Abstract
The Kaposi’s sarcoma-associated herpes virus gene product K3 (KK3) subverts the MHC class I antigen presentation pathway by downregulating MHC class I from the plasma membrane. We now show that KK3 associates with MHC class I molecules and promotes ubiquitylation of class I after export from the endoplasmic reticulum. Ubiquitylation requires the KK3 N-terminal plant homeodomain and provides the signal for class I internalization at the plasma membrane. Once internalized, ubiquitylated MHC class I is targeted to the late endocytic pathway, where it is degraded. Depletion by small interfering RNA of TSG101, a ubiquitin enzyme 2 variant protein involved in late endosomal sorting, prevents class I degradation and preserves cell surface class I expression in KK3-expressing cells. These results suggest a mechanism by which the KK3-induced class I ubiquitylation provides a signal for both internalization and sorting to the late endosomal pathway for degradation. KK3 is the first viral gene product that subverts the trafficking of a host protein via the ubiquitin-dependent endosomal sorting machinery.
Keywords: HHV8 K3/MHC class I/TSG101/ubiquitin/viral evasion
Introduction
The MHC class I antigen presentation pathway plays a critical role in defence against intracellular pathogens. MHC class I molecules display the intracellular protein content of the cell in the form of small peptides to cytotoxic T lymphocytes (CTL). Cells that present peptides derived from viral proteins are recognized and destroyed by CTL. The presentation of peptide to CTL at the cell surface is the final step in a complex intracellular process (reviewed in Pamer and Cresswell, 1998). Follow ing the cytosolic degradation of protein to peptide by the proteasome, peptides are transported from the cytosol to the endoplasmic reticulum (ER) via the transporters associated with antigen processing (TAP) molecules. Delivery of peptide to the lumen of the ER, and subsequent binding to its cognate class I molecule, allows the class I molecule to fold, dissociate from TAP and be transported through the secretory pathway to the cell surface.
The importance of the class I antigen presentation pathway is emphasized by the many strategies used by different viruses to subvert the class I machinery. This is particularly important for herpesviruses, which produce infectious virus even in the presence of an immunocompetent host (Krmpotic et al., 1999). Indeed, it is probable that all herpesviruses have developed mechanisms for class I evasion, and viral subversion of the class I pathway appears to be a prerequisite for successful viral persistence. This is well illustrated by the human cytomegalovirus (HCMV), which encodes at least six gene products that affect class I antigen presentation (Tortorella et al., 2000). As a result of co-evolution with their host’s immune system, even related viruses have evolved distinct mechanisms to evade MHC class I presentation. While both human and murine CMV encode class I evasion genes, they use unrelated host-specific gene products that affect different targets of the class I pathway.
In a recent study we showed that the murine K3 (MK3) gene product from the murine γ-herpes virus 68 reduces cell surface class I expression (Stevenson et al., 2000). Murine γ-herpes virus 68 is closely related to the human γ2-herpes virus, Kaposi’s sarcoma herpes virus (KSHV), also known as human herpes virus 8 (HHV8). KSHV is the causative agent of Kaposi’s sarcoma as well as two rare lymphoproliferative disorders: primary effusion lymphoma and multicentric Castleman’s disease (Cesarman and Knowles, 1999). A homology search on the MK3 protein identified two KSHV proteins, KK3 and KK5, which we and others have shown to decrease cell surface class I expression (Coscoy and Ganem, 2000; Ishido et al., 2000b; Stevenson et al., 2000; Haque et al., 2001). These three proteins represent the first example of homologous gene products from related viruses affecting the class I presentation pathway. However, there are clear functional differences between the murine and human K3 proteins. MK3 and KK3 have so far only been reported to decrease MHC class I expression. KK5 can only efficiently downregulate HLA-A and -B alleles (Ishido et al., 2000b), but also downregulates cell surface B7.2 and ICAM-1 (Ishido et al., 2000a; Coscoy and Ganem, 2001).
Analysis of the intracellular transport of MHC class I molecules suggests a very different site of action for the murine and human viral inhibitory molecules. MK3 expression causes rapid proteasome-dependent loss of nascent class I molecules before they exit the ER (Stevenson et al., 2000; Boname and Stevenson, 2001), while KK3 and KK5 promote the endocytosis of mature class I molecules from the cell surface for destruction in a bafilomycin-sensitive endosomal compartment (Coscoy and Ganem, 2000; Ishido et al., 2000b). Closer inspection of the murine and human viral proteins shows that they have homologous N-termini that encode a plant homeodomain (PHD) or leukaemia-associated protein (LAP) motif (Nicholas et al., 1997). PHD sequence motifs are characterized by seven cysteines and one histidine in the order Cys4HisCys3 and have been implicated in a diverse variety of cellular processes (Aasland et al., 1995). Most significantly, PHD motifs share both sequence and structural homology with RING finger domains (Aasland et al., 1995; Joazeiro and Weissman, 2000; Zheng et al., 2000; Capili et al., 2001). RING finger proteins also have a disparate set of functions but share a common theme of E3 ubiquitin ligase activity (Joazeiro and Weissman, 2000). E3 ubiquitin ligases confer specificity in the ubiquitylation process by interacting with the E2 ubiquitin-conjugating enzyme and directing ubiquitin onto the substrate. Depending on the targeted protein and the site of ubiquitylation, degradation may be proteasome or lysosome mediated.
We therefore wondered whether the murine and human viral proteins used a similar approach: that of tagging class I molecules with ubiquitin but inducing degradation by different pathways. We recently showed that ubiquitylation of murine class I molecules by MK3 in the ER is the primary event leading to a rapid, proteasome-dependent class I degradation (Boname and Stevenson, 2001). In this study we demonstrate that KK3 promotes the addition of ubiquitin onto MHC class I molecules in a post-ER compartment. At the plasma membrane, ubiquitin acts as an internalization signal, targeting MHC class I molecules for endosomal degradation via a pathway dependent on the tumour susceptibility gene 101 (TSG101).
Results
KK3 promotes the ubiquitylation of MHC class I molecules
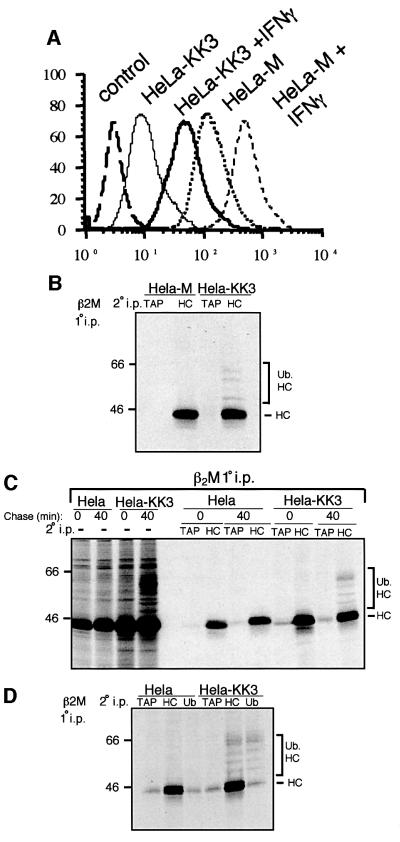
We and others have shown that KK3 causes a decrease in cell surface expression of MHC class I molecules (Coscoy and Ganem, 2000; Ishido et al., 2000b; Stevenson et al., 2000). KK3 promotes the rapid internalization of MHC class I molecules from the plasma membrane (Ishido et al., 2000b) but the molecular basis for this downregulation is not understood. Stable cell lines expressing cDNA encoding KK3 tagged with the FLAG epitope at the N-terminus (HeLa-KK3) were generated and all showed a dramatic decrease in cell surface class I expression; a representative clone is shown in Figure 1A. Our initial attempts to visualize ubiquitylated class I molecules following primary class I immunoprecipitations were unsuccessful. To clarify our gels, we immunoprecipitated class I molecules from metabolically labelled HeLa-KK3 cells and re-precipitated 1% SDS eluted proteins with the class I heavy chain-specific antibody HC10. The immunoprecipitation from HeLa-KK3 cells, but not control HeLa-M cells, revealed an additional faint ladder of bands above the expected 46 kDa class I heavy chain band, suggestive of ubiquitylated class I heavy chains (Figure 1B). To analyse these bands further required an increase in the MHC class I signal; this was achieved by stimulating Hela-KK3 cells with interferon (IFN)-γ 24 h prior to metabolic labelling. IFN-γ treatment of Hela-KK3 cells increased cell surface expression of MHC class I, but this was still below the level in unstimulated HeLa-M cells (Figure 1A). Pulse–chase analysis of these IFN-γ-treated HeLa-KK3 cells showed normal synthesis of class I molecules, but after a 40 min chase additional high molecular weight proteins (52–75 kDa) were precipitated with the β2M antisera (Figure 1C). To characterize these bands further, the class I immunoprecipitate was dissociated in 1% SDS and re-precipitation with a class I heavy chain-specific antibody revealed a ladder of four discrete bands above the 44 kDa class I heavy chain (Figure 1C), consistent with those observed in the primary MHC class I immunoprecipitation. When the products of the primary immunoprecipitation were re-precipitated with the ubiquitin antibody FK2, the identical ladder of four bands was seen (Figure 1D), confirming their identity as ubiquitylated forms of the class I heavy chain. The laddered bands were absent from control immunoprecipitations. Limited, non-specific recovery of the class I heavy chain was seen in all lanes and probably represents the vast amount of excess class I heavy chain brought down in the primary β2M immunoprecipitation. Ubiquitylated forms of class I heavy chain were seen on primary immunoprecipitation with both the β2M antisera and the conformation-specific class I antibody w6/32, but not with the antibody HC10, which only binds free class I heavy chains (data not shown). Further primary immunoprecipitations were performed with the β2M antisera.
Fig. 1. KK3 promotes the ubiquitylation of MHC class I heavy chains. (A) Cytofluorometric analysis of MHC class I expression in HeLa-M and HeLa-KK3 cells. HeLa-M and HeLa-KK3 cells were cultured for 24 h in the absence or presence of 200 U/ml IFN-γ and stained with the mAb W6/32 and a FITC-conjugated secondary antibody. (B) High molecular weight laddering of class I molecules in KK3-expressing cells. Hela-M or Hela-KK3 cells (2 × 107) were pulse-labelled for 15 min and chased for 40 min. Following 1% Triton X-100 detergent solubilization, MHC class I molecules were immunoprecipitated with a β2M-specific antiserum, and after dissociation in 1% SDS, re-precipitated with either the TAP2-specific antibody 435.3 or the heavy chain- specific antibody HC10, and resolved on a 10% SDS–PAGE gel prior to autoradiography. (C and D) The high molecular weight laddering following class I immunoprecipitations in KK3-expressing cells represents ubiquitylated class I molecules. (C) IFN-γ-stimulated Hela-M and Hela-KK3 cells (1.2 × 107) were pulse-labelled for 15 min and chased for 0 or 40 min. Following solubilization in Triton X-100, MHC class I molecules were immunoprecipitated with a β2M-specific antiserum. One-third of the β2M immunoprecipitate was resolved on a 10% SDS–PAGE gel, and the remainder dissociated in 1% SDS and re-precipitated with either the TAP2-specific mAb 435.3 or the heavy chain- specific mAb HC10. (D) IFN-γ-stimulated cells (6 × 106) were pulse-labelled as in (C), but following dissociation in 1% SDS, lysates were re-precipitated with either the TAP2-specific mAb 435.3, the heavy chain-specific mAb HC10 or the ubiquitin mAb FK2.
Ubiquitylation of MHC class I molecules occurs in a post-ER compartment
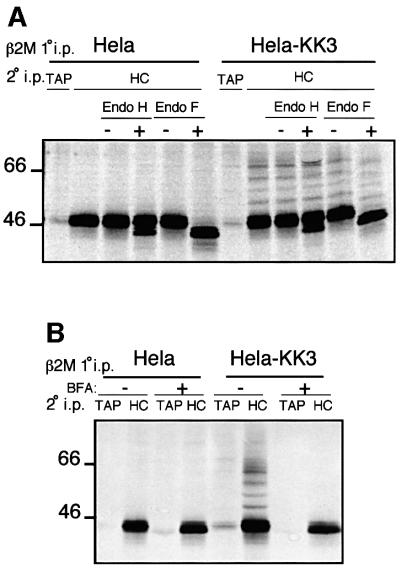
Previous work has shown that KK3 downregulates MHC class I from the plasma membrane, and we therefore wanted to identify the site of class I ubiquitylation. Following metabolic labelling of HeLa-KK3 cells, the glycosylation status of the ubiquitylated class I molecules was determined at the 40 min chase point using the endoglycosidases (Endo) H and F (Figure 2A). Endo F cleaves between the innermost β-d-N-acetylgalactosamine and asparagine residues of N-linked glycoproteins and caused a reduction in size of the class I heavy chain (Figure 2A). This was also observed with the four ubiquitylated forms of the heavy chain, indicating that the ubiquitylated class I remained glycosylated (Figure 2A). In contrast, Endo H cannot cleave oligosaccharides from N-linked glycoproteins once they have been processed in the medial-Golgi by the enzymes N-acetylglucosamine transferase I and mannosidase II. At the 40 min chase point, the majority of the 44 kDa class I heavy chains in HeLa-M and HeLa-KK3 cells were resistant to Endo H, although a small proportion of the heavy chain remained sensitive to Endo H digestion. Therefore, after the 40 min chase most of the MHC class I has been exported from the ER and progressed at least as far as the medial-Golgi. The ubiquitylated heavy chains, despite being glycosylated, were unaffected by Endo H digestion, indicating that these ubiquitylated class I molecules had progressed at least as far as the medial-Golgi.
Fig. 2. Ubiquitylated class I molecules are glycosylated, resistant to Endo H digestion and ubiquitylation is inhibited by BFA. (A) Ubiquitylated class I molecules are Endo H resistant and Endo F sensitive. IFN-γ-treated Hela-M and Hela-KK3 cells (107) were pulse-labelled for 30 min and chased for 40 min. MHC class I molecules were immunoprecipitated from Triton X-100 lysates with a β2M- specific antiserum, dissociated in 1% SDS and re-precipitated with either the TAP2-specific mAb 435.3 or the heavy chain-specific mAb HC10. The HC10 re-precipitate was either directly resolved, digested (+) or mock digested (–) with Endo H or Endo F prior to SDS–PAGE. (B) In the presence of BFA, MHC class I ubiquitylation is abolished. Hela-M and Hela-KK3 cells were pulse-labelled as in (A), but in the presence or absence of 4 µg/ml BFA. MHC class I molecules were immunoprecipitated with a β2M-specific antiserum, dissociated in 1% SDS and re-precipitated with either the TAP2-specific mAb 435.3 or the heavy chain-specific mAb HC10.
Although the above data show that ubiquitylated MHC class I molecules escape the ER, this does not preclude the ER as the site of KK3-mediated ubiquitylation. To determine whether KK3-mediated ubiquitylation occurs in the ER, HeLa-M and HeLa-KK3 cells were treated with brefeldin A (BFA), which prevents export from the ER through the secretory pathway, causing redistribution of Golgi resident enzymes to the ER (Lippincott-Schwartz et al., 1991). Cells were again pulse-labelled and chased for 40 min in the presence of BFA. Ubiquitylation of the MHC class I heavy chain was not observed in BFA-treated HeLa-KK3 cells (Figure 2B), suggesting that KK3- mediated class I ubiquitylation occurs in a post-ER compartment.
KK3 associates with and mediates ubiquitylation of MHC class I in a post-ER compartment
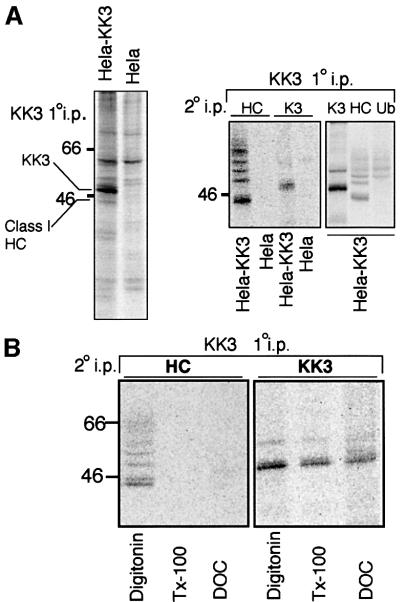
To understand better the mechanism of KK3-mediated ubiquitylation we looked for KK3 protein interactions. KK3 was immunoprecipitated from IFN-γ-stimulated, metabolically labelled cells solubilized in 1% digitonin to preserve weak protein interactions. Primary KK3 immunoprecipitations revealed both the 48 kDa KK3 band and an additional faint 44 kDa band not seen in control HeLa-M cell lysates (Figure 3A, left panel). Re-immunoprecipitation with the heavy chain-specific HC10 antibody following dissociation of the protein complex identified this band as class I heavy chain (Figure 3A). Furthermore, the KK3 associated class I heavy chains were ubiquitylated (Figure 3A, right panel), consistent with a role for KK3 in MHC class I ubiquitylation. Interaction of KK3 with MHC class I was detergent sensitive, and was disrupted by Triton X-100 and deoxycholate (DOC) (Figure 3B).
Fig. 3. KK3 associates with and promotes ubiquitylation of MHC class I molecules. (A) KK3 associates with MHC class I molecules. IFN-γ-stimulated Hela-M and Hela-KK3 cells (2 × 107) were pulse- labelled for 12 min and chased for 40 min. The KK3 viral protein was immunoprecipitated from digitonin cell lysates with the FLAG mAb M2. Ten percent of the FLAG mAb immunoprecipitate was directly resolved on a 10% SDS–PAGE gel (left panel), and the remainder dissociated in 1% SDS and re-precipitated with the heavy chain-specific mAb HC10 and the FLAG mAb M2 (for KK3) (middle panel). The experiment was repeated with HeLa-KK3 cells and, following SDS dissociation, was re-precipitated with the same two mAbs as well as the ubiquitin (Ub)-specific mAb FK2 (right panel). (B) The KK3 interaction with MHC class molecules I is detergent sensitive. IFN-γ-stimulated Hela-KK3 cells were pulse-labelled as in (A) and extracted in 1% digitonin. After removal of the nuclear fraction, the lysate was divided into three aliquots. Triton X-100 or DOC (1% final) was added to each of two aliquots. Cell lysates were immunoprecipitated for the KK3 protein with the FLAG mAb M2, dissociated in 1% SDS and re-precipitated with either the heavy chain-specific mAb HC10 (left panel) or the FLAG mAb M2 (for KK3) (right panel).
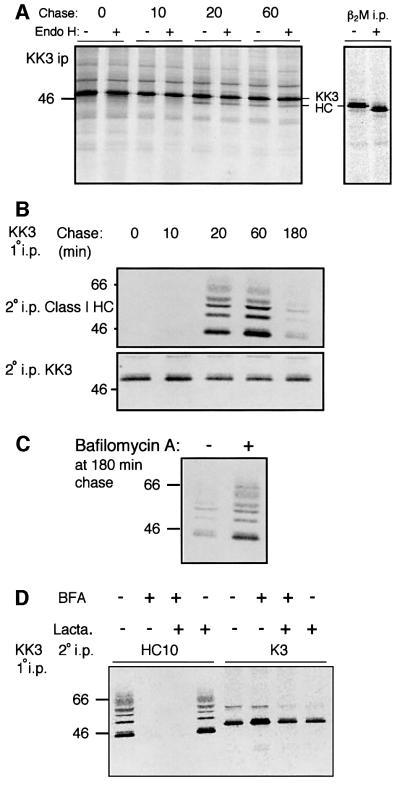
The kinetics of KK3 association with MHC class I during a 3 h chase period were further examined (Figure 4A). Immediately after a 5 min pulse, and even 10 min into the chase period, no association between MHC class I and KK3 was detected. After 20 min chase, however, MHC class I heavy chain and at least four ubiquitylated class I heavy chain species are seen in association with KK3, and the associated class I heavy chains are all Endo H resistant (Figure 4A and B). The KK3–class I association peaked at 60 min and by 3 h the majority of class I molecules had dissociated from KK3, presumably due to removal and subsequent degradation (Figure 4B). In contrast, there was no significant degradation of KK3 during the 3 h chase period (Figure 4B), suggesting that KK3 does not share the same fate as its associated class I molecules. Bafilomycin, a vacuolar proton pump inhibitor, has previously been shown to prevent degradation of MHC class I in cells expressing KK3 (Coscoy and Ganem, 2000). In the presence of bafilomycin, significantly more ubiquitylated class I molecules remained associated with KK3 at the 3 h chase point, suggesting that bafilomycin prolongs the interaction between KK3 and ubiquitylated class I molecules (Figure 4C).
Fig. 4. KK3-induced ubiquitylation of MHC class I molecules occurs in a post-ER compartment. (A and B) KK3 associates with Endo H-resistant class I molecules. (A) IFN-γ-stimulated Hela-KK3 cells (2.8 × 107) were pulse-labelled for 5 min and chased for the indicated time periods. The KK3 protein was immunoprecipitated from digitonin cell lysates with the FLAG mAb M2, digested (+) or mock digested (–) with Endo H prior to resolution by SDS–PAGE and subsequent autoradiography. To control for Endo H activity, MHC class I molecules were immunoprecipitated from Triton X-100 lysates with the β2M- specific antiserum and subjected to Endo H digestion as above (right panel). (B) HeLa-KK3 cells were pulse-labelled as in (A) for the indicated time periods, and the KK3 protein immunoprecipitated from digitonin lysates with the FLAG mAb M2. Following dissociation in 1% SDS, lysates were re-precipitated with either the heavy chain- specific mAb (HC10) (upper panel) or the FLAG mAb M2 (for KK3) (lower panel), and resolved on a 10% SDS–PAGE gel. (C) Bafilomycin prevents degradation of KK3-associated ubiquitylated class I molecules. HeLa-KK3 cells were pulse-labelled as in (A) and chased for 3 h in the presence (+) or absence (–) of bafilomycin. The KK3 protein was immunoprecipitated from digitonin lysates with the FLAG mAb M2, dissociated in 1% SDS, and lysates re-precipitated with the heavy chain-specific mAb (HC10). (D) BFA inhibits the association between KK3 and class I. HeLa-KK3 cells were pulse-labelled as in (A) and chased for 1 h in the presence (+) or absence (–) of BFA and lactacystin (Lacta.). The KK3 protein was immunoprecipitated from digitonin cell lysates with the FLAG mAb M2, dissociated in 1% SDS, and lysates were re-precipitated with either the heavy chain-specific antibody (HC10) or the FLAG mAb M2 (for KK3), and resolved on a 10% SDS–PAGE gel.
In Figure 2 we showed that ubiquitylation of MHC class I is inhibited by BFA, suggesting that ubiquitylation occurs either late in the secretory pathway, or at the plasma membrane. Following BFA treatment of HeLa-KK3 cells we were unable to detect any association between KK3 and class I molecules (Figure 4D). In the presence of BFA, the proteasome inhibitor clasto-lactacystin β-lactone was also included, to ensure that any class I molecules that were ubiquitylated and retained in the ER were not rapidly degraded by the proteasome. However, addition of the proteasome inhibitor with the BFA did not preserve the interaction between class I and KK3 (Figure 4D). Taken together, these results suggest that KK3 and class I are unable to form a stable association in the ER, despite the majority of KK3 being visualized in this compartment (data not shown; Coscoy and Ganem, 2000). KK3 must form a stable association with, and promote ubiquitylation of, class I molecules in the medial-Golgi or more distal compartment.
The PHD domain of KK3 is required for ubiquitylation but not association with MHC class I
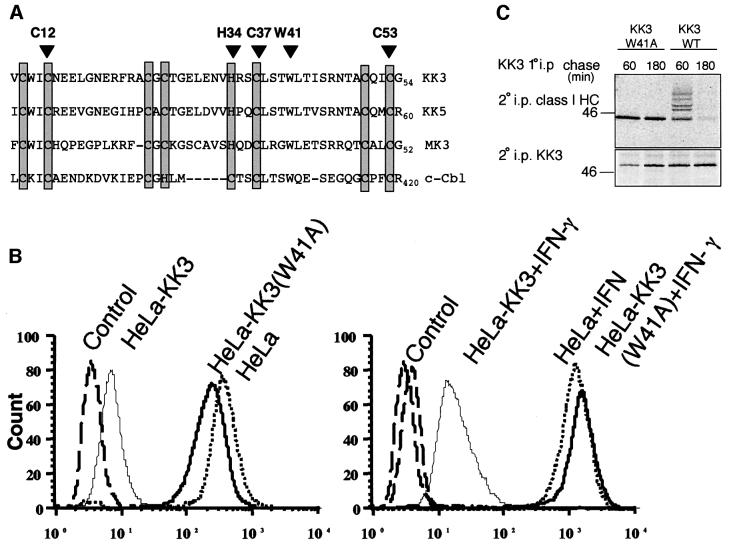
The sequence and structural homology between the KK3 PHD motif and RING finger domains suggest that the PHD domain may recruit an E2 ubiquitin-conjugating enzyme. To determine whether the N-terminal PHD motif was required for KK3 function, amino acid substitutions were made in the conserved residues of the PHD motif. Initially, three of the canonical cysteines (Cys12, Cys37, Cys53) and histidine (His34) of the PHD motif were substituted with serines and leucine, respectively (Figure 5A). Each of these mutations resulted in an unstable protein that was not amenable to further characterization (data not shown). In addition to the characteristic arrangement of the cysteines and histidine, KK3 shares a number of other conserved residues with the RING finger domain of the E2-dependent ubiquitin ligase c-Cbl (Figure 5A). Trp41 is conserved in the PHD motif of KK3 and in the RING finger domain of c-Cbl, where it corresponds to Trp408. Mutation of c-Cbl Trp408 to alanine reduces the affinity of c-Cbl for the E2 Ubc4, abolishing the ability of c-Cbl to promote substrate ubiquitylation (Joazeiro et al., 1999). We therefore reasoned that mutation of this tryptophan residue should interfere with the function of the PHD motif of KK3 without causing the structural perturbation and subsequent instability associated with the cysteine mutations.
Fig. 5. Mutation of the PHD motif of KK3 preserves cell surface class I expression by preventing ubiquitylation but not class I association. (A) Sequence alignment of the N-terminal PHD motifs of KK3, KK5 and MK3 with the RING domain of c-Cbl. The canonical cysteine and histidine residues are boxed. Residues mutated in this study are indicated with arrows. (B) Cytofluorometric analysis of cell surface MHC class I expression in HeLa-M cells stably transduced with wild-type or mutant (W41A) KK3. IFN-γ-stimulated (right panel) or non-stimulated (left panel) cells were stained with the mAb W6/32 and a FITC-conjugated secondary antibody. Control cells were only stained with the secondary antibody. (C) The KK3(W41A) PHD domain mutant preserves the association of KK3 with class I, but is not ubiquitylated. IFN-γ-stimulated HeLa-M cells (1 × 107) stably transduced with wild-type (WT) or mutant (W41A) KK3 were pulse-labelled for 5 min and chased for 60 and 180 min, as indicated. The KK3 protein was immunoprecipitated from digitonin cell lysates with the FLAG mAb M2, dissociated in 1% SDS and lysates re-precipitated with either the heavy chain-specific mAb (HC10) (upper panel) or the FLAG mAb M2 (for KK3) (lower panel).
We generated a HeLa-M cell line stably expressing the KK3(W41A) mutant tagged with an N-terminal FLAG epitope [HeLa-KK3(W41A)]. Both the mutant and wild-type KK3 proteins were expressed at similar levels (data not shown). In contrast to wild-type KK3, surface expression of MHC class I was unaffected by KK3(W41A) (Figure 5B), showing that the PHD motif is required for KK3 activity. Pulse–chase analysis demonstrated that MHC class I molecules still associated with the mutant KK3(W41A) protein (Figure 5C). In comparison with cells expressing wild-type KK3, the association between mutant KK3 and MHC class I was clearly prolonged, remaining visible even after a 3 h chase. However, at no point did we detect ubiquitylation of the class I heavy chain. Therefore, the PHD motif is not required for interaction with MHC class I but is necessary for ubiquitylation and degradation of class I. In the absence of ubiquitylation, MHC class I cell surface expression is unaffected.
Ubiquitylation by KK3 promotes the internalization of MHC class I molecules
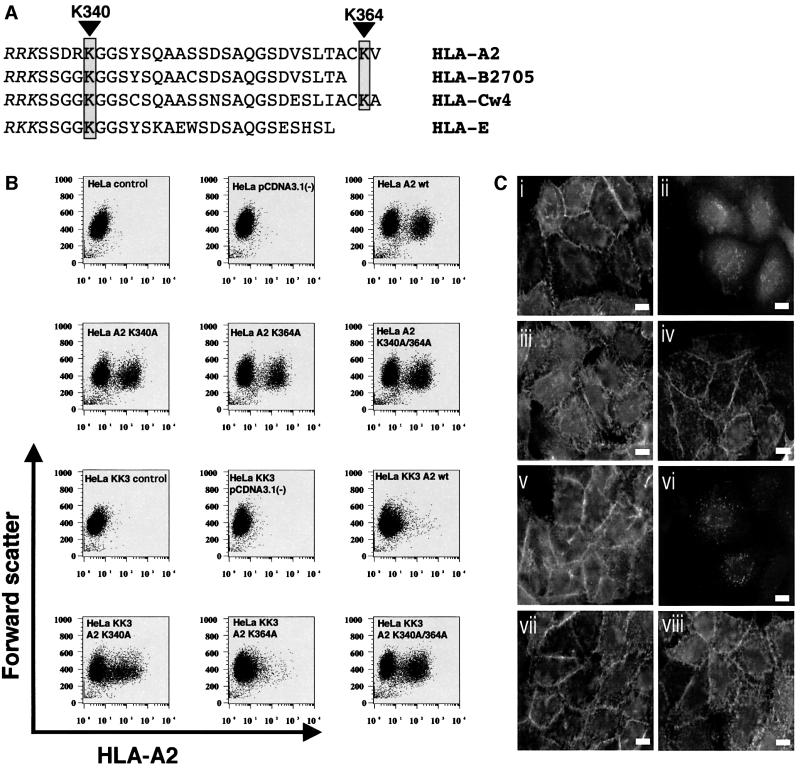
Ubiquitin is covalently attached to the ε-amino group of lysine residues. To determine whether ubiquitylation of the class I molecule might signal internalization, we made mutants of the HLA-A2 class I allele in which the cytoplasmic tail lysines were substituted with alanine (K340A and K364A mutants). Lysine at position 340 is highly conserved among class I alleles, while the lysine at position 364 is less common (Figure 6A). HeLa-M and HeLa-KK3 cells were transiently transfected with wild-type HLA-A2 and the HLA-A2 lysine mutants and examined by both flow cytometry (Figure 6B) and an immunofluorescence microscopy-based endocytosis assay (Figure 6C) (Ishido et al., 2000b). Control HeLa-M cells expressed equivalent levels of wild-type HLA-A2 and the HLA-A2 lysine mutants on the cell surface (Figure 6B). In the presence of KK3, cell surface expression of wild-type HLA-A2 as well as the non-conserved lysine mutant HLA-A2(K364A) was reduced, while surface expression of the conserved lysine K340A mutant as well as the double lysine mutant HLA-A2(K340A/K360A) was unaffected by KK3 (Figure 6B). Similar findings were seen by immunofluorescence microscopy. Cells were incubated with the HLA-A2-specific BB7.2 antibody for 1 h on ice and after washing allowed to internalize the bound antibody for 30 min at 37°C prior to fixation. In HeLa-M cells, both wild-type and mutant HLA-A2 proteins remained localized to the plasma membrane (Figure 6C). However, in HeLa-KK3 cells, wild-type HLA-A2 as well as the HLA-A2(K364A) mutant were localized in internal punctate structures, consistent with endosomes (Figure 6C), as previously described (Ishido et al., 2000b). In contrast, both the HLA-A2(K340A) mutant and the double lysine mutant remained confined to the plasma membrane (Figure 6C). Although we cannot exclude the possibility that mutation of Lys340 prevents KK3 binding to HLA-A2, we suggest that the conserved cytoplasmic tail Lys340 is the dominant residue required for KK3-mediated ubiquitylation and hence internalization of MHC class I from the plasma membrane.
Fig. 6. A cytoplasmic tail lysine residue is required for the internalization of HLA-A2 mediated by KK3. (A) Sequence alignment of the cytoplasmic tails of HLA-A2, HLA-B2705, HLA-Cw2 and HLA-E. The stop transfer sequence is shown in italics; lysine residues in the cytoplasmic tail are boxed with K340 and K364 corresponding to the HLA-A2 sequence. (B) Cytofluorometric analysis of MHC class I expression in HeLa (upper six panels) and HeLa-KK3 cells (lower six panels) transiently transfected with either wild-type HLA-A2 or the lysine mutants HLA-A2-K340A, HLA-A2 K364A and HLA-A2 K340A/K364A. Transfected cells were stained with the mAb BB7.2 and a FITC-conjugated secondary antibody. (C) Internalization of HLA-A2. HeLa-M (i, iii, v and vii) and HeLa-KK3 cells (ii, iv, vi and viii) were transiently transfected with either wild-type HLA-A2 (i and ii) or the lysine mutants HLA-A2-K340A (iii and iv), HLA-A2 K364A (v and vi) and HLA-A2 K340A/K364A (vii and viii). The HLA-A2-specific mAb BB7.2 was bound to the transfectants on ice for 1 h and the cells subsequently incubated at 37°C for 30 min before fixation. BB7.2 staining was detected with an anti-mouse FITC antibody and visualized with a Zeiss Axioplan microscope. The scale bar is 10 µm.
Depletion of TSG101 inhibits MHC class I degradation
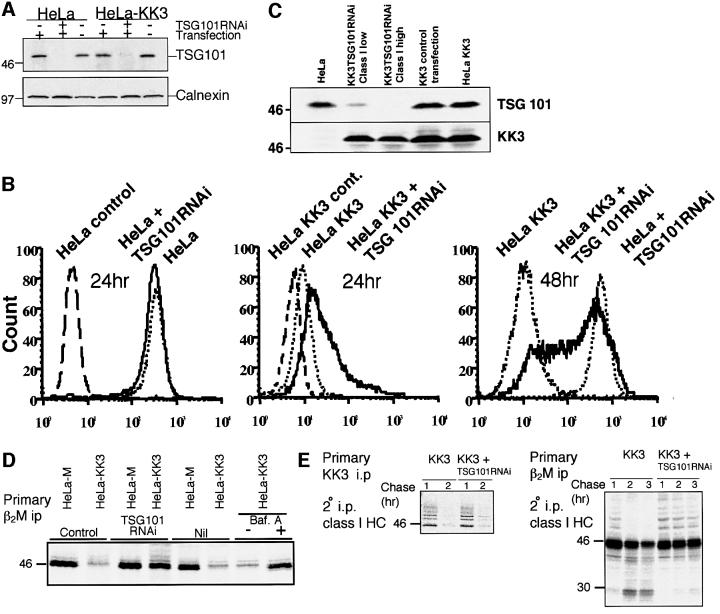
Bafilomycin, an inhibitor of endosomal/lysosomal acidification, prevents MHC class I degradation in cells expressing KK3 (Figure 4C; Coscoy and Ganem, 2000). However, we know little about the machinery that sorts ubiquitylated class I molecules from the plasma membrane to the late endocytic/lysosomal pathway for degradation. Multi-vesicular bodies (MVBs) represent an intermediate organelle in the trafficking of proteins from early endosomes to lysosomes (Lemmon and Traub, 2000; Dupre et al., 2001; Piper and Luzio, 2001). In MVBs, the limiting membrane invaginates to form internal vesicles into which proteins destined for degradation are sorted, and fusion of MVBs with lysosomes delivers these internal vesicles to the lysosomal lumen. In yeast, ubiquitylation can act as a signal to recruit proteins into the MVB internal vesicles (Katzmann et al., 2001; Reggiori and Pelham, 2001; Urbanowski and Piper, 2001). The yeast protein Vps23 is involved in the recognition of ubiquitylated proteins in MVB sorting (Katzmann et al., 2001) and its mammalian homologue TSG101 is also involved in late endosomal sorting (Babst et al., 2000). To determine whether TSG101 is also required for the targeting of MHC class I molecules for degradation, we used small interfering RNAs (siRNAs) to deplete TSG101 from HeLa-M and HeLa-KK3 cells (Elbashir et al., 2001; Garrus et al., 2001). Cells were transfected twice at 24 h intervals with TSG101 homologous siRNA, and TSG101 expression determined by immunoblot analysis. A dramatic reduction in TSG101 expression, but not of a control protein calnexin, is seen in cells transfected with TSG101 siRNA (Figure 7A). The effect of TSG101 depletion on MHC class I cell surface expression was examined at 24 and 48 h post-siRNA transfection (Figure 7B). Class I expression was unaffected in TSG101-depleted HeLa-M cells. In HeLa-KK3 cells, an increase in class I expression was seen 24 and 48 h post-transfection, 75% of the cells expressed wild-type levels of class I. These 48 h post-transfection HeLa-KK3 cells were enriched by sorting into MHC class I ‘high’ and ‘low’ populations and analysed by immunoblotting for TSG101 and KK3 expression (Figure 7C). In the class I ‘high’ population, no TSG101 could be detected but the cells retained expression of KK3. A small amount of TSG101 was detected in the class I ‘low’ population, presumably corresponding to non-transfected cells. However, TSG101 depletion did not affect MHC class I internalization, as in the immunofluorescence microscopy-based endocytosis assay the TSG101-depleted MHC class I ‘high’ cells still internalized class I from the cell surface (data not shown). Pulse–chase analysis showed that depletion of TSG101 from HeLa-KK3 cells prevented the degradation of MHC class I molecules normally seen by 3 h (Figure 7D and E) and the class I molecules remained ubiquitylated (Figure 7E, right panel). In addition, although the TSG101 depletion protected the class I molecules from degradation, they did not remain associated with KK3 (Figure 7E, left panel). Taken together, these results show that TSG101 is required for the degradation of internalized class I, and suggest that, in the absence of TSG101, class I molecules are recycled back to the plasma membrane.
Fig. 7. Cellular depletion of the TSG101 protein inhibits KK3-induced MHC class I degradation. (A) Depletion of TSG101 from HeLa-M and HeLa-KK3 using siRNA. Cells were co-transfected twice with 50 nM RNAi (lanes 2 and 5) or mock transfected (lanes 1 and 4) at 24 h intervals. Forty-eight hours after the first transfection, 2 × 105 cells were extracted in 1% Triton X-100, and lysates separated by 10% SDS–PAGE, transferred to Immobilon P membranes, and probed with the A410 anti-TSG101-specific mAb (upper panel) or control anti-calnexin antisera (lower panel). (B) Cytofluorometric analysis of cell surface MHC class I expression in HeLa-M and HeLa-KK3 cells following TSG101 depletion. Cells (2 × 105) were stained with the monoclonal mAb W6/32 and a FITC-conjugated secondary antibody at 24 h (left and middle panel) and 48 h (right panel) post-TSG101RNAi transfection. (C) TSG101-depleted HeLa-KK3 cells were sorted at the 48 h time point [as in (B), right panel] into class I ‘high’ and ‘low’ populations and probed with the A410 anti-TSG101-specific mAb (upper panel) or FLAG mAb M2 (for KK3) (lower panel). (D) TSG101 depletion prevents class I degradation. HeLa-M or Hela-KK3 cells (5 × 105) were pulse-labelled for 10 min and chased for 3 h. Triton X-100 cell lysates were immunoprecipitated with the β2M antiserum and resolved by SDS–PAGE and subsequent autoradiography. (E) Class I remains ubiquitylated in TSG101-depleted KK3 cells. IFN-γ-stimulated HeLa-KK3 or TSG101-depleted HeLa-KK3 cells (1.4 × 107) were pulse-labelled for 8 min and chased for 60–180 min. The KK3 protein was immunoprecipitated with the FLAG mAb M2, dissociated in 1% SDS and lysates re-precipitated with the heavy chain-specific mAb (HC10) (left panel). Proteins not precipitated with the FLAG mAb were re-precipitated with the rabbit β2M-specific antiserum, dissociated in 1% SDS and re-precipitated with the heavy chain-specific mAb HC10 (right panel), and resolved on a 10% SDS–PAGE gel. The 29 kDa band represents a class I degradation product.
Discussion
In this study we have characterized the molecular basis for the downregulation of MHC class I molecules by the KSHV K3 gene product (KK3). KK3 subverts the normal trafficking of class I by enhancing the endocytosis of MHC class I molecules (Coscoy and Ganem, 2000; Ishido et al., 2000b). We now show that KK3 associates with and promotes the ubiquitylation of class I molecules. Ubiquitylation is the signal for the internalization and, via a TSG101-dependent sorting step, the subsequent degradation of MHC class I molecules.
Ubiquitylation plays a critical role in determining the fate of many mammalian cell surface receptors. Receptor protein tyrosine kinases, seven-membrane-spanning receptors, G protein-coupled receptors and cytokine receptors are physiologically regulated via ligand-induced ubiquitylation, internalization and subsequent degradation (Hicke, 1999, 2001; Pickart 2001). We now provide several lines of evidence that KK3-induced ubiquitylation of MHC class I molecules is the signal for endocytosis and degradation of this protein via the endocytic pathway.
In KK3-expressing cells, MHC class I molecules reach the cell surface as inhibition of clathrin-mediated endocytosis, by overexpression of AP180, preserves cell surface class I expression (data not shown). Association with KK3 in the absence of ubiquitylation is insufficient to target class I molecules for destruction, as shown by the PHD motif mutant KK3(W41A). Targeting of HLA-A2 into the endocytic pathway is dependent on the conserved lysine at position 340 in the cytoplasmic tail, and this lysine is found in all the class I alleles downregulated by KK3. Bafilomycin also preserves the association of KK3 with ubiquitylated class I molecules. Finally, depletion of TSG101 from KK3-expressing cells demonstrates a role for this protein in MHC class I degradation.
It was surprising that ubiquitylated class I molecules were difficult to visualize and represented <10% of the total labelled class I. We know little about either the stoichiometry of the KK3–class I complex or the rate-limiting step in the ubiquitylation process, but it seems likely that ubiquitylated class I molecules represent an unstable intermediate in a dynamic process and will be rapidly degraded. During preparation of this paper, a complementary study by Ganem’s group also showed that KK3 and the related KK5 protein cause ubiquitylation of class I and, in the case of KK5, the B7.2 co-stimulatory molecule (Coscoy et al., 2001). Although this group were unable to demonstrate an association between the viral protein and class I molecules, they showed an absolute requirement for lysines in the cytoplasmic tail of the target protein. Furthermore, the introduction of a triple lysine motif to the tail of the KK5-insensitive B7.1 molecule was sufficient for KK5-induced endocytosis of B7.1, showing that lysine residues are not only necessary, but also sufficient for KK5-induced endocytosis of B7.1.
A critical role for the N-terminal PHD motif in the ubiquitylation and subsequent degradation of class I molecules was demonstrated by the KK3(W41A) mutant. PHD motifs have a characteristic arrangement of seven cysteines and a histidine (Cys4HisCys3), binding zinc in a cross-brace topology (Capili et al., 2001), a protein fold that is homologous to that of RING domains (Zheng et al., 2000). The recognition of RING domains as ubiquitin E3 ligases provided the impetus to determine whether KK3 promotes class I ubiquitylation. Substitution of the canonical zinc co-ordinating cysteines and histidine resulted in unstable proteins, suggesting that zinc binding may be important for the structural integrity of KK3. We therefore employed an alternative strategy and mutated the tryptophan at position 41 (W41), which is predicted to interfere with the function of the KK3 PHD motif. The KK3(W41A) PHD motif mutant resulted in a loss of class I ubiquitylation and a prolonged interaction between KK3 and MHC class I molecules, suggesting that ubiquitylation of class I may trigger dissociation of class I from KK3. Furthermore, the KK3 mutant was unable to downregulate MHC class I and demonstrates that association in the absence of ubiquitylation is insufficient to promote class I internalization.
Since disruption of the PHD domain did not affect association of the two proteins, the recognition and binding of MHC class I by the KK3 molecule must involve other domains of KK3. Analysis of the KK3 protein suggests it to be an integral membrane protein with both the N- and C-termini in the cytoplasm (data not shown). We have found that the transmembrane domain of HLA-A2 is necessary for KK3-mediated downregulation (data not shown), suggesting that the KK3 and class I interaction occurs through their respective transmembrane domains. This is in agreement with Coscoy and colleagues, who found that the transmembrane region of class I molecules is critical for recognition by the KK5 viral protein (Coscoy et al., 2001).
Immunofluorescence microscopy of epitope-tagged KK3, or with KK3-specific antibodies, consistently localizes KK3 to the ER (data not shown; Coscoy and Ganem, 2000). However, unlike the murine MK3 protein, we are unable to detect either association of KK3 with MHC class I, or MHC class I ubiquitylation in the ER. Our kinetic data and the sensitivity of KK3-mediated ubiquitylation to BFA argue that KK3 only associates with and ubiquitylates class I molecules that have progressed through the secretory pathway. Since KK3-associated class I molecules are Endo H resistant, the class I must have trafficked at least as far as the medial-Golgi and is likely to be at, or close to, the plasma membrane. Therefore, although at steady state KK3 is predominantly ER localized, a proportion of the protein must be exported to interact with MHC class I. Since the ER is the site of MHC class I assembly, this raises the question of why we are unable to detect a stable association between KK3 and MHC class I in this compartment. The interaction may require additional components that can only facilitate the KK3–class I association following ER export. One candidate is an E2 ubiquitin transferase. We have so far been unable to detect an associated E2, and the stable association of the KK3(W41A) mutant with MHC class I in the presumed absence of an E2 makes it unlikely that this component is limiting. Another possibility is that class I molecules may require post-translational modification later in the secretory pathway before binding to KK3, and this possibility is under investigation. This is unlikely to involve phosphorylation, as KK3 is able to downregulate MHC class I molecules lacking the conserved phosphorylation site (Paulson et al., 2001).
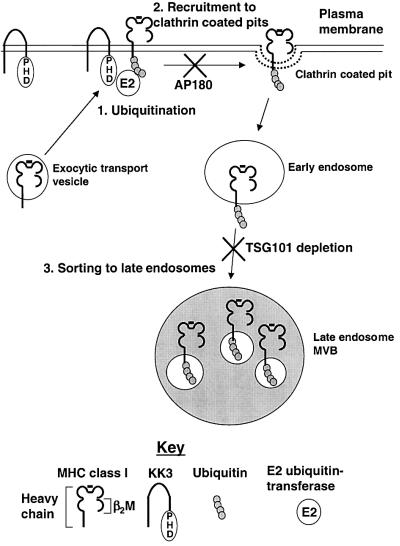
Ubiquitylation is not only a signal for the internalization of plasma membrane proteins, but may also target proteins to the late endocytic pathway. A key step in late endosomal sorting is the formation of the MVB, in which the endosomal membrane invaginates forming internal vesicles into which proteins destined for lysosomal degradation are sorted (Piper and Luzio, 2001). This sorting process is better understood in yeast, where Vps23, the homologue of TSG101, is a component of the endosome-localized protein complex (ESCRT-1) involved in the recognition of ubiquitylated proteins in MVB sorting (Katzmann et al., 2001). Both Vps23 and TSG101 contain a ubiquitin E2 variant (UEV) domain that resembles E2s but is not catalytically active, as it lacks the active site cysteine and is unable to make a transient thioester bond during ubiquitin transfer. A model for the role of TSG101 is that, like Vps23, it binds ubiquitin, and sorts ubiquitylated proteins into the internal vesicles of MVBs (Dupre et al., 2001; Pickart, 2001). TSG101 is also involved in late endosomal sorting as TSG101 mutant cells are defective in late endosomal sorting of ligand-bound epidermal growth factor receptors and recycle rather than degrade these activated receptors (Babst et al., 2000). We found that depletion of TSG101 did not affect internalization of class I but prevented KK3-induced class I degradation, presumably allowing class I molecules to recycle back to the plasma membrane. Assuming that the yeast and mammalian MVB sorting machinery are conserved, we suggest that the preservation of class I molecules following TSG101 depletion occurs because ubiquitylated MHC class I molecules are not sorted to the internal vesicles of MVBs, preventing their degradation. Therefore, we propose that KK3, by causing MHC class I to be ubiquitylated, not only provides a signal to internalize the MHC class I molecule from the plasma membrane, but also to target it for degradation in the late endocytic pathway (Figure 8).
Fig. 8. Model for the downregulation of MHC class I by KK3. (1) KK3 promotes the ubiquitylation of MHC class I molecules that have trafficked through the secretory pathway at least as far as the medial-Golgi. For simplicity, the plasma membrane is represented as the site of ubiquitylation. KK3 presumably recruits via its PHD motif an E2 ubiquitin-transferase facilitating ubiquitylation of the MHC class I cytoplasmic tail. (2) Ubiquitylation recruits the MHC class I molecule into clathrin-coated pits and the class I is internalized into early endosomes. This step can be inhibited by overexpression of AP180. (3) Once within the endosomal pathway, TSG101-dependent sorting targets the ubiquitylated MHC class I molecule to late endosomes. Assuming conservation of the yeast and mammalian MVB sorting machinery, we propose that ubiquitin sorts the MHC class I molecules into the internal vesicles in the MVB.
A large number of viral inhibitors of the class I pathway have been described. Of the viral proteins that associate with class I molecules, KK3 superficially resembles the gp48 protein of MCMV, which redirects class I to an endocytic compartment (Reusch et al., 1999), and the HIV-1 Nef protein, which downregulates and redirects cell surface class I molecules to the trans-Golgi network (TGN) (Piguet et al., 2000). However, these proteins use very different mechanisms to degrade class I. The two leucines in the cytoplasmic tail of gp48 act as a lysosomal targeting motif, while HIV Nef connects class I to the PACS-1-based TGN retrieval pathway. The HCMV encoded US2 and US11 gene products also utilize ubiquitin for class I-induced degradation (Tortorella et al., 2000) and HIV Vpu uses ubiquitin for CD4 degradation (Schubert et al., 1998). In addition, ubiquitylation plays an essential role in retroviral budding as HIV-1 Gag binds TSG101 to facilitate HIV-1 budding and depletion of TSG101 prevents this process (Garrus et al., 2001).
In summary, KK3 is the first viral gene product that subverts the trafficking of a host protein via the ubiquitin-dependent endosomal sorting machinery. Through acquisition of a PHD motif, the KK3 viral protein has adopted a novel mechanism to remove class I molecules from the plasma membrane and subvert class I-mediated antigen presentation.
Materials and methods
Antibodies
The following antibodies were used: HC10, a monoclonal antibody (mAb) recognizing free class I heavy chains kindly provided by Dr H.Ploegh (Stam et al., 1986); 435.3, an anti-TAP.2 mAb kindly provided by Dr P.Van Endert (van Endert et al., 1994); BB7.2, an HLA-A2-specific mAb (a kind gift from Dr P.Cresswell); w6/32, an anti-human class I mAb (Parham et al., 1979). The M2 (anti-FLAG) mAb, FK2 mAb (anti-ubiquitin), A410 mAb (anti-TSG101) and the rabbit anti-β2M antisera were purchased from Sigma, Affiniti, Abcam and Dako, respectively.
Constructs
pCDNA3.1(-)Pac HLA-A2. The HLA-A2 open reading frame (ORF) was amplified by the PCR from pRSV-Neo-HLA-A2 (a kind gift from Dr P.Cresswell) with the primers A2-5′ and A2-3′ and cloned into pCDNA3.1(-)Pac (Hewitt et al., 2001) with EcoRI and BamHI.
pCDNA3.1(-)Pac HLA-A2-K340A. The lysine/alanine (K340A) substitution was made using the QuikChange site-directed mutagenesis kit (Stratagene). The sense oligonucleotide was A2-K340A and the antisense primer was complementary.
pCDNA3.1(-)Pac HLA-A2-K364A. The lysine/alanine (K364A) substitution was made by PCR amplification of the HLA-A2 ORF with the primers A2-5′ and A2-3′-K364A and cloned into pCDNA3.1(-)Pac with EcoRI and BamHI.
pCDNA3.1(-)Pac HLA-A2-K340A/K364A. The double lysine mutant was generated by amplifying the HLA-A2-K340A mutant ORF with primers A2-5′ and A2-3′-K364A and cloning into pCDNA3.1(-)Pac with EcoRI and BamHI.
pCDNA3 KK3. The KK3 ORF was cloned as an EcoRI–SalI fragment from pMSCV-GFP-KSHV K3 (Stevenson et al., 2000) into the EcoRI–XhoI sites of pCDNA3.
pCDNA3 KK3-W41A. The tryptophan/alanine (W41A) substitution was made using the QuikChange site-directed mutagenesis kit (Stratagene). The sense primer was KK3 W41A and the antisense primer was complementary.
pMCSV-IRES-Neo KK3 and KK3-W41A. The KK3 and KK3-W41A ORFs were cloned as NotI (filled in with Klenow fragment and dNTPs)–EcoRI fragments from pCDNA3 KK3 into the XhoI (filled in with Klenow fragment and dNTPs)–EcoRI sites of pMCSV-IRES-Neo retroviral vector (Clontech).
Oligonucleotides. The following oligonucleotides were used: A2-5′, ATATGAATTCACCATGGCCGTCATGGCGCCCCGA; A2-3′, ATA TGGATCCTCACACTTTACAAGCTGTGAG; A2-K340A, AAGAGC TCAGATAGAGCCGGAGGGAGCTACTCT; A2-3′-K364A, ATATGG ATCCTCACACTGCACAAGCTGTGAGAGACACATC; KK3 W41A, GAAGTTGTTTAAGCACCGCGCTCACTATCTCTAGAA.
TSG101 depletion using siRNA
A siRNA duplex specific for TSG101 (TSG101 coding nucleotides 413–433) was synthesized (Dharmacon Research) and annealed as described by Elbashir et al. (2001). RNA sequences: sense, 5′ CCUCCAGUCUUCUCUCGUCTT; antisense, 5′ GACGAGAGAAGACUGGAGGTT. Cells were transfected with the TSG101 siRNA essentially as described previously (Garrus et al., 2001). Briefly, HeLa-M and HeLa-KK3 cells were plated into 6-well plates in the absence of antibiotics and transfected twice at 24 h intervals with 300 pmol of TSG101 siRNA per well with Oligofectamine reagent (Invitrogen) as per the manufacturer’s instructions. Cells were analysed 24 and 48 h after the second transfection.
Cell lines and culture
The HeLa-M cervical carcinoma cell line was grown in RPMI 1640 (Gibco-BRL) supplemented with 10% fetal calf serum (FCS). Where indicated, treatment with IFN-γ (Peprotech) was at 200 U/ml for 24 h.
Generation of the HeLa-M KK3 stable cell line
HeLa-M cells were transfected in 6-well plates with 1 µg of pCDNA3 KK3 DNA using LipofectAmine plus reagent (Gibco-BRL) and transfectants selected with 750 µg/ml G418 (Gibco-BRL). Clones expressing the FLAG-tagged KK3 were screened by immunoblotting.
Retroviral preparation and transduction
Retroviral preparation and transduction were performed as described previously (Kelly et al., 2000). 293T cells (5 × 105) were seeded onto 6-well plates in RPMI complete medium. After 24 h, 293T cells were co-transfected with 6 µl of Fugene and 1 µg of plasmid: (i) pRDF (a plasmid encoding the gene for the RD114 envelope protein of the feline endogenous virus RD114, a kind gift from Dr Elio Vanin); (ii) pEQPAM3-E (a plasmid containing the GAG and POL genes of murine leukaemia virus, a kind gift from Dr Elio Vanin); and (iii) either the wild-type or W41A mutant version of KK3 in the pMCSV-Ires-neo retroviral vector. On day 3, 20 h post-transfection of the 293T cells, the medium was changed to 2.5 ml of fresh complete RPMI. HeLa cells were seeded at 2.5 × 105 cells per 6-well plate. On day 4, the 293T supernatants containing the retroviral particles were filtered through a 0.4 µm High-flow filter and supplemented with 4 µg/µl Polybrene (Sigma). Each infection cocktail was used to replace the medium on the HeLa cells. Fresh medium was added to the 293T cells and the above process was repeated at 52 h post-transfection, replacing the infectious cocktail on the same HeLa cells. Fresh RPMI medium was added to the transduced HeLa cells 4 h following infection. Transduced HeLa cells were selected after 24 h by addition of 1–2 mg/ml G418.
Immunoprecipitation and Endo F and H digestion
For pulse–chase analysis, cells were starved for 1 h in methionine, cysteine-free medium and labelled with [35S]methionine and [35S]cysteine (Amersham) for the indicated times, chased in media containing excess cold methionine and cysteine, and extracted on ice at 2 × 106 cells/ml in 1% detergent in 10 mM Tris, 150 mM NaCl (TBS) pH 7.4, containing 0.5 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma), 0.1 mM N-α-tosyl-l-lysyl-chloromethyl ketone (TLCK; Sigma), 5 mM iodoacetamide (IAA; Sigma) (extraction buffer). The post-nuclear supernatant was pre-cleared with protein A–Sepharose beads, incubated with the primary antibody for 1 h followed by protein A–Sepharose. Primary immunoprecipitations were washed in 0.1% digitonin/TBS, separated by SDS–PAGE, dried and processed for autoradiography. For re-precipitations, the washed primary immunoprecipitate was incubated for 60 min at 37°C in 50 µl of TBS containing 1% SDS and dissociated proteins diluted 20-fold in 0.5% Triton X-100/TBS and re-precipitated for 12 h with the appropriate antibody and protein A–Sepharose beads. The beads were washed in 0.1% Triton X-100/TBS and processed for SDS–PAGE and autoradiography. For Endo H and Endo F digestions, the washed immunoprecipitate was divided into two and the Endo H/F (New England Biolabs) added to one of the samples (according to the manufacturer’s instructions), and incubated for 1 h at 37°C before analysis by SDS–PAGE and autoradiography.
Antibody feeding and immunofluorescence microscopy
HeLa-M and HeLa-M KK3 cells were grown overnight onto 13 mm glass coverslips, transiently transfected the next day with 0.5 µg of the HLA-A2 construct DNA using Fugene (Roche) and analysed 48 h later. Tissue culture medium was removed by washing with ice-cold PBS, and coverslips incubated for 1 h with the BB7.2 antibody. The coverslips were washed four times with ice-cold PBS and then either fixed in 4% paraformaldehyde in PBS for 20 min or incubated at 37°C in pre-warmed RPMI/FCS for 30 min prior to fixation. After fixation, coverslips were washed three times with PBS, incubated twice with 15 mM glycine in PBS for 5 min, washed again three times with PBS and then permeabilized with 0.2% saponin and 1% fish skin gelatin in PBS. The cells were then stained with a goat anti-mouse FITC-conjugated antibody (Jackson) with 0.2% saponin and 1% fish skin gelatin in PBS for 30 min. Coverslips were then washed four times with 0.2% saponin and 1% fish skin gelatin in PBS, twice with PBS for 5 min each and then mounted. Antibody fluorescence was visualized with a Zeiss Axioplan fluorescence microscope.
Flow cytometric analysis
Stable HeLa-M transfectants were stained with the MHC class I mAb w6/32 in PBS, 0.1% bovine serum albumin and visualized with a goat anti-mouse FITC-conjugated secondary antibody (Jackson).
Immunoblotting
Transfer of SDS–PAGE gels onto PVDF membranes and incubation with specific antibodies was performed as described previously (Lehner et al., 1997).
Acknowledgments
Acknowledgements
We thank Elio Vanin for retroviral constructs, J.Paul Luzio and John Trowsdale for advice and helpful discussion. This work was supported by The Wellcome Trust and the Medical Research Council (to P.G.S.).
References
- Aasland R., Gibson,T.J. and Stewart,A.F. (1995) The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem. Sci., 20, 56–59. [DOI] [PubMed] [Google Scholar]
- Babst M., Odorizzi,G., Estepa,E.J. and Emr,S.D. (2000) Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic, 1, 248–258. [DOI] [PubMed] [Google Scholar]
- Boname J.M. and Stevenson,P.G. (2001) MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity, 15, 627–636. [DOI] [PubMed] [Google Scholar]
- Capili A.D., Schultz,D.C., Rauscher,I.F. and Borden,K.L. (2001) Solution structure of the PHD domain from the KAP-1 corepressor: structural determinants for PHD, RING and LIM zinc-binding domains. EMBO J., 20, 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E. and Knowles,D.M. (1999) The role of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Semin. Cancer Biol., 9, 165–174. [DOI] [PubMed] [Google Scholar]
- Coscoy L. and Ganem,D. (2000) Kaposi’s sarcoma-associated herpes virus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl Acad. Sci. USA, 97, 8051–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscoy L. and Ganem,D. (2001) A viral protein that selectively down regulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Invest., 107, 1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscoy L., Sanchez,D.J. and Ganem,D. (2001) A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J. Cell Biol., 155, 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre S., Volland,C. and Haguenauer-Tsapis,R. (2001) Membrane transport: ubiquitylation in endosomal sorting. Curr. Biol., 11, R932–R934. [DOI] [PubMed] [Google Scholar]
- Elbashir S.M., Harborth,J., Lendeckel,W., Yalcin,A., Weber,K. and Tuschl,T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 494–498. [DOI] [PubMed] [Google Scholar]
- Garrus J.E. et al. (2001) Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell, 107, 55–65. [DOI] [PubMed] [Google Scholar]
- Haque M., Ueda,K., Nakano,K., Hirata,Y., Parravicini,C., Corbellino,M. and Yamanishi,K. (2001) Major histocompatibility complex class I molecules are down-regulated at the cell surface by the K5 protein encoded by Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8. J. Gen. Virol., 82, 1175–1180. [DOI] [PubMed] [Google Scholar]
- Hewitt E.W., Gupta,S.S. and Lehner,P.J. (2001) The human cyto megalovirus gene product US6 inhibits ATP binding by TAP. EMBO J., 20, 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L. (1999) Gettin’ down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol., 9, 107–112. [DOI] [PubMed] [Google Scholar]
- Hicke L. (2001) A new ticket for entry into budding vesicles—ubiquitin. Cell, 106, 527–530. [DOI] [PubMed] [Google Scholar]
- Ishido S., Choi,J.K., Lee,B.S., Wang,C., DeMaria,M., Johnson,R.P., Cohen,G.B. and Jung,J.U. (2000a) Inhibition of natural killer cell-mediated cytotoxicity by Kaposi’s sarcoma-associated herpesvirus K5 protein. Immunity, 13, 365–374. [DOI] [PubMed] [Google Scholar]
- Ishido S., Wang,C., Lee,B.S., Cohen,G.B. and Jung,J.U. (2000b) Down regulation of major histocompatibility complex class I molecules by Kaposi’s sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol., 74, 5300–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joazeiro C.A. and Weissman,A.M. (2000) RING finger proteins: mediators of ubiquitin ligase activity. Cell, 102, 549–552. [DOI] [PubMed] [Google Scholar]
- Joazeiro C.A., Wing,S.S., Huang,H., Leverson,J.D., Hunter,T. and Liu, Y.C. (1999) The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science, 286, 309–312. [DOI] [PubMed] [Google Scholar]
- Katzmann D.J., Babst,M. and Emr,S.D. (2001) Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell, 106, 145–155. [DOI] [PubMed] [Google Scholar]
- Kelly P.F., Vandergriff,J., Nathwani,A., Nienhuis,A.W. and Vanin,E.F. (2000) Highly efficient gene transfer into cord blood nonobese diabetic/severe combined immunodeficiency repopulating cells by oncoretroviral vector particles pseudotyped with the feline endo genous retrovirus (RD114) envelope protein. Blood, 96, 1206–1214. [PubMed] [Google Scholar]
- Krmpotic A., Messerle,M., Crnkovic-Mertens,I., Polic,B., Jonjic,S. and Koszinowski,U.H. (1999) The immunoevasive function encoded by the mouse cytomegalovirus gene m152 protects the virus against T cell control in vivo. J. Exp. Med., 190, 1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner P.J., Karttunen,J.T., Wilkinson,G.W. and Cresswell,P. (1997) The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc. Natl Acad. Sci. USA, 94, 6904–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon S.K. and Traub,L.M. (2000) Sorting in the endosomal system in yeast and animal cells. Curr. Opin. Cell Biol., 12, 457–466. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan,L., Tipper,C., Amherdt,M., Orci,L. and Klausner,R.D. (1991) Brefeldin A’s effects on endosomes, lysosomes and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell, 67, 601–616. [DOI] [PubMed] [Google Scholar]
- Nicholas J., Ruvolo,V., Zong,J., Ciufo,D., Guo,H.G., Reitz,M.S. and Hayward,G.S. (1997) A single 13-kilobase divergent locus in the Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genome contains nine open reading frames that are homologous to or related to cellular proteins. J. Virol., 71, 1963–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer E. and Cresswell,P. (1998) Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol., 16, 323–358. [DOI] [PubMed] [Google Scholar]
- Parham P., Barnstable,C.J. and Bodmer,W.F. (1979) Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J. Immunol., 123, 342–349. [PubMed] [Google Scholar]
- Paulson E., Tran,C., Collins,K. and Fruh,K. (2001) KSHV-k5 inhibits phosphorylation of the major histocompatibility complex class I cytoplasmic tail. Virology, 288, 369–378. [DOI] [PubMed] [Google Scholar]
- Pickart C.M. (2001) Ubiquitin enters the new millennium. Mol. Cell, 8, 499–504. [DOI] [PubMed] [Google Scholar]
- Piguet V., Wan,L., Borel,C., Mangasarian,A., Demaurex,N., Thomas,G. and Trono,D. (2000) HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nature Cell Biol., 2, 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper R.C. and Luzio,J.P. (2001) Late endosomes: sorting and partitioning in multivesicular bodies. Traffic, 2, 612–621. [DOI] [PubMed] [Google Scholar]
- Reggiori F. and Pelham,H.R. (2001) Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J., 20, 5176–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch U., Muranyi,W., Lucin,P., Burgert,H.G., Hengel,H. and Koszinowski,U.H. (1999) A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J., 18, 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert U., Anton,L.C., Bacik,I., Cox,J.H., Bour,S., Bennink,J.R., Orlowski,M., Strebel,K. and Yewdell,J.W. (1998) CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. J. Virol., 72, 2280–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam N.J., Spits,H. and Ploegh,H.L. (1986) Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit bio chemical characterization of certain HLA-C locus products. J. Immunol., 137, 2299–2306. [PubMed] [Google Scholar]
- Stevenson P.G., Efstathiou,S., Doherty,P.C. and Lehner,P.J. (2000) Inhibition of MHC class I-restricted antigen presentation by γ2-herpesviruses. Proc. Natl Acad. Sci. USA, 97, 8455–8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella D., Gewurz,B.E., Furman,M.H., Schust,D.J. and Ploegh,H.L. (2000) Viral subversion of the immune system. Annu. Rev. Immunol., 18, 861–926. [DOI] [PubMed] [Google Scholar]
- Urbanowski J.L. and Piper,R.C. (2001) Ubiquitin sorts proteins into the intralumenal degradative compartment of the late-endosome/vacuole. Traffic, 2, 622–630. [DOI] [PubMed] [Google Scholar]
- van Endert P.M., Tampe,R., Meyer,T.H., Tisch,R., Bach,J.F. and McDevitt,H.O. (1994) A sequential model for peptide binding and transport by the transporters associated with antigen processing. Immunity, 1, 491–500. [DOI] [PubMed] [Google Scholar]
- Zheng N., Wang,P., Jeffrey,P.D. and Pavletich,N.P. (2000) Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell, 102, 533–539. [DOI] [PubMed] [Google Scholar]