Abstract
Cyclic nucleotide-gated (CNG) channels play a central role in the conversion of sensory information, such as light and scent, into primary electrical signals. We have purified the CNG channel from bovine retina and have studied it using electron microscopy and image processing. We present the structure of the channel to 35Å resolution. This three-dimensional reconstruction provides insight into the architecture of the protein, suggesting that the cyclic nucleotide-binding domains, which initiate the response to ligand, ‘hang’ below the pore-forming part of the channel, attached by narrow linkers. The structure also suggests that the four cyclic nucleotide-binding domains present in each channel form two distinct domains, lending structural weight to the suggestion that the four subunits of the CNG channels are arranged as a pair of dimers.
Keywords: CNG channel/electron microscopy/pair of dimers/structure
Introduction
Cyclic nucleotide-gated (CNG) channels play a central role in the conversion of sensory stimuli into electrical signals. Stimulation of a G-protein-coupled receptor initiates a signalling cascade that leads to an alteration in the intracellular concentration of a cyclic nucleotide. This alters the open probability of a CNG channel and leads to a change in the membrane potential of the cell, initiating the signalling processes that allow us to see and smell (Fesenko et al., 1985; Nakamura and Gold, 1987). Therefore, when these channels fail, conditions such as colour blindness (Kohl et al., 1998; Sundin et al., 2000) or retinitis pigmentosa (Dryja et al., 1995) result. Similar channels are expressed in other tissues, where their function is less well understood (Kaupp and Seifert, 2002).
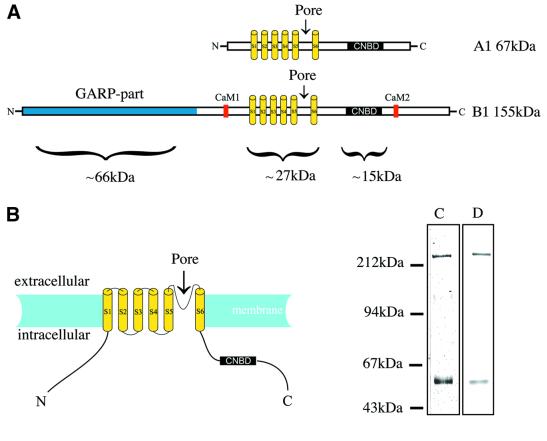
The CNG channel from the rod outer segment of the bovine retina is a hetero-oligomer of A1 (or α) and B1 (or β) subunits (Cook et al., 1987; Chen et al., 1993; Körschen et al., 1995). Cloning, sequence analysis and mutagenesis of the A1- (Kaupp et al., 1989) and B1-subunits (Körschen et al., 1995) have led to a model for their topology and the role of different regions of the channel subunit (Figure 1A and B). Both share a transmembrane topology similar to the voltage-gated potassium channels, with six putative transmembrane helices in each subunit (Kaupp et al., 1989; Wohlfart et al., 1992; Henn et al., 1995). The CNG channel pore is formed from the fifth and sixth of these helices and the intervening loop (Goulding et al., 1993; Seifert et al., 1999; Flynn and Zagotta, 2001) in a similar way to the pore of the potassium channel (Heginbotham et al., 1992). The known structure of the bacterial potassium channel, KcsA (Doyle et al., 1998), therefore has been used to model the pore of the CNG channel (Liu and Siegelbaum, 2000) and has led to suggestions about its mode of opening (Johnson and Zagotta, 2001; reviewed in Flynn et al., 2001).
Fig. 1. The topology of the CNG channel from retinal rod outer segment and its purification. (A) The sequences of the A1- and B1-subunits share a transmembrane topology with six membrane-spanning segments (S1–S6), a pore between S5 and S6 and a cyclic nucleotide-binding domain (CNBD) at the C-terminus. The B1-subunit alone has a GARP domain at the N-terminus and two calmodulin-binding sites (CaM1 and CaM2) that are lacking in the A1-subunit. (B) The proposed topology of the A1- and B1-subunits. Purified CNG channel was studied by (C) SDS–PAGE and Coomassie Blue staining and (D) by western blotting with antibodies against the A1- and B1-subunits. The A1- and B1-subunits run at apparent molecular masses of 63 and 240 kDa, respectively. The high content of glutamic acid residues in the GARP part of the B1-subunit is suggested to be responsible for its anomalous migration on an SDS–polyacrylamide gel (Körschen et al., 1995).
The similarity in membrane topology between CNG channels, tetrameric voltage-gated potassium channels and pseudotetrameric voltage-gated sodium channels led to the suggestion that CNG channels are also functional as tetramers. This was supported by studies in which channel subunits with different conductance properties were co-expressed (Liu et al., 1996).
Regions in the C-terminal domain of each CNG channel subunit show sequence homology to the cyclic nucleotide-binding domains of the bacterial transcriptional activator, catabolite activator protein (CAP), and to protein kinase A and G (Kaupp et al., 1989). Indeed, when the cyclic nucleotide-binding domain of CAP is replaced by that of the A1-subunit of the retinal CNG channel, the chimera displays cyclic nucleotide-dependent DNA-binding properties of the transcriptional activator (Scott et al., 2001). The low-resolution structure of this chimera (Scott et al., 2001) shows the CNG channel cyclic nucleotide-binding domains to adopt the same secondary and tertiary structure as those of CAP (Weber and Steitz, 1987). Indeed, mutagenesis studies of the CNG channel, guided by the structure of CAP, have allowed the properties of mutations in the channel cyclic nucleotide-binding domain to be predicted (Altenhofen et al., 1991; Varnum et al., 1995), suggesting that the domain also adopts a similar structure to that of CAP when in the channel. The cyclic nucleotide-binding domain is dimeric, both in CAP (Weber and Steitz, 1987) and in the chimera (Scott et al., 2001), leading to the suggestion that the four ligand-binding domains present in each CNG channel are arranged as two dimers. Indeed, during the gating transitions that accompany ligand binding, CNG channels appear to behave as two functional dimers (Liu et al., 1998).
The A1- and B1-subunits of the retinal CNG channel differ most extensively at the N-terminus, where a 571 residue glutamic acid- and proline-rich (GARP) domain is found only in the B1-subunit (Körschen et al., 1995). This domain interacts with the peripherin-2 complex in the disc membrane (Poetsch et al., 2001). This leads to a link between the plasma membrane and the disc membrane that may be important for the formation and maintenance of the structure of the rod outer segment. The retinal B1-subunit also has binding sites for calcium–calmodulin (Hsu and Molday, 1993; Grunwald et al., 1998; Weitz et al., 1998) that are lacking in the A1-subunit. Although the fully assembled channel appears to be a tetramer (Liu et al., 1996), the stoichiometry and arrangement of the A1- and B1-subunits are less clear. Different co-expression studies have led to the conflicting suggestions that the order of subunits around the pore of the rod CNG channel is A1–A1–B1–B1 (Shammat and Gordon, 1999) or A1–B1–A1–B1 (He et al., 2000).
Despite our understanding of the role of different parts of the channel subunit, our knowledge of the position of different domains in the correctly assembled channel is hampered by lack of structural information. Structural analysis of eukaryotic ion channels is often hindered by the scarcity of these channel proteins in natural sources, and the difficulty of their overexpression in heterologous systems. In the few cases when large quantities of channel protein are available, medium- to high-resolution structures have been achieved (Miyazawa et al., 1999; Murata et al., 2000). Otherwise, our knowledge of the structures of ion channels comes from atomic structures of bacterial channels (Chang et al., 1998; Doyle et al., 1998; Fu et al., 2000; Mindell et al., 2001; Dutzler et al., 2002), the soluble domains of eukaryotic channels (Armstrong et al., 1998; Kreusch et al., 1998; Jiang et al., 2001; Schumacher et al., 2001) and low-resolution structures of intact eukaryotic ion channels, determined by electron microscopy and image processing (Orlova et al., 1996; Sato et al., 2001; Sokolova et al., 2001). In the absence of a source of milligram quantities of CNG channels, their study is presently limited to crystallography of the cyclic nucleotide-binding domain (Scott et al., 2001) and to electron microscopy and image processing.
In the present study, we have purified the heteromeric CNG channel from bovine rod outer segments in microgram quantities and have studied it by electron microscopy and image processing of single particles. The resultant 35 Å resolution structure shows three distinct domains. The larger domain has four corners and a width similar to that of reconstructions of voltage-gated ion channels (Sato et al., 2001; Sokolova et al., 2001). We propose that this forms the membrane-spanning region of the channel. Attached to this, by two narrow linkers, are two smaller domains. These are related to one another by approximate 2-fold symmetry and we propose that they contain the ordered parts of the cytoplasmic regions of the channel. These include the cyclic nucleotide-binding domains and the ordered part of the N-terminus. The presence of two distinct domains formed from four channel subunits supports the proposal that the ligand-binding domains of CNG channels are arranged as a functional dimer in which each monomer contains two domains. The structure also suggests an architecture for the channel in which the cyclic nucleotide-binding domains ‘hang’ in the cytosol, below the pore-forming part of the channel, modulating gating of the pore through narrow linkers without comprising part of the ion pathway of the channel.
Results
Purification of the bovine rod CNG channel
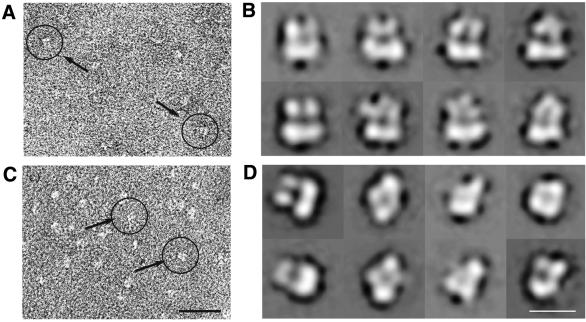
The CNG channel is abundant in bovine retinal membranes, where it can comprise as much as 6% of the total protein. Various purification schemes have been used to generate pure channel preparations from this source (reviewed in Molday and Molday, 1999). In our hands, calmodulin affinity chromatography (Hsu and Molday, 1993) yielded a preparation of channel protein that appeared homogeneous when studied by SDS–PAGE with Coomassie Blue staining (Figure 1C). Western blotting with antibodies specific to the A1- and B1-subunits showed both subunits to be present (Figure 1D). Indeed, channel prepared using a similar method was functional when reconstituted into liposomes (Cook et al., 1987). However, analysis of this material by electron microscopy of a negatively stained sample showed it to be far from homogeneous in size. Particles of ∼100 Å in diameter were observed, together with larger particles. The addition of glycerol and soybean asolectin to the purification buffers reduced the proportion of larger particles, presumably by limiting the degree of aggregation of the channel. By further separation of the calmodulin column eluant on a sucrose gradient, a preparation was obtained that appeared pure by SDS–PAGE and western blotting, and homogeneous in size when studied by electron microscopy (Figure 2A). The particles obtained in this way appeared mainly as square or wedge-shaped projections and were similar in size to particles seen when the Shaker potassium channel was studied by negative stain electron microscopy (Sokolova et al., 2001).
Fig. 2. Electron microscope images of negatively stained CNG channels. (A) CNG channels imaged with the normal of the electron microscope grid parallel to the electron beam. (B) Class averages generated by classification and averaging of the particles as in (A). (C) CNG channels imaged with the normal of the electron microscope grid at 45° to the beam direction. (D) Particles imaged with the normal of the grid at 0, 15, 30 and 45° to the beam direction were combined, classified and averaged to generate these class averages. The scale bars are 25 nm in (A) and (C) and 10 nm in (B) and (D).
Electron microscopy and image processing
The purified channel protein was applied to carbon-coated electron microscope grids and stained with uranyl acetate. Images were taken under low-dose conditions (Figure 2A) and scanned and digitized. The XIMDISP software (Crowther et al., 1996) was used to select 3000 individual particles. These images were band-pass filtered to include information only within the resolution range of 30–140 Å and were centred by alignment to a rotationally averaged sum using IMAGIC software (van Heel et al., 1996). The aligned images were classified into 24 distinct classes using reference-free multivariant statistical analysis (MSA) and images within each class were averaged together. The similar appearance of these ‘class averages’ (a selection of which are shown in Figure 2B) suggested that they represent closely related views of the channel, with a larger and two smaller domains visible. We concluded that they are views of the channel from a single direction, suggesting that the particle adopts a preferred orientation on the surface of a carbon-coated electron microscope grid.
To obtain views of the particle from different directions, we imaged the grid with the normal tilted at 0, 15, 30 and 45° to the beam direction. An image taken with the normal of the grid at 45° to the beam direction is shown in Figure 2C. A total of 11 742 particles were selected from these micrographs and filtered and centred as above. These images were subjected to MSA to sort them into classes of like views, and images within each class were averaged together to generate class averages representing a variety of different views of the channel. These class averages were used to align the images translationally and rotationally in the entire data set, and the aligned images were again subjected to MSA. The cycle of MSA, generation of class averages and realignment of the images was repeated until the resultant class averages were stable, as judged by the degree of similarity between particles placed into each class. A selection of these class averages is shown in Figure 2D.
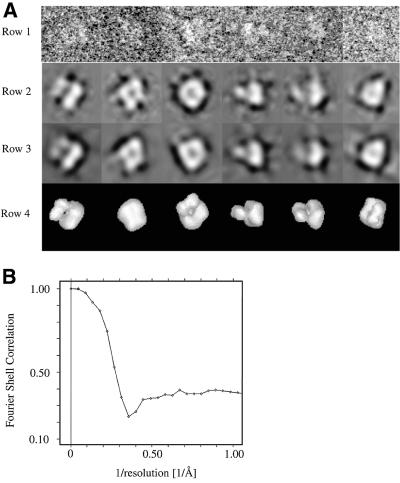
The euler angles that relate these class averages to one another in the three-dimensional structure of the particle were determined using a common lines approach in IMAGIC (van Heel et al., 1996). No symmetry was imposed during this or subsequent image processing. Knowledge of the euler angles was used to generate a three-dimensional model of the channel. Reprojections of this model were used to realign the entire data set of original images translationally and rotationally, and MSA, classification, euler angle determination and model building were repeated. The quality of each model was determined by statistical comparison of the class averages used to generate the model and the reprojections of the model in the directions of the euler angles. The cycle of realignment and regeneration of the model was repeated until this measure of quality improved no further. A total of five cycles was carried out and the gross appearance of the model did not alter during the last three cycles. The final structure was generated from 183 of a total of 192 class averages. A plot of the euler angles of these 183 averages (not shown) confirmed the suggestion that the particle adopts a preferred orientation, as some views were under-represented. Figure 3A shows the original particles used in the reconstruction, the class averages into which they were placed and the model, in projection and in surface representation, along the directions of the euler angles of the original class averages.
Fig. 3. Single particle averaging of the CNG channel. (A) Raw images of the channel with different euler angles (row 1) are compared with the corresponding class averages (row 2), the projections of the three-dimensional reconstruction (row 3) and the surface representations of the reconstruction (row 4). All of the images in one column share the same euler angles. (B) A comparison of two half data sets by FSC suggests a resolution of 35 Å, as measured by the resolution at which the FSC falls below 0.5.
To assess the validity of the structure, we first compared pairs of tilted images. The same area of the micrograph was imaged with the normal of the electron microscope grid tilted at 45 and –45° to the beam direction. Both micrographs were digitized and particles were selected using XIMDISP, allowing a single particle to be followed through the image processing procedure. The particles were combined together, filtered, aligned and subjected to MSA as above. The euler angles of the resultant class averages were determined using the three-dimensional reconstruction described above as a reference particle. If the model is correct, then two images of a single particle, taken at angles of 45 and –45°, respectively, should be assigned to classes with euler angles separated by 90°. Indeed, when we analysed a series of particles in this way, >90% displayed this behaviour.
As a second test of the structure, and to determine its resolution, we generated two independent three-dimensional reconstructions of the particle, using the method described above. These reconstructions were generated from the even- and odd-numbered particles, respectively. Both showed the same overall architecture as the original reconstruction. A comparison by Fourier shell correlation (FSC) of these two reconstructions shows a resolution of ∼35 Å (Figure 3B). The resolution of the structure was not limited by the resolution cut-off applied in the IMAGIC software as the inclusion of information within the resolution range of 25–140 Å also generated a model with a resolution close to 35 Å. Instead, the resolution is likely to be limited by the preferred orientation adopted by the particle on the electron microscope grid that leads to under-representation of some views in the structure. The presence of negative stain also limits the resolution, but attempts to study the particle in vitreous ice were hampered primarily by the low concentration of channel protein available.
Discussion
The structure of the CNG channel at35 Å resolution
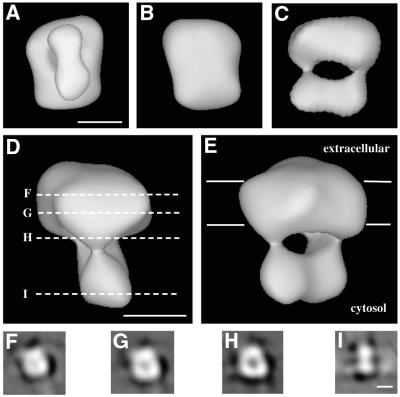
A surface representation of the channel that contains a mass equivalent to the conserved parts of four subunits (Figure 4) shows three distinct domains. The larger domain has a width of ∼100 Å, a thickness of 50 Å and four corners (see Figure 4A and B). This domain is similar in size to the putative membrane-spanning domain of the Shaker potassium channel (Sokolova et al., 2001) and has a diameter similar to that of the voltage-gated sodium channel (Sato et al., 2001). We therefore suggest that it contains the membrane-spanning parts of the four CNG channel subunits and we indicate the putative position of the lipid bilayer in Figure 4E. The slightly rectangular appearance of this domain may be the consequence of preferential orientation of the particle in combination with stain flattening in the direction perpendicular to the plane of the electron microscope grid, leaving one dimension flattened by 15%. Alternatively, the rectangular appearance may result from under-representation of views in a direction parallel to the plane of the grid, leading to decreased resolution and apparent stretching of the model in this direction. The distortion is, however, minor and the presence of four corners reveals the tetrameric arrangement of the channel. In addition, a cross-section through the channel in this region (Figure 4F and G) shows a decrease in electron density at the centre of this domain. This may be a consequence of the contrast transfer function of the electron microscope. However, this decreased region of density is in the putative location of the pore of the channel.
Fig. 4. Surface representations of the CNG channel at a contour level that includes the mass of four subunits. (A–E) Five different views of the molecule. We propose that (A) is viewed from the cytosolic side of the membrane while (B) is viewed from the extracellular side. (C), (D) and (E) are viewed from a direction parallel to the membrane, and (E) shows the putative position of the lipid bilayer, represented by two lines separated by 40 Å. (F–I) Sections through the electron density along the lines indicated in (D). The scale bars are 50 Å in length.
Attached at the cytosolic side of the transmembrane domain, by two regions of density, are two smaller domains, each with dimensions of 40 × 50 × 50 Å. Again, these may be slightly flattened by the negative stain in the direction perpendicular to the electron microscope grid. Assuming the degree of flattening of the large and small domains to be equivalent, these two smaller domains each have dimensions of 50 × 50 × 50 Å. We suggest that they contain the ordered cytoplasmic parts of the channel, including four cyclic nucleotide-binding domains and the ordered parts of four N-terminal regions. It therefore appears as though the four ligand-binding domains fold into two dimers, and that these two dimers ‘hang’ below the transmembrane part of the channel.
This model is in agreement with the structure of the cyclic nucleotide-binding domain homologue, CAP (Weber and Steitz, 1987), and a low-resolution structure of a chimera of the CNG channel ligand-binding domain with the DNA-binding domain of CAP (Scott et al., 2001). These structures show a dimer of cyclic nucleotide-binding domains to have dimensions of 30 × 45 × 50 Å (Weber and Steitz, 1987). Therefore, the volume of one of the small domains of the channel is similar to that of a dimer of the cyclic nucleotide-binding domain of CAP.
In addition to their membrane-spanning and cyclic nucleotide-binding domains, the B1-subunits of the CNG channels have a large, ∼570 residue GARP domain. This would generate a region of electron density three-quarters the size of the large transmembrane domain of the tetrameric channel. A region of electron density of this size is not apparent in the reconstruction. This may be due to several different causes. First, the GARP domain is rich in glutamic acid and proline residues and may not form a compact globular structure. Secondly, the GARP domain interacts with the peripherin-2 complex in the disc membrane of the photoreceptor cell over a distance of ∼100 Å (Poetsch et al., 2001). This suggests that the domain has an extended structure. When these interactions are disrupted during purification, further disorder may occur. Thirdly, the GARP domain may be attached to the channel by a flexible linker. In any of these cases, the domain would adopt a different position relative to the channel in each image and would be treated as noise during the averaging procedure. Indeed, large flexible domains can be completely missing from reconstructions of electron microscope images. In the case of the yeast pyruvate dehydrogenase E2 complex, the 220 residue N-terminal domains of the 60 subunits are not seen in a 30 Å resolution reconstruction from cryoelectron microscopy images (Zhou et al., 2001).
The absence of clear density for the GARP domain means that the A1- and B1-subunits cannot be distinguished at the resolution achieved. Therefore, we cannot comment on the subunit stoichiometry or the arrangement of subunits around the pore.
The CNG channel has a ‘hanging gondola’ architecture
Many of the ion channels of the nervous system have an architecture that, in the case of the Shaker potassium channel, has been described as a ‘hanging gondola’ (Sokolova et al., 2001). This describes an arrangement of cytoplasmic domains that ‘hang’ underneath the transmembrane part of the channel without forming part of the channel pore. Such an arrangement has been observed in the acetylcholine receptor (Miyazawa et al., 1999) where narrow transverse openings in the cytoplasmic parts of the channel may serve as filters to limit access to the channel pore. The structure of the voltage-gated potassium channel (Sokolova et al., 2001) also shows a domain ‘hanging’ under the pore-forming part of the channel. In this case, the hanging structure is formed from the tetramerization domains of the channel. This ‘T1 domain’ plays a role in determining which potassium channel subunits interact together in functional complexes (Li et al., 1992) and provides a docking site for the B1-subunits that modulate the channel gating properties (Rettig et al., 1994; Gulbis et al., 2000). In the case of the CNG channel, we propose the ‘hanging gondola’ to contain the ligand-binding domains.
The CNG channel is arranged as a dimer of dimers
The domains that ‘hang’ below the transmembrane part of the CNG channel are arranged as two regions of density, related to one another by approximate 2-fold symmetry. This change from an approximate 4-fold to 2-fold symmetry is seen clearly in cross-sections of the channel (Figure 4F–I). We propose that each of these smaller domains contains a dimer of ligand-binding domains. However, as the resolution obtained does not allow us to distinguish between the A1- and B1-subunits of the channel, we are unable to say whether these two dimers are formed from the ligand-binding domains of like subunits and the channel is a dimer of A1-subunits and a dimer of B1-subunits, or whether another arrangement of subunits is adopted.
Although the structure presented here is the first view of an intact CNG channel, the idea that the cyclic nucleotide-binding domains of the channel are arranged as a pair of dimers is not new. The structure of a chimera of the CNG channel cyclic nucleotide-binding domain fused to the DNA-binding domain of CAP (Scott et al., 2001) shows the ligand-binding domain to form a dimer. This is also the case in the homologous cyclic nucleotide-binding domain of CAP (Weber and Steitz, 1987).
The gating properties of CNG channels also indicate that the subunits are arranged as two functional dimers (Liu et al., 1998). The conductance properties of channels that have been constrained to have different ligand occupancies are consistent with a model in which subunits function together as pairs. Within each pair of subunits, the gating transition occurs in a cooperative manner. However, the two pairs of subunits go through the gating transition independently. This is similar to the CAP, in which the two subunits of the dimer display cooperativity (Takahashi et al., 1980).
The CNG channels are not alone as tetrameric channels that have two pairs of dimeric ligand-binding domains arranged in the cytoplasm. Recent structures of the gating domains of the small and large conductance calcium-activated potassium channels at atomic resolution also show ligand-binding domains that fold as dimers (Jiang et al., 2001; Schumacher et al., 2001). Whether these features are common to ion channels that are gated by the binding of intracellular ligands, and what the consequences of this arrangement are for the high-resolution structure and the function of the channel as a whole, remain to be investigated.
Materials and methods
Purification of the CNG channel
Rod outer segments (ROS) were prepared from dark-adapted retina as described in Schnetkamp and Daemen (1982). ROS membranes were stripped of soluble protein by hypotonic lysis and washed under dim red light. The rhodopsin content of washed membranes was determined spectrophotometrically and the membranes were diluted with hypotonic buffer [10 mM HEPES–NaOH pH 7.4, 2 mM dithiothreitol (DTT) and 2 mM EDTA] to a final rhodopsin content of 1 mg/ml. Membranes were recovered by centrifugation for 18 min at 100 000 g and washed a further three times. The final membrane pellet was solubilized in a buffer containing 18 mM CHAPS, 10 mM HEPES–NaOH pH 7.4, 200 mM NaCl, 2 mM DTT, 2 mM CaCl2, 25% (v/v) glycerol and 2 mg/ml soybean asolectin (Sigma) for 5 min. Unsolubilized material was removed by centrifugation for 60 min at 100 000 g.
The CNG channel was purified from solubilized membranes using calmodulin affinity chromatography (Hsu and Molday, 1993). A 1 ml aliquot of calmodulin–agarose (Sigma) was equilibrated with 10 vols of running buffer [10 mM HEPES–NaOH pH 7.4, 15 mM CHAPS, 150 mM NaCl, 2 mM DTT, 1 mM CaCl2, 25% (v/v) glycerol and 1.3 µg/ml soybean asolectin]. The sample was loaded at 0.5 ml/min onto the equilibrated column and washed with 10 column vols of running buffer. The channel protein was eluted with a gradient of EDTA of 0–0.25 mM in elution buffer (elution buffer being running buffer with the omission of CaCl2). The eluted protein was checked by SDS–PAGE with Coomassie Blue staining and western blotting.
Channel protein was concentrated in a Centricon-100 filter (Amicon) to a final concentration of 80 µg/ml. A total of 300 µl of the concentrated channel was placed onto the top of a gradient of 5–25% sucrose (made up in running buffer) and were centrifuged for 14 h at 175 000 g (40 000 r.p.m. in a Beckman SW55 rotor). The centrifuge was stopped without using the brake; fractions were collected using a Brandel gradient collector and analysed by SDS–PAGE and western blotting using antibodies against the A1- and B1-subunits.
Electron microscopy
A 5 µl droplet of the CNG channel preparation was applied to a glow-discharged, carbon-coated electron microscope grid. After 1 min, excess solution was removed and the grid was washed twice with elution buffer lacking lipids [10 mM HEPES–NaOH pH 7.4, 15 mM CHAPS, 150 mM NaCl, 2 mM DTT and 25% (v/v) glycerol] and negatively stained with three washes of 2% uranyl acetate containing 0.0025% polyacrylic acid. Grids were examined with a Philips CM12 electron microscope under low-dose conditions. Images were taken at a magnification of 40 000× and a defocus of 1.5 µm, with the normal of the grid at 0, 15, 30 and 45° to the beam direction. To limit beam damage, each tilted image was the first to be taken from that area of the micrograph.
Image processing
Twenty-four micrographs (with 12 at 0°, three at 15°, three at 30° and six at 45°) were digitized on an SCAI scanner (Zeiss) with a 7 µm pixel size and were compressed by averaging together groups of two pixels to give a final pixel size of 3.5 Å on the sample. A total of 11 742 particle images were selected manually using XIMDISP (Crowther et al., 1996) and image processing was carried out using IMAGIC-V (van Heel et al., 1996). A standard procedure was followed (Orlova and van Heel, 1997; Sokolova et al., 2001). The channel images were band-pass filtered to include information only within a resolution range of 30–140 Å and were normalized and centred by translational alignment to a rotationally averaged sum of all images. MSA was used to sort the centred images into classes of like views. The particles within each class were summed together and the resulting class averages were used to align the entire data set translationally and rotationally. Four cycles of MSA classification and the generation of new references for subsequent classification led to stable classes. Further cycles did not lead to a decrease in the variance within each class.
A common-lines procedure in IMAGIC was used to determine the euler angles of this final set of class averages, and a three-dimensional reconstruction was built. The agreement between the class averages and the corresponding reprojections of the reconstruction along the same euler angles were used to assess the quality of the reconstruction. The projections of the reconstruction were used as references in MSA. After four cycles of MSA, euler angle determination, model building and preparation of references by projection of the model, a stable model was obtained. Further cycles did not improve the quality of the agreement of the class sums used to generate the model and the model reprojections.
The resolution of the model was determined by splitting the original data set of particles into two halves and generating independent models using the method described above for the two half data sets. The FSC was used to compare the two models and the resolution was taken as the point at which the FSC fell below 0.5 (Bottcher et al., 1997). The approximate position of the first zero of the contrast transfer function was at a higher resolution than the filter used to remove high-resolution information. We therefore did not correct for this function.
Acknowledgments
Acknowledgements
We thank R.Esser and D.Höppner-Heitmann for preparing rod outer segments, G.Büldt and N.Unwin for supporting the project, and J.Smith, P.Rosenthal, A.Roseman and R.Henderson for helpful and constructive advice in image processing and reconstruction. This work was supported by a Strategy Fund Project of the Hermann von Helmholtz Association.
References
- Altenhofen W., Ludwig,J., Eismann,E., Kraus,W., Bönigk,W. and Kaupp,U.B. (1991) Control of ligand specificity in cyclic nucleotide-gated channels from rod photoreceptors and olfactory epithelium. Proc. Natl Acad. Sci. USA, 88, 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong N., Sun,Y., Chen,G.Q. and Gouaux,E. (1998) Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature, 395, 913–917. [DOI] [PubMed] [Google Scholar]
- Bottcher B., Wynne,S.A. and Crowther,R.A. (1997) Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature, 386, 88–91. [DOI] [PubMed] [Google Scholar]
- Chang G., Spencer,R.H., Lee,A.T., Barclay,M.T. and Rees,D.C. (1998) Structure of the MscL homologue from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science, 282, 2220–2226. [DOI] [PubMed] [Google Scholar]
- Chen T.-Y., Peng,Y.-W., Dhallan,R.S., Ahamed,B., Reed,R.R. and Yau,K.-W. (1993) A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature, 362, 764–767. [DOI] [PubMed] [Google Scholar]
- Cook N.J., Hanke,W. and Kaupp,U.B. (1987) Identification, purification and functional reconstitution of the cyclic GMP-dependent channel from rod photoreceptors. Proc. Natl Acad. Sci. USA, 84, 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R.A., Henderson,R. and Smith,J.M. (1996) MRC image processing programs. J. Struct. Biol., 116, 9–16. [DOI] [PubMed] [Google Scholar]
- Doyle D.A., Morais Cabral,J., Pfuetzner,R.A., Kuo,A., Gulbis,J.M., Cohen,S.L., Chait,B.T. and MacKinnon,R. (1998) The structure of the potassium channel: molecular basis of K+ channel conduction and selectivity. Science, 280, 69–77. [DOI] [PubMed] [Google Scholar]
- Dryja T.P., Finn,J.T., Peng,Y.-W., McGee,T.L., Berson,E.L. and Yau,K.-W. (1995) Mutations in the gene encoding the α subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc. Natl Acad. Sci. USA, 92, 10177–10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzler R., Campbell,E.B., Cadene,M., Chait,B.T. and MacKinnon,R. (2002) X-ray structure of a ClC chloride channel at 3 Å reveals the molecular basis of anion selectivity. Nature, 415, 287–294. [DOI] [PubMed] [Google Scholar]
- Fesenko E.E., Kolesnikov,S.S. and Lyubarsky,A.L. (1985) Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature, 313, 310–313. [DOI] [PubMed] [Google Scholar]
- Flynn G.E. and Zagotta,W.N. (2001) Conformational changes in S6 coupled to the opening of cyclic nucleotide-gated channels. Neuron, 30, 689–698. [DOI] [PubMed] [Google Scholar]
- Flynn G.E., Johnson,J.P.,Jr and Zagotta,W.N. (2001) Cyclic nucleotide-gated channels: shedding light on the opening of a channel pore. Nature Rev. Neurosci., 2, 643–651. [DOI] [PubMed] [Google Scholar]
- Fu D., Libson,A., Miercke,L.J.W., Weitzmann,C., Nollert,P., Krucinski,J. and Stroud,R.M. (2000) Structure of a glycerol-conducting channel and the basis for its selectivity. Science, 290, 481–486. [DOI] [PubMed] [Google Scholar]
- Goulding E.H., Tibbs,G.R., Liu,D. and Siegelbaum,S.A. (1993) Role of H5 domain in determining pore diameter and ion permeation through cyclic nucleotide-gated channels. Nature, 364, 61–64. [DOI] [PubMed] [Google Scholar]
- Grunwald M.E., Yu,W.-P., Yu,H.-H. and Yau,K.-W. (1998) Identification of a domain on the β-subunit of the rod cGMP-gated cation channel that mediates inhibition by calcium–calmodulin. J. Biol. Chem., 273, 9148–9157. [DOI] [PubMed] [Google Scholar]
- Gulbis J.M., Zhou,M., Mann,S. and MacKinnon,R. (2000) Structure of the cytoplasmic β subunit–T1 assembly of voltage-dependent K+ channels. Science, 289, 123–127. [DOI] [PubMed] [Google Scholar]
- He Y., Ruiz,M.L. and Karpen,J.W. (2000) Constraining the subunit order of rod cyclic nucleotide-gated channels reveals a diagonal arrangement of like subunits. Proc. Natl Acad. Sci. USA, 97, 895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., Abramson,T. and MacKinnon,R. (1992) A functional connection between the pores of distantly related ion channels as revealed by mutant K+ channels. Science, 258, 1152–1155. [DOI] [PubMed] [Google Scholar]
- Henn D.K., Baumann,A. and Kaupp,U.B. (1995) Probing the transmembrane topology of cyclic nucleotide-gated ion channels with a gene fusion approach. Proc. Natl Acad. Sci. USA, 92, 7425–7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y.-T. and Molday,R.S. (1993) Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature, 361, 76–79. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Pico,A., Cadene,M., Chait,B.T. and MacKinnon,R. (2001) Structure of the RCK domain from the E.coli K+ structure and demonstration of its presence in the human BK channel. Neuron, 29, 593–601. [DOI] [PubMed] [Google Scholar]
- Johnson J.P.,Jr and Zagotta,W.N. (2001) Rotational movement during cyclic nucleotide-gated channel opening. Nature, 412, 917–921. [DOI] [PubMed] [Google Scholar]
- Kaupp U.B. and Seifert,R. (2001) Cyclic nucleotide-gated ion channels. Physiol. Rev., in press. [DOI] [PubMed] [Google Scholar]
- Kaupp U.B. et al. (1989) Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature, 342, 762–766. [DOI] [PubMed] [Google Scholar]
- Kohl S., Marx,T., Giddings,I., Jägle,H., Jacobson,S.G., Apfelstedt-Sylla,E., Zrenner,E., Sharpe,L.T. and Wissinger,B. (1998) Total colourblindness is caused by mutations in the gene encoding the α-subunit of the cone photoreceptor cGMP-gated cation channel. Nature Genet., 19, 257–259. [DOI] [PubMed] [Google Scholar]
- Körschen H.G. et al. (1995) A 240 kDa protein represents the complete β subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron, 15, 627–636. [DOI] [PubMed] [Google Scholar]
- Kreusch A., Pfaffinger,P.J., Stevens,C.F. and Choe,S. (1998) Crystal structure of the tetramerization domain of the Shaker potassium channel. Nature, 392, 945–948. [DOI] [PubMed] [Google Scholar]
- Li M., Jan,Y.N. and Jan,L.Y. (1992) Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel. Science, 257, 1225–1230. [DOI] [PubMed] [Google Scholar]
- Liu D.T. and Siegelbaum,S.A. (2000) Change of pore helix conformational state upon opening of cyclic nucleotide-gated channels. Neuron, 28, 899–909. [DOI] [PubMed] [Google Scholar]
- Liu D.T., Tibbs,G.R. and Siegelbaum,S.A. (1996) Subunit stoichiometry of cyclic nucleotide-gated channels and effects of subunit order on channel function. Neuron, 16, 983–990. [DOI] [PubMed] [Google Scholar]
- Liu D.T., Tibbs,G.R., Paoletti,P. and Siegelbaum,S.A. (1998) Constraining ligand-binding site stoichiometry suggests that a cyclic nucleotide-gated channel is composed of two functional dimers. Neuron, 21, 235–248. [DOI] [PubMed] [Google Scholar]
- Mindell J.A., Maduke,M., Miller,C. and Grigorieff,N. (2001) Projection structure of a ClC-type chloride channel at 6.5 Å resolution. Nature, 409, 219–223. [DOI] [PubMed] [Google Scholar]
- Miyazawa A., Fujiyoshi,Y., Stowell,M. and Unwin,N. (1999) Nicotinic acetylcholine receptor at 4.6 Å resolution: transverse tunnels in the channel wall. J. Mol. Biol., 288, 765–786. [DOI] [PubMed] [Google Scholar]
- Molday R.S. and Molday,L.L. (1999) Purification, characterization and reconstitution of cyclic nucleotide-gated channels. Methods Enzymol., 294, 246–260. [DOI] [PubMed] [Google Scholar]
- Murata K., Mitsuoka,K., Hirai,T., Walz,T., Agre,P., Heymann,J.B., Engel,A. and Fujiyosji,Y. (2000) Structural determinants of water permeation through aquaporin-1. Nature, 407, 599–606. [DOI] [PubMed] [Google Scholar]
- Nakamura T. and Gold,G.H. (1987) A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature, 325, 442–444. [DOI] [PubMed] [Google Scholar]
- Orlova E.V., Dube,P., Harris,J.R., Beckmann,E., Zemlin,F., Markl,J. and van Heel,M. (1997) Structure of keyhole limpet hemocyanin type 1 (KLH1) at 15 Å resolution by electron cryomicroscopy and angular reconstitution. J. Mol. Biol., 271, 417–437. [DOI] [PubMed] [Google Scholar]
- Orlova E.V., Serysheva,I.I., van Heel,M., Hamilton,S.L. and Chiu,W. (1996) Two structural configurations of the skeletal muscle calcium release channel. Nature Struct. Biol., 3, 547–552. [DOI] [PubMed] [Google Scholar]
- Poetsch A., Molday,L.L. and Molday,R.S. (2001) The cGMP-gated channel and related glutamic acid-rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J. Biol. Chem., 276, 48009–48016. [DOI] [PubMed] [Google Scholar]
- Rettig J., Heinemann,S.H., Wunder,F., Lorra,C., Parcej,D.N., Dolly,J.O. and Pongs,O. (1994) Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature, 369, 289–294. [DOI] [PubMed] [Google Scholar]
- Sato C., Ueno,Y., Asai,K., Takahashi,K., Sato,M., Engel,A. and Fujiyoshi (2001) The voltage-sensitive sodium channel is a bell-shaped molecule with several cavities. Nature, 409, 1047–1051. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P.P.M. and Daemen,F.J.M. (1982) Isolation and characterization of osmotically sealed bovine rod outer segments. Methods Enzymol., 81, 110–116. [DOI] [PubMed] [Google Scholar]
- Schumacher M.A., Rivard,A.F., Bächinger,H.P. and Adelman,J.P. (2001) Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+–calmodulin. Nature, 410, 1120–1123. [DOI] [PubMed] [Google Scholar]
- Scott S.-P., Weber,I.T., Harrison,R.W., Carey,J. and Tanaka,J.C. (2001) A functioning chimera of the cyclic nucleotide-binding domain from the bovine retinal rod ion channel and the DNA-binding domain from catabolite gene-activating protein. Biochemistry, 40, 7464–7473. [DOI] [PubMed] [Google Scholar]
- Seifert R., Eismann,E., Ludwig,J., Baumann,A. and Kaupp,U.B. (1999) Molecular determinants of a Ca2+-binding site in the pore of cyclic nucleotide-gated channels: S5/S6 segments control affinity of intrapore glutamates. EMBO J., 18, 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammat I.M. and Gordon,S.E. (1999) Stoichiometry and arrangement of subunits in rod cyclic nucleotide-gated channels. Neuron, 23, 809–819. [DOI] [PubMed] [Google Scholar]
- Sokolova O., Kolmakova-Partensky,L. and Grigorieff,N. (2001) Three-dimensional structure of a voltage-gated potassium channel at 2.5 nm resolution. Structure, 9, 215–220. [DOI] [PubMed] [Google Scholar]
- Sundin O.H., Yang,J.-M., Li,Y., Zhu,D., Hurd,J.N., Mitchell,T.N., Silva,E.D. and Maumenee,I.H. (2000) Genetic basis of total colourblindness among the Pingelapese islanders. Nature Genet., 25, 289–293. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Blazy,B. and Baudras,A. (1980) An equilibrium study of the cooperative binding of adenosine cyclic 3′,5′-monophosphate and guanosine cyclic 3′,5′-monophosphate to the adenosine cyclic 3′,5′-monophosphate receptor protein from Escherichia coli. Biochemistry, 19, 5124–5130. [DOI] [PubMed] [Google Scholar]
- van Heel M., Harauz,G., Orlova,E.V., Schmidt,R. and Schatz,M. (1996) A new generation of the IMAGIC image processing system. J. Struct. Biol., 116, 17–24. [DOI] [PubMed] [Google Scholar]
- Varnum M.D., Black,K.D. and Zagotta,W.N. (1995) Molecular mechanism for ligand discrimination of cyclic nucleotide-gated channels. Neuron, 15, 619–625. [DOI] [PubMed] [Google Scholar]
- Weber I.T. and Steitz,T.A. (1987) Structure of a complex of catabolite gene activator protein and cyclic AMP refined at 2.5 Å resolution. J. Mol. Biol., 198, 311–326. [DOI] [PubMed] [Google Scholar]
- Weitz D., Zoche,M., Müller,F., Beyermann,M., Körschen,H.G., Kaupp,U.B. and Koch,K.-W. (1998) Calmodulin controls the rod photoreceptor CNG channel through an unconventional binding site in the N-terminus of the β-subunit. EMBO J., 17, 2273–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfart P., Haase,W., Molday,R.S. and Cook,N.J. (1992) Antibodies against synthetic peptides used to determine the topology and site of glycosylation of the cGMP-gated channel from bovine rod photoreceptors. J. Biol. Chem., 267, 644–648. [PubMed] [Google Scholar]
- Zhou Z.H., Liao,W., Cheng,R.H., Lawson,J.E., McCarthy,D.B., Reed,L.J. and Stoops,J.K. (2001) Direct evidence for the size and conformational variability of the pyruvate dehydrogenase complex revealed by three-dimensional electron microscopy. The ‘breathing’ core and its functional relationship to protein dynamics. J. Biol. Chem., 276, 21704–21713. [DOI] [PubMed] [Google Scholar]