For most of human history, salt was a precious commodity. People prized it for flavoring and preserving food and for use in religious ceremonies and burials. The Roman occupation of Britain peppered the English language with a legacy of salt. We retain those Latin links in words such as “salary” and “salami” and in place names like Greenwich and Sandwich, their suffix denoting a salt-works. Today salt is no longer precious. The U.S. mines ≈36 million metric tons [1 metric ton = 1 megagram (Mg)] of rock salt a year (1). Eighteen million Mg is spread on paved surfaces for deicing, making winter roads safer for people and vehicles (2). However, once the salt dissolves, it washes into streams or soil and is forgotten. A new article by Kaushal et al. (3) in a recent issue of PNAS suggested that it should not be.
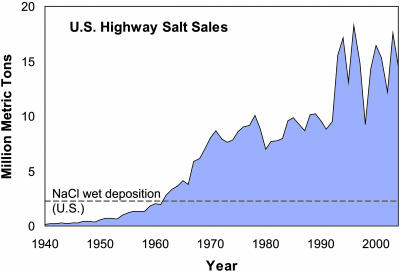
The use of rock salt (NaCl) on U.S. roads has skyrocketed in the last 65 years (Fig. 1), and chloride (Cl) concentrations in waters of the northeast have risen as a consequence (4-6). The mobility of salt in water leads to its potential problems in the environment. These problems include toxicity to plants and fish, groundwater contamination, and human health interactions, particularly salt intake and hypertension (7-9). In consequence, researchers have been monitoring increased salt concentrations in streams and groundwater for decades (4-6, 10).
Fig. 1.
Sales of rock salt for highway use in the U.S. from 1940 to 2004 in millions of metric tons (Mg) (1, 2). The dashed line denotes our estimate of the calculated annual wet deposition of Na and Cl in the U.S., derived primarily from sea salt. The amount of Na and Cl in road salt topped Na and Cl deposition for the continental U.S. some time in the early 1960s. We estimated U.S. wet deposition of NaCl based on data from 1999-2003 using deposition isopleth maps from ref. 15. The product of mean area and deposition rates for each isopleth interval was calculated by state and summed. For Na and Cl, rates of dry deposition should be smaller than rates of wet deposition.
The research by Kaushal et al. (3) documenting increased Cl concentrations in streams of the northeastern U.S. is important for several reasons. One is the long-term nature of their data sets. They analyzed Cl concentrations for 20-40 years in seven streams and rivers in Maryland, New York, and New Hampshire, showing steady increases over time. The most dramatic changes were seen in New Hampshire, where Cl concentrations have increased by more than an order of magnitude since the 1960s, sometimes topping 100 mg·liter-1. Even more importantly, if results are extrapolated into the next century, the data suggest that many rural streams in the Northeast will have baseline salt concentrations >250 mg·liter-1, the generally accepted cutoff for potable water and a level at which chronic toxicity occurs for many freshwater species.
A second aspect is their intensive focus on streams in the greater Baltimore area. In this rapidly urbanizing region, they found a logarithmic relationship between the proportion of pavement in a watershed and the mean annual Cl concentration in streams. Above 15% impervious cover, Cl concentrations were strong enough to damage some plants, and, above 40%, the streams crossed the threshold of 250 mg·liter-1 Cl. Maximum winter concentrations reached >4,600 mg·liter-1 Cl, approximately one-quarter of the amount in seawater.
Not surprisingly, the data of Kaushal et al. (3) show strong seasonal effects, with the highest concentrations in winter. More surprisingly, Cl concentrations in the rural streams did not return to baseline levels in summer, even when no salt was being applied. One reason is that salt concentrations build up over many years and remain high in the soil and groundwater. Groundwater seeping into streams often keeps water flowing during the driest periods, typically in summer. If the groundwater is salty, the stream will be salty. Increases in groundwater salinity have indeed been observed in the northeastern U.S. and Canada (11, 12). For example, a survey of 23 springs in the greater Toronto area found Cl concentrations topping 1,200 mg·liter-1 arising from road salt use (11). This groundwater salinity is the primary concern for long-term potable water supply. Once groundwater becomes salty, it typically will take decades to centuries for the salts to disappear, even when road salt use ends.
Where Is the Sodium?
The current study focused on the fate of Cl, providing clear evidence of its link to road salt and build-up in streams. Two unanswered questions are (i) how the road salt gets into the streams and (ii) what happens to the accompanying sodium (Na). Na is important for its health effects on wildlife and people and also as a biogeochemical tracer. If road salt takes a fairly direct path to streams through surface runoff or drainage systems, the amounts of Na and Cl reaching the streams should be roughly similar (0.65:1 mass ratio). If instead the dominant flow path involves underground transport through soils, where Cl anions are more mobile than Na cations, then the ratio of Na to Cl will be lower. Na will gradually displace Ca, Mg, K, and protons in the soil, altering soil fertility and uncoupling the flow of Na from Cl (13). We suggest that the ratio of changes in Na and Cl concentrations over time in the stream waters described by Kaushal et al. (3) could help determine the importance of surface vs. underground pathways of road salt transport to streams. Lower Na:Cl ratios would suggest proportionally greater fluxes through soil.
Human Use Versus Natural Deposition
To understand how much rock salt is now being applied in the U.S., we compared the amount to estimated natural deposition rates of Na and Cl. In 1940, sales of rock salt for highway use were only 149,000 Mg (Fig. 1). Today, the value is a hundred times higher, ≈18 million Mg. This amount dwarfs our calculated estimate for natural wet deposition of Na and Cl for the continental U.S. each year, 2.2 Mg·yr-1 derived primarily from sea salt (Fig. 1).
Most of the ≈18 million Mg of NaCl used on roads each year is applied in northeastern and midwestern states, with six states using three quarters of the total: New York, Ohio, Michigan, Illinois, Pennsylvania, and Wisconsin. On a statewide basis, applications of deicing salt are 200 kg·ha-1·yr-1 for New York and Ohio and 400 kg·ha-1·yr-1 in the District of Columbia (14). In rural states, such as Vermont, salt loads are still 136 kg·ha-1·yr-1 (14). Focusing just on Cl, the average input from road salt in Vermont is therefore 80 kg·ha-1·yr-1, two orders of magnitude higher than estimated atmospheric Cl inputs of 0.88 kg·ha-1·yr-1 (1999-2003 average; ref. 15).
There are real, long-term consequences to rock salt's use for freshwater systems and soils.
The increases are equally large for Na, a cation that is often abundant in rocks but tends not to be retained as much as other cations in soils (16). An average Vermont forest soil receiving an annual Na load of 50 kg·ha-1 from rock salt has the potential to displace other cations and load its entire exchangeable complex with Na in the top 10 cm of the soil in 80 years [based on an effective cation exchange capacity of 15 milliequivalents (meq)/100 g of soil and a bulk density of 1.2 g·cm3]. Obviously, rock salt is not applied evenly across a state; some areas will have higher inputs and other areas will have lower or no inputs. The key point is that, compared with natural deposition of Na and Cl, inputs of road salt are now enormous, and water moves that salt around locally and regionally as Kaushal et al. (3) highlight.
The most difficult aspect of road salt use is knowing what to do about it.
Kaushal et al. (3) do not discuss policy solutions or suggest alternatives to its use. Scandinavian countries are studying alternatives to traditional road salt, including mixing it with sand or sugar and replacing it with other chemicals, such as potassium formate. Canada took the controversial step in 1995 of placing salt on its Priority Substances List for assessment under the Canadian Environmental Protection Act, and, in 2004, it released a Code of Practice for the Environmental Management of Road Salts. Concerns for drinking water quality in New York have led some cities to use chemical alternatives, including potassium acetate (C2H3KO2) and calcium magnesium acetate (CaxMgy(C2H3O2)2(x+y)) (17). However, these chemicals are an order of magnitude more expensive than NaCl. The beauty of road salt is that it works well and is cheap.
In summary, no one is suggesting that society should instantly ban rock salt use. Nonetheless, the results of Kaushal et al. (3) do suggest that there are real, long-term consequences to its use, particularly for freshwater systems and soils. Understanding which environments are more likely to transfer salt from roads, streams, and groundwater could help managers identify sensitive species and highway segments that need alternative methods of deicing. More generally, a prudent step would be to adopt a “less is more” policy, reducing the amounts of salt applied and considering alternatives where economically feasible. As is so often the case today, society is left to balance a discrete, positive benefit (safer roads) with more dilute environmental costs that build over decades and take decades to recover (18).
R.B.J. and E.G.J. wrote the paper.
See companion article on page 13517 in issue 38 of volume 102.
References
- 1.Ewell, M. E. (2003) Mining and Quarrying Trends 2003 (U.S. Geological Survey, U.S. Department of the Interior, Washington, DC).
- 2.The Salt Institute (2004) Salt Mining Statistics (The Salt Institute, Alexandria, VA).
- 3.Kaushal, S. S., Groffman, P. M., Likens, G. E., Belt, K. T., Stack, W. P., Kelly, V. R., Band, L. E. & Fisher, G. T. (2005) Proc. Natl. Acad. Sci. USA 102, 13517-13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters, N. E. & Turk, J. T. (1981) Water Resour. Bull. 17, 586-598. [Google Scholar]
- 5.Siver, P. A., Canavan, R. W., Field, C. K., Marsicano, L. J. & Lott, A. M. (1996) J. Environ. Qual. 25, 334-345. [Google Scholar]
- 6.Godwin, K. S., Hafner, S. D. & Buff, M. F. (2003) Environ. Pollut. 124, 273-281. [DOI] [PubMed] [Google Scholar]
- 7.Forman, R. T. T. & Alexander, L. E. (1998) Annu. Rev. Ecol. Syst. 29, 207-231. [Google Scholar]
- 8.Howard, K. W. F. & Haynes, J. (1993) Geosci. Can. 20, 1-8. [Google Scholar]
- 9.Wegner, W. & Yaggi, M. (2001) Stormwater 2, No. 5.
- 10.Thunqvist, E. L. (2004) Sci. Total Environ. 325, 29-37. [DOI] [PubMed] [Google Scholar]
- 11.Williams, D. D., Williams, N. E. & Cao, Y. (2000) Water Res. 34, 127-138. [Google Scholar]
- 12.Foos, A. (2002) Environ. Geol. 44, 14-19. [Google Scholar]
- 13.Norrstrom, A. C. & Bergstedt, E. (2001) Water Air Soil Pollut. 127, 281-299. [Google Scholar]
- 14.Kostick, D. S. (1993) Bureau of Mines Information Circular 9343 (Bureau of Mines, U.S. Department of the Interior, Washington, DC).
- 15.Illinois State Water Survey, NADP Office (2005) National Atmospheric Deposition Program-National Research Support Program-3 Report (Illinois State Water Survey, NADP Office, Champaign, IL).
- 16.Jobbágy, E. G. & Jackson, R. B. (2001) Biogeochemistry 53, 51-77. [Google Scholar]
- 17.National Research Council (1991) Highway Deicing: Comparing Salt and Calcium Magnesium Acetate (Transportation Research Board, Washington, DC), Report 235.
- 18.Jackson, R. B., Carpenter, S. R., Dahm, C. N., McKnight, D. M., Naiman, R. J., Postel, S. L. & Running, S. W. (2001) Ecol. Appl. 11, 1027-1045. [Google Scholar]