Abstract
Malonyl-CoA functions as a mediator in the hypothalamic sensing of energy balance and regulates the neural physiology that governs feeding behavior and energy expenditure. The central administration of C75, a potent inhibitor of the fatty acid synthase (FAS), increases malonyl-CoA concentration in the hypothalamus and suppresses food intake while activating fatty acid oxidation in skeletal muscle. Closely correlated with the increase in muscle fatty acid oxidation is the phosphorylation/inactivation of acetyl-CoA carboxylase, which leads to reduced malonyl-CoA concentration. Lowering muscle malonyl-CoA, a potent inhibitor of carnitine/palmitoyl-CoA transferase 1 (CPT1), releases CPT1 from inhibitory constraint, facilitating the entry of fatty acids into mitochondria for β oxidation. Also correlated with these events are C75-induced increases in the expression of skeletal muscle peroxisome proliferator-activated receptor α (PPARα), a transcriptional activator of fatty acid oxidizing enzymes, and uncoupling protein 3 (UCP3), a thermogenic mitochondrial uncoupling protein. Phentolamine, an α-adrenergic blocking agent, prevents the C75-induced increases of skeletal muscle UCP3 and whole body fatty acid oxidation and C75-induced decrease of skeletal muscle malonyl-CoA. Thus, the sympathetic nervous system is implicated in the transmission of the “malonyl-CoA signal” from brain to skeletal muscle. Consistent with the up-regulation of UCP3 and PPARα is the concomitant increase in the expression of PGC1α, transcriptional coactivator of the UCP3 and PPARα-activated genes. These findings clarify the mechanism by which the hypothalamic malonyl-CoA signal is communicated to metabolic systems in skeletal muscle that regulate fatty acid oxidation and energy expenditure.
Keywords: acetyl-CoA carboxylase, malonyl-CoA, obesity, uncoupling protein 3
The hypothalamus monitors peripheral neural and hormonal signals that reflect changes in the energy status of higher animals (1). These signals are transmitted to higher brain centers, where the information is integrated and appropriate adjustments are made to alter feeding behavior. Recent evidence suggests that, in addition to signals that affect food intake, signals are also transmitted from the CNS to peripheral tissues, e.g., liver and skeletal muscle, to alter metabolic pathways involved in energy storage and expenditure (2, 3).
Previous investigations have shown that intracerebroventricular (i.c.v.) administration of C75, a potent inhibitor of fatty acid synthase (FAS) (4), rapidly (<2 h) increases hypothalamic malonyl-CoA concentration (5), suppresses expression of orexigenic neuropeptides (e.g., NPY and AgRP), and activates expression of anorexigenic neuropeptides (e.g., αMSH and CART) (6). These changes correlate closely with an abrupt curtailment of food intake and a loss of body weight (7). Compelling evidence indicates that changes in hypothalamic [malonyl-CoA], provoked by inhibition of FAS or by refeeding after fasting, serve as an intermediary in the signaling pathways that regulate feeding behavior (5).
C75-induced weight loss is due not solely to the suppression of food intake but also to increased energy expenditure (8, 9). Both lean and obese mice treated with C75 lose considerably more body weight than fasted or pair-fed controls whose food intake was limited to that of C75-treated mice (8). This finding was not anticipated, because the normal response to fasting is to increase appetite and decrease energy expenditure, a mechanism to conserve energy during times of nutritional stress. Consistent with these findings, administration of inhibitors of FAS to obese mice exhibits increased oxygen consumption, as assessed by whole-body calorimetry (9, 10).
Here we report that the C75 signal is rapidly transmitted from the CNS to skeletal muscle, apparently via the sympathetic nervous system, to provoke increased fatty acid oxidation and expression of uncoupling protein 3 (UCP3) and peroxisome proliferator-activated receptor α (PPARα). These effects appear to account for the C75-induced rise in whole-body energy expenditure.
Experimental Procedures
Animals. Male Balb/C and C57BL/6J-Lepob (Ob/Ob) mice (8 weeks of age, 20–25 g) were purchased from Charles River Laboratories, individually housed at 22°C, maintained on a 12-h/12-h light/dark cycle (lights off at 12:00 p.m.), and given access to standard rodent chow and water ad libitum. After acclimatization for 1 week, mice were randomly divided into three treatment groups: C75-treated, refed, and fasted continuously. The basic protocol for C75 and/or phentolamine (from Sigma) treatment involved an initial 23-h fast before the start of the dark cycle, then i.c.v. injection of C75 (10 μg in RPMI medium 1640 as vehicle (Invitrogen) or vehicle alone, followed by i.p. administration of phentolamine (10 mg/kg body weight) or vehicle. i.c.v. injections into the lateral ventricle under isoflurane anesthesia. After i.c.v. injection, mice were given free access to food (refed), or fasting was continued. Skeletal muscle (gastrocnemius muscle) was removed for analysis within 30–60 sec of death. After treatment, tissues were snap-frozen in liquid nitrogen and stored at -80°C until analysis. Procedures were approved by guidelines of the Johns Hopkins University School of Medicine Institutional Animal Care and Use Committee.
Measurement of Glucose, Insulin, Nonesterified Fatty Acids (NEFA), Ketones, and Triglyceride (TG) Levels in Blood and Malonyl-CoA Levels in Skeletal Muscle. Blood was drawn at approximately 12:00 p.m. from the orbital sinus from overnight-fasted (12 h) or ad lib-fed mice. Plasma was frozen and stored at -70°C. Plasma insulin was determined by RIA (Linco Research, St. Charles, MO), plasma NEFA and ketones with a kit from Wako Biochemicals (Neuss, Germany), and plasma TG and glucose with a kit from Sigma. Tissues were rapidly (≤1 min) frozen in liquid nitrogen and stored at -80°C. For analysis of cAMP and noradrenaline, frozen tissue was extracted with 0.1 N HCl and analyses performed according to the supplier's instructions (Amersham Pharmacia Biotech and ALPCO, Windham, NH, respectively). Determination of malonyl-CoA was as described (5).
Isolation of RNA and Real-Time PCR. Skeletal muscle was homogenized with a Polytron in TRIzol reagent (Invitrogen Life Technologies) and total RNA isolated according to the manufacturer's instructions. RNA was further purified by using affinity columns (Qiagen, Valencia, CA). The quality and concentration of RNA were assessed at A260 nm/280 nm and its integrity by gel electrophoresis. Real-time RT-PCR measurements were performed with a Mx3000 Multiplex Quantitative PCR System (Stratagene) by using the recommended buffers supplied by the manufacturer (Syber Green QRT-PCR master mix). RNA standard template was replaced with water for all no-template controls. Cycling conditions were as follows: initial denaturation at 95°C for 5–10 min, followed by 40 cycles of 95°C for 30 s, 1-min annealing (annealing temperature adapted for each specific primer set used), and 30-s extension at 72°C. Data analysis was performed with mx3000 software (Stratagene). The sequences of the primers were as follows: UCP3 forward primer, 5′-TGGCCCAACATCACAAGAAAT-3′, and UCP3 reverse primer, 3′-ACGCAGAAAGGAGGGCACAAAT-5′. Data were analyzed with DNASTAR (Madison, WI) lasergene software. The amounts of each transcript were calculated from the standard curve and levels of UCP3 normalized to the level of 18S rRNA (UCP3/18S rRNA).
Western Blot Analysis. Frozen gastrocnemius muscle samples (≈80 mg) were homogenized in a buffer containing 50 mM Hepes, 150 mM NaCl, 10 mM NaF, 1 mM Na3VO4, 5 mM EDTA, 0.5% Triton-X, 10% glycerol, 2 μg/ml leupeptin, 100 μg/ml PMSF, 2 μg/ml aprotinin, and 10 mg/μl pepstatin A. Equal amounts of protein (40–50 μg per lane) were subjected to SDS/PAGE. Proteins were transferred to poly(vinydifluoride) membranes, blocked with nonfat milk, and incubated overnight with polyclonal goat anti-UCP3, PGC1α, or PPARα antibody at 1:1,000 (Santa Cruz Biotechnology), anti-phospho (Ser-79)-acetyl-CoA carboxylase β (ACCβ), or a polyclonal goat anti-ACCβ antibody (diluted 1:1,000; Cell Signaling Technology, Beverly, MA).
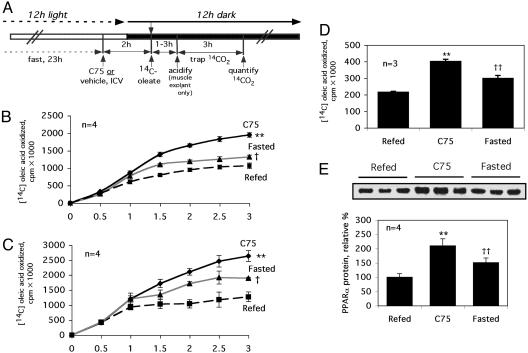
Fatty Acid Oxidation. Fatty acid ([1-14C] oleic acid) oxidation was measured either in vivo and in vitro with skeletal muscle explants. The in vivo rate of 14C-fatty acid oxidation [3 μCi (1 Ci = 37 GBq) of [1-14C] oleic acid injected intraperitonally] to 14CO2 was determined after treatment of mice as described in the legend of Fig. 2. After acclimatization in metabolic chambers fitted with NaOH traps to recover expired 14CO2, oxidation of [1-14C] oleic acid to 14CO2 was measured at 30-min intervals over the next 3 h.
Fig. 2.
Central administration of C75 rapidly activates fatty acid oxidation measured in vivo and in muscle explants. (A) Treatment protocol. (B–E) Obese (Ob/Ob) (B) or lean (C) mice were fasted for 23 h and then given an i.c.v. injection of 10 μg of C75 (C75) or vehicle. Fasting was continued (Fasted), or mice were given access to food (Refed). Fatty acid oxidation ([1-14C] oleic acid) was measured in vivo (B and C) or with skeletal muscle explants from lean mice (D). Two hours after i.c.v. injection of lean mice with C75, skeletal (gastrocnemius) muscle tissue was subjected to immunoblotting with antibody directed against mouse PPARα (E). The results are expressed as the mean ± SEM. Differences between treatment groups were analyzed by Student's t test. *, P < 0.01; **, P < 0.001; †, P < 0.01 compared with refed mice; ††, P < 0.001 compared with C75-treated mice.
Skeletal muscle explants (≈80 mg of gastrocnemius muscle) were quickly (≤20 sec) excised from mice treated and placed in 50-ml flasks fitted with center wells containing 1 N NaOH and a filter paper strip to trap 14CO2. Flasks were capped with serum bottle stoppers. Incubation media contained 3 ml of Krebs–Ringer phosphate buffer and 2 μCi (51 mCi/mmol) of [1-14C] oleic acid (Amersham Pharmacia Biosciences). Incubations were at 37°C for 1 h with oscillation (100 strokes/min), after which 1 ml of 2 N sulfuric acid was injected into the medium to release 14CO2. Flasks were then held at 50°C for 3 h to allow transfer of 14CO2 to the NaOH in the center well. After acid treatment, the contents of the center well were transferred to scintillation fluid and counted.
Results
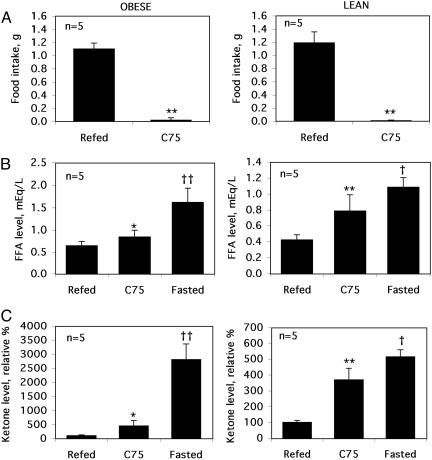
Central Administration of C75 Rapidly Lowers Blood Fatty Acid and Ketone Levels of Fasted Mice. Increased blood fatty acid and ketone levels normally accompany fasting. Unexpectedly however, i.c.v. injection of C75, which blocks food intake (Fig. 1A) and in effect produces a fasted state, rapidly (≤2 h) lowers blood fatty acids and ketones of fasted mice, the effects being most dramatic in obese mice (Fig. 1 B and C). It should be noted that these effects of C75 treatment were the same whether or not the C75-treated mice had been fasted (Fig. 1 B and C) or had had access to food (results not shown). That both fatty acid and ketone levels were rapidly decreased compared with those of fasted mice suggested that fatty acid oxidation by peripheral tissues might be increased.
Fig. 1.
Central administration of C75 rapidly decreases food intake and blood fatty acid and ketone levels. Obese (Ob/Ob) or lean mice were fasted for 23 h and then given an i.c.v. injection of 10 μg of C75 (C75) or vehicle. Fasting was continued (Fasted), or mice were given access to food (Refed). Food intake (A), blood fatty acid (B), and ketone (C) levels were measured 2 h after i.c.v. injection. Each bar represents the mean ± SEM. Differences between treatment group were assessed by Student's t test. *, P < 0.01; **, P < 0.001 relative to refed mice or †, P < 0.01; ††, P < 0.001 relative to C75-treated mice.
The hyperglycemia (530–550 mg of glucose/dl) of fasted or refed obese (Ob/Ob) mice was also reduced by ≈40% within 2 h after i.c.v. injection of C75 (results not shown). Neither fasting for 23 h ± subsequent i.c.v. injection of C75 nor refeeding fasted WT lean mice had a significant short-term effect on the blood glucose level, which ranged between 160 and 170 mg/dl. Although refeeding after a 23-h fast rapidly (≤2 h) increased blood insulin and triglyceride levels of both lean and obese (Ob/Ob) mice, C75 treatment had no effect on the levels of these blood constituents (results not shown).
Central Administration of C75 Rapidly Activates Fatty Acid Oxidation in Skeletal Muscle. Because C75 increases energy expenditure, the possibility was considered that an increased rate of fatty acid oxidation might be responsible for the C75-induced lowering of blood fatty acid and ketone levels. To test this possibility, the rate of oxidation of [1-14C] oleic acid to 14CO2 was assessed in lean or Ob/Ob mice that had been fasted and then (i) were given C75 by i.c.v. injection or (ii) were refed, or (iii) fasting was continued. The treatment protocol is illustrated in Fig. 2A. After acclimatization in metabolic chambers fitted with NaOH traps to recover expired 14CO2, the oxidation of [14C] oleic acid to form 14CO2 was measured at 30-min intervals over the next 3 h. As illustrated in Fig. 2 B and C, the rates of oxidation of the labeled fatty acid by lean and obese (Ob/Ob) mice, respectively, were increased by fasting. Central administration (i.c.v. injection) of C75 to fasted mice, however, caused far greater increases in the rate of [14C] oleic acid oxidation. It should be noted that the pattern of body fatty acid oxidation in vivo was inversely correlated with the decrease in blood fatty acid and ketone levels prompted by fasting or C75 administration (Fig. 1 B and C). These findings suggested a causal relationship.
To determine whether the C75-induced increase of fatty acid oxidation in vivo was the result of increased fatty acid oxidation by skeletal muscle, [14C] oleic acid oxidation by muscle tissue explants in vitro was assessed by using the same animal treatment protocol described above (Fig. 2 A). Immediately after the various treatments, gastrocnemius muscle explants from lean mice were quickly (≤20 sec) excised and incubated in flasks fitted with center wells containing NaOH to trap the 14CO2 produced. The effects of fasting, C75 treatment, and refeeding were similar (Fig. 2D) to those observed above for whole-body fatty acid oxidation (Fig. 2 B and C). Thus, the rate of [14C] oleic acid oxidation by muscle explants in vitro were increased by fasting relative to that by explants from refed mice (Fig. 2D). However, a much greater increase of [14C] oleic acid oxidation resulted with muscle explants from fasted mice given i.c.v. injections of C75 (Fig. 2D), a pattern identical to that observed for whole-body fatty acid oxidation (Fig. 2 B and C). Consistent with the rapid increase of fatty acid oxidation by skeletal muscle after the central administration of C75, the expression of PPARα, a transcriptional activator of genes that encode enzymes of the fatty acid oxidation pathway, occurs concomitantly (Fig. 2E).
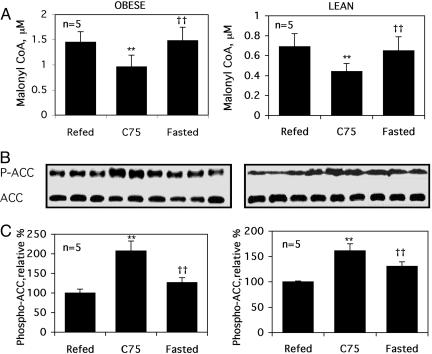
Central Administration of C75 Rapidly Alters Malonyl-CoA Concentration and Phosphorylation of ACC in Skeletal Muscle. It is established (11, 12) that the rate-limiting regulated step of fatty acid oxidation is the translocation of fatty acids into the mitochondrion, the site of β oxidation (12). This step is catalyzed by carnitine/palmitoyl-CoA transferase 1 (CPT1). Because malonyl-CoA is a potent allosteric inhibitor of CPT1, the possibility was tested that the increased rate of fatty acid oxidation caused by centrally administered C75 (Fig. 2 B–D) was due to a change in malonyl-CoA concentration in skeletal muscle. As shown in Fig. 3A, centrally administered C75 rapidly decreased the level of malonyl-CoA in muscle tissue compared with that in muscle tissue from either fasted or refed (lean or obese) mice. It can be concluded, therefore, that the “C75 signal” is rapidly transmitted from the CNS to skeletal muscle, leading to a decreased [malonyl-CoA]. Lowering malonyl-CoA concentration would release CPT1 from inhibitory constraint, thereby promoting the entry of fatty acids into mitochondria for oxidation.
Fig. 3.
Effect of C75 on the malonyl-CoA concentration and phosphorylation state ACC in skeletal muscle. Effect of i.c.v. administration of C75 on malonyl-CoA (A) and the level (B) and phosphorylation state of ACC (at Ser-79) (C) in the gastrocnemius muscle of Ob/Ob and lean mice 2 h after i.c.v. injection of C75 (10 μg/mouse). The treatment protocols for Refed, C75, and Fasted were as described in the legend of Fig. 1. Protein and phosphoprotein levels were quantified from Western blots and are expressed as percent change relative to Refed controls. Each bar represents the mean ± SEM. Differences between the treatment groups were analyzed by Student's t test. **, P < 0.001 compared with refed mice; ††, P < 0.001 compared with C75-treated mice.
The formation of malonyl-CoA in skeletal muscle, catalyzed by ACC2, is regulated through phosphorylation of ACC2 on Ser-79 by 5′-AMP kinase. Therefore, to determine the effect of centrally administered C75 on ACC activity, skeletal muscle tissue was quickly (≤20 sec) excised, subjected to SDS/PAGE, and immunoblotted with anti-phospho-ACC (Ser-79) antibody.
As illustrated in Fig. 3 B and C, phosphorylation of muscle ACC on Ser-79 was significantly higher in C75-treated mice than in fasted control or refed mice. This change occurred without a change in ACC content (Fig. 3 B and C). These findings indicate that the phosphorylation of ACC on Ser-79 by 5′-AMP kinase, which lowers ACC activity and as a consequence lowers malonyl-CoA concentration, leads to increased fatty acid oxidation. The question of how the signal to activate fatty acid oxidation is transmitted from the hypothalamus to skeletal muscle is addressed below.
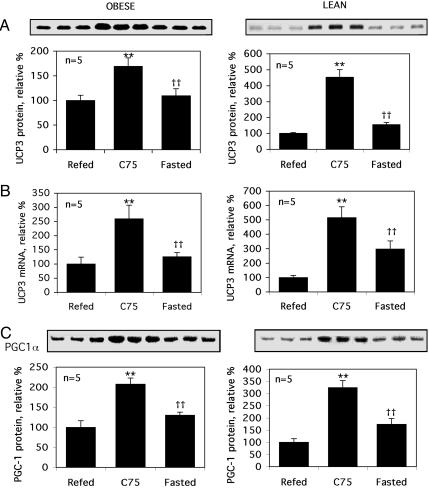
Centrally Administered C75 Increases the Expression of UCP3 and PGC1α in Skeletal Muscle. Like the effect of i.c.v. C75, disruption of the ACC2 gene lowers malonyl-CoA and increases O2 consumption, fatty acid oxidation and UCP3 in skeletal muscle (13). Moreover, the overexpression of UCP3, the principal uncoupling protein in skeletal muscle, increases oxygen consumption and mitochondrial proton motive force in muscle, leading to a reduction of body fat (14). The possibility was considered, therefore, that UCP3 participates in the C75-induced increase in energy expenditure. As illustrated in Fig. 4 A and B, centrally administered C75 activated the expression of the UCP3 gene in skeletal muscle of lean and obese (Ob/Ob) mice. The levels of both UCP3 protein (Fig. 4A) and mRNA (Fig. 4B) were rapidly (≤2 h) increased in gastrocnemius muscle after i.c.v. injection of C75. Concomitant with the up-regulation of the UCP3 gene, expression of PGC1α, transcriptional coactivator of the UCP3 and PPARα genes, was also up-regulated. Thus, central administration of C75 up-regulated the expression of PCG1α in skeletal muscle of obese (Ob/Ob) and lean mice (Fig. 4C). Of importance, these events correlated closely with the increased fatty acid oxidation in skeletal muscle provoked by C75 described above (Fig. 2).
Fig. 4.
Effect of C75 on the expression of UCP3 and PGC1α in skeletal muscle. UCP3 protein and/or mRNA and PGC-1α protein levels were quantified by real-time PCR of RNA or by Western blotting of skeletal muscle extracts obtained 2 h after i.c.v. administration of C75 to lean and obese (Ob/Ob) mice. The protocols for Refed, C75, and Fasted treatments were as described in the legend of Fig. 1. Each bar represented the mean ± SEM. Differences between treatment groups were assessed by Student's t test. **, P < 0.001 compared with refed mice; ††, P < 0.001 compared with C75-treated mice.
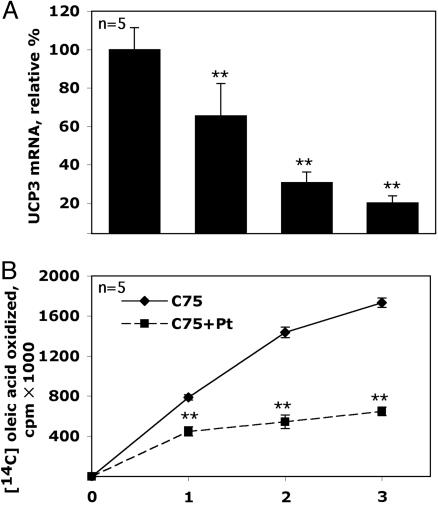
Role of the α Adrenergic System in the Response of Skeletal Muscle to Centrally Administered C75. The rapidity with which the C75 signal is transmitted from the CNS to skeletal muscle suggested neural transmission is involved. To explore this possibility, we tested the effect of phentolamine, an α blocker, on the C75-induced expression of muscle UCP3 and malonyl-CoA levels and in vivo fatty acid oxidation in lean mice. Phentolamine blocked C75-induced increase of skeletal muscle UCP3 and whole-body fatty acid oxidation rate (Fig. 5), as well as the decrease of skeletal muscle malonyl-CoA level (results not shown). These findings implicate the sympathetic nervous system in the signal transmission mechanism.
Fig. 5.
Role of the α adrenergic system in the response of skeletal muscle to centrally administered C75. The protocols for Refed, C75, and Fasted treatments were as described in the legend of Fig. 1. (A) UCP3 mRNA in skeletal muscle was analyzed by real-time PCR 2 h after i.c.v. injection of C75 (10 μg) in mice that had received an i.p. injection of the α blocker, Phentolamine (10 mg/kg body weight; C75 + Pt) 1 h before C75. (B) Lean mice were treated as described in A and then subjected to the fatty acid oxidation ([1-14C] oleic acid) protocol described in the legend to Fig. 2. Each bar represented the mean ± SEM. Statistical significance between treatment groups (Student's t test). **, P < 0.001 compared with C75-treated mice.
It is of interest that, in addition to the α blocker phentolamine (Fig. 5A), a β blocker, propranolol (results not shown), also prevents the increased expression of UCP3 in skeletal muscle induced by centrally administered C75. These findings are consistent with the role of the sympathetic nervous system in signaling via the CNS–muscle axis. Also in support of a role for the β adrenergic receptor in this process are the findings that centrally administered C75 up-regulates cAMP and expression of the β3-adrenergic receptor in skeletal muscle (results not shown), as well as the expression of PGC1α and UCP3 (Fig. 4).
Discussion
Physiological (e.g., fasting and refeeding) and pharmacological (e.g., administration of FAS or ACC inhibitors) perturbations that alter hypothalamic malonyl-CoA levels induce changes in the expression of hypothalamic neuropeptides that modulate feeding behavior (6, 7). The present investigation shows that these changes in hypothalamic malonyl-CoA concentration are also rapidly communicated from the CNS to skeletal muscle to alter energy expenditure. The rate at which this signal is transmitted from the brain to muscle suggests neural signaling. Previous reports from this and other laboratories are consistent with neural signaling from feeding centers in the hypothalamus to peripheral tissues, e.g., to the stomach altering ghrelin (an orexigen) secretion (3, 15) and to liver altering glucose production (16). Metabolites of fatty acid metabolism, notably malonyl-CoA (5) and, indirectly, long-chain fatty acids (17), have been implicated in this hypothalamic signaling pathway (3). Blocking fatty acid synthesis with the FAS inhibitor, C75 (by i.c.v. injection) or refeeding fasted mice rapidly increases malonyl-CoA, down-regulates expression of the oxigens NPY and AgRP and up-regulates expression of the anorexigens αMSH and CART in the hypothalamus (5). Likewise, administration of fatty acid by i.c.v. injection suppresses food intake (18). These perturbations have profound effects on energy metabolism (3).
In this paper, we report that central administration of C75 to obese or lean mice rapidly activates fatty acid oxidation in skeletal muscle (as measured by the conversion of [1-14C] oleic acid to 14CO2) both in the intact animal (Fig. 2 B and C) and in muscle explants from C75-treated animals (Fig. 2D). Skeletal muscle is the most abundant fatty acid metabolizing tissue in the animal. Because fatty acids are the major physiological fuel for muscle, an increase in the rate of fatty acid oxidation in this tissue would be expected to have a major impact on whole-body energy expenditure.
Although the mechanism by which the malonyl-CoA signal, initiated by centrally administered C75, is transmitted from the CNS to muscle tissue is presently unknown, the signal appears to be mediated through the sympathetic nervous system (SNS) to α adrenergic receptors in skeletal muscle. This is indicated by the fact that phentolamine, a potent α blocking agent, prevented C75-induced increases of skeletal muscle UCP3 and whole-body fatty acid oxidation (Fig. 5) and decrease of skeletal muscle malonyl-CoA. induction of both the expression of UCP3 in skeletal muscle (Fig. 5A) and whole-body fatty acid oxidation (Fig. 5B). Like the central action of leptin through the hypothalamic–SNS axis, which activates the 5′-AMP kinase (AMPK)-catalyzed phosphorylation of ACC and fatty acid oxidation in skeletal muscle (2, 19), i.c.v. C75 causes phosphorylation of ACC (on Ser-79, a target site of AMPK) and the decrease of [malonyl-CoA] in skeletal muscle (Fig. 3).
A possible explanation for the increase of energy expenditure induced by C75 is increased thermogenesis caused by up-regulation of skeletal muscle UCP3. Like the other UCPs, UCP3 is thought to dissipate the proton gradient across the inner mitochondrial membrane, producing heat rather than ATP. This thermogenic response may contribute to whole-body energy expenditure. Because UCP3 is expressed primarily in skeletal muscle, a major tissue mass in higher animals, up-regulation of UCP3 may be responsible for the C75-induced increase in energy expenditure. Fatty acids most likely fuel this thermogenic response. Closely correlated to the up-regulation of UCP3 provoked by centrally administered C75 is an increase of fatty acid oxidation by skeletal muscle (Fig. 2 B–D). Presumably, this increase is the result of the accompanying decrease in malonyl-CoA concentration (Fig. 3A) caused by the phosphorylation-induced inactivation of ACC (Fig. 3B). The decrease in malonyl-CoA level in skeletal muscle normally leads to activation of CPT1, allowing translocation of fatty acids into the β oxidation compartment of mitochondria.
Acknowledgments
This research was supported by Astellas Pharma, Inc., Tsukuba, Japan.
Abbreviations: i.c.v., intracerebroventricular; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; PPAR, peroxisome proliferator-activated receptor; UCP, uncoupling protein; CPT1, carnitine/palmitoyl-CoA transferase 1.
References
- 1.Schwartz, M. W., Woods, S. C., Porte, D., Jr., Seeley, R. J. & Baskin, D. G. (2000) Nature 404, 661-671. [DOI] [PubMed] [Google Scholar]
- 2.Minokoshi, Y. & Kahn, B. B. (2003) Biochem. Soc. Trans. 31, 196-201. [DOI] [PubMed] [Google Scholar]
- 3.Dowell, P., Hu, Z. & Lane, M. D. (2005) Annu. Rev. Biochem. 74, 515-534. [DOI] [PubMed] [Google Scholar]
- 4.Kuhajda, F. P., Pizer, E. S., Li, J. N., Mani, N. S., Frehywot, G. L. & Townsend, C. A. (2000) Proc. Natl. Acad. Sci. USA 97, 3450-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu, Z., Cha, S. H., Chohnan, S. & Lane, M. D. (2003) Proc. Natl. Acad. Sci. USA 100, 12624-12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimokawa, T., Kumar, M. V. & Lane, M. D. (2002) Proc. Natl. Acad. Sci. USA 99, 66-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loftus, T. M., Jaworsky, D. E., Frehywot, G. L., Townsend, C. A., Ronnett, G. V., Lane, M. D. & Kuhajda, F. P. (2000) Science 288, 2379-2381. [DOI] [PubMed] [Google Scholar]
- 8.Kumar, M. V., Shimokawa, T., Nagy, T. R. & Lane, M. D. (2002) Proc. Natl. Acad. Sci. USA 99, 1921-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thupari, J. N., Landree, L. E., Ronnett, G. V. & Kuhajda, F. P. (2002) Proc. Natl. Acad. Sci. USA 99, 9498-9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu, Y., Thupari, J. N., Kim, E. K., Pinn, M. L., Moran, T. H., Ronnett, G. V. & Kuhajda, F. P. (2005) Endocrinology 146, 486-493. [DOI] [PubMed] [Google Scholar]
- 11.McGarry, J. D., Leatherman, G. F. & Foster, D. W. (1978) J. Biol. Chem. 253, 4128-4136. [PubMed] [Google Scholar]
- 12.McGarry, J. D. (1995) Biochem. Soc. Trans. 23, 481-485. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Elheiga, L., Oh, W., Kordari, P. & Wakil, S. J. (2003) Proc. Natl. Acad. Sci. USA 100, 10207-10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Son, C., Hosoda, K., Ishihara, K., Bevilacqua, L., Masuzaki, H., Fushiki, T., Harper, M. E. & Nakao, K. (2004) Diabetologia 47, 47-54. [DOI] [PubMed] [Google Scholar]
- 15.Kahn, B. B., Alquier, T., Carling, D. & Hardie, D. G. (2005) Cell Metab. 1, 15-25. [DOI] [PubMed] [Google Scholar]
- 16.Andersson, U., Filipsson, K., Abbott, C. R., Woods, A., Smith, K., Bloom, S. R., Carling, D. & Small, C. J. (2004) J. Biol. Chem. 279, 12005-12008. [DOI] [PubMed] [Google Scholar]
- 17.Morgan, K., Obici, S. & Rossetti, L. (2004) J. Biol. Chem. 279, 31139-31148. [DOI] [PubMed] [Google Scholar]
- 18.Obici, S., Feng, Z., Karkanias, G., Baskin, D. G. & Rossetti, L. (2002) Nat. Neurosci. 5, 566-572. [DOI] [PubMed] [Google Scholar]
- 19.Minokoshi, Y., Kim, Y. B., Peroni, O. D., Fryer, L. G., Muller, C., Carling, D. & Kahn, B. B. (2002) Nature 415, 339-343. [DOI] [PubMed] [Google Scholar]