Abstract
Three types of tissue samples—umbilical cord (UC), umbilical cord serum (CS), and maternal serum (MS)—have often been used to assess fetal exposure to chemicals. In order to know the relationship of contamination between mothers and fetuses, we measured persistent chemicals in comparable sets of the three tissue samples. Also, we analyzed the association between the chemicals in maternal and fetal tissues to know which tissue is the best sample for fetal exposure assessment. On a wet basis, the chemical concentrations were of the order MS > CS > UC, except for some chemicals such as cis-chlordane and endosulfan. On a lipid basis, the concentrations in UC were nearly equal or often higher than in MS, but the concentrations in CS were usually lower than in others. Hexachlorocyclohexanes and penta-, hexa-, and heptachlorinated biphenyls showed an association between the concentrations in UC versus MS, and UC versus CS. These chemicals also showed high correlation coefficients between the chemical concentrations in UC of first babies and maternal age. These chemicals were closely related to each other when grouped on the basis of their concentrations using cluster analysis. In conclusion, we insist that UC is the best sample to assess fetal contamination status of persistent chemicals. There is a possibility that the assessment based on the contamination levels in CS result in an underestimation.
Keywords: cord blood, maternal blood, organochlorine pesticides, polychlorinated biphenyls (PCBs), umbilical cord
It is believed that humans are exposed to multiple chemicals from food, air, water, and so forth, including natural products; industrial products, such as polychlorinated biphenyls (PCBs), pesticides, and pharmaceuticals; and nonintentional products, such as dioxins. Human fetuses are exposed to multiple chemicals through placenta in Japan (Mori 2001; Mori et al. 2003; Todaka and Mori 2002), and infants are exposed to these chemicals through milk (Borgert et al. 2003). A number of persistent organochlorine pollutants have been detected in human follicular fluid (De Felip et al. 2004) and amniotic fluid (Foster et al. 2000). Because human fetuses and infants are considered significantly more sensitive to a variety of environmental toxicants compared with adults (Branum et al. 2003; Charnley and Putzrath 2001; Needham and Sexton 2000), the adverse effects of chemicals on these fetuses and infants are of concern.
Three types of tissue samples—umbilical cord (UC), umbilical cord serum (CS), and maternal serum (MS)—have often been used to assess fetal exposure to chemicals. There are several reports indicating that the chemical concentrations were higher in maternal blood than in cord blood (Sarcinelli et al. 2003; Waliszewski et al. 2000; Walker et al. 2003). The assessments using cord blood have suggested fetal contamination. However, the chemical concentrations in fetal tissues are still unclear. There are only a few reports using fetal tissues such as UC (Covaci et al. 2002; Grandjean et al. 2001).
In order to know the relationship of contamination between mothers and fetuses, we measured persistent chemicals in comparable sets of the three tissue samples (UC, CS, and MS). Also, we analyzed the association between the chemicals in maternal and fetal tissues to know which tissue is the best sample for fetal exposure assessment.
Materials and Methods
Sample.
Thirty-two pregnant women who were general citizens and lived in the cities of Chiba and Yamanashi, near Tokyo, Japan, were surveyed in 2002 and 2003. UC (~ 20 cm), maternal blood (10 mL), and cord blood (10 mL) were collected from the cases delivered by cesarean section. The deliveries were conducted at least 12 hr after the last meal. UC without cord blood and MS and CS were stored at −20°C until use in glassware that had been checked to be without contamination. In the whole-study subjects, 20 mothers had complete samples (MS, CS, and UC), and their average age at delivery was 32.8 ± 4.0 years. There were 12 mothers without CS samples, and their average age was 31.9 ± 4.9 years. In total, 32 cases were used for the analysis of correlation between maternal age and chemical concentrations. This study has been approved by the Congress of Medical Bioethics of Chiba University and the University of Yamanashi, and all the samples were obtained after receipt of written informed consent.
Chemicals measured.
We measured 19 organochlorine pesticides: dichlorodiphenyl-trichloroethane (DDT) and its metabolites [dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane (DDD): p,p′-DDT, o,p′-DDT, p,p′-DDE, o,p′-DDE, p,p′-DDD, o,p′-DDD], chlordane and its metabolites (cis-chlordane, trans-chlordane, trans-nonachlor, oxychlordane), heptachlor and its metabolites (heptachlor, heptachlor epoxide), methoxychlor, “drins” (dieldrin, aldrin, endrin), endosulfan isomers (mixture of α- and β-endosulfan), hexachlorobenzene (HCB), and hexachlorocyclohexane (HCH) isomers (mixture of α-, β-, γ-, and δ -HCH). We also measured 10 groups of PCB congeners grouped by their number of chlorines from 1 to 10.
Pretreatment.
MS (4–5 mL), CS (3–4 mL), and UC (17–27 g) were used for the preparation of samples for gas chromatography–mass spectrometry. The details were revealed to the public through the homepage of the Ministry of Environment of the Government of Japan (2002). Briefly, the UC samples were homogenized with ethanol/hexane (1:3) and sodium sulfuric anhydride by a Polytron PT3100 (Kinematica AG, Littau-Lucerne, Switzerland) after 13C12-labeled PCB, 13C6-labeled β-HCH, 13C6-labeled HCB, 13C9-labeled endosulfan-I, 13C12-labeled pentaCB, and 13C12-labeled p,p′-DDT had been added as quantitative standards. After filtration, the filtrate and an additional filtrate of the rehomogenate of the residue were washed with water twice. The resulting hexane extract was dehydrated using sodium sulfuric anhydride and concentrated by evaporation. One-sixth of the concentrated extract was used for measurement of PCBs, another sixth for that of organochlorine pesticides, and half for the gravimetric fat determination.
The MS and CS samples were extracted twice using an ether/hexane (3:1) mixture after addition of the quantitative standards. The resulting ether/hexane extract was dehydrated using sodium sulfuric anhydride and concentrated by evaporation (crude extract). A fourth the crude extract was used for measurement of PCBs, and another fourth for organochlorine pesticides.
Measurement of PCBs.
After the crude extract was treated with 1 mol/L KOH/ethanol for 18 hr, it was extracted using hexane three times and was concentrated with nitrogen. The concentrate was then eluted through a silica gel 60 column (70-230 ASTM-mesh; Merck, Darmstadt, Germany) with 10 mL hexane, evaporated to a final volume of 0.1 mL, and analyzed after the addition of 13C12-labeled PCB.
PCBs were quantitated by gas chromatography–mass spectrometry. Gas chromatography was performed using a Hewlett Packard HP6800 series equipped with a Micromass AutoSpec Ultima mass spectrometer (Micromass Ltd., Manchester UK). An HT8 fused silica capillary column [0.25 mm inner diameter (i.d.) × 25 m with a 0.33-mm film thickness; SGE International Pty Ltd., Austin, TX, USA] was used to separate each PCB congener. The column temperature was maintained at 100°C for 2 min, raised to 180°C at a rate of 5°C/min, maintained at 180°C for 0.5 min, raised to 270°C at a rate of 20°C/min, then to 300°C at a rate of 5°C/min, and finally maintained at 300°C for 2 min. The carrier gas (helium) flow rate was 1 mL/min. The ionizing current was 600 μA, the ionizing energy was 38 eV, and the accelerating voltage was 8 kV. The resolution of the mass spectrometer was maintained at approximately > 10,000 (10% valley) throughout, and the analysis was carried out according to selected ion monitoring.
Measurement of organochlorine pesticides.
The crude extract was evaporated to a final volume of 0.5 mL and extracted twice with hexane-saturated acetonitrile. The resulting acetonitrile extract was added to water and extracted with hexane twice, then dehydrated using sodium sulfuric anhydride, and evaporated with nitrogen. The concentrate was eluted through a Florisil column (1 g/6 cc, Seppak Vac Florisil; Waters, Milford, MA, USA) using 10 mL hexane, evaporated to a final volume of 0.1 mL, and analyzed after the addition of fluoranthene-d10.
Organochlorine pesticides were quantitated by gas chromatography-mass spectrometry in the same manner as for PCBs, except that the column was a BPX-25 fused silica capillary column, 0.22 mm i.d. × 30 m with a 0.25-mm film thickness (SGE International Pty Ltd.). The column temperature was maintained at 60°C for 1 min, raised to 300°C at a rate of 10°C/min, and finally maintained at 300°C for 10 min.
Lipid contents.
Lipid contents in the UC samples were determined gravimetrically, and lipid contents in MS and CS were determined enzymatically as the sum of the total cholesterol, triglycerides, and phospholipids.
Statistical analysis.
The statistical analysis was performed using Microsoft Excel 2002 (Microsoft, Redmond, WA, USA). Cluster analysis was performed by the cosine correlation method using GeneMaths software (version 1.50; Applied Maths BVBA, Sint-Martens-Latem, Belgium).
Results
Detection rate.
Tables 1–4 show the concentration of organochlorine pesticides (Tables 1 and 2) and PCBs (Tables 3 and 4) in the three types of tissues (MS, CS, and UC). It became clear that human fetuses were contaminated with multiple chemicals in Japan. However, o,p′-DDE, o,p′-DDD, aldrin, endrin, and methoxychlor were not detected in any of the tissues in this study. Other chemicals were detected in MS and/or UC, but the detection rate was very low in the CS (Tables 1 and 2). In particular, cis-chlordane, endosulfan, p,p′-DDT, dieldrin, p,p′-DDD, and heptachlor were not detected in CS; however, both were detected in MS (detection rate > 70%) and UC. PCB congeners with five to seven chlorines were detected in all samples, whereas other congeners showed a relatively low detection rate in CS (Tables 3 and 4).
Table 1.
Organochlorine pesticide concentrations (pg/g-lipid) in three types of tissue.
| Organochlorine pesticide, tissue | Detectiona (%) | Mean ± SD | Minimum | 25th percentile | Median | 75th percentile | Maximum |
|---|---|---|---|---|---|---|---|
| HCB | |||||||
| MS | 100 | 15,500 ± 6,220 | 3,600 | 10,000 | 16,000 | 17,800 | 31,000 |
| CS | 100 | 10,900 ± 3,680** | 5,200 | 8,800 | 11,000 | 12,000 | 18,000 |
| UC | 95 | 17,700 ± 6,360## | ND | 16,000 | 18,000 | 20,000 | 28,000 |
| HCHs | |||||||
| MS | 100 | 27,400 ± 10,630 | 13,000 | 22,000 | 26,000 | 30,000 | 55,000 |
| CS | 100 | 33,800 ± 19,300 | 12,000 | 24,000 | 28,000 | 39,000 | 100,000 |
| UC | 100 | 36,200 ± 14,920** | 18,000 | 26,000 | 30,000 | 45,000 | 69,000 |
| p,p′-DDT | |||||||
| MS | 80 | 3,380 ± 3,240 | ND | 1,000 | 2,400 | 5,100 | 11,000 |
| CS | 0 | — | — | — | — | — | |
| UC | 50 | 5,550 ± 7,160** | ND | ND | 1,100 | 9,300 | 19,000 |
| o,p′-DDT | |||||||
| MS | 15 | 38 ± 104 | ND | ND | ND | ND | 340 |
| CS | 0 | — | — | — | — | — | — |
| UC | 0 | — | — | — | — | — | — |
| p,p′-DDE | |||||||
| MS | 100 | 89,700 ± 33,600 | 19,000 | 71,000 | 93,000 | 110,000 | 150,000 |
| CS | 100 | 33,000 ± 16,500** | 14,000 | 22,000 | 28,000 | 42,000 | 75,000 |
| UC | 100 | 79,600 ± 26,200## | 29,000 | 64,000 | 78,000 | 90,000 | 140,000 |
| p,p′-DDD | |||||||
| MS | 85 | 766 ± 982 | ND | 200 | 360 | 950 | 3,800 |
| CS | 0 | — | — | — | — | — | |
| UC | 15 | 215 ± 527 | ND | ND | ND | ND | 1,600 |
| cis-Chlordane | |||||||
| MS | 100 | 240 ± 165 | 63 | 110 | 200 | 330 | 660 |
| CS | 0 | — | — | — | — | — | |
| UC | 70 | 1,220 ± 1,230** | ND | ND | 1,200 | 1,700 | 4,400 |
| trans-Chlordane | |||||||
| MS | 95 | 320 ± 257 | ND | 160 | 240 | 430 | 1,200 |
| CS | 20 | 198 ± 449 | ND | ND | ND | ND | 1,400 |
| UC | 55 | 644 ± 848** | ND | ND | 290 | 1,200 | 3,000 |
| Oxychlordane | |||||||
| MS | 85 | 2,640 ± 4,160 | ND | 620 | 1,200 | 3,900 | 19,000 |
| CS | 40 | 978 ± 1,630 | ND | ND | ND | 1,600 | 6,300 |
| UC | 60 | 2,120 ± 2,100 | ND | ND | 1,900 | 3,700 | 6,100 |
| trans-Nonachlor | |||||||
| MS | 100 | 7,230 ± 2,840 | 2,000 | 5,600 | 7,000 | 9,000 | 14,000 |
| CS | 80 | 3,780 ± 6,470 | ND | 1,400 | 1,900 | 3,800 | 30,000 |
| UC | 100 | 7,660 ± 2,580 | 2,500 | 6,200 | 6,700 | 8,300 | 14,000 |
| Dieldrin | |||||||
| MS | 70 | 495 ± 454 | ND | ND | 440 | 770 | 1,600 |
| CS | 0 | — | — | — | — | — | — |
| UC | 45 | 1,970 ± 3,170* | ND | ND | ND | 2,200 | 9,600 |
| Endosulfan | |||||||
| MS | 90 | 380 ± 267 | ND | 280 | 340 | 460 | 1,100 |
| CS | 0 | — | — | — | — | — | — |
| UC | 70 | 2,090 ± 2,440** | ND | ND | 1,600 | 2,900 | 9,400 |
| Heptachlor | |||||||
| MS | 25 | 150 ± 272 | ND | ND | ND | 100 | 700 |
| CS | 0 | — | — | — | — | — | |
| UC | 10 | 505 ± 1,620 | ND | ND | ND | ND | 6,500 |
| Heptachlor epoxide | |||||||
| MS | 100 | 142 ± 729 | 310 | 950 | 1,200 | 1,700 | 3,000 |
| CS | 95 | 1,580 ± 844 | ND | 1,100 | 1,500 | 1,900 | 3,400 |
| UC | 100 | 2,790 ± 1,280**,## | 160 | 2,000 | 2,700 | 3,500 | 6,000 |
ND, not detected.
Detection rate is shown as a percentage (n = 20).
p < 0.05 and
p < 0.001 compared with MS.
p < 0.001 compared with CS.
Table 4.
PCB concentrationsa (pg/g-wet) in three types of tissue.
| PCB, tissue | Detectiona (%) | Mean ± SD | Minimum | 25th percentile | Median | 75th percentile | Maximum |
|---|---|---|---|---|---|---|---|
| MonoCBs | |||||||
| MS | 25 | 0.10 ± 0.21 | ND | ND | ND | 0.02 | 0.68 |
| CS | 0 | — | — | — | — | — | — |
| UC | 95 | 0.52 ± 0.47 | ND | 0.16 | 0.36 | 0.82 | 1.7 |
| DiCBs | |||||||
| MS | 70 | 8.65 ± 0.28 | ND | ND | 0.35 | 0.52 | 0.89 |
| CS | 0 | — | — | — | — | — | — |
| UC | 80 | 0.38 ± 0.30 | ND | 0.24 | 0.36 | 0.50 | 1.2 |
| TriCBs | |||||||
| MS | 100 | 13.0 ± 6.14 | 6.1 | 8.5 | 10 | 15 | 31 |
| CS | 65 | 3.78 ± 4.42** | ND | ND | 3.6 | 4.8 | 17 |
| UC | 90 | 1.38 ± 1.15**,# | ND | 0.45 | 1.3 | 1.8 | 4.4 |
| TetraCBs | |||||||
| MS | 100 | 53.2 ± 17.75 | 22 | 40 | 50 | 65 | 91 |
| CS | 95 | 9.59 ± 8.32** | ND | 4.2 | 6.9 | 13 | 35 |
| UC | 95 | 3.69 ± 3.13**,## | ND | 2.4 | 2.9 | 4.5 | 15 |
| PentaCBs | |||||||
| MS | 100 | 115 ± 42.3 | 51 | 81 | 110 | 140 | 190 |
| CS | 100 | 27.5 ± 12.8** | 8.6 | 19 | 26 | 34 | 54 |
| UC | 100 | 14.6 ± 7.54**,## | 5.2 | 10 | 13 | 18 | 37 |
| HexaCBs | |||||||
| MS | 100 | 195 ± 68.4 | 90 | 140 | 210 | 240 | 340 |
| CS | 100 | 69.3 ± 25.9** | 26 | 51 | 68 | 88 | 130 |
| UC | 100 | 36.1 ± 15.3**,## | 16 | 26 | 35 | 43 | 78 |
| HeptaCBs | |||||||
| MS | 100 | 73.3 ± 26.7 | 34 | 55 | 73 | 94 | 120 |
| CS | 100 | 27.9 ± 13.8** | 6.2 | 19 | 26 | 32 | 61 |
| UC | 100 | 17.1 ± 8.26**,## | 8.6 | 12 | 16 | 20 | 43 |
| OctaCBs | |||||||
| MS | 100 | 14.5 ± 5.57 | 4.6 | 11 | 15 | 18 | 26 |
| CS | 75 | 4.03 ± 3.96** | ND | 1.4 | 3.4 | 5.1 | 16 |
| UC | 100 | 3.57 ± 1.86**,# | 1.4 | 2.1 | 3.4 | 4.3 | 8.3 |
| NonaCBs | |||||||
| MS | 70 | 1.54 ± 1.26 | ND | ND | 1.6 | 2.5 | 3.8 |
| CS | 35 | 0.32 ± 0.64 | ND | ND | ND | 0.52 | 2.7 |
| UC | 50 | 0.17* ± 0.27* | ND | ND | 0.065 | 0.26 | 1.1 |
| DecaCBs | |||||||
| MS | 85 | 0.87 ± 0.50 | ND | 0.73 | 0.86 | 1.0 | 1.7 |
| CS | 35 | 0.29 ± 0.58 | ND | ND | ND | 0.49 | 2.5 |
| UC | 55 | 0.18 ± 0.23** | ND | ND | 0.094 | 0.33 | 0.85 |
| Total PCBs | |||||||
| MS | 100 | 467 ± 154 | 220 | 340 | 490 | 570 | 780 |
| CS | 100 | 139 ± 56.4** | 56 | 100 | 130 | 180 | 270 |
| UC | 100 | 77.4 ± 35.5**,## | 35 | 57 | 73 | 91 | 190 |
ND, not detected.
Detection rate is shown as a percentage (n = 20).
p < 0.05 and
p < 0.001 compared with MS.
p < 0.05 and
p < 0.001 compared with CS.
Table 2.
Organochlorine pesticide concentrations (pg/g-wet) in three types of tissue.
| Organochlorine pesticide, tissue | Detectiona (%) | Mean ± SD | Minimum | 25th percentile | Median | 75th percentile | Maximum |
|---|---|---|---|---|---|---|---|
| HCB | |||||||
| MS | 100 | 120 ± 55.2 | 20 | 81 | 120 | 140 | 230 |
| CS | 100 | 23.9 ± 8.42** | 13 | 20 | 23 | 25 | 46 |
| UC | 95 | 19.6 ± 6.52** | ND | 17 | 20 | 23 | 33 |
| HCHs | |||||||
| MS | 100 | 208 ± 84.7 | 100 | 160 | 190 | 240 | 430 |
| CS | 100 | 74.5 ± 39.3** | 33 | 49 | 70 | 82 | 190 |
| UC | 100 | 39.8 ± 7.8**,## | 17 | 29 | 35 | 48 | 95 |
| pp′-DDT | |||||||
| MS | 80 | 26.40 ± 26.5 | ND | 8.0 | 17 | 42 | 90 |
| CS | 0 | — | — | — | — | — | — |
| UC | 50 | 5.76 ± 7.53* | ND | ND | 1.0 | 11 | 27 |
| op′-DDT | |||||||
| MS | 15 | 0.32 ± 0.89 | ND | ND | ND | ND | 3 |
| CS | 0 | — | — | — | — | — | — |
| UC | 0 | — | — | — | — | — | — |
| pp′-DDE | |||||||
| MS | 100 | 680.0 ± 277 | 190 | 480 | 640 | 900 | 1,200 |
| CS | 100 | 71.9 ± 30.7** | 26 | 50 | 72 | 95 | 130 |
| UC | 100 | 86.5 ± 26.1**,# | 31 | 76 | 88 | 94 | 150 |
| pp′-DDD | |||||||
| MS | 85 | 6.11 ± 7.99 | ND | 1.6 | 2.7 | 7.1 | 30 |
| CS | 0 | — | — | — | — | — | — |
| UC | 15 | 0.26 ± 0.63 | ND | ND | ND | ND | 2.1 |
| cis-Chlordane | |||||||
| MS | 100 | 1.81 ± 1.25 | 0.54 | 0.70 | 1.3 | 2.6 | 4.8 |
| CS | 0 | — | — | — | — | — | — |
| UC | 70 | 1.20 ± 1.19 | ND | ND | 1.1 | 2.0 | 4.4 |
| trans-Chlordane | |||||||
| MS | 95 | 2.46 ± 2.45 | ND | 1.2 | 1.9 | 3.1 | 12 |
| CS | 20 | 0.41 ± 0.90 | ND | ND | ND | ND | 2.9 |
| UC | 55 | 0.68 ± 0.91* | ND | ND | 0.25 | 1.1 | 2.9 |
| Oxychlordane | |||||||
| MS | 85 | 22.80 ± 45.4 | ND | 4.9 | 11 | 24 | 210 |
| CS | 40 | 2.19 ± 3.45 | ND | ND | ND | 3.6 | 12 |
| UC | 60 | 2.48 ± 2.50 | ND | ND | 2.3 | 4.5 | 6.9 |
| trans-Nonachlor | |||||||
| MS | 100 | 55.00 ± 23.2 | 15 | 38 | 53 | 68 | 100 |
| CS | 80 | 8.02 ± 12.1** | ND | 3.1 | 4.3 | 9.7 | 56 |
| UC | 100 | 8.52 ± 3.17** | 2.9 | 6.8 | 7.9 | 10.3 | 17 |
| Dieldrin | |||||||
| MS | 70 | 3.75 ± 3.30 | ND | ND | 3.4 | 5.9 | 10 |
| CS | 0 | — | — | — | — | — | — |
| UC | 45 | 2.02 ± 3.01 | ND | ND | 2.7 | 2.6 | 8.5 |
| Endosulfan | |||||||
| MS | 90 | 2.90 ± 2.07 | ND | 2 | 2.6 | 3.9 | 8.4 |
| CS | 0 | — | — | — | — | — | — |
| UC | 70 | 2.83 ± 2.61 | ND | ND | 1.9 | 3.3 | 10 |
| Heptachlor | |||||||
| MS | 25 | 1.44 ± 2.58 | ND | ND | ND | 1.2 | 7.2 |
| CS | 0 | — | — | — | — | — | — |
| UC | 10 | 0.57 ± 1.82 | ND | ND | ND | ND | 7.2 |
| Heptachlor epoxide | |||||||
| MS | 100 | 10.70 ± 5.67 | 2.3 | 6.9 | 9 | 13 | 24 |
| CS | 95 | 3.51 ± 1.80** | ND | 2.4 | 3.3 | 4.2 | 7.3 |
| UC | 100 | 2.89 ± 1.03** | 0.3 | 2.2 | 3.1 | 3.5 | 5.1 |
ND, not detected.
Detection rate is shown as a percentage (n = 20).
p < 0.05 and
p < 0.001 compared with MS.
p < 0.05 and
p < 0.001 compared with CS.
Table 3.
PCB concentrations (pg/g-lipid) in three types of tissue.
| PCB, tissue | Detectiona (%) | Mean ± SD | Minimum | 25th percentile | Median | 75th percentile | Maximum |
|---|---|---|---|---|---|---|---|
| MonoCBs | |||||||
| MS | 25 | 8.4 ± 30.5 | ND | ND | ND | 28 | 110 |
| CS | 0 | — | — | — | — | — | — |
| UC | 95 | 480 ± 405* | ND | 100 | 470 | 640 | 1,400 |
| DiCBs | |||||||
| MS | 70 | 44 ± 37 | ND | ND | 44 | 67 | 120 |
| CS | 0 | — | — | — | — | — | — |
| UC | 80 | 354 ± 276** | ND | 220 | 370 | 450 | 1,200 |
| TriCBs | |||||||
| MS | 100 | 1,630 ± 639 | 720 | 1200 | 1400 | 2,000 | 2,900 |
| CS | 65 | 1,630 ± 1770 | ND | ND | 1,500 | 2,200 | 6,700 |
| UC | 90 | 1,210 ± 977 | ND | 560 | 1100 | 1,800 | 3,900 |
| TetraCBs | |||||||
| MS | 100 | 7,000 ± 2120 | 4,000 | 7,400 | 7,100 | 8,000 | 12,000 |
| CS | 95 | 4,360 ± 3750** | ND | 1,800 | 3,300 | 5,600 | 14,000 |
| UC | 95 | 3,190 ± 2,200** | ND | 2,100 | 3,000 | 4,000 | 10,000 |
| PentaCBs | |||||||
| MS | 100 | 15,000 ± 4,630 | 7,700 | 11,000 | 15,000 | 18,000 | 25,000 |
| CS | 100 | 12,700 ± 6,080** | 3,700 | 8,000 | 13,000 | 17,000 | 24,000 |
| UC | 100 | 13,200 ± 6,100* | 5,100 | 7,900 | 12,000 | 18,000 | 25,000 |
| HexaCBs | |||||||
| MS | 100 | 25,600 ± 8,410 | 11,000 | 20,000 | 26,000 | 30,000 | 42,000 |
| CS | 100 | 31,200 ± 11,000** | 14,000 | 23,000 | 31,000 | 38,000 | 51,000 |
| UC | 100 | 32,600 ± 12,000** | 13,000 | 24,000 | 35,000 | 40,000 | 53,000 |
| HeptaCBs | |||||||
| MS | 100 | 9,640 ± 3,610 | 3,900 | 7,400 | 8,600 | 12,000 | 17,000 |
| CS | 100 | 12,300 ± 5,420** | 3,500 | 8,000 | 12,000 | 13,500 | 23,000 |
| UC | 100 | 15,200 ± 5,860**,## | 7,700 | 11,000 | 14,000 | 20,000 | 29,000 |
| OctaCBs | |||||||
| MS | 100 | 1,750 ± 718 | 590 | 1,500 | 1,800 | 2,500 | 3,100 |
| CS | 75 | 1,700 ± 1,380 | ND | 830 | 1,400 | 2,600 | 4,400 |
| UC | 100 | 3,130 ± 1,360**,## | 1,700 | 2,100 | 2,700 | 3,900 | 5,900 |
| NonaCBs | |||||||
| MS | 70 | 212 ± 174 | ND | ND | 220 | 290 | 530 |
| CS | 35 | 146 ± 291 | ND | ND | ND | 200 | 1,200 |
| UC | 50 | 148 ± 221 | ND | ND | 60 | 210 | 910 |
| DecaCBs | |||||||
| MS | 85 | 114 ± 65 | ND | 91 | 115 | 150 | 240 |
| CS | 35 | 130 ± 257 | ND | ND | ND | 220 | 1,100 |
| UC | 55 | 148 ± 173* | ND | ND | 96 | 255 | 570 |
| Total PCBs | |||||||
| MS | 100 | 61,500 ± 18,400 | 29,000 | 46,000 | 61,000 | 72,000 | 96,000 |
| CS | 100 | 63,800 ± 23,300 | 31,000 | 44,000 | 63,000 | 77,000 | 110,000 |
| UC | 100 | 70,000 ± 26,100*,# | 34,000 | 47,000 | 73,000 | 88,000 | 130,000 |
ND, not detected.
Detection rate is shown as a percentage (n = 20).
p < 0.05 and
p < 0.001 compared with MS.
p < 0.05 and
p < 0.001 compared with CS.
Contamination levels.
The highest concentrations found were p,p′-DDE, HCHs, and HCB in all three tissues both on a lipid basis (Table 1) and wet basis (Table 2). Generally, the chemical concentrations on a wet basis were of the order MS > CS > UC. This is due to the difference in lipid content. Lipid content in MS, CS, and UC (20 complete samples) was 0.76 ± 0.13%, 0.23 ± 0.04%, and 0.11 ± 0.02%, respectively. Remarkably, the concentrations of some chemicals in UC on a wet basis, such as cis-chlordane and endosulfan, were almost equal to those in MS (Table 2). On the other hand, on a lipid basis, the concentrations of the following chemicals in UC were nearly equal or often higher than in MS: HCHs, p,p′-DDT, cis-chlordane, trans-chlordane, endosulfan, and heptachlor epoxide (p < 0.001, paired t-test; Table 1). The chemical concentrations in CS were usually lower than in other tissues.
PCB congeners grouped on the basis of their number of chlorines showed different patterns of distribution depending on the number of chlorines. TetraCB and pentaCB concentrations were higher in MS than in UC (p < 0.05, paired t-test), whereas hexaCB and heptaCB concentrations were higher in UC than in MS (p < 0.001, paired t-test) on a lipid basis (Table 3); particularly, heptaCBs and octaCBs showed a high UC:MS ratio. The UC:MS ratio varied according to the number of chlorines in the range of 3–8: UC < MS for congeners with 3–5 chlorines, and UC > MS for congeners with 6–8 chlorines (Table 3). The detection rates of congeners with 1 or 2 chlorines were higher in UC than in MS, whereas those with of 9 or 10 chlorines were higher in MS than in UC. The concentrations (average and median, on a lipid basis) of congeners with 9 or 10 chlorines were higher than those with 1 or 2 chlorines in MS, whereas concentrations of congeners with 1 or 2 chlorines were higher than those with 9 or 10 chlorines in UC. These facts suggest that the accumulation of PCBs in UC is different depending on the number of chlorines; PCB congeners with 1, 2, 6, 7 and 8 chlorines easily accumulate in UC compared with other congeners.
Association between the chemical concentrations among chemicals.
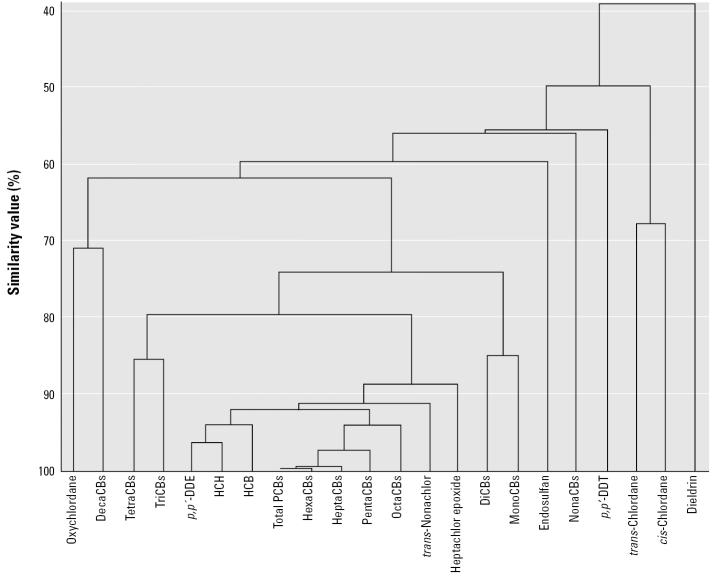
We reported that correlation existed between total PCBs and other persistent chemicals, such as p,p′-DDE, HCB, and HCHs, in human UC (Mori et al. 2003). To confirm our previous findings, we applied a cluster analysis technique in the present study. We used the cluster analysis to discover “natural” groupings of objects that reflect evolutionary or functional relationships among the objects; some of the cluster analyses often done in toxicogenomics research have this objective (Immermann and Huang 2003). The cluster analysis was performed using cosine correlation matrix for chemical concentrations in UC for UC, and the clustering results were represented in the dendrogram (Figure 1). Consequently, we found that PCBs with 5–8 chlorines and some organochlorines, such as p,p′-DDE, HCB, and HCHs, were closely related to each other (Figure 1).
Figure 1. Cluster analysis of chemical concentrations among UC samples. Similarity values were calculated by cosine correlation matrix for chemical concentrations in UC for UC; the results are shown as a dendrogram.
Association between the chemical concentrations among the three types of tissues.
Correlation of organochlorine pesticide concentrations among the three types of tissues is shown in Table 5. Some organochlorine pesticides showed no association between MS versus CS and/or between CS versus UC. Between MS versus CS, HCB, HCHs, heptachlor epoxide, and PCBs with chlorines showed a relatively high correlation coefficient (r > 0.7). Between CS versus UC, HCHs, p,p′-DDE, and PCBs with 5–8 chlorines showed relatively high correlation (r > 0.7). Comparing MS and UC, HCHs and PCBs with 4–7 chlorines showed a relatively high correlation coefficient (r > 0.7).
Table 5.
Correlation coefficients (r) of chemicals among tissues and between maternal age and chemical concentrations in UC of first babies.
| Among tissuesa |
Between maternal age and concentrations in UC of first babies (age vs. UC) | |||
|---|---|---|---|---|
| MS vs. CS | CS vs. UC | MS vs. UC | ||
| HCB | 0.73 | 0.30 | 0.39 | −0.16 |
| HCHs | 0.72 | 0.76 | 0.80 | 0.83 |
| p,p′-DDE | 0.46 | 0.76 | 0.29 | 0.28 |
| cis-Chlordane | — | — | 0.03 | −0.02 |
| trans-Nonachlor | 0.18 | 0.20 | 0.11 | 0.47 |
| Endosulfan | — | — | 0.19 | −0.20 |
| Heptachlor epoxide | 0.72 | 0.22 | 0.02 | 0.10 |
| TriCBs | — | — | 0.35 | −0.03 |
| TetraCBs | 0.51 | 0.41 | 0.73 | 0.37 |
| PentaCBs | 0.83 | 0.89 | 0.84 | 0.75 |
| HexaCBs | 0.87 | 0.87 | 0.82 | 0.76 |
| HeptaCBs | 0.68 | 0.85 | 0.72 | 0.85 |
| OctaCBs | 0.32 | 0.70 | 0.47 | 0.81 |
| NonaCBs | — | — | −0.25 | — |
| DecaCBs | — | — | 0.40 | — |
| Total PCBs | 0.82 | 0.82 | 0.81 | 0.80 |
| Correlation coefficients (r) b between “among tissues” and age vs. UC | 0.04 | 0.75 | 0.64 | — |
—, not calculated.
The values shown were calculated when the detection rate in the tissue was ≥70%.
Total PCBs were excluded from this calculation.
Association between the chemical concentrations in UC and maternal age.
Several studies have reported that chemical concentrations were dependent upon maternal age at delivery (Mori et al. 2003; Rhainds et al. 1999). Our present results confirmed that HCHs, pentaCBs, hexaCBs, heptaCBs, and octaCBs showed such correlation (Table 5). A significant correlation was found between CS versus UC and age versus UC (r = 0.75; Table 5). Also, relatively significant correlation was found between MS versus UC and age versus UC (r = 0.64; Table 5). However, we found no correlation between MS versus CS and age versus UC (r = 0.04; Table 5). That is, HCHs, pentaCBs, hexaCBs, and heptaCBs tended to show relatively high association of concentrations in CS versus UC and MS versus UC. Also, these chemicals showed high correlation coefficients between the chemical concentrations in UC of first babies and maternal age.
Discussion
We investigated the distribution of organo-chlorine pesticides and PCBs in three types of tissues (UC, CS, and MS). We analyzed the chemical contamination status mainly on a lipid basis because the liposolubility rate is thought to be a major factor influenced by rates of accumulation and elimination from tissues and organs (Parham et al. 1997) and because the existing differences depend principally on lipid content of the tissues (Henriksen et al. 1998). Several studies have reported that the concentration levels of persistent chemicals showed association between cord blood and maternal blood (Sala et al. 2001; Waliszewski et al. 2000; Walker et al. 2003). In our study, we found strong correlation between MS versus CS (Table 5) in some organochlorine pesticides and PCB congeners. Also, Grandjean et al. (2001) showed high associations between cord blood and UC. The tendency was confirmed in our study of HCHs, p,p′-DDE, and some PCB congeners (Table 5). However, we found no report that compared the concentration levels among UC, CS, and MS. Hence, we compared the data among these three tissues.
In the present study, we found that the chemical concentrations were often higher in UC than in CS on a lipid basis, and the detection rates and the concentrations in CS were often lower than in MS and UC. In past studies, chemical concentrations were higher in adipose tissues than in serum (López-Carrillo et al, 1999; Pauwels et al. 2000), and in other studies, concentrations were higher in serum lipid than in breast tissues (Waliszewski et al. 2003). Moreover, as suggested by Pauwels et al. (2000), the concentration levels of persistent chemicals varied dramatically depending on the tissues (Tables 1 and 3). One of the reasons for the confusion may be the pharmaco-kinetics of chemicals in blood. Mohammed et al. (1990) and Norén et al. (1999) reported that chemicals in blood are bound to lipoproteins and albumin rather than being dissolved in lipid, and the distribution in plasma vary according to the chemicals. It is possible that a free form of chemicals is distributed by simple equilibrium, but distribution or transport of bound form of chemicals to protein in blood is more complicated, so the chemical concentration in CS might be lower than in MS and UC. Further studies on the distribution of contaminants in different body tissues and fetal tissues are required.
In conclusion, we believe that UC is the best sample to assess fetal contamination status of persistent chemicals. There is a possibility that assessment based on the contamination levels in CS result in an underestimation.
References
- Borgert CJ, LaKind JS, Witorsch RJ. A critical review of methods for comparing estrogenic activity of endogenous and exogenous chemicals in human milk and infant formula. Environ Health Perspect. 2003;111:1020–1036. doi: 10.1289/ehp.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branum AM, Collman GW, Correa A, Keim SA, Kessel W, Kimmel CA, et al. The National Children’s Study of environmental effects on child health and development. Environ Health Perspect. 2003;111:642–646. doi: 10.1289/ehp.111-1241458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnley G, Putzrath RM. Children’s health, susceptibility, and regulatory approaches to reducing risks from chemical carcinogens. Environ Health Perspect. 2001;109:187–193. doi: 10.1289/ehp.01109187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covaci A, Jorens P, Jacquemyn Y, Schepens P. Distribution of PCBs and organochlorine pesticides in umbilical cord and maternal serum. Sci Total Environ. 2002;298:45–53. doi: 10.1016/s0048-9697(02)00167-5. [DOI] [PubMed] [Google Scholar]
- De Felip E, di Domenico A, Miniero R, Silvestroni L. Polychlorobiphenyls and other organochlorine compounds in human follicular fluid. Chemosphere. 2004;54:1445–1449. doi: 10.1016/j.chemosphere.2003.10.040. [DOI] [PubMed] [Google Scholar]
- Foster W, Chan S, Platt L, Hughee C. Detection of endocrine disrupting chemicals in samples of second trimester human amniotic fluid. Clin Endocrinol Metab. 2000;85:2954–2957. doi: 10.1210/jcem.85.8.6850. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Burse VW, Needham LL, Storr-Hansen E, Heinzow B, et al. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol. 2001;23:305–317. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- Henriksen EO, Gabrielsen GW, Skaare JU. Validation of the use of blood samples to assess tissue concentrations of organochlorines in glaucous gulls, Larus hyperboreus. Chemosphere. 1998;37:2627–2643. [Google Scholar]
- Immermann F, Huang Y. 2003. An introduction to cluster analysis. In: An Introduction to Toxicogenomics (Burczynski ME, ed). Boca Raton:CRC Press LLC, 45–78.
- López-Carrillo L, Torres-Sánchez L, López-Cervantes M, Blair A, Cebrián ME, Uribe M. The adipose tissue to serum dichlorodiphenyldichloroethan (DDE) ratio: some methodological considerations. Environ Res A. 1999;81:142–145. doi: 10.1006/enrs.1999.3961. [DOI] [PubMed] [Google Scholar]
- Ministry of Environment, Government of Japan 2002. Research on Chemicals Detected in Human Umbilical Cord [in Japanese]. Tokyo:Ministry of Environment. Available: http://www.env.go.jp/chemi/end/kento1402/mat/mat04-1.pdf [accessed 7 February 2005].
- Mohammed A, Eklund A, Ostlund-Lindgvist AM, Slanina P. Distribution of toxaphene, DDT, and PCB among lipoprotein fractions in rat and human plasma. Arch Toxicol. 1990;64:567–571. doi: 10.1007/BF01971836. [DOI] [PubMed] [Google Scholar]
- Mori C. Possible effects of endocrine disruptors on male reproductive function. Acta Anatomica Nippon. 2001;76:361–368. [PubMed] [Google Scholar]
- Mori C, Komiyama M, Adachi T, Sakurai K, Nishimura D, Takashima K, et al. Application of toxicogenomic analysis to risk assessment of delayed long-term effects of multiple chemicals including endocrine disruptors in human fetuses. Environ Health Perspect. 2003;111:803–809. [PubMed] [Google Scholar]
- Needham LL, Sexton K. Assessing children’s exposure to hazardous environmental chemicals: an overview of selected research challenges and complexities. J Expo Anal Environ Epidemiol. 2000;10:611–629. doi: 10.1038/sj.jea.7500142. [DOI] [PubMed] [Google Scholar]
- Norén K, Weistrand C, Karpe F. Distribution of PCB congeners, DDE, hexachlorobenzene, and methylsulfonyl metabolites of PCB and DDE among various fractions of human blood plasma. Arch Environ Contam Toxicol. 1999;37:408–414. doi: 10.1007/s002449900532. [DOI] [PubMed] [Google Scholar]
- Parham FM, Kohn MC, Matthews HB, DeRosa C, Portier CJ. Using structural information to create physiologically based pharmacokinetic models for all polychlorinated biphenyls. I. Tissue:blood partition. Toxicol Appl Pharmacol. 1997;144:340–347. doi: 10.1006/taap.1997.8139. [DOI] [PubMed] [Google Scholar]
- Pauwels A, Covaci A, Weyler J, Delbeke L, Dhont M, De Sutter P, et al. Comparison of persistent organic pollutant residues in serum and adipose tissue in a female population in Belgium, 1996–1998. Arch Environ Contam Toxicol. 2000;39:265–270. doi: 10.1007/s002440010104. [DOI] [PubMed] [Google Scholar]
- Rhainds M, Levallois P, Dewailly E, Ayotte P. Lead, mercury, and organochlorine compound levels in cord blood in Quebec, Canada. Arch Environ Health. 1999;54:40–47. doi: 10.1080/00039899909602235. [DOI] [PubMed] [Google Scholar]
- Sala M, Ribas-Fitó N, Cardo E, de Muga ME, Marco E, Mazón C, et al. Levels of hexacholorobenzene and other organochlorine compounds in cord blood: exposure across placenta. Chemosphere. 2001;43:895–901. doi: 10.1016/s0045-6535(00)00450-1. [DOI] [PubMed] [Google Scholar]
- Sarcinelli PN, Pereira ACS, Mesquita SA, Oliveira-Silva JJ, Meyer A, Menezes MAC, et al. Dietary and reproductive determinants of plasma organochlorine levels in pregnant women in Rio de Janeiro. Environ Res. 2003;91:143–150. doi: 10.1016/s0013-9351(02)00053-1. [DOI] [PubMed] [Google Scholar]
- Todaka E, Mori C. Necessity to establish new risk assessment and risk communication for human fetal exposure to multiple endocrine disruptors in Japan. Congent Anom Kyto. 2002;42:87–93. doi: 10.1111/j.1741-4520.2002.tb00857.x. [DOI] [PubMed] [Google Scholar]
- Waliszewski SM, Aguirre AA, Infanzón RM, Siliceo J. Carry-over of persistent organochlorine pesticides through placenta to fetus. Salud Publica Mex. 2000;42:384–390. doi: 10.1590/s0036-36342000000500003. [DOI] [PubMed] [Google Scholar]
- Waliszewski SM, Infanzon RM, Hart M. Differences in persistent organochlorine pesticides concentration between breast adipose tissue and blood serum. Bull Environ Contam Toxicol. 2003;70:920–926. doi: 10.1007/s00128-003-0070-9. [DOI] [PubMed] [Google Scholar]
- Walker JB, Seddon L, McMullen E, Houseman J, Tofflemire K, Corriveau A, et al. Organochlorine levels in maternal and blood plasma in Arctic Canada. Sci Total Environ. 2003;302:27–52. doi: 10.1016/s0048-9697(02)00319-4. [DOI] [PubMed] [Google Scholar]