Abstract
It is firmly believed that ancestral primates were nocturnal, with nocturnality having been maintained in most prosimian lineages. Under this traditional view, the opsin genes in all nocturnal prosimians should have undergone similar degrees of functional relaxation and accumulated similar extents of deleterious mutations. This expectation is rejected by the short-wavelength (S) opsin gene sequences from 14 representative prosimians. We found severe defects of the S opsin gene only in lorisiforms, but no defect in five nocturnal and two diurnal lemur species and only minor defects in two tarsiers and two nocturnal lemurs. Further, the nonsynonymous-to-synonymous rate ratio of the S opsin gene is highest in the lorisiforms and varies among the other prosimian branches, indicating different time periods of functional relaxation among lineages. These observations suggest that the ancestral primates were diurnal or cathemeral and that nocturnality has evolved several times in the prosimians, first in the lorisiforms but much later in other lineages. This view is further supported by the distribution pattern of the middle-wavelength (M) and long-wavelength (L) opsin genes among prosimians.
Keywords: color vision, diurnal, nocturnal, primate life style, prosimian
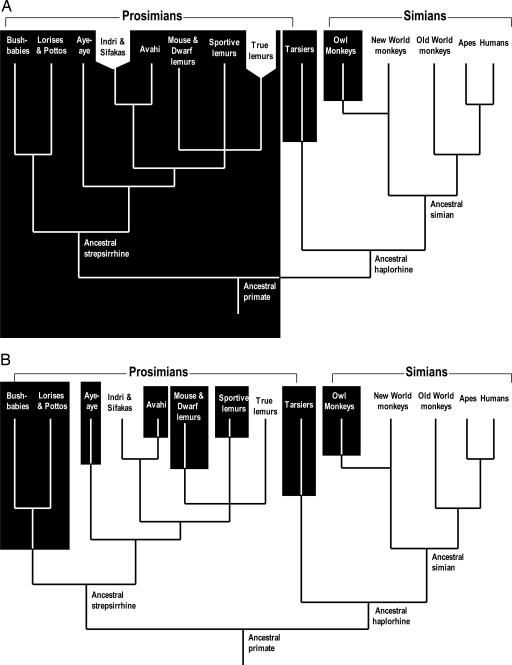
The traditional view of primate evolution (Fig. 1A) holds that the last common ancestor of living primates was nocturnal and that nocturnality has continued uninterrupted in most prosimian lineages. There are three major arguments for this view. First, the vast majority of extant prosimians are nocturnal. Therefore, the two lemuriform families, Indridae (sifakas) and Lemuridae (true lemurs), are assumed to have become either diurnal or cathemeral only in recent times. Second, the tapetum lucidum, which is a reflecting layer that enhances the ability of the eye to collect light, is found in many strepsirrhines, including some diurnal ones (1). This observation has been taken as evidence that the common ancestor of strepsirrhines had a tapetum and was, therefore, nocturnal. Third, the orbital convergence that is diagnostic of the primate clade is believed to be the result of a nocturnal visual-predator lifestyle (2). Paleontological data, however, are not conclusive about the lifestyle of early primates and suggest the existence of both nocturnal and diurnal forms (3).
Fig. 1.
The traditional view (A) and our view (B) of the evolution of nocturnality in primates. The black background indicates nocturnality. A is modified from Martin (27).
To explore this issue, we study the opsin genes in prosimians. It is well known that under nocturnality either the short-wavelength (S) or middle-/long-wavelength (M/L) opsin will experience relaxed selective constraints and may thus become nonfunctional. Indeed, a nonfunctional S opsin has been found in many nocturnal mammals (4-9). For example, the loss of the S opsin in the raccoon and the kinkajou might have occurred rather quickly because their close diurnal relative, the coati (5, 6), has a functional S opsin. Such a quick loss also is found in Aotus, the only nocturnal simian genus. Aotus diverged from its closest diurnal relatives, the callitrichines (marmosets and tamarins), only ≈13-15 million years ago (10). Yet, molecular studies indicate that the loss of the S opsin predates the radiation of Aotus (unpublished data and ref. 9). Such a strong correlation between opsin gene defects and lifestyles enables us to examine the traditional view of ancestral primate nocturnality by studying opsin evolution.
Materials and Methods
For the avahi, genomic DNA was obtained from hair follicles by using a DNA extraction kit (QIAamp DNA mini kit, Qiagen, Valencia, CA). For the rest of prosimians, genomic DNA was isolated from tissue samples by using the PureGene kit (Gentra Systems). Exons 1-5 of the S opsin in each species were amplified by the PCR from the genomic DNA. The primers initially used for the fat-tailed dwarf lemur and tarsiers were based on New World monkey S-opsin sequences (11). Overlapping PCR products were purified with the Wizard PCR purification system (Promega) or the QIAquick gel extraction kit (Qiagen) and directly sequenced on a 377 automatic sequencer (Applied Biosystems). After obtaining partial sequences of the fat-tailed dwarf lemur, prosimian-specific primers were designed to amplify the remaining species. Exons 3-5 of the X-linked opsin gene were amplified and sequenced for the avahi, the sportive lemur, Demidoff's bushbaby, and the pygmy loris according to our previous work described in ref. 12. The identification of M and L opsins was based on the amino acids at the critical residue sites responsible for spectral tuning (12-17).
Results and Discussion
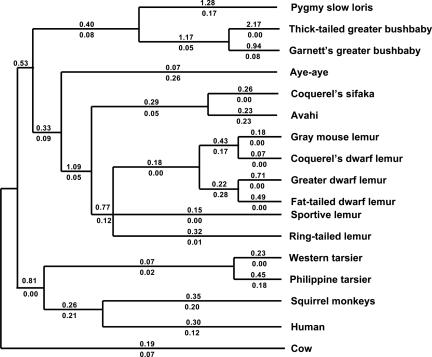
We first studied the M/L opsin gene. We had previously sequenced this gene in 21 prosimian species (12). In the present study, we sequenced four additional nocturnal species (the avahi, the sportive lemur, Demidoff's bushbaby, and the pygmy loris). We found no defect of this gene in any of these 25 species, which represent a broad sample across the prosimians. Moreover, the ratio of nonsynonymous (dN)/synonymous (dS) substitution rates is <0.1 in the majority of prosimian branches, which is less than half of the ratios (0.20 and 0.21) in the squirrel monkey branch and its ancestral branch (Fig. 2). Such a low value of dN/dS strongly indicates that these genes have been under purifying selection (and are thus functional) in the lemuriforms, including those that are presently nocturnal. In only a few branches (e.g., aye-aye and avahi) are the dN/dS ratios comparable with or slightly higher than that in the squirrel monkey lineage, which is about the average for mammalian genes. In summary, there is no evidence of functional relaxation in the prosimian M/L opsin. We might, therefore, expect functional relaxation in the S opsin.
Fig. 2.
The dN/dS ratio on each branch of the primate phylogeny (nonscaled branches) for the S opsin gene (above the branch) and the M/L opsin gene (below the branch). The primate phylogeny is based on the current view (28-31). The cow is included as an outgroup. The dN/dS ratios were estimated from DNA sequences of the first four exons of the S opsin gene and exons 3-5 of the M/L opsin gene by the codeml program of the paml software package, in which the free-ratio model was used (32). The cow, squirrel monkey, and human sequences are from GenBank (accession nos. U92557, U53875, and U53874).
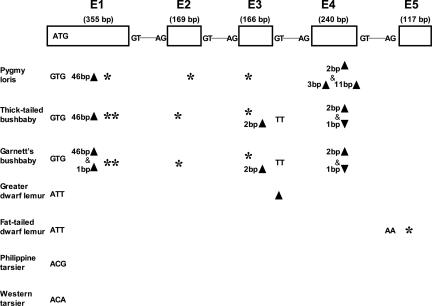
Under the traditional view, we should expect similar levels of functional relaxation in the S opsin gene in all nocturnal prosimian lineages, presumably with some shared defects. On the contrary, our data show drastically different levels of functional relaxation in the S opsin gene among the nocturnal prosimians, with some showing strong evidence of functional relaxation and some showing none at all. We sequenced the S opsin gene in 14 representative prosimians, which include 12 nocturnal species and 2 diurnal species and belong to three major clades: Malagasy lemuriforms (aye-aye, sifakas, avahi, mouse lemurs, dwarf lemurs, sportive lemurs, and ring-tailed lemurs), Afro-Asian lorisiforms (bushbabies and slow lorises), and Asian tarsiiforms. In the three lorisiform species examined, the S opsin gene has become a pseudogene due to several severe defective mutations (Fig. 3), some of which had been found in two previous studies (18, 19). Many of these mutations are shared by the three lorisiform species, indicating that they were acquired in their common ancestor. Exon 1 of all three species has a 46-nt deletion, which produces a frame-shift and introduces premature stop codons in the first exon. Another shared defect is a 2-nt deletion in exon 4, which also results in a frame-shift and premature stop codons. The same defect was found in our screening of exon 4 in three more species of lorises and three more species of bushbabies. The shared defects also include two nonsense mutations in exons 1 and 3, respectively, and the mutation of the ATG start codon to GTG. In addition to the shared defects described above, defective mutations specific to each lineage also are found within this group, including the 1-nt insertion in exon 4 in the bushbaby lineage, a defect found previously (refs. 18 and 19 and Fig. 3).
Fig. 3.
Defective mutations in the coding regions of the S opsin gene of bushbabies, loris, lemurs, and tarsiers. The S opsin gene contains five exons, with a total length of 1,047 bp for the coding regions. Except for the loris, the two bushbabies and the sportive lemur, where only the first four exons (930 bp) were sequenced and the PCR amplification of the last exon (117 bp) failed, the DNA sequences for the entire coding region of all other 10 species were obtained. Defective mutations include deletions (▴) or insertions (▾), resulting in a frame-shift and premature stop codons, deletion of the splicing sites, point mutations at the start codon or splicing sites, and nonsense mutations (*).
In sharp contrast, among the nine lemurs we studied, only two nocturnal lemur species have defective mutations, none of which cause a frame-shift (Fig. 3). Thus, the majority of nocturnal lemuriforms, as well as the two diurnal species sampled, show a functional S opsin gene. For diurnal lemurs, the S opsin-containing cones had previously been detected by electroretinographic flicker photometry (20), and an intact coding sequence of the S opsin has been found (19). What is unexpected is that the majority of nocturnal lemurs examined also have an intact S opsin gene. For example, although defects were found in the fat-tailed and greater dwarf lemurs (genus Cheirogaleus), no defect was found in their close relatives, the mouse and Coquerel's dwarf lemurs. For the two tarsier species, the only defective mutations are at the start codon (Fig. 3). The defective mutations found in one tarsier species, the western tarsier, are consistent with the sequence in a study described in ref. 19. In any case, such mutations are probably not incapacitating, because two codons after this original start codon, there is another ATG that can presumably be used as an alternative start. Indeed, a recent immunochemical study provides evidence for S-opsin expression in the retina of another tarsier species, Tarsius spectrum (21). For the lemurs and tarsiers, each defect found was screened for multiple individuals in the same species. In all cases, we found the defects in all individuals (at least 2) studied, suggesting that the mutations are diagnostic of the species.
Such different levels of functional relaxation in the S opsin gene were further supported by our estimation of the dN/dS ratios in different lineages (Fig. 2). The dN/dS ratios along the branches of the bushbaby/loris group are much higher than those of lemurs (Fig. 2). In contrast, the dN/dS ratios along the branches of the two tarsiers are similar to those in the squirrel-monkey and human lineages. For the greater and fat-tailed dwarf lemurs, which are nocturnal and have minor S-opsin gene defects, the dN/dS ratios are higher than those of the other lemurs but still much lower than those of the bushbaby/loris group. Although the dN/dS ratios in some nocturnal lemurs (aye-aye and sportive lemur) are lower than those in diurnal lemurs (sifaka and ring-tailed lemur), the differences are not statistically significant.
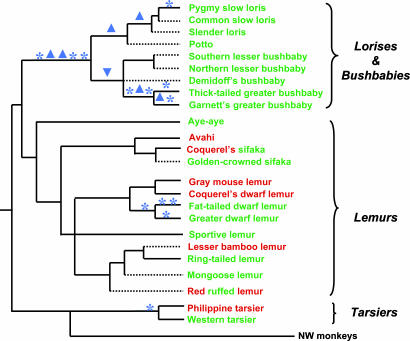
The above findings of different levels of functional relaxation in the prosimian S opsin gene, no sharing of defective mutation among the three major prosimian clades (Fig. 4) and the presence of an intact S opsin gene in many prosimians are incompatible with the traditional view. We, therefore, propose that the ancestral primate was diurnal or cathemeral, that routine nocturnality evolved early in the bushbaby/loris lineage but relatively recently in other prosimians, and that Indridae and Lemuridae have always been diurnal (or cathemeral) or at least have not undergone any long period of strictly nocturnal life (Fig. 1B). According to this view, different levels of functional relaxation in nocturnal prosimian lineages simply reflect different time points of shift to nocturnality. Specifically, the very high levels of functional relaxation in lorisiforms, as revealed by many severe defects and high dN/dS ratios, result from an early shift to nocturnality. Tarsiers and nocturnal lemurs, on the other hand, shifted to nocturnality more recently and, therefore, have started to accumulate only minor defects, if any, and the recent functional relaxation has largely not yet been reflected in the dN/dS ratio.
Fig. 4.
Distributions of the S-opsin gene defects and of the M (green) and L (red) opsins among prosimians. Defective mutations were mapped with macclade software (33). The phylogenetic tree is based on the current view (28-31) (nonscaled branches), and the New World (NW) monkey lineage is included to show its relationship with the prosimians. *, Mutations that could cause defects, including nonsense mutations, mutations at the start codon, and deletion of splicing sites. ▴, Frame-shifting deletions; ▾, frame-shifting insertions. Solid branches lead to species where the S opsin gene has been sequenced, and dashed branches denote those that have not been sequenced. Both green and red are used for Coquerel's sifaka and the red ruffed lemur, where the M/L polymorphism has been found. The X-linked opsins for the avahi, the sportive lemur, Demidoff's bushbaby, and the pygmy loris were sequenced in this study; the data for the rest of species were from a previous study (12).
This view is supported by our studies on the X-linked M/L opsin of prosimians (refs. 12 and 22 and the present study). From the distribution of M/L opsin among primates (Fig. 4), a parsimony inference indicates that the common ancestor of primates was polymorphic for the M and L alleles. This inference requires at most 12 losses of the L or M allele (Fig. 4). Such a loss can easily occur by random drift because the M/L polymorphism has no advantage for a nocturnal animal. In contrast, the assumption of monomorphism for the M allele in the primate ancestor would require seven gains of the L allele, five for the strepsirrhines, one for Tarsius syrichta, and one for the New World monkeys. Each gain requires two events: (i) the specific mutation causing a spectral shift from M to L opsin and (ii) the establishment of the L allele or even the replacement of M by L in the population. The chances for either event are low. For example, the probability for a mutation with a 1% selective advantage over the existing allele to become established in the population is only ≈2%. At any rate, the seven gains require at least 2 × 7 = 14 events. In the same manner, we can calculate that the assumption of L monomorphism in the ancestral primates requires even more events. Note that the calculations assume that those diurnal lemurs not yet screened for the M/L polymorphism are monomorphic for the M or L allele. If any of these species are actually polymorphic, the assumption of M/L polymorphism for ancestral primates would be even more parsimonious than the assumption of monomorphism.
The M/L polymorphism, together with a functional S opsin, should confer trichromacy in female ancestors heterozygous for the M and L alleles. We note that anatomical and physiological studies have revealed many similarities in the organization of prosimian and simian visual systems (23). For instance, the parvocellular (P cell) system, which is specialized for trichromacy by mediating red-green color opponency, was found in all primates, including the strictly nocturnal bushbabies (24). These findings suggest that the common ancestor of primates already had the proper neural system to support trichromacy. Because trichromacy is of no demonstrated use to nocturnal animals, its existence implies that the ancestral primates were diurnal or cathemeral.
Our view provides a simple scenario for the M and L opsin-distribution patterns among prosimians (Fig. 4). The pattern in the bushbaby/loris group suggests an early loss of the L allele in the ancestor of this group, because among the nine representative species studied, only the M opsin has been found (refs. 12 and 22 and the present study). Such an early loss of the M/L polymorphism indicates an early shift from diurnal to nocturnal life. On the other hand, nocturnal lemurs and tarsiers may have experienced the loss of the M/L polymorphism only recently. For instance, the fat-tailed dwarf lemur has the M allele, whereas the closely related mouse dwarf lemur has the L allele. These observations are consistent with recent shifts from diurnality to nocturnality in the tarsiers and nocturnal lemurs.
Our view also can explain the presence of tapeta in many prosimians, which has been perceived as strong evidence for primate ancestral nocturnality, if we assume that the ancestral primate was active both day and night, i.e., cathemeral. To maintain this lifestyle, the ancestral primate should have had a dual-purpose eye; namely, it should have an M/L opsin polymorphism and a functional S opsin to achieve good vision during daytime and a tapetum to increase visual sensitivity in the night. As the primates radiated, nocturnal features were strengthened and some diurnal features were lost in nocturnal lineages, whereas the opposite was true for the diurnal lineages. For lemurs that still maintain a cathemeral lifestyle, both types of features may be found. This observation and the following suggest that the presence/absence of a tapetum might not be the best indicator for nocturnality/diurnality. First, many cathemeral strepsirrhines (e.g., Eulemur species) have no tapeta, although some diurnal species (e.g., ring-tailed lemur and bamboo lemur) do (1). Second, not all nocturnal primates have tapeta. For instance, all tarsiers and owl monkeys lack tapeta. In fact, the absence of a tapetum in tarsiers has been viewed as evidence that the tarsier lineage was originally diurnal for a period before becoming nocturnal (refs. 25 and 26 and Fig. 1B). Our data from the opsin gene system strongly uphold this hypothesis.
Acknowledgments
We thank K. Neiswanger, P. Wright, B. Adriamihaja, and the Duke Primate Center (Durham, NC) for DNA or tissue samples; Z. Yang for his kind help in using paml software; and M. Cartmill, P. Kappeler, M. Rasmussen, and C. Ross for discussion. This work was supported by National Institutes of Health grants, a seed grant from the University of Massachusetts (Boston), and the Research Grants Council of Hong Kong.
Abbreviations: S, short-wavelength; M, middle-wavelength; L, long-wavelength.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. DQ191893-DQ191958).
References
- 1.Pariente, G. (1979) in The Study of Prosimian Behavior, eds. Doyle, G. A. & Martin, R. D. (Academic, London), pp. 411-459.
- 2.Allman, J. M. (1999) Evolving Brains (Freeman, New York).
- 3.Fleagle, J. G. (1999) Primate Adaptation and Evolution (Academic, San Diego), 2nd Ed., pp. 330-396.
- 4.Jacobs, G. H. (1993) Biol. Rev. 68, 413-471. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs, G. H. & Deegan, J. F., II (1992) J. Comp. Physiol. A 171, 351-358. [DOI] [PubMed] [Google Scholar]
- 6.Chausseil, M. (1992) Anim. Learn. Behav. 20, 259-265. [Google Scholar]
- 7.Deegan, J. F., II, & Jacobs, G. H. (1994) Am. J. Primatol. 33, 205. [Google Scholar]
- 8.Deegan, J. F., II, & Jacobs, G. H. (1996) Am. J. Primatol. 40, 55-66. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs, G. H., Deegan, J. F., II, Neitz, J., Crognale, M. A. & Neitz, M. (1993) Vision Res. 33, 1773-1783. [DOI] [PubMed] [Google Scholar]
- 10.Schneider, H., Schneider, M. P., Sampaio, I., Harada, M. L., Stanhope, M., Czelusniak, J. & Goodman, M. (1993) Mol. Phylogenet. Evol. 2, 225-242. [DOI] [PubMed] [Google Scholar]
- 11.Shimmin, L. C., Mai, P. & Li, W.-H. (1998) J. Mol. Evol. 44, 378-382. [DOI] [PubMed] [Google Scholar]
- 12.Tan, Y. & Li, W.-H. (1999) Nature 402, 36. [DOI] [PubMed] [Google Scholar]
- 13.Neitz, M., Neitz. J. & Jacobs, G. H. (1991) Science 252, 971-974. [DOI] [PubMed] [Google Scholar]
- 14.Merbs, S. L. & Nathans, J. (1993) Photochem. Photobiol. 58, 706-710. [DOI] [PubMed] [Google Scholar]
- 15.Asenjo, A. B., Rim, J. & Oprian, D. D. (1994) Neuron 12, 1131-1138. [DOI] [PubMed] [Google Scholar]
- 16.Shyue, S.-K., Boissinot, S., Schneider, H., Sampaio, I., Schneider, M. P., Abee, C. R., Williams, L., Hewett-Emmett, D., Sperling, H. G., Cowling, J. A., et al. (1998) J. Mol. Evol. 46, 697-702. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura, S. & Kubotera, N. (2003) Gene 321, 131-135. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs, G. H., Neitz, M. & Neitz, J. (1996) Proc. Roy. Soc. B. 263, 705-710. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura, S. & Kubotera, N. (2004) J. Mol. Evol. 58, 314-321. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs, G. H. & Deegan, J. F., II (1993) Am. J. Primatol. 30, 243-256. [DOI] [PubMed] [Google Scholar]
- 21.Hendrickson, A., Djajadi, H. R., Nakamura, L., Possin, D. E. & Sajuthi, D. (2000) J. Comp. Neurol. 424, 718-730. [DOI] [PubMed] [Google Scholar]
- 22.Zhou, Y.-H., Hewett-Emmett, D., Ward, J. P. & Li, W.-H. (1997) J. Mol. Evol. 45, 610-618. [DOI] [PubMed] [Google Scholar]
- 23.Casagrande, V. A. & Kaas, J. H. (1994) in Primary Visual Cortex in Primates, Cerebral Cortex, eds. Peters, A. & Rockland, K. S. (Plenum, New York), Vol. 10, pp. 201-259. [Google Scholar]
- 24.Yamada, E. S., Marshak, D. W., Silverira, L. C. L. & Casagrande, V. A. (1998) Vision Res. 38, 3345-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin, R. D. (1973) Symp. Zool. Soc. London 33, 301-337. [Google Scholar]
- 26.Cartmill, M. (1980) in Evolutionary Biology of the New World Monkeys and Continental Drift, eds. Ciochon, R. L. & Chiarelli, A. B. (Plenum, New York), pp. 243-274.
- 27.Martin, R. D. (1994) Primate Origins and Evolution (Princeton Univ. Press, Princeton).
- 28.Rumpler, Y., Crovella, S. & Montagnon, D. (1994) Folia Primatol. 63, 149-155. [DOI] [PubMed] [Google Scholar]
- 29.Yoder, A. D., Cartmill, M., Ruvolo, M., Smith, K. & Vilgalys, R. (1996) Proc. Natl. Acad. Sci. USA 93, 5122-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoder, A. D., Vilgalys, R. & Ruvolo, M. (1996) Mol. Biol. Evol. 13, 1339-1350. [DOI] [PubMed] [Google Scholar]
- 31.Goodman, M., Porter, C. A., Czelusniak, J., Page, S. L., Schneider, H., Shoshani, J., Gunnell, G. & Groves, C. P. (1998) Mol. Phylogenet. Evol. 9, 585-598. [DOI] [PubMed] [Google Scholar]
- 32.Yang, Z. (1997) Comput. Appl. Biosci. 13, 555-556. [DOI] [PubMed] [Google Scholar]
- 33.Maddison, D. R. & Maddison, W. P. (2001) MACCLADE 4, Analysis of Phylogeny and Character Evolution (Sinauer, Sunderland, MA), Version 4.03.