Abstract
Zebrafish fin regeneration requires the formation and maintenance of blastema cells. Blastema cells are not derived from stem cells but behave as such, because they are slow-cycling and are thought to provide rapidly proliferating daughter cells that drive regenerative outgrowth. The molecular basis of blastema formation is not understood. Here, we show that heat-shock protein 60 (hsp60) is required for blastema formation and maintenance. We used a chemical mutagenesis screen to identify no blastema (nbl), a zebrafish mutant with an early fin regeneration defect. Fin regeneration failed in nbl due to defective blastema formation. nbl also failed to regenerate hearts. Positional cloning and mutational analyses revealed that nbl results from a V324E missense mutation in hsp60. This mutation reduced hsp60 function in binding and refolding denatured proteins. hsp60 expression is increased during formation of blastema cells, and dysfunction leads to mitochondrial defects and apoptosis in these cells. These data indicate that hsp60 is required for the formation and maintenance of regenerating tissue.
Keywords: blastema, regeneration, zebrafish, genetics, stress response
Humans have the ability to renew lost or damaged tissues and organs. Homeostatic renewal of components of blood, skeletal muscle, and epithelia is well characterized. These constant renewal processes are believed to be mediated by the action of resident stem cells, pluripotent cells of a specific lineage. Humans also have the capacity to regenerate acutely injured tissues. Prominent among these are liver, digit-tip, and corneal regeneration. In contrast to chronic renewal, these acute regenerative processes are not mediated by the action of stem cells. Instead, they are thought to be mediated through the partial dedifferentiation and proliferation of parenchymal cells, such as hepatocytes or mesenchymal cells.
Teleost fish and urodele amphibians have remarkable regenerative capabilities. Zebrafish acutely regenerate heart (1), fins (2), optic nerve (3), scales (4) and spinal cord (5). To identify the molecular mechanisms of regeneration, we performed a genetic screen for mutant zebrafish with defects in fin and cardiac regeneration (1, 6, 7). As a result, we have begun to identify regenerative genes at specific stages of regeneration.
Zebrafish fin regeneration takes 1 week to complete at 33°C. The first step of regeneration is the closure of the wound. This is a nonproliferative event, involving the migration of existing epithelial cells to cover the wound (8). The formation of wound epidermis is completed within the first 6–12 h postamputation (hpa). The second step is blastema formation, the creation of regeneration cells that drive regeneration (8). Shortly after the wound epidermis is formed, mesenchymal cells immediately beneath this epithelium become disorganized. In urodele amphibians, this disorganizational step requires the action of matrix metalloproteinases (9). Next, a number of cells beneath the amputation plane begin to proliferate and migrate toward the wound epidermis to form a nascent blastema (8). Third, this early blastema matures to form a distal blastema consisting of slow-cycling cells that behave like stem cells even though they are derived from mesenchymal cells (8). These cells express msxb and msxc, transcriptional repressors that may help maintain a pluripotent state (8, 10, 11). These distal blastema cells are thought to give rise to proximal blastema cells that proliferate intensely and drive regenerative outgrowth (7, 8).
During regeneration, cells are exposed to a variety of stresses originating from the wound environment. Environmental stress, injury, disease, and even growth and differentiation place organisms under stress. A highly conserved cellular stress response is the induction of heat-shock proteins (Hsps), initially so-named because they were induced in Drosophila larvae after a slight elevation of temperature (12). Hsps are essential for cell survival in all species subjected to stress. Hsps have a chaperone function, demonstrated by their ability to bind proteins and mediate conformational changes during protein folding (13).
Hsp60 is a well characterized chaperone mainly localized in mitochondria of eukaryotic cells (14–16). Hsp60, also known as phage growth λ E large (GroEL) in bacteria, is involved in the folding and assembly of polypeptide chains into oligomeric complexes. Although Hsp60 has an essential role in the folding of many proteins, it also has additional functions (17). Interestingly, hsp60 is up-regulated in stem cells (18) and is highly expressed in germ cells (19–21). However, it is not known whether Hsp60 is important for the function of these cells.
We performed a mutagenesis screen in zebrafish to identify temperature-sensitive mutants defective in caudal fin regeneration (6, 7). The no blastema (nbl) mutant showed a defect in blastema formation and maintenance in fin regeneration and failed to regenerate heart. Instead of forming an early blastema, mesenchymal cells in nbl did not express msx genes and died through apoptosis. Through positional cloning and mutational analyses, we discovered that nbl results from a loss-of-function missense mutation in hsp60. We found that hsp60 is up-regulated in mesenchymal cells during blastema formation and that ultimately overlaps the msxb-positive distal blastema during regenerative outgrowth. These cells are the stem-cell-like regeneration cells required for subsequent regenerative outgrowth. Thus, hsp60 function is required for protecting stem-cell-like regeneration cells from stress and apoptosis during regeneration.
Materials and Methods
Surgery. Fin amputation (8) and cardiac surgery were performed as described (1).
Mutagenesis and Screen for Regeneration Mutants. Mutagenesis and screen were performed as described (6).
Immunohistochemistry and in Situ Hybridization. Hematoxylin staining and whole-mount in situ hybridization were performed as described (22). Antisense RNA probes were generated by using a 2.4-kb hsp60 cDNA (EST fp49d05) and msxb and msxc sequences (11). BrdUrd incorporation assay was performed as described (8). Immunohistochemistry and acid fuchsin-orange G staining was performed as described (1, 6). To label cardiomyocytes, we used an anti-rabbit myocyte enhancer factor 2 antibody (Santa Cruz Biotechnology). Apoptotic cells were detected by using an antiactive Caspase-3 antibody (Calbiochem).
Northern Blot Analyses. Northern analyses were performed as described (6). We used Hsp60 (EST fp49d05) and β-actin cDNAs to generate probes.
Genetic Mapping and Positional Cloning. Genetic mapping and positional cloning were performed as described (6, 7). A 578-bp PCR fragment containing the mutation was amplified from hsp60 cDNA by using the following primers: forward (Fwd), 5′-AGAGGAGTCATGATGGCCGTAG-3′ and reverse (Rev), 5′-AAGAGCAAGACCCATAGCCTCA-3′. hsp60 genomic DNA fragment containing the mutation was amplified: Fwd, 5′-TCTCATTTAAAGGCGTAGTTCACAG-3′ and Rev, 5′-CCTTAATGACGGCCACTCCATCTGA-3′. The GenBank accession number for hsp60 is NM_181330.
EM. EM was performed as described (6).
Phenotype Rescue and Morpholino Antisense Knockdown. WT hsp60 cDNA or mutated hsp60 cDNA (V324E) was subcloned into the pCS2+ expression vector (23). Rescue experiments were performed as described (6). Antisense morpholino oligonucleotides against hsp60 5′-CATGACACTGGGTAAACGCAGCATT-3′ (Gene Tools, Carvalis, OR) were injected at 300 μM into one-cell-stage WT embryos and screened at 3 days postfertilization (dpf) for the hsp60 phenotype.
In Vitro Functional Assay. The mutant GroEL V300E was constructed by using the QuikChange site-directed mutagenesis kit (Stratagene). WT GroEL, phage growth λ E small (GroES), and GroEL V300E proteins were purified as described (24). In vitro functional assay were performed as described (24, 25).
Statistics. BrdUrd-positive nuclei were counted, and results were expressed as mean ± SEM. Statistical significance determined through two-tailed Student's t test.
Results
Genetic Screen for Regeneration Mutants and Identification of nbl. To identify mutants that failed to regenerate, we treated male zebrafish with N-ethyl-N-nitrosourea and generated mutagenized families by early pressure parthenogenesis (6, 7). We assumed that many regeneration genes would also be required for development, so we screened for temperature-sensitive mutants. We raised 431 families to adulthood at 25°C, amputated caudal fins, and shifted the temperature to 33°C for 7 days before assessing regeneration. Of note, WT fin regeneration is completed normally at 33°C and proceeds approximately two times faster than at 25°C (2). To enhance the specificity of our screen, we selected for fish that survived the restrictive condition (33°C) for at least 2 weeks. To increase the likelihood of identifying genes critical for the early phases of regeneration, we selected for fish that failed to express msxb, a marker of early blastema formation. Through these processes, we identified nbl, a temperature-sensitive zebrafish fin regeneration mutant.
The nbl phenotype was inherited as a recessive trait and was identified in a family in which 4 of 17 members displayed regenerative defects. nbl regenerates did not form new bone and failed to grow beyond the amputation plane after 7 days at 33°C (Fig. 1A). nbl regenerated fins at the permissive temperature, 25°C.
Fig. 1.
nbl, a temperature-sensitive regeneration mutant. (A) Whole-mount WT and nbl caudal fin regenerates at 0 and 7 dpa. nbl shows a clear block in fin regeneration. Red dashed lines demarcate amputation plane. Red arrows show clotted blood in nbl.(B) Sections of regenerating fins stained with hematoxylin at 3, 6, 16, and 24 hpa (33°C). A fluid- or bloodfilled space separates the wound epidermis from mesenchyme in nbl (black arrow). nbl fins fail to form blastema (red arrow; 24 hpa). (C) Heart sections 7 dpa stained with acid fuchsin-orange G to stain fibrin (orange) and collagen (blue) deposits. nbl ventricle did not initiate cardiac regeneration. Yellow dashed line demarcates the amputation plane. (D) Heart regeneration at 12 dpa. Myocyte enhancer factor 2 staining marks cardiomyocytes (red), and BrdUrd (green) identifies cycling cells. A low percentage of cardiomyocytes incoporate BrdUrd (orange) in WT. No evidence of myocyte or nonmyocyte proliferation was observed in nbl hearts.
To further define the nbl regeneration defect, we performed histologic analyses on fin regenerates obtained from sequential stages of regeneration at 33°C. Wound healing appeared normal (Fig. 1B). However, a fluid- or bloodfilled space separated the wound epidermis from mesenchyme in nbl mutant as early as 6 hpa (Fig. 1B). Occasionally, this space was filled by collapsing wound epidermis. This phenotypic abnormality was more pronounced at 24 hpa, at which point WT zebrafish form the initial blastema. In contrast, nbl regenerates developed large acellular areas where the blastema is normally formed (Fig. 1B). The name nbl was derived from this no blastema phenotype.
To determine whether nbl was essential for cardiac regeneration, we surgically removed ≈20% of the ventricular myocardium from 1-year-old adults and examined hearts histologically. WT fish formed fibrin clots by 7 days postamputation (dpa; Fig. 1C), and cardiac myofibers penetrated the clot and constructed a new muscle around the wound by 17 days (1). The restoration of cardiac muscle resulted from cardiomyocyte proliferation (1) (Fig. 1D). In contrast, nbl could not initiate cardiac regeneration. nbl failed to fill in the wound area with fibrin clots or collagen scar (Fig. 1C, n = 10). BrdUrd studies showed no evidence of myocyte or nonmyocyte proliferation around the wound at 12 dpa (Fig. 1D, n = 5).
Blastema Defects in nbl. To determine the cellular mechanism of the nbl regenerative failure, we examined the histology of nbl fin regenerates. During blastema formation (24 hpa) msxb is expressed among mesenchymal cells that ultimately form the blastema (Fig. 2A). In contrast to WT, msxb expression was absent in nbl and there was no evidence of blastema formation (Fig. 2A).
Fig. 2.
Failed blastema formation in nbl. (A) Whole-mount in situ hybridization and longitudinal sections of msxb in WT and nbl fin regenerates at 24 hpa. msxb (red arrowhead) marks early blastema cells. Note the absence of msxb expression in nbl, indicating failure of early blastema formation. (B) Sections from WT and nbl regenerates immunostained for BrdUrd (green) and DAPI (blue). Fish were treated with BrdUrd for 6 h before harvesting at 24 hpa. No blastema is observed in nbl regenerates at 33°C. However, mesenchymal cell proliferation in nbl is slightly increased. Epidermal cell proliferation in nbl is comparable to WT at 33°C. Data in graph are the mean ± SEM; n = 7; *, P < 0.01. (C) Sections from WT and nbl regenerates immunostained for active Caspase-3 (red) and DAPI (blue). WT and nbl fish regenerated for 16 h at 25°C followed by a 4-h incubation at 33°C. Note there are Caspase-3-positive cells proximal to the amputation plane in nbl. Normally, this is where mesenchymal tissue disorganization occurs, a process that immediately precedes early blastema formation.
To further dissect blastema formation in nbl, we assessed the proliferation of mesenchymal cells by using BrdUrd incorporation (Fig. 2B). BrdUrd is a thymidine analog that is incorporated into DNA during replication. Interestingly, the proliferation level of mesenchyme tissue proximal to the amputation plane was slightly increased in nbl regenerates (Fig. 2B; WT 46.2 ± 4.0%, nbl 62.6 ± 3.4%, P = 0.0086, n = 7). Proliferation of epidermal cells in nbl regenerates did not differ from WT (Fig. 2B). However, the lack of mesenchymal cells above the amputation plane in nbl is further evidence for failed blastema formation (Fig. 2B).
To determine whether programmed cell death accounted for lost tissue in nbl mutants, WT and nbl fish were allowed to regenerate for 16 hpa at 25°C before a 4-h incubation at the restrictive temperature of 33°C. We detected a small number of active Caspase-3-positive cells in the mesenchyme beneath the wound epidermis, where mesenchymal disorganization occurs (Fig. 2C). These data indicate that a small number of cells in the region of blastema formation undergo apoptosis in nbl.
Distal Blastema Defects in nbl. The blastema in regenerating zebrafish fins consists of two compartments, the distal and proximal blastemas. The distal blastema is a small number of msxb-positive cells immediately beneath the wound epidermis (8). Although derived from mesenchymal cells during blastema formation, these distal blastema cells behave like stem cells, because they are slow-cycling and are thought to give rise to proximal blastema cells (8). In contrast, proximal blastema cells do not express msxb and proliferate intensely (7, 8). To determine the effect of the nbl mutation on the mature blastema during regenerative outgrowth (beyond 48 hpa at 33°C), we performed temperature-shift experiments. WT and nbl fish were allowed to regenerate for 5 days at 25°C before an 8-h incubation at 33°C. The temperature shift led to a loss of msxb expression in nbl distal blastema cells (Fig. 3A, n = 15). However, in WT regenerates, msxb expression was maintained (Fig. 3A). This observation was confirmed by using other distal blastema markers, including msxc (data not shown).
Fig. 3.
Failed blastema maintenance in nbl.(A) Whole-mount in situ hybridization of msxb in WT and nbl fin regenerates. Fish were incubated for 5 days at 25°C before an 8-h incubation at 33°C. msxb expresson is absent in the nbl distal blastema (red arrowhead). (B) Sections of WT and nbl regenerates immunostained for BrdUrd (green) and DAPI (blue). Fish were incubated with BrdUrd during the final 2 h at 33°C. Note that an 8-h incubation at 33°C does not affect mesenchymal and epidermal cell proliferation levels during regenerative outgrowth but distal blastemal cells disappear in nbl (red arrowhead). (C) Apoptosis of blastema cells in nbl. WT and nbl fish regenerated for 5 days at 25°C followed by 4 h at 33°C. Fins were immunostained with active Caspase-3 (red) and DAPI (blue). Note that distal blastema cells undergo apoptosis in nbl. (D) Electron micrographs of distal blastema in WT and nbl fish. Fins regenerated for 5 days at 25°C followed by 8-h incubations at 33°C. Note the larger mitochondria with empty matrix (red arrowhead) in nbl mutants. (Bars, 2 μm.)
During regenerative outgrowth, the proximal blastema cells proliferate intensely. To determine the effect of nbl on cell proliferation, fish were treated with BrdUrd during the final 2 h of the 8 h incubation at 33°C. Mesenchymal and epidermal cell proliferation in nbl fish were unaffected during regenerative outgrowth. However, distal blastemal cells were absent in nbl mutants after the brief heat shock (Fig. 3B; red arrowhead).
To determine whether apoptosis accounted for the absent distal blastema cells in nbl, we examined active Caspase-3 immunostaining. After a 4-hour heat shock, we identified activated Caspase-3 staining in the distal blastema (Fig. 3C). These data demonstrate that nbl is required for the survival and maintenance of msxb-positive distal blastema cells.
To determine the subcellular defect in nbl, we examined distal blastema cells of WT and nbl regenerates using EM. WT distal blastema cells had large nuclei with pronounced nucleoli, abundant ribosomes, rough endoplasmic reticulum, and clusters of mitochondria (Fig. 3D). The presence of apoptotic cells was detected after 4-h incubation at 33°C in nbl regenerates (data not shown). After 8 h at 33°C, nbl distal blastema cells showed dilated mitochondria with empty matrix (Fig. 3D). Of note, nbl mitochondrial shape was normal in regions other than the distal blastema (data not shown). These data indicate that the nbl mutation causes dilated mitochondria and apoptosis selectively in distal blastema cells.
Hsp60 Is the nbl Gene. To define the nbl gene, we scored 2,302 fish from nbl × nbl/+ mapping crosses for regeneration defects. Initial genotypic mapping placed nbl between z54324 and z50394 on chromosome 9. To refine localization of nbl, we initiated a chromosomal walk using bacterial artificial chromosome clones from z54324 and z7144, which flanked the 0.61-cM nbl region (Fig. 4A). Single-strand conformation polymorphism markers from the z06z013214 and zC117M22z genomic clones confined nbl to a 0.13-cM critical region (Fig. 4A). Three markers, fi27b05, fi98e03, and zK31k6tA1, were nonrecombinant (Fig. 4A).
Fig. 4.
hsp60 is the nbl gene. (A) Genetic and physical map of nbl. Linkage map (Top), transcripts (Middle), and physical map (Bottom) places nbl between z54324 and z7144. Numbers in parentheses show recombination events from 2,302 meioses between nbl phenotype and linked genetic markers. (B) DNA sequence analysis from nbl and WT strains revealed a unique thymidine to adenosine mutation in nbl, causing valine-324 to glutamic acid missense mutation in Hsp60 (red arrows). (C) Multiple species alignment of the hsp60 region containing the V324E mutation. Note complete conservation of valine-324 among species, including E. coli. (D) Ribbon drawing structure of one subunit in the cis GroEL (Hsp60) ring. Figures were generated by using molmol (32) and coordinates from Protein Data Bank file 1AON (33). The location of the mutation site is shown in red space-filling form in the apical domain (light blue). Intermediate (green) and equatorial domains (blue) are also shown. (E) Northern blot analysis of hsp60 expression in regenerating caudal fin. hsp60 levels are up-regulated during blastema formation at both 25°C and 33°C. β-actin expression shown as a control. (F) Whole-mount in situ hybridization of hsp60 during regeneration. Fish were incubated at 25°C to ensure expression is not induced by heat-shock treatment. hsp60 expression is first induced beneath the wound epidermis, where mesenchymal tissue disorganization occurs at 24 hpa. At 48 hpa, hsp60 is expressed in the newly formed blastema. At 120 hpa, hsp60 expression is up-regulated in the distal blastema where msxb is expressed.
Four transcripts were identified in the nbl critical region: hsp60, hsp10, preimplantation protein 3, and 322240M22 Rik protein. DNA sequence analysis of hsp10, preimplantation protein 3, and 322240M22 Rik protein failed to reveal mutations (data not shown). DNA sequence analysis of cDNAs from nbl, and several WT strains revealed a thymidine to adenosine mutation in hsp60 (Fig. 4B), causing a valine-324 to glutamic acid substitution. Multiple species alignment of hsp60 showed complete conservation of valine-324 among species, including Escherichia coli (Fig. 4C). A ribbon diagram showing the structure of GroEL (E. coli homolog of Hsp60) showed that the analogous valine (red) is located in the apical domain (light blue; Fig. 4D). This apical domain is important for Hsp10 and nonnative protein binding (24).
To define the timing and localization of hsp60 expression during fin regeneration and to determine whether hsp60 expression is consistent with the nbl phenotype, we performed Northern blot and in situ hybridization analyses. Fish were incubated at 25°C to ensure that expression was not induced by heat-shock treatment. We found that zebrafish hsp60 was expressed in all adult somatic tissues examined, including unamputated caudal fins. The highest expression was detected in testis and ovary (data not shown). The level of hsp60 mRNA in the unamputated caudal fin was low (Fig. 4E). However, hsp60 was up-regulated during blastema formation (48 hpa at 25°C, 24 hpa at 33°C; Fig. 4E). The high level of hsp60 expression declined to levels similar to unamputated fins during subsequent stages of regeneration (Fig. 4E). In situ hybridization analyses revealed that hsp60 expression was first observed at 12 hpa at 25°C (data not shown). At 12 and 24 hpa, hsp60 was localized to the mesenchymal cells immediately beneath the wound epidermis (Fig. 4F). These cells demarcate the region undergoing tissue disorganization. At this timepoint, the cell proliferation, which ultimately forms the rudimentary blastema, had not yet begun (8). Thus, hsp60 expression is up-regulated before blastema formation.
Later, when the rudimentary blastema is formed (48 hpa at 25°C), hsp60 was highly expressed in blastemal cells (Fig. 4F). hsp60 expression was overlapped with msxb, a blastemal marker. During regenerative outgrowth (120 hpa at 25°C), hsp60 expression was up-regulated in the distal blastema (Fig. 4F). These data indicate that hsp60 is up-regulated in msxb-positive blastema cells during fin regeneration, and hsp60 expression patterns are consistent with the nbl phenotype.
Rescue of nbl by Overexpression of Hsp60. To determine whether hsp60 was essential for embryonic development, we raised embryos at 33°C. Although 81% (97/120) of WT or heterozygous zebrafish reached the swimming stage, no nbl mutants attained this stage at 33°C. nbl defects included cardiac hypertrophy and progressive pericardial effusion (data not shown). nbl fish raised at 25°C appeared to grow normally, because 87% (515/593) of nbl mutants survived to adulthood.
To confirm that hsp60 was the nbl gene, we injected mRNA encoding either WT or mutant (V324E) hsp60 transcripts into nbl embryos. After injection, embryos were transferred to 33°C. We scored for ability to swim and for pericardial effusion, because nbl embryos consistently demonstrated pericardial effusion at 33°C (536/536; Fig. 5A). nbl embryos injected with the enhanced GFP control showed pericardial effusion (158/158) and did not reach the swimming stage (0/158; Fig. 5B). In contrast, 25% (44/173) of nbl embryos injected with WT hsp60 mRNA were able to swim, and 86% (149/173) did not display the nbl pericardial effusion phenotype (Fig. 5 A and B). We conclude that WT hsp60 overexpression rescues nbl mutants from embryonic lethality.
Fig. 5.
hsp60 rescues nbl.(A) nbl embryos raised at 33°C develop pericardial effusion and die at 5 dpf. Embryos from homozygous nbl crosses were injected with 1 nl of 200 ng/μl mRNA (hsp60WT, hsp60V324E, or enhanced GFP) and then transferred to 33°C. Blue arrows mark pericardial effusion; red arrow shows normal cardiac anatomy. Note that hsp60WT mRNA rescues the pericardial effusion phenotype. (B) nbl embryos were scored for the presence of pericardial effusion and ability to swim at 5 dpf. Hsp60WT mRNA rescues the nbl phenotype. (C) WT embryos injected with morpholino against hsp60 show pericardial effusion at 3 dpf at 28.5°C. Blue arrows mark pericardial effusion. These results indicate that hsp60 dysfunction, and not heat shock, caused the nbl phenotype.
To determine whether the embryonic pericardial effusion resulted specifically from hsp60 dysfunction rather than heat shock, we injected WT embryos with hsp60 morpholinos. Most (74%; 189/255) of these embryos showed pericardial effusion at 28.5°C (Fig. 5C). These data indicate that Hsp60 dysfunction, and not heat shock, caused the nbl phenotype. Taken together, our linkage, mutation, expression, mRNA rescue and morpholino data all indicate that hsp60 is the nbl gene.
Functional Consequences of the nbl Mutation. Hsp60 is a molecular chaperone that prevents misfolding and promotes refolding of nonnative proteins (15). This protein is a member of the highly conserved family of molecular chaperones which includes GroEL, the E. coli homolog of Hsp60. Hsp60 depends upon its cochaperone, Hsp10 (GroES in E. coli). Before GroEL binds ATP and GroES, the nonnative protein is held simultaneously by multiple GroEL apical domains (Fig. 4D; light blue).
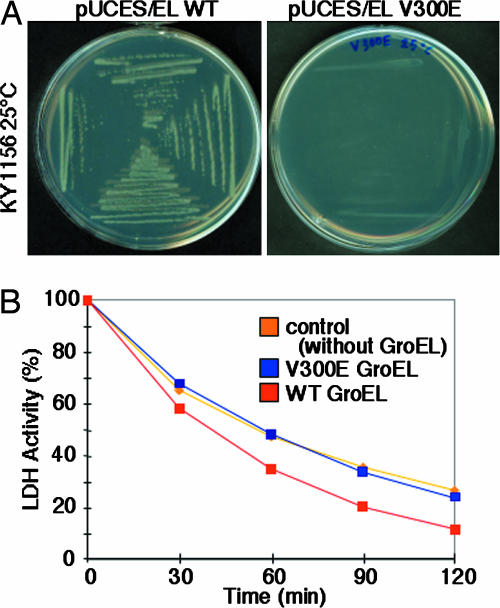
To determine whether Hsp60 function was affected by the V324E mutation, we evaluated the analogous mutation (V300E) in GroEL in a bacterial growth assay using E. coli strain KY1156, which requires functional GroEL for growth (26). We transformed KY1156 cells with a plasmid that produces WT GroES and GroEL V300E. As a control, we also transformed KY1156 cells with WT GroES and WT GroEL. At 25°C, growth of the mutant transformed colonies was extremely slow (Fig. 6A), suggesting that insufficient quantities of functional GroEL protein was present. These data indicate that at 25°C, GroEL V300E was not able to function as a replacement for WT GroEL. These data suggest that GroEL V300E is a loss-of-function mutation.
Fig. 6.
GroEL V300E causes reduced chaperone function. (A) The E. coli strain KY1156 (26) requires functional GroEL for growth. GroEL V300E is analogous to Hsp60 V324E. KY1156 transfected with GroEL V300E decreased growth at 25°C, suggesting this mutation reduces GroEL function. (B) GroEL V300E shows decreased affinity for nonnative form of LDH. LDH activity was measured after addition of nonpermissive solution (without ATP at 42°C) at time 0 in the presence of mutant (blue) or WT GroEL (red). Negative control was without GroEL (orange).
To perform chaperone function, GroEL must bind nonnative proteins. Lactate dehydrogenase (LDH) activity was measured following addition of nonpermissive solution (without ATP at 42°C) at time 0 in the presence of mutant or WT GroEL. WT GroEL binds nonnative LDH, which changed the equilibrium between native and nonnative LDH in solution. This binding, in turn, drove the equilibrium to lower native and increased nonnative LDH, thus lowering LDH activity. GroEL V300E did not lower LDH activity, similar to the control (without GroEL). These data suggest that GroEL V300E has lower binding affinity for the nonnative form of LDH at 42°C (Fig. 6B).
Discussion
In this study, we used a genetic approach to study regeneration with a mutagenesis screen for zebrafish fin regeneration mutants. We identified the nbl mutant, which displayed a defect in blastema formation and maintenance. The nbl defect was caused by a mutation in hsp60. Evidence indicating that hsp60 is the nbl gene include: (i) genetic linkage of nbl to a 0.13-cM region containing hsp60, (ii) absence of mutations in other genes within the critical region, (iii) identification of a V324E substitution in a conserved amino acid in Hsp60, (iv) absence of this mutation in all common zebrafish strains, (v) rescue of nbl embryonic lethality by hsp60 mRNA, (vi) hsp60 expression consistent with the nbl phenotype, and (vii) hsp60 morpholino phenocopies the nbl embryonic phenotype.
Our data indicate that hsp60 expression is up-regulated in blastema cells during zebrafish fin regeneration, and that Hsp60 function is required for blastema formation and viability. In previous studies, we have demonstrated that distal blastema cells behave like stem cells (8). Perhaps Hsp60 has a unique role in pluripotent cells. Interestingly, other studies have demonstrated that stem cells exhibit increased Hsp expression (18). Additionally, during rat spermatogenesis, Hsp60 is expressed in spermatogonia and early spermatocytes but not in postmeiotic germ cells (19–21). Furthermore, knockdown of hsp60 in Caenorhabditis elegans showed a sterile phenotype (27), indicating that Hsp60 is required for gametogenesis. It is possible that blastema cells are under increased stress because of their unique niche and proximity to highly proliferative cells, which make them sensitive to Hsp60 dysfunction. Alternatively, blastema cells may have increased sensitivity to normal or even low levels of stress due to the importance of high fidelity in stem cells.
nbl mutants show an early and complete regeneration block in both fin and heart. It is difficult to conclude that these phenotypes are caused solely by reduced chaperone function. A possible explanation is that Hsp60 is the latest addition to the growing list of Toll-like receptor ligands (17, 28–30). It is tempting to consider Toll-like receptors as a general danger signal receptors (31) involved in tissue repair. Trauma triggers an immunogenic environment, and Hsp60 may play a vital role in these processes.
In summary, we have discovered that Hsp60 is required for blastema formation and maintenance during fin regeneration. These findings suggest that Hsp60 may play a role in undifferentiated cells.
Acknowledgments
We thank K. Poss and A. Nechiporuk for protocols, advice, and helpful suggestions; A. Hillam, L. Wilson, A. Sanchez, C. Richards, and O. Paugois for fish care; H. Mulhern for the EM; W. Zhu for genotyping; L. Zon, S. Odelberg, E. Kaji, I. Splawski, F. Engel, M. Schebesta, and K. Tseng for critique of the manuscript; and Keating laboratory members for helpful discussions. This work was supported by National Heart, Lung, and Blood Institute Grant SCCOR RFA HL 02-027; the Uehara Memorial Foundation; and the Donald W. Reynolds Foundation.
Abbreviations: Hsp, heat-shock protein; hpa, hours postamputation; dpf, days postfertilization; dpa, days postamputation; GroEL, phage growth λ E large; GroES, phage growth λ E small; LDH, lactate dehydrogenase.
References
- 1.Poss, K. D., Wilson, L. G. & Keating, M. T. (2002) Science 298, 2188-2190. [DOI] [PubMed] [Google Scholar]
- 2.Johnson, S. L. & Weston, J. A. (1995) Genetics 141, 1583-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhardt, R. R., Tongiorgi, E., Anzini, P. & Schachner, M. (1996) J. Comp. Neurol. 376, 253-264. [DOI] [PubMed] [Google Scholar]
- 4.Bereiter-Hahn, J. & Zylberberg, L. (1993) Comp. Biochem. Physiol. 105A, 625-641. [Google Scholar]
- 5.Becker, T., Wullimann, M. F., Becker, C. G., Bernhardt, R. R. & Schachner, M. (1997) J. Comp. Neurol. 377, 577-595. [DOI] [PubMed] [Google Scholar]
- 6.Nechiporuk, A., Poss, K. D., Johnson, S. L. & Keating, M. T. (2003) Dev. Biol. 258, 291-306. [DOI] [PubMed] [Google Scholar]
- 7.Poss, K. D., Nechiporuk, A., Hillam, A. M., Johnson, S. L. & Keating, M. T. (2002) Development (Cambridge, U.K.) 129, 5141-5149. [DOI] [PubMed] [Google Scholar]
- 8.Nechiporuk, A. & Keating, M. T. (2002) Development (Cambridge, U.K.) 129, 2607-2617. [DOI] [PubMed] [Google Scholar]
- 9.Vinarsky, V., Atkinson, D. L., Stevenson, T. J., Keating, M. T. & Odelberg, S. J. (2005) Dev. Biol. 279, 86-98. [DOI] [PubMed] [Google Scholar]
- 10.Odelberg, S. J., Kollhoff, A. & Keating, M. T. (2000) Cell 103, 1099-1109. [DOI] [PubMed] [Google Scholar]
- 11.Akimenko, M. A., Johnson, S. L., Westerfield, M. & Ekker, M. (1995) Development (Cambridge, U.K.) 121, 347-357. [DOI] [PubMed] [Google Scholar]
- 12.Ashburner, M. & Bonner, J. J. (1979) Cell 17, 241-254. [DOI] [PubMed] [Google Scholar]
- 13.Rye, H. S., Roseman, A. M., Chen, S., Furtak, K., Fenton, W. A., Saibil, H. R. & Horwich, A. L. (1999) Cell 97, 325-338. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, M. Y., Hartl, F. U., Martin, J., Pollock, R. A., Kalousek, F., Neupert, W., Hallberg, E. M., Hallberg, R. L. & Horwich, A. L. (1989) Nature 337, 620-625. [DOI] [PubMed] [Google Scholar]
- 15.Martin, J., Horwich, A. L. & Hartl, F. U. (1992) Science 258, 995-998. [DOI] [PubMed] [Google Scholar]
- 16.Soltys, B. J. & Gupta, R. S. (1996) Exp. Cell Res. 222, 16-27. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi, K., Burkart, V., Flohe, S. & Kolb, H. (2000) J. Immunol. 164, 558-561. [DOI] [PubMed] [Google Scholar]
- 18.Ramalho-Santos, M., Yoon, S., Matsuzaki, Y., Mulligan, R. C. & Melton, D. A. (2002) Science 298, 597-600. [DOI] [PubMed] [Google Scholar]
- 19.Paranko, J., Seitz, J. & Meinhardt, A. (1996) Differentiation 60, 159-167. [DOI] [PubMed] [Google Scholar]
- 20.Sarge, K. D. & Cullen, K. E. (1997) Cell Mol. Life Sci. 53, 191-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werner, A., Meinhardt, A., Seitz, J. & Bergmann, M. (1997) Cell Tissue Res. 288, 539-544. [DOI] [PubMed] [Google Scholar]
- 22.Poss, K. D., Shen, J., Nechiporuk, A., McMahon, G., Thisse, B., Thisse, C. & Keating, M. T. (2000) Dev. Biol. 222, 347-358. [DOI] [PubMed] [Google Scholar]
- 23.Turner, D. L. & Weintraub, H. (1994) Genes Dev. 8, 1434-1447. [DOI] [PubMed] [Google Scholar]
- 24.Kawata, Y., Kawagoe, M., Hongo, K., Miyazaki, T., Higurashi, T., Mizobata, T. & Nagai, J. (1999) Biochemistry 38, 15731-15740. [DOI] [PubMed] [Google Scholar]
- 25.Kawata, Y., Nosaka, K., Hongo, K., Mizobata, T. & Nagai, J. (1994) FEBS Lett 345, 229-232. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki, T., Yoshimi, T., Furutsu, Y., Hongo, K., Mizobata, T., Kanemori, M. & Kawata, Y. (2002) J. Biol. Chem. 277, 50621-50628. [DOI] [PubMed] [Google Scholar]
- 27.Rual, J. F., Ceron, J., Koreth, J., Hao, T., Nicot, A. S., Hirozane-Kishikawa, T., Vandenhaute, J., Orkin, S. H., Hill, D. E., van den Heuvel, S., et al. (2004) Genome Res. 14, 2162-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. M. & Hoffmann, J. A. (1996) Cell 86, 973-983. [DOI] [PubMed] [Google Scholar]
- 29.Vabulas, R. M., Ahmad-Nejad, P., da Costa, C., Miethke, T., Kirschning, C. J., Hacker, H. & Wagner, H. (2001) J. Biol. Chem. 276, 31332-31339. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A., Jr. (1997) Nature 388, 394-397. [DOI] [PubMed] [Google Scholar]
- 31.Matzinger, P. (2002) Science 296, 301-305. [DOI] [PubMed] [Google Scholar]
- 32.Koradi, R., Billeter, M. & Wuthrich, K. (1996) J. Mol. Graphics 14, 29-32, 51-55. [DOI] [PubMed] [Google Scholar]
- 33.Xu, Z., Horwich, A. L. & Sigler, P. B. (1997) Nature 388, 741-750. [DOI] [PubMed] [Google Scholar]