Abstract
Progression through vertebrate oocyte maturation requires that pre-existing, maternally derived mRNAs be translated in a strict temporal order. The mechanism that controls the timing of oocyte mRNA translation is unknown. In this study we show that the early translational induction of the mRNA encoding the Mos proto-oncogene is mediated through a novel regulatory element within the 3′ untranslated region of the Mos mRNA. This novel element is responsive to the MAP kinase signaling pathway and is distinct from the late acting, cdc2-responsive, cytoplasmic polyadenylation element. Our findings suggest that the timing of maternal mRNA translation is controlled through signal transduction pathways targeting distinct 3′ UTR mRNA elements.
Keywords: MAP kinase/Mos/mRNA/oocyte/translation
Introduction
Regulated mRNA translation plays a key role in early developmental processes, particularly during periods when gene transcription is inactivated (Wickens et al., 2000). One such example is during oocyte maturation, where required changes in protein levels are mediated through the translational regulation of pre-existing, maternally derived mRNAs. The translational induction of maternal mRNAs has been correlated with an increase in mRNA poly(A) tail length. This evolutionarily conserved process, termed cytoplasmic polyadenylation, can be directed through a uridine-rich regulatory element in the 3′ untranslated region (3′ UTR) of the mRNA, designated the cytoplasmic polyadenylation element (CPE) (Richter, 1999). CPE sequences and the CPE-binding protein (CPEB) (Hake and Richter, 1994) appear to function both in translational repression of the stored mRNAs in immature oocytes and in facilitating mRNA cytoplasmic polyadenylation and translational induction in maturing oocytes (Fox et al., 1989; McGrew et al., 1989; McGrew and Richter, 1990; Paris and Richter, 1990; Sallès et al., 1992; Standart and Dale, 1993; Gebauer et al., 1994; Stebbins-Boaz et al., 1996; Stutz et al., 1998; de Moor and Richter, 1999; Minshall et al., 1999; Barkoff et al., 2000; Charlesworth et al., 2000; Tay et al., 2000).
In the frog Xenopus laevis, translational induction of the mRNA encoding the MAP kinase kinase kinase, Mos, occurs soon after exposure to the maturation stimulus progesterone and prior to oocyte germinal vesicle breakdown (GVBD). This early translation of the Mos mRNA is essential for progesterone-stimulated maturation (Sagata et al., 1988, 1989; Sheets et al., 1995). In contrast, translational induction of mRNAs encoding Wee1 and cyclin B1 have been shown to occur temporally later in maturation, around the time of GVBD (Kobayashi et al., 1991; Murakami and Vande Woude, 1998; Charlesworth et al., 2000). The translational induction of ‘late’ mRNAs requires the prior translational induction of the ‘early’ Mos mRNA (Ballantyne et al., 1997). The temporal order of Mos, Wee1 and cyclin B1 mRNA translation is necessary to ensure hormone-dependent progression through oocyte meiotic maturation (Freeman et al., 1991; Sheets et al., 1995; Murakami and Vande Woude, 1998; Howard et al., 1999; Nakajo et al., 2000). Since the Mos, Wee1 and cyclin B1 mRNA 3′ UTRs all contain CPEs, it is not clear how CPE sequences alone control the temporal order of mRNA translation.
The CPE-directed translational activation of the cyclin B1 mRNA has been shown to be regulated by maturation promoting factor (cdc2 kinase) signaling (Ballantyne et al., 1997; de Moor and Richter, 1997; Howard et al., 1999). In contrast to the cyclin B1 mRNA, while both the aurora kinase Eg2 and the cdc2 signaling pathways have been implicated in the control of Mos protein levels (Nebreda et al., 1995; Mendez et al., 2000; Castro et al., 2001), Mos mRNA translation can be stimulated by the MAP kinase pathway independently of cdc2 activity (Howard et al., 1999). A role for the CPE in the regulation of Mos mRNA translation has been suggested by the inhibitory effects of a dominant-negative CPEB protein upon Mos protein accumulation in response to progesterone stimulation (Mendez et al., 2000). However, the necessity for the Mos CPE in the initiation of Mos mRNA polyadenylation and translational activation has not been previously tested. In this study we sought to determine how MAP kinase signaling targets Mos mRNA translational activation. We identify a novel element within the Mos mRNA 3′ UTR, distinct from the CPE, which links MAP kinase signaling to the early progesterone-stimulated induction of Mos mRNA translation.
Results
Mos mRNA translation occurs in a CPEB-independent manner
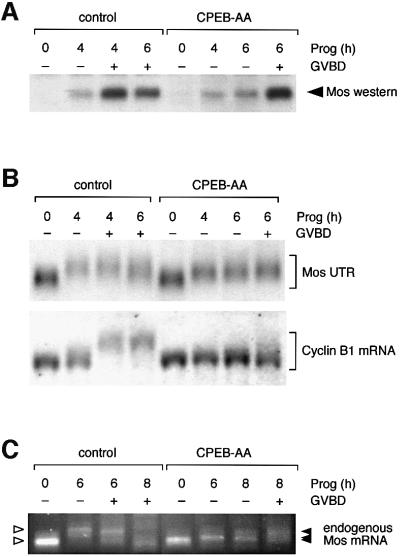
CPE and CPEB function have been implicated indirectly in the control of Mos mRNA translation (Mendez et al., 2000). However, this Eg2-dependent regulation of Mos protein accumulation did not explain the previously characterized stimulation of Mos mRNA cytoplasmic polyadenylation and translation by the MAP kinase signaling pathway (Howard et al., 1999). Consequently, we re-examined the requirement for Eg2 and CPEB function in the regulation of early Mos mRNA translational activation prior to GVBD. Using a dominant-negative form of Xenopus CPEB (CPEB-AA, encoding alanine substitutions at the sites of Eg2 phosphorylation), we observed no maturation in dominant-negative CPEB-expressing oocytes at the time when 50% of the water-injected oocytes had completed GVBD, consistent with previous findings (Mendez et al., 2000). However, the initial translation of Mos protein is not inhibited in these dominant-negative CPEB-expressing oocytes (Figure 1A), indicating that the early translational activation of the Mos mRNA can occur in a CPEB-independent manner.
Fig. 1. CPEB-independent control of Mos mRNA translational activation. (A) Western blot of endogenous Mos protein. Immature oocytes were injected with 3–6 ng RNA encoding Xenopus CPEB-AA or water as a negative control, and left for 16 h to express the protein. Oocytes were stimulated by addition of progesterone. Samples were taken for both control and CPEB-AA-treated oocytes when the control oocytes had reached GVBD50 (4 h) and GVBD100 (6 h). Control oocytes were segregated at GVBD50 based on whether they had (+) or had not (–) completed GVBD. Expression of CPEB-AA significantly delayed GVBD but did not prevent low-level Mos protein accumulation prior to GVBD. This effect on Mos accumulation was observed in four independent experiments. At later time points some CPEB-AA-expressing oocytes underwent GVBD. Segregation of these oocytes based on GVBD status revealed that oocytes escaping the CPEB-AA block to maturation then accumulated high levels of Mos protein equivalent to control, post-GVBD, oocytes. (B) Northern blot showing the differential effect of CPEB-AA on polyadenylation of Mos UTR and cyclin B1 mRNA. Total RNA was prepared from the same oocyte samples described in (A) and analyzed for the polyadenylation status of either the co-injected wild-type terminal 48-nt Mos 3′ UTR (Mos UTR) or the endogenous cyclin B1 mRNA. Polyadenylation (retarded mobility) of the Mos UTR was observed in CPEB-AA-expressing oocytes. This experiment was repeated three times with similar results. In contrast, cyclin B1 polyadenylation was inhibited in CPEB-AA-expressing oocytes. (C) Polyadenylation of endogenous Mos mRNA analyzed by RT–PCR. In this experiment GVBD50 was at 6 h and GVBD100 was at 8 h. Polyadenylation (retarded mobility) of the Mos mRNA was observed in CPEB-AA-expressing oocytes following progesterone stimulation. A representative experiment is shown.
In seven independent experiments, expression of CPEB-AA resulted in a delay of oocyte maturation (defined here as the time for the oocyte population to reach 50% GVBD) from an average of 4–5 h to an average of 7–8 h. Segregation of oocytes into those that either had or had not completed GVBD (GVBD+ or GVBD–, respectively) demonstrated that completion of GVBD is correlated with elevated Mos protein levels (Figure 1A). We also found that expression of the dominant-negative CPEB protein significantly delayed the accumulation of elevated Mos protein levels and oocyte GVBD (Figure 1A). It has been shown previously that Mos protein levels must reach a critical level for the commitment of oocytes to undergo GVBD and complete meiotic maturation (Chen and Cooper, 1997; Chen et al., 1997). We propose that the dominant-negative CPEB protein does not block the initial translation of the Mos mRNA as had been previously suggested (Mendez et al., 2000), but rather inhibits oocyte GBVD through the attenuation of Mos protein accumulation.
Since we observed translation of the Mos mRNA in CPEB-AA-expressing oocytes, we wished to determine whether this Mos mRNA translation was correlated with polyadenylation of the Mos 3′ UTR. We found that expression of CPEB-AA did not prevent the temporally early (4 h, no GVBD) Mos 3′ UTR polyadenylation (Figure 1B, upper panel). This suggests that CPEB-AA does not inhibit the cytoplasmic polyadenylation or translational activation of the Mos mRNA. By contrast, CPEB-AA expression did prevent the cytoplasmic polyadenylation of the endogenous CPE-regulated cyclin B1 mRNA (Figure 1B, lower panel).
To confirm that the Mos reporter UTR utilized in Figure 1B reflected the behavior of the endogenous Mos mRNA, RT–PCR was employed to assess in vivo polyadenylation status (Rassa et al., 2000). Figure 1C demonstrates that the endogenous Mos mRNA is polyadenylated in the presence of CPEB-AA, although the progesterone-stimulated poly(A) tail extension is shorter in oocytes expressing CPEB-AA when compared with control oocytes. The reduced length of the Mos mRNA poly(A) tail extension in CPEB-AA-expressing oocytes nonetheless appears sufficient to allow Mos mRNA translational activation (Figure 1A). A similar reduction in poly(A) tail extension in CPEB-AA-expressing oocytes was also observed with the Mos UTR reporter RNA (Figure 1B). We conclude that the endogenous Mos mRNA is subject to CPEB-independent polyadenylation and translational activation in progesterone-stimulated oocytes.
Mos mRNA cytoplasmic polyadenylation occurs in a CPE-independent manner
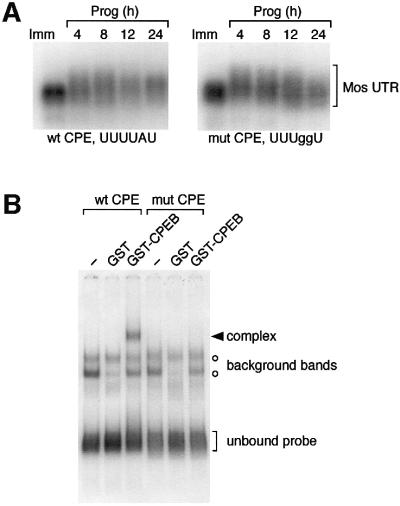
To address directly the requirement for the Mos CPE sequence in regulating Mos mRNA polyadenylation, the CPE sequence was disrupted by nucleotide substitution (UUUUAU to UUUggU) (McGrew and Richter, 1990; Stebbins-Boaz et al., 1996). Disruption of the Mos CPE prevented interaction of the Mos 3′ UTR with the Xenopus CPEB protein (Figure 2B). Consistent with Mos mRNA translation occurring in a CPEB-independent fashion (Figure 1), disruption of the CPE did not prevent progesterone-stimulated cytoplasmic polyadenylation of the Mos 3′ UTR (Figure 2A). This finding suggests that an element distinct from the CPE regulates the progesterone-stimulated cytoplasmic polyadenylation of the Mos mRNA. At later time points, deadenylation of the CPE-disrupted Mos UTR was consistently observed (Figure 2A), indicating that the CPE may play a role in maintaining the extended poly(A) tail in mature oocytes.
Fig. 2. CPE-independent polyadenylation of the Mos 3′ UTR. (A) Wild-type (Howard et al., 1999) and CPE-disrupted Mos UTRs correlating to the terminal 321 nt of the Mos mRNA 3′ UTR were injected into immature oocytes, and the ability of progesterone to induce polyadenylation (retarded mobility) was analyzed by northern blot. At the times indicated, pools of 10 oocytes were taken and RNA was extracted. Half of the oocytes had undergone GVBD after 4 h of culture. (B) Wild-type or mutant Mos UTR probes were analyzed for interaction with Xenopus CPEB by RNA electrophoretic mobility shift assay (EMSA). A specific CPEB binding complex was only observed with the wild-type Mos UTR probe.
A novel element controls the cytoplasmic polyadenylation of the Mos mRNA
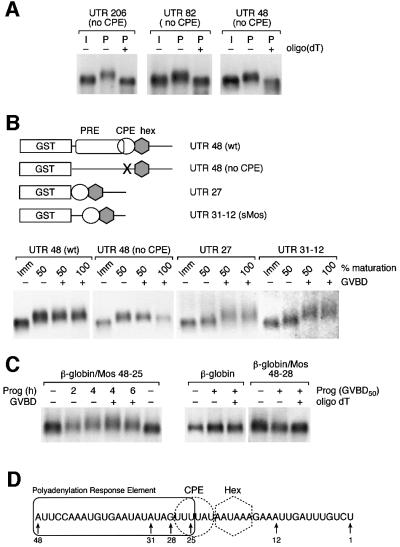
Deletion analysis of the Mos 3′ UTR revealed that the sequence within the region encompassing the terminal 48 nucleotides (nt) of the Mos UTR was sufficient to mediate the CPE-independent Mos mRNA cytoplasmic polyadenylation in response to progesterone (Figure 3A). This region conferred temporally early polyadenylation (prior to GVBD) to both wild-type and CPE-disrupted Mos terminal 48-nt UTR transcripts (Figure 3B). Further deletion of the reporter UTR to include only the terminal 27 nt of the wild-type Mos UTR (Figure 3B, UTR 27), which retains an intact CPE, resulted in dramatically attenuated polyadenylation prior to GVBD (Figure 3B). Significant polyadenylation of the UTR 27 RNA occurred after GVBD. Similar to the UTR27 reporter RNA, polyadenylation of a construct encoding nucleotides 31–12 of the wild-type Mos UTR, termed sMos (Stebbins-Boaz et al., 1996) was temporally late and occurred at GVBD, indicating that nucleotides 5′ of position 31 within the wild-type Mos UTR are important for temporally early polyadenylation in response to progesterone stimulation. We conclude that a novel polyadenylation response element (PRE), distinct from the previously characterized CPE sequence, includes sequence 5′ of the CPE and directs pre-GVBD Mos mRNA polyadenylation. CPE-directed polyadenylation, by contrast, occurs predominantly after GVBD.
Fig. 3. Identification of a novel Mos 3′ UTR regulatory element. (A) Truncation analysis of the Mos 3′UTR. Immature oocytes were injected with different lengths of the CPE-disrupted Mos 3′ UTR, and the ability of progesterone to induce polyadenylation (retarded mobility) was analyzed by northern blot. Total RNA was prepared from pools of oocytes when 50% of progesterone-stimulated oocytes had undergone GVBD. CPE-independent polyadenylation was retained within the last 48 nt of the Mos UTR. (B) Upper panel, schematic representation of the UTR constructs employed. The GST open reading frame (open box) was fused to the Mos 3′ UTR. The polyadenylation hexanucleotide (gray hexagon), CPE (open circle) and PRE (open rectangle) are also indicated. Mutational disruption of the CPE is indicated by an ‘X’. UTR 27 lacks Mos UTR sequence 5′ of the CPE, and sMos encompasses nucleotides 31–12 of the wild-type Mos UTR (Stebbins-Boaz et al., 1996). Lower panel, northern blot of wild-type and mutant Mos UTR-injected oocytes. When 50% of the oocytes had undergone GVBD, oocytes were segregated into pools that had either had a white spot at the animal pole (GVBD+) or had not (GVBD–), and total RNA was extracted. Total RNA was also prepared when all the oocytes had matured (100% GVBD) and from time-matched immature (Imm) oocytes. Wild-type 48 UTR and 48 UTR (no CPE) polyadenylation (retarded mobility) occurred prior to GVBD. CPE-directed polyadenylation of the Mos UTR 27 RNA and sMos RNA was observed predominantly after GVBD. (C) Northern blot of β-globin/Mos UTR chimeras. Total RNA was prepared from wild-type and mutant β-globin UTR-injected oocytes at the indicated times. Fifty percent of the oocytes had undergone GVBD (GVBD50) at 4 h. Where indicated, oligo(dT) and RNase H were used to remove any poly(A) tails from the UTR reporter constructs (oligo dT +). (D) Schematic representation of the relative positions of the polyadenylation response element (rectangle), CPE (circle) and polyadenylation hexanucleotide (dotted hexagon) sequences within the last 48 nt of the Mos UTR. The numbers indicate the position relative to the site of poly(A) addition (designated 1).
To define further the position of the PRE within the Mos 3′ UTR, we determined if nucleotides 48 to 28, immediately 5′ of the CPE sequence in the Mos 3′ UTR, were sufficient to mediate CPE-independent polyadenylation of the Mos mRNA prior to GVBD. These nucleotides were inserted into the β-globin 3′ UTR, which does not undergo cytoplasmic polyadenylation (Figure 3C) (Hyman and Wormington, 1988; Varnum and Wormington, 1990), at an equivalent position relative to the polyadenylation hexanucleotide sequence as found in the Mos 3′ UTR. This 21-nt region, which lacks a functional CPE sequence, did not direct progesterone-dependent cytoplasmic polyadenylation (Figure 3C, β-globin/Mos 48–28). However, inclusion of the first three 5′ uridines of the CPE (nucleotides 27–25), which precede the diguanosine nucleotide substitution mutations in the CPE-disrupted Mos UTR, was sufficient to confer progesterone- dependent cytoplasmic polyadenylation to the chimeric β-globin/Mos UTR prior to GVBD (Figure 3C, β-globin/Mos 48–25). We conclude that the PRE sequence is present within the 24-nt region spanning nucleotides 48–25 of the Mos 3′ UTR and partially overlaps the CPE sequence (Figure 3D).
The PRE directs early translational induction in response to progesterone stimulation
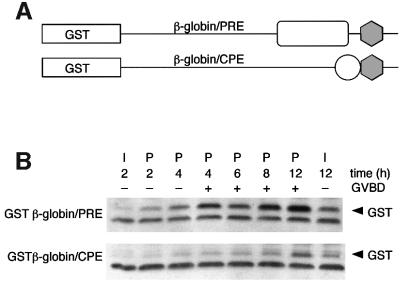
We utilized a glutathione S-transferase (GST) reporter RNA assay (Charlesworth et al., 2000) to confirm that the PRE directs early translation of the Mos mRNA. The Mos PRE sequence was inserted into the β-globin 3′ UTR (at equivalent positions relative to the polyadenylation hexanucleotide as found in the Mos 3′ UTR) fused downstream of the GST coding region (Figure 4A). Injection of oocytes with the Mos PRE-containing GST reporter RNA resulted in significant progesterone-stimulated accumulation of GST protein prior to GVBD (Figure 4B). In contrast, injection of a Mos CPE-containing GST reporter RNA directed GST translation at later times during maturation, but gave rise to very little translation prior to GVBD (Figure 4B). These results correlate with the timing of PRE- and CPE-directed Mos 3′ UTR cytoplasmic polyadenylation (Figure 3). Deletion of the six 5′ nucleotides of the PRE (nucleotides 48–43) ablated PRE-directed translational activation (data not shown), indicating that the PRE may encompass the entire 24 nt region indicated in Figure 3D (nucleotides 48–25 of the Mos 3′ UTR).
Fig. 4. The PRE directs temporally early mRNA translation in response to progesterone. (A) Schematic representation of the GST reporter RNA constructs. The relative positions of the polyadenylation hexanucleotide (gray hexagon), CPE (open circle) and PRE (open rectangle) are indicated. (B) GST western blot of protein lysates prepared from oocytes injected with equivalent amounts of GST β-globin/PRE or GST β-globin/CPE reporter RNAs.
The PRE sequence is responsive to MAP kinase signaling
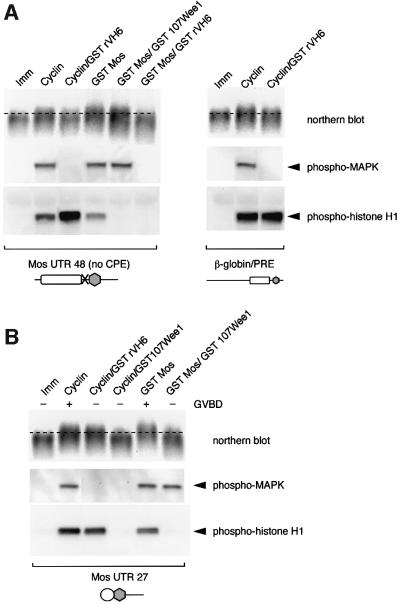
Xenopus p42 MAP kinase activation has been shown to be an early response to progesterone stimulation (Fisher et al., 1999, 2000). We have previously shown that inhibition of MAP kinase signaling attenuates Mos mRNA polyadenylation and translation in response to progesterone stimulation (Howard et al., 1999). We wished to determine if the PRE was a target of MAP kinase signaling. We found that activation of the MAP kinase pathway, through the injection of exogenous Mos RNA (encoding a GST Mos fusion protein) into immature oocytes resulted in polyadenylation of the Mos UTR reporter RNA containing the PRE [Figure 5A, left panel, Mos UTR 48 (no CPE)]. Similar to the effect on the wild-type Mos UTR (Howard et al., 1999), the GST Mos-induced polyadenylation of the PRE-containing (CPE-disrupted) Mos UTR was ablated with the MAP kinase phosphatase rVH6, but was not inhibited by the cdc2 inhibitor Wee1 (Figure 5A, left panel). Activation of cdc2 by injection of cyclin B1 protein (Solomon et al., 1990; Freeman et al., 1991) induced polyadenylation of the PRE containing (CPE-disrupted) Mos UTR, but this PRE-mediated polyadenylation was inhibited by co-expression of rVH6 (Figure 5A, left panel). This result indicates that cdc2-induced PRE polyadenylation requires activation of MAP kinase signaling. The ability of active cdc2 to subsequently stimulate the MAP kinase pathway via an indirect feedback mechanism has been reported previously (Gotoh et al., 1991). To verify that it was the PRE and not other sequences within the last 48 nt of the Mos 3′ UTR that was MAP kinase responsive, the β-globin/PRE construct was utilized (Figure 5A, right panel). Similar to the Mos UTR 48 (no CPE), cyclin-stimulated polyadenylation of the β-globin/PRE UTR was attenuated by rVH6 co-expression. By contrast, a Mos UTR retaining the CPE, but not the PRE, undergoes cdc2-stimulated cytoplasmic polyadenylation that is not inhibited by rVH6 (Figure 5B, Mos UTR 27), indicating that the CPE is not a direct target of MAP kinase signaling. Furthermore, MAP kinase-stimulated polyadenylation of Mos UTR 27 is inhibited by Wee1 co-expression, demonstrating that the CPE-mediated polyadenylation requires cdc2 activity (Figure 5B, GST Mos/GST 107Wee1). Taken together, our data indicate that the PRE is the target of MAP kinase signaling while the CPE is the target of cdc2-dependent signaling.
Fig. 5. The polyadenylation response element (PRE) is the target of MAP kinase signaling. Immature oocytes were injected with RNA encoding GST107Wee1 to inhibit cdc2 activity or GSTrVH6 to inhibit MAP kinase activity, and left overnight to express the protein (Howard et al., 1999). Oocytes were then injected with RNA specifying PRE- or CPE-containing Mos UTRs (A and B, respectively) or a PRE-containing β-globin UTR (A, right panel), and left untreated (Imm) or co- injected with RNA encoding GST Mos to stimulate MAP kinase signaling, or cyclin B1 protein to activate cdc2 signaling (Howard et al., 1999). Pools of oocytes injected with the PRE-containing construct were prepared when 50% of the stimulated oocytes had undergone GVBD. Because PRE-mediated polyadenylation is temporally early, only oocytes that had not undergone GVBD were selected. Pools of oocytes injected with the CPE-containing construct were prepared when 100% of the stimulated oocytes had undergone GVBD, since CPE-mediated polyadenylation occurs predominantly after GVBD. Total RNA was prepared and protein lysates were taken from these pools. The polyadenylation status of the injected Mos UTRs was assessed by northern blot analyses (retarded mobility is indicative of polyadenylation). MAP kinase activity was assessed by western blot using phosphoMAP kinase antibodies, and cdc2 activity was assessed by western blot with phosphohistone H1 antibodies following an in vitro kinase assay (see Materials and methods) from the same samples utilized for northern blot analyses. The presence of the CPE sequence is represented schematically by an open circle, the PRE by a rectangle and the polyadenylation hexanucleotide by a gray hexagon.
Polyadenylation of the Mos mRNA correlates with progesterone-stimulated MAP kinase activation
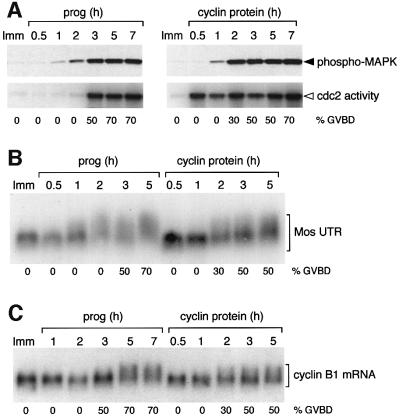
Previous studies have examined the signaling dependency of maternal mRNA translation using ectopic MAP kinase and cdc2 activators. To address the role of signaling pathways in regulating the timing of progesterone-stimulated Mos mRNA translational activation in vivo, we determined the timing of Mos and cyclin B1 cytoplasmic polyadenylation relative to the activation of the MAP kinase and cdc2 pathways. In progesterone-stimulated oocytes, MAP kinase was activated prior to cdc2 activation and before GVBD (Figure 6A, left panel). Polyadenylation of the Mos UTR coincided with MAP kinase activation (Figure 6B). In contrast, polyadenylation of the cyclin B1 mRNA occurred after cdc2 activation and after GVBD (Figure 6C), consistent with previous reports demonstrating CPE- and cdc2-dependent polyadenylation of cyclin B1 mRNA (Ballantyne et al., 1997; de Moor and Richter, 1997; Howard et al., 1999).
Fig. 6. Mos polyadenylation temporally correlates with MAP kinase activation. Oocytes were injected with the terminal 321 nt Mos UTR reporter RNA and then left either untreated (Imm) or stimulated to mature (prog or cyclin protein) as indicated. Maturation kinetics of each oocyte population is indicated (% GVBD). Protein lysates and RNA extraction were prepared from pools of oocytes at the indicated times. Immature (Imm) oocyte samples were taken after 4 h. (A) Time course of progesterone- (prog) or cyclin-stimulated MAP kinase activation (upper panel), and cdc2 activation (lower panel). The MAP kinase activity in the sample was assessed by western blot using phosphoMAP kinase antibodies and cdc2 activity was measured by radiolabel incorporation into histone H1 (see Materials and methods). Samples were also analyzed by northern blot for polyadenylation (retarded mobility) of the 321-nt wild-type Mos UTR (B) and for endogenous cyclin B1 mRNA polyadenylation (C). While cyclin B1 injection advanced cdc2 activation and cyclin B1 mRNA polyadenylation, the injected oocytes reached 50% GVBD at the same rate as progesterone-treated oocytes (3 h).
To determine if these correlations were causal, we reversed the relative order of MAP kinase and cdc2 activation by injecting recombinant cyclin B protein into immature oocytes in the absence of progesterone. Utilizing this technique, robust cdc2 activity is observed before MAP kinase activity (Figure 6A, right panel). The timing of polyadenylation of endogenous cyclin B1 mRNA was advanced in oocytes that were injected with cyclin B protein when compared with progesterone-treated oocytes (Figure 6C). By contrast, the onset of Mos UTR polyadenylation was delayed in cyclin B protein-injected oocytes compared with progesterone-treated oocytes (Figure 6B). These results demonstrate that initiation of Mos mRNA polyadenylation correlates with activation of the MAP kinase, but not the cdc2 pathway, while cyclin B1 mRNA polyadenylation occurs subsequent to the activation of the cdc2 pathway during progesterone-stimulated Xenopus oocyte maturation.
Discussion
Mos protein levels must reach a critical threshold within progesterone-stimulated oocytes for the commitment to transition through GVBD and complete meiotic maturation (Chen and Cooper, 1997; Chen et al., 1997). Mos protein levels are controlled at the level of Mos mRNA translation (Sagata et al., 1988, 1989; Sheets et al., 1995). It has hitherto been assumed that the translational activation of the Mos mRNA is regulated through the CPE sequence in the Mos mRNA 3′ UTR (Sheets et al., 1995; Mendez et al., 2000), although this had not been tested directly. In the present study, we report that the initial translational activation of the Mos mRNA occurs in a CPE- and CPEB- independent manner. The initial translational activation of the Mos mRNA is mediated by a novel regulatory element, the PRE, which resides within nucleotides 48–25 [relative to the site of poly(A) addition] of the Mos mRNA 3′ UTR. Our data demonstrate that while the Mos PRE and CPE sequences are partially overlapping within the Mos 3′ UTR, the two sequences are functionally separable. Progesterone-stimulated, PRE- directed cytoplasmic polyadenylation and translational induction temporally precede CPE-directed cytoplasmic polyadenylation and translational induction (Figures 3 and 4). We report here that the PRE is the target of MAP kinase signaling, while CPE-directed cytoplasmic polyadenylation is stimulated by cdc2 activity (Figure 5). Since progesterone-stimulated MAP kinase pathway activation is temporally distinct from cdc2 activation (Figure 6), our results establish a mechanism by which the timing of PRE-dependent and CPE-dependent mRNA translation can be differentially regulated in response to hormonal stimulation.
Several lines of evidence have implicated the CPE sequence in the translation of the Mos mRNA. Consistent with earlier work, we found that expression of a dominant-negative CPEB-AA protein attenuates high level Mos protein accumulation and oocyte cell cycle progression. However, we found that CPEB-AA does not block the initial polyadenylation and translation of the Mos mRNA in response to progesterone stimulation (Figure 1). We propose that the apparent discrepancy between our findings and those of a prior report (Mendez et al., 2000) is due to variation in both the time of sample preparation and the Mos UTR reporter constructs utilized in the two studies. We examined Mos protein accumulation at early times following progesterone stimulation (prior to GVBD). Since Mos protein is unstable prior to cdc2 activation and GVBD (Nebreda et al., 1995; Castro et al., 2001), degradation of Mos may have precluded detection at the time point analyzed by Mendez et al. Moreover, the sMos UTR reporter used in the prior study lacked the PRE sequence and so was not capable of undergoing the temporally early CPE- and CPEB-independent polyadenylation we observed with the PRE-containing, endogenous Mos mRNA and wild-type Mos UTR reporter RNAs (Figures 1 and 3B). A role for CPE-directed Mos mRNA translational activation has also been inferred from experiments utilizing antisense oligonucleotide-directed truncation of endogenous Xenopus Mos mRNA with subsequent prosthetic Mos 3′ UTR RNA rescue (Sheets et al., 1995). However, while elegantly demonstrating a requirement for Mos mRNA polyadenylation in vivo, the prosthetic rescue construct (containing the terminal 83 nt of the Mos 3′ UTR) did not distinguish between the PRE and the CPE in mediating the early cytoplasmic polyadenylation and translational activation of the Mos mRNA.
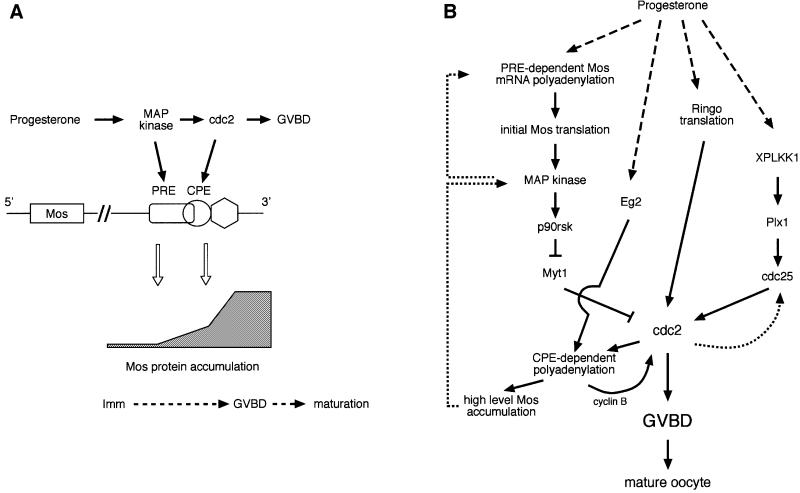
The findings reported in this study suggest a molecular explanation for the biphasic accumulation of Mos protein observed following progesterone stimulation (Gotoh et al., 1995). We propose that progesterone stimulates temporally early, PRE-mediated Mos mRNA translational activation via MAP kinase signaling (Figure 7A). Since this first phase can occur in the presence of CPEB-AA (Figure 1A), we conclude that it is independent of CPEB and the Eg2 signaling pathway. Subsequently, and prior to GVBD, Eg2-dependent regulation of CPEB, cdc2 activation and CPE-mediated mRNA translation are required to enhance Mos protein accumulation to levels necessary for GVBD and to commit oocytes to the all-or-none transition through meiotic maturation (Figure 7A). Our findings indicate that in the absence of CPEB-dependent mRNA translation, PRE-mediated translational activation of the Mos mRNA is not sufficient to elevate Mos protein to levels necessary to progress through meiotic maturation. This may be a consequence of the transient nature of PRE-mediated cytoplasmic polyadenylation in the absence of a functional CPE sequence (Figures 2 and 3). The PRE and CPE sequences may thus function sequentially to initiate and maintain Mos protein accumulation in vivo in response to progesterone stimulation. The model shown in Figure 7A depicts cdc2 targeting the Mos CPE sequence later in maturation. We do not, however, rule out a contribution from other CPE-regulated maternal mRNAs in the enhanced, CPEB-dependent accumulation of Mos protein just prior to GVBD. Such CPE-containing mRNAs may encode activators of cdc2, since cdc2 activity has been demonstrated to be required for stable Mos protein accumulation (Nebreda et al., 1995; Castro et al., 2001).
Fig. 7. (A) Sequential model of Mos mRNA translational control in response to progesterone stimulation. In this model, PRE-directed Mos mRNA translational activation precedes CPE-directed translation as a consequence of progesterone-stimulated MAP kinase activation preceding cdc2 activation. Solid arrows denote causal relationships between processes. (B) Signal transduction pathways that converge upon cdc2 activation and GVBD in progesterone-stimulated Xenopus oocytes. Positive feedback loops are indicated by dotted lines. See text for details.
Progesterone-stimulated translational activation of the Mos mRNA leads to the establishment of a Mos/MAP kinase positive feedback loop (Figure 7B, dotted line) that is important for progression through meiosis (Matten et al., 1996; Roy et al., 1996; Howard et al., 1999). Based on the data presented in this study, we extend these previous findings and demonstrate that the ability of MAP kinase to induce Mos mRNA translation is mediated by PRE-directed cytoplasmic polyadenylation. Within the context of this feedback loop, MAP kinase activity is both upstream and downstream of Mos mRNA translation. MAP kinase activation also leads to cdc2 activation, through p90 Rsk-mediated inactivation of the cdc2 inhibitory kinase Myt1 (Palmer et al., 1998). While our data indicate that the PRE is the early temporal determinant of Mos mRNA cytoplasmic polyadenylation and translational activation, it remains to be determined if MAP kinase mediates the initial progesterone-stimulated induction of Mos mRNA translation. While MAP kinase activation was reported to occur prior to Mos protein accumulation (Fisher et al., 2000), this temporally early MAP kinase activity still required the translation of unidentified maternal mRNAs. It is possible that a novel progesterone-stimulated signaling pathway initially targets the Mos PRE sequence. Further work will be required to elucidate the signaling pathway that triggers the initial translation of the Mos mRNA prior to establishment of the Mos/MAP kinase feedback loop.
In addition to the Mos/MAP kinase pathway, additional signaling pathways have been implicated in cdc2 activation (Figure 7B). These pathways include: (i) the aurora kinase family member Eg2, which has been implicated in the activation of CPE/CPEB-mediated maternal mRNA translation (Mendez et al., 2000); (ii) Ringo (aka Speedy), a cyclin-independent activator of cdc2 (Ferby et al., 1999; Lenormand et al., 1999); and (iii) the polo-like protein kinase signaling pathway (PLKK1 and Plx1), which leads to activation of the pre-MPF cdc2 pool through cdc25-dependent dephosphorylation of cdc2 (Kumagai and Dunphy, 1996; Abrieu et al., 1998; Qian et al., 1998a,b; Karaiskou et al., 1999). It appears that these pathways may cooperate with the Mos/MAP kinase pathway since inhibition of any one pathway delays or inhibits progesterone-stimulated oocyte maturation.
Based on the findings of this study, we conclude that the early temporal activation of MAP kinase signaling targets PRE-directed mRNA translation, while subsequent cdc2 activation results in later CPE-directed mRNA translation and thereby establishes a mechanism to control the temporal order of maternal mRNA translational recruitment. A search of the 3′ UTR database (Pesole et al., 2002) has revealed that additional Xenopus maternal mRNAs contain PRE-related sequences including FGF receptor 1, DNA topoisomerase 1, Ringo and cyclin B3. Like the Mos mRNA, the FGF receptor 1 and Ringo mRNAs have been shown to be translationally activated early, prior to GVBD, in response to progesterone stimulation (Culp and Musci, 1998; Ferby et al., 1999). Thus, PRE sequences may present a common mechanism through which to induce the early translational activation of maternal mRNAs during progesterone-stimulated oocyte maturation.
Materials and methods
Plasmid constructions and RNA synthesis
Standard PCR mutagenesis was employed to introduce disruptive nucleotide substitutions into the Mos CPE sequence (TTTTAT to TTTggT) within the terminal 321 bp of the Mos 3′ UTR (Howard et al., 1999). A series of truncations in the CPE-disrupted Mos 321 UTR were generated by inserting a 5′ XhoI site by standard PCR mutagenesis to make UTRs containing the last 206, 82 and 48 nt. CPE-independent polyadenylation was retained in the last 48 nt. The Mos UTR 27 construct encompasses nucleotides 27 to 1 of the wild-type Mos UTR sequence [where 1 is designated as the site of poly(A) addition]. The sMos construct (Stebbins-Boaz et al., 1996) encompasses nucleotides 31–12 of the wild-type Mos UTR sequence (CPE, polyadenylation hexanucleotide and four flanking nucleotides to side) and was generated using PCR primers incorporating a 5′ XbaI site and a 3′ PstI site. The Mos PRE and CPE sequences were introduced into the β-globin 3′ UTR by PCR mutagenesis. The Mos and β-globin UTR constructs were subcloned downstream of the GST coding region in the pGEM GST vector (Charlesworth et al., 2000). For in vitro transcription, all Mos UTR plasmids were linearized with XbaI, except the sMos UTR plasmid which was linearized with PstI. The sequence integrity of all mutant UTR constructs was confirmed by DNA sequencing. The construction of the GST–Xenopus CPEB expression plasmid as well as Mos UTR wild-type and CPE-disrupted EMSA probe plasmids has been described elsewhere (Welk et al., 2001). The dominant-negative Xenopus CPEB was constructed using PCR mutagenesis with S174A to S180A mutations essentially as described previously (Mendez et al., 2000). All constructs were transcribed with SP6 RNA Polymerase (Promega), as described previously (Melton et al., 1984). The EMSA probes were synthesized without a 5′ diGTP cap. The GST Mos, GST 107Wee1 and GST rVH6 expression constructs have been described previously (Howard et al., 1999).
Oocyte cultures and injections
Dumont stage VI immature oocytes were isolated as described previously (Charlesworth et al., 2000) and polyadenylation was analyzed following injection with 0.5–1 ng of reporter RNA. To reveal progesterone-inducible translation, GST reporter RNAs were injected at 0.05–0.1 ng of RNA per oocyte in order to reduce the background level of translation (Charlesworth et al., 2000). Oocytes were stimulated with 2 µg/ml progesterone (Sigma) and the rate of GVBD was monitored morphologically by the appearance of a white spot on the animal hemisphere. Except where indicated, pools of oocytes were harvested in proportion to the percentage GVBD in each sample, and immature control samples were prepared at the same time as the progesterone-stimulated oocyte samples. Recombinant human cyclin B1 protein (lacking the D-box) was expressed and purified as described previously (Kumagai and Dunphy, 1995). Results shown are representative experiments that were typically repeated three times with similar results.
Western blot analyses
Preparation of protein lysates and western blot analyses were performed as previously described (Howard et al., 1999). Rabbit polyclonal antibody against GST (Z-5) and Xenopus c-Mos (C237) were obtained from Santa Cruz Biotechnology, Inc. MAPK activation was visualized with an antibody specific for the activated, phosphorylated form of the enzyme obtained from New England Biolabs. For analysis of both protein and RNA from the same oocyte samples, pools of oocytes were rapidly lysed in Nonidet P-40 buffer as described above; a portion was then removed and mixed immediately with RNA STAT-60 (see below).
Cdc2 kinase assays
To assay the activity of cdc2 specifically, cyclin-dependent kinase complexes were affinity purified using GST–p13suc1 beads (Upstate Biotech Inc., Lake Placid, NY) prior to the kinase assay as described previously (Howard et al., 1999). The beads were then incubated with 30 µl of histone H1 and 20 µCi of [γ-32P]ATP. Samples were resolved by SDS–PAGE on 12% polyacrylamide gels (Novex), and the phosphoproteins were visualized by autoradiography. Alternatively, the beads were incubated in 30 µl containing 50 µM histone H1 and 66 µM ATP. Samples were then resolved by SDS–PAGE on 12% polyacrylamide gels (Novex), and the phosphorylation status of the histone H1 protein substrate determined by western blotting with phosphohistone H1 antibodies (06-597; Upstate Biotech Inc.).
Polyadenylation assay using northern blot analyses
Total RNA was purified from oocytes using RNA STAT-60 (Tel-Test B), and the polyadenylation status of injected reporter RNAs and endogenous cyclin B1 mRNA was assayed by northern blot as described previously (Howard et al., 1999). Northern blots were hybridized with a GST-specific probe, a Mos UTR probe or a Xenopus cyclin B1 probe as indicated, and visualized using the AlkPhos Direct system with CDP-Star™ chemiluminescence (Amersham Life Science). Retarded mRNA mobility is indicative of 3′ UTR polyadenylation. To confirm that any increase in mRNA size was specifically due to polyadenylation, RNA samples were treated with RNase H and oligo(dT) to eliminate any poly(A) tail prior to gel analysis. RNase H reactions were performed as described previously (Howard et al., 1999)
Polyadenylation assay using RT–PCR
To assay for endogenous Mos mRNA polyadenylation, the PolyA Assay (Rassa et al., 2000) was utilized. The assay was performed essentially as described by Rassa et al. (2000) with the following modifications. Fifty picomoles of oligonucleotide P1 (Rassa et al., 2000) were ligated to 600 ng of total RNA using T4 RNA ligase (New England Biolabs). The ligase was then heat inactivated at 65°C for 15 min. Reverse transcription was performed in a 50 µl reaction with 50 pmol P1′ (Rassa et al., 2000) using Superscript II (Invitrogen), following the manufacturer’s instructions. After inactivation of Superscript II, 2 U of RNase H (Promega) were added for 30 min at 37°C. The cDNA preparation (2 µl) and a gene-specific primer that anneals to nucleotides –73 to –50 of the Mos 3′ UTR (5′-GTTGCATTGCTGTTTAAGTGGTAA) was used in a 50 µl Pfu (Stratagene) PCR containing a total of 3 mM Mg2+. The PCR consisted of 30 cycles under the following conditions: 94°C for 30 s, 56°C for 1 min, 72°C for 1 min. For each condition, two reactions were performed that were pooled and purified using the PCR purification protocol from QIAquick (Qiagen). PCR products were resolved on a 2% agarose (Invitrogen) gel and visualized using ethidium bromide staining. The size of the Mos UTR PCR product in immature control or CPEB-AA-expressing oocytes was 125 bp (Figure 1C, lower open arrowhead, which represents 73 nt of amplified 3′ UTR prior to the site of poly(A) addition, 24 nt of the 3′ PCR anchor primer P1, plus the poly(A) tail which appears to be ∼28 nt in immature oocytes). In progesterone-stimulated control oocytes, the Mos PCR product increased to 156 nt in a distinct subpopulation of the mRNAs (Figure 1C, upper open arrowhead, representing an increase in the size of the Mos mRNA poly(A) from 28 nt to ∼60 nt). In progesterone-stimulated CPEB-AA-expressing oocytes, several populations of polyadenylated Mos mRNAs were observed (Figure 1C, closed arrowheads), representing Mos mRNAs with mean poly(A) tails of 35 and 50 nt.
Electrophoretic mobility gel shift assay
Protein for gel shift assays was prepared by coupled transcription/translation in a rabbit reticulocyte lysate system and RNA binding activity determined as described previously (Charlesworth et al., 2000; Welk et al., 2001).
Acknowledgments
Acknowledgements
We thank Paul Mueller for the human cyclin B1 baculovirus expression vector. This work was supported by grants from the National Institutes of Health (No. HD35688) and the Arkansas Biosciences Institute to A.M.M., and National Research Service Award Predoctoral Fellowships to J.A.R. and L.A.K.
References
- Abrieu A., Brassac,T., Galas,S., Fisher,D., Labbe,J.C. and Doree,M. (1998) The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci., 111, 1751–1757. [DOI] [PubMed] [Google Scholar]
- Ballantyne S., Daniel,D.L.J. and Wickens,M. (1997) A dependent pathway of cytoplasmic polyadenylation reactions linked to cell cycle control by c-mos and CDK1 activation. Mol. Biol. Cell, 8, 1633–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoff A.F., Dickson,K.S., Gray,N.K. and Wickens,M. (2000) Translational control of cyclin B1 mRNA during meiotic maturation: coordinated repression and cytoplasmic polyadenylation. Dev. Biol., 220, 97–109. [DOI] [PubMed] [Google Scholar]
- Castro A., Peter,M., Magnaghi-Jaulin,L., Vigneron,S., Galas,S., Lorca,T. and Labbe,J.C. (2001) Cyclin B/cdc2 induces c-Mos stability by direct phosphorylation in Xenopus oocytes. Mol. Biol. Cell, 12, 2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth A., Welk,J. and MacNicol,A. (2000) The temporal control of Wee1 mRNA translation during Xenopus oocyte maturation is regulated by cytoplasmic polyadenylation elements within the 3′ untranslated region. Dev. Biol., 227, 706–719. [DOI] [PubMed] [Google Scholar]
- Chen M. and Cooper,J.A. (1997) The β subunit of CKII negatively regulates Xenopus oocyte maturation. Proc. Natl Acad. Sci. USA, 94, 9136–9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Li,D., Krebs,E.G. and Cooper,J.A. (1997) The casein kinase II β subunit binds to Mos and inhibits Mos activity. Mol. Cell. Biol., 17, 1904–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp P.A. and Musci,T.J. (1998) Translational activation and cytoplasmic polyadenylation of FGF receptor-1 are independently regulated during Xenopus oocyte maturation. Dev. Biol., 193, 63–76. [DOI] [PubMed] [Google Scholar]
- de Moor C.H. and Richter,J.D. (1997) The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes. Mol. Cell. Biol., 17, 6419–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moor C.H. and Richter,J.D. (1999) Cytoplasmic polyadenylation elements mediate masking and unmasking of cyclin B1 mRNA. EMBO J., 18, 2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferby I., Blazquez,M., Palmer,A., Eritja,R. and Nebreda,A.R. (1999) A novel p34cdc2-binding and activating protein that is necessary and sufficient to trigger G2/M progression in Xenopus oocytes. Genes Dev., 13, 2177–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D.L., Brassac,T., Galas,S. and Doree,M. (1999) Dissociation of MAP kinase activation and MPF activation in hormone-stimulated maturation of Xenopus oocytes. Development, 126, 4537–4546. [DOI] [PubMed] [Google Scholar]
- Fisher D.L., Mandart,E. and Doree,M. (2000) Hsp90 is required for c-Mos activation and biphasic MAP kinase activation in Xenopus oocytes. EMBO J., 19, 1516–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C.A., Sheets,M.D. and Wickens,M.P. (1989) Poly(A) addition during maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev., 3, 2151–2162. [DOI] [PubMed] [Google Scholar]
- Freeman R.S., Ballantyne,S.M. and Donoghue,D.J. (1991) Meiotic induction by Xenopus cyclin B is accelerated by coexpression with mosXe. Mol. Cell. Biol., 11, 1713–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F., Xu,W., Cooper,G.M. and Richter,J.D. (1994) Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J., 13, 5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y. et al. (1991) Xenopus M phase MAP kinase: isolation of its cDNA and activation by MPF. EMBO J., 10, 2661–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Masuyama,N., Dell,K., Shirakabe,K. and Nishida,E. (1995) Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein cascade. J. Biol. Chem., 270, 25898–25904. [DOI] [PubMed] [Google Scholar]
- Hake L.E. and Richter,J.D. (1994) CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell, 79, 617–627. [DOI] [PubMed] [Google Scholar]
- Howard E.L., Charlesworth,A., Welk,J. and MacNicol,A.M. (1999) The mitogen-activated protein kinase signaling pathway stimulates mos mRNA cytoplasmic polyadenylation during Xenopus oocyte maturation. Mol. Cell. Biol., 19, 1990–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman L.E. and Wormington,W.M. (1988) Translational inactivation of ribosomal protein mRNAs during Xenopus oocyte maturation. Genes Dev., 2, 598–605. [DOI] [PubMed] [Google Scholar]
- Karaiskou A., Jessus,C., Brassac,T. and Ozon,R. (1999) Phosphatase 2A and polo kinase, two antagonistic regulators of cdc25 activation and MPF auto-amplification. J. Cell Sci., 112, 3747–3756. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Minshull,J., Ford,C., Golsteyn,R., Poon,R. and Hunt,T. (1991) On the synthesis and destruction of A- and B-type cyclins during oogenesis and meiotic maturation in Xenopus laevis. J. Cell Biol., 114, 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy,W.G. (1995) Control of the Cdc2/cyclin B complex in Xenopus egg extracts arrested at a G2/M checkpoint with DNA synthesis inhibitors. Mol. Biol. Cell, 6, 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy,W.G. (1996) Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science, 273, 1377–1380. [DOI] [PubMed] [Google Scholar]
- Lenormand J.L., Dellinger,R.W., Knudsen,K.E., Subramani,S. and Donoghue,D.J. (1999) Speedy: a novel cell cycle regulator of the G2/M transition. EMBO J., 18, 1869–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matten W.T., Copeland,T.D., Ahn,N.G. and Van de Woude,G.F. (1996) Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev. Biol., 179, 485–492. [DOI] [PubMed] [Google Scholar]
- McGrew L.L. and Richter,J.D. (1990) Translational control by cytoplasmic polyadenylation during Xenopus oocyte maturation: characterization of cis and trans elements and regulation by cyclin/MPF. EMBO J., 9, 3743–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew L.L., Dworkin-Rastl,E., Dworkin,M.B. and Richter,J.D. (1989) Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev., 3, 803–815. [DOI] [PubMed] [Google Scholar]
- Melton D.A., Krieg,P.A., Rebagliati,M.R., Maniatis,T., Zinn,K. and Green,M.R. (1984) Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res., 12, 7035–7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez R., Hake,L.E. andresson,T., Littlepage,L.E., Ruderman,J.V. and Richter,J.D. (2000) Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature, 404, 302–307. [DOI] [PubMed] [Google Scholar]
- Minshall N., Walker,J., Dale,M. and Standart,N. (1999) Dual roles of p82, the clam CPEB homolog, in cytoplasmic polyadenylation and translational masking. RNA, 5, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M.S. and Vande Woude,G.F. (1998) Analysis of the early embryonic cell cycles of Xenopus; regulation of cell cycle length by Xe-wee1 and Mos. Development, 125, 237–248. [DOI] [PubMed] [Google Scholar]
- Nakajo N., Yoshitome,S., Iwashita,J., Iida,M., Uto,K., Ueno,S., Okamoto,K. and Sagata,N. (2000) Absence of wee1 ensures the meiotic cell cycle in Xenopus oocytes. Genes Dev., 14, 328–338. [PMC free article] [PubMed] [Google Scholar]
- Nebreda A.R., Gannon,J.V. and Hunt,T. (1995) Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes. EMBO J., 14, 5597–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A., Gavin,A.C. and Nebreda,A.R. (1998) A link between MAP kinase and p34cdc2/cyclin B during oocyte maturation: p90rsk phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J., 17, 5037–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J. and Richter,J.D. (1990) Maturation-specific polyadenylation and translational control: diversity of cytoplasmic polyadenylation elements, influence of poly(A) tail size and formation of stable polyadenylation complexes. Mol. Cell. Biol., 10, 5634–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesole G., Liuni,S., Grillo,G., Licciulli,F., Mignone,F., Gissi,C. and Saccone,C. (2002) UTRdb and UTRsite: specialized databases of sequences and functional elements of 5′ and 3′ untranslated regions of eukaryotic mRNAs. Update 2002. Nucleic Acids Res., 30, 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y.W., Erikson,E., Li,C. and Maller,J.L. (1998a) Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol. Cell. Biol., 18, 4262–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y.W., Erikson,E. and Maller,J.L. (1998b) Purification and cloning of a protein kinase that phosphorylates and activates the polo-like kinase Plx1. Science, 282, 1701–1704. [DOI] [PubMed] [Google Scholar]
- Rassa J.C., Wilson,G.M., Brewer,G.A. and Parks,G.D. (2000) Spacing constraints on reinitiation of paramyxovirus transcription: the gene end U tract acts as a spacer to separate gene end from gene start sites. Virology, 274, 438–449. [DOI] [PubMed] [Google Scholar]
- Richter J.D. (1999) Cytoplasmic polyadenylation in development and beyond. Microbiol. Mol. Biol. Rev., 63, 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy L.M., Haccard,O., Izumi,T., Lattes,B.G., Lewellyn,A.L. and Maller,J.L. (1996) Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene, 12, 2203–2211. [PubMed] [Google Scholar]
- Sagata N., Oskarsson,M., Copeland,T., Brumbaugh,J. and Van de Woude,G.F. (1988) Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature, 335, 519–525. [DOI] [PubMed] [Google Scholar]
- Sagata N., Daar,I., Oskarsson,M., Showalter,S.D. and Van de Woude,G.F. (1989) The product of the mos proto-oncogene as a candidate initiator for oocyte maturation. Science, 245, 643–646. [DOI] [PubMed] [Google Scholar]
- Sallès F.J., Darrow,A.L., O’Connell,M.L. and Strickland,S. (1992) Isolation of novel murine maternal mRNAs regulated by cytoplasmic polyadenylation. Genes Dev., 6, 1202–1212. [DOI] [PubMed] [Google Scholar]
- Sheets M.D., Wu,M. and Wickens,M. (1995) Polyadenylation of c-mos mRNA as a control point in Xenopus meiotic maturation. Nature, 374, 511–516. [DOI] [PubMed] [Google Scholar]
- Solomon M.J., Glotzer,M., Lee,T.H., Philippe,M. and Kirschner,M.W. (1990) Cyclin activation of p34cdc2. Cell, 63, 1013–1024. [DOI] [PubMed] [Google Scholar]
- Standart N. and Dale,M. (1993) Regulated polyadenylation of clam maternal mRNAs in vitro. Dev. Genet., 14, 492–499. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B., Hake,L.E. and Richter,J.D. (1996) CPEB controls the cytoplasmic polyadenylation of cyclin, Cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J., 15, 2582–2592. [PMC free article] [PubMed] [Google Scholar]
- Stutz A., Conne,B., Huarte,J., Gubler,P., Volkel,V., Flandin,P. and Vassalli,J.D. (1998) Masking, unmasking and regulated polyadenyl ation cooperate in the translational control of a dormant mRNA in mouse oocytes. Genes Dev., 12, 2535–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay J., Hodgman,R. and Richter,J.D. (2000) The control of cyclin B1 mRNA translation during mouse oocyte maturation. Dev. Biol., 221, 1–9. [DOI] [PubMed] [Google Scholar]
- Varnum S.M. and Wormington,W.M. (1990) Deadenylation of maternal mRNAs during Xenopus oocyte maturation does not require specific cis-sequences: a default mechanism for translational control. Genes Dev., 4, 2278–2286. [DOI] [PubMed] [Google Scholar]
- Welk J., Charlesworth,A., Smith,G.D. and Macnicol,A.M. (2001) Identification of the gene encoding human cytoplasmic polyadenyl ation element binding protein. Gene, 263, 113–121. [DOI] [PubMed] [Google Scholar]
- Wickens M., Goodwin,E.B., Kimble,J., Strickland,S. and Hentze,M.W. (2000) Translational control of developmental decisions. In Sonenberg,N., Hershey,J. and Mathews,M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.