Abstract
IFN-γ secretion by natural killer (NK) cells is pivotal to several tumor and viral immune responses, during which NK and dendritic cells cooperation is required. We show here that macrophages are mandatory for NK cell IFN-γ secretion in response to erythrocytes infected with Plasmodium falciparum (Pf), a causative agent of human malaria. In addition, direct sensing of Pf infection by NK cells induces their production of the proinflammatory chemokine CXCL8, without triggering their granule-mediated cytolytic programs. Despite their reported role in Pf recognition, Toll-like receptor (TLR) 2, TLR9, and TLR11 are individually dispensable for NK cell activation induced by Pf-infected erythrocytes. However, IL-18R expression on NK cells, IL-18 production by macrophages, and MyD88 on both cell types are essential components of this previously undescribed pathway of NK cell activation in response to a parasite infection.
Keywords: innate immunity
In mammals, natural killer (NK) cells are found in blood, in secondary lymphoid organs, and in peripheral nonlymphoid tissues such as liver and lungs (1, 2). NK cells participate in host innate responses that occur upon viral and intracytoplasmic bacterial infections but also during the course of tumor development and allogeneic transplantation (3). These lymphocytes are not only important players of innate effector responses but also participate to the initiation and development of antigen-specific responses (4, 5). An involvement of NK cells in the control of parasitic infections has been reported, but the molecular and functional basis of their activation in response to parasites and/or parasite-infected cells remains unknown (6).
Plasmodium spp. are apicomplexan parasites responsible for malaria infection, a major cause of human disease that leads to 300 million acute cases and at least 1 million deaths every year. Among Plasmodium spp., Plasmodium falciparum (Pf) causes the most severe form of malaria (7). In human hosts, malaria starts with the bite of an infected mosquito. The injected parasites migrate from the skin to the liver in which they mature and multiply. The incubation period usually lasts 5-7 days and ends with release of parasites into the blood circulation. Subsequently, cyclic proliferation of parasites starts in red blood cells (RBC), quickly followed by the clinical symptoms.
Despite major efforts to decipher the host response to Pf infection, immunity to malaria is still poorly understood. This is in part due to the highly sophisticated mechanisms used by Pf to generate enormous antigenic variability and in part to the unknown function of 60% of the 5,400 genes present in Pf genome (7). In mouse models of malaria, NK cells represent an important early source of IFN-γ, and NK-cell depletion leads to an increased parasitemia and higher mortality (6). In addition, the NK complex (NKC), a genetic region of highly linked genes encoding several receptors involved in NK cell function, plays a role in immunological responses during murine malaria infection (8). During experimental Pf infection of nonimmune human volunteers, several cytokines including IFN-γ are produced in the serum 1-2 days prior to clinical symptoms and detectable parasitemia, suggesting that cells from the innate immune system detect the presence of the parasite very early during the course of infection (9). In vitro experiments with human peripheral blood mononuclear cells (PBMC) have also shown that NK cells may be the first source of IFN-γ after exposure to blood stage Pf in an IL-12-dependent manner (10, 11). However, these pioneering studies have been performed by using a single laboratory Pf strain, and have raised a number of unresolved issues regarding the extension of these observations to other Pf strains and the factors required for NK cell activation. Here we dissect the cellular and molecular requirements as well as the functional consequences of NK cell activation in response to blood stage Pf.
Materials and Methods
Parasites. Pf was cultured by a slightly modified standard procedure in human RBC in RPMI medium 1640 containing bicarbonate, glutamine, 0.2% glucose, 50 μM hypoxanthine, 10 μg/ml gentamicin, and 5% Albumax (Invitrogen) (12). The parasites used in this study were FcR3, 3D7, FcR3-CSA, FcR3-CD36, IPL/BRE1-ICAM1 (BC2), IPL/BRE1-CD36 (B3A4) (13), C9, and BXII strains from Brazil, and 42-CSA and 193-CSA placental isolates from Cameroon also adapted to culture (14). CSA (FCR3-CSA), CD36 (FCR3-CD36, IPL/BRE1-CD36), and ICAM1 (IPL/BRE1-ICAM1) adhesive phenotypes were selected from the FCR3 strain and maintained by panning as described in ref. 15. Cultures were routinely tested for mycoplasma contamination by ELISA and proved to be mycoplasma-free in the laboratory of J.G. Trophozoites and mature schizont-infected erythrocytes (Pf-RBC) were enriched by incubation with modified fluid gelatin-Plasmion. After enrichment, parasitemia was comprised between 60% and 90%. Uninfected erythrocytes (RBC) were treated in the same conditions and used as controls.
Cell Isolation and Stimulation. PBMC were isolated by Ficoll/Hypaque density gradient centrifugation (Amersham Pharmacia Biotech) from cytopheresis or whole blood samples obtained from healthy volunteers (Etablissement Français du Sang, Marseille, France). Human NK cells, plasmacytoid BDCA2+ dendritic cells (PDC), dendritic cells (DC), and monocytes were sorted by magnetic cell sorting (MACS), respectively, with human NK cell isolation kit II, BDCA-4 cell isolation kit, human blood dendritic cell isolation kit II, and human monocytes isolation kit II (Miltenyi Biotec). The purity of all sorted populations ranged from 85% to 97%. In some experiments, purified cells were sorted by flow cytometry (FACSVantage SE, BD Biosciences) to reach a purity of 99%. Mouse splenocytes or human PBMC were seeded at 5 × 105 per well into 96-well round-bottom tissue culture plates. Purified human PDC, DC, or monocytes were seeded at 4 × 104 per well. For mixed splenocytes experiments, 2.5 × 105 splenocytes of each mouse strain were added to the wells. Purified human NK cells and mouse Ky.2 NK cell lines were seeded at 105 per well. When indicated, 5 × 105 splenocytes were added to Ky.2. Cells were cultured in the presence or absence of Pf-RBC or RBC (2 × 106). K562 tumor cells (2 × 105 per well) and phorbol 12-myristate 13-acetate (PMA)/ionomycin (50 ng/ml PMA and 500 ng/ml ionomycin) were used as positive controls for human and mouse NK activation. When mentioned, human rIL-12 and mouse rIL-12 (PeproTech) were added to cell cultures at 1 ng/ml and 20 pg/ml, respectively. For intracellular IFN-γ assay, Golgi stop (BD Biosciences) was added to cultures immediately when the analysis was performed at 4 h, or during the last 6 h of the incubation when the analysis was performed at 20-24 h. Other tests were performed as described in Supporting Methods, which is published as supporting information on the PNAS web site.
Results
NK Cell Activation by Pf-RBC. In vivo, NK cells can encounter Pf infected RBC (Pf-RBC) in the blood and in the spleen, in the presence of other leukocytes. We therefore coincubated Pf-RBC (trophozoite/schizont stage, 3D7 Pf strain) with freshly isolated human PBMC, to investigate whether primary NK cells are activated under these circumstances. As shown in Fig. 1A (and in Fig. 6, which is published as supporting information on the PNAS web site), Pf-RBC induced the up-regulation of activation markers, CD25 and CD69, on NK cells within PBMC. This up-regulation was observed in all individuals tested upon 20-24 h of coculture with Pf-RBC, although its intensity was variable among individuals. Moreover, IFN-γ was produced by NK cells upon 24 h of treatment with Pf-RBC, and as soon as 4 h in the presence of suboptimal concentration of IL-12 (1 ng/ml) (Fig. 1B). In the later setting, the frequency of IFN-γ-producing NK cells was increased by 1.3- to 4.3-fold in the presence of Pf-RBC compared with RBC (mean 2.1 ± 0.5, P < 0.0001). Despite the high variability of Plasmodium spp., the increase in CD25 and CD69 surface expression (data not shown), and IFN-γ production was induced by all nine Pf strains tested, including clinical isolates (Fig. 1C). CD25 and CD69 expression were within ranges comparable with those induced by the prototypical human tumor cell target K562 (Fig. 1 A). In contrast to K562, we could not detect signs of NK cell cytotoxicity upon encounter with Pf-RBC, as shown by the absence of cytotoxic granule exocytosis detected by Lamp1 (CD107a) and Lamp2 (CD107b) cell-surface redistribution (Fig. 1D) (16). Therefore, RBC infected by multiple Pf strains induced the up-regulation of CD25 and CD69 expression as well as IFN-γ production by human NK cells within PBMC.
Fig. 1.
Pf-RBC-induced NK cell activation within PBMC. Freshly isolated human PBMC were cultured alone (0) or in the presence of the NK cell target K562, Pf-RBC, or uninfected RBC (RBC). NK cell activation was analyzed by flow cytometry after gating on CD3-CD56+ NK cells (A-C) or on CD56+ lymphocytes (D). Statistical analyses were done by using a one-tailed Wilcoxon signed rank test. (A) After 20-24 h of coculture, the percentage of CD25+ cells within NK cells (Left) and the mean fluorescence intensity (MFI) of CD69 staining on NK cells (Right) are indicated for 30 healthy donors. Each dot represents the result obtained from one donor. (B) IFN-γ production by NK cells in PBMC was assessed by flow cytometry after coculture with 3D7-RBC or RBC during 20-24 h (Left) or 4 h in the presence of suboptimal doses of IL-12 (Right). Each dot represents the results from one donor. (C) IFN-γ production by NK cells was determined after 4 h of PBMC exposure to RBC infected with various Pf strains in the presence of IL-12. For each experiment, NK cell activation with the 3D7 strain was used as a control and represents 100% of NK cell IFN-γ production. Depending on the strains, 2-14 experiments were performed. Means ± SEM are represented. (D) Lamp1 and Lamp2 mobilization by NK cells (CD3-) or CD56+ T cells (CD3+) in PBMC was assessed by flow cytometry after 4 h of culture in the presence of monensin. One representative experiment of five is shown.
Full NK Cell Activation Induced by Pf-RBC Requires Help from Macrophages. Purified NK cells were responsive to Pf-RBC, as shown by the up-regulation of CD69 cell-surface expression (Fig. 2A). Importantly, Pf-RBC also induce purified NK cells to produce CXCL8 (IL-8), a proinflammatory cytokine characterized by its leukocyte chemotactic activity and its NK stimulating functions (Fig. 2 A) (17). Incubation of Pf-RBC but not uninfected RBC with human NK cell lines (NKL, NK92) also led to an up-regulated expression of activation markers, to the production of CXCL8 (data not shown), and to a direct interaction evoking “rosettes” (see Fig. 7, which is published as supporting information on the PNAS web site). Therefore, human NK cells are activated upon direct Pf-RBC recognition. However, the production of IFN-γ after coculture with Pf-RBC for 20 h, or for 4 h in the presence of suboptimal dose of IL-12, was extinguished when NK cells were purified from PBMC (Fig. 2B), indicating that full NK cell activation induced by Pf-RBC requires help from another cell type present in PBMC. Reciprocal interactions between NK cells and DC have been documented and are critical for NK cell activation, DC maturation, and appropriate control of murine cytomegalovirus infection and tumor cell elimination (5). DC respond to pathogens by undergoing a maturation process characterized by increased surface expression of MHC molecules. HLA class I and class II molecule expression on DC were up-regulated upon coculture with Pf-RBC (see Fig. 8, which is published as supporting information on the PNAS web site), consistent with previous reports (18). Yet, addition of purified autologous DC (CD19-, CD14-, BDCA1+, or BDCA2+ or BDCA3+) or purified plasmacytoid BDCA2+ dendritic cells (PDC) did not restore NK cell capacity to produce IFN-γ upon encounter with Pf-RBC (Fig. 2B). In addition, depletion of DC in PBMC had no effect on NK cell activation under these settings (data not shown). In contrast, the selective depletion of CD14+ monocytes from PBMC abolished IFN-γ production by NK cells in response to Pf-RBC (Fig. 2B). Reciprocally, addition of sorted autologous monocytes to purified NK cells restored their IFN-γ production capacity (Fig. 2B), with significant effects observed with monocyte/NK ratios as low as 1/10 (data not shown). Because monocytes readily differentiate toward a macrophagic state upon in vitro culture, it is impossible to formally restrict the helper NK cell function to monocytes or macrophages, which will both be referred to as macrophages hereafter. Our results thus reveal an early innate response in which Pf-RBC directly activate NK cells (leading to CXCL8 production) and indirectly activate NK cells (leading to IFN-γ production) via a specific collaboration with macrophages.
Fig. 2.
Full NK cell activation induced by Pf-RBC requires monocytes/macrophages. (A) Purified or FACS-sorted NK cells were exposed to medium alone (0), 3D7-RBC, or RBC for 24 h. (Upper) CD69 expression by NK cell assessed by flow cytometry (mean ± SEM of 10 experiments). (Lower) CXCL8 production in the supernatant of culture (mean ± SEM of 3 experiments). (B) After 4 h of exposure to RBC infected with 3D7 strain, in the presence of suboptimal doses of IL-12, the frequency of IFN-γ-producing NK cells was assessed by flow cytometry. In each experimental condition, the IFN-γ production was calculated as the proportion of IFN-γ-producing NK cells subtracted from background, as compared with IFN-γ-producing NK cells within PBMC in the presence of Pf-RBC (this “100% value” corresponds to 8.4% in this representative experiment). Purified NK cells were cultured alone or together with purified autologous DC, plasmacytoid DC (Upper) or FACS-sorted monocytes (Lower). CD14+ monocytes were also depleted from PBMC by FACS sorting before stimulation (Lower, NK in PBMC w/o monocytes). One representative experiment of three is shown.
Mandatory Role of MyD88 in NK Cell Activation Induced by Pf-RBC. A feature shared by many innate sensors is their conservation through evolution (19, 20). We thus reasoned that the signals provided by Pf-RBC to human NK cells and macrophages might be conserved across species, and we tested whether human RBC infected by various Pf strains could activate mouse splenic NK cells in the presence of macrophages. Remarkably, coculture of human RBC infected by Pf with mouse splenocytes led to IFN-γ production and up-regulation of CD69 cell-surface expression by mouse NK cells (Fig. 3A). As for human NK cells, the level of IFN-γ induced by the coculture with Pf-RBC was greater in the presence of suboptimal concentration of IL-12 (data not shown). The reactivity of mouse splenocytes to Pf-RBC allowed us to further dissect the signaling pathways involved in this innate recognition strategy by using various mutant mouse models. NK cell reactivity to Pf-RBC was not extinguished when splenocytes were isolated from RAG-1-/- mice, thus showing that B and T cells were not essential partners for this innate response (Fig. 3A). Many innate sensors are wired to the downstream signaling machinery via specialized adaptors. In particular, adaptors with immunoreceptor tyrosine-based activation motif (ITAM) (e.g., DAP12) associate with multiple innate sensors such as the triggering receptors expressed on myeloid cells (TREM) (21). However, NK cell activation still occurred when splenocytes were prepared from mice deficient for the ITAM-bearing polypeptides expressed by cells of the innate immune system (CD3ζ, FcRγ, and DAP12; ZGK-RAG mice) (Fig. 3B). Other adaptors include MyD88 that acts downstream of the IL-1R, the IL-18R, as well as all TLR except TLR3, although its role in mouse TLR12 and TLR13 signaling is still unknown (22). Yet, no substantial decrease in NK cell IFN-γ production upon Pf-RBC stimulation was observed in TLR1-, TLR2-, TLR9-deficient (Fig. 3B) or TLR3-, TLR4-, TLR6-, IL-1R-deficient (data not shown) mice. In contrast, no production of IFN-γ by NK cells was observed when splenocytes were isolated from MyD88 and IL-18R-deficient mice (Fig. 3B). Mouse NK cells are thus activated by Pf-infected human RBC via ITAM-independent but MyD88-dependent and IL-18R-dependent pathways.
Fig. 3.
Mouse NK cells are activated by Pf-RBC via an ITAM-independent and MyD88-dependent pathway. (A and B) Freshly isolated mouse splenocytes (from WT or genetically deficient mice for the indicated molecules) were cocultured with Pf-RBC (3D7-RBC) or uninfected RBC (RBC) for 20-24 h. NK cell activation was analyzed by flow cytometry after gating on CD3-NK1.1+ NK cells. Each dot represents the result from one individual mouse. Statistical analyses were done by using a one-tailed Wilcoxon signed rank test. (A Left and B) NK cell IFN-γ production was determined by intracytoplasmic staining after activation in the presence of suboptimal doses of IL-12. (A Right) CD69 expression on WT NK cell surface was assessed after indicated stimulation in the absence of exogenous cytokines (B) shows the percentage of IFN-γ-producing NK cells in the presence of 3D7-RBC (the background signals detected in the presence of uninfected RBC were subtracted).
To identify the cells on which these molecules were required, we performed mixed splenocyte experiments. These experiments revealed that only Ly5.1+ WT NK cells but neither Ly5.1- MyD88-/- NK cells nor Ly5.1- IL-18R-/- NK cells produce IFN-γ in the presence of Pf-RBC, indicating that expression of MyD88 and IL-18R by NK cells is mandatory for this activation (Fig. 4A). In addition, when the mouse NK cell line Ky.2 (23) was cocultured with Pf-RBC, no IFN-γ production was observed (Fig. 4B). Coincubation with WT splenocytes enabled IFN-γ production by Ky.2 NK cells (Fig. 4B). These data show that mouse NK cells require cellular help for full activation in the presence of Pf-RBC, similar to our results obtained with human NK cells and macrophages (Fig. 2). No alteration in NK cell IFN-γ production was detected when we used splenocytes from IL-18R-deficient mice, consistent with a specific role of IL-18R on NK cells (Fig. 4B). In contrast, splenocytes from MyD88-/- mice were impaired in their capacity to help Ky.2 NK cells to produce IFN-γ when they were exposed to Pf-RBC stimulation (Fig. 4B) but not to PMA/ionomycin (data not shown). Therefore, mouse NK cell activation induced by Pf-RBC requires MyD88-dependent, IL-18R-independent help.
Fig. 4.
MyD88 is required for NK cell activation induced by Pf-RBC. (A) Splenocytes from Ly5.1+ WT mice were mixed with splenocytes from either Ly5.1- MyD88-/- mice (Top), Ly5.1- IL-18R-/- mice (Middle), or Ly5.1- WT mice (Bottom) at a 1/1 ratio. Mixed splenocytes were then stimulated for 20-24 h in the presence of IL-12 with 3D7-RBC, RBC, or PMA/ionomycin. Two parameter flow cytometry contour plots, gated on NK cells, are shown. The frequencies of IFN-γ+ NK cells expressing Ly5.1+ or Ly5.1- are indicated in the upper and lower quadrant of each plot, respectively. Similar results were obtained in three experiments. (B) The Ly5.1+ mouse NK cell line Ky.2 was cultured in the absence (0) or presence of freshly isolated splenocytes from Ly5.1- WT or deficient mice and stimulated with 3D7-RBC for 20-24 h in the presence of IL-12. The percentages of Ly5.1+ Ky.2 cells producing IFN-γ are indicated. Results are expressed as means ± SEM of three experiments. Statistical analyses were performed by using a one-tailed Wilcoxon signed rank test. When cocultured with uninfected RBC, the percentage of IFN-γ+ Ky.2 cells was 1.9 ± 1.5 in the absence of splenocytes (n = 4), 4.4 ± 1.2 with WT splenocytes (n = 5), 2.9 ± 1.9 with MyD88 knockout (KO) splenocytes (n = 5), and 6.8 ± 2.0 with IL-18R knockout splenocytes (n = 3).
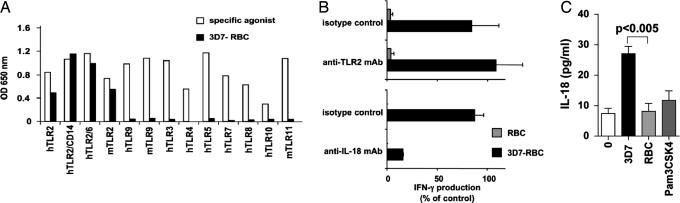
Molecular Requirements for Macrophage/NK Cell Activation by Pf-RBC. We further addressed the nature of the MyD88-associated human innate sensors that could be implicated in the recognition of Pf-RBC. Several TLR have been shown to recognize Pf-RBC. TLR9 recognizes hemozoin, an hydrophobic heme polymer produced by Pf digestion of hemoglobin (24). TLR11 interacts with profilin-like molecules expressed by apicomplexan parasites such as Toxoplasma gondii, Cryptosporidium parvum, and Pf (25). Finally, Pf GPI have been shown to interact with TLR2, leading to TNF-α secretion by macrophages (26). Using a large panel of HEK293 reporter cell transfectants expressing various human and mouse TLR, we found that Pf-RBC were selectively recognized by human and mouse TLR2 but not by the other TLR tested, including TLR9 and TLR11 (Fig. 5A). As expected, the recognition of Pf-RBC by TLR2 was more efficient in the presence of CD14 and TLR6 (20). However, a blocking anti-TLR2 mAb failed to impair NK cell activation induced by Pf-RBC (Fig. 5B) despite its ability to extinguish the activation of hTLR2+ HEK293 cell transfectants by Pf-RBC (see Fig. 9, which is published as supporting information on the PNAS web site). Therefore, our results obtained with mouse and human cells show that NK cell activation induced by Pf-RBC is not critically dependent on the engagement of TLR2 and TLR9 individually.
Fig. 5.
Human TLR2 is not mandatory for NK cell activation induced by Pf-RBC, but IL-18 is required. (A) HEK293 reporter cells transfected with indicated human (h) or mouse (m) TLR were stimulated with a specific agonist for each TLR (open bars) or 3D7-RBC (filled bars) for 18-24 h. Results are represented as optical density (OD) after subtraction of the background signal obtained with RBC or medium alone. (B) The production of IFN-γ by NK cells within human PBMC in response to 3D7-RBC versus RBC was assessed in the presence of anti-TLR2 blocking mAb, compared with the isotype control (Upper, n = 5), and in the presence of anti-IL-18 blocking mAb, compared with the isotype control (Lower, n = 2). Results (means ± SD) are presented as a percentage of control calculated as in Fig. 2. (C) IL-18 production was assayed in the supernatant of purified human macrophages after 24 h of culture with Pam3CSK4, 3D7-RBC, or RBC, in the presence of medium alone.
We then addressed the role of IL-18 in the human system. First, incubation of Pf-RBC with human macrophages led to their production of IL-18 (Fig. 5C). Second, blocking anti-IL-18 mAb extinguished IFN-γ production by human NK cells stimulated with Pf-RBC (Fig. 5B). In contrast, blocking anti-IL-1β mAb had no effect on IFN-γ production by human NK cells stimulated with Pf-RBC (data not shown). Saturating concentration of TLR2 agonists (Pam3CSK4 and FSL-1) induced the secretion of only marginal levels of IL-18 by macrophages and no detectable IFN-γ production by NK cells, although they trigger the production of substantial amounts of TNF-α by macrophages (Fig. 5C and data not shown). Thus, TLR2 triggering is not sufficient for IL-18 secretion by macrophage, which is essential to NK cell IFN-γ production via the IL-18R/MyD88 pathway.
Discussion
Although NK cells have been reported to play a role during the course of parasite infection, numerous issues are still poorly understood. Our data provide evidence that sensing Pf infection by NK cells is a very general phenomenon, not only conserved for a variety of Pf strains in human but also in the mouse, suggesting that the pathways implicated in that process are conserved. We also show that human NK cells present in PBMC secrete IFN-γ upon interaction with Pf-RBC, consistent with previous reports (10, 11). However, in these earlier studies, the NK cell response to Pf-RBC was not detected in all individuals (10, 11). We also found heterogeneity in the strength of NK cell reactivity to Pf-RBC among donors, but this might result in part from variations in the kinetics of the response because CD25 and CD69 were up-regulated at the NK cell surface for all donors tested. We also document a direct interaction between Pf-RBC and NK cells by the rapid formation of “rosettes.” This direct interaction between NK cells and Pf-RBC induces CD69 expression and CXCL8 production but not NK cell degranulation, suggesting that the major role of NK cell against Pf infection is not direct cytotoxicity. NK cell cytotoxicity against Pf-RBC has been previously reported, but differences in NK cell purification and in NK cell cytolytic assays could account for these apparently discordant findings (27, 28). Our results, rather, support a role for NK cell cytokines in the recruitment and the activation of other cells during malaria infection.
Importantly, the direct sensing of Pf-RBC is not sufficient for full NK cell activation, as shown by a strong reduction in IFN-γ secretion, when the response of purified NK cells is compared with that of NK cells within PBMC. This requirement for another cell type that cooperates with NK cells is also conserved in the mice, emphasizing the importance of this phenomenon. Despite the numerous studies that have highlighted the role of DC-NK interactions in the immune responses to viruses and tumors (5), and despite DC activation induced by Pf-RBC (18), DC are not sufficient to help NK cells to produce IFN-γ in response to Pf-RBC. However, we cannot rule out a role of these cells in producing IL-12 because we add suboptimal doses of this cytokine in our experimental system. In these latter conditions, help to NK cells is selectively provided by macrophages with a mandatory role for their capacity to secrete IL-18. Very few reports document a collaboration between macrophage and NK cells (29). Our results reveal the pivotal and selective role for macrophages in the secretion of NK cell IFN-γ induced by Pf-RBC. It remains to be addressed whether macrophages are also important in NK cell response to other parasites, such as Toxoplasma gondii, Leishmania major, or Trypanosoma cruzi, and whether the resulting immune response differs from that initiated by the DC-NK cross-talks during tumor development or viral infection.
The innate sensors potentially involved in the initiation of NK and/or macrophage activation and cooperation include TLR2 (26), TLR9 (24), and TLR11 (25). Despite the reported involvement of TLR9 and TLR11 in the sensing of Pf, we could not detect an activation of neither TLR9+ nor TLR11+ HEK293 transfectants induced by Pf-RBC. It is possible that hemozoin interacting with TLR9 (24) and Pf profiling-like molecules interacting with TLR11 (25) may not be present in sufficient amount in our Pf-RBC preparation, and/or that these Pf molecules need a processing of Pf-RBC in endocytic compartments after phagocytosis. However, NK cells from TLR9-deficient mice were fully responsive to Pf-RBC, and TLR11 is not expressed in human, suggesting that those receptors are not required for human NK cell activation induced by Pf-RBC. Using a series of deficient mice, we identified MyD88 as a mandatory adaptor involved in at least two cell types and in association with at least two distinct receptors. On NK cells, the MyD88-associated IL-18R is a major indirect sensor for Pf infection. On macrophages, MyD88 is required in the help provided to NK cells, but no known TLR is responsible on its own for this role. Yet, human and mouse TLR2 were able to recognize Pf-RBC, with a minor contribution of hTLR6 when coexpressed with hTLR2. TLR2 is involved in the triggering of cytokine secretion by macrophages in response to Pf-RBC (26, 30) (data not shown). However, TLR2 agonists induce neither a strong IL-18 secretion by macrophages nor IFN-γ production by NK cells. Moreover, blocking TLR2-mediated signals does not affect human NK cell IFN-γ secretion, and TLR2 is not mandatory to provide a help to mouse NK cells. The molecular basis of the MyD88 involvement in macrophage stimulation leading to NK cell activation thus remains an open question. We excluded the requirement of each individual TLR tested, but it is still possible that a combination of several TLR may be involved and redundant in that process. Another possibility is the existence of a still unknown MyD88-linked pathway involved directly or indirectly in Pf recognition by macrophages. Interestingly, the other MyD88-associated cytokine receptor IL-1R is required neither in mice nor in humans (data not shown) for NK cell activation induced by Pf-RBC. The pathway leading to full NK cell activation by parasitized erythrocytes is thus highly specific and requires MyD88 signaling and IL-18 production by macrophages.
Our study documents a selective and functional interaction between macrophages and NK cells during parasite infection for the production of IFN-γ and establishes that a conserved component of the pathogen, or a consistent alteration induced by the pathogen, must be detected by the macrophage/NK cell “tandem.” Importantly, Pf-RBC directly triggers the production of CXCL8 by NK cells and the production of IL-18 by macrophages. The clinical outcome of this coordinated innate immune response to Pf infection remains to be evaluated during the course of natural Pf infection. Yet, the present study provides insights for previously reported clinical and epidemiologic studies. IFN-γ and CXCL8 were indeed detected in the serum in the early phases of the infection, during experimental Pf infection of nonimmune volunteers (9), as well as during natural infection (31, 32). In addition, previous studies have pointed to a role of IL-18 during the course of malaria in endemic areas (33-35). However, it is obvious that the immune response to a highly sophisticated and rapidly evolving parasite like Pf is the result of >100,000 years of interaction history with its obligatory human host. It is thus predictable that the NK cell-macrophage-Pf triangle interactions might either be somehow beneficial for the parasite or might have a significant cost for the host, such as immunopathology.
Supplementary Material
Acknowledgments
We thank F. Vély, P. Rihet, and A. Stewart for critical review of the manuscript; F. Fumoux for initial help in this study; E. Tomasello for the kind gift of mice and advice; D. Drocourt, A. Moretta, B. Pouvelle, and M. Dalod for stimulating discussions, help, and reagents; W. Yokoyama for the Ky.2 cell line; F. Navarro (Becton Dickinson) and D. Bossy (Beckman Coulter) for partnership in reagents; and the Centre d'Immunologie de Marseille-Luminy flow cytometry facility. E.V. is supported in part by specific grants from the European Union (ALLOSTEM) and the Ligue Nationale contre le Cancer (Equipe Labellisée “La Ligue”), and by institutional grants from the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, and the Ministère de l'Enseignement Supérieur et de la Recherche. M.B. is supported by ALLOSTEM. C.F. is supported in part by Deutsche Forschungsgemeinschaft Grant SFB571 and Deutsche Krebdhilfe Grant 70-3344-Schm8. J.G. is supported in part by the European Union BiomalPar Network of Excellence, LSHP Grant CT 2004 503578, and Bill and Melinda Gates Foundation Grant 29202.
Abbreviations: DC, dendritic cells; NK, natural killer; PBMC, peripheral blood mononuclear cells; Pf, Plasmodium falciparum; PMA, phorbol 12-myristate 13-acetate; RBC, red blood cells; TLR, Toll-like receptor.
References
- 1.Dokun, A. O., Chu, D. T., Yang, L., Bendelac, A. S. & Yokoyama, W. M. (2001) J. Immunol. 167, 5286-5293. [DOI] [PubMed] [Google Scholar]
- 2.Walzer, T., Dalod, M., Robbins, S. H., Zitvogel, L. & Vivier, E. (2005) Blood 106, 2252-2258. [DOI] [PubMed] [Google Scholar]
- 3.Moretta, L. & Moretta, A. (2004) EMBO J. 23, 255-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raulet, D. H. (2004) Nat. Immunol. 5, 996-1002. [DOI] [PubMed] [Google Scholar]
- 5.Degli-Esposti, M. A. & Smyth, M. J. (2005) Nat. Rev. Immunol. 5, 112-124. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson, M. M. & Riley, E. M. (2004) Nat. Rev. Immunol. 4, 169-180. [DOI] [PubMed] [Google Scholar]
- 7.Hall, N., Karras, M., Raine, J. D., Carlton, J. M., Kooij, T. W., Berriman, M., Florens, L., Janssen, C. S., Pain, A., Christophides, G. K., et al. (2005) Science 307, 82-86. [DOI] [PubMed] [Google Scholar]
- 8.Hansen, D. S., Evans, K. J., D'Ombrain, M. C., Bernard, N. J., Sexton, A. C., Buckingham, L., Scalzo, A. A. & Schofield, L. (2005) Infect. Immun. 73, 2288-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermsen, C. C., Konijnenberg, Y., Mulder, L., Loe, C., van Deuren, M., van der Meer, J. W., van Mierlo, G. J., Eling, W. M., Hack, C. E. & Sauerwein, R. W. (2003) Clin. Exp. Immunol. 132, 467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Artavanis-Tsakonas, K. & Riley, E. M. (2002) J. Immunol. 169, 2956-2963. [DOI] [PubMed] [Google Scholar]
- 11.Artavanis-Tsakonas, K., Eleme, K., McQueen, K. L., Cheng, N. W., Parham, P., Davis, D. M. & Riley, E. M. (2003) J. Immunol. 171, 5396-5405. [DOI] [PubMed] [Google Scholar]
- 12.Trager, W. & Jensen, J. B. (1976) Science 193, 673-675. [DOI] [PubMed] [Google Scholar]
- 13.Robert, C., Pouvelle, B., Meyer, P., Muanza, K., Fujioka, H., Aikawa, M., Scherf, A. & Gysin, J. (1995) Res. Immunol. 146, 383-393. [DOI] [PubMed] [Google Scholar]
- 14.Gysin, J., Pouvelle, B., Fievet, N., Scherf, A. & Lepolard, C. (1999) Infect. Immun. 67, 6596-6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pouvelle, B., Fusai, T., Lepolard, C. & Gysin, J. (1998) Infect. Immun. 66, 4950-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alter, G., Malenfant, J. M. & Altfeld, M. (2004) J. Immunol. Methods 294, 15-22. [DOI] [PubMed] [Google Scholar]
- 17.Sivori, S., Falco, M., Della Chiesa, M., Carlomagno, S., Vitale, M., Moretta, L. & Moretta, A. (2004) Proc. Natl. Acad. Sci. USA 101, 10116-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichyangkul, S., Yongvanitchit, K., Kum-arb, U., Hemmi, H., Akira, S., Krieg, A. M., Heppner, D. G., Stewart, V. A., Hasegawa, H., Looareesuwan, S., et al. (2004) J. Immunol. 172, 4926-4933. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann, J. A. (2003) Nature 426, 33-38. [DOI] [PubMed] [Google Scholar]
- 20.Beutler, B. (2004) Nature 430, 257-263. [DOI] [PubMed] [Google Scholar]
- 21.Colonna, M. (2003) Nat. Rev. Immunol. 3, 445-453. [DOI] [PubMed] [Google Scholar]
- 22.Akira, S. & Takeda, K. (2004) Nat. Rev. Immunol. 4, 499-511. [DOI] [PubMed] [Google Scholar]
- 23.Karlhofer, F. M., Orihuela, M. M. & Yokoyama, W. M. (1995) J. Exp. Med. 181, 1785-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coban, C., Ishii, K. J., Kawai, T., Hemmi, H., Sato, S., Uematsu, S., Yamamoto, M., Takeuchi, O., Itagaki, S., Kumar, N., et al. (2005) J. Exp. Med. 201, 19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarovinsky, F., Zhang, D., Andersen, J. F., Bannenberg, G. L., Serhan, C. N., Hayden, M. S., Hieny, S., Sutterwala, F. S., Flavell, R. A., Ghosh, S. & Sher, A. (2005) Science 308, 1626-1629. [DOI] [PubMed] [Google Scholar]
- 26.Krishnegowda, G., Hajjar, A. M., Zhu, J., Douglass, E. J., Uematsu, S., Akira, S., Woods, A. S. & Gowda, D. C. (2005) J. Biol. Chem. 280, 8606-8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orago, A. S. & Facer, C. A. (1991) Clin. Exp. Immunol. 86, 22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mavoungou, E., Luty, A. J. & Kremsner, P. G. (2003) Eur. Cytokine Network 14, 134-142. [PubMed] [Google Scholar]
- 29.Dalbeth, N., Gundle, R., Davies, R. J., Lee, Y. C., McMichael, A. J. & Callan, M. F. (2004) J. Immunol. 173, 6418-6426. [DOI] [PubMed] [Google Scholar]
- 30.Zhu, J., Krishnegowda, G. & Gowda, D. C. (2005) J. Biol. Chem. 280, 8617-8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgmann, H., Hollenstein, U., Wenisch, C., Thalhammer, F., Looareesuwan, S. & Graninger, W. (1995) Clin. Immunol. Immunopathol. 76, 32-36. [DOI] [PubMed] [Google Scholar]
- 32.Kremsner, P. G., Winkler, S., Brandts, C., Wildling, E., Jenne, L., Graninger, W., Prada, J., Bienzle, U., Juillard, P. & Grau, G. E. (1995) Am. J. Trop. Med. Hyg. 53, 532-538. [DOI] [PubMed] [Google Scholar]
- 33.Kojima, S., Nagamine, Y., Hayano, M., Looareesuwan, S. & Nakanishi, K. (2004) Acta Trop. 89, 279-284. [DOI] [PubMed] [Google Scholar]
- 34.Perkmann, T., Winkler, H., Graninger, W., Kremsner, P. G. & Winkler, S. (2005) Cytokine 29, 153-158. [DOI] [PubMed] [Google Scholar]
- 35.Nagamine, Y., Hayano, M., Kashiwamura, S., Okamura, H., Nakanishi, K., Krudsod, S., Wilairatana, P., Looareesuwan, S. & Kojima, S. (2003) Trans. R. Soc. Trop. Med. Hyg. 97, 236-241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.