Abstract
Previously, we identified that a majority of patients with anorexia nervosa (AN) and bulimia nervosa (BN) as well as some control subjects display autoantibodies (autoAbs) reacting with α-melanocyte-stimulating hormone (α-MSH) or adrenocorticotropic hormone, melanocortin peptides involved in appetite control and the stress response. In this work, we studied the relevance of such autoAbs to AN and BN. In addition to previously identified neuropeptide autoAbs, the current study revealed the presence of autoAbs reacting with oxytocin (OT) or vasopressin (VP) in both patients and controls. Analysis of serum levels of identified autoAbs showed an increase of IgM autoAbs against α-MSH, OT, and VP as well as of IgG autoAbs against VP in AN patients when compared with BN patients and controls. Further, we investigated whether levels of these autoAbs correlated with psychological traits characteristic for eating disorders. We found significantly altered correlations between α-MSH autoAb levels and the total Eating Disorder Inventory-2 score, as well as most of its subscale dimensions in AN and BN patients vs. controls. Remarkably, these correlations were opposite in AN vs. BN patients. In contrast, levels of autoAbs reacting with adrenocorticotropic hormone, OT, or VP had only few altered correlations with the Eating Disorder Inventory-2 subscale dimensions in AN and BN patients. Thus, our data reveal that core psychobehavioral abnormalities characteristic for eating disorders correlate with the levels of autoAbs against α-MSH, suggesting that AN and BN may be associated with autoAb-mediated dysfunctions of primarily the melanocortin system.
Keywords: anorexia, autoimmunity, behavior, bulimia, hypothalamus
Neuropeptides are important transmitters in the brain limbic and neuroendocrine systems involved in the regulation of different modalities of feeding, social and defensive behaviors, the stress response, and homeostasis (1). These roles of neuropeptides suggest a possible implication in some neuropsychiatric conditions primarily manifested by behavioral abnormalities (2, 3), including anorexia nervosa (AN) and bulimia nervosa (BN) (4). Although changes in concentrations of some neuropeptides in plasma or in cerebrospinal fluid have been noted in patients with eating disorders (5, 6), the mechanisms underlying these changes are unknown, and the modern therapy of eating disorders adopts the symptomatic approach (7).
In search of possible peptidergic mechanisms underlying eating disorders, we recently found that a majority of a group of Swedish AN and BN patients display autoAbs against α-melanocyte-stimulating hormone (α-MSH) (8), a melanocortin peptide involved in appetite control (9-12). AutoAbs against adrenocorticotropic hormone (ACTH) and luteinizing hormone-releasing hormone (LHRH) also were identified in some patients and controls (8). The growing evidence of the immune system's critical involvement in some neurological and psychiatric disorders (13) suggests that autoAbs reacting with neuropeptides responsible for the central control of appetite may partake in the pathogenesis of eating disorders.
In the present work, we further studied the occurrence of these autoAbs in AN and BN using a patient sample from Estonia. In these sera we in addition identified autoAbs reacting with oxytocin (OT) and vasopressin (VP), two neurohormones primarily involved in the regulation of water balance as well as in several motivated behaviors, including central mechanisms of social interactions (14, 15). Furthermore, to clarify the relevance of identified autoAbs reacting with neuropeptides to the symptoms of eating disorders, we tested our hypothesis that the levels of such autoAbs may correlate with the range of psychological problems in AN and BN patients. For this purpose, the widely used and reliable Eating Disorder Inventory (EDI-2) test (16, 17) was used to assesses the cognitive and behavioral characteristics commonly found in individuals with AN and BN, while autoAbs levels were measured by ELISA.
Methods
Human Subjects. Sera from Estonian female patients with AN [mean age 19.5 yr, n = 12, body mass index (BMI) ± SD, 16.3 ± 1.99] or with BN (mean age 21.5 yr, n = 42, BMI 20.8 ± 1.97) were used in this study. AN and BN were diagnosed by a psychiatrist and a clinical psychologist according to the 4th Ed. of Diagnostic and Statistical Manual of Mental Disorders (18). Sera from female volunteers (mean age 21.4 yr, n = 41, BMI 20.2 ± 2.41) served as the control group. Serum samples from AN and BN patients and controls were taken on the day of completing the EDI-2 test, instantly frozen, and kept at -70°C until processed for immunohistochemistry or ELISA.
Immunohistochemical Screening of Human Sera. To detect presence of IgG autoAbs against hypothalamic neuropeptides, sera from patients and controls were applied on rat hypothalamic and pituitary sections and processed immunohistochemically (8). Experiments were designed in accordance with guidelines on animal care and approved by the local ethical committee, Stockholms norra djurförsöksetiska nämnd. One day before they were killed, some rats were treated by intracerebroventricular injection of colchicine (120 μg in 20 μl of 0.9% NaCl) to cause accumulation of neuropeptides in the cell soma due to interruption of axonal transport. To test the specificity of autoAb binding, the targeted molecules were identified by preadsorption of human sera with synthetic neuropeptides, as described in ref. 8 (for details see Supporting Methods, which is published as supporting information on the PNAS web site).
Competitive α-MSH Binding. α-MSH receptor binding was performed as described in ref. 19. Total IgG was purified from AN patient serum with high levels of α-MSH IgG Abs. Incubations of rat brain sections with 0.1 nM 125I-labeled α-MSH (PerkinElmer) were done with and without purified IgG. Resulting autoradiographs were quantified for the relative optical density in the hypothalamus (for details see Supporting Methods).
ELISA. Levels of IgG and IgM autoAbs against α-MSH, ACTH, OT, or VP were measured by using the ELISA technique (for details see Supporting Methods). Each determination was done in duplicate. The coefficient of variation between duplicate values was <5%. F(ab′) fragments were prepared by pepsin digestion (20).
EDI-2 Test. The EDI-2 (17) is a self-report instrument and an expanded version of the EDI (16). The revision retained the original 64 items measuring eight cognitive and behavioral dimensions common in AN and BN, such as Drive for Thinness, Bulimia, Body Dissatisfaction, Ineffectiveness, Perfectionism, Interpersonal Distrust, Interoceptive Awareness, and Maturity Fears. To the EDI-2, 27 items were added to form three new subscale symptoms such as Ascetism, Impulse Regulation, and Social Insecurity. Each item responded to a 6-point scale, ranging from 0 (never) to 5 (always), which was recorded to a 4-point scale from 0 to 3 (original 0,1, and 2 are all reduced to 0; whereas 3,4, and 5 correspond to 1, 2, and 3, respectively). The validity of the Estonian version of the EDI-2 test was previously confirmed (21).
Statistics. Data were analyzed in statistica program (StatSoft, Tulsa, OK). For analysis of the levels of autoAbs we used the nonparametric ANOVA and the Kruskal-Wallis tests. Cochran-C test was used for the analysis of homogeneity of sample distribution. Data are presented in the graphs (Fig. 3) with median OD values (Kruskal-Wallis, *, P < 0.05; **, P < 0.001 vs. controls).
Fig. 3.
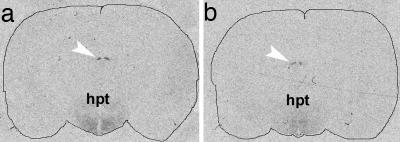
Autoradiographs of 125I-labeled α-MSH binding to the rat brain sections. (a) Control. (b) Preincubation with IgG, purified from the serum of an AN patient with a high α-MSH IgG level, significantly reduced signal intensity. Hpt, hypothalamus; arrowhead, medial habenula.
To test our hypothesis that the plasma levels of autoAbs against α-MSH, ACTH, OT, and/or VP correlate with the degree of psychological problems in AN and BN patients, we compared correlations between the plasma levels of each Abs and the EDI-2 total as well as each EDI-2 subscale score among the controls, AN, and BN patients. A general linear model of multiple regression analysis was used to compare the possible correlations. Full factorial analysis between the categorical predictor (groups: AN, BN, and controls), the continuous predictor (Ig), and the dependent factor (EDI score) was computed, resulting in the invariant test of significance (sigma-restricted parameterization). If this test showed significant differences (P < 0.05) between the groups, we recorded β-correlations and their P values, which illustrate alterations in direction and magnitude of correlations for each EDI/Ig pair in AN or BN vs. controls. Additionally, Pearson's r moment correlation factors with P values were computed for each EDI-total/Ig and EDI-subscale/Ig pairs as well as for each BMI/Ig pairs. Single-factor ANOVA and the Fisher least significant difference test were used to compare means of BMI and total EDI-2 score between controls and AN and BN patients. Unpaired, two-tailed Student's t test was used to compare the relative optic density levels of α-MSH binding on the rat brain sections.
Results
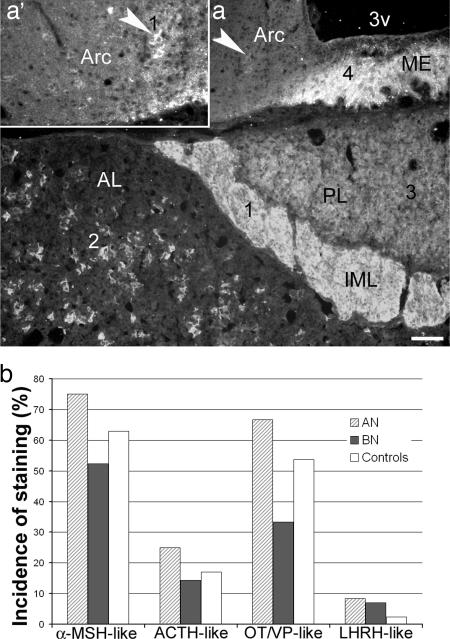
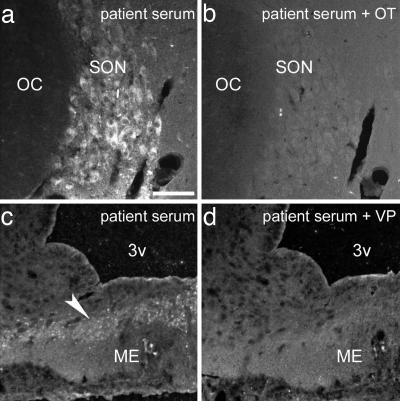
Immunohistochemical Identification of AutoAbs. Previously, by using immunohistochemistry, we identified the presence of IgG autoAbs against α-MSH, ACTH, and LHRH in Swedish patients with eating disorders and controls (8). In the present work, by using the same approach in an Estonian sample, we also detected a distinct pattern of immunostaining of the rat brain and pituitary sections produced by some, but not all, sera from patients and controls. Most commonly, this pattern included α-MSH-like staining in the hypothalamic arcuate nucleus and the intermediate pituitary lobe, ACTH-like staining in the anterior pituitary lobe, and/or OT/VP-like staining in the supraoptic and paraventricular nuclei and their projections by means of the median eminence to the posterior pituitary lobe. Less commonly, LHRH-like staining in the external layer of the median eminence also was detected. An example of this pattern and its incidence among AN, BN, and controls is illustrated in Fig. 1. By using absorption of sera with synthetic α-MSH, ACTH, and LHRH we confirmed our previous data on the presence of IgG autoAbs reacting with the corresponding peptides (data not shown). Abolishment or reduction of OT/VP-like staining was found after absorption of sera with either synthetic OT or VP (10-6 M), providing evidence that patients and controls also display circulating IgG autoAbs reacting with these two neuropeptides (Fig. 2).
Fig. 1.
Immunohistochemical screening for autoAbs. (a) A characteristic pattern of immunostaining of the rat mediobasal hypothalamus and pituitary by the serum from an AN patient showing α-MSH-like staining (1) in the intermediate pituitary lobe (IML) and the arcuate nucleus (Arc, arrowhead, same in the Inset a*′), ACTH-like staining (2) in the anterior pituitary lobe (AL), OT/VP-like staining (3) in the posterior pituitary lobe (PL), and LHRH-like staining (4) in the median eminence (ME). (Scale bars: a, 100 μm; a′, 50 μm.) (b) Incidence of this neuropeptide-like immunostaining among AN and BN patients and controls.
Fig. 2.
Absorption of a patient serum with either synthetic OT (b) or VP (d) resulted in abolishment of OT/VP-like immunostaining in the rat supraoptic nucleus (SON, b vs. a) or the median eminence (ME, d vs. c). OC-optic chiasm, 3v-3rd brain ventricle. Arrowhead points to OT/VP-like immunopositive fibers in the ME. (Scale bar: 100 μm.)
α-MSH Binding. The autoradiographic analysis of α-MSH binding revealed a characteristic distribution of the signal in the hypothalamus and in the medial habenula (Fig. 3) as reported in ref. 22. Quantification of the signal intensity in the hypothalamus showed a 25% decrease in the α-MSH IgG group vs. control group (relative optic density ± SD, 0.024 ± 0.007 vs. 0.018 ± 0.006, respectively; P < 0.01).
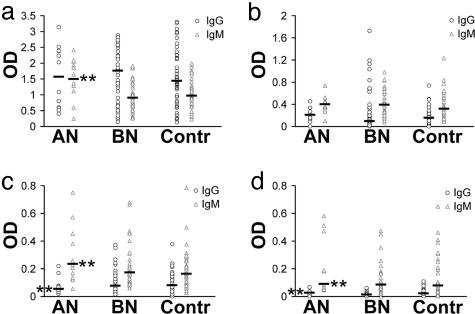
Levels of AutoAbs by ELISA. We used ELISA to measure levels of serum IgG autoAbs as well as to verify the presence and measure levels of IgM autoAbs reacting with α-MSH, ACTH, OT, or VP in patients and controls. The true Ab nature of autoAbs binding to synthetic peptides in microplates was proven, because F(ab)2 fragment of IgG retained 75% of the binding shown by IgG. The binding was inhibitable in all four assay systems in a dose-dependent manner by competition with antigen. Large variations in the levels of autoAbs against all four neuropeptides were found in both patients and controls. Because of a nonhomogenous distribution of OD values within the groups as assessed by the Cohran-C test, a nonparametric statistical analysis was used to test for possible differences between the groups. Significantly increased (Kruskal-Wallis, P < 0.01) levels of IgM autoAbs against α-MSH, OT, and VP, as well as IgG autoAbs against VP were found in AN patients vs. either controls or BN patients, whereas IgG autoAbs levels against OT were found to be lower (Fig. 4).
Fig. 4.
Levels (OD, optic density in ELISA) of IgM and IgG autoAbs against α-MSH (a), ACTH (b), OT (c), and VP (d) in AN and BN patients and controls. Bars show median values (Kruskal-Wallis, **, P < 0.001 vs. controls).
Correspondence Between Immunohistochemical and ELISA Data. To analyze whether the immunohistochemical detection of autoAbs corresponded to the levels of autoAbs as measured by ELISA, the intensity of the neuropeptide-like staining was subjectively rated from 0 (no staining) to 3 (very strong). It appeared that only in the AN group the intensity of α-MSH-like staining correlated positively with the ELISA values for α-MSH autoAbs (r = 0.76, P < 0.01), whereas no such correlations were found in other groups or for other neuropeptide autoAbs. Accordingly, in the three AN sera that did not show α-MSH-like immunostaining, the OD values for α-MSH IgG levels were in the lowest range (0.3-0.8). Surprisingly, in the control sera, a similar low range of OD often was accompanied by visible α-MSH-like staining, whereas higher levels of OD did not correspond to the strong α-MSH-like staining. However, in our routine immunohistochemical studies, we also have sometimes experienced lack of correlation between titer and stainability. This phenomenon may be due to several factors, for example that not all Abs may react equally well on formalin-fixed tissue.
BMI and Neuropeptide AutoAbs. The BMI was significantly different among the patients and controls (ANOVA, P < 0.01). The BMI in AN patients was lower vs. controls and vs. BN patients (Fisher's least significant difference, mean BMI ± SD, 16.3 ± 1.99 vs. 20.2 ± 2.41 and vs. 20.8 ± 1.97, respectively; P < 0.01 for both), but the BMI of BN patients and of controls was not significantly different. Among all possible combinations evaluated between the BMI and neuropeptide Ig levels, significant Pearson's correlations were found between the BMI and α-MSH IgG levels in BN (r = -0.46, P < 0.01) and between the BMI and VP IgG levels in AN (r = -0.6, P < 0.05).
EDI-2 and Neuropeptide AutoAbs. The total EDI-2 scores differed significantly among the three groups (ANOVA, P < 0.01): AN patients (mean EDI-2 score ± SD, 71.2 ± 40.8), BN patients (94.6 ± 42.2), and controls (34.7 ± 23.5). The total EDI-2 score was not significantly different between AN and BN patients, but both patient groups scored higher than controls (Fisher's least significant difference, P < 0.01 for both).
Screening of all of the combinations between the subscale score of the EDI-2 and the Abs levels as measured by ELISA, using our statistical model, we found several pairs that significantly differed among the controls and AN and BN patients. Most of the significant differences between patients and controls (β-correlations) were found in various psychological dimensions of EDI-2 vs. levels of autoAbs against α-MSH (Table 1). These differences were also significant for the total EDI-2 score (Table 1 and Fig. 5). Some significant β-correlations also were noted for autoAbs against ACTH, OT, and VP; such as a strong link between the levels of IgG autoAbs against ACTH and the score for Maturity Fears (AN, β = 1.01; BN, β = -1.15, P < 0.01); and a less strong link between levels of OT and VP IgM autoAbs and score for Bulimia (AN, OT, β = -0.37; VP, β = -0.33, P < 0.05 for both; BN, OT, β = 0.37, P < 0.05, VP, β = 0.42, P < 0.01). Remarkably, all of the β-correlations in AN were opposite from those in BN. To further analyze the origin of these differences, we searched for the correlation factors (Pearson's r) between Abs levels and EDI-2 total and subscale score. No significant Pearson's correlations for these pairs existed among controls, but many of them were present in AN and BN patients (Table 2). In contrast, for the pairs without significant β-correlations, some significant Pearson's correlations were found in all groups (Table 3).
Table 1. The β-correlations (AN and BN vs. controls) for pairs that showed significant differences among patients and controls.
| AutoAbs
|
||
|---|---|---|
| EDI-2 subscale | α-MSH IgG (β) | α-MSH IgM (β) |
| Drive for thinness | ||
| AN | 0.70** | −0.64* |
| BN | −0.76** | 0.51* |
| Bulimia | ||
| AN | 0.54* | −0.57* |
| BN | −0.43* | 0.65** |
| Ineffectiveness | ||
| AN | 0.62* | n.s. |
| BN | −0.53** | n.s. |
| Interpersonal distrust | ||
| AN | n.s. | −0.59* |
| BN | n.s. | 0.85** |
| Ascetism | ||
| AN | 0.62* | n.s. |
| BN | −0.71* | n.s. |
| Impulse regulation | ||
| AN | 0.73** | −0.64* |
| BN | −0.5 n.s. | 0.66* |
| Social insecurity | ||
| AN | 0.64* | n.s. |
| BN | −0.64* | n.s. |
| Total EDI-2 | ||
| AN | 0.67** | −0.60* |
| BN | −0.58* | 0.60* |
β-correlations are for AN and BN vs. controls. Pairs are EDI-2 score/α-MSH autoAbs level. *, P < 0.05; **, P < 0.01; n.s., not significant.
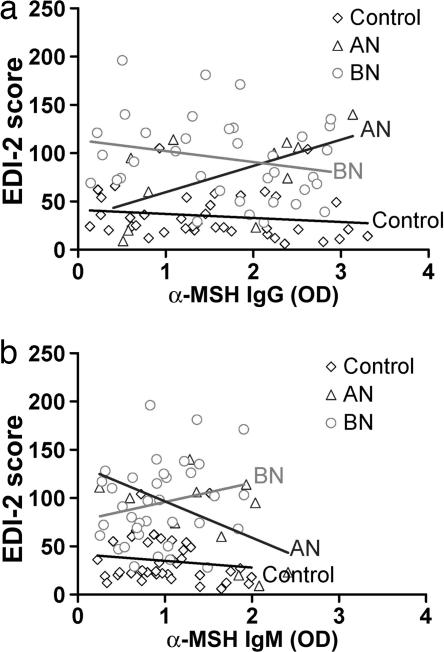
Fig. 5.
Linear trend lines between the total EDI-2 score and levels of α-MSH IgG autoAbs (a) or α-MSH IgM autoAbs (b) in AN and BN patients and control subjects. The angles between the trend lines of the control group and those of AN or BN patients reflect β-correlations of the total EDI-2 score (Table 1). Note the opposite directions of the trend lines between the AN and BN groups as well as between IgG and IgM autoAbs.
Table 2. The correlation coefficients, Pearson's r, for pairs that showed significant β-correlations among AN and BN patients and controls.
| EDI-2 subscale/autoAbs | AN | BN | Contr |
|---|---|---|---|
| α-MSH IgG | |||
| DT | 0.55* | −0.36* | −0.01 |
| B | 0.45 | −0.13 | −0.14 |
| IE | 0.51* | −0.19 | −0.22 |
| A | 0.61* | −0.28* | −0.04 |
| IR | 0.59* | −0.11 | −0.17 |
| SI | 0.46 | −0.26 | −0.10 |
| α-MSH IgM | |||
| DT | −0.66* | 0.08 | −0.12 |
| B | −0.41 | 0.3* | −0.01 |
| ID | −0.55* | 0.3* | −0.19 |
| IR | −0.54* | 0.18 | −0.11 |
| ACTH IgG | |||
| MF | 0.69** | −0.29* | −0.01 |
| OT IgM | |||
| B | −0.40 | 0.32* | 0.23 |
| VP IgM | |||
| B | −0.26 | 0.43** | 0.22 |
| IA | 0.17 | 0.45** | 0.09 |
The correlation coefficients, Pearson's r (*, P < 0.05; **, P < 0.01) for those pairs (EDI-2 score/autoAbs level), that showed significant β-correlations among AN and BN patients and controls (Contr). A, Ascetism; DT, Drive for Thinness; B, Bulimia; BD, Body Dissatisfaction; IE, Ineffectiveness; IR, Impulse Regulation; P, Perfectionism; ID, Interpersonal Distrust; IA, Interoceptive Awareness; MF, Maturity Fears; and SI, Social Insecurity.
Table 3. The correlation coefficients, Pearson's r, for pairs that did not show significant β-correlations among AN and BN patients and controls.
| EDI-2 subscale/autoAbs | AN | BN | Contr |
|---|---|---|---|
| α-MSH IgG | |||
| BD | 0.34 | −0.35* | −0.05 |
| α-MSH IgM | |||
| MF | −0.06 | −0.09 | −0.33* |
| A | −0.58* | 0.18 | −0.05 |
| ACTH IgG | |||
| IA | 0.65* | 0.14 | 0.09 |
| IR | 0.40 | 0.08 | 0.30* |
| EDI-2 total | 0.54* | −0.13 | 0.04 |
| ACTH IgM | |||
| MF | −0.02 | −0.34* | 0.28 |
| OT IgG | |||
| DT | 0.04 | −0.29* | 0.13 |
| BD | −0.23 | −0.30* | 0.16 |
| OT IgM | |||
| IA | −0.10 | 0.28 | 0.45** |
| MF | 0.12 | −0.19 | 0.29* |
| EDI-2 total | −0.13 | 0.04 | 0.33* |
| VP IgG | |||
| BD | −0.16 | −0.17 | 0.31* |
| SI | −0.24 | −0.34* | −0.18 |
| EDI-2 total | −0.31 | −0.29* | 0.17 |
| VP IgM | |||
| DT | −0.04 | 0.29* | −0.09 |
| ID | −0.02 | 0.15 | 0.28* |
| IR | −0.31 | 0.30* | −0.09 |
The correlation coefficients, Pearson's r (*, P < 0.05; **, P < 0.01) for those pairs (EDI-2 score/autoAbs level), which did not show significant β-correlations among AN and BN patients and controls. For abbreviations, see Table 2.
Discussion
Possible Physiological Significance of Neuropeptide AutoAbs. Our work shows that autoAbs (IgG and IgM) against α-MSH, ACTH, OT, and VP can be detected in patients with eating disorders as well as in control subjects. The occurrence of neuropeptide autoAbs in control subjects suggests that autoAbs reacting with these neuropeptides/neurohormones might constitute a neuro-immunoendocrine mechanism that could be beneficial for the homeostatic control. The postulated functional role of neuropeptide autoAbs generally corresponds to the concept of chemical homeostasis proposed for natural autoAbs reacting with small molecules including some neurotransmitters and cytokines (23-26). Igs are relatively long-lived molecules compared with neuropeptides, i.e., the in vivo plasma half-lives for IgM and IgG are 5 and 23 days, respectively (27) vs. minutes for neuropeptides (e.g., 8 min for ACTH and 15 min for VP) (28). In this way, in normal conditions, autoAbs could serve as long-term regulatory factors, similar to well-known hormone-binding proteins, stabilizing/protecting and maintaining the levels of secreted neuropeptides/neurohormones.
Relevance of Neuropeptide AutoAbs to AN and BN. Our findings that the levels of IgM or IgG subclasses of autoAbs reacting with α-MSH, OT, or VP significantly differ between AN and BN patients or controls suggest that these autoAbs could be relevant to the development of eating disorders. However, because some control subjects displayed higher levels of autoAbs than some AN and BN patients, it is clear that their merely elevated serum levels are not sufficient to cause eating disorders. Nevertheless, our data revealing altered correlations between autoAbs levels and the psychological dimensions of the EDI-2 in AN and BN patients indicate that normally nonharmful autoAbs may become pathogenic. The necessary factor for this switch could be an increased blood-brain barrier permeability triggered by various stressful events, such as starvation, infection, or psychological stress. In fact, it has recently been shown that circulating NMDA Abs are not pathogenic until they cross the blood-brain barrier, resulting in cognitive impairment (29).
We show that autoAbs levels are associated with discrete psychological dimensions that constitute psychological phenotypes of eating disorders. As such, the majority of defining psychological traits in AN and BN patients were found to be associated with autoAb levels against α-MSH, including Drive for Thinness, Bulimia, Ineffectiveness, Interpersonal Distrust, Ascetism, Impulse Regulation, and Social Insecurity. Additionally, a significant Pearson's correlation was found between α-MSH IgG level and Body Dissatisfaction in BN. Indeed, the first three dimensions of the EDI have been reported to constitute the core psychological symptoms in both AN and BN patients (30). Hence, autoAbs against α-MSH appear to be relevant to these symptoms, and the most likely route seems to be interference with the central melanocortin system. In fact, the dysregulation of the central melanocortin system may result in psychobehavioral abnormalities characteristic for eating disorders, because critical involvement of melanocortins in the central control of appetite and body weight is well known (9-12, 31), and, besides the control of appetite, melanocortins are involved in variety of motivated behavior (32) and affective states (33-35). The mechanism of possible autoAbs interference with the melanocortin system may include a direct blockage of melanocortin receptors (as we observed in our in vitro study) by α-MSH-autoAbs complexes, rendering them inaccessible for orexigenic agouti-related protein (36) or by complement-mediated damage to the cells bearing the peptide-receptor complex.
Interestingly, our data show that correlations for α-MSH IgG and IgM isotypes were opposite for a given psychological dimension. Moreover, these correlations in AN patients often were “mirrored” in BN patients. Because AN and BN are both characterized by preoccupation with appetite and by other psychopathological traits, as reflected in the elevated EDI scores in both groups, it was surprising that in AN patients the elevated EDI-2 scores were associated with higher levels of α-MSH IgG autoAbs isotype but were associated in BN with the IgM isotype. Although the origin of this phenomenon needs further investigation, we can speculate that some characteristics of autoAbs reacting with α-MSH could be associated with development of either anorexia or bulimia.
In addition to α-MSH autoAbs, some significant β-correlations between EDI-2 subscale dimensions and other neuropeptide autoAbs were found in AN and BN patients. Among them, the score for Bulimia was associated with the levels of OT and VP IgM autoAbs. Concentrations of both OT and VP were reported to be altered in anorectic patients (5, 6), and both peptides are known for their suppressive effect on food intake (37, 38), suggesting that OT and VP autoAbs also could be relevant to appetite control. However, it is perhaps more likely that a possible derangement of OT- and VP-ergic systems by autoAbs primarily underlie elevated anxiety and difficulties in establishing social contacts in patients with eating disorders (39). Both OT and VP are known for their anxiolytic and anxiogenic effects, respectively (40, 41), as well as for their role in brain mechanisms of social interactions (14, 15). Supporting these roles of OT and VP, Social Insecurity correlated with levels of VP IgG (Table 3) in BN patients, whereas levels of OT IgG in this correlation was close to significance (P = 0.05). Further, Maturity Fears in both patient groups were strongly associated only with ACTH autoAbs, suggesting that this psychological trait is primarily related to the activity of the hypothalamic-pituitary-adrenal axis. Maturity Fears, as defined by Garner et al. (16), measures one's wish to retreat to the security of the preadolescent years as a response to the overwhelming demands of adulthood. Thus, ACTH autoAbs may represent a pathophysiological link for a range of stress-related disorders characterized by failure of adaptation mechanisms (42).
Interestingly, some significant Pearson's correlations between autoAb levels and the EDI-2 subscale score were found also within the control group (Table 3), suggesting that already in normal subjects autoAbs against neuropeptides might influence some psychological traits. As such, a significant correlation found between OT IgM levels and the total EDI-2 score supports the idea of OT being the key messenger in the normal stress response in females (43, 44).
To the Origin of Neuropeptide AutoAbs. The large variation in the levels of autoAbs against α-MSH, ACTH, OT, and VP from zero to high OD values found in some apparently normal control subjects indicates a complex origin. The concept of molecular mimicry (45) between pathogenic and nonpathogenic microorganisms and neuropeptides can be applied to explain putative mechanisms. In fact, fragments of both α-MSH and ACTH primary protein sequences display at least five consecutive amino acids identical to some bacterial and viral proteins, such as those from Escherichia coli, Helicobacter pylori and Influenza A virus (46). It is therefore possible that intestinal microflora or some infections may be responsible for the appearance of Abs that will be able to bind neuropeptides. The inductive role of the intestinal microflora is of particular interest, because it installs early in life and is influenced by hygienic standards. An example of this important role is the isohemagglutinins of the ABO blood group induced by a variety of the normal gut flora (47). Indeed, if the intestinal route is the main source of autoAbs reacting with neuropeptides, it might explain the higher incidence (63%) of α-MSH-like staining by sera from Estonian controls in the present study vs. 16% in previously studied Swedish controls (8), because significant differences in intestinal microflora have been found between Swedish and Estonian populations (48). The incidence of H. pylori, for instance, was reported to be as high as 56% in Estonian children ages 9-15 years (49) vs. only 10% in Swedish children of the same age (50). Certainly, a direct comparison of neuropeptide autoAbs levels with relation to gut microflora would be interesting.
Conclusion
In conclusion, we found that α-MSH autoAbs are associated with core psychobehavioral abnormalities, whereas autoAbs reacting with ACTH, OT, or VP are associated with fewer psychopathological traits characteristic for patients with eating disorders. Our work provides previously undescribed evidence for a role of autoAbs reacting with hypothalamic neuropeptides in the origin of neuropsychiatric conditions characterized by distinct psychobehavioral abnormalities such as eating disorders. Based on our data and the known role of hypothalamic neuropeptides, we propose that autoAb-mediated dysfunctions of primarily the melanocortin system may contribute to the development of AN and BN. Further investigation of this previously undescribed concept in animal models could clarify the role of neuropeptide autoAbs in pathophysiology of AN and BN, with the aim of developing novel treatment strategies of eating disorders.
Supplementary Material
Acknowledgments
This work was supported in particular by Torsten and Ragnar Söderberg's Foundations as well as by Swedish Medical Research Council Grants 04X-2887 and 4145, Marianne and Marcus Wallenberg's Foundation, European Union Grants QLG3-CT-2000-00237 and LSHM-CT-2003-503474, the Söderström-Königs Foundation, Estonian Ministry of Education and Science Grant 2643, and Estonian Science Foundation Grant 5450.
Abbreviations: α-MSH, α-melanocyte-stimulating hormone; ACTH, adrenocorticotropic hormone; AN, anorexia nervosa; BMI, body mass index; BN, bulimia nervosa; EDI-2, Eating Disorder Inventory-2; LHRH, luteinizing hormone-releasing hormone; OT, oxytocin; VP, vasopressin.
References
- 1.De Wied, D. (1987) Prog. Brain Res. 72, 93-108. [PubMed] [Google Scholar]
- 2.Swaab, D. F. (2004) Int. Rev. Cytol. 240, 305-375. [DOI] [PubMed] [Google Scholar]
- 3.Panksepp, J. & Harro, J. (2004) in Textbook of Biological Psychiatry, ed. Panksepp, J. (Wiley-Liss, Hoboken, NJ).
- 4.Bailer, U. F. & Kaye, W. H. (2003) Curr. Drug Targets CNS Neurol. Disord. 2, 53-59. [DOI] [PubMed] [Google Scholar]
- 5.Gold, P. W., Kaye, W., Robertson, G. L. & Ebert, M. (1983) N. Engl. J. Med. 308, 1117-1123. [DOI] [PubMed] [Google Scholar]
- 6.Demitrack, M. A., Lesem, M. D., Listwak, S. J., Brandt, H. A., Jimerson, D. C. & Gold, P. W. (1990) Am. J. Psychiatry 147, 882-886. [DOI] [PubMed] [Google Scholar]
- 7.Södersten, P., Bergh, C. & Ammar, A. (2003) Eur. J. Pharmacol. 480, 67-74. [DOI] [PubMed] [Google Scholar]
- 8.Fetissov, S. O., Hallman, J., Oreland, L., Af Klinteberg, B., Grenbäck, E., Hulting, A. L. & Hökfelt, T. (2002) Proc. Natl. Acad. Sci. USA 99, 17155-17160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. (1997) Nature 385, 165-168. [DOI] [PubMed] [Google Scholar]
- 10.Marks, D. L. & Cone, R. D. (2001) Recent Prog. Horm. Res. 56, 359-375. [DOI] [PubMed] [Google Scholar]
- 11.Seeley, R. J., Drazen, D. L. & Clegg, D. J. (2004) Annu. Rev. Nutr. 24, 133-149. [DOI] [PubMed] [Google Scholar]
- 12.MacNeil, D., Howard, A., Guan, X., Fong, T., Nargund, R., Bednarek, M., Goulet, M., Weinberg, D., Strack, A., Marsh, D., et al. (2002) Eur. J. Pharmacol. 450, 93-109. [DOI] [PubMed] [Google Scholar]
- 13.Steinman, L. (2004) Nat. Immunol. 5, 575-581. [DOI] [PubMed] [Google Scholar]
- 14.Choleris, E., Gustafsson, J. A., Korach, K. S., Muglia, L. J., Pfaff, D. W. & Ogawa, S. (2003) Proc. Natl. Acad. Sci. USA 100, 6192-6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim, M. M., Wang, Z., Olazabal, D. E., Ren, X., Terwilliger, E. F. & Young, L. J. (2004) Nature 429, 754-757. [DOI] [PubMed] [Google Scholar]
- 16.Garner, D. M., Olmsted, M. P. & Polivy, J. (1983) Int. J. Eating Disord. 2, 15-34. [Google Scholar]
- 17.Garner, D. M. (1991) Eating Disorder Inventory-2 Professional Manual (Psychological Assessment Resources, Odessa, FL).
- 18.American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders (Am. Psychiatric Assoc., Washington, DC), 4th Ed.
- 19.Melander, T., Köhler, C., Nilsson, S., Hökfelt, T., Brodin, E., Theodorsson, E. & Bartfai, T. (1988) J. Chem. Neuroanat. 1, 213-233. [PubMed] [Google Scholar]
- 20.Stanworth, D. R. & Turner, M. W. (1986) in Handbook of Experimental Immunology, eds. Weir, D. M., Herzenberg, L. A. & Blackwell, C. (Blackwell Scientific, Oxford), pp. 12.10-12.46.
- 21.Podar, I., Hannus, A. & Allik, J. (1999) J. Personality Assessment 73, 133-147. [DOI] [PubMed] [Google Scholar]
- 22.Tatro, J. B. (1990) Brain Res. 536, 124-132. [DOI] [PubMed] [Google Scholar]
- 23.Boyden, S. V. (1966) Adv. Immunol. 5, 1-28. [DOI] [PubMed] [Google Scholar]
- 24.Grabar, P. N. (1975) Ontogenez 6, 115-126. [PubMed] [Google Scholar]
- 25.Avrameas, S. (1991) Immunol. Today 12, 154-159. [DOI] [PubMed] [Google Scholar]
- 26.Bykova, A. & Baker, E. (1998) Nat. Immun. 16, 198-206. [DOI] [PubMed] [Google Scholar]
- 27.Goldsby, R. A., Kindt, T. J., Osborne, B. A. & Kuby, J. (2003) Immunology (Freeman, New York).
- 28.Wilson, J. D. & Foster, D. W. (1992) (Saunders, Philadelphia).
- 29.Kowal, C., DeGiorgio, L. A., Nakaoka, T., Hetherington, H., Huerta, P. T., Diamond, B. & Volpe, B. T. (2004) Immunity 21, 179-188. [DOI] [PubMed] [Google Scholar]
- 30.Kaye, W. H., Klump, K. L., Frank, G. K. & Strober, M. (2000) Annu. Rev. Med. 51, 299-313. [DOI] [PubMed] [Google Scholar]
- 31.Hillebrand, J. J., Kas, M. J. & Adan, R. A. (2005) Peptides, 10.1016/j.peptides.2004.11.027. [DOI] [PubMed]
- 32.De Wied, D. & Jolles, J. (1982) Physiol. Rev. 62, 976-1059. [DOI] [PubMed] [Google Scholar]
- 33.Adan, R. A., Szklarczyk, A. W., Oosterom, J., Brakkee, J. H., Nijenhuis, W. A., Schaaper, W. M., Meloen, R. H. & Gispen, W. H. (1999) Eur. J. Pharmacol. 378, 249-258. [DOI] [PubMed] [Google Scholar]
- 34.Chaki, S., Hirota, S., Funakoshi, T., Suzuki, Y., Suetake, S., Okubo, T., Ishii, T., Nakazato, A. & Okuyama, S. (2003) J. Pharmacol. Exp. Ther. 304, 818-826. [DOI] [PubMed] [Google Scholar]
- 35.Kishi, T. & Elmquist, J. K. (2005) Mol. Psychiatry 10, 132-146. [DOI] [PubMed] [Google Scholar]
- 36.Ollmann, M. M., Wilson, B. D., Yang, Y. K., Kerns, J. A., Chen, Y., Gantz, I. & Barsh, G. S. (1997) Science 278, 135-138. [DOI] [PubMed] [Google Scholar]
- 37.Olson, B. R., Drutarosky, M. D., Chow, M. S., Hruby, V. J., Stricker, E. M. & Verbalis, J. G. (1991) Peptides 12, 113-118. [DOI] [PubMed] [Google Scholar]
- 38.Langhans, W., Delprete, E. & Scharrer, E. (1991) Physiol. Behav. 49, 169-176. [DOI] [PubMed] [Google Scholar]
- 39.Brambilla, F. (2001) Physiol. Behav. 73, 365-369. [DOI] [PubMed] [Google Scholar]
- 40.Kosfeld, M., Heinrichs, M., Zak, P. J., Fischbacher, U. & Fehr, E. (2005) Nature 435, 673-676. [DOI] [PubMed] [Google Scholar]
- 41.Griebel, G., Simiand, J., Serradeil-Le Gal, C., Wagnon, J., Pascal, M., Scatton, B., Maffrand, J. P. & Soubrie, P. (2002) Proc. Natl. Acad. Sci. USA 99, 6370-6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheatland, R. (2005) Med. Hypotheses 65, 287-295. [DOI] [PubMed] [Google Scholar]
- 43.Neumann, I. D. (2002) Prog. Brain Res. 139, 147-162. [DOI] [PubMed] [Google Scholar]
- 44.Taylor, S. E., Klein, L. C., Lewis, B. P., Gruenewald, T. L., Gurung, R. A. & Updegraff, J. A. (2000) Psychol. Rev. 107, 411-429. [DOI] [PubMed] [Google Scholar]
- 45.Oldstone, M. B. (1998) FASEB J. 12, 1255-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fetissov, S. O. (2004) in Neuropsychiatric Disorders and Infection, ed. Fatemi, S. H. (Taylor & Francis, London), p. 296.
- 47.Springer, G. F., Williamson, P. & Brandes, W. C. (1961) J. Exp. Med. 113, 1077-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Björksten, B., Sepp, E., Julge, K., Voor, T. & Mikelsaar, M. (2001) J. Allergy Clin. Immunol. 108, 516-520. [DOI] [PubMed] [Google Scholar]
- 49.Oona, M., Rago, T. & Maaroos, H. I. (2004) Scand. J. Gastroenterol. 39, 1186-1191. [DOI] [PubMed] [Google Scholar]
- 50.Granquist, A., Bredberg, A., Sveger, T. & Axelsson, I. (2002) Acta Paediatr. 91, 636-640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.