Abstract
Pressures on normal human acetabular cartilage have been collected from two implanted instrumented femoral head hemipros-theses. Despite significant differences in subjects' gender, morphology, mobility, and coordination, in vivo pressure measurements from both subjects covered similar ranges, with maximums of 5-6 MPa in gait, and as high as 18 MPa in other movements. Normalized for subject weight and height (nMPa), for free-speed walking the maximum pressure values were 25.2 for the female subject and 24.5 for the male subject. The overall maximum nMPa values were 76.2 for the female subject during rising from a chair at 11 months postoperative and 82.3 for the male subject while descending steps at 9 months postoperative. These unique in vivo data are consistent with corresponding cadaver experiments and model analyses. The collective results, in vitro data, model studies, and now corroborating in vivo data support the self-pressurizing “weeping” theory of synovial joint lubrication and provide unique information to evaluate the influence of in vivo pressure regimes on osteoarthritis causation and the efficacy of augmentations to, and substitutions for, natural cartilage.
Osteoarthritis, manifested by cartilage degradation in the major load-bearing joints of the human, is prevalent, debilitating, and costly, affecting over 40 million Americans (1). The etiology of this disease is unknown (2, 3). To quote from the web page of the National Institute of Arthritis and Musculo-skeletal and Skin Diseases (3): “The mechanisms responsible for cartilage destruction, however, are not well understood. Current therapeutic modalities provide marginal symptomatic relief and with few exceptions have no effect on the preservation of cartilage or on disease progression.”
A decade or more likely passes before cartilage has fissured to the point where bone contact instigates pain, the clinical sign of arthritis. That mechanical factors may contribute to the initiation or progression of cartilage destruction (4, 5) is suggested both by the high loads the joints support and the absence in cartilage of the usual mediators of physiological communication: the tissue is avascular as well as aneural. Again quoting from the web page of the National Institute of Arthritis and Musculo-skeletal and Skin Diseases (3): “Mechanical load across joints is critically important for the maintenance of cartilage, and abnormal mechanical loading of joints can lead to pathological conditions.”
Thus, cartilage cells (chondrocytes) appear to depend on the mechanical environment to control their function as well as to transport nutrients and metabolites (6-9). The pressures on and in cartilage are the manifestation of the applied load in the environment of the cartilage matrix. Pressure and pressure-time cycling thus appear to be “critically important for the maintenance of cartilage” (3). As a result, the pressure cartilage experiences appears central to the etiology of osteoarthritis, acting either directly, e.g., collagen fiber rupture, or through mechanical/biological coupling, e.g., the influence of the mechanical microenvironment on chondrocyte metabolism. Thus, it is surprising that our project has produced, to our knowledge, the only experimental in vivo mammalian pressures, and these in humans.
Pressure distribution information is also a crucial element in scientific characterization of how, in healthy cartilage, the mechanical and biological properties of cartilage, bone, and synovial fluid synergize locally and globally to achieve high-load-capacity, low-friction, long-wearing skeletal bearings. Normal synovial joints exhibit very little friction (measured coefficients of friction in vitro for whole joints range from 0.002 to 0.02), with modest gliding speeds as in gait (0.0-0.3 m/s), even when subjected to high loading; five times body weight has been measured across the human hip joint (10).** To explain this remarkable tribology the mechanisms of human-engineered bearings (hydrodynamic, elastohydrodynamic, boundary, and squeeze-film lubrication) have been invoked, as well as other hypotheses (“weeping” and “boosted”). Over two dozen theories for the extraordinary performance of synovial joints have been proposed (11); however, “although numerous theories have been put forth to attempt to explain joint lubrication, the mechanisms involved are still far from being understood” (12).
Thus, quantifying cartilage pressures and pressure distributions, in actual joints in vivo during routine daily activities, appears to be central both to elucidate the pressure regimes that cartilage and its biological components experience and to define the lubrication mechanism of synovial joints. Addressing the global synovial joint, uniquely shaped as it has been by evolution and ontogeny, loaded and moving as it does in life, has been the overriding aspect of our research program from its outset.
By contrast, virtually all other cartilage researchers focus their study on small samples of cartilage, excised from joints with or without bone, under uniaxial load with permeable or impermeable platen and/or perimeter restraints (see, for example, ref. 13). This approach poses uncertainties both analytically and experimentally as to the actual boundary conditions on the loaded surfaces and at the perimeter of the sample. The imposed loading and restraint conditions are not those experienced by cartilage in the global joint, either in vivo or in vitro.
To pursue our “whole joint” approach, we first had to determine whether acquiring in vivo pressure data was feasible. We demonstrated a design incorporating pressure transducers and telemetry, based on the standard hemi- or endoprosthesis routinely implanted to replace the defective femoral head (14). Otherwise identical to the standard implant in function and biocompatibility, the instrumented femoral head would bear against the natural cartilage of the acetabulum.
Experiments were conducted on cadaver acetabula to assure future patient safety in the absence of any cartilage pressure data and to establish the calibration range for the transducers. A pseudo hemiprosthesis with similar pressure instrumentation, but hard wired, was installed in a custom-designed hip-joint simulator. Theoretical load vectors for human gait, taken from the literature, were applied to acetabula cartilage. These experiments produced direct measurements of local pressures and pressure distribution on cartilage in a synovial joint (15). With the feasibility and safety of the pressure data collection method demonstrated, the research focus shifted to evaluation of the hip-joint environment in vivo.
Whereas the nonuniform pressure distributions from our first human subject (16-18) were similar to those measured in vitro, the magnitudes of in vivo cartilage pressure were considerably higher than the in vitro pressure magnitudes. One possibility was that the literature values for forces at the human hip were low. Thus, confirmation of our de novo in vivo data required further in vivo study. Herein we present pressure data from our second subject, of different gender, morphology, and coordination than our first subject, and compare data from both subjects performing similar activities.
Methods
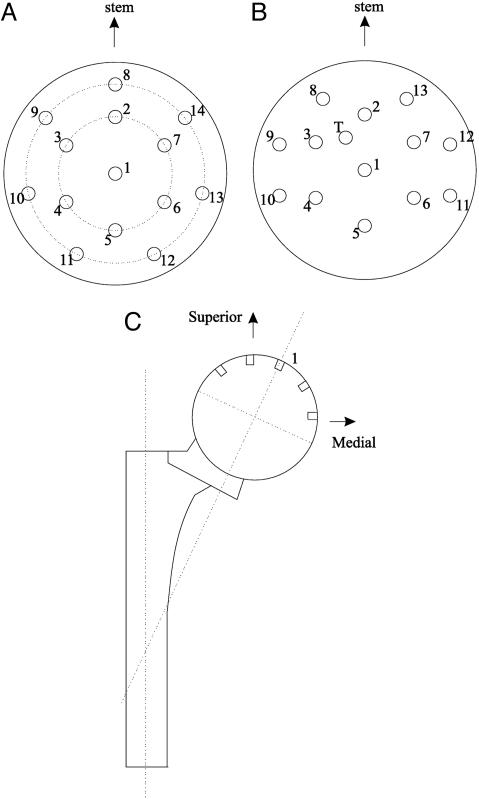
For both subjects the contact pressures on acetabular cartilage were measured by instrumented endoprostheses (14, 19).†† The implants are fabricated of the same cobalt chromium alloy and employ the same geometry as standard endoprostheses of which over 200,000 are implanted annually in the United States (20). The upper hemisphere of the pseudo femoral head, which contacts acetabular cartilage, encloses 14 pressure transducers for the first implant and 13 for the second. Fig. 1 A and B shows the respective locations of transducers.‡‡ The diaphragm of each transducer is integral with the spherical surface of the head and is connected to a silicon cantilever-beam on which is fused a four-arm strain gauge sensor, for an overall sensitivity of 0.28 μm of deflection per MPa applied to the diaphragm. Inside the hermetically sealed head, electronic circuits sequentially sample the transducer outputs and multiplex a frame of 14 pressures at 250 Hz for the first subject and 13 pressures and temperature at 500 Hz for the second subject, transmitted as a radio-telemetry signal in the FM band at 100 MHz. An antenna at the distal end of the stem serves to transmit the data and as a power-receiving antenna from an external 100-kHz source, which is an induction coil garter worn around the thigh during data acquisition.
Fig. 1.
Locations of pressure transducers on pseudo femoral heads of hemipros-theses. (A and B) Views along the axis of the head through transducer 1. A is the implant in the first subject, and B is the implant in the second subject. (C) Location of central transducer 1.
Our first subject was a 73-year-old female, 1.68 m in height (H) and of body weight (BW) 663 N, with excellent coordination of movement. She fractured her right femoral neck. Our second subject was an 82-year-old male, 1.6 m in H and of BW 565 N, with coordination difficulties. He experienced a left neck fracture. The Institutional Review Board of the Massachusetts Institute of Technology approved the prostheses, and the Institutional Review Board of the Massachusetts General Hospital approved the surgical procedure. Both subjects gave informed consent. By using a gauge to measure the excised natural femoral heads (21), both subjects were implanted with instrumented endoprostheses with 47.5-mm-diameter heads.§§ Both subjects' acetabular cartilage was deemed normal by radiographs and, during surgery, by visual observation.
At the Biomotion Laboratory of the Massachusetts General Hospital, pressure data transmission was synchronized with the 3D kinematics of the subject's pelvis, thigh, shank, and foot segments. These data were acquired electro-optically by employing our system called TRACK (22, 23) from arrays of light-emitting diodes (see Fig. 2). Foot-floor forces were measured from two force plates. Because the transducers are in the pseudo femoral head, but we are interested in the locations of the pressure readings on acetabular cartilage on the pelvis, coordinate transformations were performed serially. First a prosthesis coordinate frame fixed the transducer locations with respect to the pseudo femoral head (see Figs. 1 A and B). Then that frame was referenced to the prosthesis intermedullary stem (see Fig. 1C). From surgical and x-ray information the stem coordinate frame was referenced to the femur. TRACK kinematic data provided the relationship between the femur and the pelvis, with a final relationship between the pelvis and acetabular coordinates based on x-ray information.
Fig. 2.
Female subject showing garter around right thigh over hip-implanted instrumented-endoprosthesis for powering prosthesis and receiving PAM-FM signal from prosthesis. Light-emitting diode arrays for TRACK kinematic acquisition are attached to the foot, shank, thigh, and pelvis. The cane is also force-instrumented. (Reproduced from ref. 59.)
Extensive pressure data were acquired during both subjects' rehabilitation (24-26) and then every several months until their natural demise, 5 years for the female subject and 3 years for the male. Protocols included various exercises, e.g., isometric abduction of the hip muscles, natural or “free-speed” walking, paced walking, negotiating stairs, rising from chairs, and single-leg standing. Here we report representative samples from the extensive data from both subject's prostheses. For details on procedures, see ref. 27; for complete data sets for both subjects, see ref. 28.
Results
The movement studied most extensively was walking, an activity performed routinely during the daily life of each subject, for which kinematic and kinetic data (but not cartilage pressure) are in the literature for comparison. In free-speed gait, the subject was instructed to walk as he or she normally would and to look ahead (rather than down at the ground). Despite significant differences in their morphology and mobility, the maximum pressures from the two subjects during the load bearing, or stance, phase of normal walking was in the 5- to 6-MPa range. Fig. 3 is a plot of the magnitudes of local pressure from all transducers versus time for the male subject during free-speed walking at 12 months postoperative.
Fig. 3.
Pressure (MPa) measured at transducers in femoral head versus time for the male subject in free-speed gait at 12 months postoperative.
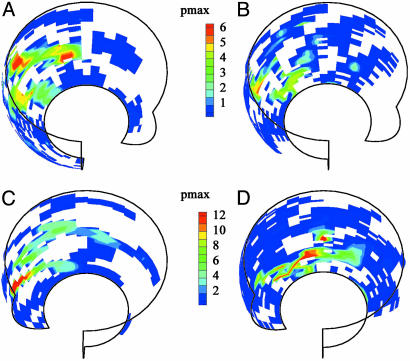
The regional pressures on acetabular cartilage for free-speed walking are presented in Fig. 4A for the female at 11 months postoperative, where the maximum pressure was 6.1 MPa, and in Fig. 4B for the male at 1 year postoperative, which shows a maximum pressure of 5.4 MPa. The display of Fig. 4 does not explicitly present temporal information but uses, for the test reported, subject kinematic data to present the locations on acetabular cartilage of pressure measurements from the transducers on the femoral head. The view is into the right hip socket (the male's data are rotated to the right hip) with the subject's anterior toward the right side of the plot. The acetabular cartilage in the socket is regionalized into bins with color-coding of the maximum pressure recorded in each bin. Regions in which no measurements were made are left blank.
Fig. 4.
Maximum pressures in regions of acetabulum. (A and B) Data for free-speed gait. A shows data from the female subject at 11 months postoperative. B shows data from the male subject at 12 months postoperative. (C) Data for the female subject rising from a chair, 11 months postoperative. (D) Data for the male subject descending steps, 12 months postoperative. The views are into the right hip socket (the male's data are rotated to the right hip) with the subject's anterior toward the right side of each plot.
The highest pressures were measured during different activities for the two subjects but were of comparable magnitudes. The maximum pressure measurements occurred during activities undertaken less frequently than walking, rising from a chair and descending stairs. The difference in which activity produced the highest pressures reflects the differing capabilities of the two subjects. In particular, the coordination and balance problems experienced by the second subject were problematic when traversing stairs. In addition, the protocol for rising from a chair was more detailed during tests with the second subject, resulting in different kinematics. However, the most important point is that the overall maximum pressures were of similar magnitude for the two subjects. This was a critical finding because the maximum pressure measurements were surprisingly high.
Data were taken for rising from different height chairs: the lower the chair height, the higher the local pressures. The highest pressure recorded from the female subject, 18 MPa at 11 months postoperative, was while rising from a chair 80% of knee height. Fig. 4C gives the regional maximum pressures for this test for the female subject. In this display, the acetabular surface has been rotated to show the regions of high pressure more clearly. For the male subject, the maximum pressures when rising from a chair were much lower and positioned more anteriorly than were the high pressures for the female subject. However, in stair descent the male subject generally generated higher pressures than did the female, possibly because of balance difficulties since the kinematic data show greater variation and higher frequency components in joint angle measurements. Fig. 4D shows his regional maximum pressures during stair descent with a maximum value of 15.5 MPa. A maximum pressure measurement of 18.2 MPa was made during a similar stair descent test at 9 months postoperative; however, those data could not be displayed in this format because of the absence of synchronized kinematic data.
As mentioned earlier the two subjects had acetabula similar enough to require the same size prosthesis, but the individuals were otherwise quite different morphologically. To account for the different height and weight of each subject the pressure data were normalized and made nondimensional over a range of 0-100 by this equation:
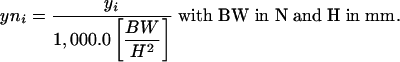 |
The maximum normalized pressure values (nMPa) for free-speed walking were 25.2 for the female subject at 6 months postoperative and 24.5 for the male subject at 12 months postoperative. The overall maximum nMPa values were 76.2 for the female subject during rising from a chair at 11 months postoperative and 82.3 for the male subject while descending steps at 9 months postoperative.
Discussion
The close correspondence of pressures measured in both subjects is reassuring, especially considering the physical differences between the two subjects. The highest pressures, during chair rise for the woman and during stair descent for the man, and even the 5- to 6-MPa pressures recorded in walking were much higher than our in vitro pressure measurements, we believe because of the experienced in vivo forces at the hip being higher than those analyzed in the literature. Co-contraction of the musculature about the hip to stabilize the joint by increasing joint stiffness or impedance (29) is likely the source of the higher hip forces. Balanced co-contraction components of forces from opposing muscles, while adding to joint force (and therefore pressures), produce no torque and therefore no rotation about the joint. As a result, joint force vectors calculated from kinetic and kinematic data using inverse Newtonian analysis as reported in the literature cannot detect the co-contraction components of joint force. The presence of significant co-contraction forces about the hip, even in normal walking, was demonstrated in a study employing acetabular pressure data from the male subject (30). The location of highest pressure on the acetabulum did not correspond, at the same time in the gait cycle, with the location of the force vector at the hip. To measure hip joint force in vivo we have in preparation hemiprostheses similar to those used in the study herein but instrumented to measure the components of hip force. Hemiprostheses do not alter the natural femoral head-acetabulum geometry and musculature as do total joint replacement prostheses, where hip-joint geometry and musculature are deliberately altered surgically, as in the case of the instrumented total joint (10).
Whereas the in vitro absolute values are lower, as explained above, the nonuniform pressure distributions in vivo are similar to those measured in vitro. This finding is relevant to characterizing the tribology of the synovial joint, in particular resolving whether, upon loading a synovial joint, interstitial fluid in the cartilage matrix is expressed from the matrix into the interarticular gap, proposed as “weeping” lubrication by McCutchen (31), or from the loaded gap into the matrix, “boosted” lubrication, as proposed by Dowson (32). The in vivo local pressures at transducers <10 mm apart vary by megapascals, consistent with our in vitro pressure data (15) and therefore our in vitro geometric studies of cartilage are germane.
Ultrasonic geometric studies of both components of the same natural hip joint show that the mating surfaces of unloaded ace-tabular and femoral head cartilage layers are sectors of spheres (33, 34) of virtually the same diameter (35) on which are superimposed undulations of ≈75-μm root mean square above and below the mean surface, with wavelengths of millimeter scale. These small variations from sphericity influence the sealing process as Kenyon (36) had shown in an analytic model for surface flow in cartilage. When loaded, the higher ridges contact first, and their patterns of contact comprise the seals, with the resulting valleys forming the passages, accounting for the nonuniform pressure distributions seen both in vitro and in vivo. Thus, our in vitro and in vivo pressure data, exhibiting high local maxima, irregular and idiosyncratic isobars, and steep pressure gradients, reflect localized sealing throughout the interarticular gap.
An in vitro experiment-based analysis (37), corroborated by and consistent with the in vivo pressure data reported herein, used a finite-element-model of the acetabulum cartilage layer, quantified by ultrasonic 3D measurements of surface shape and thickness distribution of the in situ layer (33) and ultrasonic determination of the permeabilities and moduli of the tissue (38). Two otherwise identical pseudoprostheses, matched to the acetabulum diameter, one with pressure instrumentation, and the other with an ultrasonic transducer to measure cartilage consolidation, were loaded into the acetabulum. The resulting experimental data were applied as boundary conditions for the finite-element-model analysis. The results show interstitial fluid pressurization in the cartilage layer and in the gap (39) with a fluid pressure distribution in the layer that produced flow from the cartilage thickness into loaded areas of the interarticular gap, and thence through the gap to unloaded, low-pressure regions. A dynamic computer simulation of the natural joint in gait, based on experimental geometric and property data from both components of the hip joint, showed osmotically induced flow (imbibitions) into both acetabular and femoral head cartilage layers occurring only during the unloaded swing phase. During the loaded stance phase of gait, pressurized fluid was expressed from the cartilage layers into the interarticular gap (40).
Thus, our corroborated in vivo data are consistent with prior in vitro pressure and geometric data and our analytic and computer modeling. All address the natural joint, and all support the 1959 “weeping” hypothesis of McCutchen (31) that synovial joints support load mainly by self-pressurized hydrostatic fluid pressure. However, the debate continues. A recent study (41) using cartilage plugs reported experimental interstitial cartilage pressure consistent with that reported in ref. 37. However, ref. 41 was extended to report experimental friction data on cartilage plugs (42), and the dispute continued. Then a study (43) modeled the “cartilage layer” as flat, smooth, and 2D, approached and penetrated by a 2D, “rippled, rigid indenter” purported to represent the complex surface of natural cartilage. This theoretical analysis concluded flow was into the cartilage, supporting the “boosted” premise. A responding letter to the editor (44) cited the experiment-based Macirowski study (37) with the “weeping” conclusion. This drew a reply (45), in which Ateshian found fault with ref. 37, which he has since retracted (46). Because cartilage in the normal joint is ≈70% liquid, which is essentially incompressible, and because the cartilage layers consolidate under load (see figure 6 of ref. 37) ≈10%, expression of liquid out of the layers into the interarticular space appears inevitable, as does “weeping” lubrication.
As for other synovial joint tribology theories, while the squeeze film hypothesis has been analyzed for short-time (several seconds) loading of a simplified model (47), it cannot explain effective joint performance during long-duration loading, such as in extended standing. Hydrodynamic lubrication is precluded by the absence of sustained, unidirectional motion in the natural joint. In vivo pressures varying over the loaded area argues convincingly against the elastohydrodynamic hypothesis of synovial joint lubrication (48). In human-designed elastohydrodynamic bearings, the load-supporting area has a uniform pressure-field over an essentially uniform clearance, surrounded by a reduced clearance perimeter, which for such bearings comprises the seal. For an analysis of elastohydrodynamic in the hip joint, see ref. 49. Full boundary lubrication is inconsistent with the very low measured coefficients of friction of natural joints, but see below.
In ref. 37 pressurized fluid in the interarticular space supports >90% of the load. Hence, most cartilage-to-cartilage contact areas are protected from high normal stresses. The very low coefficients of friction measured on cartilage plugs or in synovial joints are thus the results of the total applied stress being partitioned between fluid support and solid-to-solid contact, combined with efficient lubrication of the actual contact areas. A boundary lubrication mechanism, which probably relies on Swann's “lubricating glycoproteins” (50), is important, because the opposing cartilage layers make contact over some 10% of the total area, and that is where most of the friction occurs. The “weeping” mechanism, producing a fluid film over most of the opposing surfaces, makes the frictional resistance of the joint at least an order of magnitude lower than would be the case for total area contact.
Of clinical significance is the relation between joint tribology and cartilage disease. A common pathway of cartilage destruction into osteoarthritis may be the gradual deterioration of the interarticular seal. Seal deterioration, allowing increased fluid exudation, will increase cartilage consolidation and strain, and therefore matrix stresses. The overstressed cartilage is likely subjected to fibrillation and crevassing (51), the morphological manifestations of osteoarthritis. These in turn will also lead to higher (ultimately an order of magnitude or more) frictional losses, with the resulting temperature rise (52) in the cartilage perhaps causing pathological cellular reactions (53). How cartilage constitutive properties, cartilage surface conditions, and cartilage layer geometry, on a macroscopic scale, affect the seal integrity and the interarticular gap resistance, as well as the detailed nature of the seal itself, needs to be studied. For analysis and experiment that estimates the average interarticular clearance in the loaded joint as small as 100 Å, see ref. 54.
Cartilage thickness and degeneration for our male subject were posthumously quantified from magnetic resonance imaging and histological analysis (55). The explanted left endoprosthesis-loaded acetabulum were compared with the right normal acetabulum as control. Pressure magnitudes measured during gait correlated negatively with cartilage thickness and positively with regional histology scores typical of early osteoarthritis. The study attributed acetabular cartilage degeneration to repetitive stress. Comparing acetabula data from the endoprosthesis and control joints, cartilage degeneration did not appear to be mediated solely by articulation with the metallic endoprosthesis.
The unique data described herein [and more extensively detailed in the theses of K.C.M. (27, 28)] on the pressures and pressure-time cycles cartilage experience in life are essential to understanding the etiology of osteoarthritis, as in research on the influence of pressure at matrix, cellular, and subcellular scales (8, 56, 57). These in vivo pressures also define the pressure environment that proposed augmentations to, and substitutions for, natural cartilage must endure for the subsequent life of the joint. More immediately these data can help orthopedic surgeons and physical therapists better plan procedures and activities less stressful to their patients' hips, especially those joints predisposed to osteoarthritis (18, 58).
Finally, since 1968 our global, whole-synovial-joint approach has proved difficult to fund. Reductionist and theoretical approaches have prevailed but have contributed little to addressing how normal cartilage in the intact joint performs so remarkably and why it fails, with endless refinement of mathematical models of the tissue and experiments on cartilage plugs. The end result is that after almost a half-century of speculation the tribology of the bearing is still debated, and the etiology of the disease remains unknown.
Acknowledgments
The research program described in this article and in related references was performed in the Mechanical Engineering Department at the Massachusetts Institute of Technology (MIT) and in the Biomotion Laboratory (BL) at the Massachusetts General Hospital (MGH) by students at MIT and BL and is detailed in their 21 bachelor's, 30 master's, and 16 doctoral theses. The design and fabrication of the instrumented prostheses at MIT were supported by fellowships from the National Science Foundation and the National Institutes of Health-General Medical Sciences; by grants from the Medical Foundation of Massachusetts, the Easter Seals Research Foundation, the National Institutes of Health (AM-6116), and the Arthritis Foundation; and by the Veterans Administration, the Smith Petersen Fund of MGH, the MIT Newman Laboratory Fund, the Sloan Fund for Basic Research, the Charles Stark Draper Laboratory, and the Germeshausen and Whitaker Professorships of MIT. The Howmedica, Zimmer, and Kulite corporations donated materials for the prostheses. Implantation at MGH and data acquisition at BL at MGH, followed by the processing and interpretation of the data at MIT, were supported by the William H. Harris Foundation of MGH; the Arthritis Foundation of Massachusetts; the June Rockwell Levy Foundation; National Institutes of Health Grants AR40036 and HD30063; National Institute on Disability and Rehabilitation Research, Department of Education Grants G008830074, 1 H133P90005, and REC-H133E80024; Veterans Administration Rehabilitation Research and Development Service Grant REUVDHT9898; the Arthritis Foundation; and the Whitaker Foundation.
Footnotes
Measured with a force-instrumented total artificial hip joint as compared with the pressure-instrumented partial-joint-replacement prostheses used in this study of cartilage.
For illustrations of the pressure-instrumented pseudo femoral head hemisphere, electronics package, and antenna at the end of the intermedullary stem, see figure 1 of ref. 16; and for a frontal plane x-ray of the pelvis-thigh region of the first subject, see figure 2 of ref. 16.
After experience with a symmetrical location of the transducers on the first implant, transducer locations were slightly changed, and one transducer cavity (T) was used for a temperature sensor.
Postmortem measurement of the respective excised pelvi showed that the female's endoprosthesis was 0.6 mm oversize, whereas the male's was 0.7 mm undersized, both within the 1-mm-diameter increment available in commercial endoprostheses.
References
- 1.Kuettner, K. E., Schleyerbach, R., Peyron, J. G. & Hascall, V. C., eds. (1991) Articular Cartilage and Osteoarthritis (Raven, New York).
- 2.Shakoor, N. & Loeser, R. F. (2004) Sci. Aging Knowl. Environ. 35, dn2. [DOI] [PubMed] [Google Scholar]
- 3.National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (2005) Cartilage and Connective Tissue Research, www.niams.nih.gov/an/stratplan/cartilage.htm.
- 4.Sokoloff, L. (1969) The Biology of Degenerative Joint Disease (Univ. of Chicago Press, Chicago).
- 5.Radin, E. L., Ehrlich, M. G. & Chernack, R. (1978) Clin. Orth. 131, 288-293. [PubMed] [Google Scholar]
- 6.Sah, R. L., Kim, Y. J., Doong, J. H., Grodzinsky, A. J., Plass, A. H. K. & Sandy, J. D. (1989) J. Orth. Res. 7, 619-636. [DOI] [PubMed] [Google Scholar]
- 7.Lee, D. A., Knight, M. M., Bolton, J. F., Idowu, B. D., Kayser, M. V. & Bader, D. C. (2000) J. Biomech. 33, 81-95. [DOI] [PubMed] [Google Scholar]
- 8.Ikenoue, T., Trindale, M.C.D., Lee, M.S., Lin, E.Y., Schurman, D.J., Goodman, S. B. & Smith, R. L. (2003) J. Orth. Res. 21, 110-116. [DOI] [PubMed] [Google Scholar]
- 9.Szafranski, J. D., Grodzinsky, A. J., Burger, B., Gaschen, V., Hung, H.-H. & Hunziker, E. B. (2004) Osteoarthritis Cartilage 12, 937-946. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann, G., Graichen, F. & Rohlman, A. (1993) J. Biomech. 26, 969-990. [DOI] [PubMed] [Google Scholar]
- 11.Furey, M. J. (1995) in The Biomedical Engineering Handbook, ed. Bronzino, J. D. (CDC Press, Boca Raton, FL), pp. 333-351.
- 12.Furey, M. J. (1995) in The Biomedical Engineering Handbook, ed. Bronzino, J. D. (CDC Press, Boca Raton, FL), p. 333.
- 13.Park, S., Hung, C. T. & Ateshian, G. A. (2004) Osteoarthritis Cartilage 12, 65-73. [DOI] [PubMed] [Google Scholar]
- 14.Carlson, C. E., Mann, R. W. & Harris, W. H. (1974) IEEE Tran. Biomed. Eng. 21, 257-264. [DOI] [PubMed] [Google Scholar]
- 15.Rushfeldt, P. D., Mann, R. W. & Harris, W. H. (1981) J. Biomech. 14, 315. [DOI] [PubMed] [Google Scholar]
- 16.Hodge, W. A., Fijan, R. L., Carlson, K .L., Burgess, R. G., Harris, W. H. & Mann, R. W. (1986) Proc. Natl. Acad. Sci. USA 83, 2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewin, R. (1986) Science 232, 1192-1193. [DOI] [PubMed] [Google Scholar]
- 18.Hodge, W. A., Carlson, K. L., Fijan, R. L., Burgess, R. G., Riley, P. O., Harris, W. H. & Mann, R. W. (1989) J. Bone Joint Surg. 71-A, 9, 1378-1386. [PubMed] [Google Scholar]
- 19.Mann, R. W. & Burgess, R. G. (1990) in Implantable Telemetry in Orthopaedics, eds. Bergmann, G., Graichen, F. & Rohlmann, A. (Free Univ. of Berlin, Berlin), pp. 65-75.
- 20.Praemer, A., Furner, S. & Rice, D. P. (1992) in Musculoskeletal Conditions in the United States (Am. Acad. of Orthopaedic Surgeons, Park Ridge, IL), p. 133.
- 21.Harris, W. H., Rushfeldt, P. D., Carlson, C. E., Scholler, J.-M. & Mann, R. W. (1975) in The Hip: Proceedings of the Third Annual Open Science Meeting of The Hip Society (Mosby, St. Louis), pp. 93-98.
- 22.Antonsson, E. A. & Mann, R. W. (1989) J. Dyn. Syst. Measurement Control 3, 31-39. [Google Scholar]
- 23.Rowell, D. & Mann, R. W. (1990) in Proceedings of the International Symposium on Gait Analysis, State-of-the-Art of Measuring Systems, and Their Implications in Prosthetic and Orthotic Technology, eds. Boenick, U., Näder, M. & Mainka, C. (Tech. Univ. of Berlin, Berlin), pp. 150-165.
- 24.Strickland, E., Fares, M., Krebs, D. E., Riley, P. O., Givens-Hess, D. L., Hodge, W. A. & Mann, R. W. (1992) Phys. Ther. 72, 691-699. [DOI] [PubMed] [Google Scholar]
- 25.Givens-Heiss, D. L., Krebs, D. E., Riley, P. O., Strickland, E., Fares, M., Hodge, W. A. & Mann, R. W. (1992) Phys. Ther. 72, 700-705. [DOI] [PubMed] [Google Scholar]
- 26.McGibbon, C. A., Krebs, D. E. & Mann, R. W. (1997) Arthritis Care Res. 10, 300-307. [DOI] [PubMed] [Google Scholar]
- 27.Carlson, K. L. (1986) M.S. thesis (Massachusetts Inst. of Technology, Cambridge).
- 28.Carlson, K. L. (1993) Ph.D. thesis (Massachusetts Inst. of Technology, Cambridge).
- 29.Hogan, N. (1984) IEEE Trans. Autom. Control 29, 681-690. [Google Scholar]
- 30.Park, S., Krebs, D. E. & Mann, R. W. (1999) Gait Posture 10, 211-222. [DOI] [PubMed] [Google Scholar]
- 31.McCutchen, C. W. (1959) Nature 184, 1284-1285. [DOI] [PubMed] [Google Scholar]
- 32.Walker, R. S., Dowson, D., Longfield, M. D. & Wright, V. (1968) Ann. Rheum. Dis. 27, 512-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rushfeldt, P. D., Mann, R. W. & Harris, W. H. (1981) J. Biomech. 14, 253-260. [DOI] [PubMed] [Google Scholar]
- 34.Tepic, S., Mann, R. W. & Harris, W. H. (1980) in Proceedings of the 26th Annual Orthopaedic Research Society, ed. Mitchell, N. S. (Orthopaedic Research Society, Rosemont, IL), p. 189.
- 35.Tepic, S. (1980) M.S. thesis (Massachusetts Inst. of Technology, Cambridge).
- 36.Kenyon, D. E. (1980) J. Biomech. 13, 129-134. [DOI] [PubMed] [Google Scholar]
- 37.Macirowski, T., Tepic, S. & Mann, R. W. (1994) Trans. ASME 116, 10-19. [DOI] [PubMed] [Google Scholar]
- 38.Tepic, S., Macirowski, T. & Mann, R. W. (1983) Proc. Natl. Acad. Sci. USA 80, 3331-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dent, J. R. (1979) Ph.D. thesis (Massachusetts Inst. of Technology, Cambridge).
- 40.Tepic, S., Macirowski, T. & Mann, R. W. (1985) in Biomechanics: Current Interdisciplinary Research, eds. Perrin, S. M. & Schneider, E. (Martinus Nijhoff, Boston), pp. 221-226.
- 41.Park, S., Krishnan, R., Nicoll, S. B. & Ateshian, G. A. (2003) J. Biomech. 36, 1785-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishman, R., Kopasz, M. & Ateshian, G. A. (2004) J. Orth. Res. 22, 565-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soltz, M. A., Basol, I. M. & Ateshian, G. A. (2003) J. Biomech. Eng. 125, 585-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mann. R. W. (2004) J. Biomech. Eng. 126, 538. [PubMed] [Google Scholar]
- 45.Ateshian, G. A. (2004) J. Biomech. Eng. 126, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ateshian, G. A. (2005) J. Biomech. Eng. 127, 726. [Google Scholar]
- 47.Fein, R. S. (1966-1967) Inst. Mech. Eng. 181, Part 3J, 125-128. [Google Scholar]
- 48.Tanner, R. I. (1966) Phys. Med. Biol. 11, 119-127. [DOI] [PubMed] [Google Scholar]
- 49.Harrigan, T. P. (1985) Sc.D. thesis (Massachusetts Inst. of Technology, Cambridge).
- 50.Swann, D. A., Slayter, H. S. & Silver, F. H. (1981) J. Biol. Chem. 256, 5921-5925. [PubMed] [Google Scholar]
- 51.Kerin, A. J., Coleman, A., Wisnom, M. R. & Adams, M. A. (2003) Clin. Biomech. 18, 960-968. [DOI] [PubMed] [Google Scholar]
- 52.Tepic, S., Macirowski, T. & Mann, R. W. (1985) J. Orth. Res. 3, 516-520. [DOI] [PubMed] [Google Scholar]
- 53.Madreperla, S. A., Louwerenburg, B., Mann, R W., Towle, C. A., Mankin, H. J. & Treadwell, B. V. (1985) J. Orth. Res. 3, 30-35. [DOI] [PubMed] [Google Scholar]
- 54.Xue, Q. (1991) Ph.D. thesis (Massachusetts Inst. of Technology, Cambridge).
- 55.McGibbon, C. A., Krebs, D. E., Trahan, C. A., Trippel, S. B. & Mann, R. W. (1999) J. Arthroplasty 14, 52-58. [DOI] [PubMed] [Google Scholar]
- 56.Chen-Tung, C., Bhargava, M., Lin, P. M. & Torzilli, P. A. (2004) J. Orth. Res. 21, 888-898. [DOI] [PubMed] [Google Scholar]
- 57.Trindade, M. C. D., Shida, J., Ikenoue, T., Lee, M. S., Lin, E. Y., Yaszay, B., Yerby, S. & Smith, R. L. (2004) Osteoarthritis Cartilage 12, 729-735. [DOI] [PubMed] [Google Scholar]
- 58.Fagerson, T. L., Krebs, D. E., Harris, B. A. & Mann, R. W. (1995) Physiotherapy (London) 81, 533-540. [Google Scholar]
- 59.Mann, R. W. (2002) J. Rehabil. Res. Dev. 39 (6), Suppl., 23-38. [PubMed] [Google Scholar]