Abstract
Here we present a detailed genetic analysis of let-512/vps34 that encodes the Caenorhabditis elegans homologue of the yeast phosphatidylinositol 3-kinase Vps34p. LET-512/VPS34 has essential functions and is ubiquitously expressed in all tissues and developmental stages. It accumulates at a perinuclear region, and mutations in let-512/vps34 result in an expansion of the outer nuclear membrane as well as in a mislocalization and subsequent complete lack of expression of LRP-1, a C.elegans LDL receptor normally associated with the apical surface of hypodermal cells. Using a GFP::2xFYVE fusion protein we found that the phosphatidylinositol 3-phosphate (PtdIns 3-P) product of LET-512/VPS34 is associated with a multitude of intracellular membranes and vesicles located at the periphery, including endocytic vesicles. We propose that LET-512/VPS34 is required for membrane transport from the outer nuclear membrane towards the cell periphery. Thus, LET-512/VPS34 may regulate the secretory pathway in a much broader range of compartments than was previously suggested for the yeast orthologue.
Keywords: Caenorhabditis elegans/membrane transport/PtdIns 3-kinase
Introduction
Phosphoinositides serve as membrane signals mediating intracellular trafficking and protein targeting. They direct the movement of membrane compartments, and control the translocation and activity of proteins that contain phosphoinositide-binding motifs such as pleckstrin homology-, FYVE-, PX- and ENTH-domains (reviewed in Simonsen et al., 2001; Wishart et al., 2001). Phosphoinositide 3-kinases (PI3Ks) are an important type of lipid kinase that provide targets for the above mentioned protein domains. PI3Ks form a large evolutionarily conserved family of enzymes that specifically phosphorylate inositol phospholipids at the D-3 position of the inositol ring. PI3Ks are key regulators of diverse cellular pathways that include cytokine and growth factor receptor signalling cascades, apoptosis, regulation of the actin cytoskeleton and intracellular membrane trafficking (Carpenter and Cantley, 1996; De Camilli et al., 1996; Toker and Cantley, 1997; Rameh and Cantley, 1999).
In the yeast Saccharomyces cerevisiae, VPS34 encodes the sole detectable PI3K activity. Vps34p is the prototype for the class III PI3Ks with its substrate specificity restricted to phosphatidylinositol (PtdIns) (Stack and Emr, 1994). Genetic and biochemical studies have identified Vps34p as part of a molecular complex required for the efficient sorting and vesicle-mediated delivery of resident vacuolar proteins from the late trans-Golgi network via an intermediate endosomal compartment to the yeast vacuole (Herman and Emr, 1990; Schu et al., 1993). Mutations in the VPS34 gene that deplete cells of phosphatidylinositol 3-phosphate (PtdIns 3-P) result in mis-sorting and secretion of Golgi-modified precursor forms of several vacuolar hydrolases, including carboxypeptidase Y, proteinase A and proteinase B (Robinson et al., 1988; Schu et al., 1993). Other characteristics of the phenotype shown by VPS34 mutants involve a temperature-sensitive growth defect and defects in osmoregulation and in vacuole segregation during mitosis (Herman and Emr, 1990). Inactivation of the Vps34p PI3K also has been shown to alter a late stage of the endocytic pathway in yeast. Reduced cellular levels of PtdIns 3-P caused by loss of Vps34p function impede the transport of the endocytosed fluorescent dye FM4-64 (Vida and Emr, 1995) to the vacuole, thus resulting in a late-stage endocytosis defect characterized by an accumulation of FM4-64 in pre-vacuolar endocytic compartments (Wurmser and Emr, 1998).
Much less is known about the function of the Vps34p orthologues in higher eukaryotes. Studies in mammalian cells, mainly based on experiments with the two non-isoform specific PI3K inhibitors wortmannin and LY294002, have suggested that mammalian PI3Ks regulate the protein traffic to the lysosomes (Brown et al., 1995; Davidson, 1995; Row et al., 2001). In plants, PI3K expression is correlated with membrane proliferation during root nodule formation (Hong and Verma, 1994) and wortmannin inhibits at least one type of vacuolar sorting (Matsuoka et al., 1995). Expression of Arabidopsis thaliana VPS34 antisense constructs revealed that this gene is essential for plant growth and development (Welters et al., 1994). Furthermore, the protein appears to be associated with nuclear and nucleolar transcription sites in plant cells (Bunney et al., 2000). Despite these numerous studies, however, a detailed genetic analysis of a Vps34p orthologue in the context of higher eukaryotes has not yet been reported. To close this gap and to learn more about the function of Vps34p proteins in multicellular organisms, we have analysed loss-of-function mutations of the only Vps34p orthologue in Caenorhabditis elegans. We show that VPS34 is encoded by let-512, a gene that is essential for development and growth. The protein product, LET-512/VPS34, is expressed in somatic cells and in the germ line of all developmental stages of both sexes. It is concentrated at the perinuclear region and loss-of-function mutations result in an expansion of the perinuclear space. Altogether these data suggest that the outer nuclear membrane is the primary location of the C.elegans VPS34. Its lipid product PtdIns 3-P, however, is much more broadly distributed in the cells. Most of the detectable PtdIns 3-P is located in endocytic compartments, implying that functional membrane trafficking machinery to deliver PtdIns 3-P from its site of synthesis to the cell periphery is present. Furthermore, we show that mutations in let-512/vps34 interfere with the secretion of LRP-1, a gp330/megalin-related member of the LDL receptor superfamily, at the apical surface of the hypodermal cells.
Results
The genome of C.elegans encodes a single Vps34p homologue
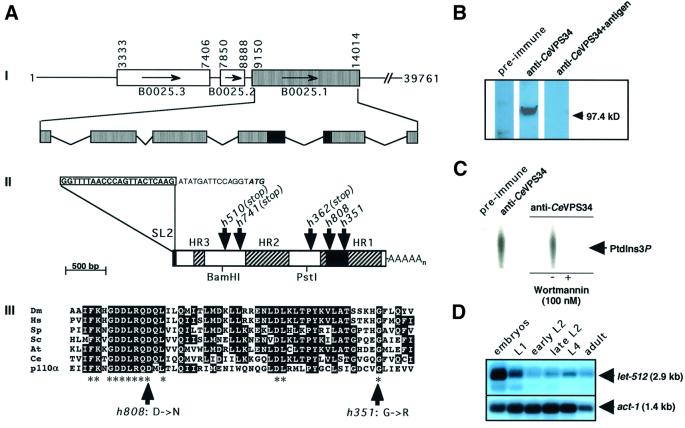
A database search revealed that the genome of C.elegans encodes a single homologue of the yeast class III PI3K, Vps34p. This protein (CeVPS34) is encoded by the gene B0025.1. We have used a PCR probe specific to the predicted B0025.1 coding region to screen a C.elegans mixed-stage cDNA library (Barstead and Waterston, 1989) and have identified 15 positive clones out of 500 000 plaques. One of them, P51Y, was randomly chosen and completely sequenced (Figure 1A). Its length of 2837 bp was consistent with the size of a single mRNA detected on a northern blot with poly(A)+ RNA from a wild-type mixed-stage C.elegans population (data not shown). The cDNA contained 36 bp of 5′ UTR, 110 bp of 3′ UTR, and a 2691 bp long open reading frame composed of six exons encoding a predicted protein of 897 amino acids (Figure 1A). A canonical AAUAAA poly(A) signal was present 11 bp upstream of the poly(A) tail. No trans-spliced leader sequence was present at the 5′ end of the cDNA clone P51Y, but a RT–PCR amplification of C.elegans wild-type RNA revealed that the transcript of B0025.1 is trans-spliced to SL2 exclusively, suggesting that its coding region is located at a downstream position within a polycistronic operon (Spieth et al., 1993; Zorio et al., 1994). This hypothesis is confirmed by the genomic sequence that encodes two upstream ORFs predicted to be in the same operon (Figure 1A). cDNAs corresponding to both genes have been isolated and sequenced (our unpublished results).
Fig. 1. The C.elegans gene let-512/vps34 (B0025.1) encodes a Vps34p homologue. (A) Structure of let-512/vps34 (B0025.1). (I) let-512/vps34 (B0025.1), encoded by cosmid B0025, is preceded by the two upstream genes B0025.2 and B0025.3, which are predicted to be transcribed as a polycistron. Numbering is according to sequences in the DDBJ/EMBL/GenBank database. let-512/vps34 is composed of six exons spanning ∼4.8 kb. The core catalytic lipid kinase domain is located on exons 4 and 5 (black box). (II) let-512/vps34 (B0025.1) mRNA. The let-512/vps34(B0025.1) mRNA is trans-spliced to the SL2 spliced-leader sequence (boxed). The putative ATG start codon is written in bold and italic. The positions of the point mutations in the alleles h351, h362, h510, h741 and h808 are indicated. The striated domains represent homology regions (HR) 1 to 3, which are conserved between the different Vps34p PI3K family members (Wymann and Pirola, 1998). The black box represents the core catalytic lipid kinase domain. The 1.1 kb BamHI–PstI fragment was subcloned for expression of a His-tagged fusion protein and polyclonal anti-LET-512/VPS34 antiserum production. (III) Alignment of the core catalytic domains of different Vps34p PI3K family members and the mammalian p110α PI3K (Hiles et al., 1992). Dm, D.melanogaster; Hs, Homo sapiens; Sp, Schizosaccharomyces pombe; Sc, S.cerevisiae; At, A.thaliana; Ce, C.elegans. Asterisks indicate highly conserved amino acid residues within the catalytic domain of all PI3Ks implicated in ATP and substrate binding (Walker et al., 1999). (B) Western blot analysis of LET-512/VPS34. Total protein extracts from C.elegans were blotted after SDS–PAGE and incubated with pre-immune serum (pre- immune), with anti-LET-512/VPS34 antiserum (anti-CeVPS34) and with anti- LET-512/VPS34 antigen that was pre-incubated with the LET-512/VPS34 antiserum (anti-CeVPS34+antigen). (C) PI3K activity was immunoprecipitated from disrupted and lysed mixed-stage cultures of N2 C.elegans strains with pre-immune or anti-LET-512/VPS34 antiserum, before PI3K-mediated PtdIns 3-P formaion was assayed. Where indicated, immobilized LET-512/VPS34 was pre-incubated with wormannin for 15 min at 30°C. (D) Developmental northern blot analysis of let-512/vps34 (B0025.1) expression. Poly(A)+ RNA was purified from staged cultures and equivalent amounts of RNA (3.8 µg/lane) were loaded on a denaturing agarose gel. A 1.1 kb C-terminal XhoI fragment of the let-512/vps34 (B0025.1) cDNA was used as hybridization probe (see Materials and methods). act-1 (Krause et al., 1989) was used as a loading control.
Over its entire length, the predicted amino acid sequence of CeVPS34 shows most sequence similarities to Vps34p-like PI3Ks of other species, namely to the S.cerevisiae Vps34p (Herman and Emr, 1990), to the Drosophila melanogaster PI3K_59F (Linassier et al., 1997) and to the human phosphatidylinositol-specific PI3K HsVPS34 (Volinia et al., 1995). To further confirm that CeVPS34 is a member of the Vps34p family of PI3Ks, we have tested its ability to phosphorylate PtdIns at the D-3 position of the inositol ring in vitro. A polyclonal antiserum raised against an N-terminal fragment of CeVPS34 (amino acid residues 197–569) lacking the conserved lipid kinase domain was used to immunoprecipitate CeVPS34. On western blots, the antibodies recognized a protein of the expected molecular mass of 100 kDa (Figure 1B). The same protein was co-precipitated from C.elegans total extracts (data not shown). The immunoprecipitate was incubated with PtdIns and [γ-32P]ATP, and the reaction products were assayed by thin layer chromatography (TLC) and autoradiography. The anti-CeVPS34 immunoprecipitate displayed a lipid kinase activity towards PtdIns, yielding PtdIns 3-P as a product. This activity was totally inhibited following addition of the fungal metabolite wortmannin (Figure 1C), a potent inhibitor of PI3Ks (Arcaro and Wymann, 1993; Wymann et al., 1996). Altogether, structural and biochemical data confirm that the C.elegans gene B0025.1 encodes a PtdIns-specific PI3K that structurally and functionally belongs to the Vps34p subfamily of PI3Ks.
CeVPS34 is ubiquitously expressed during development and accumulates at a perinuclear localization
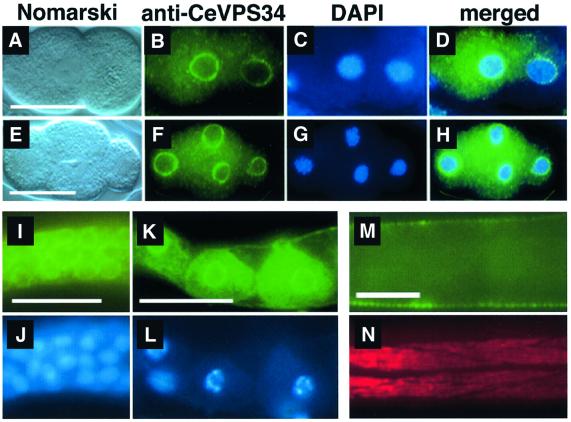
We have tested the developmental expression profile of B0025.1. On northern blots with poly(A)+ RNA isolated from staged wild-type animals, B0025.1 mRNA was detected at all developmental stages; however, strongest expression occurred during embryogenesis until the first larval stage (L1). During the L2 larval stage, B0025.1 expression level was low, but it increased again in L4 larvae and adults (Figure 1D). The subcellular distribution of CeVPS34 was tested by using polyclonal anti-CeVPS34 antibodies (see above). We found that the protein was ubiquitously present in all major tissues and at all developmental stages in C.elegans. Most interestingly, CeVPS34 was strongly concentrated at a perinuclear localization in all blastomeres of the embryo, but some weak granular and diffuse cytoplasmic anti-CeVPS34 staining was also evident (Figure 2A–H). In larval and adult stages of both sexes, the protein was also found at the nuclear envelope of the cells from most major tissues such as the hypodermis or the intestine (results not shown). Furthermore, we observed that CeVPS34 was abundantly present in the germline of adult hermaphrodites. Both the mitotically dividing germ line precursor nuclei and the differentiated oocytes of the more proximal germ line showed bright perinuclear and cytoplasmic staining (Figure 2I and K). Altogether, these results point to the nuclear envelope and possibly the perinuclear endoplasmic reticulum (ER) as the innermost localization sites for CeVPS34.
Fig. 2. Immunostainings of different developmental stages of C.elegans with the anti-LET-512/VPS34 polyclonal antiserum. LET-512/VPS shows a strong perinuclear accumulation in all blastomeres of developing embryos. (A–D) Two-cell stage embryo. (E–H) Four-cell stage embryo. Blue, DAPI staining; green, anti-CeVPS34 staining. (I, J) Microdissected distal gonad: (I) fluorescent micrograph, (J) DAPI staining. (K, L) Growing oocytes in a microdissected proximal gonad: (K) Anti-CeVPS34 staining, (L) DAPI staining. (M, N) Late L3 homozygous segregant of the let-512(h510) strain. Anterior is to the left. (M) Anti-LET-512/VPS staining. (N) Same specimen as in (M) stained with the mAb5-6 monoclonal antibody specific for myosin heavy-chain as control. Scale bars: 20 µm.
CeVPS34 is encoded by the essential gene let-512
B0025.1 is located on chromosome I and maps between the two genes dpy-5 and bli-4. This region contains several lethal mutations (Howell et al., 1987) that were tested for rescue with the construct B0025ΔSacII. B0025ΔSacII is a 3′ truncated derivative of cosmid B0025 containing the CeVPS34 encoding region B0025.1, and the two upstream genes B0025.2 and B0025.3 of the operon (Figure 1A). Two different alleles of one of these candidate genes, let-512(h351) and let-512(h510), could be rescued, indicating that CeVPS34 must be encoded by one of the three genes contained in B0025ΔSacII. To determine if B0025.1 corresponded to let-512, we partially sequenced the five available alleles of let-512. We found that three of them introduced stop codons in the CeVPS34 coding region at amino acid positions 165(h510), 249(h741) and 574(h362) that are predicted to result in truncated protein products lacking the putative core catalytic domain (Figure 1A; Table I). The two other alleles of let-512 caused amino acid substitutions at positions 651(h808) and 687(h351), two highly conserved amino acid residues within the catalytic domain of the Vps34p family of proteins (Figure 1A; Table I). In summary, our data demonstrate that the CeVPS34 PI3K is encoded by the gene let-512, and we therefore re-named the gene let-512/vps34.
Table I. let-512/vps34 mutant alleles.
| Allele | Protein alteration | Sequence |
||
|---|---|---|---|---|
| Wild type | Mutanta | Fertilityb | ||
| h351 | G687R | GGA | AGA | 0% |
| h362 | R574-opal | CGA | TGA | 0% |
| h510 | W165-amber | TGG | TAG | 0% |
| h741 | R249-opal | CGA | TGA | 0% |
| h808 | D651N | GAT | AAT | 11% (n = 949) |
aSequence alterations found in the different let-512/vps34 mutant alleles are underlined. Altered amino acids are abbreviated using the single-letter code.
bAllele fertility was judged by scoring the percentage of homozygous animals that reach adulthood and were fertile in each generation. For alleles h351, h362, h510 and h741, homozygous dpy-5; let-512; unc-13 animals that had lost the free duplication sDp2(I;f) were never fertile, whereas, although at a low percentage, animals homozygous for let-512(h808) were fertile and could be maintained without sDp2(I;f).
let-512/vps34 mutants arrest development at the L3 or L4 molt
We have analysed the phenotype of let-512/vps34 mutant animals. Four out of the five alleles (h510, h741, h362 and h351) appeared to have the same sterile or lethal phenotype, suggesting that they represent strong loss of function or null alleles. Homozygous dpy-5;let-512; unc-13 animals segregating from sDp2(I;f) balanced mothers variably arrested growth and development either during or shortly after the molts from the L3 to L4 or from the L4 to the adult stage (Table I). A few hermaphrodites arresting after the final molt were able to develop into young adults with up to six disorganized and arrested embryos in their uterus, whereas others were marked by the presence of abnormal oocytes. Animals homozygous for the fifth allele (h808) had a weaker phenotype. These animals exhibited the same general arrest phenotype as those carrying the strong alleles, but ∼11% (n = 949) of them reached adulthood and were fertile (Table I), permitting the maintenance of this strain in the absence of sDp2(I;f).
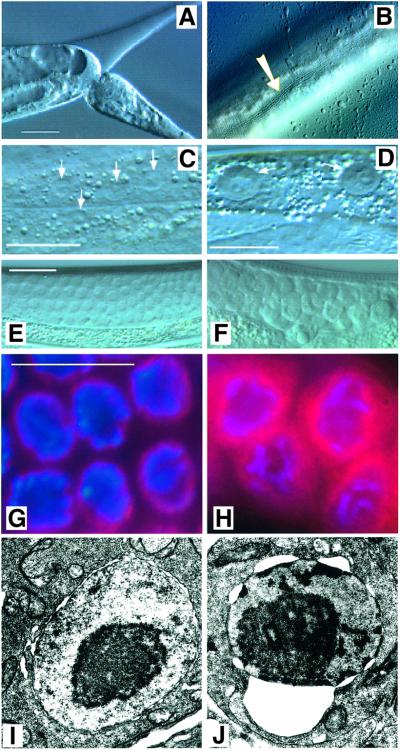
To analyse the let-512/vps34 mutant phenotype in more detail we have used the allele h510. The marker mutations dpy-5 and unc-13 were crossed out from the h510-bearing chromosome (see Materials and methods). Homozygous let-512(h510) animals from heterozygous mothers showed the same developmental arrest during or shortly after the molts from the L3 to L4 or from the L4 to the adult stage, like homozygous dpy-5;let-512;unc-13 triple mutants. Prior to the onset of arrest, homozygous let-512/vps34 animals did not differ phenotypically from wild-type animals at the same developmental stage. At the L3/L4 or L4/adult molts, however, let-512(h510) homozygous worms failed to shed their old cuticles, which were usually displaced from the anterior and sometimes from the most posterior end, but not from the rest of the body (Figure 3A). In h510 mutant animals arresting at the L4/adult molt, however, the alae of the newly synthesized adult cuticle were clearly visible (Figure 3B). This indicates that the molting defects cannot be attributed to a failure in new cuticle formation. No differences between animals incubated at 15, 20 or 25°C were observed. Obviously, the phenotype of let-512 mutation worms is not temperature dependent, which is opposite to results obtained in yeast (Herman and Emr, 1990).
Fig. 3. Phenotype of homozygous let-512/vps34(h510) mutant worms. (A) Severe constriction caused by an unshed cuticle in the tail region of an arrested young adult animal. (B) Alae (arrow) of the newly synthesized adult cuticle of mutant animal arrested at the L4 to adult molt. (C and E) Wild-type nuclei: (C) hypodermis, (E) germ line. (D and F) Homozygous let-512/vps34(h510) segregants arrested at the L4/adult stage molt showing an extension of the outer nuclear membrane: (D) hypodermal and (F) germ line cells. Scale bars: 20 µm. (G, H) DAPI (blue) and mAb414 (red) double staining of germ cell nuclei in the distal gonad: (G) a wild-type animal, (H) a homozygous let-512(h510) mutant hermaphrodite. mAb414 specifically recognizes proteins of the nuclear pore complex (see text). Scale bars: 20 µm. (I, J) Transmission electron microscopic pictures of nuclei from the gonad: (I) wild-type animal and (J) let-512(h510) mutant animal, showing an enlargement of the nuclear membrane space.
Mutations in let-512/vps34 result in an expansion of the perinuclear space
Besides the molting defects, arrested homozygous let-512(h510) segregants were frequently marked by an expansion of the nuclear envelope in various somatic cells, including the cells of the hypodermis, the intestine and the body wall muscles, and in germ cells (Figure 3E and F). Staining of micro-dissected gonads isolated from homozygous let-512(h510) hermaphrodites with DAPI or with the antibody mAb414, which specifically recognizes several nuclear pore components of C.elegans (Davis and Blobel, 1986; Browning and Strome, 1996; Pitt et al., 2000), revealed that this defect was restricted to the outer nuclear membrane, whereas the size of the inner nuclear membrane remained unchanged (Figure 3G and H). The diffuse distribution of the mAb414 epitopes within the enlarged perinuclear space suggests that the dissociation of the outer and the inner nuclear membrane may lead to a disintegration of the nuclear pore complexes in let-512 mutants. An enlarged perinuclear space caused by the expansion of ribosome-free sheets of the outer nuclear membrane in arrested let-512(h510) homozygote animals, finally, was also seen in transmission electron microscopy (Figure 3I and J). Interestingly, the defects in the outer nuclear membrane correlate well with the perinuclear localization of LET-512/VPS34 protein.
PtdIns 3-P is absent from intracellular membranes and vesicles in let-512/vps34 mutant animals
We next assessed the intracellular distribution of PtdIns 3-P, the product of LET-512/VPS34. Therefore, we made a fusion protein containing two FYVE RING finger domains coupled to green fluorescent protein (GFP) [gfp::2xT10G3.5(FYVE)]. The FYVE RING finger domain is a conserved 70-residue protein module that was shown to interact specifically with PtdIns 3-P (reviewed in Gillooly et al., 2001). The FYVE RING finger domains used for our GFP fusion construct were derived from the yet uncharacterized C.elegans protein T10G3.5. T10G3.5 is a putative coiled-coil protein displaying 22% overall identity and 42% overall similarity to the human EEA1 (early endosome antigen 1) that is required for membrane docking and early endosome fusion (Mills et al., 1998; Simonsen et al., 1998; Christoforidis et al., 1999a). The FYVE finger domain of T10G3.5 contains both a RING motif, characterized by the spacing of eight cysteine and/or histidine residues (Cx2Cx9–39Cx1–3C/Hx2–3Cx2Cx4–48 Cx2C) that permits coordination of two zinc atoms (Saurin et al., 1996), and the highly conserved FYVE signature motif (R/K)(R/K)HHCR surrounding the third zinc-coordinating cysteine that is critical for PtdIns 3-P binding (Gillooly et al., 2001).
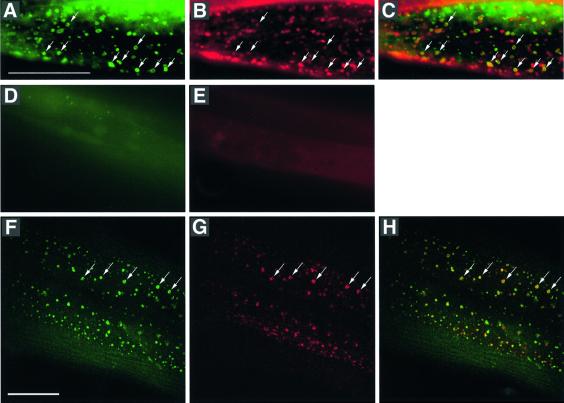
The specificity of the GFP::2xT10G3.5(FYVE) fusion protein for binding PtdIns 3-P was tested in a yeast mutant strain deleted for the VPS34 gene (Schu et al., 1993). In wild-type yeast cells, the GFP::2xT10G3.5(FYVE) fusion protein was bound to endosomal/vacuolar membranes, whereas in VPS34 mutant cells devoid of PtdIns 3-P, the fusion protein was cytosolic (data not shown). We have transformed C.elegans with the heat shock promoter driven gfp::2xT10G3.5(FYVE) fusion construct and analysed its expression pattern. Animals wild type for let-512 showed the strongest expression in the intestinal and hypodermal cells. In these cells, the GFP::2xT10G3.5 (FYVE) fusion protein was targeted to a multitude of intracellular membranes and vesicles, most of them being localized at peripheral regions (Figure 4A and F; data not shown). In arrested let-512(h510) mutant worms the fusion protein was also expressed, but it remained diffusely distributed in the cytosol (Figure 4D). This indicates that PtdIns 3-P was absent from all microscopically detectable membranes and vesicles from mutant animals.
Fig. 4. PtdIns 3-P, the product of LET-512/VPS34, and the LDL-receptor LRP-1 co-localize to endocytic intermediates in the hypodermal syncytium hyp7. (A–D) Fluorescent microscopy images of a GFP–FYVE fusion protein [GFP::2xT10G3.5(FYVE)] expressing wild-type hermaphrodite stained with the endocytic marker FM 4-64. (A) GFP::2xT10G3.5(FYVE), (B) FM 4-64, and (C) merged images. Scale bar: 20 µm. (D) Expression of GFP::2xT10G3.5(FYVE) in the hypodermis of a let-512/vps34(h510) mutant animal. GFP::2xT10G3.5(FYVE) remains completely cytosolic and does not bind to any defined structure within the hypodermal cells of let-512(h510) mutant animals. (E) FM 4-64 staining of a let-512/vps34(h510) mutant animal. No endocytic vesicles or other structures are stained, suggesting that the dye cannot be endocytosed by the hypodermal cells of let-512/vps34(h510) mutant animals. (F–H) Confocal fluorescent images of a GFP::2xT10G3.5(FYVE) expressing wild-type hermaphrodite stained with the anti-LRP-1 antibodies 1H6 and 4H5. (F) GFP::2xT10G3.5(FYVE), (G) anti-LRP-1, and (H) merged images. Scale bar: 20 μm.
PtdIns 3-P is located to the endocytic pathway
In the hypodermis of let-512/vps34 wild-type animals, we observed a strong localization of GFP::2xT10G3.5(FYVE) to apical peripheral vesicular structures within the main hypodermal syncytium hyp7 (Figure 4A and F). To test whether these peripheral structures could represent endocytic compartments, we stained GFP::2xT10G3.5 (FYVE)-expressing worms with the fluorescent dye FM 4-64. This dye specifically labels the yeast plasma membrane and the endocytic pathway upon internalization (Vida and Emr, 1995) and is endocytosed by C.elegans embryos when added to the medium (Rappleye et al., 1999). We found that FM 4-64 is rapidly taken up by the hypodermal syncytium hyp7 in larvae and adult worms following perforation of the cuticle using an injection needle. Upon internalization, FM 4-64 stains punctuated structures that are frequently co-labelled with the GFP::2xT10G3.5(FYVE) fusion protein (Figure 4A–C). Thus, these data clearly demonstrate an association of PtdIns 3-P with endocytic intermediates in hypodermal cells of C.elegans. A possible requirement for PtdIns 3-P and hence for LET-512/VPS34 in the endocytic pathway of C.elegans is suggested by our observation that arrested homozygous animals are no longer capable of endocytosing the FM 4-64 dye (Figure 4E).
LET-512/VPS34 is required for the expression and localization of LRP-1 at the apical surface of hyp7
The severe defects in molting and in the proper shedding of the old cuticle displayed by arrested homozygous let-512(h510) mutant worms resembled the defects caused by mutations in the C.elegans gene lrp-1 (Yochem et al., 1999). Mutations in the lrp-1 gene cause an arrest in growth and development, usually during the molt from L3 to L4, and result in a striking inability of homozygous animals to shed and degrade the old cuticle during the molt (Yochem et al., 1999). lrp-1 encodes a gp330/megalin-related member of the LDL receptor superfamily and is predominately located to the apical surface of the hypodermal syncytia hyp6 and hyp7 (Yochem et al., 1999). It was suggested that LRP-1 plays a major role in receptor-mediated uptake of cholesterol through the hypodermis (Yochem and Greenwald, 1993; Yochem et al., 1999). The strikingly similar phenotype of let-512 and lrp-1 mutant animals prompted us to compare the intracellular localization of LRP-1 with that of LET-512/VPS34 and its lipid product PtdIns 3-P. The cellular distribution of LRP-1 was investigated by confocal immunofluorescence microscopy using the two monoclonal anti-LRP-1 antibodies 1H6 and 4H5 (Yochem et al., 1999). In wild-type animals the antibodies stained vesicles at the apical surface of the hyp7 syncytium (Figure 4G) (Yochem et al., 1999). Co-staining with the monoclonal antibody MH27, which is specific for the hypodermal adherens junctions (Francis and Waterston, 1985), demonstrated that the LRP-1 marked structures are confined to the dorsal and ventral ridges of hyp7 and wider sections on either side of the lateral seam cells (Figure 5A). Double staining with the GFP::2xT10G3.5(FYVE) fusion protein revealed a co-localization of the LRP-1 receptor with PtdIns 3-P in vesicles (Figure 4F–H), showing that they represent endocytic structures. However, no co-localization of LRP-1 and LET-512/VPS34, which mainly reside at a perinuclear position (see above), could be observed.
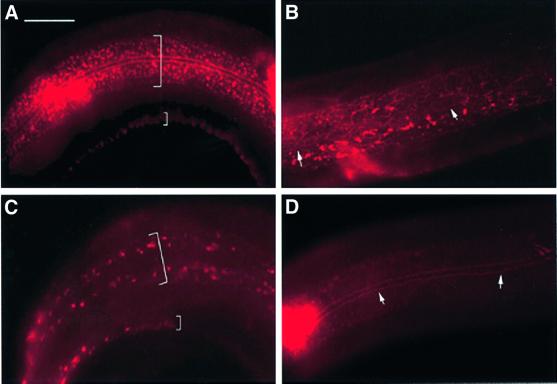
Fig. 5. Aberrant localization of the lrp-1 gene product in let-512/vps34(h510) mutant animals. Each specimen has been stained for LRP-1 with the 1H6 and 4H5 monoclonal antibodies, and for apical adherens junctions in epithelial cells with the monoclonal antibody MH27. (A) Late L4 larva [dpy-5(e61)]. Two wide bands of apical, punctate LRP-1 staining (large parentheses) flank the hypodermal adherence junctions between hyp7 and the lateral seam syncytium (MH27 staining). A small parenthesis depicts the band of LRP-1 staining in the ventral ridge of hyp7. (B) dpy-5(e61);let-512/vps34(h510) L3 larvae. Unlike the punctate staining pattern evident in wild-type and dyp-5 animals, LRP-1 is less regularly distributed in the dpy-5;let-512/vps34 background, adopting a tubular and mesh-like appearance. (C) Anterior part of a late L3/early L4 dpy-5;let-512/vps34 larvae showing a disappearing aberrant LRP-1 signal. The large bracket indicates the two bands of LRP-1 staining on either side of the lateral seam syncytium, whereas the small bracket depicts LRP-1 in the ventral ridge of hyp7. (D) An arrested late L4 mutant dyp-5;let-512/vps34 animal. The LRP-1 signal has almost completely disappeared at the apical surface of hyp7; a faint and diffuse LRP-1 signal remains in the cytoplasm. White arrows in (B) and (D) indicate MH27 staining of the lateral hypodermal seam. The anterior of the animals is to the left in all pictures. Scale bar: 20 µm.
Co-localization of the LRP-1 receptor with PtdIns 3-P in endocytic vesicles suggests a possible role of PtdIns 3-P (and hence LET-512/VPS34) in receptor-mediated endocytosis at the hyp7 plasma membrane of C.elegans. To investigate this issue further, we looked at the distribution of LRP-1 at the apical surface of hyp7 in dpy-5(e61); let-512(h510) double mutant animals. The dpy-5(e61) phenotype permitted the identification of homozygous let-512(h510) segregants prior to L3/L4 arrest. As control we used dpy-5(e61) single mutant animals, which had an LRP-1 expression pattern corresponding to wild-type animals (Figure 5A). During the developmental arrest of dpy-5(e61);let-512(h510) homozygous animals, LRP-1 gradually became less abundant and less regularly distributed at the apical surface of hyp7 than in control animals that were wild type for let-512. Furthermore, the endocytic vesicles changed their form and adopted a more tubular or mesh-like appearance (Figure 5B and C). In arrested dpy-5(e61); let-512(h510) worms, the apical LRP-1 signals disappeared almost completely, although some faint and diffuse LRP-1 signal remained in the cytoplasm of hyp7 (Figure 5D). From these experiments we concluded that the function of LET-512/VPS34 is required for the secretion and localization of LRP-1 at the apical surface of the hypodermal syncytium of C.elegans. Thus, let-512/vps34 mutant animals have defects in the secretion and/or in the production of LRP-1.
Discussion
Here we report on the identification and the genetic analysis of let-512/vps34, which encodes the only structural homologue of the S.cerevisiae Vps34p PI3K in the genome of C.elegans. We show that LET-512/VPS34 is strongly concentrated in a perinuclear location and we present evidence that it is vital for vesicle budding and membrane transport from the outer nuclear membrane towards the cell periphery in the worm.
We have characterized five recessive mutations of let-512/vps34. Four of them confer an identical lethal phenotype and sequence data indicate that they represent strong loss-of-function or null alleles. Homozygotes segregating from heterozygous mothers were able to hatch and initiate development but they arrested development at or shortly after the molts from L3 to L4 or from L4 to adult. Only a few animals were able to reach adulthood and to produce a small number of disorganized and arrested embryos. The lethality of let-512/vps34 is in contrast to the situation in yeast, where the deletion of the VPS34 gene is essential for vegetative growth only at elevated temperatures (Herman and Emr, 1990). One of the severe alleles of let-512/vps34, h351 causes an amino acid substitution at a highly conserved position within the catalytic domain of LET-512/VPS34. This is in line with earlier data from yeast showing that Vps34p proteins carrying point mutations in the catalytic domain are unable to rescue the phenotype of a VPS34 deletion (Schu et al., 1993) and suggests that the lipid kinase activity is essential for the biological function of the Vps34p class of proteins.
Polyclonal antibodies detected LET-512/VPS34 in all major tissues and all developmental stages of C.elegans. It is expressed abundantly in the germ line and is maternally contributed to the early embryo. This maternal contribution explains why let-512/vps34(h510) homozygotes segregating from heterozygous mothers can develop until the L3/L4 or L4/adult molt. During the development of wild-type animals, LET-512/VPS34 is present at high levels until the L1 larval stage, decreases in L2 and L3 larvae, and peaks again as animals reach the L4 larval and adult stages. The elevated expression levels of LET-512/VPS34 in L4 larvae and adults could reflect a requirement for the protein in the developing gonad.
Using a GFP::2xFYVE fusion protein that specifically binds PtdIns 3-P, we found that PtdIns 3-P is associated with a multitude of intracellular membranes and vesicles located at peripheral positions. In arrested let-512/ vps34 mutant animals, however, the GFP::2xFYVE fusion protein was completely cytosolic, indicating that PtdIns 3-P was absent from all microscopically detectable membranes and vesicles in mutant animals lacking LET-512/VPS34. The most striking labelling with the GFP::2xFYVE probe in WT animals, however, was associated with peripheral vesicles located at the apical surface of the hypodermal main syncytium. Co-staining with the fluorescent dye FM 4-64, a marker for endocytic intermediates (Vida and Emr, 1995), identified them as being of endosomal origin. The association of PtdIns 3-P with endocytic vesicles suggests a role for LET-512/VPS34 in the endocytic pathway of C.elegans. This view is supported by the finding that arrested homozygous let-512/vps34 animals were no longer capable of internalizing FM 4-64 by endocytosis, although other interpretations are also possible (see below). In yeast, PtdIns 3-P is highly enriched on the intralumenal vesicles of endosomes and vacuoles, and recent studies in yeast and mammalian cells have implicated PI3K activity as an essential component of the endosomal system (for a recent review see Gillooly et al., 2001). The yeast enzyme Vps34p plays a role in sorting events that follow the initial stages of endocytosis, i.e. in the transport of endocytic cargo from a pre-vacuolar endocytic compartment to the vacuole (Würmser and Emr, 1998; reviewed in Simonsen et al., 2001), and inhibition of PI3Ks in mammalian cells by the drug wortmannin blocks early endosomal trafficking (reviewed in Rameh and Cantley, 1999). The specific roles of LET-512/VPS34 in the endocytic pathway of C.elegans remain to be determined.
LET-512/VPS34 has a completely different cellular distribution than its PtdIns 3-P product, in that it is strongly concentrated at a perinuclear localization. This is particularly evident in the nuclei of the distal gonad that lack rough ER and that are not surrounded by Golgi bodies (D.Hall, personal communication). Loss-of-function mutations in let-512/vps34 result in an expansion of the perinuclear space, and our data suggest that LET-512/VPS34 is located to and acts directly at the outer nuclear membrane. The biochemical composition of this membrane is very similar to that of the ER, and in many cells it is a site of membrane-bound protein synthesis, as shown by the attachment of ribosomes (Franke et al., 1981). We propose that LET-512/VPS34 produces PtdIns 3-P required for vesicle budding and membrane transport from the outer nuclear membrane towards the cell periphery. Inactivation of LET-512/VPS34 may block vesicle budding, thereby causing a retention of secretory and biosynthetic material resulting in an expansion of the perinuclear space between the inner and the outer nuclear membrane, and possibly the interconnected lumen of the ER. This hypothesis is consistent with the fact that the nuclear expansion observed in let-512/vps34 mutant animals is most obvious in the cells of the hypodermis and the gut, two tissues characterized by a high secretory activity (White, 1988). The expansion of the perinuclear space may eventually lead to the disintegration of the nuclear pores and subsequently to a complete breakdown of nuclear RNA export and protein synthesis. Thus, the deficiency in LRP-1 localization at the apical surface of the hyp7 syncytium in let-512/vps mutant animals may be caused by both a general defect in the secretory pathway and a subsequent breakdown of protein synthesis in these cells. Differences in the cellular distribution between Vps34p and PtdIns 3-P were also found in mammalian cells. Indirect immunoflorescence microscopy demonstrated that the majority of VPS34 localizes to the trans-Golgi network, but some of it is also distributed to late endosomes (Kihara et al., 2001). PtdIns 3-P, however, is highly enriched on early endosomes and in the internal vesicles of multivesicular endosomes (Gillooly et al., 2000).
Besides the distensions of the perinuclear space, let-512/vps34 homozygote worms often develop abnormal and large vacuoles, although the degree of this vacuolarization varies greatly among individuals (data not shown). The accumulation of these abnormal vacuoles may be explained by the fact that the different membrane compartments along the vesicle transport pathways are still capable of receiving input from transport vesicles, but fail to form, package and bud outgoing vesicles correctly. Weak antibody staining, however, suggests that LET-512/VPS34 may not only be located and function at the nuclear periphery, but also at vesicles throughout the cytoplasm (see Figure 2). In mammalian cells, the small GTPase Rab5 has been proposed to recruit VPS34 to the early endosomes via its binding to the Vps15p homologue p150 (Christoforidis et al., 1999b). Homologues of Rab5 (Grant and Hirsh, 1999) and Vps15p are both encoded by the genome of C.elegans, but a possible interaction of these two proteins in a complex with LET-512/VPS34 has yet to be established by genetic and biochemical studies.
In summary, we show that the C.elegans PI3K LET-512/VPS34, like Vps34p from yeast, is required for a functional membrane trafficking machinery. There are, however, important differences between the two organisms, the most striking one concerning the subcellular localization of their VPS34 proteins. Some preliminary evidence suggests that VPs34p could be associated with the cytoplasmatic phase of the trans-Golgi apparatus (Herman et al., 1991; Stack et al., 1993), rather than being located at the nuclear envelope like LET-512/VPS34. Correspondingly, Vps34p functions in the vesicle-mediated transport of newly synthesized soluble proteins from the late trans-Golgi network via an intermediate endosomal compartment to the vacuole (Herman and Emr, 1990; Schu et al., 1993), whereas the early secretory pathway from the ER to the Golgi apparatus does not require PI3K activity, since it is not interrupted in vps34 loss-of-function mutants (Klionsky et al., 1990). Thus, LET-512/VPS34 is involved in the regulation of the secretory membrane and protein trafficking pathways in a broader range of compartments than was previously suggested for the yeast homologue Vps34p. This points towards fundamental differences in the role of VPS34 in the membrane trafficking system between yeast and C.elegans (and perhaps higher eukaryotes in general).
Materials and methods
Strains and general methods
Caenorhabditis elegans strains were cultured using standard conditions (Brenner, 1974). Wild-type worms correspond to C.elegans var. Bristol strain N2. The following mutations and rearrangements were used: LG I, let-512(h351, h362, h510, h741, h808), dpy-5(e61), unc-13(e450), sDp2(I;f). The genotype of the strain FR359 used for rescue of the let-512/vps34 lethal phenotype was swEx236[rol-6(su1006), B0025ΔSacII]. The strain FR480 is of the genotype swEx312[rol-6(su1006), hsp16-2:: GFP::2xT10G3.5(FYVE)].
Identification and isolation of the let-512/vps34 cDNA
A PCR amplified probe corresponding to positions 740–774 of the LET-512/VPS34 amino acid sequence was used to screen a C.elegans mixed-stage cDNA library (Barstead and Waterston, 1989). Sequence comparisons and alignments were obtained by use of the University of Wisconsin Genetics Computer Group (GCG) software package (version 10.1; Devereux et al., 1984) and BLAST (Altschul et al., 1997). The DDBJ/EMBL/GenBank database accession number for the let-512/vps34 nucleotide sequence reported in this paper is Y12543.
Developmental northern blot analysis
mRNA extraction from staged C.elegans cultures and northern blot analysis was performed as described by Puoti and Kimble (1999). The let-512/vps34-specific probe was obtained by XhoI digestion of the P51Y plasmid releasing a 1.1 kb-long terminal 3′ fragment of the let-512/vps34 cDNA.
Isolation and transgenic rescue of let-512
The EMS-induced let-512/vps34 mutations balanced by the free duplication sDp2(I:f) described in this study were initially characterized by Howell et al. (1987).
To identify the point mutations associated with the let-512/vps34 alleles, genomic DNA from homozygous dpy-5; let-512; unc-13 segregants was PCR-amplified and sequenced directly. To eliminate potentially confounding effects, the dpy-5 and unc-13 marker mutations were outcrossed from the h510-bearing chromosome and the presence of the let-512(h510) point mutation was confirmed by PCR in homozygous animals segregating from heterozygous mothers. For transgenic rescue, the let-512/vps34-containing cosmid B0025 was shortened by ∼21 kb of genomic DNA downstream of the polycistron by SacII digestion and religation (B0025ΔSacII). The strain FR359 (swEx236[rol-6(su1006), B0025ΔSacII]) was generated by microinjection of 50 µg/ml of B0025ΔSacII and 200 µg/ml of pRF4 rol-6(su1006) DNA into the gonads of wild-type hermaphrodites (Mello and Fire, 1995). For transgenic rescue of let-512/vps34 mutants, heterozygote dpy-5 let-512 unc-13/+++ males that had lost sDp2(I:f) (Howell et al., 1987; McKim and Rose, 1990) were mated to FR359 hermaphrodites. The L4 hermaphrodite rollers resulting from this cross were individually plated and their progeny screened for the presence of non-lethal Dpy Unc animals.
Antibody production
To generate a His6-tagged LET-512/VPS34 fusion protein, a BamHI–PstI fragment encoding amino acid residues 197–569 from the let-512/vps34 cDNA (see Figure 1A) was cloned into the expression vector pQE. The recombinant protein was expressed in Escherichia coli M15 cells and purified by nickel-nitrilo-triacetic acid (Ni-NTA) metal affinity chromatography under denaturing conditions according to the manufacturer’s instructions (Qiagen). Rabbit polyclonal antibodies were raised against the purified fusion protein according to standard protocols (Harlow and Lane, 1988). The specificity of the resulting serum was investigated by western blotting on total C.elegans protein extracts separated by 8% SDS–PAGE. Whereas the anti-CeVPS34 antiserum recognized a single band with the expected size of ∼100 kDa, pre-immune serum did not. Preincubation of the antiserum overnight at 4°C with 8.4 µg of the purified antigen completely eliminated the observed signal (Figure 1B), confirming that the antiserum is specific to CeVPS34. The anti-CeVPS34 antiserum was used at a 1:2000 dilution, whereas the pre-immune serum was used at a 1:1000 dilution. Western blots were developed using enhanced chemiluminescence (ECL kit, Amersham). The specificity of the anti-CeVPS34 antiserum is demonstrated further by the fact that animals homozygous for the allele let-512(h510) fail to stain with the anti-CeVPS34 antiserum (Figure 2M).
PtdIns 3-kinase assay
Mixed stage cultures of the C.elegans N2 strain were suspended in a hypotonic buffer (50 mM NaCl, 5% glycerol, 1 mM EDTA, 5 mM DTT, 50 mM HEPES, supplemented with a protease inhibitor cocktail containing leupeptin, pepstatin, PMSF and aprotinin). Worms were sonicated with a tip sonicator on ice, and disruption was followed microscopically. At ∼90% disruption, the buffer was supplemented with Triton X-100 to 1%. After 15 min, debris was sedimented at 1000 g for 5 min at 4°C. CeVPS34 was immunoprecipitated with anti-CeVPS34 antiserum from the supernatant (see above), or alternatively pre-immune serum was used as a control. After incubation with antibodies for 2 h, immunocomplexes were immobilized on protein A–Sepharose (Pharmacia) for 1 h. After three washes with 0.5 M LiCl/20 mM Tris pH 7.4 and three washes with 5 mM MgCl2/20 mM HEPES pH 7.4, beads were suspended in 5 mM MgCl2/5 mM MnCl2/20 mM HEPES pH 7.4 (40 µl), before 10 µl of sonicated PtdIns/PS (1 mg/ml each) and 10 µl of 60 µM/10 µCi of [γ-32P]ATP (Hartmann) was added to start the reaction. After 30 min at 30°C, lipids were extracted and separated on two TLC systems as described previously (Walsh et al., 1991; Wymann et al., 1996). The latter, borate-based system discriminates between PtdIns 3-P and PtdIns 4-P. Recombinant p85/p110 was used to produce PtdIns 3-P standards (Wymann et al., 1996).
Antibody stainings and fluorescence microscopy
Whole-mount immunostainings on larvae and adult worms were performed as described previously (Bettinger et al., 1996). For anti-CeVPS34 immunostainings, adults and larvae were permeabilized by freeze-fracture, and fixed in –20°C methanol for 5 min and –20°C acetone also for 5 min. Embryos and microdissected gonads were immunostained on poly-l-lysine coated slides. A coverslip was placed over the samples and the slides were frozen on dry ice for at least 20 min. Coverslips were flicked off and the specimens fixed in methanol and acetone as above. Stainings of microdissected gonads with the mAb414 antibody (BABCO) were performed as described in Pitt et al. (2000). All fixed specimens were washed three times for 15 min in phosphate-buffered saline with 0.1% Tween-20 (PBST) at room temperature prior to incubation with primary or secondary antibodies overnight at 4°C. Primary antibodies were used at the following dilutions: anti-CeVPS34, 1:100; MH27 (Francis and Waterston, 1985), 1:1000; mAb5-6 (Miller et al., 1983), 1:50 to 1:100; anti-LRP-1 antibodies 1H6 and 4H5 (Yochem et al., 1999), 1:100 each; and mAb414, 1:200. FITC- or Cy3-conjugated goat secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were used at 1:1000 to 1:2000 dilutions. Nuclei were visualized by staining with 2 µg/ml DAPI (Sigma). For FM 4-64 (Molecular Probes Inc., Eugene, OR) labelling, living worms were incubated in M9 buffer (Brenner, 1974) containing 32 µM FM 4-64. Specimens were observed using a Leica DM RXA epifluorescence microscope, and images were taken with a Hamamatsu colour chilled 3CCD camera and processed with the Hamamatsu colour chilled 3CCD camera controller (C5810). Confocal images were obtained using a Bio-Rad MRC1024 confocal microscope.
2xT10G3.5(FYVE) construct and PtdIns 3-P localization
The FYVE finger used in this study is encoded by nucleic acid residues 2702–2928 of the Kohara cDNA clone yk24a5 encoding the C.elegans FYVE finger protein T10G3.5. 2xT10G3.5(FYVE) consists of this domain in duplicate separated by the linker QGQGS. 2xT10G3.5(FYVE) was cloned behind GFP into the vector pPD117.01 (provided by A.Fire) and a fragment coding for GFP::2xT10G3.5(FYVE) coupled to the let-858 3′ UTR was recloned into the ectopic expression vector pPD49.78 (provided by A.Fire) driven by the hsp16-2 heat shock promotor (Stringham et al., 1992). The transgenic line FR480 was generated by microinjection of 50 µg/ml of the final 2xT10G3.5(FYVE) construct and 200 µg/ml of pRF4 rol-6(su1006) DNA into wild-type hermaphrodites (Mello and Fire, 1995). To induce expression of the GFP:: 2xT10G3.5(FYVE) fusion construct, transgenic worms were heat shocked at 30°C for time periods varying between 15 and 30 min. The extrachromosomal array swEx312[rol-6(su1006), hsp16-2::GFP:: 2xT10G3.5(FYVE)] was crossed into both a dpy-5(e61) unc-13(e450) and a dpy-5(e61) let-512(h510) unc-13(e450) background to analyse the specificity of PtdIns 3-P recognition in the presence and absence of functional LET-512/VPS34 PI3K activity in C.elegans.
Electron microscopy
Individual animals were cut open under a dissecting microscope in a drop of fixative containing 2.7% glutaraldehyde and 1.33% formaldehyde in 0.13 M cacodylate buffer. After an overnight fixation at 4°C the fixative was changed into washing buffer (0.1 M cacodylate) and the samples were embedded in agar, post-fixed with 0.5% cacodylate buffered OsO4, stained with 2% uranyl acetate, dehydrated in ethanol and propylene oxide, and embedded in Durcupan (Fluka). Thereafter samples were cut with a Reichert–Jung Ultracut-E Type ultramicrotome, stained with lead citrate and examined in a JEM100CX II electron microscope.
Acknowledgments
Acknowledgements
We thank R.Barstead for providing the C.elegans cDNA library, A.Coulson for cosmids, A.Fire for the vector kits, S.Gasser for the mAb414 antibody, Y.Kohara for cDNAs, D.Miller for the mAb5-6 antibody, A.Puoti for performing developmental northern blot analysis, R.Waterston for the MH27 antibody, J.Yochem for the anti-LRP-1 antibodies 1H6 and 4H5, and H.Stenmark for advice concerning the design of FYVE probes. We are also grateful to Y.Molleyres, L.Bulliard and G.Pizzimento for excellent technical assistance and all the members of our laboratory for helpful discussions. This work was supported by the Swiss National Science Foundation grant numbers 3100-40.776 and 3100-056953.99 (to F.M.) and 3100-50506.97 (to M.P.W.).
References
- Altschul S.F., Madden,T.L., Schäffer,A.A., Zhang,J., Zhang,Z., Miller,W. and Lipman,D.J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res., 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro A. and Wymann,M.P. (1993) Wortmannin is a potent phophatidylinositol 3-kinase inhibitor: the role of phosphatidyl 3,4,5- trisphosphate in neutrophil responses. Biochem. J., 296, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barstead R.J. and Waterston,R.H. (1989) The basal component of the nematode dense body viniculin. J. Biol. Chem., 264, 10177–10185. [PubMed] [Google Scholar]
- Bettinger J.C., Lee,K. and Rougevie,A.E. (1996) Stage-specific accumulation of the terminal differentiation factor LIN-29 during Caenorhabditis elegans development. Development, 122, 2517–2527. [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974) The genetics of Caenorhabditis elegans. Genetics, 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W.J., DeWald,D.B., Emr,S.D., Plutner,H. and Balch,W.E. (1995) Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J. Cell Biol., 130, 781–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning H. and Strome,S. (1996) A sperm-supplied factor required for embryogenesis in C. elegans. Development, 122, 391–404. [DOI] [PubMed] [Google Scholar]
- Bunney T.D., Watkins,P.A.C., Beven,A.F., Shaw,P.J., Hernandez,L.E., Lomonossoff,G.P., Shanks,M., Peart,J. and Drøbak,B.K. (2000) Association of phosphatidylinositol 3-kinase with nuclear transcrip tion sites in higher plants. Plant Cell, 12, 1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C.L. and Cantley,L.C. (1996) Phosphoinositide kinases. Curr. Opin. Cell Biol., 8, 153–158. [DOI] [PubMed] [Google Scholar]
- Christoforidis S., McBride,H.M., Burgoyne,R.D. and Zerial,M. (1999a) The Rab5 effector EEA1 is a core component of endosome docking. Nature, 397, 621–625. [DOI] [PubMed] [Google Scholar]
- Christoforidis S., Miaczynska,M., Ashman,K., Wilm,M., Zhao,L., Yip,S.C., Waterfield,M.D., Backer,J.M. and Zerial,M. (1999b) Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nature Cell. Biol., 1, 249–252. [DOI] [PubMed] [Google Scholar]
- Davidson H.W. (1995) Wortmannin causes mistargeting of procathepsin D. Evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J. Cell Biol., 130, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L.I. and Blobel,G. (1986) Identification and characterization of a nuclear pore complex protein. Cell, 45, 699–709. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Emr,S.D., McPherson,P.S. and Novick,P. (1996) Phospho inositides as regulators in membrane traffic. Science, 271, 1533–1539. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli,P. and Smithies,O. (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res., 12, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G.R. and Waterston,R.H. (1985) Muscle organization in Caenorhabditis elegans: localization of proteins implicated in thin filament attachment and I-band organization. J. Cell Biol., 101, 1532–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W.W., Scheer,U., Krohne,G. and Jarasch,E.D. (1981) The nuclear envelope and the architecture of the nuclear periphery. J. Cell Biol., 91, 39S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly D.J., Morrow,I.C., Lindsay,M., Gould,R., Bryant,N.J., Gaullier,J.M., Parton,R.G. and Stenmark,H. (2000) Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J., 19, 4577–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly D.J., Simonsen,A. and Stenmark,H. (2001) Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem. J., 355, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. and Hirsh,D. (1999) Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell, 10, 4311–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E. and Lane,D. (1988) Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Herman P.K. and Emr,S.D. (1990) Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol. Cell. Biol., 10, 6742–6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P.K., Stack,J.H., DeModena,J.A. and Emr,S.D. (1991) A novel protein kinase homolog essential for protein sorting to the yeast lysosome-like vacuole. Cell, 64, 425–437. [DOI] [PubMed] [Google Scholar]
- Hiles I.D. et al. (1992) Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell, 70, 419–429. [DOI] [PubMed] [Google Scholar]
- Hong Z. and Verma,D.P. (1994) A phosphatidylinositol 3-kinase is induced during soybean nodule organogenesis and is associated with membrane proliferation. Proc. Natl Acad. Sci. USA, 91, 9617–9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A.M., Gilmour,S.G., Mancebo,R.A. and Rose,A.M. (1987) Genetic analysis of a large autosomal region in Caenorhabditis elegans by the use of a free duplication. Genet. Res. Camb., 49, 207–213. [Google Scholar]
- Kihara A., Kabeya,Y., Ohsumi,Y. and Yoshimori,T. (2001) Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep., 2, 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D.J., Herman,P.K. and Emr,S.D. (1990) The fungal vacuole: composition, function, and biogenesis. Microbiol. Rev., 54, 266–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Wild,M., Rosenzweig,B. and Hirsh,D. (1989) Wild-type and mutant actin genes in Caenorhabditis elegans. J. Mol. Biol., 208, 381–392. [DOI] [PubMed] [Google Scholar]
- Linassier C., MacDougall,L.K., Domin,J. and Waterfield,M.D. (1997) Molecular cloning and biochemical characterization of a Drosophila phosphatidylinositol-specific phosphoinositide 3-kinase. Biochem. J., 321, 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K., Bassham,D.C., Raikhel,N.V. and Nakamura,K. (1995) Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol., 130, 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim K.S. and Rose,A.M. (1990) Chromosome I duplications in Caenorhabditis elegans. Genetics, 124, 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. and Fire,A. (1995) DNA transformation. In Epstein,H.F. and Shakes,D.C. (eds), Caenorhabditis elegans: Modern Biological Analysis of an Organism. Academic Press, San Diego, CA, pp. 451–482.
- Miller D.D., Ortiz,I., Berliner,G.C. and Epstein,H.F. (1983) Differential localization of two myosins within nematode thick filaments. Cell, 34, 477–490. [DOI] [PubMed] [Google Scholar]
- Mills I.G., Jones,A.T. and Clague,M.J. (1998) Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr. Biol., 8, 881–884. [DOI] [PubMed] [Google Scholar]
- Pitt J.N., Schisa,J.A. and Priess,J.R. (2000) P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA. Dev. Biol., 219, 315–333. [DOI] [PubMed] [Google Scholar]
- Puoti A. and Kimble,J. (1999) The Caenorhabditis elegans sex determination gene mog-1 encodes a member of the DEAH-box protein family. Mol. Cell. Biol., 19, 2189–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameh L.E. and Cantley,L.C. (1999) The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem., 274, 8347–8350. [DOI] [PubMed] [Google Scholar]
- Rappleye C.A., Paredez,A.R., Smith,C.W., McDonald,K.L. and Aroian,R.V. (1999) The coronin-like protein POD-1 is required for anterior–posterior axis formation and cellular architecture in the nematode Caenorhabditis elegans. Genes Dev., 13, 2838–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.S., Klionsky,D.J., Banta,L.M. and Emr,S.D. (1988) Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol., 8, 4936–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row P.E., Reaves,B.J., Domin,J., Luzio,J.P. and Davidson,H.W. (2001) Overexpression of a rat kinase-deficient phosphoinositide 3-kinase, Vps34p, inhibits cathepsin D maturation. Biochem. J., 353, 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Saurin A.J., Borden,K.L., Boddy,M.N. and Freemont,P.S. (1996) Does this have a familiar RING? Trends Biochem. Sci., 21, 208–214. [PubMed] [Google Scholar]
- Schu P.V., Takegawa,K., Fry,M.J., Stack,J.H., Waterfield,M.D. and Emr,S.D. (1993) Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science, 260, 88–91. [DOI] [PubMed] [Google Scholar]
- Simonsen A. et al. (1998) EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature, 394, 494–498. [DOI] [PubMed] [Google Scholar]
- Simonsen A., Wurmser,A.E., Emr,S.D. and Stenmark,H. (2001) The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol., 13, 485–492. [DOI] [PubMed] [Google Scholar]
- Spieth J., Brooke,G., Kuersten,S., Lea,K. and Blumenthal,T. (1993) Operons in C. elegans: polycistronic mRNA precursors are processed by trans-splicing of SL2 downstream coding regions. Cell, 73, 521–532. [DOI] [PubMed] [Google Scholar]
- Stack J.H. and Emr,S.D. (1994) Vps34p required for yeast vacuolar protein sorting is a multiple specificity kinase that exhibits both protein kinase and phosphatidylinositol-specific PI 3-kinase activities. J. Biol. Chem., 269, 31552–31562. [PubMed] [Google Scholar]
- Stack J.H., Herman,P.K., Schu,P.V. and Emr,S.D. (1993) A membrane-associated complex containing the Vps15 protein kinase and the Vps34p PI 3-kinase is essential for protein sorting to the yeast lysosome-like vacuole. EMBO J., 12, 2195–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringham E.G., Dixon,D.K., Jones,D. and Candido,E.P.M. (1992) Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol. Biol. Cell, 3, 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A. and Cantley,L.C. (1997) Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature, 387, 673–676. [DOI] [PubMed] [Google Scholar]
- Vida T.A. and Emr,S.D. (1995) A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol., 128, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volinia S., Dhand,R., Vanhaesebroeck,B., MacDougall,L.K., Stein,R., Zvelebil,M.J., Domin,J., Panaretou,C. and Waterfield,M.D. (1995) A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p–Vps15p protein sorting system. EMBO J., 14, 3339–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E.H., Perisic,O., Ried,C., Stephens,L. and Williams,R.L. (1999) Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature, 402, 313–320. [DOI] [PubMed] [Google Scholar]
- Walsh J.P., Caldwell,K.K. and Majerus,P.W. (1991) Formation of phosphatidylinositol 3-phosphate by isomerization from phosphatidyl inositol 4-phosphate. Proc. Natl Acad. Sci. USA, 88, 9184–9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welters P., Takegawa,K., Emr,S.D. and Chrispeels,M.J. (1994) AtVPS34, a phosphatidylinositol 3-kinase of Arabidopsis thaliana, is an essential protein with homology to a calcium-dependent lipid binding domain. Proc. Natl Acad. Sci. USA, 91, 11398–11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. (1988) The anatomy. In Wood,W.B. (ed.), The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 81–122.
- Wishart M.J., Taylor,G.S. and Dixon,J.E. (2001) Phoxy lipids. revealing px domains as phosphoinositide binding modules. Cell, 105, 817–820. [DOI] [PubMed] [Google Scholar]
- Würmser A.E. and Emr,S.D. (1998) Phosphoinositide signaling and turnover: PtdIns(3)P, a regulator of membrane traffic, is transported to the vacuole and degraded by a process that requires lumenal vacuolar hydrolase activities. EMBO J., 17, 4930–4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymann M.P. and Pirola,L. (1998) Structure and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta, 1436, 127–150. [DOI] [PubMed] [Google Scholar]
- Wymann M.P., Bulgarelli-Leva,G., Zvelebil,M.J., Pirola,L., Vanhaesebroeck,B., Waterfield,M.D. and Panayotou,G. (1996) Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol. Cell. Biol., 16, 1722–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J. and Greenwald,I. (1993) A gene for a low density lipoprotein receptor-related protein in the nematode Caenorhabditis elegans. Proc. Natl Acad. Sci. USA, 90, 4572–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J., Tuck,S., Greenwald,I. and Han,M. (1999) A gp330/megalin-related protein is required in the major epidermis of Caenorhabditis elegans for completion of molting. Development, 126, 597–606. [DOI] [PubMed] [Google Scholar]
- Zorio D.A.R., Cheng,N.N., Blumenthal,T. and Spieth,J. (1994) Operons as a common form of chromosomal organization in C. elegans. Nature, 372, 270–272. [DOI] [PubMed] [Google Scholar]