Abstract
Adult stem cells are essential for tissue renewal, regeneration, and repair, and their expansion in culture is of paramount importance for regenerative medicine. Using the whisker follicle of the rat as a model system, we demonstrate that (i) clonogenicity is an intrinsic property of the adult stem cells of the hair follicle; (ii) after cultivation for >140 doublings, these stem cells, transplanted to the dermo-epidermal junction of newborn mouse skin, form part or all of the developing follicles; (iii) the stem cells incorporated into follicles are multipotent, because they generate all of the lineages of the hair follicle and sebaceous gland; (iv) thousands of hair follicles can be generated from the progeny of a single cultivated stem cell; (v) cultured stem cells express the self-renewal genes Bmi1 and Zfp145;(vi) several stem cells participate in the formation of a single hair bulb; and (vii) there are many more stem cells in whisker follicles than could be anticipated from label-retaining experiments.
Keywords: skin, transplantation
Mammalian skin contains specialized adult stem cells to maintain a proper protective function (1-3). Skin stem cells, like other adult stem cells, are thought to reside at precise locations, termed niches, where they benefit from a unique environment that favors self-renewal through symmetrical or asymmetrical divisions (1, 4, 5). Hair follicles and epidermis contain keratinocytes that are clonogenic (6, 7). Some of these clonogenic keratinocytes, termed holoclones, can be serially propagated in culture for >180 doublings (7-9). Hence, it is theoretically possible to generate the entire epithelial compartment of the epidermis of an adult human (≈8 × 1010 cells) with the progeny of a single holoclone. Perhaps the best proof of “stemness” of holoclones is their capacity to generate and renew an epidermis for years when transplanted onto patients with extensive, deep burn wounds (10).
Numerous clonogenic keratinocytes are present in whisker follicles, which, like all hair follicles, are constituted of a core of concentric epithelial sheaths, the outer root sheath, the inner root sheath, and the hair fibers (3, 11, 12). The epithelial core is entirely surrounded by a mesenchymal sheath that is in continuity with the follicular papilla of the hair bulb. Most clonogenic keratinocytes of a whisker follicle (up to 1,500 in the largest follicles) are located in the upper region where the nerves end, whereas their number in the lower-intermediate region and the hair bulb depends on the phase of the hair cycle (12, 13). During the growth phase (anagen), few clonogenic keratinocytes are present in the hair bulb, and none are present in the lower-intermediate region despite an active multiplication and differentiation of the keratinocytes located in the hair matrix at a rate sufficient to sustain whisker growth in the rat of 1.5 mm/day. At regular intervals, a whisker stops growing, the hair bulb regresses so much or to an extent that it cannot be identified as an organized structure, and the lower-intermediate region of the follicle shortens, although not so much as in pelage follicles. At this time, the number of clonogenic keratinocytes increases up to 700 in the lower-intermediate region of the largest whisker follicles (12). A new hair bulb soon forms, and whisker growth resumes. At that point, the number of clonogenic keratinocytes progressively decreases during anagen, until the next cycle. Hence, the distribution of clonogenic keratinocytes varies in a spatiotemporal manner. There are opposing opinions on the nature of the clonogenic keratinocytes. Some view them as progenitor cells (14, 15) that are incapable of sustaining long-term hair follicle renewal, whereas others view them as stem cells (8, 9, 12). Here, we demonstrate that clonogenic keratinocytes are bona fide multipotent stem cells.
Materials and Methods
Animals. Fischer 344 rats were from the Curie Institute (Paris) or Charles River Breeding Laboratories, as were the athymic mice. Sprague-Dawley rats that constitutively expressed EGFP (green rat CZ-004) were from Japan SLC (Hamamatsu, Japan) (16). Animal housing and experiments were performed according to Swiss guidelines.
Cultivation of Single Cells. Rat whisker follicles were microdissected as described in refs. 12 and 13. Single cells were isolated with a Pasteur pipet as described in ref. 17, either from freshly dissociated whisker follicles or from cultivated whisker keratinocytes. Cells were then individually cultivated on a feeder layer of lethally irradiated 3T3-J2 cells (6). Cultures were fed every 3-4 days with medium supplemented with 10 ng/ml human recombinant EGF and as described in ref. 7.
Retroviral Infection. Defective amphotropic retroviruses bearing a nuclear localization signal 3′ to an Escherichia coli lacZ (β-gal) gene under the control of a retroviral LTR were produced by a human myosarcoma cell line (TeFly). Keratinocytes were infected by cultivation onto a lethally irradiated feeder layer constituted in equal parts of TeFly and 3T3 cells. To determine whether infectious recombinant retroviruses were produced by the transduced keratinocytes, 3T3 cells were cultivated in medium conditioned by the transduced keratinocytes. None of the 3T3 cells expressed β-gal, indicating that no recombinant viruses were produced by the transduced keratinocytes.
Transplantation. Pieces of newborn mouse or rat skin were incubated in 2% EDTA for 150 min at 37°C. After rinsing in medium, the epidermis was gently separated from the dermis to form a small pocket with the help of a 30-gauge needle. Donor cells (5 × 105) were then carefully injected into the pocket and allowed to attach for 1 hr at room temperature (Fig. 6, which is published as supporting information on the PNAS web site). Grafts were kept overnight at 4°C before they were transplanted onto the back of athymic mice with their dermal side facing the mouse fascia, stitched in place, and covered with a mouse skin flap to prevent drying. Necrosis of the flap usually occurred a few days later, and the graft became air-exposed. Dense hairs covered the graft within days. The duration of the first cycle was usually extended from 30 to 50 days as a consequence of the experimental procedure.
Histology. β-gal staining was performed according to standard protocols. Samples were then embedded in resin (Historesin, Leica, Vienna), and 4-μm sections were obtained.
RT-PCR. Primers, the sequences of which are given in Table 1, which is published as supporting information on the PNAS web site, were selected to anneal with mRNA of each of the 23 genes. Total RNAs were extracted by using TRIzol reagent (Invitrogen). Total RNA (10 ng) was amplified by using the OneStep RT-PCR kit (Qiagen, Valencia, CA). PCR products were resolved by agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV light. β-Actin control for equal loading was used throughout the experiments.
FISH Analysis. Karyotypes and FISH analyses were carried out by Chrombios (Raubling, Germany). Rat genomic DNA was labeled with FITC-dUTP (green fluorescence) by degenerate oligonucleotide primer PCR, and mouse satellite DNA was labeled with carboxytetramethyl-rhodamine-dUTP (red fluorescence). Diploid cells (42 chromosomes) were observed in most clones and subclones tested (9/10), whether the cells originated from the upper or lower part of the whisker follicle of the rat.
Results
Clonogenic Keratinocytes of the Upper Region of the Hair Follicle Are Multipotent Stem Cells. The upper constant region of the whisker follicle of the rat contains up to 1,500 clonogenic keratinocytes, whereas their number in the lower variable region depends on the phase of the hair cycle (12, 13). To investigate the fate of clonogenic keratinocytes, 100 single cells were randomly isolated from the constant region of a rat whisker follicle (YR25) with a Pasteur pipet by using an inverted microscope and individually cultivated on a feeder layer of irradiated 3T3 cells as described in refs. 6 and 17. From the 100 isolated cells, 14 clones were identified (14% cloning efficiency). Twelve clones were grown to mass culture. Each was then infected with defective amphotropic retroviruses bearing a β-gal gene. The percentage of transduced cells varied from clone to clone. Clone 4 (YR25P3-cl4), which has the largest number of transduced cells, was subcloned. Three subclones (RA5, RA9, and RA15), all cells of which expressed β-gal, were selected. β-gal-labeled keratinocytes of each clone were injected into a small pocket made at the dermo-epidermal junction of the back skin of a newborn mouse when pelage hair follicles were still being formed (Fig. 6). Grafts were then transplanted onto the back of athymic mice and harvested at various periods of time. A variable number of cells incorporated into developing follicles, but some follicles were constituted almost entirely of labeled cells (Fig. 1A). Each clone contributed to the formation of eight lineages of the hair follicle, including those of outer root sheath, inner root sheath, and hair shaft and to the sebaceous gland and the epidermis.
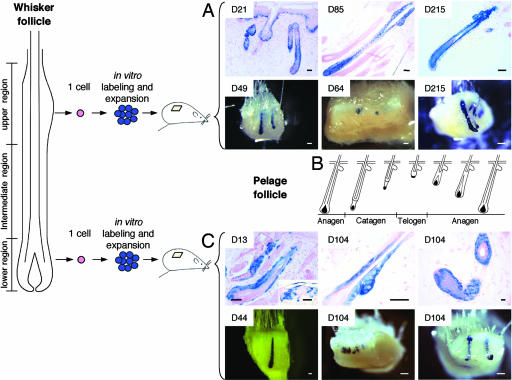
Fig. 1.
Clonogenic keratinocytes are long-term multipotent stem cells. Single keratinocytes were isolated from the upper or lower regions of whisker follicles of adult rats. Clones were labeled by using a defective retrovirus bearing a β-gal gene and subcloned, and labeled cells (passages 11-12) were injected into newborn mouse skin (Fig. 6). Transplanted clonogenic keratinocytes, whether from the upper or lower regions, contributed to all epithelial lineages of hairy skin, including sebaceous glands, and participated in the hair cycle of pelage follicles for months. Note the variable degree of chimerism and that the epidermis is not labeled in long-term grafts (Lower in A and C). Insets show duration of transplantation in days in A and C. D21-D85 follicles were from subclone RA15 of clone YR25P3-cl4; D215 follicles were from subclone RA9 of clone YR25P3-cl4. D49 (anagen) and D64 (early anagen) follicles were from subclone RA15 of clone YR25P3-cl4. D215 follicles (anagen and telogen) were from subclone RA9 of clone YR25P3-cl4. In Lower, all follicles were from clone YR207P1-cl16. (B) Schematic representation of the hair cycle phases of a pelage follicle. (Scale bars: biopsies stained for β-gal in toto, 100 μm; microscopic sections, 50 μm.)
Pelage follicles undergo phases of growth (anagen), regression (catagen), and rest (telogen) throughout the entire life of a mammal (Fig. 1B). Cells are recruited from the bulge at the onset of each growth phase to form a hair germ, from which a new hair bulb develops (11, 18, 19). We examined the capacity of our transplanted cells to participate in the hair cycle of pelage follicles over long periods of time. Labeled cells of these follicles were found at all phases of growth and regression during the hair cycle for the life of the recipient athymic mouse (Fig. 1 A). After up to seven hair cycles, the follicles continued to contain a contribution of labeled cells. These experiments demonstrate that the three labeled clones, and therefore their founding cells, were multipotent stem cells. Similar results were obtained with 4 other YR25P3 clones, as well as with 10 clones isolated from whiskers of rats constitutively expressing EGFP, demonstrating that each of 17 tested clones was derived from a multipotent stem cell.
The Lower Region of the Hair Follicle also Contains Multipotent Stem Cells. The lower-intermediate region of the follicle deep to the bulge is the variable region of a whisker follicle. Although the number of clonogenic keratinocytes in this region depends on the stage of the hair cycle, up to 700 clonogenic cells can be isolated from this region at the onset of a new anagen phase (12). Clonogenic cells isolated from the lower region of cycling whisker follicles were labeled with a retrovirus bearing a β-gal gene and were transplanted as described above. The labeled cells behaved identically to the clonogenic stem cells isolated from the upper constant region of the follicle. The formation of outer root sheath, inner root sheath, and cortical lineages of sebaceous glands and epidermis; the capacity to participate in several hair cycles (Fig. 1C); and their gene expression pattern (see below) were identical. A total of 17 experiments was also performed with clonogenic cells from whiskers of EGFP rats with similar results.
Transplanted Cultured Stem Cells Retain Stemness. To further evaluate the self-renewing capacity of the transplanted cells, we isolated EGFP+ cells from chimeric pelage follicles several months after the labeled cells were transplanted. A second transfer was carried out, consisting of expansion of a single cell in culture followed by transplantation of its progeny. The EGFP+ cells participated for a second time in the formation of all epithelial lineages of the hair follicle and sebaceous glands and were retained through several hair cycles (Fig. 2 A and B). Stem cell character persisted through the first and second transfers for a period of 313 days with no decline in any aspect of hair follicle formation.
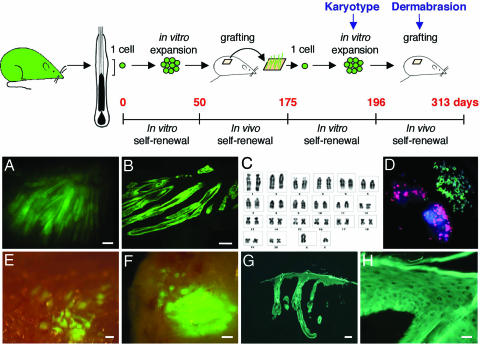
Fig. 2.
Long-term persistence of hair follicles generated from a single multipotent stem cell. A single cell (clone YR219P3-cl7) was directly isolated from the upper region of a whisker follicle of an EGFP rat. After cultivation for 50 days, it was transplanted. One hundred twenty-five days later, a biopsy was obtained from the graft, and clonogenic EGFP+ cells were isolated, subcloned, and cultivated for 3 weeks before they were again transplanted. (A) UV illumination of a biopsy 117 days after the second transplantation; numerous EGFP+ follicles were present. The epidermis was not labeled. (B) Frozen section of A. (C) Cells of subclone 14 of clone YR219P3-cl7 were diploid (2n = 42). (D) FISH analysis shows that no fusion occurred between rat (green probe) and mouse (red probe) cells during the transplantation; cells labeled in red are irradiated 3T3 feeder cells. Nuclei are blue (DAPI). (E) Graft of subclone 14 of clone YR219P3-cl7 at 54 days after the second transplantation. Labeled follicles appeared as green spots under UV illumination. The epidermis was not labeled. (F) The same graft 4 days after dermabrasion with sandpaper. The surface of the graft was bright green (compare with A). (G) Microscopic appearance of F. An EGFP+ epidermis formed, indicating that EGFP+ cells moved out of the hair follicles. (H) Detail is shown. (Scale bars: A and E-G, 100 μm; B, 50 μm; H, 20 μm.)
Because it has been shown that cell fusion can occur between transplanted donor cells and recipient cells (20, 21), we examined the karyotype of β-gal and EGFP+ cells isolated and cultivated from chimeric pelage follicles. Normal diploid rat karyotypes were observed (Fig. 2C). Most importantly, no fusion between mouse and rat cells was observed (Fig. 2D), indicating that the involvement of labeled cells in the long-term regeneration of the follicles did not result from an in vivo fusion between the transduced rat cells and the resident stem cells of the mouse. Furthermore, cells of a subclone of RA15, the karyotype of which was aneuploid, were rapidly eliminated after transplantation, suggesting that stem cells must be diploid to permanently engraft.
Repairing the Epidermis. There are differing opinions about the contribution of hair follicle stem cells to epidermal renewal; some view the epidermis as self-renewing and containing its own stem cells (8, 9, 22), whereas others view it as being renewed by the migration of stem cells or progenitor cells originating from adjacent hair follicles (11). To clarify this point, we examined the contribution of hair follicle stem cells to the formation of epidermis in long-term transplantation experiments. Labeled cells, whether labeled by means of β-gal defective retroviruses or constitutively expressing EGFP, were observed in the epidermis adjacent to labeled hair follicles only for the first 3 weeks of transplantation (Fig. 1 A, day 21). Thereafter, the infundibulum clearly marked the boundary between labeled follicles and the adjacent epidermis, which remained devoid of labeled cells for the entire duration of the transplantation, up to 7 months (Figs. 1 A and C, 2 A and E, and 3B). A similar pattern was observed when the labeled rat cells were transplanted onto newborn rat skin, indicating that the result was not due to a species difference (Fig. 7 D-F, which is published as supporting information on the PNAS web site). However, when the epidermis was wounded by dermabrasion, the labeled cells quickly moved out of the infundibulum to restore the epidermal integrity in agreement with previous observations (Fig. 2 F-H) (23). This contribution of the hair follicle cells to the epidermis again lasted for only a short period, and the epidermal cells of the mouse progressively replaced the EGFP+ cells (data not shown). These results demonstrate that in the steady state, hair follicle stem cells do not contribute to epidermal renewal and hence that the epidermis must contain its own dedicated stem cells. They also indicate that the transplanted cells, which initially contributed to the formation of epidermis, were either epidermal progenitors with a finite lifespan or stem cells that did not find a favorable environment to remain engrafted permanently.
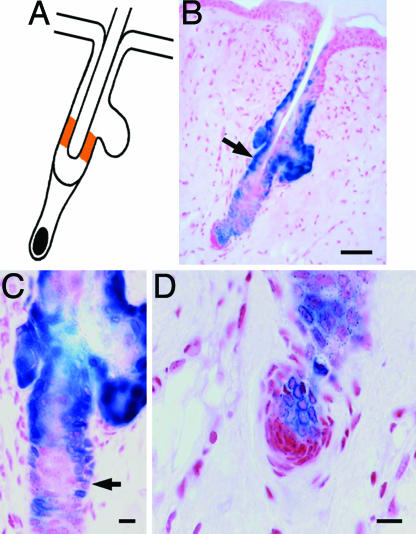
Fig. 3.
Homing to the niche. (A) Location of the niche (orange) in a pelage follicle below the sebaceous gland. (B) Transplanted stem cells (subclone RA15 of clone YR25P3-cl4) correctly homed to the bulge region (arrow) of a mouse pelage hair follicle 64 days after transplantation. The follicle shown was in early anagen. Note the sharp boundary between the follicle and the unlabeled epidermis. Note also that the sebaceous glands and the lower part of the infundibulum contain labeled cells. (C) Labeled stem cells intermingled with unlabeled resident stem cells (arrow). (D) The hair germ was mostly constituted of labeled cells; the dermal papilla cells located below were unlabeled. (Scale bars: B, 50 μm; C and D, 20 μm.)
Homing to the Bulge of Pelage Follicles. In pelage follicles, the stem cells are located only in the upper bulge region near the site of insertion of the arrector pili muscle (Fig. 3A) (11, 19). Remarkably, transplanted labeled stem cells originating from the rat whisker follicle were always observed in the bulge region of mouse chimeric pelage follicles, whether the stem cells were initially derived from the upper (Fig. 3 B and C) or lower (not shown) region of the whisker follicle. This result demonstrates that transplanted stem cells from either region home to the bulge and that the basis for this homing is conserved among different hair follicles (pelage and whisker) and related species (mouse and rat). Labeled cells were constantly present in sebaceous glands of labeled follicles. Our experiments could not distinguish between direct homing of stem cells to the sebaceous glands and secondary migration of sebaceous progenitors originating from the bulge.
Stem Cells Contribute a Variable Number of Progenitors. We then examined to what extent the transplanted β-gal stem cells and the recipient stem cells contributed to the formation of the different epithelial lineages in chimeric hair follicles that underwent the hair cycle. The degree of mosaicism was variable, ranging from small to high. Transplanted cells contributed a part of a sheath, several sheaths (Fig. 4), or the entire hair matrix (Figs. 1 A and C and 3D). Similar results were obtained with transplanted cells constitutively expressing EGFP (not shown), indicating that the mosaicism observed with β-gal-labeled cells did not result from a shutting off of the transduced β-gal gene. Similar results were obtained when clonogenic rat keratinocytes were transplanted onto newborn rat skin, indicating that the mosaicism did not result from a species difference (Fig. 7). These results demonstrated that a minimum of two stem cells, one of the donor type and one of the recipient type, were recruited from the bulge to form a hair bulb and therefore that hair bulbs are polyclonal. Moreover, mosaicism within a single cell sheath demonstrated that a minimum of two stem cells, one of donor type and one of the recipient mouse, contributed progenitors to this sheath. Stem cells can express only a restricted number of their potential lineages during the formation of the hair bulb, as previously suggested by lineage analysis using defective retroviruses or allophenic mice (24-26).
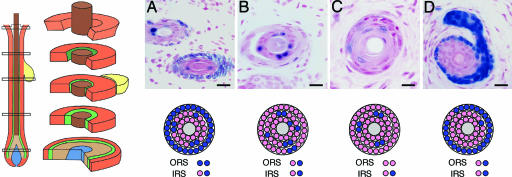
Fig. 4.
Mosaicism indicates that the hair bulb is polyclonal. (Left) Schematic drawing of cross sections of an anagen follicle modified from ref. 44. (A-D) Labeled follicles were often mosaic, indicating that several stem cells participated in their formation or renewal (see Figs. 1 and 3). Mosaicism was often observed within a single follicular sheath, indicating that the committed progenitors that participated in the formation of that particular sheath were generated by different stem cells. (Scale bars: 20 μm.)
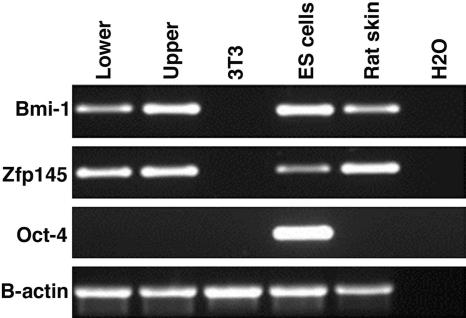
Cultivated Stem Cells Express Bmi1 and Zfp145. Gene profiling of hematopoietic and neural stem cells has led to the identification of genes that are commonly expressed in stem cells. These so-called stemness genes encode proteins ranging from transcription factors to adhesion molecules, from signaling to cell cycle-related molecules. The mRNA profile of stem cells isolated from mouse pelage follicles was recently determined by using elegant transgenic approaches (14, 27). Several candidate genes were identified, some of which were common to hematopoietic and neural stem cells. We have examined the expression of these genes in cultivated multipotent stem cells by RT-PCR. All of them remained expressed in cultured cells derived from individual multipotent stem cells (Fig. 8, which is published as supporting information on the PNAS web site). However, it would be premature to draw definite conclusions as to their importance without thoroughly evaluating the impact of the genes on stem cell function. RNA interference experiments in cultured rat follicle stem cells coupled with in vivo functional analysis should distinguish the genes that are important for stemness from those that are expressed in response to proliferation, migration, or a different environment. Recent work indicated that Bmi1, a member of the polycomb group, and the transcriptional repressor Zfp145 (Plzf) were important for the renewal of hematopoietic stem cells and germ cells (28-31). Interestingly, both Bmi1 and Zfp145 were expressed in clonogenic multipotent stem cells (Fig. 5), supporting the idea that the products of these genes may be important for the renewal and maintenance of the follicle stem cells.
Fig. 5.
Cultivated stem cells express Bmi1 and Zfp145. Clones were obtained from the upper and lower regions of whisker follicles of EGFP rats (clones YR219P5-cl7 and YR221P1-cl10.3). EGFP+ cells were sorted from 3-day-old cultures at passages 7 and 11 to eliminate the irradiated 3T3 cells. mRNAs were then extracted, and RT-PCRs were performed using specific primers (see Table 1). Mouse ES cells were used as a positive control for the expression of Oct-4, Bmi1, and Zfp145.
Discussion
Clonogenicity is an important property of adult epithelial stem cells of the hair follicle, as is true for other adult stem cells, including neural, hematopoietic, muscle, and epidermal stem cells, as well as for embryonic stem cells. This property is essential if one wants to expand stem cells for regenerative medicine. Not all dividing cells are clonogenic, as in the hair bulb, which contains hundreds of nonclonogenic dividing cells and few clonogenic cells (12, 13, 32). There is now compelling evidence that epidermal stem cells express higher levels of α6, β1 integrins, or CD34 than progenitor cells (22, 33-35). However, the number of clonogenic keratinocytes in populations enriched for these markers by cell sorting is always smaller than that of stem cells simply sorted on small size with a Pasteur pipet (up to 28%) (10, 17), indicating that other cell surface molecules may be important or that the cell-sorting procedure severely affects stem cell behavior. The latter possibility is supported by the low colony-forming efficiency of these cells (0.3%) obtained in recent experiments (14, 36).
There are many differences between multipotent progenitors and stem cells as illustrated by hematopoiesis, in which long-term multipotent stem cells can permanently engraft but multipotent progenitors cannot. These cells do not express the same genes, do not respond to the same growth factors, and may not behave similarly in neoplasia (37, 38). Two laboratories have recently demonstrated that pelage hair follicles contain clonogenic keratinocytes that participate in the formation of different hair follicle lineages, but these studies could not distinguish between stem cells and multipotent progenitor cells, because transplantation experiments were carried out for only short periods of time (14, 36). Our experiments unambiguously demonstrate that clonogenic keratinocytes present in hair follicles are long-term multipotent stem cells. There are many more stem cells in the upper region than anticipated by label-retaining experiments (19, 39). We calculate that there are enough clonogenic stem cells in a whisker follicle (up to 1,500) to provide for whisker renewal for >50 years. Why does the upper region contain so many stem cells, when even a few rounds of symmetrical divisions of a small number of stem cells would be sufficient to maintain them at a constant number? Do epithelial stem cells have a role other than tissue maintenance? Answers most likely relate to the sensory function of whisker follicles, because the upper region is where the nerves terminate.
Skin stem cells, like other adult stem cells, are thought to reside at precise locations, so-called niches, where they supposedly benefit from a unique environment favoring self-renewal through symmetrical or asymmetrical divisions (1, 4, 5). The cellular and molecular composition of niches likely involves a specific combination of nurse cells, growth factors, and extracellular matrix. For instance, osteoblasts and stromal cells appear instrumental for bone marrow stem cells, whereas astrocytes and oligodendrocytes are important for neural stem cells (40, 41). Consequently, niches have different cellular and molecular signatures. The upper constant region of the hair follicle located below the sebaceous glands is the niche for the multipotent follicle stem cells (3, 11, 12, 42) and the melanocyte stem cells (43). It has been recently demonstrated that this niche encompasses a region larger than the site of insertion of the arrector pili muscle (14, 36, 42) and that adherence to the basement membrane is not necessary to maintain stemness (36). Our results demonstrate that stem cells cultivated for long periods of time and then transplanted into pelage hair follicles retain the capacity to recognize and home to the niche. Once there, they participate in hair cycle along with the resident stem cells. There are at least two mechanisms by which a stem cell could recognize a location as a niche: through specific adhesion molecules or through specific cell-cell contact. RNA interference experiments in cultured rat follicle stem cells followed by transplantation of the mutated cells should discriminate between these hypotheses.
It is commonly thought that committed progenitors are generated in the upper region of the hair follicle by stem cells dividing asymmetrically and that stem cells exiting the niche quickly lose their self-renewing capacities (11, 14). Our results formally demonstrate that the clonogenic keratinocytes located in the lower region or in the hair bulb, millimeters away from the upper region, are bona fide multipotent stem cells that can divide symmetrically for months in culture and participate in long-term hair follicle renewal when transplanted through more than a single transplantation. Hence, keratinocyte stem cells can migrate, self-renew, and proliferate outside the bulge and participate in the hair cycle. It has been suggested recently that committed progenitors can be reprogrammed to become multipotent stem cells during cell cultivation (14, 35, 36); this hypothesis is highly improbable because our results, corroborating previous observations (12), demonstrate a strict correlation between the ability of a region of a whisker follicle to respond to skin morphogenetic signals and the number of clonogenic keratinocytes that it contains. It is also unlikely that adult hair follicles contain stem cells that cannot undergo clonal growth in culture and yet still demonstrate long-term self-renewal and multipotentiality if transplanted.
A rapid and transitory expansion of the population of the stem cells that have exited the upper constant region of a hair follicle at the onset of anagen is a key step in the formation of a hair bulb. The pattern of mosaicism observed in the different epithelial layers of the chimeric follicles strongly supports the hypothesis that the generation of committed progenitors is a phenomenon that depends on soluble factors and on the position occupied by each stem cell in the developing hair germ (26). The segregation of self-renewing stem cells from the site of formation of late progenitors by separating the two in different locations of the hair follicle would be a way to protect the stem cells from receiving undesired signals that could otherwise result in a rapid exhaustion of the stem cell pool (13). A similar mechanism has been suggested for the pelage follicle (18). The functional difference between a whisker follicle and a pelage follicle is reflected in the distance that the stem cells must migrate before they are within the reach of inductive signals. The presence of a rigid collagenous capsule in a whisker follicle would prevent an extensive shortening of the lower region of the follicle during catagen and hence prevent the follicular papilla from approaching the stem cells located in the upper region, which explains why these stem cells must migrate a long distance before receiving inductive signals from the papilla. Conversely, the extensive shortening of pelage follicles in catagen coupled with an upward migration of the follicular papilla only necessitates that stem cells migrate a short distance. When does a stem cell decide to migrate out of the upper region? Is it a stochastic or controlled phenomenon? Is there a continuous flux or not? What is the rate of stem cell migration? The answers to these questions are likely to lead to better understanding of the molecular control of stem cell trafficking and, consequently, of hair growth.
Supplementary Material
Acknowledgments
We are grateful to N. Kumagai for his support, O. Danos (Genethon, Evry, Francy) for TeFly cells, A. Smith (University of Edinburgh, Edinburgh) for mouse ES cells, M. Okabe (Osaka University, Osaka) for EGFP rats, R. Luthi-Carter for advice with microarrays, and L. Schnell and J. Vannod for excellent technical help. This work was supported by grants from the Swiss National Science Foundation (PNR46 4046-101111 to A.R.), Ecole Polytechnique Fédérale de Lausanne (EPFL), and Lausanne University Hospital (to Y.B.) and by the Institut National de la Santé et de la Recherche Médicale, the Association pour la Recherche Contre le Cancer, and the Association Française Contre les Myopathies. S.C. was supported by a graduate fellowship from the Oise and Doubs chapters of the French League against Cancer and by EPFL.
References
- 1.Watt, F. M. & Hogan, B. L. (2000) Science 287, 1427-1430. [DOI] [PubMed] [Google Scholar]
- 2.Gambardella, L. & Barrandon, Y. (2003) Curr. Opin. Cell Biol. 15, 771-777. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs, E., Tumbar, T. & Guasch, G. (2004) Cell 116, 769-778. [DOI] [PubMed] [Google Scholar]
- 4.Schofield, R. (1978) Blood Cells 4, 7-25. [PubMed] [Google Scholar]
- 5.Nilsson, S. K. & Simmons, P. J. (2004) Curr. Opin. Hematol. 11, 102-106. [DOI] [PubMed] [Google Scholar]
- 6.Rheinwald, J. G. & Green, H. (1975) Cell 6, 331-343. [DOI] [PubMed] [Google Scholar]
- 7.Rochat, A., Kobayashi, K. & Barrandon, Y. (1994) Cell 76, 1063-1073. [DOI] [PubMed] [Google Scholar]
- 8.Barrandon, Y. & Green, H. (1987) Proc. Natl. Acad. Sci. USA 84, 2302-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathor, M. B., Ferrari, G., Dellambra, E., Cilli, M., Mavilio, F., Cancedda, R. & De Luca, M. (1996) Proc. Natl. Acad. Sci. USA 93, 10371-10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rochat, A. & Barrandon, Y. (2004) in Handbook of Stem Cells: Adult and Fetal Stem Cells, ed. Lanza, R. (Elsevier, Amsterdam), Vol. 2, pp. 763-772. [Google Scholar]
- 11.Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T. T. & Lavker, R. M. (2000) Cell 102, 451-461. [DOI] [PubMed] [Google Scholar]
- 12.Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. (2001) Cell 104, 233-245. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi, K., Rochat, A. & Barrandon, Y. (1993) Proc. Natl. Acad. Sci. USA 90, 7391-7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris, R. J., Liu, Y., Marles, L., Yang, Z., Trempus, C., Li, S., Lin, J. S., Sawicki, J. A. & Cotsarelis, G. (2004) Nat. Biotechnol. 22, 411-417. [DOI] [PubMed] [Google Scholar]
- 15.Lavker, R. M. & Sun, T. T. (2000) Proc. Natl. Acad. Sci. USA 97, 13473-13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito, T., Suzuki, A., Imai, E., Okabe, M. & Hori, M. (2001) J. Am. Soc. Nephrol. 12, 2625-2635. [DOI] [PubMed] [Google Scholar]
- 17.Barrandon, Y. & Green, H. (1985) Proc. Natl. Acad. Sci. USA 82, 5390-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panteleyev, A. A., Jahoda, C. A. & Christiano, A. M. (2001) J Cell Sci. 114, 3419-3431. [DOI] [PubMed] [Google Scholar]
- 19.Cotsarelis, G., Sun, T. T. & Lavker, R. M. (1990) Cell 61, 1329-1337. [DOI] [PubMed] [Google Scholar]
- 20.Medvinsky, A. & Smith, A. (2003) Nature 422, 823-825. [DOI] [PubMed] [Google Scholar]
- 21.Wagers, A. J., Sherwood, R. I., Christensen, J. L. & Weissman, I. L. (2002) Science 297, 2256-2259. [DOI] [PubMed] [Google Scholar]
- 22.Jones, P. H., Harper, S. & Watt, F. M. (1995) Cell 80, 83-93. [DOI] [PubMed] [Google Scholar]
- 23.Al-Barwari, S. E. & Potten, C. S. (1976) Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 30, 201-216. [DOI] [PubMed] [Google Scholar]
- 24.Ghazizadeh, S. & Taichman, L. B. (2001) EMBO J. 20, 1215-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamimura, J., Lee, D., Baden, H. P., Brissette, J. & Dotto, G. P. (1997) J. Invest. Dermatol. 109, 534-540. [DOI] [PubMed] [Google Scholar]
- 26.Kopan, R., Lee, J., Lin, M. H., Syder, A. J., Kesterson, J., Crutchfield, N., Li, C. R., Wu, W., Books, J. & Gordon, J. I. (2002) Dev. Biol. 242, 44-57. [DOI] [PubMed] [Google Scholar]
- 27.Tumbar, T., Guasch, G., Greco, V., Blanpain, C., Lowry, W. E., Rendl, M. & Fuchs, E. (2004) Science 303, 359-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buaas, F. W., Kirsh, A. L., Sharma, M., McLean, D. J., Morris, J. L., Griswold, M. D., de Rooij, D. G. & Braun, R. E. (2004) Nat. Genet. 36, 647-652. [DOI] [PubMed] [Google Scholar]
- 29.Costoya, J. A., Hobbs, R. M., Barna, M., Cattoretti, G., Manova, K., Sukhwani, M., Orwig, K. E., Wolgemuth, D. J. & Pandolfi, P. P. (2004) Nat. Genet. 36, 653-659. [DOI] [PubMed] [Google Scholar]
- 30.Iwama, A., Oguro, H., Negishi, M., Kato, Y., Morita, Y., Tsukui, H., Ema, H., Kamijo, T., Katoh-Fukui, Y., Koseki, H., et al. (2004) Immunity 21, 843-851. [DOI] [PubMed] [Google Scholar]
- 31.Park, I. K., Qian, D., Kiel, M., Becker, M. W., Pihalja, M., Weissman, I. L., Morrison, S. J. & Clarke, M. F. (2003) Nature 423, 302-305. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds, A. J. & Jahoda, C. A. (1991) J. Cell Sci. 99, Part 2, 373-385. [DOI] [PubMed] [Google Scholar]
- 33.Trempus, C. S., Morris, R. J., Bortner, C. D., Cotsarelis, G., Faircloth, R. S., Reece, J. M. & Tennant, R. W. (2003) J. Invest. Dermatol. 120, 501-511. [DOI] [PubMed] [Google Scholar]
- 34.Tani, H., Morris, R. J. & Kaur, P. (2000) Proc. Natl. Acad. Sci. USA 97, 10960-10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva-Vargas, V., Lo Celso, C., Giangreco, A., Ofstad, T., Prowse, D. M., Braun, K. M. & Watt, F. M. (2005) Dev. Cell 9, 121-131. [DOI] [PubMed] [Google Scholar]
- 36.Blanpain, C., Lowry, W. E., Geoghegan, A., Polak, L. & Fuchs, E. (2004) Cell 118, 635-648. [DOI] [PubMed] [Google Scholar]
- 37.Wagers, A. J. & Weissman, I. L. (2004) Cell 116, 639-648. [DOI] [PubMed] [Google Scholar]
- 38.Goodell, M. A., Brose, K., Paradis, G., Conner, A. S. & Mulligan, R. C. (1996) J. Exp. Med. 183, 1797-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavker, R. M., Cotsarelis, G., Wei, Z. G. & Sun, T. T. (1991) Ann. N.Y. Acad. Sci. 642, 214-225. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, J., Niu, C., Ye, L., Huang, H., He, X., Tong, W. G., Ross, J., Haug, J., Johnson, T., Feng, J. Q., et al. (2003) Nature 425, 836-841. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez-Buylla, A. & Lim, D. A. (2004) Neuron 41, 683-686. [DOI] [PubMed] [Google Scholar]
- 42.Braun, K. M., Niemann, C., Jensen, U. B., Sundberg, J. P., Silva-Vargas, V. & Watt, F. M. (2003) Development (Cambridge, U.K.) 130, 5241-5255. [DOI] [PubMed] [Google Scholar]
- 43.Nishimura, E. K., Jordan, S. A., Oshima, H., Yoshida, H., Osawa, M., Moriyama, M., Jackson, I. J., Barrandon, Y., Miyachi, Y. & Nishikawa, S. (2002) Nature 416, 854-860. [DOI] [PubMed] [Google Scholar]
- 44.Krstic, R. (1997) in Human Microscopy (Springer, Berlin), pp. 456-457.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.