Abstract
Mice lacking the AP-1 transcription factor c-jun die at mid-gestation showing heart defects and impaired hepatogenesis. To inactivate c-jun in hepatocytes, mice carrying a floxed c-jun allele were generated. Perinatal liver-specific c-jun deletion caused reduced hepatocyte proliferation and decreased body size. After partial hepatectomy, half of the mutants died and liver regeneration was impaired. This phenotype was not present in mice lacking the N-terminal phosphorylation sites of c-Jun. The failure to regenerate was accompanied by increased cell death and lipid accumulation in hepatocytes. Moreover, cyclin-dependent kinases and several cell cycle regulators were affected, resulting in inefficient G1–S phase progression. These studies identify c-Jun as a critical regulator of hepatocyte proliferation and survival during liver development and regeneration.
Keywords: AP-1/c-jun/hepatocyte growth factor/liver regeneration
Introduction
In rodents, liver mass increases by several fold in the first postnatal weeks until there is a sharp drop in hepatocyte proliferation in the fourth week of life when the liver/body weight ratio approaches adult levels. The adult hepatocyte is a quiescent, highly differentiated cell that executes a variety of metabolic functions (Fausto, 2000). Hepatocytes have a very long half-life (∼180 days in the mouse) and minimal replicative activity, i.e. mitosis is observed only in approximately one out of 20 000 hepatocytes (Steer, 1995). However, in contrast to most other differentiated cell types, the differentiated hepatocyte has retained the capability to re-enter the cell cycle and resume cell division. After 70% partial hepatectomy (PH), in which the large and the median lobes of the liver are surgically removed, the normally quiescent liver cells rapidly proliferate, restoring liver mass within a few days (Michalopoulos and DeFrances, 1997; Fausto, 2000). In response to PH, >95% of the mature hepatocytes synchronously exit the G0 phase and enter the cell cycle (Steer, 1995). The residual lobes grow until they are equal in size and volume to the original liver and then abruptly stop any further growth. The events involved in the regenerative response are precise, carefully orchestrated and highly regulated.
Several growths factors and cytokines have been implicated in liver regeneration. Hepatocyte growth factor (HGF) and transforming growth factor (TGF)-α are potent hepatic mitogens in vitro and highly expressed after hepatectomy (Webber et al., 1994; Fausto, 2000). In addition, mice lacking tumour necrosis factor receptor type 1 (TNFR-1) and interleukin (IL)-6 knock-out mice show impaired liver regeneration (Cressman et al., 1996; Yamada et al., 1997). After PH, the activation of growth factor and cytokine signalling pathways leads to the induction of transcription factor complexes such as nuclear factor (NF)-κB, c-Myc, STAT3, CREM and AP-1, which have been implicated in the control of liver regeneration (Cressman et al., 1996; Taub, 1996a; Heim et al., 1997; Servillo et al., 1998).
In response to PH, the DNA binding activity of the heterodimeric sequence-specific transcription factor AP-1, which is composed mainly of the products of the jun and fos families of genes, is rapidly induced (Heim et al., 1997; Brenner, 1998). Transcription of c-jun and c-fos mRNA is strongly induced in regenerating hepatocytes and PH also activates the c-Jun N-terminal kinases (JNKs, also known as SAPKs), which phosphorylate and modify the activity of a number of transcription factors including c-Jun (Westwick et al., 1995; Whitmarsh and Davis, 1996; Brenner, 1998). c-Jun N-terminal phosphorylation (JNP) at the serine residues 63 and 73 within its transactivation domain is thought to increase transcription by stimulating the recruitment of the coactivator proteins CBP and p300 to target gene promoters, including the c-jun gene itself (Angel et al., 1988; Arias et al., 1994; Bannister et al., 1995).
Fetuses lacking c-jun die at mid-gestation with defects in heart morphogenesis and increased apoptosis of both hepatoblasts and hematopoietic cells in the fetal liver, thus preventing the analysis of c-Jun function at later stages of liver development (Hilberg et al., 1993; Johnson et al., 1993; Eferl et al., 1999). A function of c-jun in hepatocyte development could also be demonstrated in chimeras generated by injection of c-jun–/– embryonic stem (ES) cells into wild-type blastocysts. ES cell derivatives could efficiently contribute to all organs analysed, but were progressively lost in the liver after birth (Hilberg et al., 1993; Eferl et al., 1999).
JNP was thought to be required for the anti-apoptotic functions of c-Jun during hepatogenesis, since mice lacking the JNK kinase SEK1 exhibit a liver defect similar to c-jun–/– fetuses (Ganiatsas et al., 1998; Nishina et al., 1999). However, jnk1 and jnk2 double mutant mice are embryonic lethal and show defects in neuronal apoptosis, but liver defects have not been reported (Kuan et al., 1999; Sabapathy et al., 1999). We have shown that JNP is not required for c-Jun’s function during embryogenesis, since mice carrying a mutant c-jun allele having the JNK phosphoacceptor serines 63 and 73 changed to alanines (junAA) are viable and fertile. However, these mutant mice show defects in stress-induced neuronal and thymocyte apoptosis (Behrens et al., 1999, 2001).
To investigate the role of c-jun in liver function and regeneration, we have generated mice harbouring an allele of c-jun flanked by loxP sites (c-junf). We have employed the albumin-cre transgenic line to inactivate c-jun in hepatocytes before birth (c-junΔli mice) and the inducible Mx-cre line to delete c-jun in the livers of adult mice (c-junΔli* mice). Moreover, to investigate the role of JNP during liver regeneration we also analysed the previously described junAA mice (Behrens et al., 1999). We found that hepatocyte proliferation was decreased, but liver function was not altered in mice lacking c-jun in the liver. In response to PH, mutant livers show impaired regeneration accompanied by increased cell death and lipid accumulation in hepatocytes, a pathological condition known as microvesicular steatosis. Moreover, cyclin-dependent kinases and several cell cycle regulatory molecules were affected in regenerating mutant hepatocytes, resulting in inefficient G1–S phase progression. This function of c-jun was independent of JNP, since liver regeneration was normal in mice harbouring a mutant c-jun allele lacking the JNK phosphoacceptor serines 63 and 73. Therefore, mice lacking c-jun in the liver identify c-Jun as a critical regulator of hepatocyte proliferation and survival after liver injury.
Results
c-jun is required for postnatal liver development
To investigate the role of c-jun in postnatal liver development and regeneration, a floxed allele of c-jun was introduced into ES cells by homologous recombination. The neomycin resistance and the thymidine kinase genes were removed by cre-mediated recombination and correct targeting was confirmed by Southern blot analysis (Figure 1). Heterozygous mice carrying a floxed c-jun allele (c-junf) were generated and intercrossed to obtain homozygous c-junf/f mice. These were fertile and did not show any phenotypical or histological abnormalities, suggesting that the two loxP sites inserted into the c-jun locus do not affect its function.
Fig. 1. Generation of mice harbouring a floxed c-jun allele. (A) Schematic representation of the targeting strategy employed to generate a floxed allele of c-jun. The c-jun open reading frame is represented by a rectangle, thin lines represent untranslated regions of the c-jun locus. The neomycine resistance gene (NeoR), the thymidine kinase gene (tk) and the diphtheria toxin alpha gene (DTα) are indicated; loxP sites are shown as triangles. X, XbaI; Xh, XhoI; P, PstI; E, EcoRI; H, HindIII. (B) Southern blot analysis of genomic DNA from the parental ES cell line E14.1 (+/+) and from three targeted (targ/+) ES cell clones. (C) After transient tranfection of cre, ES cell clones were selected for the loss of the NeoR and tk genes. Southern blot analysis of genomic DNA from the parental ES cell line E14.1 (+/+) and from three floxed (f/+) ES cell clones.
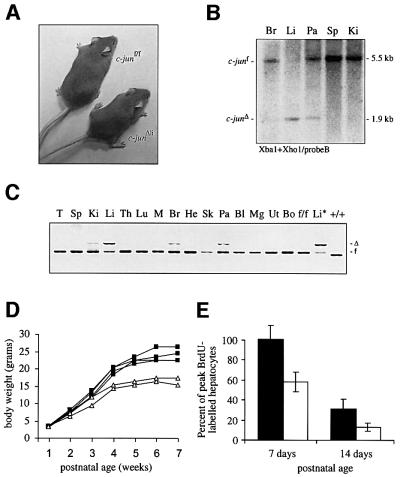
To analyse the function of c-jun during peri- and postnatal liver development, c-jun was inactivated tissue-specifically in hepatocytes using a transgenic line that expresses the cre recombinase under the control of the liver-specific albumin promoter and the albumin and alpha feto-protein enhancers (Alfp-cre) (Kellendonk et al., 2000). Mice lacking c-jun in the liver (c-junΔli) were born with Mendelian frequencies; however, at the age of 2 weeks they had reduced body weight, which persisted throughout adulthood (Figure 2A and D). Alfp-cre- mediated recombination of c-junf was already observed at embryonic day (E) 17.5 and completed postnatally in the liver, although some recombination could be detected in pancreas, brain and kidney by Southern blot and PCR analysis (Figure 2B and C). Bromodeoxyuridine (BrdU) labelling experiments revealed a significantly decreased number of S-phase hepatocytes in mutant livers of 7- and 14-day-old mice, indicating that c-jun is required for hepatocyte proliferation during postnatal development (Figure 2E). Apart from the reduction in body size, c-junΔli mice were phenotypically normal and did not develop any signs of impaired liver function (data not shown).
Fig. 2. Analysis of mice lacking c-jun in the liver. (A) Photograph of 8-week-old female c-junΔli and littermate control mice. (B) Southern blot of genomic DNA isolated from various organs of a c-junf/f Alfp-cre mouse (c-junΔli). Br, brain; Li, liver; Pa, pancreas; Sp, spleen; Ki, kidney. (C) PCR analysis of genomic DNA isolated from various organs of a c-junΔli mouse. T, tail; Sp, spleen; Ki, kidney; Li, liver; Th, thymus; Lu, lung; M, skeletal muscle; Br, brain, He, Heart; Sk, skin; Pa, pancreas; Bl, bladder; Mg, mammary gland; Ut, uterus; Bo, bone; f/f, c-junf/f control; Li*, liver of c-junf/f Mx-cre (c-junΔli*) mouse; +/+, wild-type control. (D) Postnatal growth rate of one litter of c-junf/f (black squares) and c-junΔli mice (white triangles). The result is representative of six litters analysed from three independent breeding cages. (E) Quantification of BrdU-immunostaining of c-junf/f (black bar) and c-junΔli livers (white bar) 7 and 14 days after birth.
Defective liver regeneration in the absence of c-jun
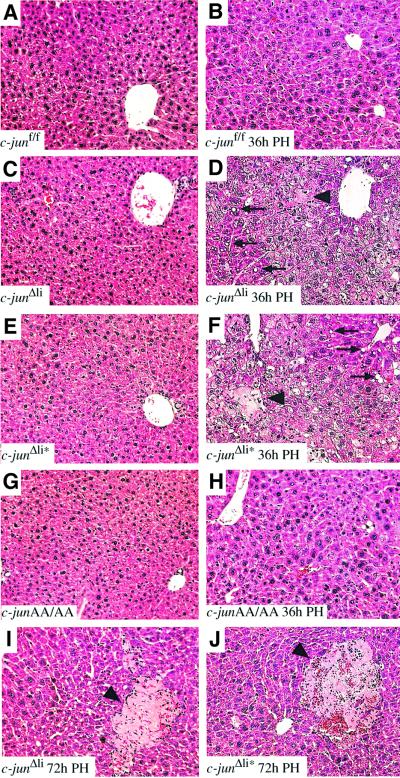
To determine whether c-jun is required for liver regeneration, PHs were performed on c-junΔli mice and control littermates. Absence of c-jun resulted in defective liver regeneration, since 11 out of 22 c-junΔli mice died within the first 3 days after surgery, whereas no mortality was observed in c-junf/f homozygous controls (zero out of 21). Between 24 and 96 h after PH, nine out of 22 c-junΔli mice showed lethargy, weakness and tremulousness, which was evident in only one control animal (out of 21). c-junΔli mice completely recovered after sham surgery, thereby excluding that surgery as a general stress response is responsible for the increased mortality observed in hepatectomized mice. The histological appearance of c-junΔli and controls was comparable before hepatectomy (Figure 3A and C). However, after PH, mutant livers contained a large number of hepatocytes with multiple cytoplasmic fat droplets (Figures 3D and 4H). This condition is histopathologically similar to microvesicular steatosis seen in a variety of storage, metabolic and toxic processes in humans, including drug reactions, fatty liver of pregnancy, Reye’s syndrome and acute foamy degeneration associated with alcohol (Taub, 1996b; Michalopoulos and DeFrances, 1997). Moreover, whereas control livers appeared healthy, large areas of necrotic tissue were detected in mutant regenerating livers (Figure 3B and D). The few surviving c-junΔli mice were capable of resolving these lesions; however, small necrotic foci remained (Figure 3I).
Fig. 3. c-jun is required for liver regeneration. Histological analysis of wild-type (A, B), c-junΔli (Alfp-cre; C, D, I), c-junΔli* (Mx-cre; E, F, J) and junAA/AA (G, H) livers before (A, C, E, G), 36 h after (B, D, F, H) and 72 h after (I, J) hepatectomy. In (D), (F), (I) and (J), arrowheads indicate areas of necrotic tissue; some fat-containing vacuoles are indicated by arrows (magnification ×20).
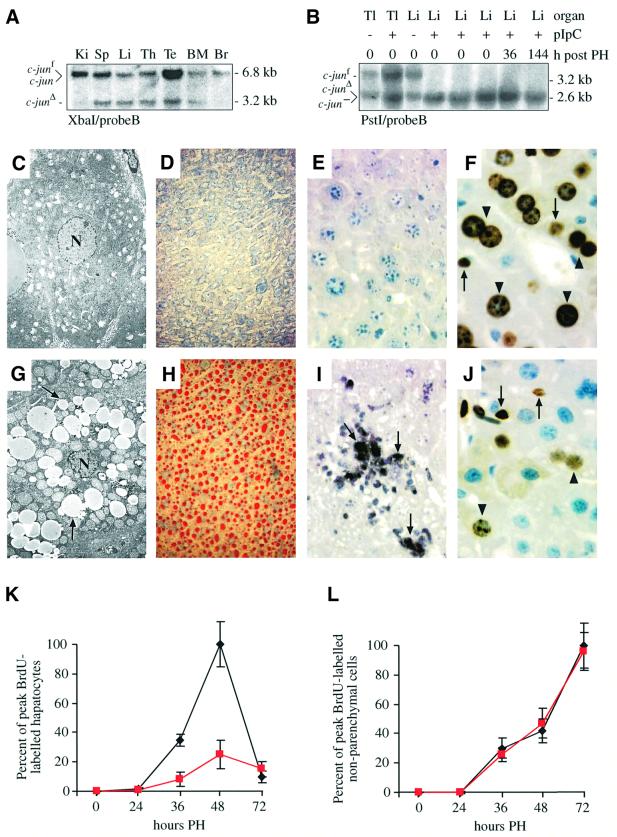
Fig. 4. Increased cell death and decreased hepatocyte proliferation after hepatectomy in the absence of c-jun. (A) Southern blot analysis of genomic DNA isolated from various organs of one pIpC-treated c-junf/+ Mx-cre+ mouse. Ki, kidney; Sp, spleen; Li, liver; Th, thymus; Te, testes; BM, bone marrow; Br, brain. (B) Southern blot analysis of genomic DNA isolated from tail (Tl) and liver (Li) of pIpC-treated (+) and untreated (–) c-junf/– heterozygous mice before and after hepatectomy. c-jun– is the null allele (Hilberg et al., 1993). (C, G) Electronmicroscope image of a control (C) and a c-junΔli mutant liver (G) 48 h after hepatectomy. The hepatocyte nucleus is indicated by ‘N’ and fat-containing vacuoles by arrows. (D, H) Oil red O-staining of a control (D) and a c-junΔli* mutant (H) liver 48 h after hepatectomy. Neutral lipids are visualized as red droplets in (H). (E, I) TUNEL-staining of a control (E) and a c-junΔli* mutant liver (I) 48 h after hepatectomy. Some TUNEL-positive cells are indicated by arrows. (F, J) BrdU-immunostaining of c-junf/f (F) and c-junΔli* (J) livers 48 h after hepatectomy. Arrows and arrowheads point to some BrdU-positive non- parenchymal cells and hepatocytes, respectively (J). (K, L) Quantification of BrdU-positive hepatocytes (K) and non-parenchymal cells (L) of c-junf/f (black lines) and c-junΔli* (red lines) mice after hepatectomy. Magnification: (C, G), ×3150; (D, H), ×20; (E, F, I, J), ×40.
To exclude the possibility that the impaired liver regeneration observed in c-junΔli mice was secondary to a defect in early postnatal hepatogenesis (see Figure 2), c-junf/f mice were intercrossed to transgenic mice expressing cre under the control of the interferon α-inducible Mx promoter (Kuhn et al., 1995). c-jun was deleted in 8-week-old mice by injection of the interferon α-inducer pIpC, and Mx-cre-mediated recombination of the floxed c-jun allele reached completion in the liver of a c-junf/– mouse (Figure 4B). In addition, significant deletion was also detected in other organs (Figure 4A) (Kuhn et al., 1995). Deletion of c-jun in adult mice did not cause apparent phenotypical alterations or premature death up to 18 months after injection (data not shown). Moreover, analysis of several parameters of liver function, including serum bilirubin, tri-glicerides, cholesterol, aspartate aminotransferase/alanine aminotransferase (AST/ALT), lactate dehydrogenase and urea/nitrogen did not reveal differences between c-jun mutant mice and control littermates 2, 6 and 12 months after pIpC injection (data not shown). These results therefore indicate that c-jun is dispensable for liver function in adult animals.
As shown for the Alfp-cre-mediated deletion, PH following Mx-cre-mediated deletion of c-jun (c-junΔli*) resulted in increased mortality within the first 3 days after surgery (six out of 12 mutants died compared with one out of 10 controls). Regenerating livers were also characterized by a disrupted architecture, hepatocyte death and accumulation of cytoplasmic fat droplets (Figure 3E and F). At later stages, these mutant livers still displayed areas of necrotic tissue and were composed entirely of hepatocytes lacking c-jun, excluding the possibility that a small proportion of wild-type cells that escaped deletion were responsible for liver regeneration (Figures 3J and 4B). These results indicate that c-jun is required cell-autonomously in hepatocytes during PH and that the phenotype observed is not a consequence of impaired postnatal hepatogenesis in the absence of c-jun.
Interestingly, JNP was not required for c-Jun function during PH, since in response to hepatectomy junAA/AA regenerating livers had a similar morphological appearance than controls (Figure 3G and H) Moreover, the absence of JNP did not result in increased mortality after PH (zero out of eight) as observed in c-jun mutant mice. Therefore, it appears that JNP is required neither for the function of c-jun in embryonic hepatogenesis nor for liver regeneration.
Decreased proliferation and increased cell death in c-jun mutant regenerating livers
We next determined whether c-jun has an anti-apoptotic role during liver regeneration similar to its function during fetal hepatogenesis (Hilberg et al., 1993; Eferl et al., 1999). Electronmicroscopic analysis of mutant hepatocytes revealed numerous fat-containing vacuoles consistent with microvesicular steatosis detected in control hepatocytes (Figure 4C and G). Staining with Oil red O confirmed the presence of high amounts of lipids in c-jun deficient hepatocytes (Figure 4D and H). Mutant hepatocytes also contained enlarged mitochondria, which were not found in controls (Figure 4C and G). Apoptotic bodies were not observed in these mutant cells, suggesting cell death by necrosis (Figure 4G). However, scattered islands of TUNEL-positive pyknotic hepatocytes could be detected in other areas of regenerating mutant, but not control livers, suggesting that an apoptotic pathway may contribute to hepatocyte death in the absence of c-Jun (Figure 4E and I).
To examine whether c-jun was required for cell cycle progression after PH, the number of S-phase cells at various time points after PH was determined by BrdU incorporation in the few c-jun mutant and control mice that appeared well after surgery. In control livers, significant BrdU incorporation was first detected 36 h after PH and was maximal after 48 h. Compared with controls, the rate of BrdU incorporation was drastically reduced in mutant livers, with a marked reduction of proliferating hepatocytes at 36 and 48 h after hepatectomy (Figure 4F, J and K). In contrast, DNA synthesis in non-parenchymal liver cells was unchanged and peaked later than in hepatocytes (Figure 4L). These results suggest that in the absence of c-Jun, a cell cycle defect rather than increased apoptosis might be responsible for impaired liver regeneration.
c-jun regulates cell cycle progression in regenerating hepatocytes
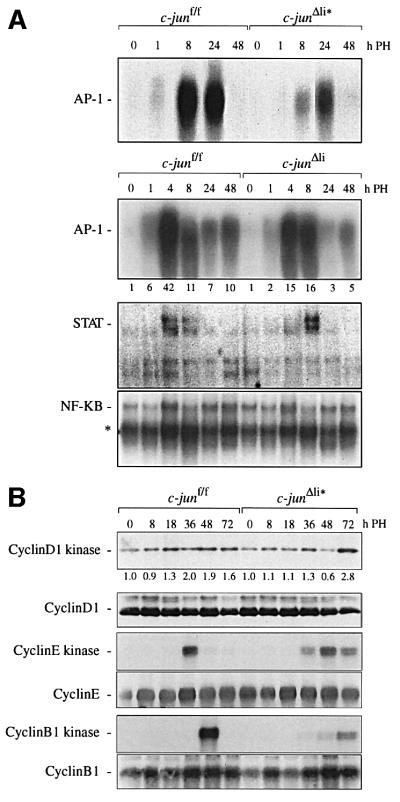
After hepatectomy, growth factors like IL-6 and TNF-α mediate regeneration by activating several transcription factor complexes including AP-1, STAT and NF-κB, which directly regulate hepatocyte proliferation (Cressman et al., 1996; Yamada et al., 1997). Deletion of c-jun by Mx-cre and by Alfp-cre resulted in reduced AP-1 DNA binding activity, confirming that c-Jun is an important component of AP-1 transcription factor complexes after PH (Figure 5A). Compared with c-junΔli*, this reduction was less dramatic in c-junΔli livers, most likely because of the presence of c-jun in non-parenchymal cells. However, quantification of the AP-1 DNA binding activity in c-junΔli livers still reveals a >2-fold reduction in the mutants 4 h after PH (Figure 5A). In contrast, the induction of STAT DNA binding activity was only slightly delayed and therefore increased at 8 h after PH, whereas the induction of NF-κB DNA binding activity was unchanged in mutant regenerating livers. This indicates that c-Jun does not regulate liver regeneration by significantly altering growth factor signalling involving STAT and NF-κB activation (Figure 5A).
Fig. 5. Molecular analysis of regenerating livers lacking c-jun. (A) AP-1, STAT and NF-κB DNA-binding in nuclear extracts from c-junf/f, c-junΔli and c-junΔli* livers; numbers below the AP-1 electrophoretic mobility-shift assay of c-junf/f and c-junΔli livers represent the fold induction of DNA-binding activity relative to the respective 0 time point; the asterisk indicates binding of p50 homodimers. (B) Immuno precipitated cyclin D1-, cyclin E- or cyclin B1-associated kinase activity in c-junf/f and c-junΔli* livers isolated at the indicated times after hepatectomy. Numbers below the cyclin D1-kinase activity represent the fold induction relative to the respective 0 time point. The presence of cyclin D1, cyclin E and cyclin B1 in the immunoprecipitates was confirmed by western blot analysis.
To analyse the molecular mechanism of c-jun-mediated hepatocyte proliferation, the expression and activity of cyclin D- and cyclin E-dependent kinases, which are key regulators of the G1–S transition, was studied in c-jun deficient livers. Active cyclin D-dependent kinases are higher order complexes consisting of a catalytic subunit, cyclin-dependent kinase (CDK) 4 or 6, a D-type cyclin, proliferating cell nuclear antigen (PCNA) and either the CDK inhibitor (CKI) p21Cip1 or p27Kip1 (Sherr and Roberts, 1999). Cyclin D1-dependent kinase activity was fully induced 36 h after PH in controls, but only after 72 h in mutant livers (Figure 5B). In addition, cyclin E-dependent kinase activity was poorly activated in the absence of c-jun, compared with a strong induction in the control (Figure 5B). Moreover, cyclin B1-dependent kinase activity, which is present in G2 and M phase, was strongly induced 48 h after PH, but was dramatically decreased and its induction delayed in c-jun-deficient hepatocytes (Figure 5B). These results indicate that c-Jun regulates cell cycle progression of regenerating hepatocytes by controlling the activities of CDKs.
We next analysed the protein levels of several cell cycle regulators after hepatectomy. In control livers, low levels of cyclin D protein could be detected which peaked between 24 and 36 h (Figure 6A and B). In contrast, PCNA protein levels were highest after 36 h and then declined (Figure 6A). In the absence of c-jun, both proteins reached highest levels only 36 h after PH and were still expressed at 72 h after surgery (Figure 6A). Therefore, c-Jun does not seem to be required for efficient induction and sustained expression of D-type cyclins and PCNA. Since the expression of PCNA is maximal in late G1, the failure of down-regulation in mutant hepatocytes may be a secondary effect of a partial cell cycle block at the G1–S transition. Cyclin B1 was detectable 48 h after PH, but in mutant regenerating livers cyclin B1 protein levels were reduced, probably reflecting the lower number of regenerating c-jun-deficient hepatocytes entering G2–M phase (Figure 6A). It was shown previously that immortalized c-jun-deficient fibroblasts show increased p53 and p21 protein levels, which are responsible for the slow proliferation (Schreiber et al., 1999; Shaulian et al., 2000). However, p53 protein levels were comparable between control and mutant regenerating hepatocytes (Figure 6A), whereas p21 induction could be detected 12 h after PH and accumulated at 48 h in the mutants (Figure 6B). In contrast, p27 protein levels were unaffected by PH and were comparable in control and mutant livers (data not shown). Both c-Jun protein and JNP were strongly induced by PH and absent in mutant livers (Figure 6A). In contrast, the kinetics of JunB induction was similar, but protein levels were slightly increased by the absence of c-jun.
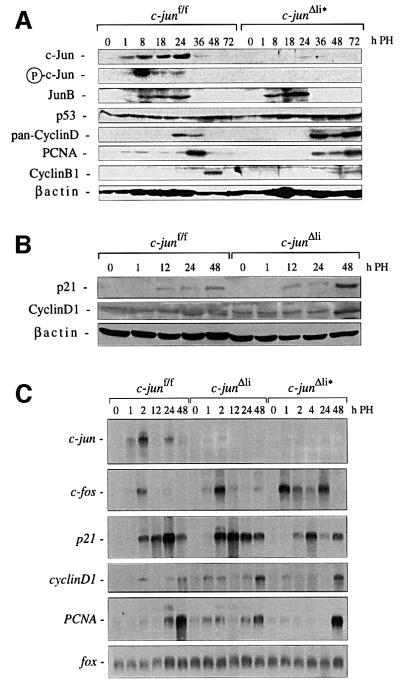
Fig. 6. Protein and RNA expression in regenerating livers lacking c-jun. (A, B) Expression of various proteins in c-junf/f, c-junΔli* (A) and c-junΔli (B) livers isolated at the indicated time points after hepatectomy. β-actin was used as a loading control. (C) Induction of c-jun, c-fos and fox mRNA (loading control), p21, PCNA and cyclinD1 in regenerating c-junf/f and c-jun-mutant livers at the indicated time points after hepatectomy.
Northern analysis revealed that c-jun mRNA was induced by hepatectomy, whereas c-jun transcription was nearly absent in regenerating mutant livers, confirming efficient c-jun deletion (Figure 6C). Transcription of c-fos was also stimulated by PH in control mice and was significantly increased in c-jun mutant livers (Figure 6C). Interestingly, up-regulation of c-fos mRNA levels has also been observed during defective liver regeneration in CREM-deficient mice (Servillo et al., 1998). Transcrip tional induction of p21 was not significantly altered in c-junΔli regenerating hepatocytes; however, 48 h after PH p21 levels were declining in controls, but not in mutant livers (Figure 6C). Likewise, the regulation of the c-jun target gene cyclinD1 (Wisdom et al., 1999; Bakiri et al., 2000) was normal at early time points in regenerating mutant hepatocytes, but cyclinD1 mRNA accumulated after 48 h in the absence of c-jun. Therefore, in c-Jun deficient livers the failure of down-regulating p21 at the mRNA and protein level could be responsible for the inefficient G1–S transition and proliferation defect of mutant hepatocytes.
Discussion
The Alfp-cre and Mx-cre transgenic lines used in this study have allowed us to bypass the embryonic lethality of c-jun knock-out mice and enabled the investigation of the function of c-jun in the liver at later stages of development. In the first postnatal weeks, the liver undergoes a dramatic expansion and reaches the adult size (Fausto et al., 1995). During this phase, c-jun expresssion is induced in liver cells, suggesting that it might play an important role in hepatocyte proliferation (Eferl et al., 1999). We have shown previously that, in chimeras, hepatocytes derived from c-jun–/– ES cells can be detected in the embryonic liver, but are progressively lost during later embryonic and postnatal development, suggesting that the absence of c-jun results in a competitive disadvantage of mutant compared with wild-type cells (Eferl et al., 1999). Perinatal deletion of c-jun in c-junΔAlfp-cre mice results in a significant reduction of liver weight and, probably as a consequence, the body weight of c-junΔAlfp-cre mice is also reduced, demonstrating that c-jun is required for early postnatal hepatocyte proliferation. However, c-jun–/– hepatocytes were capable of achieving liver expansion at a sufficient rate to support and sustain liver function.
Growth factors like epidermal growth factor (EGF) and HGF and cytokines including TNF-α and IL-6 are produced after PH and are believed to synergically orchestrate the cell cycle re-entry of quiescent hepatocytes. These two signalling cascades co-operate, since TNF-α stimulates DNA replication in cultured hepatocytes only in the presence of growth factors (Kirillova et al., 1999). Both growth factor and cytokine signalling pathways induce AP-1 activity, although through different mechanisms. TNFR-1 stimulation leads to the sequential activation of NF-κB, IL-6 and STAT3, resulting in transcriptional induction of the c-fos gene (Fausto, 2000). In mice lacking TNFR-1 or IL-6, c-fos induction is compromised, but c-jun expression is normal (Cressman et al., 1996; Yamada et al., 1997). Despite normal TNF-α and IL-6 signalling in c-Jun deficient hepatocytes, as judged by NF-κB activation, the liver regeneration defect is strikingly similar in all three lines of mutant mice: increased mortality after hepatectomy, microvesicular steatosis and reduced hepatocyte proliferation (Cressman et al., 1996; Yamada et al., 1997). In all mutant lines, AP-1 DNA binding activity was markedly reduced, indicating that the decrease in AP-1 activity is significantly contributing to the defect observed in TNF-α/IL-6-deficient regenerating livers. It appears that two independent pathways are required for complete AP-1 induction in the regenerating liver: a TNF-α/IL-6-dependent signal that stimulates c-Fos, and a second mechanism, potentially emanating from growth factor receptors, resulting in c-Jun stimulation. The pathway leading to c-jun induction has not yet been identified, although potential candidates are the well-known EGF- and HGF-dependent signalling cascades. A possible mechanistic link between HGF signalling and c-jun is supported by the similarities of their knock-out phenotypes, as both HGF–/– and c-jun–/– fetuses die at mid-gestation with increased hepatocyte apoptosis (Hilberg et al., 1993; Schmidt et al., 1995). The embryonic and early postnatal lethality of mice lacking the EGF receptor, HGF and the HGF receptor c-Met has prevented loss-of-function approaches to studying the role of EGF and HGF signalling during liver regeneration and c-jun induction (Bladt et al., 1995; Schmidt et al., 1995; Sibilia and Wagner, 1995).
We have shown previously that fibroblasts lacking c-jun exhibit a cell cycle defect due to elevated p53 and p21 protein levels under normal culture conditions as well as following UV irradiation (Schreiber et al., 1999; Shaulian et al., 2000). c-Jun was also shown to directly regulate the cyclin D1 and PCNA promoters in fibroblasts (Liu et al., 1998; Wisdom et al., 1999; Bakiri et al., 2000). However, in hepatocytes lacking c-Jun, p53 mRNA and protein levels are unchanged (Figure 6A; data not shown). p21, PCNA and cyclin D1 mRNAs are normally induced, but mRNA and protein levels accumulate at later time points after hepatectomy (Figure 6A and B). Elevated p21 levels could account for the delayed cell cycle progression of hepatocytes lacking c-jun, as transgenic overexpression of p21 has been shown to impair liver regeneration severely (Wu et al., 1996). Recently, it has been demonstrated that c-Jun can regulate p21 protein levels by inhibiting p53 binding to the p21 promoter, a mechanism that could also account for p21 overproduction in spite of normal p53 protein levels (Shaulian et al., 2000). Therefore, the failure to progress through the cell cycle might be caused by inefficient activation of cyclin-dependent kinases which are essential for an efficient proliferative response (Sherr and Roberts, 1999).
N-terminal phosphorylation is not necessary for hepatocyte proliferation and prevention of cell death, since liver regeneration after PH was normal in mice harbouring an allele of c-jun lacking the JNP sites. Although surprising given the robust activation of JNKs by PH, this result is in agreement with our previous finding that JNP is also not required for c-Jun function in hepatogenesis (Behrens et al., 1999). The proliferation defect and the increased cell death of c-jun–/– regenerating hepatocytes may be independent events, since mice lacking the transcription factor CREM or the 40S ribosomal protein S6 display similar cell cycle defects to c-Jun mutants, although they do not show increased hepatocyte death and survive PH (Servillo et al., 1998; Volarevic et al., 2000). c-Jun may exert its mitogenic and anti-apoptotic effects by regulating two classes of target genes that are independently required for hepatocyte proliferation and the prevention of hepatocyte apoptosis during liver regeneration. However, there is precedence for a coupling of cell proliferation and apoptosis, because failure to respond to a proliferation signal can trigger apoptosis. Forced expression of the proliferation-inducing gene c-myc in serum-starved quiescent cells results in cell death. It has been suggested that the conflict between two opposing signals, in the latter case quiescence by serum starvation and c-myc expression, cannot be tolerated by the cell and activates a death program (Evan et al., 1994). It is conceivable that hepatocytes lacking c-jun may be unable to respond to the proliferation signals elicited by hepatectomy, and that the strong proliferation signal would trigger cell death in c-jun–/– cells that fail to enter the cell cycle.
In the absence of c-jun, a microvesicular steatosis-like phenotype was also observed after PH in the liver. Microvesicular steatosis accompanies and may contribute to liver failure, coma and death in a wide variety of pathological conditions, including alcoholism, toxicity of several medications, delta hepatitis, sudden child hood death, congenital defects of fatty acid β oxidation and cholesterol ester storage diseases (Taub, 1996b; Michalopoulos and DeFrances, 1997). Despite their great clinical importance, the aetiology of microvesicular steatosis and the molecular mechanism of liver injury are still elusive. It will be interesting to determine whether the appearance of microvesicular steatosis correlates with impaired AP-1 activity in human patients as it does in mutant mice.
Materials and methods
Generation of c-junf mice
The genomic c-jun locus derived from a λ FIX 129 library (Stratagene) was cloned and the 5′ loxP site was inserted into an EcoRI site present in the 5′ untranslated region (UTR) of c-jun. A floxed neomycin resistance and thymidine kinase gene selection cassette was inserted into 3′ flanking sequence 2.5 kb downstream of the translation stop codon. A diphtheria toxin (DTα) gene was inserted for selection against random integrants. The linearized targeting construct was electroporated into E14.1 ES cells and the identification of homologous recombinants by PCR using two sets of primers was performed as described previously (Behrens et al., 1999). The neomycin and thymidine kinase genes were deleted by transient transfection of a vector expressing cre recombinase. Two ES cell clones carrying a floxed allele of c-jun were injected into C57BL/6 blastocysts and several chimeras from one ES cell clone transmitted the mutant allele to their offspring.
Histological analysis, BrdU immunohistochemistry and TUNEL assay
To analyse cell proliferation, mice were injected intraperitonially with 100 µg of BrdU per gram of body weight 1 h prior to killing. Liver tissues were fixed in 4% formaldehyde, dehydrated, embedded in paraffin and sectioned (5 µm). Sections were stained with Harris haematoxylin and eosin (Sigma). BrdU, Oil red O and TUNEL stainings were performed as described previously (Behrens et al., 1999, 2000). The number of BrdU-positive hepatocytes was determined by manual counting of six high-power fields per liver analysed. The mean for each time point was plotted as a percentage of the number of labelled cells at the peak time point of control c-junf/f mice. The data represent the mean values ± SEM of three control and three mutant livers per time point.
PH and pIpC injection
PH was performed under anaesthesia as described previously (Servillo et al., 1998). The abdominal cavity was entered through a transverse incision just below and parallel to the rib cage. The large left lateral lobe and median lobes were ligated and removed. The mortality of wild-type animals following PH was <5%. To delete c-jun in the adult liver, 8-week-old c-junf/f mice were injected three times at 3 day intervals with 400 µg pIpC in 200 µl phosphate buffered saline (PBS).
Northern and western blot analysis
Mice were killed at the indicated times and poly(A)+ liver RNA isolation, northern blots, and hybridizations were performed as described previously (Behrens et al., 1999). The c-fos probe used also recognizes the fox transcript, which serves as an internal loading control. Western blot analysis was performed according to standard procedures using antibodies specific for c-Jun (Transduction Laboratories); cyclin D1 (Zymed), β-actin (Sigma), phospho-c-Jun (Ser63), JunB (from M.Yaniv); p53, p21, cyclin D, cyclin E, cyclin B1, PCNA (Santa Cruz) (Behrens et al., 1999).
Electrophoretic mobility-shift assay
Nuclear extracts of liver cells were prepared and DNA binding activity was measured using oligonucleotide probes specific for AP-1 (5′-GGGTGACTCACCGGGTGAA-3′), STAT (5′-GTGCATTTCCCGTAAATCTTGTCTCA-3′) and NF-κB (5′-TCGAGGGCTGGGGATTCCCCATCTC-3′) as described previously (Yamada et al., 1997). AP-1 binding activity was quantified with a phosphoimager (data not shown).
Immunocomplex kinase assay
For kinase assays, 250 µg of whole-cell extracts were immunoprecipitated with cyclin D1–, cyclin E– or cyclin B1–agarose conjugated antibodies (Santa Cruz), and immune complexes were incubated with glutathione S-transferase–Rb (C-pocket 769–921; Santa Cruz) or histone H1 (Boehringer Mannheim) substrates as described previously (Passegue and Wagner, 2000).
Acknowledgments
Acknowledgements
We are grateful to S.Pekez for maintaining our mouse colony. We thank R.Johnson for the genomic c-jun λ phage clone; R.Kühn and K.Rajewsky for providing Mx-cre transgenic mice; M.Yaniv for antibodies; R.Eferl for PCR analysis; R.Ricci and R.Eferl for supplying additional c-junΔli* and c-junΔli mice; H.E.Gustafson and B.Pfister for technical assistance; R.Eferl, W.Jochum, E.Passegue, R.Ricci, P.Schirmacher and K.Zatloukal for critical reading of the manuscript; and H.Tkadletz for help with preparing the illustrations. The IMP is funded by Boehringer Ingelheim, and this work was supported by the Austrian Research Foundation (S74-MOB) and the Austrian Industrial Research Promotion Fund.
References
- Angel P., Hattori,K., Smeal,T. and Karin,M. (1988) The jun proto-oncogene is positively autoregulated by its product, Jun/AP1. Cell, 55, 875–885. [DOI] [PubMed] [Google Scholar]
- Arias J., Alberts,A.S., Brindle,P., Claret,F.X., Smeal,T., Karin,M., Feramisco,J. and Montminy,M. (1994) Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature, 370, 226–229. [DOI] [PubMed] [Google Scholar]
- Bakiri L., Lallemand,D., Bossy-Wetzel,E. and Yaniv,M. (2000) Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. EMBO J., 19, 2056–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister A.J., Oehler,T., Wilhelm,D., Angel,P. and Kouzarides,T. (1995) Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene, 11, 2509–2514. [PubMed] [Google Scholar]
- Behrens A., Sibilia,M. and Wagner,E.F. (1999) Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nature Genet., 21, 326–329. [DOI] [PubMed] [Google Scholar]
- Behrens A., Jochum,W., Sibilia,M. and Wagner,E.F. (2000) Oncogenic transformation by ras and fos is mediated by c-Jun N-terminal phosphorylation. Oncogene, 19, 2657–2663. [DOI] [PubMed] [Google Scholar]
- Behrens A., Sabapathy,K., Graef,I., Cleary,M., Crabtree,G.R. and Wagner,E.F. (2001) Jun N-terminal kinase 2 modulates thymocyte apoptosis and T cell activation through c-Jun and nuclear factor of activated T cell (NF-AT). Proc. Natl Acad. Sci. USA, 98, 1769–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladt F., Riethmacher,D., Isenmann,S., Aguzzi,A. and Birchmeier,C. (1995) Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature, 376, 768–771. [DOI] [PubMed] [Google Scholar]
- Brenner D.A. (1998) Signal transduction during liver regeneration. J. Gastroenterol. Hepatol., 13 (Suppl.), S93–S95. [DOI] [PubMed] [Google Scholar]
- Cressman D.E., Greenbaum,L.E., DeAngelis,R.A., Ciliberto,G., Furth,E.E., Poli,V. and Taub,R. (1996) Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science, 274, 1379–1383. [DOI] [PubMed] [Google Scholar]
- Eferl R., Sibilia,M., Hilberg,F., Fuchsbichler,A., Kufferath,I., Guertl,B., Zenz,R., Wagner,E.F. and Zatloukal,K. (1999) Functions of c-Jun in liver and heart development. J. Cell Biol., 145, 1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G., Harrington,E., Fanidi,A., Land,H., Amati,B. and Bennett,M. (1994) Integrated control of cell proliferation and cell death by the c-myc oncogene. Philos. Trans. R. Soc. Lond. B Biol. Sci., 345, 269–275. [DOI] [PubMed] [Google Scholar]
- Fausto N. (2000) Liver regeneration. J. Hepatol., 32, 19–31. [DOI] [PubMed] [Google Scholar]
- Fausto N., Laird,A.D. and Webber,E.M. (1995) Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J., 9, 1527–1536. [DOI] [PubMed] [Google Scholar]
- Ganiatsas S., Kwee,L., Fujiwara,Y., Perkins,A., Ikeda,T., Labow,M.A. and Zon,L.I. (1998) SEK1 deficiency reveals mitogen-activated protein kinase cascade crossregulation and leads to abnormal hepatogenesis. Proc. Natl Acad. Sci. USA, 95, 6881–6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim M.H., Gamboni,G., Beglinger,C. and Gyr,K. (1997) Specific activation of AP-1 but not Stat3 in regenerating liver in mice. Eur. J. Clin. Invest., 27, 948–955. [DOI] [PubMed] [Google Scholar]
- Hilberg F., Aguzzi,A., Howells,N. and Wagner,E.F. (1993) c-jun is essential for normal mouse development and hepatogenesis. Nature, 365, 179–181. [DOI] [PubMed] [Google Scholar]
- Johnson R.S., van Lingen,B., Papaioannou,V.E. and Spiegelmann,B.M. (1993) A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev., 7, 1309–1317. [DOI] [PubMed] [Google Scholar]
- Kellendonk C., Opherk,C., Anlag,K., Schutz,G. and Tronche,F. (2000) Hepatocyte-specific expression of Cre recombinase. Genesis, 26, 151–153. [DOI] [PubMed] [Google Scholar]
- Kirillova I., Chaisson,M. and Fausto,N. (1999) Tumor necrosis factor induces DNA replication in hepatic cells through nuclear factor κB activation. Cell Growth Differ., 10, 819–828. [PubMed] [Google Scholar]
- Kuan C.Y., Yang,D.D., Samanta Roy,D.R., Davis,R.J., Rakic,P. and Flavell,R.A. (1999) The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron, 22, 667–676. [DOI] [PubMed] [Google Scholar]
- Kuhn R., Schwenk,F., Aguet,M. and Rajewsky,K. (1995) Inducible gene targeting in mice. Science, 269, 1427–1429. [DOI] [PubMed] [Google Scholar]
- Liu Y.C., Chang,H.W., Lai,Y.C., Ding,S.T. and Ho,J.L. (1998) Serum responsiveness of the rat PCNA promoter involves the proximal ATF and AP-1 sites. FEBS Lett., 441, 200–204. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G.K. and DeFrances,M.C. (1997) Liver regeneration. Science, 276, 60–66. [DOI] [PubMed] [Google Scholar]
- Nishina H. et al. (1999) Defective liver formation and liver cell apoptosis in mice lacking the stress signaling kinase SEK1/MKK4. Development, 126, 505–516. [DOI] [PubMed] [Google Scholar]
- Passegue E. and Wagner,E.F. (2000) JunB suppresses cell proliferation by transcriptional activation of p16INK4a expression. EMBO J., 19, 2969–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabapathy K., Jochum,W., Hochedlinger,K., Chang,L., Karin,M. and Wagner,E.F. (1999) Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech. Dev., 89, 115–124. [DOI] [PubMed] [Google Scholar]
- Schmidt C., Bladt,F., Goedecke,S., Brinkmann,V., Zschiesche,W., Sharpe,M., Gherardi,E. and Birchmeier,C. (1995) Scatter factor/hepatocyte growth factor is essential for liver development. Nature, 373, 699–702. [DOI] [PubMed] [Google Scholar]
- Schreiber M., Kolbus,A., Piu,F., Szabowski,A., Mohle-Steinlein,U., Tian,J., Karin,M., Angel,P. and Wagner,E.F. (1999) Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev., 13, 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servillo G., Della Fazia,M.A. and Sassone-Corsi,P. (1998) Transcription factor CREM coordinates the timing of hepatocyte proliferation in the regenerating liver. Genes Dev., 12, 3639–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E., Schreiber,M., Piu,F., Beeche,M., Wagner,E.F. and Karin,M. (2000) The mammalian UV response: c-Jun induction is required for exit from p53-imposed growth arrest. Cell, 103, 897–907. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. and Roberts,J.M. (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev., 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Sibilia M. and Wagner,E.F. (1995) Strain-dependent epithelial defects in mice lacking the EGF receptor. Science, 269, 234–238. [DOI] [PubMed] [Google Scholar]
- Steer C.J. (1995) Liver regeneration. FASEB J., 9, 1396–1400. [DOI] [PubMed] [Google Scholar]
- Taub R. (1996a) Liver regeneration. 4. Transcriptional control of liver regeneration. FASEB J., 10, 413–427. [PubMed] [Google Scholar]
- Taub R. (1996b) Liver regeneration in health and disease. Clin. Lab. Med., 16, 341–360. [PubMed] [Google Scholar]
- Volarevic S., Stewart,M.J., Ledermann,B., Zilberman,F., Terracciano,L., Montini,E., Grompe,M., Kozma,S.C. and Thomas,G. (2000) Proliferation, but not growth, blocked by conditional deletion of 40S ribosomal protein S6. Science, 288, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Webber E.M., Wu,J.C., Wang,L., Merlino,G. and Fausto,N. (1994) Overexpression of transforming growth factor-α causes liver enlargement and increased hepatocyte proliferation in transgenic mice. Am. J. Pathol., 145, 398–408. [PMC free article] [PubMed] [Google Scholar]
- Westwick J.K., Weitzel,C., Leffert,H.L. and Brenner,D.A. (1995) Activation of Jun kinase is an early event in hepatic regeneration. J. Clin. Invest., 95, 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh A.J. and Davis,R.J. (1996) Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med., 74, 589–607. [DOI] [PubMed] [Google Scholar]
- Wisdom R., Johnson,R.S. and Moore,C. (1999) c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J., 18, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Wade,M., Krall,L., Grisham,J., Xiong,Y. and Van Dyke,T. (1996) Targeted in vivo expression of the cyclin-dependent kinase inhibitor p21 halts hepatocyte cell-cycle progression, postnatal liver development and regeneration. Genes Dev., 10, 245–260. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Kirillova,I., Peschon,J.J. and Fausto,N. (1997) Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc. Natl Acad. Sci. USA, 94, 1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]