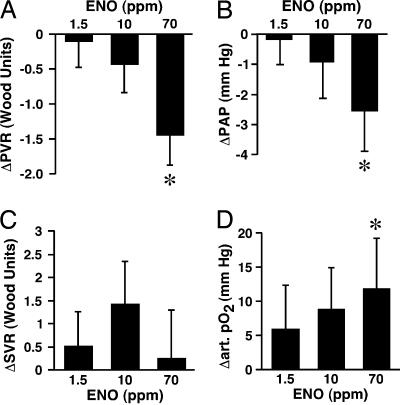
Hemodynamics and oxygenation. ENO produced dose-dependent decreases in PAP and PVR (Fig. 7 A and B; P < 0.05; see also Table 2, which is published as supporting information on the PNAS web site). Nine of 10 patients responded (Table 2). After a washout period, hemodynamics returned to baseline without rebound (P was not significant vs. baseline values). Mean systemic arterial pressure (Table 2), systemic vascular resistance (Fig. 7C), and cardiac index (not shown) were unchanged by ENO. Arterial pO2 improved dose-dependently (P < 0.05; Fig. 7D). Individual responses are provided in Table 2.
Fig. 7.
Effects of ENO on hemodynamics and oxygenation in PAH patients. Changes in PVR (A), PAP (B), systemic vascular resistance (C), and arterial pO2 (D) in response to inhaled ENO at ≈1.5, 10, or 70 ppm (0.0025%, 0.025%, or 0.125%, 10 min each) are shown. Data represent the mean ± SEM from 5-10 patients. *, significant difference vs. baseline by mixed procedures (in A, B, and D).
Discussion
Sustained hypoxemia is a common consequence of lung disease and a common cause of pulmonary hypertension. Current ideas surrounding the mechanism(s) by which pathological hypoxia raises PAP are focused mainly on the vasculature and do not include an active role for RBCs. We now demonstrate that (i) hypoxia depletes RBCs of SNO-Hb and consequently impairs their ability to counteract pulmonary vasoconstriction and improve oxygenation, (ii) RBCs from hypoxemic patients with elevated PAPs exhibit depletion of SNO-Hb and consequent impairment in pO2-regulated vasodilation, and (iii) in situ repletion of SNO-Hb corrects these physiological deficits in both animals and humans. More generally, our data suggest that blood-borne SNO bioactivity may play a physiological role in V/Q matching and that hypoxia-induced defects in NO processing by RBCs may contribute to pulmonary hypertension.
We find that sustained hypoxemia alters the disposition of NO within Hb (heme vs. thiol and α- vs. β-hemes) and thereby limits the production of SNO-Hb. Parallel analysis by EPR spectroscopy revealed a loss of the reactive β-chain FeNO [that exists in equilibrium with β-SNO (3)] and accumulation over time of Hb[αFeIINO] [from which NO cannot be readily dislodged upon oxygenation (3)], thus rationalizing the lower SNO-Hb yield. We emphasize that the six-coordinate, rather than the five-coordinate, Hb[αFeIINO] species that predominates with supraphysiological amounts of NO accumulates under these more physiological conditions and that additional chemical processes, including the binding of O2 to the β-subunit vacated by NO, may operate to suppress S-nitrosylation (i.e., O2 blocks the access of NO to the β-heme; for details, see ref. 3). In addition, SNO-Hb production from native RBC Hb[FeNO] was more sensitive to pO2 and more readily impaired by hypoxia than cell-free Hb[FeNO] (Fig. 1 A vs. B), likely reflecting the presence in RBCs of allosteric effectors such as 2,3-diphosphoglycerate and suggesting a different Hb[FeNO] microcomposition [i.e., the ligation and oxidation state of the accompanying hemes in the NO-containing Hbs are different in RBCs vs. purified Hb (3)]. More generally, we observed a requirement that pO2 exceed the P50 (pO2 at which Hb is 50% saturated with O2) for efficient production of SNO-Hb, consistent with the crystallographic studies showing that SNO-Hb forms only within the R structure (19). Thus, effective NO processing by RBCs depends on both the composition of the NO micropopulation and the concentration and duration of the O2 exposure.
We suggest the following model. The endothelium of the pulmonary artery is a rich source of NO (30). Thus, NO scavenging by RBCs does not increase PVR unless NO synthesis is reduced by hypoxia (3, 31). RBCs thereby prevent blood from perfusing poorly ventilated alveolar units in HPV (27) (decrease in V/Q mismatch). A coordinated increase in flow to better-ventilated units (while mitigating excessive HPV) would be of added physiological benefit (increase in V/Q match). RBC-SNO entering the lung at mixed venous pO2 is poised to serve this function. NO synthesis may also decline as a result of endothelial dysfunction. However, our results suggest that decreased NO synthesis in PAH does not account entirely for the deficiency in RBC-SNO, because the amount of Hb[FeNO] entering the lungs is only slightly depleted. Rather, the much larger decline in SNO vs. FeNO (Fig. 4 B and C) points to a decrease in the pO2-governed intramolecular transfer of NO from heme iron (FeNO) to cysteine thiol (SNO) across the lung. A deficiency in RBC-SNO may thus contribute to the NO insufficiency state of PAH, promoting pathological HPV.
Endothelium-derived NO can exert vasodilatory effects by raising cGMP. We have now demonstrated that potent relaxations of endothelium-denuded arteries by RBCs are also mediated, at least partly, by cGMP (Fig. 4E). cGMP-dependent relaxations of endothelium-denuded vessels excludes an obvious role for ATP that might be released from RBCs under hypoxia (3). Moreover, our results strongly suggest that ENO (in contrast with inhaled NO) mainly elicits its effect through this RBC-based mechanism. Specifically, the changes in oxygenation and PVR mediated by ENO were correlated precisely over time with levels of RBC-SNO (a duration of effects that greatly exceeds the lifetime of NO), and infusions of RBC-SNO reproduced the effects of ENO (on PVR and oxygenation) (Fig. 3). Thus, SNO-containing RBCs clearly mediate improvements in V/Q matching and decreases in PVR.
There has been recent confusion in the literature over the putative role of nitrite in hypoxia-mediated relaxations. Gladwin, Schechter, and colleagues (11) have suggested that nitrite is a principal effector of hypoxic vasodilation in vivo. Two physiological lines of evidence have been provided. (i) An A-V nitrite gradient. (ii) Vasodilation by infused nitrite. However, plasma nitrite (and thus any A-V nitrite gradient) is in fact principally a measure of endothelial nitric-oxide synthase (eNOS) activity (i.e., NO conversion to nitrite), which is higher in arteries than veins, rather than a measure of NO bioactivity (nitrite conversion to NO) (12, 32). Hypoxic vasodilation in vivo is largely eNOS-independent and thus obligatorily independent of nitrite (3, 12). In addition, vasodilation by infused nitrite in vivo [a pharmacological effect that is characterized by an increase in venous O2 saturation (11)] does not bear on physiological hypoxic vasodilation (which is defined by a decrease in venous O2 saturation). Indeed, the in vitro relaxations attributed to nitrite (11) are far too slow to meet the temporal requirements for hypoxic relaxations in vivo (3, 12, 33). Finally, although we (3, 6, 24) and, subsequently, others (11) have reported that Hb can, under certain conditions, transform inorganic nitrite into bio-active SNO-Hb by means of an FeNO intermediate [which itself exerts no vasodilatory activity (4, 7, 12)], we emphasize that we do not supplement RBCs with nitrite (or NO) here and find no effect of added nitrite on either RBC or Hb vasoactivity under standard conditions. Similarly, Cosby et al. (11) did not observe an effect of nitrite on either the extent or rate of RBC relaxations in vitro. Perhaps infused nitrite mediates its reported vasodilator effect in vivo (11) through formation of SNO-Hb (24) or through its metabolism to NO or SNO in vascular smooth muscle (34), but in our in vitro systems and in our patients, it is the level of SNO-Hb that predicts RBC vasoactivity.
It has been reported recently that RBCs from patients with diabetes (7) and sickle cell disease (15) exhibit impaired NO vasodilator activity, attributed to Hb glycosylation (7) and genetic (sickle) mutation (15), respectively, which alter the allosteric properties of the Hb tetramer. Taken together with our findings of hypoxia-induced alterations in the composition of Hb-NO species that subserve RBC vasodilation, these data point to the general possibility that defects in RBC NO processing (and/or a deficiency of RBC-SNO) may underlie a range of impairments, including systemic and pulmonary hypertension, the vascular diatheses associated with hemoglobinopathies, and the ischemic morbidity of RBC transfusion (35, 36). Strategies aimed at normalizing RBC NO bioactivity may provide an innovative approach to treatment of vascular disorders.
Supplementary Material
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL42444 (to J.S.S.) and HL04014 (to T.J.M.) and National Science Foundation Grant MCB 00981228 (to D.J.S.).
Abbreviations: pO2, partial pressure of O2; PAP, pulmonary arterial pressure; PVR, pulmonary vascular resistance; SNO-Hb, S-nitrosohemoglobin; SNO, S-nitrosothiol; HPV, hypoxic pulmonary vasoconstriction; V/Q, ventilation-perfusion; ENO, ethyl nitrite; PAH, pulmonary arterial hypertension.
References
- 1.Gonzalez-Alonso, J., Richardson, R. S. & Saltin, B. (2001) J. Physiol. 530, 331-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyton, A. C. (1986) Textbook of Medical Physiology (Saunders, Philadelphia).
- 3.Singel, D. J. & Stamler, J. S. (2005) Annu. Rev. Physiol. 67, 99-145. [DOI] [PubMed] [Google Scholar]
- 4.Pawloski, J. R., Hess, D. T. & Stamler, J. S. (2001) Nature 409, 622-626. [DOI] [PubMed] [Google Scholar]
- 5.Stamler, J. S., Jia, L., Eu, J. P., McMahon, T. J., Demchenko, I. T., Bonaventura, J., Gernert, K. & Piantadosi, C. A. (1997) Science 276, 2034-2037. [DOI] [PubMed] [Google Scholar]
- 6.McMahon, T. J., Moon, R. E., Luchsinger, B. P., Carraway, M. S., Stone, A. E., Stolp, B. W., Gow, A. J., Pawloski, J. R., Watke, P., Singel, D. J., et al. (2002) Nat. Med. 8, 711-717. [DOI] [PubMed] [Google Scholar]
- 7.James, P. E., Lang, D., Tufnell-Barret, T., Milsom, A. B. & Frenneaux, M. P. (2004) Circ. Res. 94, 976-983. [DOI] [PubMed] [Google Scholar]
- 8.Datta, B., Tufnell-Barrett, T., Bleasdale, R. A., Jones, C. J., Beeton, I., Paul, V., Frenneaux, M. & James, P. (2004) Circulation 109, 1339-1342. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich, H. H., Ellsworth, M. L., Sprague, R. S. & Dacey, R. G., Jr. (2000) Am. J. Physiol. Heart Circ. Physiol. 278, H1294-1298. [DOI] [PubMed] [Google Scholar]
- 10.Doctor, A., Platt, R., Sheram, M. L., Eischeid, A., McMahon, T., Maxey, T., Doherty, J., Axelrod, M., Kline, J., Gurka, M., et al. (2005) Proc. Natl. Acad. Sci. USA 102, 5709-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosby, K., Partovi, K. S., Crawford, J. H., Patel, R. P., Reiter, C. D., Martyr, S., Yang, B. K., Waclawiw, M. A., Zalos, G., Xu, X., et al. (2003) Nat. Med. 9, 1498-1505. [DOI] [PubMed] [Google Scholar]
- 12.Luchsinger, B. P., Rich, E. N., Yan, Y., Williams, E. M., Stamler, J. S. & Singel, D. J. (2005) J. Inorg. Biochem. 99, 912-921. [DOI] [PubMed] [Google Scholar]
- 13.Gow, A. J. & Stamler, J. S. (1998) Nature 391, 169-173. [DOI] [PubMed] [Google Scholar]
- 14.Herold, S. & Rock, G. (2003) J. Biol. Chem. 278, 6623-6634. [DOI] [PubMed] [Google Scholar]
- 15.Pawloski, J. R., Hess, D. T. & Stamler, J. S. (2005) Proc. Natl. Acad. Sci. USA 102, 2531-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia, L., Bonaventura, C., Bonaventura, J. & Stamler, J. S. (1996) Nature 380, 221-226. [DOI] [PubMed] [Google Scholar]
- 17.Liu, L., Yan, Y., Zeng, M., Zhang, J., Hanes, M. A., Ahearn, G., McMahon, T. J., Dickfeld, T., Marshall, H. E., Que, L. G. & Stamler, J. S. (2004) Cell 116, 617-628. [DOI] [PubMed] [Google Scholar]
- 18.McMahon, T. J., Stone, A. E., Bonaventura, J., Singel, D. J. & Stamler, J. S. (2000) J. Biol. Chem. 275, 16738-16745. [DOI] [PubMed] [Google Scholar]
- 19.Chan, N. L., Kavanaugh, J. S., Rogers, P. H. & Arnone, A. (2004) Biochemistry 43, 118-132. [DOI] [PubMed] [Google Scholar]
- 20.Lipton, A. J., Johnson, M. A., Macdonald, T., Lieberman, M. W., Gozal, D. & Gaston, B. (2001) Nature 413, 171-174. [DOI] [PubMed] [Google Scholar]
- 21.Pezacki, J. P., Ship, N. J. & Kluger, R. (2001) J. Am. Chem. Soc. 123, 4615-4616. [DOI] [PubMed] [Google Scholar]
- 22.Funai, E. F., Davidson, A., Seligman, S. P. & Finlay, T. H. (1997) Biochem. Biophys. Res. Commun. 239, 875-877. [DOI] [PubMed] [Google Scholar]
- 23.Sonveaux, P., Kaz, A. M., Snyder, S. A., Richardson, R. A., Cardenas-Navia, L. I., Braun, R. D., Pawloski, J. R., Tozer, G. M., Bonaventura, J., McMahon, T. J., et al. (2005) Circ. Res. 96, 1119-1126. [DOI] [PubMed] [Google Scholar]
- 24.Luchsinger, B. P., Rich, E. N., Gow, A. J., Williams, E. M., Stamler, J. S. & Singel, D. J. (2003) Proc. Natl. Acad. Sci. USA 100, 461-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosaka, H., Sawai, Y., Sakaguchi, H., Kumura, E., Harada, N., Watanabe, M. & Shiga, T. (1994) Am. J. Physiol. 266, C1400-C1405. [DOI] [PubMed] [Google Scholar]
- 26.Hille, R., Palmer, G. & Olson, J. S. (1977) J. Biol. Chem. 252, 403-405. [PubMed] [Google Scholar]
- 27.Weissmann, N., Grimminger, F., Walmrath, D. & Seeger, W. (1995) Respir. Physiol. 100, 159-169. [DOI] [PubMed] [Google Scholar]
- 28.Moya, M. P., Gow, A. J., McMahon, T. J., Toone, E. J., Cheifetz, I. M., Goldberg, R. N. & Stamler, J. S. (2001) Proc. Natl. Acad. Sci. USA 98, 5792-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moya, M. P., Gow, A. J., Califf, R. M., Goldberg, R. N. & Stamler, J. S. (2002) Lancet 360, 141-143. [DOI] [PubMed] [Google Scholar]
- 30.Ignarro, L. J., Byrns, R. E., Buga, G. M. & Wood, K. S. (1987) Circ. Res. 61, 866-879. [DOI] [PubMed] [Google Scholar]
- 31.Kantrow, S. P., Huang, Y. C., Whorton, A. R., Grayck, E. N., Knight, J. M., Millington, D. S. & Piantadosi, C. A. (1997) Am. J. Physiol. 272, L1167-L1173. [DOI] [PubMed] [Google Scholar]
- 32.Kleinbongard, P., Dejam, A., Lauer, T., Rassaf, T., Schindler, A., Picker, O., Scheeren, T., Godecke, A., Schrader, J., Schulz, R., et al. (2003) Free Radical Biol. Med. 35, 790-796. [DOI] [PubMed] [Google Scholar]
- 33.Allen, B. W. & Piantadosi, C. A. (2004) Circ. Res. 94, e105. [PubMed] [Google Scholar]
- 34.Chen, Z., Foster, M. W., Zhang, J., Mao, L., Rockman, H. A., Kawamoto, T., Kitagawa, K., Nakayama, K. I., Hess, D. T. & Stamler, J. S. (2005) Proc. Natl. Acad. Sci. USA 102, 12159-12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao, S. V., Jollis, J. G., Harrington, R. A., Granger, C. B., Newby, L. K., Armstrong, P. W., Moliterno, D. J., Lindblad, L., Pieper, K., Topol, E. J., et al. (2004) J. Am. Med. Assoc. 292, 1555-1562. [DOI] [PubMed] [Google Scholar]
- 36.Hebert, P. C., Yetisir, E., Martin, C., Blajchman, M. A., Wells, G., Marshall, J., Tweeddale, M., Pagliarello, G. & Schweitzer, I. (2001) Crit. Care Med. 29, 227-234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.