Abstract
We raised monoclonal antibodies against senile plaque (SP) amyloid and obtained a clone 9D2, which labeled amyloid fibrils in SPs and reacted with ∼50/100 kDa polypeptides in Alzheimer’s disease (AD) brains. We purified the 9D2 antigens and cloned a cDNA encoding its precursor, which was a novel type II transmembrane protein specifically expressed in neurons. This precursor harbored three collagen-like Gly–X–Y repeat motifs and was partially homologous to collagen type XIII. Thus, we named the 9D2 antigen as CLAC (collagen-like Alzheimer amyloid plaque component), and its precursor as CLAC-P/collagen type XXV. The extracellular domain of CLAC-P/collagen type XXV was secreted by furin convertase, and the N-terminus of CLAC deposited in AD brains was pyroglutamate modified. Both secreted and membrane-tethered forms of CLAC-P/collagen type XXV specifically bound to fibrillized Aβ, implicating these proteins in β-amyloidogenesis and neuronal degeneration in AD.
Keywords: Alzheimer’s disease/amyloid/collagen
Introduction
Alzheimer’s disease (AD) is a progressive dementing neurodegenerative disorder of the elderly characterized pathologically by a massive deposition of β-amyloid as senile plaques (SPs) and cerebral amyloid angiopathy (Selkoe, 2001). The principal component of AD amyloid is the amyloid β peptide (Aβ), which is proteolytically produced from a transmembrane precursor, βAPP, as 39–43 amino acid fragments (Masters et al., 1985; Kang et al., 1987). Currently, the notion that production and deposition of Aβ are closely related to the pathogenesis of AD is widely accepted because (i) Aβ deposition occurs in only a few pathological conditions including AD, Down’s syndrome (DS) and pathological aging; (ii) deposition of Aβ (and especially that of highly aggregable Aβ42 species ending at the 42nd residue) as diffuse plaques is one of the earliest pathological changes in AD brains (Iwatsubo et al., 1994); and, most importantly, (iii) missense mutations in βAPP or presenilin genes linked to early-onset, autosomally dominant inherited forms of AD increase the production of Aβ42 (Suzuki et al., 1994; Duff et al., 1996; Tomita et al., 1997), leading to its enhanced tissue deposition (Mann et al., 2001). However, several lines of evidence, including those from human neuropathological (Katzman et al., 1988) and transgenic mice studies (Irizarry et al., 1997), suggest that the deposition of Aβ per se is insufficient to cause neuronal death and symptomatic manifestations of dementia.
A number of non-Aβ proteinacious components are deposited in SPs associated with Aβ (Dickson, 1997). Notably, apolipoprotein E (apoE), whose polymorphism determines a major genetic risk factor for AD (Strittmatter et al., 1993), is also associated with Aβ in AD brains (Namba et al., 1991). Despite some disagreements between in vitro data (Ma et al., 1994; Naiki et al., 1997), recent results in transgenic mice show that overexpression of βAPP under an apoE null background causes a decrease in deposition and fibrillization of Aβ, and that transgenic supplementation of human apoE (especially E4) restores and facilitates deposition of fibrillized Aβ and neuritic degeneration (Holtzman et al., 2000). Such data strongly implicate apoE in the formation and fibrillization of β-amyloid and further AD-specific alterations in vivo. In addition, complement components are also known to co-deposit with plaque amyloid and are implicated in inflammatory changes in AD brains (Webster et al., 1994). Thus, there is a compelling need to search for additional non-Aβ components of plaque amyloid that may affect or promote SP formation and thereby facilitate the development of AD-specific neurodegenerative changes.
Here, we describe a novel SP amyloid component, CLAC (collagen-like Alzheimer amyloid plaque component) and its precursor CLAC-P/collagen type XXV (Col XXV) that we have identified through our search for SP components by raising antibodies to SP amyloid. CLAC-P/Col XXV showed a unique, membrane-bound collagen-like structure harboring three Gly–X–Y repeat motifs that may define a novel class of neuronal collagens. The collagen-like extracellular domain was secreted by furin to be destined to deposit with extracellular β-amyloid. Specific binding of fibrillized Aβ with secreted and membrane-tethered forms of CLAC-P/Col XXV harboring a unique collagen-like structure strongly implicates these polypeptides in fibrillization, protection against proteolysis and cell toxicity of β-amyloid in AD brains.
Results
Establishment and characterization of a monoclonal antibody 9D2 that specifically labels senile plaques in AD brains
To identify novel components of amyloid plaques in AD brains, we partially purified insoluble fractions from AD cortices by sucrose density gradient centrifugation followed by extraction with Triton X-100 and urea. Using the Triton/urea-insoluble materials as immunogens, we generated monoclonal antibodies (mAbs) and screened them by immunostaining unfixed smears of the post-sucrose pellets of AD brains. Among ∼300 mAbs generated, ∼10 immunostained amyloid fragments, and we further characterized a clone 9D2 (mAb 9D2), which labeled amyloid deposits most intensely.
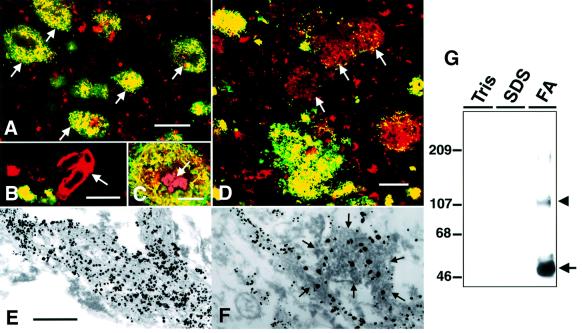
mAb 9D2 strongly immunostained SP amyloid in tissue sections from cerebral cortices of patients with AD (Figure 1A). Neuritic or primitive types of plaques, especially, showed strong and discrete immunolabeling in a thick bundle- or coarse granule-like fashion (Figure 1A, C and D), whereas vascular amyloid deposits never showed positive staining (Figure 1B). 9D2 did not stain diffuse plaques in the cerebral cortices of non-demented individuals or in the striatum or cerebellar cortices of AD brains, and the core portion of classical SP often failed to show a positive reaction (Figure 1C). Quantitative analysis of areas covered by Aβ or 9D2 immunoreactivities in frontal cortices of 15 AD patients showed that 15.8 ± 5.3% (average ± SD) of total cortical area was Aβ-positive, whereas 9.3 ± 5.9% was 9D2-positive. Thus, 60 ± 26% of the total Aβ-positive area was positive for 9D2. 9D2 did not label any normal structures in AD or control brains. Double immunofluorescence labeling showed that 9D2-positive portions of SPs always overlapped with Aβ immunoreactivities (Figure 1A, C and D), but not with neuritic or glial labeling (data not shown), suggesting that 9D2 antigens are directly associated with amyloid. Immunoelectron microscopic analysis confirmed that bundles of amyloid fibrils are directly labeled by 9D2 (Figure 1E), and double labeling showed that a fraction of Aβ-positive amyloid bundles are co-labeled by 9D2 (Figure 1F).
Fig. 1. Immunohistochemical and biochemical characterization of mAb 9D2. (A) SPs in the frontal cortex of an AD brain doubly immunolabeled by mAb 9D2 (green) and anti-AβN3(pyroGlu) (red). Note that all SPs show areas of homogenous yellow immunofluorescence (arrows), indicating that Aβ and 9D2 antigen are both present. (B) Aβ-positive amyloid angiopathy (arrow; red) in AD cortex is not labeled by mAb 9D2. (C) The core portion of a typical SP (arrow) often lacks 9D2 immunoreactivity (9D2-IR). (D) A subset of Aβ-positive SPs in AD neocortex lack 9D2-IR (arrows, red) although a few dot-like areas of 9D2-IR are present in some SPs. Scale bars in (A–D) are equivalent to 100, 50, 20 and 50 µm, respectively. (E) Immunoelectron microscopic observation of amyloid bundles in SPs of AD neocortex labeled by mAb 9D2. Thick bundles of amyloid fibrils are decorated by coarse particles of 1 nm gold enhanced by silver intensification. The scale bar in (E) is equivalent to 400 nm in (E) and (F). (F) Amyloid fibrils doubly immunolabeled by anti-AβN3(pyroGlu) (1 nm gold particles with silver intensification) and mAb 9D2 (immunoperoxidase labeling). Note that 9D2/immunoperoxidase-positive amyloid bundles (encircled by arrows) were also Aβ-positive (decorated by black particles), indicating that 9D2- and Aβ-IRs are co-localized. (G) Immunoblot analysis of 9D2-positive polypeptides in AD brains. Frozen AD cortices were extracted sequentially by Tris saline (Tris), 2% SDS and 70% formic acid (FA). Note that 9D2-positive polypeptides were extracted exclusively in the FA-soluble fraction as ∼50 (arrow) and ∼100–110 kDa (arrowhead) proteins. Molecular mass standards are shown in kilodaltons.
We then characterized the antigen recognized by 9D2 by immunoblotting of fractions of AD brains. Tris saline-soluble or SDS-soluble fractions of AD brains showed no immunoreactive bands for 9D2, whereas 9D2 strongly labeled a major ∼50 kDa and a minor ∼100–110 kDa polypeptide in formic acid extracts of SDS-insoluble fractions (Figure 1G). No 9D2-reactive substances were detected in any fractions of normal brains.
Purification of 9D2 antigen polypeptides and identification of their partial amino acid sequences
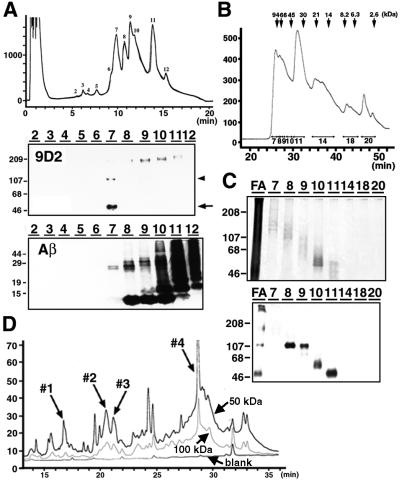
To identify the amino acid sequences of 9D2 antigen, we further purified 9D2-positive polypeptides from the 70% formic acid extracts of SDS-insoluble fractions of AD brains. After removal of formic acid, the fraction was resolved in 6 M guanidine HCl and separated by reverse phase HPLC (RP-HPLC). 9D2-positive 50/100–110 kDa polypeptides were recovered at an acetonitrile (ACN) concentration of ∼30%, whereas Aβ was fractionated at higher ACN concentrations (Figure 2A). We next subjected RP-HPLC-purified fractions to gel filtration, and recovered 9D2-positive 50, 60, 70 and 100–110 kDa polypeptides, respectively (Figure 2B and C), that were purified to near homogeneity as confirmed by protein silver staining (Figure 2C). We then digested the major 50 or 100–110 kDa 9D2 antigen by Achromobacter lyticus protease I (API; lysylendopeptidase), and separated the peptide fragments by RP-HPLC. The peak patterns of digested peptides derived from 50 and 100–110 kDa polypeptides were very similar (Figure 2D), suggesting that the 100–110 kDa species is a dimer of the 50 kDa polypeptide. By analyzing the amino acid sequences of API-digested peptide fragments using a protein sequencer, we obtained the following two sequences: INHGFLSADQQLIK (peptide 2-1: peak 4 in Figure 2D) and GEQGDQGPRMVFOK (where O represents hydroxyproline; peptide 2-2: peak 3). In addition, 22 amino acid (peak 1: RGRRGESGPPGQOGPQGPOGPK) and 31 amino acid (peak 2: RRLIJGDQGQAGPOGPOGPOGPOGPOGDTGK; where J represents hydroxylysine) sequences composed of Gly–X–Y collagen-like repeats were also identified. MALDI-TOF mass analysis of peaks 1–4 gave mass numbers of 2215, 3304, 1562 and 1584, respectively, that corresponded exactly to the molecular mass of the sequences obtained by a protein sequencer (data not shown). We then digested the 9D2 antigen by another endoprotease, Asp-N, separated the peptide fragments and obtained the following amino acid sequence: DQGPRMVFO(or P)KINHGFLSA, which clearly indicated that peptides 2-2 and 2-1 are contiguous in a tandem fashion. We raised polyclonal antibodies against synthetic peptides corresponding to the peptides 2-2 and 2-1, respectively, and confirmed that both antibodies label SPs in AD brains and react with 50 and 100–110 kDa polypeptides in a similar manner to mAb 9D2 (see Figure 5A and B), as well as with a 70 kDa polypeptide, which, occasionally, is labeled faintly by 9D2 (see Figure 2C, lower panel, lane 9), strongly suggesting that the 28 amino acid sequence comprised of peptides 2-1 and 2-2 is a partial sequence of 9D2 antigen.
Fig. 2. Purification of 9D2 antigen polypeptide from AD brains. (A) Separation of 9D2 antigen from Aβ in formic acid extracts of AD brains by RP-HPLC. Peaks 2–12 shown in the elution profile (upper panel) were analyzed by immunoblotting with 9D2 (middle panel) or BAN50 (anti-Aβ; lower panel). 9D2-positive ∼50 (arrow) and ∼100–110 kDa (arrowhead) polypeptides were separated in fraction 7, whereas monomeric (∼4 kDa) or oligomeric forms of Aβ were eluted in fractions 8–12. (B) Separation of 9D2 antigen by size exclusion chromatography. The elution profile of fraction 7 in (A) is shown with fraction numbers. Elution positions of molecular mass standard proteins are shown above the panel. (C) Silver-stained gel replica of fractions in (B) separated by SDS–PAGE (upper panel). FA: fraction 7 of formic acid extracts in (A) prior to gel filtration. Immunoblot analysis of fractions 7–20 in (B) with 9D2 (lower panel). Molecular mass standards are shown in kilodaltons. (D) Separation of API-digested peptide fragments derived from ∼50 and ∼100 kDa 9D2 antigen polypeptides by RP-HPLC. Fractions 1–4 that gave partial amino acid sequences of CLAC protein are indicated by arrows. The ordinates in (A) (upper panel), (B) and (D) are shown in milliabsorbance (mA) at 215 nm.
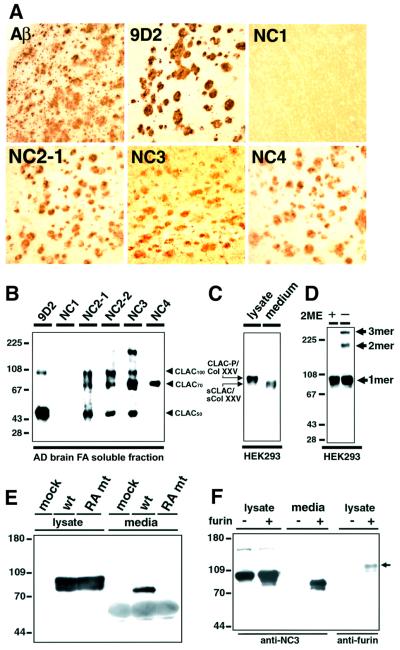
Fig. 5. Characterization of CLAC polypeptide deposited in AD brains and CLAC-P/Col XXV expressed in HEK293 cells, and secretion of sCLAC/sCol XXV by furin convertase. (A) Immunohistochemistry of AD neocortex stained by anti-AβN3(pyroGlu), 9D2 and anti-CLAC-P/Col XXV antibodies. (B) Immunoblot analysis of formic acid extracts of AD brains probed by mAb 9D2 and anti-CLAC-P/Col XXV antibodies. Note that anti-NC2-1, NC2-2 and NC3 label a set of polypeptides (CLAC50, CLAC70 and CLAC100), whereas anti-NC4 exclusively labeled CLAC70. (C) Immunoblot analysis of HEK293 cells stably expressing human CLAC-P/Col XXV with anti-NC3. Left lane, cell lysate; right lane, culture media. Note that sCLAC/sCol XXV in culture media co-migrates with CLAC70 at ∼70 kDa. Molecular mass standards are common to (B) and (C). (D) Formation of trimers (3mer) and dimers (2mer) of CLAC-P/Col XXV under non-reducing conditions. Cell lysates of HEK293 cells stably transfected with human CLAC-P/Col XXV were analyzed by immunoblotting with anti-NC3 with (left lane) or without (right lane) 2-mercaptoethanol (2ME) pre-treatment. (E) COS-1 cells were transiently transfected with cDNAs encoding wild-type (wt) or RA mutant (mt) human CLAC-P/Col XXV, the latter being substituted with alanine at two arginine residues, at positions –1 and –4 to the putative furin cleavage site, or with an empty vector (mock). Cell lysate (left panel) and culture media (right panel) were analyzed by immunoblotting with anti-NC3. (F) RPE.40 mutant CHO cells that lack furin were stably transfected with human CLAC-P/Col XXV. Lysates and culture media from these stable cells with (+) or without (–) transient co-transfection of mouse furin were analyzed by immunoblotting with anti-NC3 (lysate and media, left panel) or anti-furin (lysate, right panel; the arrow shows transfected furin polypeptide). Molecular mass standards are shown in kilodaltons.
Precursor of 9D2 antigen (CLAC-P/Col XXV) is a novel transmembrane collagen
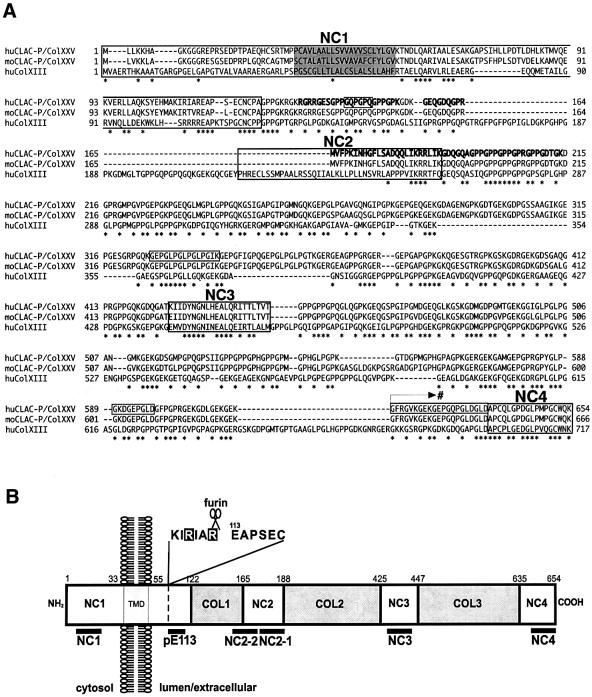
Based on the amino acid sequences of 9D2 antigen, we cloned an 80 bp partial cDNA by PCR using a human brain-derived cDNA library as a template. We cloned a cDNA encoding the entire open reading frame (ORF) for the precursor of 9D2 antigen by the rapid amplification of cDNA ends (RACE) method, which coded for a 654 amino acid protein with a possible type II transmembrane topology, harboring three Gly–X–Y collagen-like repeat motifs (COL1–COL3) in the putative extracellular domain that are flanked by four non-collagenous domains (NC1–NC4) as well as one putative transmembrane domain at the N-terminus (Figure 3A and B). The partial amino acid sequences derived from AD amyloid fractions corresponded to those within the COL1 to NC2 subdomains (Figure 3A). In addition, we detected at least four alternatively spliced isoforms that confer variability at the extracellular collagen-like repeats (boxed in Figure 3A), in addition to another splice variant that codes for an alternative NC4 domain (indicated by an arrow with # in Figure3A; see legend). Because of the unique collagen-like characteristic structure, we named the 9D2 antigen as CLAC. The domain structure and amino acid sequences of the precursor protein of CLAC were partially homologous to those of collagen type XIII α1 (Col XIII), a non-fibril-forming transmembrane collagen (∼43.0% at the amino acid level and ∼49.9% at the nucleic acid level; see Figure 3A; Hagg et al., 1998). Thus, we named the transmembrane CLAC precursor as CLAC-P/Col XXV (DDBJ/EMBL/GenBank accession No. AF293340), considering the recent reports on novel types of collagens XXIII (Gordon et al., 2000) and XXIV (Gordon et al., 2002). A search of human genome data from the International human genome project of the NIH located the CLAC-P/Col XXV gene on Homo sapiens chromosome 4q (4q22–24: AP002021 or 4q25: AC004051). We also cloned a cDNA encoding the entire ORF of murine CLAC-P/Col XXV (DDBJ/EMBL/GenBank accession No. AF315290), which was comprised of 666 amino acids and was highly homologous to human CLAC-P/Col XXV (90% at the amino acid level and 83% at the nucleic acid level; Figure 3A).
Fig. 3. Amino acid sequence, domain structure and topology of CLAC-P/Col XXV. (A) Predicted sequence of human (upper lane) and mouse (middle lane) CLAC-P/Col XXV, as well as of human Col XIII (lower lane). Peptide fragments identified by amino acid sequencing (peaks 1–4 in Figure 2D) are shown in bold. Four non-collagenous domains are boxed with the names of NC1–NC4, and a putative transmembrane domain is shaded. Amino acid residues that undergo alternative splicing in variants of human CLAC-P/Col XXV (i.e. residues 141–146, 326–340, 589–597 and 616–636) are also boxed, and an arrow with # indicates the starting point of a C-terminal splice variant that replaces the C-terminus including the NC4 domain by the amino acid sequence: VTSPSQHVPCLILLLLSALLFSLCDSI (DDBJ/EMBL/GenBank accession No. AF293341; registered as CLAC-P type II). Amino acid residues identical among the three molecular species are marked by asterisks. (B) Schematic representation of the domain structure and topology of human CLAC-P/Col XXV. The NC1 domain composed of cytoplasmic, transmembrane (TMD) and extracellular portions, three extracellular NC domains (NC2–NC4) and the three collagenous domains (COL1–COL3) are shown with amino acid residue numbers. The locations of the epitopes of antibodies used in this study and a putative furin cleavage site within NC1 are shown below and above the column, respectively.
Specific expression of CLAC-P/Col XXV in neurons
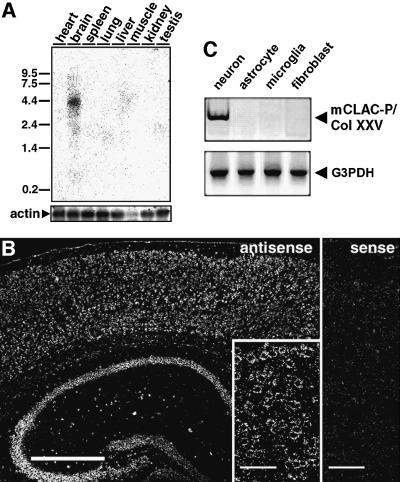
We next examined the regional and cellular expression of CLAC-P/Col XXV mRNA in murine tissues. By northern blotting, CLAC-P/Col XXV mRNA, encompassing an ORF of ∼2 kb, was expressed specifically in brain as an ∼4.4 kb transcript (Figure 4A). RT–PCR analysis showed low level expression in heart, testis and eye in addition to high expression in brain (data not shown). In situ hybridization of murine brain tissue showed specific expression of CLAC-P/Col XXV mRNA in neurons of cerebral neocortices, hippocampus and other subcortical neuronal nuclei (Figure 4B). RT–PCR of mRNA extracted from murine primary brain cells confirmed specific expression of CLAC-P/Col XXV in neurons but not in astrocytes, microglia or meningeal fibroblasts (Figure 4C). RT–PCR analysis of human tissue-derived mRNA also confirmed brain-specific expression of CLAC-P/Col XXV (data not shown).
Fig. 4. mRNA expression patterns of CLAC-P/Col XXV. (A) Northern blot analysis of CLAC-P/Col XXV mRNA expression in various mouse tissues. The blots were rehybridized using the actin probe as a control of mRNA loading. Standards are shown in kilobases. (B) In situ hybridization of CLAC-P/Col XXV mRNA in mouse cerebral neocortex and hippocampus (left, antisense probe), and a higher magnification of cerebral neocortex showing neuronal localization of signals (inset). Hybridization with a sense probe gave no specific signals (cerebral neocortex, right). Scale bars in (B) are equivalent to 500 µm (left panel) and 50 µm (inset and right panel). (C) RT–PCR analysis of expression of CLAC-P/Col XXV in cell type-specific primary cultures from mouse brains, showing neuron-specific expression of CLAC-P/Col XXV (upper panel). Expression of G3PDH as a control is shown in the lower panel.
The extracellular domain of CLAC-P/Col XXV is secreted by furin and deposited in senile plaques of AD brains
To determine which of the molecular subdomains of CLAC-P/Col XXV are deposited in AD brains, we raised antibodies against each subdomain and examined the reactivity of SPs as well as CLAC proteins extracted from AD brains. On immunohistochemistry of AD cortices, all antibodies directed to the putative extracellular portion of CLAC-P/Col XXV (i.e. anti-NC2-2, NC2-1, NC3 and NC4) immunolabeled SPs in a similar pattern to those with mAb 9D2, whereas anti-NC1 never labeled SPs (Figure 5A). Immunoblotting of formic acid extracts of AD cortices with anti-NC2-1, NC2-2 and NC3 showed three major positive bands migrating at 50, 70 and ∼100–110 kDa (CLAC50, CLAC70 and CLAC100, respectively), whereas anti-NC4 reacted exclusively with CLAC70, and mAb 9D2 preferentially labeled CLAC50 and CLAC100 (Figure 5B). In contrast, anti-NC1 never labeled positive bands. These results strongly suggested (i) that CLAC70 encompasses a large part of the extracellular portion of CLAC-P/Col XXV including the NC2–NC4 domains that may be secreted from CLAC-P/Col XXV; (ii) that CLAC50 is truncated somewhere between NC3 and NC4 domains; and (iii) that CLAC100 may be a dimer of CLAC50 (see the ‘purification’ section and Figure 2D).
To determine whether the extracellular portion of CLAC-P/Col XXV is secreted, we constructed expression plasmids encoding human CLAC-P/Col XXV and expressed them in cultured cells. Human CLAC-P/Col XXV stably transfected in HEK293 cells was expressed as an ∼80 kDa polypeptide that is positive for anti-NC1, NC2-1, NC2-2, NC3 and NC4 under reducing conditions, whereas ∼160 or ∼240 kDa high molecular weight species were also observed under non-reducing conditions, which may represent dimer or trimer forms, suggesting that CLAC-P/Col XXV forms a triple helix structure like other collagens (Figure 5D). In contrast, an ∼70 kDa polypeptide immunoreactive with anti-NC2, NC3 and NC4 but not with anti-NC1, that co-migrated with CLAC70 (compare Figure 5B and C), was detected in the culture media of transfected cells, which may be the ectodomain of CLAC-P/Col XXV liberated from the membrane as a secreted form (sCLAC/sCol XXV) (Figure 5C).
To learn more about the mechanism of secretion of CLAC-P/Col XXV ectodomain, we looked carefully at the amino acid sequences of the NC1 extracellular portion, and found a cluster of basic amino acids, 107KIRIAR112(E113), preceding E113. The presence of basic amino acids at the –1, –4 and –6 positions to E113 fulfills the consensus sequence for cleavage by furin convertase (Nakayama et al., 1997) between R112 and E113. To examine whether furin cleaves CLAC-P/Col XXV at the predicted site, we substituted two basic amino acid residues at positions –1 (R112) and –4 (R109) by alanine (RA mutant). RA mutant CLAC-P/Col XXV was expressed as an ∼80 kDa polypeptide in cell lysates, whereas sCLAC/sCol XXV was not detected in the culture media (Figure 5E). We then stably expressed human CLAC-P/Col XXV in RPE.40 mutant CHO cells that are known to lack furin (Duguay et al., 1995). Cell-associated CLAC-P/Col XXV was expressed as an ∼80 kDa protein, whereas sCLAC/sCol XXV was not secreted, in sharp contrast to the robust secretion of 70 kDa sCLAC/sCol XXV from wild-type CHO cells transfected with CLAC-P/Col XXV (Figure 5F). Finally, transfection of mouse furin cDNA into RPE.40 cells expressing CLAC-P/Col XXV restored secretion of sCLAC/sCol XXV, demonstrating that CLAC-P/Col XXV is cleaved by furin between R112 and E113, and the extracellular domain is secreted as sCLAC/sCol XXV (Figure 5F).
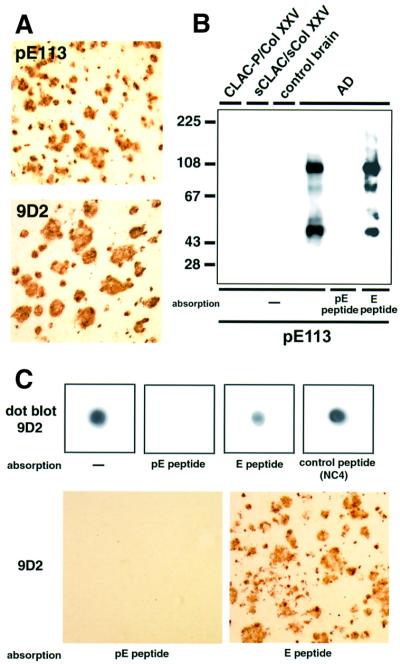
mAb 9D2 that initially detected CLAC polypeptide in AD brains reacted neither with full-length CLAC-P/Col XXV nor with sCLAC/sCol XXV (data not shown). We suspected that the N-terminus of CLAC in AD brain may undergo some post-translational modification, e.g. pyroglutamate modification, because (i) the N-terminus of sCLAC/sCol XXV created by furin cleavage is glutamic acid that can undergo pyroglutamate modification similarly to Aβ (Saido et al., 1995); and (ii) the N-terminus of CLAC recovered from AD brains was blocked upon amino acid sequencing (data not shown). To verify this possibility, we raised an antibody (anti-pE113) against a synthetic peptide corresponding to residues 113–118 of CLAC-P/Col XXV harboring pyroglutamate at position 113 (pE peptide). Anti-pE113 reacted exclusively with pE peptide, but not with a CLAC-P/Col XXV 113–118 peptide beginning with unmodified glutamic acid (E peptide), on dot blotting (data not shown). Anti-pE113 immunolabeled SPs in AD brains in a similar fashion to anti-NC2, NC3 and NC4 or mAb 9D2 (Figure 6A), and reacted with CLAC50, CLAC70 and CLAC100 on immunoblots, but not with CLAC-P/Col XXV or sCLAC/sCol XXV (Figure 6B), suggesting that CLAC deposited in AD brains starts at E113, which is pyroglutamate modified. We suspected that mAb 9D2 exhibited similar characteristics to anti-pE113, and performed absorption with pE and other peptides. Pre-absorption of mAb 9D2 with pE peptide, but not with E peptide or an irrelevant peptide, completely abolished immunolabeling of CLAC on dot blots as well as of SP in tissue sections (Figure 6C), suggesting that mAb 9D2 is also directed to the pyroglutamate modification of the N-terminus of CLAC that occurs in AD brains.
Fig. 6. The N-terminus of CLAC deposited in AD brains undergoes pyroglutamate modification. (A) Immunolabeling of SP in the frontal neocortex of AD brains with anti-pE113 (upper panel) and 9D2 (lower panel). (B) Immunoblot analysis of formic acid extracts of AD and control brains, as well as of CLAC-P/Col XXV and sCLAC/sCol XXV in HEK293 cells stably expressing CLAC-P/Col XXV, with anti-pE113. Note that pre-absorption of anti-pE113 with an immunogen peptide (pE peptide), but not with E peptide, abolished positive immunoreaction of CLAC. (C) Characterization of 9D2 as an N-terminal pyroglutamate modification-specific antibody. Dot blot analysis of formic acid extracts (upper panels) or tissue sections (lower panels) of AD brains by 9D2, showing abolition of positive reaction by absorption with pE peptide, but not with E peptide or irrelevant control peptides.
sCLAC/sCol XXV and CLAC-P/Col XXV specifically bind to a fibrillized form of Aβ
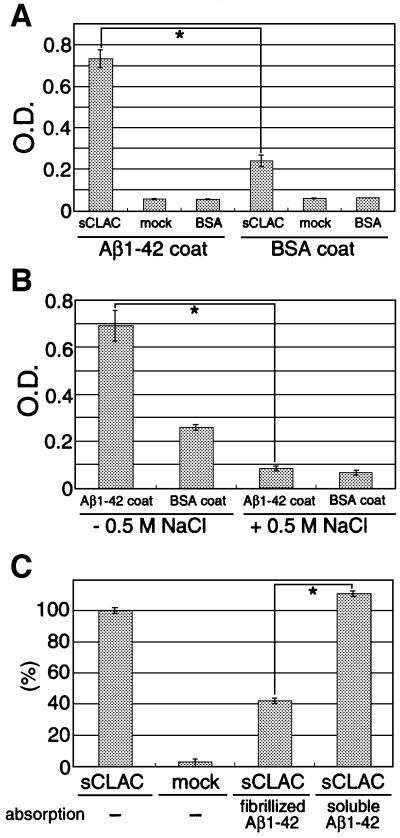
To determine whether CLAC directly binds to Aβ, we first studied the interaction between synthetic Aβ and sCLAC/sCol XXV secreted into media from HEK293 cells stably transfected with human CLAC-P/Col XXV by an enzyme-linked immunosorbent assay (ELISA)-based method. sCLAC/sCol XXV was bound specifically to fibrillized Aβ pre-coated onto well surfaces, but not to bovine serum albumin (BSA), suggesting that sCLAC/sCol XXV and Aβ bind directly to each other in vitro (Figure 7A). This binding was completely blocked in the presence of 0.5 M NaCl, implying an ionic interaction (Figure 7B). We next examined whether sCLAC/sCol XXV binds to either fibrillized or soluble forms of Aβ. When culture media containing sCLAC/sCol XXV pre-absorbed with soluble Aβ were loaded onto wells pre-coated with fibrillized Aβ, sCLAC/sCol XXV was bound to a similar extent as with non-absorbed media, whereas binding of sCLAC/sCol XXV to pre-coated Aβ was significantly reduced when media were pre-absorbed with fibrillized Aβ (Figure 7C), suggesting that sCLAC/sCol XXV binds specifically to the fibrillized form of Aβ.
Fig. 7. Binding of sCLAC/sCol XXV to Aβ. (A) Bound sCLAC on multi-well plates pre-coated with fibrillized Aβ1–42 or BSA after incubation with conditioned media from HEK293 cells stably transfected with CLAC-P/Col XXV (sCLAC), or that from mock-transfected cells (mock) or BSA as controls, was detected by incubation with anti-NC3 followed by immunoperoxidase reaction. (B) Binding of sCLAC/sCol XXV onto multi-well plates pre-coated with Aβ1–42 or BSA after incubation with conditioned media containing sCLAC/sCol XXV in the presence (right lanes) or absence (left lanes) of 0.5 M NaCl. Mean optical densities ± SD in four independent experiments are shown and *P <0.01 by Student’s t-test in (A) and (B). (C) Binding of sCLAC/sCol XXV to pre-coated Aβ1–42 after incubation with conditioned media of CLAC-P/Col XXV stable (sCLAC) or mock-transfected cells pre-absorbed with fibrillized or soluble Aβ1–42. Percentages ± SD of optical densities in four independent experiments relative to those from incubation of sCLAC/sCol XXV without absorption are shown.
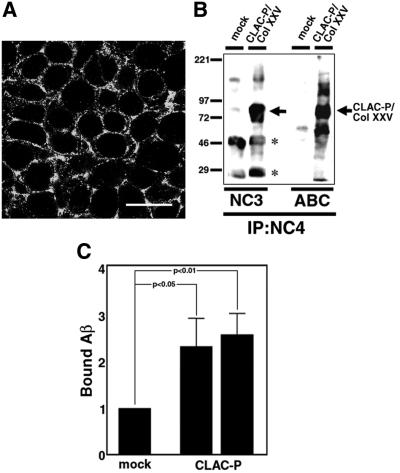
To examine whether membrane-tethered CLAC-P/Col XXV can also bind Aβ, we examined the interaction of CLAC-P/Col XXV with Aβ at the cell surface of transfected cells. For this purpose, we stably expressed human CLAC-P/Col XXV in HEK293 cells, and confirmed the cell surface distribution of CLAC-P/Col XXV by immunocytochemistry (Figure 8A) and cell surface biotin labeling (Figure 8B). We then incubated the HEK293 cells expressing CLAC-P/Col XXV with fibrillized Aβ, and quantitated cell-bound Aβ by immunoblotting. Two independent HEK293 stable cell lines transfected with CLAC-P/Col XXV bound ∼2.5-fold more Aβ compared with control cells, suggesting that CLAC-P/Col XXV binds to fibrillized Aβ at the cell surface (Figure 8C).
Fig. 8. Cell surface expression of CLAC-P/Col XXV and binding of Aβ therewith. (A) Immunofluorescence labeling of HEK293 cells stably transfected with human CLAC-P/Col XXV in a confluent state by anti-NC4. Note that cell surface and subplasmalemmal areas are labeled. Scale bar = 10 µm. (B) Surface biotin labeling of CLAC-P/Col XXV in HEK293 stable cells. After surface labeling of CLAC-P/Col XXV with biotin, cell lysates of CLAC-P/Col XXV stable cells or mock-transfected cells were immunoprecipitated by anti-NC4, followed by immunoblotting with anti-NC3 (left panels), or directly visualized by avidin–biotin complex (ABC). Note that CLAC-P/Col XXV migrating at ∼80 kDa is labeled by both anti-NC3 and ABC (arrow). Asterisks show non-specific reaction of immunoglobulin heavy and light chains. (C) Relative levels of fibrillized Aβ1–42 bound to the cell surface CLAC-P/Col XXV in two HEK293 stable cell lines expressing CLAC-P/Col XXV as assayed by quantitative immunoblotting with BC05 (relative to the level in mock-transfected cells as 1.0). Mean ± SD in six independent experiments for each cell line is shown. *P <0.01 and <0.05, respectively, by Student’s t-test in the two cell lines.
Discussion
In this present study, we have identified and characterized a novel proteinaceous component of SP amyloid in AD brains harboring collagen-like motifs, which we have designated CLAC, that is proteolytically derived from a brain-specific, novel transmembrane collagen, CLAC-P/Col XXV. Among numerous non-Aβ amyloid components thus far identified in AD brains, CLAC exhibits a number of unique characteristics, in addition to its collagen-like structure, implicating its role in AD pathogenesis as follows. (i) CLAC is deposited preferentially in the primitive or neuritic types of SPs in cerebral cortices that are highly characteristic of AD brains (Selkoe, 2001) but not in the diffuse plaques of non-demented aged individuals, diffuse deposits in subcortical structures and cerebellum in AD nor in cerebral amyloid angiopathy. This relative specificity of CLAC deposition in AD brain parenchym strongly argues for its significance in the neuro degenerative process of AD. (ii) CLAC is derived from a secreted form of CLAC-P/Col XXV (sCLAC/sCol XXV) through proteolytic cleavage by furin convertase, and the N-terminus of CLAC is pyroglutamate modified. (iii) sCLAC/sCol XXV or its precursor CLAC-P/Col XXV specifically binds to the fibrillized form of Aβ. (iv) CLAC is massively deposited in AD brains, and is one of the few SP components to be identified by protein chemical analysis and not simply by immunohistochemistry.
The co-existence of Aβ and CLAC immunoreactivities, as revealed by double fluorescence immunostaining, direct labeling of amyloid fibrils by immunoelectron microscopy and extraction of CLAC by formic acid, strongly supports the idea that CLAC is an integral component of amyloid deposits in AD brains. Immunoelectron microscopy showed that CLAC is associated with amyloid fibrils that form bundles intermixed with cellular processes. Taken together with the selective binding of fibrillized Aβ with sCLAC/sCol XXV in vitro, it seems possible that deposition of CLAC in SPs is closely related to the fibrillization of Aβ. However, amyloid cores at the central portions of classical type SPs and amyloid angiopathy, both of which are composed of highly fibrillized Aβ, were devoid of CLAC. The reason for this selectivity of CLAC incorporation into a subset of Aβ deposits is unknown at present. The neuron-specific expression of CLAC-P/Col XXV may explain the selective deposition of CLAC in brain parenchyma, and deposition of CLAC may need close interaction of amyloid fibrils with neuritic components. It is also possible that CLAC has affinity with a specific conformation of Aβ fibrils only exhibited in neuritic/primitive plaques, and promotes further fibrillization by binding to Aβ. CLAC has a collagen-like triple helix structure that hampers attacks by a number of proteases (Bruckner and Prockop, 1981), and this may protect against degradation of amyloid deposits, as shown with other Aβ-associated proteins (e.g. amyloid P component; Tennent et al., 1995), thereby promoting accumulation of Aβ. Further in vitro experiments as well as in vivo studies using transgenic mice are needed to elucidate the role of CLAC in Aβ deposition and AD pathology.
CLAC-P/Col XXV, the precursor of CLAC, exhibits a unique structure as a type II single-pass transmembrane protein with collagen-like motifs. A subset of proteins harboring collagen-like motifs with a similar transmembrane structure have been documented. Among these, Col XIII α1 chain shows homology to CLAC-P/Col XXV, and has a similar domain structure harboring three collagen-like and four non-collagenous domains, as well as a putative transmembrane domain in the NC1 portion (Hagg et al., 1998; Snellman et al., 2000). Col XIII is one of the non-fibril-forming collagens expressed in a number of systemic tissues including brain, but the expression level in brain seems to be lower than in other organs (Sund et al., 2001a). Recently it has been shown that Col XIII localizes to adherens junctions and plays a critical role in the maintenance of this type of junction (Sund et al., 2001b). Although we have not yet confirmed the distribution of endogenous CLAC-P/Col XXV polypeptides, because of its relatively low levels of protein expression and/or detection sensitivity, it is tempting to speculate that CLAC-P/Col XXV plays a crucial role in the formation and function of adherens junctions in neurons, which have been identified as puncta adherens (Peters et al., 1991) or synaptic adherens junction (Uchida et al., 1996). In addition, the apparent molecular size of CLAC-P/Col XXV on SDS–PAGE (∼80 kDa) was slightly larger than that predicted from the actual amino acid length (654 amino acids), implying the occurrence of some post-translational modification, e.g. glycosylation.
Another type II transmembrane collagen-like protein is scavenger receptor type A (SRA) with a shorter collagen-like stretch compared with CLAC-P/Col XXV (Kodama et al., 1990). SRA has been shown to bind to the fibrillized form of Aβ and contribute to the scavenging of Aβ by microglial cells (El Khoury et al., 1996). Our findings that CLAC-P/Col XXV binds to fibrillized Aβ at the cell surface and that CLAC-P/Col XXV is expressed specifically in neurons may favor the view that CLAC-P/Col XXV is involved in the cellular effects of fibrillized Aβ (e.g. by serving as a cell surface receptor that mediates Aβ neurotoxicity), rather than its scavenging. Another protein harboring collagen-like Gly–X–Y repeats associated with amyloid deposits in AD brains is complement component C1q A chain that also forms a triple helix and binds to the fibrillized form of Aβ. Notably, in vitro experiments have shown that C1q promotes fibrillization of Aβ (Webster et al., 1994). Direct effects of CLAC binding on Aβ fibrillization should also be examined using purified sCLAC/sCol XXV.
We have shown unequivocally that the extracellular portion of CLAC-P/Col XXV is secreted by furin convertase and that CLAC70 and CLAC50 correspond to the entire length and the N-terminal two-thirds of the CLAC-P/Col XXV extracellular portion (i.e. sCLAC/sCol XXV), respectively. The observation that CLAC polypeptides in AD brains are labeled positively by anti-NC2 or NC3 (and also by anti-NC4 in CLAC70) but not by anti-NC1 suggests that CLAC is derived from sCLAC/sCol XXV secreted by furin. Specific labeling of CLAC polypeptides by pyroglutamate-modified end-specific antibody at the furin cleavage site strongly supports this view. The identity of CLAC100 is not known, but is most probably an SDS-resistant dimeric form of CLAC50, because (i) CLAC100 showed immunoreactivity similar to CLAC50 (i.e. positive for anti-NC2 and NC3 but negative for anti-NC4); (ii) the peptide mapping pattern of API-digested CLAC50 was very similar to that of CLAC100; and (iii) no CLAC-P/Col XXV mRNA isoform that may yield an extracellular domain of a size of ∼100 kDa has been identified. It is interesting to note that a similar ∼100 kDa protein termed ‘AMY antigen’ has been documented in the insoluble fractions of AD brains using mAbs raised against the same fraction (Schmidt et al., 1997; Lemere et al., 1999). Although the molecular sizes of the AMY antigens are not completely identical to those of CLAC, similar immunolabeling patterns of SP with AMY mAbs (i.e. staining of a subset of SPs and lack of labeling of amyloid angiopathy) suggest that AMY antigens may correspond to a subset of CLAC polypeptides that we have detected in AD brains.
Furin is involved in the proteolytic processing of a number of peptide hormones or transmembrane proteins (Nakayama, 1997). Notably, it has been shown recently that the liberation of the C-terminus of a type II integral membrane protein, BRI, as ABri peptide (the major component of amyloid deposits in familial British dementia, FBD) is also mediated by furin (Kim et al., 1999). Moreover, we have shown that the N-terminus of CLAC deposited in AD brains that starts at the furin cleavage site (E113) of CLAC-P/Col XXV is pyroglutamate modified. Cyclization by intramolecular dehydration of glutamate is a non-enzymatic modification of an N-terminal glutamate that is observed physiologically in a number of polypeptides as well as in pathological conditions including Aβ deposits in AD brains (Saido et al., 1995) and ABri in FBD (Vidal et al., 1999). It has been suggested that this modification confers a resistance to proteolysis on Aβ deposited in AD brains (Saido et al., 1995). A similar stabilization of pyroglutamate-modified CLAC polypeptide may in turn intensify the structural integrity of β-amyloid deposits that contributes further to the progression of AD. However, it should be noted that immunoreactivities of antibodies against the pyroglutamate-modified N-terminus of CLAC (especially that of mAb 9D2) for CLAC70 was faint, suggesting that this modification may be a late phenomenon that takes place in concert with the processing/dimerization of CLAC. Interestingly, the proteolytic cleavage of BRI by furin has been shown to be up-regulated in FBD mutant BRI that has an elongated C-terminus with altered structure (Kim et al., 1999). Although any missense mutations or polymorphisms in the human CLAC-P/Col XXV gene linked to AD have not yet been detected, it should be determined whether the expression of CLAC-P/Col XXV or the proteolytic release of sCLAC/sCol XXV is increased in sporadic or familial forms of AD brains.
In summary, we have identified here CLAC, a novel amyloid component of SPs in AD brains with a unique collagen-like structure, that is proteolytically released by furin from its neuron-specific precursor, CLAC-P/Col XXV, and binds to the fibrillized form of Aβ. Further characterization of CLAC/CLAC-P/Col XXV will facilitate the elucidation of the structural and functional roles of this novel class of transmembrane collagen-like proteins (including Col XIII) in the brain, as well as furthering our understanding of the mechanism of β-amyloid formation and the development of novel therapeutic strategies for AD through intervening in the pathological actions of CLAC/CLAC-P/Col XXV.
Materials and methods
Generation of monoclonal antibodies against insoluble AD brain fractions
Amyloid-rich insoluble fractions from AD brains as immunogens were prepared as follows. Cortical gray matter was homogenized in TSI buffer [50 mM Tris–HCl pH 7.6, 150 mM NaCl, 0.5 mM diisopropyl fluorophosphate (DIFP), 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM EGTA, 1 µg/ml antipain, 1 µg/ml leupeptin, 1 µg/ml pepstatin, 1 µg/ml Nα-p-tosyl-L-lysine chloromethyl ketone (TLCK)], ultracentrifuged at 260 000 g for 30 min, and the pellets were separated by discontinuous sucrose density gradient centrifugation as described (Iwatsubo et al. 1996). The 1.5–2.2 M interface was extracted by 1% Triton X-100 containing 1.5 M KCl and the pellet was resuspended in 5 M urea and passed through a glass bead column to remove blood vessels. The pellets of eluates were resuspended in 50 mM Tris–HCl pH 7.6 containing 1% SDS, and injected subcutaneously into Balb/c mice to generate mAbs as described (Iwatsubo et al., 1996). Screening of hybridoma supernatants was performed by immunostaining smears of the 1.5–2.2 M interface of AD brains.
Purification and amino acid sequence analysis of CLAC polypeptide
Cortical gray matter from the brains of patients with AD or DS with severe AD pathology was homogenized in 3 vols of TSI buffer and centrifuged at 260 000 g for 20 min. The pellet was homogenized again in 4 vols of TSI buffer containing 1 M sucrose and centrifuged at 260 000 g for 20 min to remove myelin and lipid-rich contaminants. The pellet was resuspended in 5 vols of TSI buffer containing 0.32 M sucrose, passed through a glass bead column (see above) and centrifuged at 260 000 g for 20 min. The resultant pellet was homogenized in 3 vols of TSI buffer containing 2% SDS and centrifuged at 260 000 g for 20 min. The SDS-insoluble pellet was dissolved in 70% formic acid, centrifuged at 260 000 g for 20 min, and the formic acid-soluble fraction was desiccated by Speed-Vac and then resuspended in 6 M guanidine HCl. The guanidine HCl-soluble fraction was fractionated by RP-HPLC (Hewlett-Packard) on an Aquapore RP300 column (2.1 × 30 mm, Applied Biosystems) with a linear gradient of 0–64% ACN in 0.1% trifluoroacetic acid (flow rate: 0.2 ml/min). The fractions positive for 9D2 were separated further by size-exclusion HPLC on a TSK gel superSW3000 column (4.6 × 300 mm × 2; Tosoh). 9D2-positive fractions were desalted on an RP300 column by HPLC, suspended in 50 mM Tris–HCl pH 9.0 and digested with API or suspended in 50 mM Tris–HCl pH 8.0 and digested with Asp-N. These digests were separated by RP-HPLC on a Superspher Select B column (2.1 × 125 mm, Merck), and the peaks thus obtained were sequenced on a protein sequencer (Applied Biosystems).
cDNA cloning of CLAC-P/Col XXV
cDNA cloning of human or murine CLAC-P/Col XXV was performed by the RACE method. Briefly, partial cDNA fragments corresponding to the NC2 region of human CLAC-P/Col XXV were amplified by PCR using LA Taq polymerase, and a pair of degenerate primers based on partial amino acid sequences of this region were used as PCR primers, and the human brain Marathon-Ready cDNA library as a template. The initially obtained PCR fragment of 80 bp was extended by RACE using the same Marathon-Ready cDNA library, and a full-length cDNA encoding human CLAC-P/Col XXV was obtained. A cDNA fragment corresponding to nucleotides –34 to +2039 of human CLAC-P/Col XXV was subcloned into a mammalian expression vector pcDNA3.1. Murine CLAC-P/Col XXV cDNA was cloned by PCR using primers based on the human CLAC-P/Col XXV cDNA sequence and a murine brain Marathon-Ready cDNA library as a template. RA mutation of human CLAC-P/Col XXV was introduced by in vitro mutagenesis using a double-stranded DNA template.
Northern blotting, RT–PCR and in situ hybridization
Mouse Multiple Tissue Northern (MTN™) blots (Clontech) were hybridized with a 32P-radiolabeled mouse CLAC-P/Col XXV cDNA fragment (nucleotides +328 to +885) for 4 h at 65°C in rapid hybrid buffer (Amersham Pharmacia). After washing in SSC containing 0.1% SDS, blots were exposed to an imaging plate for 24 h and visualized with a bioimaging analyzer BAS-1800 (Fuji Film). For RT–PCR analysis of CLAC-P/Col XXV mRNA expression in cell type-specific murine brain cell cultures, primary neurons, astrocytes, microglia and meningeal fibroblasts were grown from E18 fetus or newborn Balb/c mice as described (Fukumoto et al., 1999), and mRNAs were extracted by Isogen and reverse-transcribed as described. RT–PCR analysis was performed as described (Tokuhiro et al., 1998) using murine CLAC-P/Col XXV-specific PCR primers. In situ hybridization was performed on paraffin sections of the brains of 8-week-old mice fixed by 4% paraformaldehyde, using a 3H-labeled single-stranded RNA probe corresponding to the sense and antisense nucleotide sequence of murine CLAC-P/Col XXV (nucleotides –267 to +333), and visualized by autoradiography using the emulsion method as previously described (Brady and Finlan, 1990).
Antibodies and immunoblot analysis
Polyclonal antibodies were raised in rabbits against synthetic peptides corresponding to the following amino acid sequences of CLAC-P/Col XXV conjugated with keyhole limpet hemocyanin (KLH): anti-NC1 against residues 14–27 (EPRSEDPTPAEQHC), anti-NC2-1 against 171–183 (GCNHGFLSADQQLIK), anti-NC2-2 against 155–169 (CKGEQGDQGPRMVFOK), anti-NC3 against 430–443 (DYNGNLHEALQRITC), anti-NC4 against 641–654 (LGPDGLPMPGCWQK) and anti-pE113 against pyroglutamate-modified 113–118 [(pyro)EAPSEC]. The antisera were affinity purified against immunogen peptides as described (Saido et al., 1995). SDS–PAGE was performed as previously described (Tomita et al., 1997) under a reducing condition unless otherwise noted. The immunoblots were developed using Immunostar reagents and visualized by LAS-1000plus as described (Tomita et al., 1997).
Immunohistochemistry and electron microscopy
Immunohistochemistry of human brain tissue was performed as described (Iwatsubo et al., 1996). Briefly, frontal cerebral cortices (Brodmann area 8/9) were obtained at autopsy, fixed in 10% formalin for 24 h and cut at 50 µm thickness. Floating sections were immunostained by the avidin–biotin method using 3,3′-diaminobenzidine (DAB) as a chromogen. The percentage areas covered by Aβ or CLAC immunoreactivities were quantitated by Mac SCOPE software, and two nearly consecutive areas (3.58 mm2/area) for each section were examined. Double fluorescence labeling and observation with a confocal laser scanning microscope were as described (Iwatsubo et al., 1996). For immunoelectron microscopy, floating sections were reacted with a primary antibody, followed by incubation with a secondary antibody tagged with 1 nm colloidal gold. After silver intensification treatment, sections were processed for electron microscopic observation. For double immunoelectron microscopic analysis, floating sections were incubated with a mixture of two primary antibodies, and then with a mixture of horseradish peroxidase (HRP)-tagged secondary antibody against one species and an antibody against the other species tagged with 1 nm colloidal gold. The reactions were visualized by DAB and silver intensification, respectively, and ultrathin sections were viewed without electron staining.
Cell culture, transfection and biotin surface labeling
HEK293 cells, monkey COS-1 cells, CHO cells and CHO derivative furin-deficient cells (RPE.40) (Moehring and Moehring, 1983) were cultured as described (Tomita et al., 1997). Transient and stable cell lines were generated by transfecting the cDNAs in pcDNA3.1 vector using Lipofectamine reagent and selection in Dulbecco’s modified Eagle’s medium (DMEM) containing hygromycine B at 167 µg/ml. Surface biotin labeling of CHO cells was performed according to a previously described method (Stephens et al., 1993). Briefly, cells on a 78 cm2 dish were incubated with 200 µg/ml of the dimethylsulfoxide (DMSO)-solubilized biotin derivative d-biotinyl-ε-aminocaproic acid N-hydroxysuccinimidester (Boehringer Mannheim) for 45 min on ice. Thus labeled cells were collected and lysed in IP buffer (TSI buffer including 0.5% NP-40 and 0.5% SDS). After pre-incubation with protein G–agarose beads, cell lysates were incubated with 5–10 µg of affinity-purified anti-NC4 at 4°C for 8 h. The resulting immunocomplexes were precipitated by incubating with protein G–agarose beads for 8 h at 4°C. Immuno precipitated proteins were visualized by reaction with HRP-tagged avidin.
Aβ binding assay
The binding assay of sCLAC/sCol XXV with Aβ immobilized on solid phase was performed according to a previously described method (Webster et al., 1997) with some modifications. Synthetic Aβ1–42 (Bachem) was solubilized in 1,1,1,3,3,3,-hexafluoro-2-propanol (HFIP) at a concentration of 1 mg/ml, and was dried and solubilized in phosphate-buffered saline (PBS) including 2% DMSO at 100 µg/ml and filtered through a 0.22 µm pore filter immediately prior to use. Fibrillized Aβ was generated by incubating Aβ at 37°C for 24 h with continuous agitation. A 50 µl aliquot of fibrillized Aβ was allowed to dry on wells of ELISA plates, blocked for 1 h with Block Ace, and washed with PBS containing 0.05% Tween-20 (PBST). Microplate wells were then incubated with culture media of HEK293 cells stably transfected with human CLAC-P/Col XXV or an empty vector for 1 h, washed with PBST, and then probed with anti-NC4. After incubation with HRP-tagged secondary antibody, bound sCLAC/sCol XXV was quantitated by development using a TMB microwell system. In a competition assay, culture media were pre-incubated with 10 µg of fibrillized or non-fibrillized Aβ at 4°C for 8 h and then loaded onto microplate wells pre-coated with fibrillized Aβ. For binding assay of Aβ with cell surface CLAC-P/Col XXV, HEK293 cells stably transfected with human CLAC-P/Col XXV or an empty vector were incubated with fibrillized Aβ1–42 (1 µg/ml) for 1 h at 37°C. Cell lysates (50 µg/lane) were analyzed by immunoblotting with BC05 together with known amounts of synthetic Aβ1–42, and bound Aβ was quantitated by measuring the intensity of Aβ bands migrating at 4 kDa using NIH image software.
Acknowledgments
Acknowledgements
The authors thank T.Pihlajaniemi for a kind suggestion on collagen nomenclature, Y.Imamura, A.Iwai, M.Yoshimoto, N.Dohmae, Y.Osada and A.Nishimura for helpful comments and discussions, T.Sakakura and M.Baba for excellent assistance in immunohistochemistry, T.C.Saido for anti-Aβ antibody, D.F.Steiner for RPE.40 cells, K.Nakayama for mouse furin cDNA, and the BF Research Institute for continuous support. This work was supported by grants-in-aid from the Ministry of Health and Welfare and the Ministry of Education, Science, Culture and Sports, Japan.
References
- Brady M.A.W. and Finlan,M.F. (1990) Radioactive labels: autoradiography and choice of emulsions for in situ hybridization. In Polak,J.M. and McGee,J.O’D. (eds), In situ Hybridization, Principles and Practice. Oxford University Press, Oxford, UK, pp. 31–57.
- Bruckner P. and Prockop,D.J. (1981) Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal. Biochem., 110, 360–368. [DOI] [PubMed] [Google Scholar]
- Dickson D.W. (1997) The pathogenesis of senile plaques. J. Neuropathol. Exp. Neurol., 56, 321–339. [DOI] [PubMed] [Google Scholar]
- Duff K. et al. (1996) Increased amyloid-β42(43) in brains of mice expressing mutant presenilin 1. Nature, 383, 710–713. [DOI] [PubMed] [Google Scholar]
- Duguay S.J., Lai-Zhang,J. and Steiner,D.F. (1995) Mutational analysis of the insulin-like growth factor I prohormone processing site. J. Biol. Chem., 270, 17566–17574. [DOI] [PubMed] [Google Scholar]
- El Khoury J., Hickman,S.E., Thomas,C.A., Cao,L., Silverstein,S.C. and Loike,J.D. (1996) Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature, 382, 716–719. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Tomita,T., Matsunaga,H., Ishibashi,Y., Saido,T.C. and Iwatsubo,T. (1999) Primary cultures of neuronal and non-neuronal rat brain cells secrete similar proportions of amyloid β peptides ending at Aβ40 and Aβ42. Neuroreport, 10, 2965–2969. [DOI] [PubMed] [Google Scholar]
- Gordon M.K., Gerecke,D.R., Hahn,R.A., Bhatt,P., Goyal,M. and Koch,M. (2000) Cloning of a new collagen, type XXIII, expressed in cornea. Invest. Ophthalmol. Vis. Sci., 41, S752. [Google Scholar]
- Gordon M.K., Hahn,R.A., Zhou,P., Kistler,A., Gerecke,D.R. and Koch,M. (2002) Structure of collagen XXIV and its expression in cornea and retina. Invest. Ophthalmol. Vis. Sci., in press. [Google Scholar]
- Hagg P., Rehn,M., Huhtala,P., Vaisanen,T., Tamminen,M. and Pihlajaniemi,T. (1998) Type XIII collagen is identified as a plasma membrane protein. J. Biol. Chem., 273, 15590–15597. [DOI] [PubMed] [Google Scholar]
- Holtzman D.M. et al. (2000) Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA, 97, 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry M.C., Soriano,F., McNamara,M., Page,K.J., Schenk,D., Games,D. and Hyman,B.T. (1997) Aβ deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J. Neurosci., 17, 7053–7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo T., Odaka,A., Suzuki,N., Mizusawa,H., Nukina,N. and Ihara,Y. (1994) Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43). Neuron, 13, 45–53. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T., Yamaguchi,H., Fujimuro,M., Yokosawa,H., Ihara,Y., Trojanowski,J.Q. and Lee,V.M.-Y. (1996) Purification and characterization of Lewy bodies from the brains of patients with diffuse Lewy body disease. Am. J. Pathol., 148, 1517–1529. [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire,H.G., Unterbeck,A., Salbaum,J.M., Masters,C.L., Grzeschik,K.H., Multhaup,G., Beyreuther,K. and Muller-Hill,B. (1987) The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature, 325, 733–736. [DOI] [PubMed] [Google Scholar]
- Katzman R., Terry,R., DeTeresa,R., Brown,T., Davies,P., Fuld,P., Renbing,X. and Peck,A. (1988) Clinical, pathological and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann. Neurol., 23, 138–144. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Wang,R., Gordon,D.J., Bass,J., Steiner,D.F., Lynn,D.G., Thinakaran,G., Meredith,S.C. and Sisodia,S.S. (1999) Furin mediates enhanced production of fibrillogenic ABri peptides in familial British dementia. Nature Neurosci., 2, 984–988. [DOI] [PubMed] [Google Scholar]
- Kodama T., Freeman,M., Rohrer,L., Zabrecky,J., Matsudaira,P. and Krieger,M. (1990) Type I macrophage scavenger receptor contains α-helical and collagen-like coiled coils. Nature, 343, 531–535. [DOI] [PubMed] [Google Scholar]
- Lemere C.A., Grenfell,T.J. and Selkoe,D.J. (1999) The AMY antigen co-occurs with Aβ and follows its deposition in the amyloid plaques of Alzheimer’s disease and Down syndrome. Am. J. Pathol., 155, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Yee,A., Brewer,H.B.,Jr, Das,S. and Potter,H. (1994) Amyloid-associated proteins α1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer β-protein into filaments. Nature, 372, 92–94. [DOI] [PubMed] [Google Scholar]
- Mann D.M.A., Pickering-Brown,S.M., Takeuchi,A. and Iwatsubo,T. (2001) Amyloid angiopathy and variability in amyloid β deposition is determined by mutation position in presenilin-1 linked Alzheimer’s disease. Am. J. Pathol., 158, 2165–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C.L., Simms,G., Weinman,N.A., Multhaup,G., McDonald,B.L. and Beyreuther,K. (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl Acad. Sci. USA, 82, 4245–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring J.M. and Moehring,T.J. (1983) Strains of CHO-K1 cells resistant to Pseudomonas exotoxin A and cross-resistant to diphtheria toxin and viruses. Infect. Immun., 41, 998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki H., Gejyo,F. and Nakakuki,K. (1997) Concentration-dependent inhibitory effects of apolipoprotein E on Alzheimer’s β-amyloid fibril formation in vitro. Biochemistry, 36, 6243–6250. [DOI] [PubMed] [Google Scholar]
- Nakayama K. (1997) Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem. J., 327, 625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba Y., Tomonaga,M., Kawasaki,H., Otomo,E. and Ikeda,K. (1991) Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt–Jakob disease. Brain Res., 541, 163–166. [DOI] [PubMed] [Google Scholar]
- Peters A., Palay,S. and Webster,H.de F. (1991) Synapses. In The Fine Structure of the Nervous System, 3rd edn. Oxford University Press, New York, pp.138–211.
- Saido T.C., Iwatsubo,T., Mann,D.M.A., Shimada,H., Ihara,Y. and Kawashima,S. (1995) Dominant and differential deposition of distinct β-amyloid peptide species, AβN3(pE), in senile plaques. Neuron, 14, 457–466. [DOI] [PubMed] [Google Scholar]
- Schmidt M.L., Lee,V.M.-Y., Forman,M., Chiu,T.S. and Trojanowski,J.Q. (1997) Monoclonal antibodies to a 100-kd protein reveal abundant Aβ-negative plaques throughout gray matter of Alzheimer’s disease brains. Am. J. Pathol., 151, 69–80. [PMC free article] [PubMed] [Google Scholar]
- Selkoe D.J. (2001) Alzheimer’s disease: genes, proteins and therapy. Physiol. Rev., 81, 741–766. [DOI] [PubMed] [Google Scholar]
- Snellman A., Tu,H., Vaisanen,T., Kvist,A.P., Huhtala,P. and Pihlajaniemi,T. (2000) A short sequence in the N-terminal region is required for the trimerization of type XIII collagen and is conserved in other collagenous transmembrane proteins. EMBO J., 19, 5051–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L.E., Sonne,J.E., Fitzgerald,M.L. and Damsky,C.H. (1993) Targeted deletion of β1 integrins in F9 embryonal carcinoma cells affects morphological differentiation but not tissue-specific gene expression. J. Cell Biol., 123, 1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W.J., Saunders,A.M., Schmechel,D., Pericak-Vance,M., Enghild,J., Salvesen,G.S. and Roses,A.D. (1993) Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl Acad. Sci. USA, 90, 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund M., Ylonen,R., Tuomisto,A., Sormunen,R., Tahkola,J., Kvist,A.P., Kontusaari,S., Autio-Harmainen,H. and Pihlajaniemi,T. (2001a) Abnormal adherence junctions in the heart and reduced angiogenesis in transgenic mice overexpressing mutant type XIII collagen. EMBO J., 20, 5153–5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund M., Vaisanen,T., Kaukinen,S., Ilves,M., Tu,H., Autio-Harmainen,H., Rauvala,H. and Pihlajaniemi,T. (2001b) Distinct expression of type XIII collagen in neuronal structures and other tissues during mouse development. Matrix Biol., 20, 215–231. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Cheung,T.T., Cai,X.-D., Odaka,A., Otvos,L.,Jr, Eckman,C., Golde,T.E. and Younkin,S.G. (1994) An increased percentage of long amyloid β protein secreted by familial amyloid β protein precursor (βAPP717) mutants. Science, 264, 1336–1340. [DOI] [PubMed] [Google Scholar]
- Tennent G.A., Lovat,L.B. and Pepys,M.B. (1995) Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer disease and systemic amyloidosis. Proc. Natl Acad. Sci. USA, 92, 4299–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuhiro S., Tomita,T., Iwata,H., Kosaka,T., Saido,T.C., Maruyama,K. and Iwatsubo,T. (1998) The presenilin 1 mutation (M146V) linked to familial Alzheimer’s disease attenuates the neuronal differentiation of NTera 2 cells. Biochem. Biophys. Res. Commun., 244, 751–755. [DOI] [PubMed] [Google Scholar]
- Tomita T. et al. (1997) The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid β protein ending at the 42nd (or 43rd) residue. Proc. Natl Acad. Sci. USA, 94, 2025–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N., Honjo,Y., Johnson,K.R., Wheelock,M.J. and Takeichi,M. (1996) The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J. Cell Biol., 135, 767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal R., Frangione,B., Rostagno,A., Mead,S., Revesz,T., Plant,G. and Ghiso,J. (1999) A stop-codon mutation in the BRI gene associated with familial British dementia. Nature, 399, 776–781. [DOI] [PubMed] [Google Scholar]
- Webster S., O’Barr,S. and Rogers,J. (1994) Enhanced aggregation and β structure of amyloid β peptide after coincubation with C1q. J. Neurosci. Res., 39, 448–456. [DOI] [PubMed] [Google Scholar]
- Webster S., Bonnell,B. and Rogers,J. (1997) Charge-based binding of complement component C1q to the Alzheimer amyloid β-peptide. Am. J. Pathol., 150, 1531–1536. [PMC free article] [PubMed] [Google Scholar]