Abstract
Voltage-dependent ion channels gate open in response to changes in cell membrane voltage. This form of gating permits the propagation of action potentials. We present two structures of the voltage-dependent K+ channel KvAP, in complex with monoclonal Fv fragments (3.9 Å) and without antibody fragments (8 Å). We also studied KvAP with disulfide cross-bridges in lipid membranes. Analyzing these data in the context of the crystal structure of Kv1.2 and EPR data on KvAP we reach the following conclusions: (i) KvAP is similar in structure to Kv1.2 with a very modest difference in the orientation of its voltage sensor; (ii) mAb fragments are not the source of non-native conformations of KvAP in crystal structures; (iii) because KvAP contains separate loosely adherent domains, a lipid membrane is required to maintain their correct relative orientations, and (iv) the model of KvAP is consistent with the proposal of voltage sensing through the movement of an arginine-containing helix-turn-helix element at the protein-lipid interface.
Keywords: membrane protein, protein-lipid interface, voltage-gated ion channel, voltage sensor
Voltage-dependent ion channels “sense” voltage differences across the cell membrane and open or close in response to its value (1). These channels contain a centrally located pore surrounded by four voltage sensors. Voltage-dependent K+ (Kv) channels are tetramers with four identical subunits, each with six transmembrane segments (S1-S6): S5 and S6 form the central pore at the interface between the subunits, and S1-S4 form the voltage sensors (2, 3). The voltage sensors have four to seven positively charged amino acids (usually arginine) on S4, known as gating charges, and fewer negatively charged amino acids (aspartate or glutamate) distributed on S1, S2, and S3. The voltage sensors undergo a conformational change when the pore gates open, coupling movement of the S4 gating charges within the membrane electric field to channel open probability.
The first structure of a Kv channel, termed KvAP, from the archeabacterium Aeropyrum Pernix (4), was determined by crystallizing the channel as a complex with a monoclonal Fab fragment attached to its voltage sensors [Protein Data Bank (PDB) ID code 1ORQ] (5). In that structure the voltage sensors are in a non-native conformation, displaced toward the intracellular side of the transmembrane pore. Together with a second structure of the voltage sensor alone, termed the isolated voltage sensor (PDB ID code 1ORS), the following ideas about voltage-dependent gating were proposed (5, 6): that the voltage sensor is a very mobile structure, presumably because it must carry charged amino acids through the membrane electric field; that the charge bearing S4 forms a helix-turn-helix with the C-terminal half of S3 (S3b), and that the helix-turn-helix moves at the protein-lipid interface a large distance. Experiments using avidin capture of biotin linked to the voltage sensor indicated that four of the S4 arginine amino acids translate 15-20 Å across the membrane. These structural and functional studies formed the basis for a conceptual model in which the helix-turn-helix “paddle” element shifts its position at the protein-lipid interface, opening the pore while moving its charged amino acids (6).
Because of the non-native conformation of the voltage sensors in the crystal, the KvAP structure left two compelling questions unanswered. The first, which is important for understanding the mechanism of voltage-dependent gating, is what is the native membrane conformation of KvAP? The second question, related to the mechanism of gating but also relevant to the more general subject of membrane protein structure, is why does KvAP not maintain a native conformation in crystals? Much speculation surrounded this second question, including the wonder whether antibody fragments can “distort” membrane protein structures.
This study attempts to address both questions. We have determined two additional structures of KvAP, bound to monoclonal Fv fragments and in the absence of antibody fragments altogether, and we have carried out cross-link studies with KvAP in lipid membranes. Analysis of these data in the context of the crystal structure of a eukaryotic relative of KvAP, known as Kv1.2 (7, 8), and in the context of published EPR data on KvAP (9), leads to a constrained model for a native structure of KvAP in lipid membranes. The results also lead to an interesting general conclusion about the structure of Kv channels: because they are comprised of separate loosely adherent domains (pore and voltage sensors) embedded in the membrane, an intact lipid membrane is required to maintain a native orientation of domains with respect to each other. We anticipate that other complex membrane proteins exhibiting this property will be identified in the future, and discernment of their structures will require a combination of structural and biochemical techniques, similar to the approaches used here.
Methods
Structure Determination. The KvAP channel was expressed and purified as described (5) with modifications (Supporting Text, which is published as supporting information on the PNAS web site). Monoclonal Fv fragments were produced by expression in Escherichia coli and purified by using Co2+ affinity and gel filtration chromatography. A first crystal form was grown by vapor diffusion in the absence antibody fragments, and maps were calculated by using density modified molecular replacement phases (pore model) (10). A second crystal form was grown by vapor diffusion in the presence of Fv fragments, phases were determined by molecular replacement and heavy atom derivitization by using Tl+ (10), and an atomic model was built and refined (Table 1, which is published as supporting information on the PNAS web site) (11, 12).
Cross-Bridge Studies. Biochemical cross-bridge studies on KvAP were carried out after introducing cysteine residues at specific locations in the pore and voltage sensor by using QuikChange (Stratagene). Inside-out membrane vesicles were prepared as described with slight modification (13). Air oxidation (no catalysts) was allowed to occur in membranes overnight at room temperature. N-ethyl-maleimide (20 mM) was added to the vesicles before SDS solubilization to block remaining free cysteines. Western blot was carried out by using an antibody raised against the KvAP channel. Further details are provided in Supporting Text.
Results
Crystal Structures With and Without Antibody Fragments. The structure determinations were carried out by using a truncated version of KvAP (36 C-terminal amino acids deleted). The deleted amino acids follow the sixth (last) membrane-spanning α-helix and were disordered in the original structure of KvAP. The function of the truncated channel in lipid membranes is very similar to the full-length KvAP channel.
Two additional crystal forms were grown by using the detergent-like lipid 1,2-diheptanoyl-sn-glycero-3-phosphocholine. The most important difference between these crystals is that one was grown in the absence of antibody fragments and the other as a complex with monoclonal Fv fragments attached to the voltage sensors. Triclinic crystals of KvAP without antibody fragments diffracted to a resolution of 8 Å. Molecular replacement using the KvAP pore without voltage sensors (from PDB ID code 1ORQ) showed two channels in the unit cell (10). An electron density map shows clear helical segments for the pore and the voltage sensors, which were absent in the model phases (Fig. 1a). The helical organization of KvAP is discernable at this resolution. In contrast to the initial structure of KvAP (PDB ID code 1ORQ), the voltage sensors are located entirely within the “plane of the lipid membrane.” The crystal lattice is supported by contacts between the helix-turn-helix voltage sensor paddle elements, which make contacts with each other from adjacent channels (Fig. 1b).
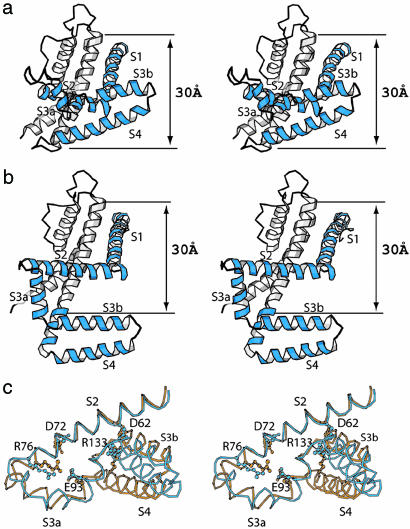
Fig. 1.
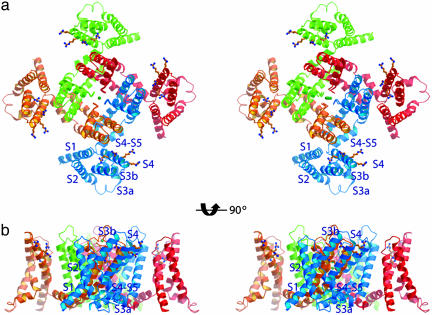
KvAP structures are in similar conformations with and without Fv fragments. (a) Four-fold averaged electron density map (1.0 σ) of KvAP at 8 Å calculated with (2 Fo - Fc) Fourier coefficients with the voltage sensor omitted. Two KvAP pores were used to calculate the map. The α-carbon traces of the KvAP structure (dark brown) were generated by superposition of the KvAP-Fv complex structure onto the KvAP pore molecular replacement solutions. (b) Contacts in the KvAP crystal. A layer of channel molecules is formed by lateral packing of voltage sensors with neighboring channel molecules within a layer and end-to-end packing between layers. Two channels (red), surrounded by black lines, define the unit cell. (c) Native sharpened 2-fold averaged electron density map (1.0 σ) of the KvAP-33H1 Fv complex at 3.9 Å calculated with Fo Fourier coefficients. Combined phases (single isomorphous replacement with anomalous scattering and partial molecular replacement phases) were used for map calculation. The α-carbon traces of the KvAP-Fv structure were colored dark brown. (d) Crystal packing in the KvAP-33H1 Fv crystal as viewed down the crystallographic 4-fold axis. Channels are colored blue, and Fv fragments are colored green. Four channels and 16 Fvs, surrounded by black lines, comprise a unit cell.
Tetragonal crystals of KvAP in complex with Fv fragments diffracted X-rays to a resolution of 3.9 Å. Molecular-replacement phases using the KvAP pore were combined with heavy atom phases from Tl+-soaked crystals (10). After solvent flattening and averaging, a good-quality electron density map (Fig. 1c) allowed building of a model and refinement to Rfree 0.39 (10-12). This crystal lattice is mediated by contacts between Fv fragments (Fig. 1d).
The α-carbon trace of KvAP in the 8-Å electron density map (Fig. 1a) is the KvAP-Fv complex structure after removal of the Fv fragments. It is evident that the channel structures in the two crystal forms are very similar even though one has Fv fragments bound to its voltage sensors and the other does not. The Fv fragments have not had a significant influence on the conformation of the voltage sensors. Because the KvAP-Fv complex structure is better defined it will be the focus of further consideration.
Comparison with Previous KvAP Structures. The structure of a single subunit from the KvAP tetramer (Fig. 2a) is shown from the side with the extracellular surface above. The essential features of this structure include voltage sensors (helices S1-S4) surrounding the centrally located pore, S1 and S2 helices wrapped around the pore, and helix-turn-helix S3b-S4 voltage sensor paddles positioned adjacent to the S2 helices. The major difference between this structure and the original KvAP crystal structure (Fig. 2b; PDB ID code 1ORQ) is the position of the paddle, which in this structure is drawn near to S2 at the level of the membrane rather than extended toward the cytoplasm.
Fig. 2.
Structure of the KvAP-33HI complex and comparison with previous KvAP structures. (a) Stereo view of a single KvAP subunit from the side with extracellular solution above. S1-S4 helices are colored blue. (b) Stereo view of a single KvAP subunit of the KvAP-6E1 Fab complex (PDB ID code 1ORQ). (c) Comparison with the isolated voltage sensor structure. Stereo view of a superposition of the isolated voltage sensor (gold, PDB ID code 1ORS) and the KvAP-Fv complex (blue). The S2 helix (Leu-55 to Tyr-75) was used for superposition, and the S1 helix is not shown. Residues Asp-62, Asp-72, Arg-76, Glu-93, and Arg-133, which are important for channel function, are shown in ball-and-stick representation.
In this structure the position of the paddle with respect to S2 is very similar to that observed in the crystal structure of the isolated voltage sensor, in which Arg-133 on S4 forms a salt bridge with Asp-62 on S2 (PDB ID code 1ORS) (Fig. 2c). The same salt bridge is present in the full-length channel structure. The presence of this salt bridge appears to be correlated with pH: crystals of the original KvAP structure (in which the paddle is pulled away from S2 and the salt bridge is broken) were grown at pH 5.0 (5), crystals of the isolated voltage sensor were grown at pH 7.5 (5), and crystals of the KvAP-Fv and KvAP (8 Å) crystals were grown at pH 8.5 and 9.0, respectively. Thus, conditions that favor salt-bridge formation (higher pH) support a conformation in which the paddle remains near S2, within the plane of the membrane. This conformation of the paddle with respect to S2 and salt-bridge formation involving Arg-133 is now observed in more than one crystal structure of KvAP. Shaker K+ channels do not function when the corresponding arginine residue (position 377) is mutated (2). For these reasons we suspect that the observed salt bridge and position of the voltage sensor paddle with respect to the S2 helix is functionally relevant.
The S1 and S2 helices in the structures have the same horizontal disposition as seen previously, although in membranes we know they must have a more vertical orientation (9, 23) (Fig. 2 a and b). In this regard the crystal structures in this study, like the original structure, do not represent a truly native conformation of the voltage sensor. The horizontal positioning of S1 and S2 cannot be attributed to the antibody fragments bound to the voltage sensor because we observe the same conformation in three structures: with Fab fragments (5), with Fv fragments, and without antibody fragments. If not antibody fragments, what accounts for the horizontal positioning of these helices?
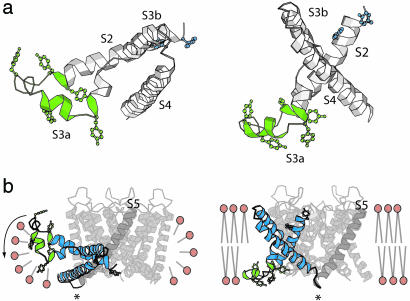
Role of the Lipid Bilayer in Voltage Sensor Orientation. Several features of the crystal structures in this study provide a likely explanation for the horizontal orientation of the voltage sensor in detergent micelles. The rather elaborate turn connecting S2 to S3 contains five tyrosine amino acids that project out from its perimeter (Fig. 3a). In the crystal this turn is located at a level corresponding to the middle of the membrane (Fig. 2a), which is unexpected because tyrosine side chains are energetically favored to reside at the interface between the hydrophobic and head group layers (e.g., near the edge of the membrane) (14-16). The detergent-like lipid 1,2-diheptanoyl-sn-glycero-3-phosphocholine used to crystallize the channel does not form planar lipid membranes, only micelles, so a planar membrane constraint is absent in the crystal. In the context of a lipid membrane with planar boundaries we should expect the tyrosine residues to be driven toward the interface layer. A rigid body rotation of a voltage sensor domain (or of helices S2-S4) around its connection to S5, as shown (Fig. 3b), would place the tyrosine residues at the interface. The same rotation would also bring the S1-S2 loop (which has near it several tyrosine residues) to the extracellular lipid-water interface. From these energetic considerations we suggest that a planar lipid membrane constrains the voltage sensor domains with respect to the pore, and when the channel is removed from the membrane, the unconstrained domains can easily adopt a non-native orientation. This behavior is interesting because it would be expected to occur only if the domains are loosely attached to the pore as fairly self-contained structural units.
Fig. 3.
The structure of the S2 to S3 turn. (a) The turn connecting the S2 to S3 helices (green, Tyr-63 to Pro-95) from two different viewpoints. Tyr residues are shown as ball-and-stick representation. (b) Possible transformation of the voltage sensor upon detergent extraction. (Left) The structure of KvAP in a detergent micelle. S2, S3, and S4 helices are colored blue, and the turn connecting S2 to S3 helices is colored green. (Right) The putative conformation of KvAP in the membrane. Asterisk marks the pivot point of proposed voltage sensor rotation.
Several lines of evidence support the idea that the voltage sensors do indeed function as nearly self-contained structural domains. First, expression of the isolated voltage sensor of KvAP (in the absence of a pore) enabled us to determine its structure, and upon expression the domain is targeted to and stable in the cell membrane (5). Second, voltage dependence is transferable by splicing a voltage sensor onto a nonvoltage-dependent K+ channel (17). And third, a voltage-dependent phosphatase with an S1-S4 sequence attached to a soluble enzyme shows that the voltage sensor can operate on a target other than an ion channel (18).
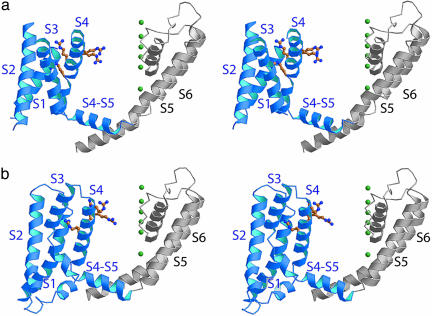
Defining the Position of the KvAP Voltage Sensor by Analogy to Kv1.2. The structure of a related Kv channel, Kv1.2 from the rat, helps to define the native position of the KvAP voltage sensor with respect to the pore (Fig. 4a) (PDB ID code 2A79) (8). Potential distortions of the Kv1.2 voltage sensor upon extraction from the membrane are restricted by the connection of the S1 helix to a cytoplasmic domain (not shown in Fig. 4a), which does not exist in KvAP. The KvAP voltage sensor (Fig. 2a) can be adjusted to have the appearance of the sensor in Kv1.2 by rotating the sensor vertically (as was done in Fig. 3b), straightening the S4-S5 linker helix, and folding the S1 helix back onto the body of the sensor, similar to its position in the KvAP isolated voltage sensor crystal structure (PDB ID code 1ORS) (Fig. 4b). In the resulting KvAP model, the S4-S5 linker is like that in Kv1.2: it forms an amphipathic helix with a hydrophobic surface (to face the membrane) and a hydrophilic surface (to face the cytoplasm) (Fig. 5a). Furthermore, in KvAP the sharp turn connecting the S4 transmembrane helix to the S4-S5 linker interfacial helix occurs at the position of a glycine amino, which is frequently found at such turns. The straightforward transformation from the crystal structures of KvAP to the model in Fig. 4b, with preservation of chemical features that correspond well to those in Kv1.2, provides good evidence that the native structure of KvAP is similar to Kv1.2.
Fig. 4.
A model of KvAP based on the structure of Kv1.2. (a) A subunit of the Kv1.2 (PDB ID code 2A79) viewed from the side. The voltage sensor region (S1-S4) is colored blue, and the pore region (S5-S6) is colored gray. A queue of K+ ions (green spheres) is shown as a reference point for comparison with the KvAP model. (b) The KvAP model. The model was constructed by tilting S2-S4 helices of the KvAP structure (PDB ID code 2A0L), adjusting the S4-S5 linker to resemble the linker in the Kv1.2 structure (PDB ID code 2A79), folding the S1 helix similar to its position in the isolated voltage sensor (PDB ID code 1ORS), and repositioning the sensor slightly to account for EPR O2 (lipid) accessibility (9).
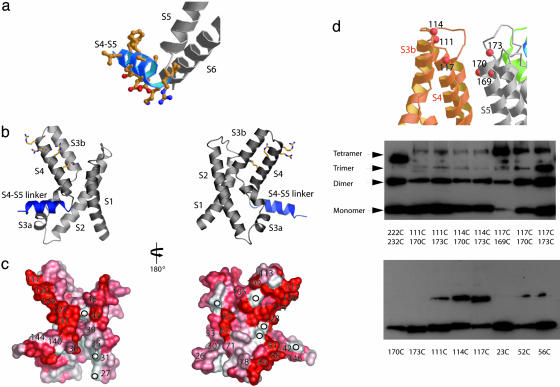
Fig. 5.
Mapping EPR O2 (lipid) accessibility data onto the voltage sensor region of the KvAP. (a) The S4-S5 linker (colored blue) of the KvAP model. The side chains of the S4-S5 linker shown as a ball-and-stick representation indicate the amphipathic nature of this linker region. (b and c) The EPR O2 (lipid) accessibility data (9) mapped onto the voltage sensor structure. O2 accessibility ranges from white (low accessibility) to red (high accessibility). Numbers in c indicate amino acid position, circles show positions where data are not available. (d) Western blot analysis of cross-linking. Membrane vesicles containing single or double Cys mutations (at numbered residues) in KvAP were subjected to air oxidation. The size of the covalent monomer, dimer, trimer, and tetramer are indicated. Residues 222 and 232 on the S6 helix were chosen as a control for cross-linking based on the crystal structure of KvAP. The 222C/232C cross-linked tetramer migrates slightly faster than the other cross-linked tetramers.
Biochemical Information on KvAP in Lipid Membranes. Perozo and colleagues (9) have used EPR spectroscopy to measure lipid exposure of protein surfaces of the KvAP voltage sensor. These data agree well with the position of the voltage sensor in Kv1.2 (8) (Fig. 4a) with one small discrepancy. In the Kv1.2 crystal structure, one edge of the S4 helix leans against the S5 helix of a neighboring subunit; however, the corresponding amino acids in KvAP exhibit high lipid exposure according to the EPR data (Fig. 5 b and c, residues 124 and 127). In addition, a face of S1 in Kv1.2 that would be expected to exhibit high lipid exposure (because it is on the lipid-facing surface) appears in KvAP to be in a protein environment (i.e., neither lipid nor water) according to the EPR data (Fig. 5 b and c, residues 31, 35, and 39). These differences between expected lipid exposure on the basis of the Kv1.2 crystal structure and observed lipid exposure of corresponding amino acids in KvAP suggest that the voltage sensor in KvAP might be rotated slightly with respect to the pore, compared with the sensor in Kv1.2. The rotation of the voltage sensor that would be required to account for the EPR data is very modest and in fact has already been made in the model shown in Fig. 4b, relative to the Kv1.2 voltage sensor in Fig. 4a. That small variations in the orientation of the voltage sensors might occur between KvAP and Kv1.2 is not surprising, especially because the constraint imposed by the linker to the T1 domain in Shaker family Kv channels (e.g., Kv1.2) does not exist in KvAP.
We carried out disulfide cross-bridge studies to further examine the relationship between KvAP and Kv1.2. Several studies have shown that a cysteine residue introduced at the position of the first arginine residue on S4 (the extracellular-most arginine, corresponding to position 117 in KvAP) in the Shaker K+ channel (which is very similar to Kv1.2) readily forms a cross-bridge with a cysteine introduced near the extracellular end of S5 of a neighboring subunit (19). We observe a qualitatively similar result with KvAP channels in membranes (Fig. 5d). In KvAP, however, we do not observe the same quantitative degree of complete covalent tetramer formation that is observed in the Shaker K+ channel (19). The data on KvAP are thus consistent with the voltage sensor being in a similar location as in Shaker (or Kv1.2), but the lower efficiency of tetramer formation is consistent with the possibility that S4 may be slightly further away from S5, as the EPR data suggest.
We note additional aspects of the disulfide cross-bridge studies on KvAP channels that parallel previous data on Shaker K+ channels. Cross-bridge formation between cysteine residues on the voltage sensor paddle and the extracellular side of S5 do not depend highly on the precise location of the cysteines (19-22), and a single cysteine residue near the extracellular “tip” of the voltage sensor paddle can result in the formation of covalent subunit dimers, presumably through linkage of voltage sensor paddles from adjacent subunits (20). The nonspecificity of cross-bridge formation and covalent subunit dimerization mediated by single cysteine residues on the voltage sensor paddle suggests that the paddle is a highly mobile unit (Fig. 5d).
Discussion
Two additional crystal structures of KvAP show the voltage sensors within the plane of the lipid membrane, but the domains still deviate from their expected vertical orientation relative to the pore. Antibody fragments per se are not the cause of this deviation because it is observed in the presence of Fab fragments and Fv fragments and in the absence of antibody fragments. Therefore, it is incorrect to conclude that antibody fragments should be avoided in the crystallization of membrane proteins. One important conclusion of this study, and the answer to the second question raised in the Introduction, is that KvAP appears to require an intact lipid membrane to maintain a native conformation, because the voltage sensors do not adhere tightly to the pore. The sensors, like the pore, have their own intrinsic structural and chemical properties to match the membrane's hydrophobic core, interface, and water layers, ensuring that they “float” properly in the lipid membrane. Kv channels might be the first examples of membrane proteins with separate, weakly attached membrane-spanning domains, but other important membrane proteins with this property will likely be identified and studied in the future.
For proteins like KvAP the detergent micelle may be an imperfect mimic of the lipid bilayer, but detergent-mediated crystallization is still a valuable approach for understanding such proteins. A non-native protein structure simply means that additional information is required to help interpret what we see. In the present analysis we have combined crystal structures of the KvAP channel with biochemical cross-bridge studies in membranes, EPR data from the literature, and comparisons with a related Kv channel structure to arrive at a plausible answer to the first question raised in the Introduction: what is the native structure of KvAP in lipid membranes? The model is shown in Fig. 6. Coordinates for the model shown in Fig. 6 are available on request from R.M.
Fig. 6.
A model of the KvAP tetramer in the open conformation. The top-down view (a) and the side view (b) of the proposed model of the KvAP tetramer in the open conformation. Each subunit is colored blue, green, gold, and red. This model is the same as in Fig. 4b but it is shown as a tetramer to show the position of the voltage sensor relative to the pore.
This proposed KvAP structure is quite similar to the Kv1.2 channel structure, which was used to help guide its construction (8). The pore conformation corresponds to that observed in two KvAP crystal structures (PDB ID codes 1ORQ and 2A0L) with the S6 inner helices adjusted slightly to accommodate their interaction with the S4-S5 linker helices. The S6 inner helices near the intracellular pore entryway have a wide diameter (≈12 Å), suggesting that the pore is open. The conformation of the voltage sensors is very similar to the sensor in the crystal structure of KvAP (PDB ID code 2A0L) but with S1 moved near to its position in the isolated voltage sensor structure (PDB ID code 1ORS) (5). The voltage sensor domains in KvAP, compared with those in Kv1.2, are rotated slightly about an axis perpendicular to the membrane plane relative to the pore in such a way that S1 is more buried in protein and S4 is more exposed to lipid to account for lipid exposed surfaces mapped by EPR analysis (9). The conformation of the voltage sensor paddle (the helix-turn-helix formed by S3b and S4) is near to an open conformation because the four conserved arginine residues on S4 are near the extracellular surface.
The turn connecting S2 to S3, which is not resolved in the structure of Kv1.2, is defined by several crystal structures of KvAP. On the basis of amino acid sequence analysis we think that this turn will be conserved in voltage-dependent K+ and Na+ channels. In particular, there is a salt-bridge pair that is always conserved, with a nearly fixed number of amino acids between them (see Fig. 7, which is published as supporting information on the PNAS web site). In KvAP, and probably other voltage-dependent channels, we propose that one of the functions of this turn is to serve as a membrane interface anchor. The various crystal structures of KvAP suggest that this turn also serves a second function, to allow the voltage sensor paddle to undergo large movements. We base this statement on the observation that the turn is different in its conformation from one crystal structure to the next, indicating that it is intrinsically flexible (PDB ID codes 1ORS, 1ORQ, and 2A0L).
The model of KvAP shown in Fig. 6, which is well constrained by crystallographic data, biochemical data, and the structure of Kv1.2, is in good agreement with the original conceptual model for KvAP gating in which an arginine-containing helix-turn-helix paddle element was proposed to move at the protein-lipid interface to control gating (6). Recent experiments on the accessibility of avidin to tethered biotin of various lengths attached throughout the KvAP protein are in excellent agreement with this model (23).
Supplementary Material
Acknowledgments
We thank the staffs for beam lines ALS 8.2.1 (Advanced Light Source, Lawrence Berkeley National Laboratory, Berkeley, CA), CHESS A1 (Cornell High Energy Synchrotron Source, Cornell University, Ithaca, NY), and NSLS X-25 (National Synchrotron Light Source, Brookhaven National Laboratory, Upton, NY); E. Perozo for EPR data; and V. Ruta, F. Valiyaveetil, and R. Jain for advice. This work was supported by National Institutes of Health Grant GM43949 (to R.M.). S.-Y.L. is a Jane Coffin Childs Memorial Fund fellow, and R.M. is an Investigator in Howard Hughes Medical Institute.
Author contributions: S.-Y.L., A.L., J.C., and R.M. performed research; S.-Y.L. and R.M. analyzed data; and S.-Y.L. and R.M. wrote the paper.
Abbreviations: KV, voltage-dependent K+; PDB, Protein Data Bank.
Data deposition: Coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2A0L).
References
- 1.Hille, B. (2001) Ion Channels of Excitable Membranes (Sinauer, Sunderland, MA).
- 2.Bezanilla, F. (2000) Physiol. Rev. 80, 555-592. [DOI] [PubMed] [Google Scholar]
- 3.Sigworth, F. J. (1994) Q. Rev. Biophys. 27, 1-40. [DOI] [PubMed] [Google Scholar]
- 4.Ruta, V., Jiang, Y., Lee, A., Chen, J. & MacKinnon, R. (2003) Nature 422, 180-185. [DOI] [PubMed] [Google Scholar]
- 5.Jiang, Y., Lee, A., Chen, J., Ruta, V., Cadene, M., Chait, B. & MacKinnon, R. (2003) Nature 423, 33-41. [DOI] [PubMed] [Google Scholar]
- 6.Jiang, Y., Ruta, V., Chen, J., Lee, A. & MacKinnon, R. (2003) Nature 423, 42-48. [DOI] [PubMed] [Google Scholar]
- 7.Long, S. B., Campbell, E. B. & MacKinnon, R. (2005) Science 309, 897-903. [DOI] [PubMed] [Google Scholar]
- 8.Long, S. B., Campbell, E. B. & MacKinnon, R. (2005) Science 309, 903-908. [DOI] [PubMed] [Google Scholar]
- 9.Cuello, L. G., Cortes, D. M. & Perozo, E. (2004) Science 306, 491-495. [DOI] [PubMed] [Google Scholar]
- 10.Collaborative Computational Project 4 (1994) Acta Crystallogr. D 50, 760-763.15299374 [Google Scholar]
- 11.Brunger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kuntsleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S., et al. (1998) Acta Crystallogr. D 54, 905-921. [DOI] [PubMed] [Google Scholar]
- 12.Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. (1991) Acta Crystallogr. A 47, 110-119. [DOI] [PubMed] [Google Scholar]
- 13.Mosher, M. E., White, L. K., Hermolin, J. & Fillingame, R. H. (1985) J. Biol. Chem. 260, 4807-4814. [PubMed] [Google Scholar]
- 14.Hessa, T., Kim, H., Bihlmaier, K., Lundin, C., Boekel, J., Andersson, H., Nilsson, I., White, S. H. & von Heijne, G. (2005) Nature 433, 377-381. [DOI] [PubMed] [Google Scholar]
- 15.White, S. H., Ladokhin, A. S., Jayasinghe, S. & Hristova, K. (2001) J. Biol. Chem. 276, 32395-32398. [DOI] [PubMed] [Google Scholar]
- 16.Ulmschneider, M. B. & Sansom, M. S. (2001) Biochim. Biophys. Acta 1512, 1-14. [DOI] [PubMed] [Google Scholar]
- 17.Lu, Z., Klem, A. M. & Ramu, Y. (2001) Nature 413, 809-813. [DOI] [PubMed] [Google Scholar]
- 18.Murata, Y., Iwasaki, H., Sasaki, M., Inaba, K. & Okamura, Y. (2005) Nature 435, 1239-1243. [DOI] [PubMed] [Google Scholar]
- 19.Laine, M., Lin, M. C., Bannister, J. P., Silverman, W. R., Mock, A. F., Roux, B. & Papazian, D. M. (2003) Neuron 39, 467-481. [DOI] [PubMed] [Google Scholar]
- 20.Elliott, D. J., Neale, E. J., Aziz, Q., Dunham, J. P., Munsey, T. S., Hunter, M. & Sivaprasadarao, A. (2004) EMBO J. 23, 4717-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broomand, A., Mannikko, R., Larsson, H. P. & Elinder, F. (2003) J. Gen. Physiol. 122, 741-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandhi, C. S., Clark, E., Loots, E., Pralle, A. & Isacoff, E. Y. (2003) Neuron 40, 515-525. [DOI] [PubMed] [Google Scholar]
- 23.Ruta, V., Chen, J. & MacKinnon, R. (2005) Cell, in press. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.