Abstract
We have cloned and characterized a new member of the voltage-dependent Ca2+ channel γ subunit family, with a novel gene structure and striking properties. Unlike the genes of other potential γ subunits identified by their homology to the stargazin gene, CACNG7 is a five-, and not four-exon gene whose mRNA encodes a protein we have designated γ7. Expression of human γ7 has been localized specifically to brain. N-type current through CaV2.2 channels was almost abolished when co-expressed transiently with γ7 in either Xenopus oocytes or COS-7 cells. Furthermore, immunocytochemistry and western blots show that γ7 has this effect by causing a large reduction in expression of CaV2.2 rather than by interfering with trafficking or biophysical properties of the channel. No effect of transiently expressed γ7 was observed on pre-existing endogenous N-type calcium channels in sympathetic neurones. Low homology to the stargazin-like γ subunits, different gene structure and the unique functional properties of γ7 imply that it represents a distinct subdivision of the family of proteins identified by their structural and sequence homology to stargazin.
Keywords: calcium channel/expression/γ subunit/stargazin/suppression
Introduction
Voltage-dependent calcium channels (VDCCs) play a fundamental role in the coupling of membrane depolarization to many cellular processes by regulating cytoplasmic Ca2+ concentration in excitable cells. They are hetero-multimers consisting of a pore-forming α1 subunit assembled with auxiliary β, α2δ and possibly γ subunits. These subunits can be encoded by several different genes with alternative splice variants and are expressed in a tissue-specific manner. Much work has concentrated on the characterization of functional properties of the α1, β and α2δ subunits (Birnbaumer et al., 1998; Dolphin, 1998; Jones, 1998; Perez-Reyes, 1998; Catterall, 2000). However, research concerning the role of the γ subunit has not been as extensive.
Until recent years, only a single gene, exclusively expressed in skeletal muscle, was believed to encode a VDCC γ subunit (Jay et al., 1990; Powers et al., 1993). Recordings of Ca2+ currents from dihydropyridine receptors (DHPRs) of skeletal myotubes from mice lacking this γ1 subunit suggest that its role is to limit calcium entry through these channels, increase the rate at which the channels inactivate and hyperpolarize the half-maximal potential for the voltage dependence of steady-state inactivation (Freise et al., 2000; Ahern et al., 2001). Subsequently, a second putative VDCC γ subunit, γ2, was identified based on its structural similarity to γ1, despite having only weak protein sequence identity (25%) (Letts et al., 1998). Mutations in the γ2 gene, cacng2, were found to underlie the absence epilepsy phenotype of the allelic stargazer (stg) and waggler (wag) mutant mice. Subsequent studies have identified six further putative γ subunits (γ3–γ8), not all of which have been cloned and expressed (Black and Lennon, 1999; Burgess et al., 1999, 2001; Klugbauer et al., 2000).
The γ2, γ3 and γ4 subunits form a subfamily exclusively localized to the central nervous system (CNS) (Letts et al., 1998; Klugbauer et al., 2000) whose interaction with VDCCs has been investigated in several studies (Letts et al., 1998; Klugbauer et al., 2000; Kang et al., 2001; Sharp et al., 2001). The γ5 and γ7 subunits (Burgess et al., 1999, 2001) are predicted to represent another subfamily of stargazin-related proteins, with extremely low sequence identity to γ1 and ∼25% identity to γ2. These subunits, like other members of the putative γ subunit superfamily, are proteins predicted to have four transmembrane segments with intracellular N- and C-termini, and were reported to be encoded by a gene assembled from four exons (Burgess et al., 2001). However, assembly of the full-length γ5 and γ7 cDNAs has not been described and there are no functional data for either of these γ subunits.
In the present study, we report the identification, cloning and functional characterization of a novel protein we have named the γ7 subunit. The first four exons of the gene encoding this protein are identical to those encoding the predicted γ7 subunit gene previously described by Burgess et al. (2001), but the transcription and translation of a final fifth exon results in the γ7 described in the present study having a very different and much longer C-terminus. Our results show that the co-expression of the γ7 subunit almost abolishes the functional expression and markedly suppresses the level of CaV2.2 subunit protein. We also report the identification of the γ5 subunit which, like γ7, is predicted to be encoded by a five-exon gene.
Results
Cloning of the γ5 and γ7 genes
The full-length mouse stargazin sequence (Letts et al., 1998) was used as a query sequence to search the DNA databases. A short 487 bp sequence was assembled from three expressed sequence tags (ESTs) and was found to contain an open reading frame (ORF) with 26% identity to stargazin, although it contained no in-frame start or stop codons.
A 487 bp fragment corresponding to this in silico sequence was amplified from human whole brain cDNA, and sequence analysis confirmed the computer predictions. Primers specific for each end of this fragment were used to amplify the missing parts of the ORF using 5′ and 3′ RACE. A 330 bp fragment containing the missing 5′ sequence and start ATG codon, and a 400 bp band containing the missing 3′ sequence and stop codon were obtained. These three DNA fragments were then used to assemble the full-length 828 bp stargazin-like ORF in a single ‘splice-overlap’ PCR.
When this full-length sequence was used to search the human high-throughput genomic sequences (HTGS) using the BLASTn algorithm, a bacterial artificial chromosome (BAC), clone AC008440, derived from human chromosome 19 was identified. Analysis of this BAC using the gene prediction program Genscan (Burge and Karlin, 1997) predicts that the 828 bp ORF is assembled from five exons and encodes a 275 amino acid protein.
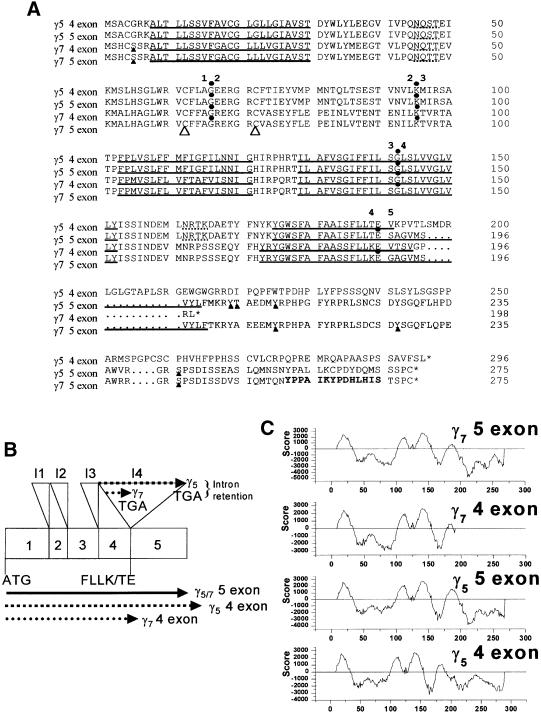
When compared with all of the previously published γ subunits, this 828 bp cDNA clone exhibited 100% identity to the previously predicted human γ7 subunit over the first four exons (Burgess et al., 2001). However, the sequence of the cDNA clone described here diverges from the γ7 sequence (Burgess et al., 2001), due to the presence of the fifth exon (Figure 1A and B). It would therefore appear that CACNG7 is in fact a five-exon gene that encodes the γ7 subunit, and the previously predicted four-exon γ7 sequence results from read-through into intron four (Figure 1B).
Fig. 1. Protein sequences, proposed splicing mechanism and hydropathy plots of a family of low homology stargazin-related genes. (A) Alignment of the five-exon γ5 and γ7 subunit sequences with the previously predicted four-exon γ5 and γ7 subunits using the Clustal_W algorithm. Dotted lines indicate consensus N-glycosylation sites and solid triangles beneath residues mark consensus sites for phosphorylation by cAMP- and cGMP-dependent protein kinase, protein kinase C, casein kinase II or tyrosine kinase. The exon–intron boundaries are marked by solid dots above the residue whose codon is interrupted by the adjacent intron. Note that the sequence identity between γ5 and γ7 is 80% conserved throughout the additional fifth exon, whereas the predicted four-exon γ5 and γ7 subunits differ greatly in sequence identities and length. The two large open triangles designate a pair of cysteine residues that are conserved amongst the putative VDCC γ subunits and may be involved in the formation of disulfide bridges. The transmembrane-spanning segments, as predicted by the TMpred program (Hofmann and Stoffel, 1993), are indicated by solid underlining. The residues highlighted in bold in the C-terminus of γ7 identify the epitope for the anti-γ7 antibody. (B) A schematic diagram of the γ5 and γ7 gene structure. The full-length genes are encoded by five exons interrupted by four introns, I1–I4. If the previously predicted four-exon γ5 and γ7 subunits were expressed, the extent of predicted read-through into intron 4 is displayed above the gene structure. The start codon, terminal amino acids of exon 4, and both normally spliced and intron-retained γ5 or γ7 are shown below the gene structure. (C) Hydropathy plots of the five-exon γ5 and γ7 subunits predicted by the TMpred program (Hofmann and Stoffel, 1993) compared with the previously predicted four-exon γ5 and γ7. Amino acid position is shown on the x-axis, and positive TMpred values indicate putative membrane-spanning regions. γ7 and γ5 are predicted to have four transmembrane-spanning α-helices with intracellular N- and C-termini.
BLAST searches of mouse HTGS identified cacng7, the mouse orthologue of the γ7 gene within BAC AC079557, which, like its human counterpart, contained five exons. Confirmation that the mouse γ7 mRNA is expressed was obtained by amplifying the complete cDNA in a single RT–PCR from mouse cerebellar total RNA. The mouse orthologue possesses 70% identity to the human γ7 at the nucleic acid level and, remarkably, 100% identity at the protein level.
BLASTn searches and Genscan analysis of human chromosome 17 BAC AC005988 identified another related gene encoding a 275 amino acid protein. This gene also possessed five exons and predicted a protein with 70.5% amino acid identity compared with the human γ7 and 27% identity compared with human γ2. It exhibited 100% identity to the previously described human γ5 subunit (Burgess et al., 1999) over its first 190 amino acids but, like the γ7 subunit, the additional fifth exon encodes an alternative C-terminus. Unlike the previously predicted four-exon γ5 and γ7, the C-termini of the five-exon γ5 and γ7 share considerable identity (80%, Figure 1A). We therefore named this subunit γ5 (gene CACNG5) and subsequently identified the mouse orthologue on mouse chromosome 16 BAC AC079424. The mouse orthologue (cacng5) exhibited 89.5% identity at the nucleotide level and 97% identity at the protein level to the human sequence.
Hydropathy plots predict that, like all the stargazin-related proteins, γ7 and γ5 have four transmembrane-spanning α-helices with predicted intracellular N- and C-termini (Figure 1C). The final transmembrane-spanning α-helices of γ7 and γ5 are predicted to be six and eight amino acids longer, respectively, than their equivalents in the previously predicted four-exon γ7 or γ5, and the full-length γ7 has a much more substantial cytosolic C-terminus than in the predicted four-exon γ7, in which only four intracellular amino acids are predicted after the final transmembrane segment.
The sequences have been deposited in the DDBJ/EMBL/GenBank database with the following accession Nos: human γ7 (AF458897), human γ5 (AF458898), mouse γ7 (AF458899) and mouse γ5 (AF458900).
Tissue distribution
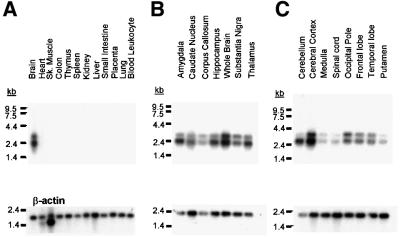
The tissue distribution of the novel γ7 mRNA was analysed by northern blot. Figure 2 shows a human multiple tissue northern blot (Figure 2A) and two brain region blots (Figure 2B and C) hybridized with a probe corresponding to nucleotides 576–763 of the γ7 ORF. This region was chosen because it contains the least identity when compared with other γ-subunits (53% to human γ2), and is unique to γ7. Thus, this probe will not detect the predicted four-exon γ7, should this be expressed. This γ7-specific probe reveals two transcripts of ∼2.4 and 3.0 kb, both of which are expressed only in brain. Both of these transcripts are expressed in all brain regions probed, although the shorter transcript is expressed at greater levels in several areas including cerebellum, amygdala, hippocampus and thalamus. These blots were stripped and re-probed using the same probe as that designed to detect the four-exon γ7 (Burgess et al., 2001). This probe, corresponding to nucleotides 80–482 of the γ7 ORF, would detect both the predicted four-exon γ7 and full-length γ7 transcripts. No additional transcripts were seen in any of the blots with this probe. Indeed, precisely the same expression profile was seen as with the full-length γ7-specific probe, including the same differential expression of the short and long transcripts seen in cerebellum, amygdala, hippocampus and thalamus (data not shown).
Fig. 2. The expression profile of the human γ7 subunit. (A) Multiple tissue northern blots probed specifically for the γ7 subunit show two mRNA species of ∼2.4 and 3.0 kb that are localized specifically to human brain. Multiple brain region blots (B and C) show that the γ7 subunit is expressed in all the individual brain regions probed. The bottom section of each panel displays the mRNA detected by the control β-actin probe for each blot.
Influence of the γ7 subunit on heterologous expression of VDCCs and KV3.1b
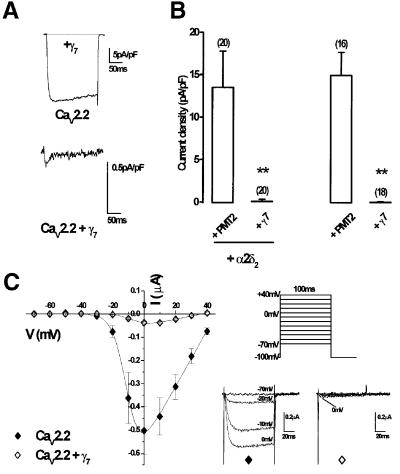
Having investigated the tissue distribution of the γ7 subunit in human brain, we next examined the effect of expression of this protein on Ba2+ currents recorded from neuronal VDCCs expressed in COS-7 cells. For comparison with immunocytochemistry data (see below), we transfected an N-terminal green fluorescent protein-tagged CaV2.2 construct (GFP–CaV2.2), previously shown to have no significant differences in its biophysical properties compared with the non-tagged channel (Raghib et al., 2001). These experiments revealed an almost total abolition of whole-cell GFP–CaV2.2 Ba2+ current (IBa) in cells co-transfected with γ7 (Figure 3A, upper trace). In 1 mM Ba2+, mean current density of cells expressing GFP–CaV2.2/β1b/α2δ2 was –13.5 ± 4.3 pA/pF at 0 mV (n = 20) (Figure 3B) but, even when extracellular [Ba2+] was increased to 10 mM, currents from GFP–CaV2.2/β1b/α2δ2/γ7-transfected COS-7 cells remained extremely small (–0.23 ± 0.08 pA/pF at 0 mV, n = 20, P <0.01) (Figure 3A, lower trace, and B). In a recent report, it was stated that inhibition of CaV2.2 currents by the γ2 subunit was dependent upon co-expression of an α2δ subunit (Kang et al., 2001). To investigate if the same is true of the much more robust suppressive effect of γ7, recordings were made from cells in the absence of co-transfected α2δ2 subunit. The histograms in Figure 3B show that the influence of the γ7 subunit on CaV2.2/β1b currents was independent of co-expression of an α2δ subunit (CaV2.2/β1b, –14.9 ± 2.7 pA/pF at 0 mV in 1 mM Ba2+, n = 16; CaV2.2/β1b/γ7, –0.02 ± 0.03 pA/pF at 0 mV in 10 mM Ba2+; n = 18, P <0.01).
Fig. 3. GFP–CaV2.2/β1b cDNAs were transiently transfected into COS-7 cells with or without α2δ2 and γ7 subunits. (A) Example traces elicited by a 200 ms step depolarization to +10 mV from a holding potential of –80 mV in the presence of 1 mM Ba2+ (upper panel). In the presence of γ7, extracellular Ba2+ solution was also increased to 10 mM (lower panel). (B) Histogram of mean current densities measured at +10 mV in 1 mM Ba2+ for controls and 10 mM Ba2+ in the presence of γ7. Co-expression of γ7 abolished currents in both the presence and absence of the α2δ2 subunit. The number of experiments (n) for each condition is given in parentheses above the columns, and data from all cells tested are included (**P <0.01, Student’s t-test). (C) Peak I–V relationships and individual representative traces for CaV2.2 (solid diamonds, n = 26) and CaV2.2 + γ7 (open diamonds, n = 24), expressed in Xenopus oocytes with the β1b auxiliary subunit were determined by measuring peak Ba2+ current amplitudes recorded during 100 ms test pulses between –70 and +40 mV (holding potential –100 mV; +10 mV increments; [Ba2+] in extracellular medium: 5 mM).
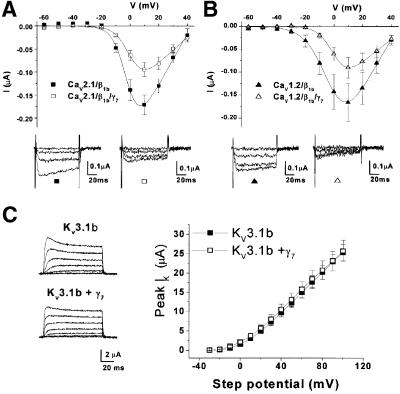
To examine whether these effects were peculiar to transfection in mammalian expression systems, we next looked at the effect of γ7 on CaV2.2 currents expressed in Xenopus oocytes, where there cannot be any question as to whether all cDNAs are present in each cell. Figure 3C shows recordings made in 5 mM Ba2+ from Xenopus oocytes expressing CaV2.2/β1b either with or without co-expression of γ7. The maximum conductance (Gmax), determined from the current–voltage (I–V) plots, was dramatically and significantly reduced when the human γ7 subunit was co-expressed compared with oocytes where it was not, with a corresponding 92.6% reduction in peak current amplitude at 0 mV (Figure 3C and Table I). The half-maximal value for the voltage dependence of activation (V50) was also shifted 3.4 mV more depolarized upon co-expression of γ7. An almost identical 94% inhibition of CaV2.2/β1b/α2δ2 currents was observed by γ7 in this system (data not shown). To examine whether the residual current represents the current induced by auxiliary subunits in Xenopus oocytes (Lacerda et al., 1994), we examined currents from oocytes transfected with only the auxiliary subunit β1b. However, we observed that these currents were also reduced from –28.1 ± 9.6 nA (n = 10) to –8.3 ± 5.3 nA (n = 9) upon co-expression of γ7. We next investigated the effects of γ7 on other CaVα1 subtypes. The γ7 subunit also significantly reduced the Gmax of CaV2.1/β1b and CaV1.2/β1b VDCCs by 48 and 52%, respectively (Figure 4A and B, Table I). There were no significant effects of γ7 on their voltage dependence of activation (Table I). Thus, the effect of γ7 on these channels is not as striking as that seen with CaV2.2.
Table I. Influence of the γ7 subunit on the activation properties of VDCCs expressed in Xenopus oocytes.
| Channel | Activation |
|
n | ||
|---|---|---|---|---|---|
| V50 (mV) | k | Gmax (µS) | Peak IBa (µA) | ||
| CaV2.2 | –7.34 ± 0.79 | 4.34 ± 0.24 | 12.7 ± 2.29 | –0.50 ± 0.10 | 26 |
| CaV2.2 + γ7 | –3.88 ± 0.92** | 4.66 ± 0.40 | 1.50 ± 0.27*** | –0.04 ± 0.01*** | 24 |
| CaV2.1 | –0.63 ± 0.73 | 5.03 ± 0.24 | 5.76 ± 0.95 | –0.17 ± 0.02 | 17 |
| CaV2.1 + γ7 | 0.92 ± 0.68 | 4.71 ± 0.41 | 2.98 ± 0.46** | –0.09 ± 0.02** | 19 |
| CaV1.2 | –0.68 ± 2.08 | 6.80 ± 0.31 | 5.88 ± 1.40 | –0.17 ± 0.04 | 10 |
| CaV1.2 + γ7 | 1.75 ± 0.70 | 5.68 ± 0.59 | 2.84 ± 0.57* | –0.10 ± 0.02 | 14 |
Data are expressed as mean ± SEM of the number of replicates, n. *P <0.05, **P <0.01 and ***P <0.001 according to an unpaired Student’s t-test. The peak IBa was at +10 mV for CaV2.1 and CaV1.2 and at 0 mV for CaV2.2.
Fig. 4. Effect of heterologous expression of γ7 with other channels. (A and B) Mean I–V relationships and individual representative traces for (A) CaV2.1 (solid squares, n = 17) and CaV2.1 + γ7 (open squares, n = 19); (B) CaV1.2 (solid triangles, n = 10) and CaV1.2 + γ7 (open triangles, n = 15), expressed in Xenopus oocytes with β1b. Peak Ba2+ current amplitudes were recorded during 100 ms test pulses between –70 and +40 mV (holding potential –100 mV; +10 mV increments; [Ba2+] 10 mM). (C) Peak I–V relationship for KV3.1b (solid squares, n = 10), and KV3.1 + γ7 (open squares, n = 10), and current traces for KV3.1 (top left panel) and KV3.1 + γ7 (bottom left panel) expressed in Xenopus oocytes. Holding potential was –100 mV, and steps were between –30 and +100 mV for 100 ms. The scale bar refers to both panels.
Together with the data generated in COS-7 cells, the near complete abolition of CaV2.2 current seen in Xenopus oocytes upon co-expression of the γ7 subunit suggests that it may be affecting the expression of these channels rather than altering their biophysical properties. However, since the effect of γ7 on the Ba2+ currents through CaV2.2 VDCCs expressed in COS-7 cells or Xenopus oocytes was so striking, we investigated the possibility that it down-regulates other heterologously expressed ion channels by co-expressing it with the Shaw-like voltage-dependent potassium channel, KV3.1b. Figure 4C shows representative traces and the peak I–V relationship of KV3.1b currents expressed in Xenopus oocytes alone and co-expressed with the γ7 subunit, which indicate that the γ7 subunit had no effect on the peak current amplitude of heterologously expressed KV3.1b channels.
Influence of the γ7 subunit on endogenous Ca2+ currents recorded from cultured sympathetic neurones
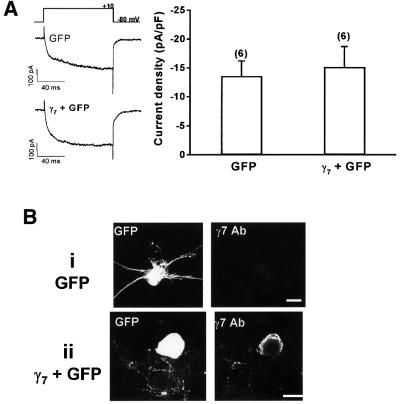
We investigated the electrophysiological consequence of the acute expression of γ7 upon the endogenous VDCC currents from sympathetic neurones of the rat superior cervical ganglion (SCG). These neurones express 80–90% N-type and 10% L-type current (Plummer et al., 1989; Delmas et al., 1998) and provided a vehicle in which we could determine if the γ7 subunit could exert its influence upon pre-existing Ca2+ channels. Whole-cell patch-clamp recordings, performed 36–48 h post-isolation and transfection from control sympathetic neurones transfected with the GFP marker, gave a mean IBa current density at 0 mV of –13.6 ± 2.6 pA/pF (n = 6). However, this was not significantly altered in neurones transfected with GFP and the γ7 subunit (–15.2 ± 3.5 pA/pF, n = 6) (Figure 5A). Expression of γ7 protein in γ7-transfected neurones was verified by immunocytochemistry (Figure 5B), which also demonstrated the presence of only a very low level of immunoreactivity for endogenous γ7 in neurones transfected with GFP alone (Figure 5B). Although the morphology of sympathetic neurones cultured for 36–48 h is quite variable, in a preliminary observation, we also noted an alteration in neurite morphology in sympathetic neurones transfected with γ7 (Figure 5B), but this will require further detailed examination in a future study. It would therefore appear that the γ7 subunit is unable to acutely influence the properties of pre-existing N-type VDCCs in these neurones, and that γ7 does not co-exist endogenously in SCG neurones with N-type channels.
Fig. 5. Effect of heterologous expression of γ7 in cultured sympathetic neurones. (A) Left: example traces from sympathetic neurones transiently transfected with GFP or GFP and the γ7 subunit, elicited by a 100 ms step depolarization to +10 mV from a holding potential of –80 mV in medium containing 10 mM Ba2+. Right: histogram of mean current densities measured from sympathetic neurones at +10 mV. The number of experiments (n) for each condition is given in parentheses above the columns. (B) Upper row: a GFP-transfected sympathetic neurone (left panel), showing lack of immunostaining for endogenous γ7 (right panel). Lower row: a GFP- plus γ7-transfected sympathetic neurone (left panel), showing immunostaining for γ7 (middle panel). Data are representative of three transfections. Scale bars = 15 µm.
Immunocytochemical analysis of the effects of the γ7 subunit
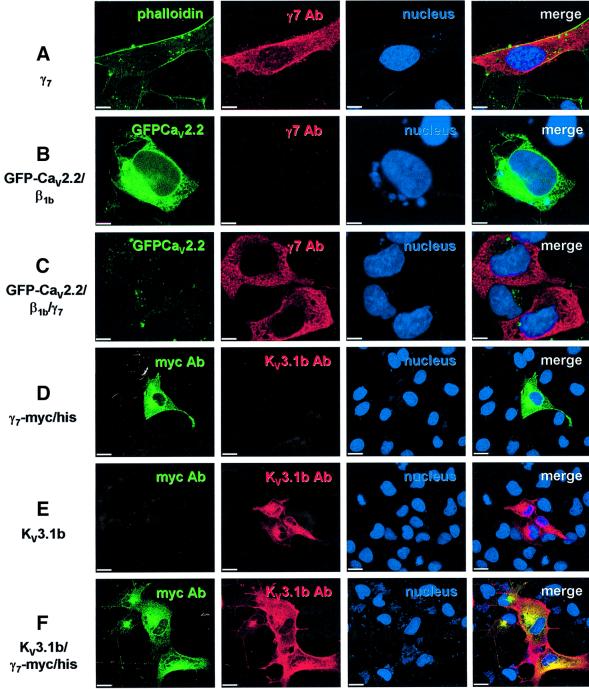
The subcellular distribution of the expressed γ7 subunit and its effects upon the expression of CaV2.2 subunits were determined using immunocytochemistry and confocal laser scanning microscopy. When the γ7 subunit alone was transiently transfected into COS-7 cells (Figure 6A), expression of γ7 protein was observed throughout the cytoplasm and not specifically associated with the plasma membrane (delineated by Oregon Green–phalloidin). Figure 6B shows the typical fluorescence pattern of a cell transiently transfected with GFP–CaV2.2/β1b. Figure 6C shows two cells where the γ7 subunit has also been co-transfected. A striking reduction in the fluorescence of GFP–CaV2.2 was observed upon co-expression of the γ7 subunit, whilst the γ7 levels remained comparable with those in cells transfected with the γ7 subunit alone.
Fig. 6. Co-expression of γ7 with GFP–CaV2.2 almost abolishes GFP fluorescence in COS-7 cells. Cells were transfected with (A) the γ7 subunit alone, (B) GFP–CaV2.2/β1b, (C) GFP–CaV2.2/β1b/γ7, (D) γ7-myc/his, (E) KV3.1b or (F) KV3.1b/γ7-myc/his. The panels in column 1 show staining with Oregon Green–phalloidin, GFP fluorescence or FITC-labelled myc Ab as stated; the panels in column 2 show Texas red staining for the γ7 subunit or KV3.1b subunit as stated; the panels in column 3 display the blue DAPI staining of the nucleus; and the panels in column 4 show the merged images. The scale bar in (A–C) represents 10 µm and in (D–F), 20 µm.
Immunocytochemical methods also confirmed the earlier electrophysiological findings that the γ7 subunit does not adversely affect the expression of the KV3.1b subunit. Figure 6D displays the expression of a γ7-myc/his fusion protein expressed alone in a COS-7 cell. The expression pattern of this construct is similar to that of the untagged γ7 (Figure 6A). Shown in Figure 6E is the expression of KV3.1b when transfected alone. The distribution and the expression level of KV3.1b were unaltered by the co-expression of the γ7-myc/his fusion protein (Figure 6F).
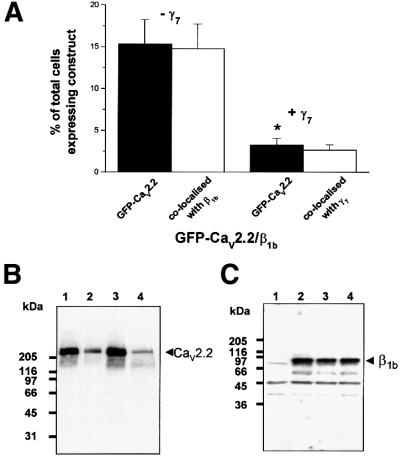
In transient transfections, co-transfected subunits are not always co-expressed in every cell. We therefore sought to quantify the extent of the reduction in observed GFP–CaV2.2 fluorescence caused by γ7 subunit co-expression. This was measured by counting the number of GFP-positive cells in both the GFP–CaV2.2/β1b- and GFP–CaV2.2/β1b/γ7-transfected cultures. The mean percentage of the total cells per 16 mm2 field of view with GFP–CaV2.2 fluorescence, which entirely co-localizes with β1b expression, was reduced from 16.4 ± 3.4% in GFP–CaV2.2/β1b-transfected cells to 3.3 ± 0.7% in cells additionally transfected with the γ7 subunit (Figure 7A). The same histogram also shows that almost all cells that exhibited GFP–CaV2.2 fluorescence in the CaV2.2/β1b/γ7 transfections were also labelled for γ7 (3.0 ± 0.7%), implying that there is some residual GFP–CaV2.2 fluorescence in some cells expressing the γ7 subunit. The specificity of the γ7 antibody (Ab) was determined by western blotting where it was observed to label a major band at ∼35 kDa when γ7 was expressed in COS-7 cells (data not shown).
Fig. 7. The effect of co-expression of γ7 subunit on the expression of CaV2.2 in COS-7 cells. (A) Histogram of the mean percentage of total cells per field of view (±SEM) expressing GFP–CaV2.2 and those which are co-localized with β1b or γ7 (visualized with Texas red), following transfection with the subunit combinations stated. (B) Western blotting and immunodetection using anti-rabbit brain CaV2.2 II–III loop Ab. Lane 1, GFP–CaV2.2; lane 2, GFP–CaV2.2 and γ7; lane 3, CaV2.2 Δ3′UTR; lane 4, CaV2.2 Δ3′UTR and γ7. The positions of molecular weight markers are shown on the left. Blots are representative of three similar experiments. (C) Western blotting and immunodetection with anti-β1b Ab. Lane 1, untransfected COS-7 cells; lane 2, GFP–CaV2.2/β1b; lane 3, GFP–CaV2.2/β1b/γ7; lane 4, GFP–CaV2.2/β1b/γ7-myc/his.
Effect of co-expression of the γ7 subunit on the CaV2.2 protein levels
GFP–CaV2.2 or untagged CaV2.2 expressed in COS-7-cells are detectable by western blot using an Ab against the II–III loop of CaV2.2 (band at ∼240 kDa). When the γ7 subunit was co-expressed with either the GFP–CaV2.2 or untagged CaV2.2, the intensity of the CaV2.2 band was greatly reduced, but expression of the protein was not completely abolished (Figure 7B). No smaller molecular weight bands were observed that might represent degradation products of the GFP–CaV2.2 or untagged CaV2.2 or partially synthesized protein. Similar results were obtained both in the absence and presence of the auxiliary β1b subunit, and also using the anti-GFP Ab (data not shown). Additionally, the co-expression of γ7 or γ7-myc/his with CaV2.2/β1b did not reduce the expression of the β1b subunit (Figure 7C). These data extend the findings from confocal imaging and electrophysiology experiments by revealing that the γ7 subunit suppresses the expression of GFP– CaV2.2 protein rather than interfering with the correct conformational folding or trafficking of the GFP-labelled protein that could result in reduced fluorescence or recorded current densities.
Discussion
We have described the identification of two genes that encode putative VDCC subunits γ7 and γ5, by their sequence and structural homology to the mouse stargazin gene (cacng2), and have cloned and expressed the cDNA for both human and mouse γ7. The γ7 subunit is predicted to be a 275 amino acid, 31 kDa protein possessing four transmembrane-spanning domains with intracellular N- and C-termini. The in silico sequence for γ5 also predicts a 275 amino acid, 31 kDa protein with identical transmembrane topology to γ7. Their overall size is much closer to that predicted for the skeletal muscle γ1 subunit than for the neuronal γ2, γ3 and γ4 subunits. Their sequence homology with the γ1 subunit is extremely low, but both the γ7 and γ5 subunits possess ∼25% identity to the human γ2 subunit, with higher degrees of conservation occurring in the predicted transmembrane regions (up to 36%). It must also be noted that the C-termini of the γ7 and γ5 subunits differ from those of γ2, γ3 and γ4 subunits, as they are much shorter and lack the classical consensus motif for interaction with PDZ domains (Kornau et al., 1997; Tomita et al., 2001). This C-terminal motif of γ2 (also present in γ3 and γ4 subunits) has been shown to play a crucial role in trafficking AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate) receptor subunits to their correct location in the postsynaptic density (Chen et al., 2000). This difference suggests that γ7 and γ5 may have a different function from that of the γ2, γ3 and γ4 subunits.
The γ7 and γ5 subunits are, however, 70.5% identical to one another and, unlike all other putative γ subunits, are assembled from five exons, the first four of which are identical to exons 1–4 of the genes encoding the previously predicted γ7 and γ5 subunits (Burgess et al., 1999, 2001). Sequence analysis of the BAC clones in which these genes were found suggests that the previously predicted four-exon γ7 and γ5 subunit sequences result from inclusion of intronic sequences at the exon 4–intron 4 boundary of CACNG7 and CACNG5 (Figure 1B). Inspection of the sequence of intron 4 of CACNG7 and CACNG5 reveals that in-frame stop codons are found 25 and 319 nucleotides, respectively, downstream of the exon 4–intron 4 boundary. The final eight C-terminal amino acids of the predicted four-exon γ7 are encoded by the first 24 bases of intron 4 before a stop codon. Similarly, the C-terminal 106 amino acids of the predicted four-exon γ5 would be encoded by the entirety of intron 4, resulting in a C-terminal γ5 sequence which has extremely low sequence homology to the other γ subunits.
The sequence of the corresponding mouse orthologues of the predicted human four-exon γ7 and γ5 can be deduced by analysis of the relevant mouse BACs. However, the stop codons within the fourth intron of each mouse gene are not in the same or even similar positions to their human equivalents, and their C-terminal regions do not retain homology to their human counterparts. In contrast, the in silico prediction of the mouse five-exon γ5 sequence exhibits 89.5% identity to that of human γ5 at the nucleic acid level and 97% identity at the protein level. Our results strongly indicate that the previously predicted four-exon γ5 and γ7 subunits are not expressed in vivo.
The γ5 and γ7 subunits represent a distinct subdivision of the γ subunit family of proteins identified by structural and sequence homology to stargazin. In addition to the lower sequence homology to the neuronal γ2, γ3 and γ4 subunits, the identification of these gene products, assembled from five exons rather than four, as in the case for the stargazin gene and its closer homologues, is evidence for further evolutionary diversion from a common ancestor. This conclusion was also reached in a recent genomic analysis of the γ subunit superfamily (Chu et al., 2001).
Northern blot analysis of the γ7 mRNA revealed it to be expressed exclusively in neuronal tissue. Two probes were used in different hybridizations, one specific for full-length γ7 and the other, used in RT–PCR-based expression analysis in the previous study (Burgess et al., 2001), which would detect mRNAs for both full-length γ7 and the putative four-exon γ7. Both probes hybridized to ∼2.4 and ∼3 kb mRNAs, with identical expression patterns in all tissues in which they are expressed. Thus the two bands cannot represent separate transcripts for the previously predicted four-exon γ7 and the five-exon γ7 described here. It would appear highly unlikely, given these data, that the four-exon γ7 is expressed in vivo.
Co-expression of the γ7 subunit almost completely abolished Ba2+ current through N-type CaV2.2 VDCCs expressed in both Xenopus oocyte and COS-7 mammalian expression systems. It also significantly reduced maximum Ba2+ conductance in the non-N type CaV2.1 and CaV1.2 VDCCs, but the effects were not so pronounced. In contrast to the effects of the neuronal γ2 subunit, which has been reported in a previous study to inhibit N-type CaV2.2/β3/α2δ1 currents by ∼37% (Kang et al., 2001), the influence of the γ7 subunit is not dependent upon co-expression of an exogenous α2δ subunit as part of the Ca2+ channel complex.
Several mechanisms could underlie the suppression of VDCC currents by the γ7 subunit, including: (i) interference with the functioning of the channel at the plasma membrane; (ii) inhibition of the delivery of the channel to the plasma membrane; (iii) prevention of the correct folding of the pore-forming subunit; (iv) more rapid protein degradation; and (v) suppression of channel protein synthesis.
Confocal imaging of COS-7 cells transiently expressing N-terminally tagged GFP–CaV2.2 Ca2+ channel complexes revealed that co-expression of the γ7 protein, which itself is highly expressed throughout the cell, resulted in an almost complete loss of GFP fluorescence. This suggested reduced synthesis or stability of GFP–CaV2.2, or interference of the γ7 subunit with the correct folding of the GFP-tagged protein. Western blot analysis of the levels of both GFP–CaV2.2 and untagged CaV2.2 revealed that co-expression of γ7 results in a much reduced steady-state level of CaV2.2. This is likely to be due to a reduction in the level of CaV2.2 protein synthesized when γ7 is co-expressed, because no smaller molecular weight products of degradation were evident. It nevertheless is also possible that γ7 induces rapid and complete targeting of CaV2.2 to a degradation pathway. Whilst northern blot analysis is required to examine whether the CaV2.2 mRNA level is reduced in the presence of γ7, we have noted in the present study that the reduction in CaV2.2 protein level still occurs following the removal of its 3′-untranslated region (UTR) (Figure 7B), which has been implicated in message stability (Schorge et al., 1999).
These results raised the possibility that the γ7 was causing a non-specific inhibition of transcription or translation. However, we have shown that the influence of γ7 does not cause a generalized down-regulation of all heterologously expressed ion channels, because the I–V relationship for the voltage-dependent potassium channel KV3.1b expressed in Xenopus oocytes was unaffected by co-expression of the γ7 subunit. Furthermore, immunocytochemistry experiments confirmed that the expression of KV3.1b in COS-7 cells was unaltered by the co-expression of the γ7 subunit and there was little effect on expression of the VDCC β1b subunit.
Our results bear a striking resemblance to the dominant-negative synthesis suppression of the CaV2.2 subunit induced by the co-expression of truncated CaV2.2 constructs (Raghib et al., 2001). In that investigation, it was proposed that the translation of full-length CaV2.2 was halted when domain I or domain I–II of CaV2.2 interacted with the initially synthesized transmembrane α-helices of the nascent full-length CaV2.2. We do not yet know if a similar mechanism might be invoked for the down-regulation of CaV2.2 by γ7.
Further evidence indicates that the influence of γ7 expression may be upon newly synthesized CaV2.2 protein. Transient expression of the γ7 subunit in sympathetic neurones did not significantly alter endogenous (largely N-type) calcium current densities. The γ7 subunit therefore does not affect the function of pre-existing functional N-type channels.
It appears that the influence of γ7 on VDCC expression and function is via a mechanism unlike that reported previously for the other stargazin-like proteins. Nevertheless, in the present study, inhibition of expression by the γ7 subunit is apparently specific for VDCC subunits, particularly CaV2.2, because its co-expression with KV3.1b did not inhibit the expression of the potassium channel.
That γ7 is actually a subunit of a functional plasma membrane calcium channel complex appears unlikely, at least for CaV2.2. This is reinforced by the absence of significant endogenous γ7 in sympathetic neurones, where the current is predominantly N-type. Future work will examine in which cell types γ7 is expressed endogenously in the brain, and whether its endogenous function is to down-regulate CaV2.2 expression.
Materials and methods
Text-based in silico cloning
The GenBank and EMBL databases were searched for sequences possessing homology to the full-length sequence of the mouse stargazin gene (Letts et al., 1998). ESTs and genomic sequences from both databases were clustered to identify overlapping identical sequence belonging to the same transcript using the GCG package (Wisconsin Package Version 9.0, Genetics Computer Group) and a program developed by GlaxoSmithKline Research and Development Bioinformatics group, termed ESTBlast (Gill et al., 1997). Multiple sequence alignments of conceptually translated proteins were aligned using the Clustal_W algorithm (Omiga 1.1, Oxford Molecular Group, Oxford, UK).
Cloning of stargazin-like genes
An RT–PCR approach was used to amplify the complete ORFs or partial sequences of predicted γ-subunits either from cDNA derived from RNA or from overlapping partial clones. Human brain total RNA (Invitrogen, Paisley, UK) or mouse cerebellar total RNA purified from the cerebella of 31-day-old mice using the RNeasy kit (Qiagen, Crawley, UK) were used to generate cDNA using the Superscript Pre-amplification System (Invitrogen) primed with random hexamers.
Cloning human γ7. The γ7, 487 bp fragment predicted by in silico cloning was amplified from 25 ng of Superscript-synthesized human brain cDNA using 25 pmol each of primer pair 5′-CGGGAGAAAGGTCGCTGT-3′ and 5′-TCATTTGGATGGACACGTCG-3′. This was then extended by 5′ and 3′ RACE protocols using Marathon Ready total human brain cDNA (BD Biosciences Clontech, Basingstoke, UK). The complete nucleotide sequence of γ7 was assembled from two of the generated RACE clones and the original 487 bp clone by a splice overlap PCR protocol using a primer pair specific for the predicted ORF. The cDNA was sequenced then subcloned into a modified pMT2 expression vector (Swick et al., 1992). Human γ7-myc/his was constructed by amplifying the complete ORF minus the stop codon from the pMT2-γ7 clone and inserting the fragment in-frame into the multiple cloning site of pCDNA3.1 myc/his A (+) (Invitrogen) using the NotI and ApaI restriction sites.
Cloning mouse γ7. The nucleotide sequence of the human γ7 was compared with the HTGS subset of GenBank using BLASTn and tBLASTn with default parameters. The complete coding sequence was found within chromosome 19 BAC AC079557 and used to design gene-specific primers 5′-ATGAGTCACTGCAGCAGCCG-3′ and 5′-TCA GCACGGCGAAGTGGAGA-3′. The complete ORF was amplified in a single PCR from 20 ng of mouse cerebellar cDNA and cloned into pCR4-TOPO for sequencing, and subcloned into pMT2 and pRK5 for expression in Xenopus oocytes/COS-7 cells or SCG neurones, respectively.
Northern blots
Human 12-lane multiple tissue blots and brain II and IV blots (BD Biosciences Clontech) were hybridized at 65°C in ExpressHyb solution (BD Biosciences Clontech) according to the manufacturer’s instructions. The α-32P-radiolabelled γ7 probes corresponding to nucleotides 576–763 or 80–482 were assembled according to the Strip-EZ DNA Probe Synthesis Removal Kit [Ambion (Europe) Ltd, Huntingdon, UK]. The final stringency wash performed was 0.1× SSC, 0.1% SDS at 65°C.
Other cDNA clones
The following cDNAs were used: rabbit CaV2.2 (D14157), N-terminal GFP-tagged rabbit CaV2.2 (Raghib et al., 2001), rabbit CaV2.2 Δ3′UTR, rabbit CaV2.1 (X57689), rat brain CaV1.2 (isoform CII, M67515), rat β1b (X61394, except R417S, V435, V449A, W492R, V511A, a gift from Dr T.P.Snutch), mouse α2δ2 (AF247139, common brain splice variant), mut-3b GFP (M62653, except S72A and S65G, a gift from Dr T.E.Hughes) and rat KV3.1b (M68880). The 3′-UTR of rabbit CaV2.2 was removed to generate CaV2.2 Δ3′UTR, by introducing an SpeI site immediately after the stop codon by PCR and subcloning the CaV2.2 back into the vector. All cDNAs were subcloned into expression vector pMT2 (Swick et al., 1992), with the exception of KV3.1b that was in pRC-CMV.
Cell culture and transfections
Monkey COS-7 cells were cultured as previously described (Brice et al., 1997). Transfection was performed with Geneporter transfection reagent (Gene Therapy Systems, San Diego, CA). Cells were plated onto coverslips 2–3 h prior to transfection. The DNA and Geneporter reagent (2 µg and 10 µl, respectively) were each diluted in 500 µl of serum-free medium, mixed, and applied to the cells. The α1, β, α2δ and γ subunits were mixed and transfected in a 1:1:1:1 ratio by DNA weight. In the absence of α2δ and/or γ7 subunit, blank pMT2 vector was substituted. After 3.5 h, 1 ml of medium containing 20% serum was added to the cells, which were then incubated at 37°C for 3 days. Prior to electrophysiological recording, cells were re-plated using a non-enzymatic cell dissociation solution (Sigma, Dorset, UK) and maintained at 27°C for between 2 and 6 h. Cells for immunocytochemistry were not re-plated.
Electrophysiology
Xenopus oocytes. Xenopus oocytes were prepared, injected and utilized for electrophysiology as previously described (Canti et al., 1999), with the following exceptions. Plasmid cDNAs for the different VDCC subunits were mixed in a weight ratio of 1:1:1:1. The recording solution for CaV2.2-injected oocytes contained: 5 mM Ba(OH)2, 80 mM TEA-OH, 2 mM CsOH and 5 mM HEPES (pH 7.4 with methanesulfonic acid). The Ba(OH)2 concentration was raised to 10 mM for recording CaV1.2 and CaV2.1 currents. KV3.1b currents were recorded 3 days after intranuclear injection of oocytes with 9 nl of cDNA together with an equal amount of γ7 in pMT2, or water for the control. The external medium contained: 96 mM NaCl, 5 mM HEPES, 2 mM KCl, 1 mM MgCl2 and 1.8 mM CaCl2 (pH 7.4).
COS-7 cells. Recordings were made from fluorescent COS-7 cells expressing GFP–CaV2.2. Because GFP–CaV2.2 was only weakly fluorescent, free GFP (ratio 0.1) was included in the transfection to identify transfected cells from which recordings were made. Whole-cell patch–clamp recording was performed as previously described (Meir et al., 2000). Ba2+ (1 mM) was used as a charge carrier, except in the presence of γ7 subunit where 10 mM Ba2+ was also used. Experiments were performed at room temperature. Data are expressed as mean ± SEM. Statistical analysis was performed using paired or unpaired Student’s t-test, as appropriate.
SCG preparation, transfection and electrophysiology
Sympathetic neurones were isolated from 17-day-old Sprague–Dawley rats killed by CO2 inhalation, following a modified procedure described by Delmas et al. (1998), in which collagenase type IA (500 U/ml, Sigma) and trypsin type XI (3000 U/ml, Sigma) were used. Aliquots of the cell suspension were transferred immediately to the electroporation cuvettes together with 10 µg of each of the cDNAs to be transfected. After electroporation (Morales et al., 2000) (250 V, 900 µF, EasyjecT Plus, EquiBio, Ashford, UK), the cells were resuspended in pre-warmed growth medium, plated on poly-l-lysine (Sigma)-coated Petri dishes and incubated at 37°C, 5% CO2, for 36–48 h before use for immunocytochemistry or electrophysiological experiments. Currents were elicited in the whole-cell patch–clamp configuration from a holding potential of –80 mV by 100 ms steps from –40 to +40 mV. Otherwise all other recording conditions were as described above, with the exception of the addition of 0.5 µM tetrodotoxin to the extracellular solution.
Antibody generation
The peptide identified in Figure 1A was synthesized by standard solid-phase techniques, purified by gel filtration chromatography and used to generate polyclonal anti-γ7 rabbit Abs as previously described (Amaya et al., 2000). His-tagged rat β1b subunit, purified essentially as previously described (Canti et al., 2001), was used to generate the polyclonal rabbit β1b Ab.
Immunocytochemistry
COS-7 cells or SCG neurones were fixed and permeabilized for immunocytochemistry essentially as previously described (Brice et al., 1997). Primary Abs, affinity-purified anti- γ7, anti-myc (Autogen-Bioclear, Calne, UK) and anti-KV3.1b (Alomone labs, Jerusalem, Israel) were used at 0.8, 4 and 1.5 µg/ml, respectively. Anti-β1b antiserum was used at 1:1000 dilution. Secondary biotin-conjugated goat anti-mouse (Molecular Probes, Eugene, OR) or goat anti-rabbit (Sigma, Dorset, UK) Abs were applied at 10 and 5 µg/ml, respectively. Texas red- or fluorescein isothiocyanate (FITC)-conjugated streptavidin were applied at 3.33 µg/ml. In co-labelling experiments, KV3.1b was labelled with goat anti-rabbit Texas red-conjugated secondary Ab at 10 µg/ml (Molecular Probes). In some experiments, cells were incubated for 20 min with Oregon Green–phalloidin (6.6 µM, Molecular Probes). The nuclear dye 4′,6-diamidino-2-phenylindole (DAPI; 300 nM, Molecular Probes) was also used to visualize the nucleus. Cells were examined on a confocal scanning laser microscope (Leica TCS SP, Milton Keynes, UK). All images were scanned sequentially to eliminate cross-talk, and photomultiplier settings were kept constant in each experiment.
Western blotting
COS-7 cells were processed as previously described (Raghib et al., 2001). Samples (50 µg of total protein/lane in Figure 7B and 10 µg of total protein/lane in Figure 7C) were separated by SDS–PAGE using 4–20% gradient gels and then transferred electrophoretically to polyvinylidene fluoride membranes. The membranes were blocked with 3% bovine serum albumin (BSA) for 5 h at 55°C and then incubated overnight at 20°C with either 1.2 µg/ml of an anti-peptide Ab raised in rabbits against residues 846–861 within the II–III loop of rabbit brain CaV2.2 (Raghib et al., 2001), or a 1:1000 dilution of anti-rat β1b antiserum. Secondary Ab (a 1:1000 dilution of goat anti-rabbit IgG–horseradish peroxidase conjugate) was added and the membranes incubated for 1 h. Following extensive washing, bound Abs were detected using enhanced chemiluminescence (ECL, Amersham Pharmacia Biotech, Little Chalfont, UK).
Acknowledgments
Acknowledgements
We thank Dr L.Kaczmarek (Yale University School of Medicine) for providing the KV3.1b cDNA, Dr M.Rees (University College London) for the α2δ2 cDNA, and Dr Y.Goda (University of California, San Diego) for advice on electroporation of neurones. We are also grateful to Dr D.Crowther (Genomics, GlaxoSmithKline, Stevenage, UK) for his comments on the bioinformatics, Ayesha Raghib for the GFP–CaV2.2 clone, and Wendy Pratt and Manuela Nieto-Rostro for technical assistance. F.J.M. was an MRC PhD student in collaboration with GlaxoSmithKline. The Wellcome Trust provided additional support.
References
- Ahern C.A. et al. (2001) Modulation of L-type Ca2+ current but not activation of Ca2+ release by the γ1 subunit of the dihydropyridine receptor of skeletal muscle. BMC Physiol., 1, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya F., Decosterd,I., Samad,T.A., Plumpton,C., Tate,S., Mannion,R.J., Costigan,M. and Woolf,C.J. (2000) Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol. Cell. Neurosci., 15, 331–342. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Qin,N., Olcese,R., Tareilus,E., Platano,D., Costantin,J. and Stefani,E. (1998) Structures and functions of calcium channel β subunits. J. Bioenerg. Biomembr., 30, 357–375. [DOI] [PubMed] [Google Scholar]
- Black J.L. III, and Lennon,V.A. (1999) Identification and cloning of putative human neuronal voltage-gated calcium channel γ-2 and γ-3 subunits: neurologic implications. Mayo Clin. Proc., 74, 357–361. [DOI] [PubMed] [Google Scholar]
- Brice N.L., Berrow,N.S., Campbell,V., Page,K.M., Brickley,K., Tedder,I. and Dolphin,A.C. (1997) Importance of the different β subunits in the membrane expression of the α1A and α2 calcium channel subunits: studies using a depolarization-sensitive α1A antibody. Eur. J. Neurosci., 9, 749–759. [DOI] [PubMed] [Google Scholar]
- Burge C. and Karlin,S. (1997) Prediction of complete gene structures in human genomic DNA. J. Mol. Biol., 268, 78–94. [DOI] [PubMed] [Google Scholar]
- Burgess D.L., Davis,C.F., Gefrides,L.A. and Noebels,J.L. (1999) Identification of three novel Ca2+ channel γ subunit genes reveals molecular diversification by tandem and chromosome duplication. Genome Res., 9, 1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess D.L., Gefrides,L.A., Foreman,P.J. and Noebels,J.L. (2001) A cluster of three novel Ca2+ channel γ subunit genes on chromosome 19q13.4: evolution and expression profile of the γ subunit gene family. Genomics, 71, 339–350. [DOI] [PubMed] [Google Scholar]
- Canti C., Page,K.M., Stephens,G.J. and Dolphin,A.C. (1999) Identification of residues in the N terminus of α1B critical for inhibition of the voltage-dependent calcium channel by Gβγ. J. Neurosci., 19, 6855–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canti C., Davies,A., Berrow,N.S., Butcher,A.J., Page,K.M. and Dolphin,A.C. (2001) Evidence for two concentration-dependent processes for β-subunit effects on α1B calcium channels. Biophys. J., 81, 1439–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W.A. (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell. Dev. Biol., 16, 521–555. [DOI] [PubMed] [Google Scholar]
- Chen L., Chetkovich,D.M., Petralia,R.S., Sweeney,N.T., Kawasaki,Y., Wenthold,R.J., Bredt,D.S. and Nicoll,R.A. (2000) Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature, 408, 936–943. [DOI] [PubMed] [Google Scholar]
- Chu P.J., Robertson,H.M. and Best,P.M. (2001) Calcium channel γ subunits provide insights into the evolution of this gene family. Gene, 280, 37–48. [DOI] [PubMed] [Google Scholar]
- Delmas P., Brown,D.A., Dayrell,M., Abogadie,F.C., Caulfield,M.P. and Buckley,N.J. (1998) On the role of endogenous G-protein βγ subunits in N-type Ca2+ current inhibition by neurotransmitters in rat sympathetic neurones. J. Physiol. (Lond.), 506, 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A.C. (1998) Mechanisms of modulation of voltage-dependent calcium channels by G proteins. J. Physiol. (Lond.), 506, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freise D. et al. (2000) Absence of the γ subunit of the skeletal muscle dihydropyridine receptor increases L-type Ca2+ currents and alters channel inactivation properties. J. Biol. Chem., 275, 14476–14481. [DOI] [PubMed] [Google Scholar]
- Gill R.W., Hodgman,T.C., Littler,C.B., Oxer,M.D., Montgomery,D.S., Taylor,S. and Sanseau,P. (1997) A new dynamic tool to perform assembly of expressed sequence tags (ESTs). CABIOS, 13, 453–457. [DOI] [PubMed] [Google Scholar]
- Hofmann K. and Stoffel,W. (1993) TMbase—a database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler, 374, 166. [Google Scholar]
- Jay S.D., Ellis,S.B., McCue,A.F., Williams,M.E., Vedvick,T.S., Harpold,M.M. and Campbell,K.P. (1990) Primary structure of the γ subunit of the DHP-sensitive calcium channel from skeletal muscle. Science, 248, 490–492. [DOI] [PubMed] [Google Scholar]
- Jones S.W. (1998) Overview of voltage-dependent calcium channels. J. Bioenerg. Biomembr., 30, 299–312. [DOI] [PubMed] [Google Scholar]
- Kang M.-G., Chen,C.-C., Felix,R., Letts,V.A., Frankel,W.N., Mori,Y. and Campbell,K.P. (2001) Biochemical and piophysical evidence for γ2 subunit association with neuronal voltage-activated Ca2+ channels. J. Biol. Chem., 276, 32917–32924. [DOI] [PubMed] [Google Scholar]
- Klugbauer N., Dai,S.P., Specht,V., Lacinov,L., Marais,E., Bohn,G. and Hofmann,F. (2000) A family of γ-like calcium channel subunits. FEBS Lett., 470, 189–197. [DOI] [PubMed] [Google Scholar]
- Kornau H.C., Seeburg,P.H. and Kennedy,M.B. (1997) Interaction of ion channels and receptors with PDZ domain proteins. Curr. Opin. Neurobiol., 7, 368–373. [DOI] [PubMed] [Google Scholar]
- Lacerda A.E., Perez-Reyes,E., Wei,X., Castellano,A. and Brown,A.M. (1994) T-type and N-type calcium channels of Xenopus oocytes: evidence for specific interactions with β subunits. Biophys. J., 66, 1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letts V.A. et al. (1998) The mouse stargazer gene encodes a neuronal Ca2+-channel γ subunit. Nature Genet., 19, 340–347. [DOI] [PubMed] [Google Scholar]
- Meir A., Bell,D.C., Stephens,G.J., Page,K.M. and Dolphin,A.C. (2000) Calcium channel β subunit promotes voltage-dependent modulation of α1B by Gβγ. Biophys. J., 79, 731–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M., Colicos,M.A. and Goda,Y. (2000) Actin-dependent regulation of neurotransmitter release at central synapses. Neuron, 27, 539–550. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. (1998) Molecular characterization of a novel family of low voltage-activated, T-type, calcium channels. J. Bioenerg. Biomembr., 30, 313–318. [DOI] [PubMed] [Google Scholar]
- Plummer M.R., Logothetis,D.E. and Hess,P. (1989) Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron, 2, 1453–1463. [DOI] [PubMed] [Google Scholar]
- Powers P., Liu,S., Hogan,K. and Gregg,R. (1993) Molecular characterization of the gene encoding the γ subunit of the human skeletal muscle 1,4-dihydropyridine-sensitive Ca2+ channel (CACNLG), cDNA sequence, gene structure and chromosomal location. J. Biol. Chem., 268, 9275–9279. [PubMed] [Google Scholar]
- Raghib A., Bertaso,F., Davies,A., Page,K.M., Meir,A., Bogdanov,Y. and Dolphin,A.C. (2001) Dominant-negative synthesis suppression of voltage-gated calcium channel Cav2.2 induced by truncated constructs. J. Neurosci., 21, 8495–8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorge S., Gupta,S., Lin,Z.X., McEnery,M.W. and Lipscombe,D. (1999) Calcium channel activation stabilizes a neuronal calcium channel mRNA. Nature Neurosci., 2, 785–790. [DOI] [PubMed] [Google Scholar]
- Sharp A.H., Black,J.L.,III, Dubel,S.J., Sundarraj,S., Shen,J.P., Yunker,A.M.R., Copeland,T.D. and McEnery,M.W. (2001) Biochemical and anatomical evidence for specialized voltage-dependent calcium channel γ isoform expression in the epileptic and ataxic mouse, stargazer. Neuroscience, 105, 599–617. [DOI] [PubMed] [Google Scholar]
- Swick A.G., Janicot,M., Cheneval-Kastelic,T., McLenithan,J.C. and Lane,M.D. (1992) Promoter-cDNA-directed heterologous protein expression in Xenopus laevis oocytes. Proc. Natl Acad. Sci. USA, 89, 1812–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S., Nicoll,R.A. and Bredt,D.S. (2001) PDZ protein interactions regulating glutamate receptor function and plasticity. J. Cell Biol., 153, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]