Abstract
Heat shock factor 2, one of the four vertebrate HSFs, transcriptional regulators of heat shock gene expression, is active during embryogenesis and spermatogenesis, with unknown functions and targets. By disrupting the Hsf2 gene, we show that, although the lack of HSF2 is not embryonic lethal, Hsf2–/– mice suffer from brain abnormalities, and meiotic and gameto genesis defects in both genders. The disturbances in brain are characterized by the enlargement of lateral and third ventricles and the reduction of hippocampus and striatum, in correlation with HSF2 expression in proliferative cells of the neuroepithelium and in some ependymal cells in adults. Many developing spermatocytes are eliminated via apoptosis in a stage-specific manner in Hsf2–/– males, and pachytene spermatocytes also display structural defects in the synaptonemal complexes between homologous chromosomes. Hsf2–/– females suffer from multiple fertility defects: the production of abnormal eggs, the reduction in ovarian follicle number and the presence of hemorrhagic cystic follicles are consistent with meiotic defects. Hsf2–/– females also display hormone response defects, that can be rescued by superovulation treatment, and exhibit abnormal rates of luteinizing hormone receptor mRNAs.
Keywords: apoptosis/brain defects/gametogenesis/HSF2/synaptonemal complex
Introduction
Heat shock proteins (Hsps) function as molecular chaperones at various stages in protein biogenesis and degradation (Mathew and Morimoto, 1998). Hsps are induced following proteotoxic stresses and in a variety of physio-pathological conditions (fever, ischemia, viral or bacterial infections, brain injury and aging). Hsps are also required for normal progression of the cell cycle and differentiation. Some HSPs are crucial in the maintenance of spermatogenesis (testis-specific Hsps; reviewed in Eddy, 1999) and for embryogenesis (see for example Voss et al., 2000).
Hsp gene transcription is regulated by heat shock factors (HSFs) (Pirkkala et al., 2001). In Drosophila, the sole HSF is necessary for larval development and oogenesis, independently of hsp gene expression (Jedlicka et al., 1997). In vertebrates, four HSFs are found. HSF1 is the major heat stress-responsive factor expressed ubiquitously. Like Drosophila HSF, HSF1 plays a role in development (McMillan et al., 1998; Xiao et al., 1999). Inactivation of the mouse Hsf1 gene leads to placental insufficiency and growth retardation. Hsf1–/– females are infertile due to the fact that HSF1 is a maternal factor present in the one-cell stage embryo and is required for development to the two-cell stage (Christians et al., 2000). Male mice expressing an active form of HSF1 in the testis are infertile due to apoptosis of pachytene spermatocytes, while female fertility is not affected (Nakai et al., 2000).
In contrast to ubiquitous HSF1, HSF2 is expressed at high levels and is only active in two developmental pathways: spermatogenesis and embryogenesis. HSF2 mRNA and protein are expressed in a stage-specific manner during adult spermatogenesis in rodents, and the protein localizes to the nuclei of early pachytene spermatocytes and round post-meiotic spermatocytes (Sarge et al., 1994; Alastalo et al., 1998). In contrast to HSF1, HSF2 is not a maternal factor, but is active from the eight-cell stage (Mezger et al., 1994a,b) and peaks around day 9.5 of gestation, with elevated levels in the developing neural tube. HSF2 levels and activity then decrease and are restricted to the developing brain in E15.5 embryos (Rallu et al., 1997). HSF2 function and targets have remained obscure, with no clear correlations with Hsp expression. Here, we have investigated the role of HSF2 by inactivating the mouse Hsf2 gene using homologous recombination.
Results
Targeted disruption of the Hsf2 gene in ES cells and generation of HSF2-deficient mice
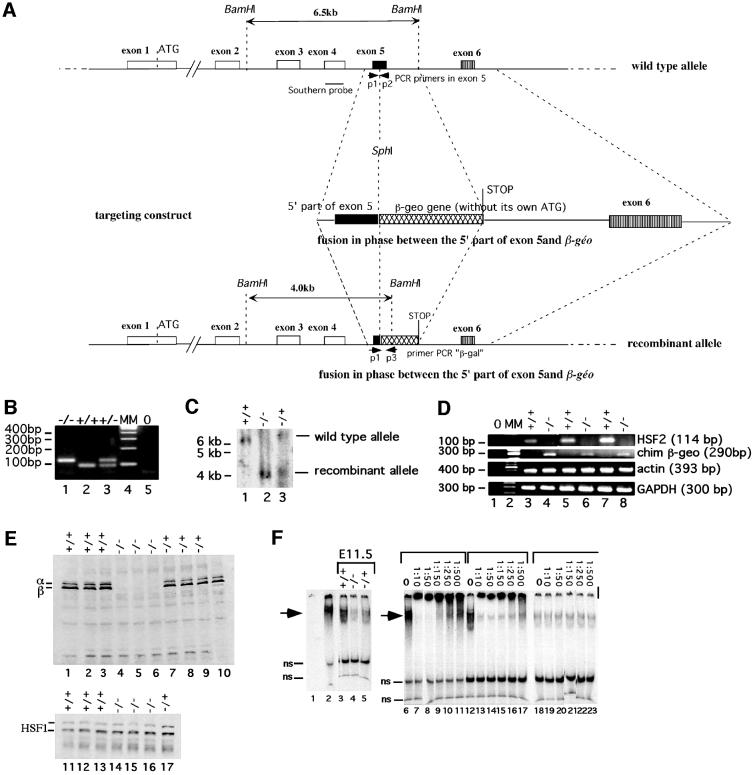
Since HSF2 is expressed in embryonic stem (ES) cells, we chose a promoterless targeting vector strategy to disrupt the Hsf2 gene, by insertion of the β-geo gene in-phase at the SphI site of exon 5, which lies in the oligomerization domain of HSF2. In the case of a homologous recombination event, a chimeric protein is produced, which retains the HSF2 DNA-binding domain, but is interrupted in the oligomerization domain at Lys167: the first hydrophobic array of six heptad repeats is conserved, but the overlapping arrays 2 and 3 are eliminated (Wu, 1995). Therefore, the expected chimeric protein cannot trimerize or bind DNA (Figure 1A). Moreover, this protein lacks NLS2, one of the two HSF2 nuclear localization signals, which lies between the residues Lys195 and Lys210. Since both NLS are necessary for the nuclear localization of HSF2, the chimeric protein is expected to be cytoplasmic and not able to perform nuclear functions (Sheldon and Kingston, 1993). The homologous recombination places the β-geo gene in-phase with the beginning of exon 5 and without a promoter in the targeting construct. The β-geo gene is a chimera between the lacZ gene and the G418 resistance gene (neo) (Friedrich and Soriano, 1991). After electroporation with the targeting vector, the G418-resistant ES clones result either from rare random insertion of the targeting construct, in-phase with a promoter, or from homologous recombination, which places the β-geo gene under the control of the Hsf2 promoter region, active in ES cells. After recombination in ES cells, the β-galactosidase expression is the reporter of the Hsf2 promoter activity.
Fig. 1. Targeted inactivation of the Hsf2 gene. (A) Schematic representation of the wild-type and mutated alleles. Horizontal small arrows show the location of the three primers used for PCR genotyping. (B) PCR genotyping of offspring from F1 heterozygous intercrosses. (C) Southern blot of BamHI-digested tail DNA. Lanes 1 and 3: wild-type allele 6.5 kb fragment. Lanes 2 and 3: disrupted allele 4 kb fragment. (D) RT–PCR analysis of HSF2, chimeric HSF2-βgeo, actin and GAPDH mRNA levels in Hsf2+/+, Hsf2+/– and Hsf2–/– tissues. Lane 1: no reverse transcription (0); lane 2, molecular markers (MM); lanes 3 and 4, testis; lanes 5 and 6, E9.5 embryos; lanes 7 and 8, E13.5 embryos. (E) Western blot analysis of whole E11.5 embryo extracts of littermates with polyclonal anti-mouse HSF2 (upper panel). Equal loading and transfer were assessed with a monoclonal anti-HSF1 (lower panel). Lanes 1–3 and 11–13, Hsf2+/+; lanes 4–6 and 14–17, Hsf2–/–; lanes 7–9 and 17, Hsf2+/–; lane 10, unstressed F9 EC cells. (F) EMSA analysis of E11.5 embryo extracts with an HSE-containing double-stranded oligonucleotide. Lane 1, no extracts; lanes 2 and 6–11, unstressed F9 EC cells; lanes 3–5 and 12–23, embryos. The dilutions of polyclonal anti-mouse HSF2 in supershift experiment are indicated. Arrowheads, specific HSF2–HSE complexes; ns, non-specific complexes.
Recombination events at the Hsf2 locus were identified among the G418-resistant colonies by Southern blot analysis of ES cell genomic DNA with a 5′-external probe. Among 27 colonies, two showed a Southern pattern compatible with recombination of one Hsf2 allele and were used for injection into C57Bl/6 blastocysts. One of them led to germline transmission. One female chimera was obtained and crossed with C57Bl/6 males. The presence of a wild-type or mutated Hsf2 allele in progeny was determined by PCR amplification and confirmed by Southern blot (Figure 1B and C).
F1 heterozygous (Hsf2+/–; C57Bl/6/129Sv genetic background) mice were viable and intercrossed. Litter size for these intercrosses was normal (8.6 ± 2.3 pups/litter, n = 15). Out of 132 genotyped F2 progeny, 30 were wild type (22.7%), 69 heterozygous (52.3%) and 33 homozygous (25%), not statistically different from a Mendelian rate (25, 50 and 25%). A similar male:female ratio was observed (17 females for 13 males in wild-type individuals; 35 males and 34 females for heterozygotes; and 14 males and 19 females for homozygotes).
To confirm that Hsf2-null mutants were devoid of wild-type HSF2, we measured the HSF2 mRNA levels by RT–PCR on adult testis and on E9.5 and E13.5 embryos. No HSF2 mRNA was detected in the samples from Hsf2–/– testis or embryos (Figure 1D). In contrast, the chimeric recombinant mRNA encoding β-geo was detected in Hsf2–/– testis and embryos, but not in the wild-type samples. Western blot analysis confirmed the absence of HSF2 protein in Hsf2–/– embryos (Figure 1E) and testes (data not shown). To exclude the fact that the chimeric recombinant protein could retain HSE-binding activity, even as a monomer, we performed electrophoretic mobility shift assay (EMSA) on E11.5 embryo whole extracts. F9 embryonal carcinoma (EC) cells, which contain high levels of active HSF2, were used as positive controls. In Hsf2+/– embryos, constitutive HSE-binding activity was reduced notably when compared with wild types (Figure 1F, lanes 3 and 5). A faint signal was detected in the Hsf2–/– embryos (Figure 1F, lane 4), but this residual HSE-binding activity was not due to HSF2. Indeed, an antibody able to induce total supershift of HSF2 complexes in F9 EC cells (Figure 1F, lanes 6–11) did not induce any supershift of the HSE complexes present in Hsf2–/– extracts, even at high concentrations (Figure 1F, lanes 18–23). A similar residual HSE-binding activity remained in wild-type embryos after supershifting with anti-HSF2 (Figure 1F, lanes 13–17). X-gal staining on testis (Figure 2E) and brain (data not shown), and immunohistochemistry on embryonic fibroblasts (data not shown), confirmed that the chimeric protein was cytoplasmic and not nuclear, suggesting that it cannot reach any DNA targets, even if it may retain a minor DNA-binding activity, undetectable by EMSA. We conclude that HSF2 deficiency was not embryonic lethal and that the phenotype associated with the homozygous mutation, as described below, is due to the lack of functional HSF2 protein.
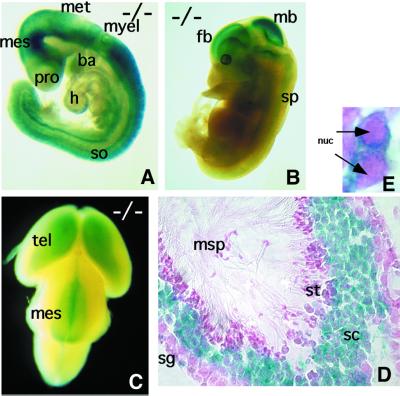
Fig. 2. LacZ expression as a reporter of the HSF2 expression profile. (A and B) Lateral view of an E9.5 and E13.5 Hsf2–/– embryo, respectively. (C) Dorsal view of an E15.5 Hsf2–/– embryo. (D) Transverse section of a seminiferous tubule showing X-gal staining of spermatocytes. (E) Cytoplasmic localization of the chimeric recombinant protein using β-galactosidase detection by X-gal staining (blue) in mouse pachytene spermatocytes (Hsf2–/– male). nuc, nucleus; h, heart; so, somites; pro, prosencephalon; mes, mesencephalon; met, metencephalon; myel, myel encephalon; tel, telencephalon; ba, branchial arches; fb, forebrain; mb, midbrain; sp, spinal chord; msp, mature spermatozoa; sg, spermatogonia; sc, spermatocytes; st, spermatids.
The lacZ reporter gene expression faithfully reproduces the HSF2 expression profile
We analyzed the β-galactosidase (β-gal) expression pattern, a reporter of Hsf2 promoter activity in embryos and adults. At all embryonic stages, the overall pattern of β-gal expression was similar in Hsf2+/– and Hsf2–/– embryos, except for signal intensity. As expected, no β-gal activity was detected in one-cell stage embryos (Mezger et al., 1994a,b; Christians et al., 1997). At E7.5, all three embryonic layers were highly stained, and at E8.5 the head fold was marked more strongly (data not shown). At E9.5, the β-gal expression was very strong in the developing nervous system, but absent from the trunk mesenchyme (Figure 2A). At E13.5, the β-gal activity was restricted to the central nervous system (CNS) (Figure 2B) and, at E15.5, this pattern was even more restricted within the telencephalic vesicles and part of the midbrain (Figure 2C). The β-gal activity profile was restrained progressively to the developing CNS and therefore parallels HSF2 DNA-binding activity profiles (Rallu et al., 1997).
In adults, strong β-gal activity was detected in the spermatocytes (Figure 2D), a known site of HSF2 expression during spermatogenesis (Sarge et al., 1994; Alastalo et al., 1998), but not in the elongated spermatids or spermatozoa. Sertoli cells did not express β-gal, but type A spermatogonia were stained.
Characteristics of HSF2 expression in the developing and adult brain
In the developing brain, β-gal was not expressed in the whole neuroepithelium, but was restricted along the lumen of the ventricles (Figure 3A). The ventricular zone (vz) is the location of the proliferating neural precursors (Takahashi et al., 1992). Comparison at E12.5 of the HSF2 profile, detected by immunohistochemistry, and of the proliferating cells of the vz, stained by anti-bromodeoxyuridine (BrdU) antibodies, confirmed that HSF2 expression corresponds to proliferating cells in the vz (Figure 3B and C). High magnification revealed that HSF2 staining was mainly nuclear and seemed to exclude the S phase cells (BrdU-positive cells located to the internal vz). Interestingly, β-gal expression was also detected at specific sites in the adult brain: in some discrete cells of the ependymal layer near the vz (Figure 3D–F).
Fig. 3. HSF2 and β-gal expression in the ventricular zone of embryonic and adult brain. (A) Parasagittal section of an E13.5 Hsf2–/– embryonic brain showing β-gal expression along the lumen of the ventricles. fb, forebrain; mb, midbrain; chp, choroid plexus. (B and C) HSF2 immunolocalization and BrdU staining, respectively, at the level of midbrain in E12.5 Hsf2+/+ embryos. vz, ventricular zone; lv, lumen of the ventricle. (D–F) β-gal detection in the ependymal layer of adult Hsf2–/– brain. st, striatum; se, septum; ep, ependymal zone; lv, lateral ventricle. Scale bar: 50 µm. (E) Detail of (D), magnification: 20×. (F) Detail of a transverse section at the level of the hippocampus (h), magnification: 20×.
Structural abnormalities and β-galactosidase expression in the adult brain
The HSF2 and β-gal expression patterns in proliferative regions of the developing brain prompted us to investigate the effects of HSF2 deficiency in the adult brain. Hsf2–/– adult brains (n = 9) systematically displayed structural abnormalities when compared with wild-type brains (n = 7); the lateral and third ventricles were enlarged and the hippocampus was dramatically reduced at all parasagittal or transverse section levels in adult brain (Figure 4).

Fig. 4. Abnormal structure of the HSF2-deficient adult brain. Brain transverse sections (A–F) from Hsf2+/+ (A, C and E) or Hsf2–/– (B, D and F) 3-month-old males, illustrating the enlargment of the lateral and third ventricles all along the brain. (G and H) Parasagittal sections of the adult brain showing the reduced size of the hippocampus in Hsf2–/– (–/–) (H) compared with Hsf2+/+ (WT) (G) brain. ST, striatum; SE, septum; H, hippocampus; LV, lateral ventricle; TV, third ventricle; CE, cerebellum.
Alteration of spermatogenesis in Hsf2–/– males
Seminiferous tubules of homozygous Hsf2–/– mice exhibit morphological changes. Testes in Hsf2–/– mice were significantly smaller (P < 0.01) than in wild-type animals (Figure 5A). The mean weight of testes isolated from the Hsf2–/– animals was 70.4 ± 7.1 mg versus 113.1 ± 4.7 mg for wild-type testes. The average diameter of seminiferous tubules in the HSF2-deficient testis (n = 5 mice, 165.5 ± 28.7 µm) was significantly smaller (P < 0.01) than in Hsf2+/+ mice (n = 5 mice, 187.3 ± 16.2 µm). Microscopic analysis of hematoxylin- and eosin-stained testis sections revealed that the Hsf2+/+ animals had tubules containing normal amounts of differentiating germ cells from different developmental steps organized into the typical layer pattern (Figure 5B). In the Hsf2–/– mice, however, many of the tubules showed signs of disruption of spermatogenesis such as degenerating cells with condensed nuclei and eosin-positive cytoplasm, absence of certain differentiating spermatocytes and spermatids, and vacuolization of the tubules. Occasionally, tubules devoid of all meiotic spermatocytes and post-meiotic haploid spermatids were observed (Figure 5B). In addition, the average weight of the epididymis of Hsf2–/– mice was less than for wild-type animals (data not shown). The number of sperm found in the epididymis of the Hsf2–/– mice (n = 5, 7.9 ± 6.5 × 106 per ml of the isolation buffer) was significantly lower (P < 0.05), in comparison with sperm counts of Hsf2+/+ animals (n = 5, 18.6 ± 11.7 × 106 per ml of the isolation buffer).
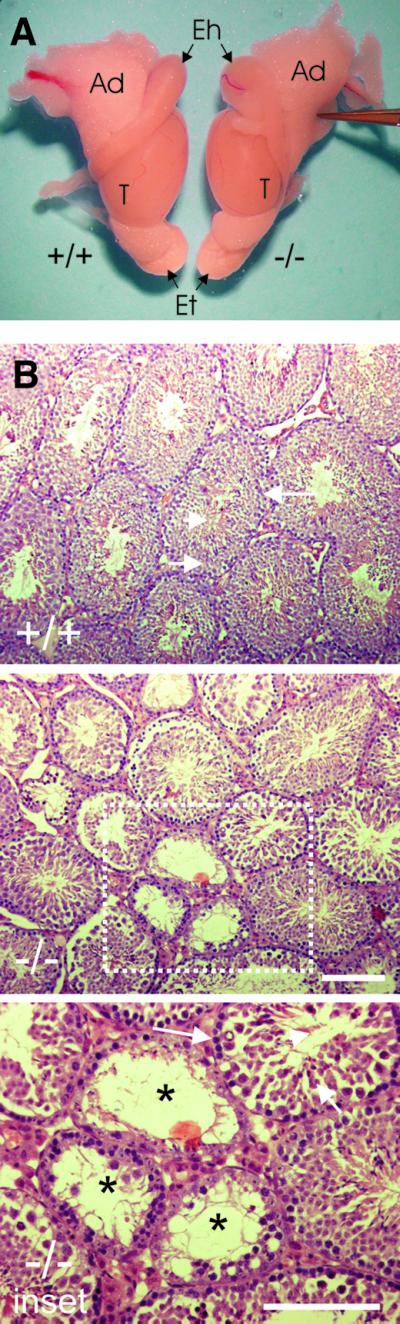
Fig. 5. Gross anatomy of male reproductive organs and analysis of testis cross-sections of adult Hsf2+/+ (+/+) and Hsf2–/– mice (–/–). (A) Testis and epididymis size. T, testis; Eh, head of epididymis; Et, tail of epididymis; Ad, adipose tissues. (B) Cross-sections from testes. For (+/+): arrowhead, elongating spermatids; short arrow, spermatocytes; long arrow, spermatogonia. For (–/–): reduction in the diameter of the seminiferous tubules and indications of disruption of spermatogenesis. For inset: lack of spermatocytes (short arrow) and spermatids (arrowhead), vacuolization of the tubules (asterisk). Bars = 100 µm.
Increased apoptosis in the testes of Hsf2–/– mice. We isolated all testicular cells and performed staining with fluorescein isothiocyanate (FITC)-conjugated annexin V, a method used successfully in the detection of apoptosis in testis and other organs by flow cytometry and on tissue sections (Vermes et al., 1995; Henriksen and Parvinen, 1998; van Engeland et al., 1998). Flow cytometric analysis of whole testis cells from Hsf2–/– and Hsf2+/+ animals showed a significant difference (P < 0.05) in the number of annexin V–FITC-positive cells: 22.6 ± 12.1% of the cells in the testes of Hsf2–/– mice (n = 5) were annexin V–FITC-positive compared with 8.1 ± 4.1% of the Hsf2+/+ mice (n = 5, Figure 6A). Clusters of apoptotic cells were detected by TUNEL assay within the seminiferous tubules of Hsf2–/– males (data not shown).
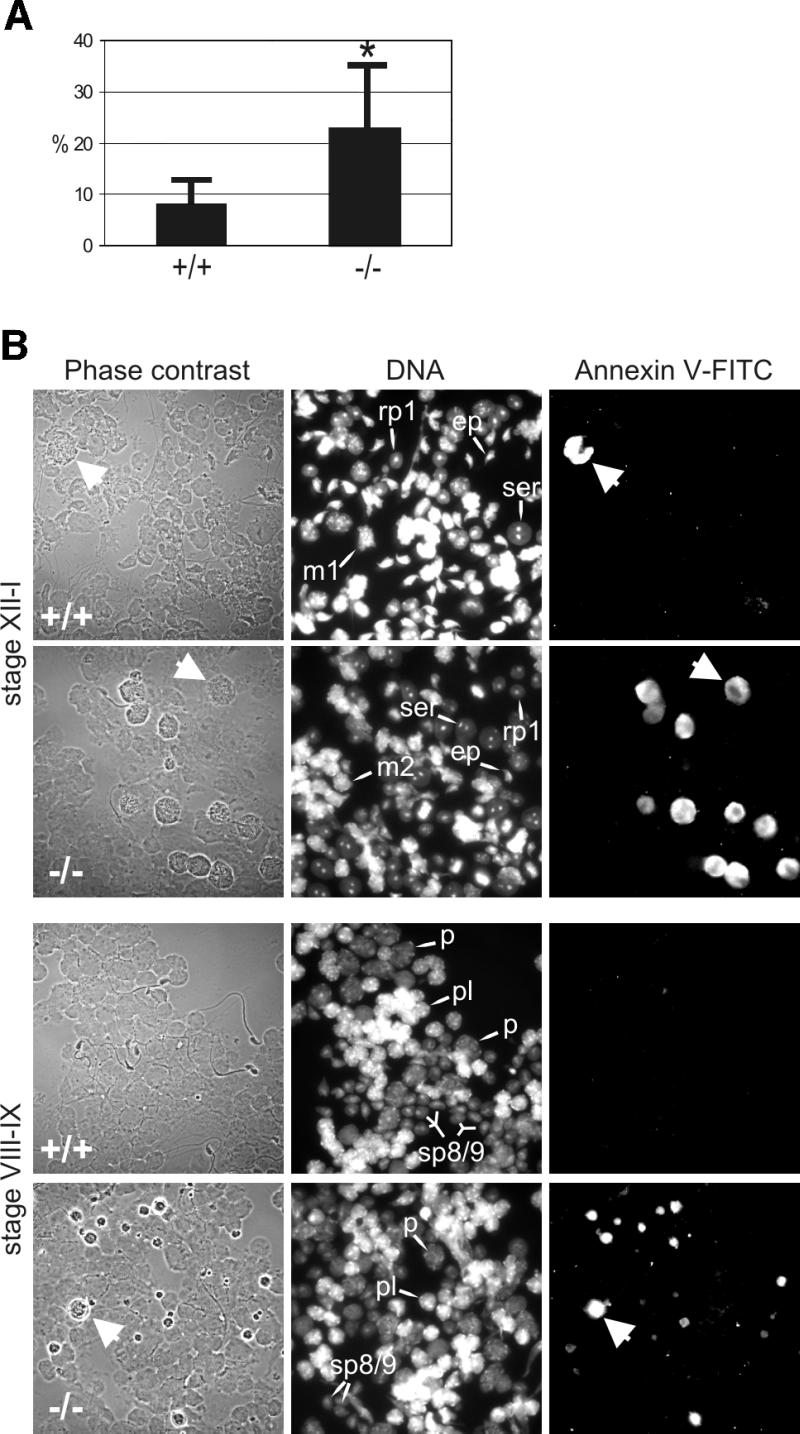
Fig. 6. Apoptosis of developing germ cells in the testes of Hsf2–/– mice. (A) Flow cytometric analysis of annexin V–FITC-stained testicular cells. (B) Stage-specific apoptosis in the testis of Hsf2–/– mice. Translumination-assisted dissection of the seminiferous tubules followed by annexin V–FITC immunofluorescence and microscopic analysis revealed two populations of dying cells in the Hsf2–/– mice at stages VIII–IX and XII–I; late pachytene and meiotically dividing spermatocytes (arrows). Tubule segments isolated from stages XII–I containing type A3 and A4 spermatogonia, early pachytene spermatocytes, meiotically dividing spermatocytes (m1, m2), round step-1 spermatids (rp1) and elongating spermatids (ep), and from stages VIII–IX containing type A1 spermatogonia, pre-leptotene spermatocytes (pl), late pachytene spermatocytes (p) and elongating step-8/9 spermatids (sp8/9). Note the reduction in the number of post-meiotic round and elongated spermatids in the Hsf2–/– mice.
Spermatocytes of the HSF2-deficient mice die at late pachytene of meiotic prophase and during meiotic divisions. We investigated what types of cell undergo apoptosis in the testis by utilizing stage-specific microdissection of seminiferous tubules (Parvinen et al., 1993). Isolated cells from individual stages of spermatogenesis were fixed on slides and stained with annexin V–FITC. The dying cells in the Hsf2–/– mice testes were always found in clusters, indicating a stage-specific elimination of the cells during spermatogenesis. Two major annexin V–FITC-positive cell populations were discovered at stages VIII–IX and XII–I in the Hsf2–/– mice testes (Figure 6B). The spermatocytes at late pachytene of meiotic prophase and at meiotic divisions together accounted for almost 90% of the dying cells in Hsf2–/– testis. The 500 annexin V–FITC-positive cells analyzed from three Hsf2–/– mice were in early stages of apoptosis: 169 (34%) were at pachytene and 277 (55%) at meiotic M phase. The third apoptotic testicular cell type was type A spermatogonia that died at mitosis (data not shown). A few apoptotic cells were also detected in the Hsf2+/+ animals (Figure 6B), most of which were late pachytene or meiotically dividing spermatocytes.
Synaptonemal complexes of pachytene spermatocytes are malformed in the Hsf2–/– mice. The synaptonemal complex (SC) forms the axis of paired chromosomes during the pachytene stage (Walker and Hawley, 2000). We investigated the structure of SCs in mid-pachytene spermatocytes of HSF2-deficient and wild-type mice using immunohistochemical detection of synaptonemal complex protein 3 (SCP3), which is localized in the lateral elements of the SC (Schalk et al., 1998).
The structure of the SC in Hsf2–/– pachytene spermatocytes was often disorganized (Figure 7A). The continuous parallel alignment of the lateral elements was disrupted in 16.5% of the Hsf2–/– spermatocytes at stages VII–IX (33 cells of a total of 200 analyzed in four Hsf2–/– animals), corresponding to the mid-pachytene stage. In Hsf2+/+ animals, only 3.5% of cells (seven cells of a total of 200 analyzed in two wild-type mice) had similar structural defects (P < 0.05). A typical cell with a defective SC had 1–4 pairs of lateral elements, along which one or a maximum of two loop-like structures were observed, indicative of defective synapsis between the pairs of homologous chromosomes (Figure 7B). The site of the loop-like structure varied along the SC from the very centromere-proximal end to the opposite end. In a few Hsf2–/– cells, the region of asynapsis was at the centromere terminus of the acrocentric mouse chromosome pairs, causing separation of the centromere pairs detected with Crest anti-centromere antisera (Figure 7C).
Fig. 7. Synapsis between the homologous chromosomes is defective in the Hsf2–/– mice. (A) Immunofluorescence labeling with anti-SCP3 antibody (Cy3 channel, red) and Crest anti-centromere sera (FITC channel, green). A part of a seminiferous tubule in developmental stage VIII (Clermont, 1972), with middle pachytene spermatocytes (mp), round spermatids (rs) and elongated spermatids (es). Loop-like configurations are present between one or more pairs of homologous chromosomes (arrow in the inset A). (B) An SC with one loop-like structure near the centromere terminus of a chromosome pair (arrow) (Z-level section). (C) Separation of the lateral elements at the centromere region (arrowheads in the inset C; stack of five Z-level sections from a confocal microscope series). Merge of anti-SCP3 (red) and Crest (green) in images (B) and (C). Bars = 10 µm.
The impact of these defects on male fertility was investigated. Five wild-type males and five homozygous males were crossed over a 5 month period with four outbred OF1 females each. The averages of pups/litter, 9.30 ± 3.02 for wild-type males and 9.33 ± 3.39 for HSF2-deficient males, were not significantly different (P = 0.997).
Complex and multiple female fertility defects
HSF2-deficient females are hypofertile. Intercrosses of Hsf2+/– females with Hsf2+/– males resulted in 8.6 ± 2.3 offspring/litter (n = 15), comparable with wild-type intercrosses (8.75 ± 0.4 pups/litter; n = 4). In contrast, out of 17 plugged Hsf2–/– females, seven gave no offspring, five gave a normal litter and five had intermediate scores (1–4 pups), resulting in an average of 3.6 ± 3.4 pups/litter. Offspring were obtained with a similar average when Hsf2–/– females were crossed with either Hsf2+/+ (2.14; n = 7 females) or Hsf2–/– males (3.43; n = 7 females) (P = 0.52). Thus, the reduced litter size of the Hsf2–/– females is not related to the male genotype and not due to lethality of Hsf2–/– embryos.
Increased embryonic lethality before E9.5. We examined the number and viability of the embryos at day E9.5 of gestation. Out of 11 Hsf2–/– plugged females, only five were pregnant at E9.5. The number of implanted embryos per pregnant female was normal (11.8 ± 1.3) and comparable with that observed in Hsf2+/– females (11.4; n = 10). However, an abnormally high number of dead embryos (resorbed or retarded) was observed (39% for Hsf2–/– females versus 18.4% for Hsf2+/– females). If considering all the plugged females, the average number of normally developing embryos at E9.5 was 3.27 ± 4.02 (instead of 9.3 for the heterozygous females) and reflected the average of offspring/litter. It was similar at E9.5, at E16.5 (3.4 ± 4.04) and at birth (3.6 ± 3.4 pups/litter), suggesting that the majority of the defects occur before E9.5.
Ovulation defects in Hsf2–/– females. We compared the ovulation performances of nine Hsf2–/– and 10 Hsf2+/+ females on the day of vaginal plug detection, after mating with wild-type outbred OF1 males. Hsf2–/– females were found to have dramatic ovulation problems. Only three Hsf2–/– females produced eggs, resulting in a total average of 3.5 eggs per Hsf2–/– female. Seven out of 10 Hsf2+/+ females ovulated with a total average of 7.4 eggs per female. The average number of fertilized eggs was 0.6 fertilized egg per Hsf2–/– female versus 4.9 per Hsf2+/+ female (P < 0.05). Indeed, Hsf2–/– females frequently produced abnormal eggs: 43.7% of the eggs were fragmented or without polar bodies (versus 5.4% for control females) or were unfertilized (40.6% with only one polar body versus 28.4% in controls).
Meiotic and hormonal problems in Hsf2–/– females. We attempted to rescue ovulation in Hsf2–/– females by administration of pregnant mare serum gonadotropin (PMSG/FSH)/human chorionic gonadotropin (hCG/LH) to 21- to 27-day-old mice, a treatment used to induce superovulation. All Hsf2–/– females were able to ovulate after this treatment. A total of 35.1 ± 22.5 eggs were ovulated by Hsf2–/– females (n = 16), statistically comparable (P = 0.853) with 37.2 ± 11.7 eggs in wild-type females (n = 5). However, while the eggs of wild-type females were able to develop in vitro to the two-cell stage with good scores (27.3 ± 1.7 eggs; 78.3% of the total ovulated eggs), eggs of Hsf2–/– females were often abnormal and only 10.2 ± 5.8 eggs (29.2% of the total) developed to the two-cell stage, which was significantly lower (P = 0.0132). The fact that ∼70% of the eggs ovulated by Hsf2–/– females were abnormal is suggestive of meiotic problems. Since superovulation treatment rescued ovulation in Hsf2–/– females, part of the ovarian defects observed in pubescent Hsf2–/– females may be secondary to disturbed hormonal physiological concentrations of gonadotropins or ovarian function alterations.
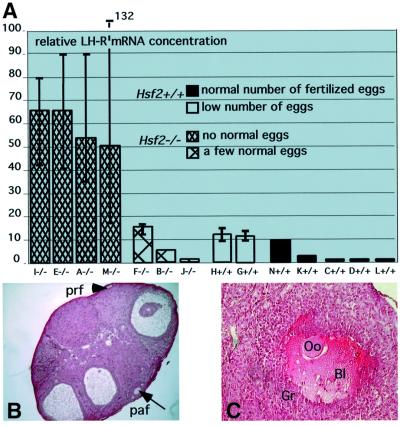
Ovarian defects in Hsf2–/– females. To determine to what extent the ovarian function was altered, the expression level of the luteinizing hormone receptor (LH-R) was examined on the day of vaginal plug detection by semi-quantitative RT–PCR on mRNAs from individual ovaries of 5-month-old females, and their ovulation scores were determined. The level of LH-R mRNAs was found to be 50–60 times higher in the ovaries of females that ovulated no eggs or only abnormal eggs. Ovaries from Hsf2–/– females that ovulated at least a few normal eggs contained amounts of LH-R mRNAs similar to the Hsf2+/+ ovaries (Figure 8A).
Fig. 8. Ovarian defects in Hsf2–/– females. (A) Abnormally elevated levels of luteinizing hormone receptor (LH-R) mRNAs in Hsf2–/– with anovulatory problems. Seven Hsf2+/+ and seven Hsf2–/– 5-month-old females were mated with OF1 males. On the day of plug detection, eggs were harvested and counted in the ampulla. In parallel, LH-R mRNA levels were analyzed by RT–PCR. The relative mRNA concentration is given in arbitrary units. Value 1 was attributed to the ovary exhibiting the lowest expression level. Each plot corresponds to the average determined from three or four PCR experiments (except for female B that was tested only once). Error bars are indicated. Dense hatched bars, Hsf2–/– females that produced no eggs or only abnormal fragmented eggs. Hatched bars, Hsf2–/– females that produced a few (an average of two) fertilized eggs. Empty bars, Hsf2+/+ females with no ovulated fertilized eggs. Filled bars, Hsf2+/+ females with normal ovulation scores and a normal number of fertilized eggs. (B) Paraffin section of an Hsf2–/– ovary with marked ovulatory problems: low number of primary (prf) and pre-antral (paf) follicles. (C) A pre-antral hemorrhagic follicle in the section of an Hsf2–/– ovary which never gave offspring. Oo, oocyte; Gr, granulosa cells; Bl, blood.
High LH-R mRNA levels suggested a disturbed hormonal response. Histological analysis of ovaries from Hsf2–/– females with ovulatory problems and high levels of LH-R mRNAs revealed a low number of follicles from each stage (Figure 8B). Analysis of ovaries from Hsf2–/– females that were unable to have progeny also revealed, instead of corpora lutea, the presence of hemorrhagic large follicles with a trapped oocyte (Figure 8C).
Discussion
Three main defects ensue from HSF2 deficiency in the mouse: defective meiotic chromosome synapsis and increased apoptosis in testis; female fertility problems; and altered brain morphology. Female hypofertility probably results from two apparently distinct phenomena: one related to meiotic alteration and the other having a hormone response component.
Meiosis is affected by HSF2 deficiency in males and females
Meiosis includes genetic recombination, pairing of homologous chromosomes and formation of the SC between the chromosome pairs (synapsis) (Walker and Hawley, 2000); all events that require timely controlled expression of meiotic proteins at specific developmental stages of gametogenesis.
Several lines of evidence show that meiosis is affected by HSF2 deficiency, in both males and females. Homozygous deletion of the Hsf2 gene causes apoptosis of nearly 25% of the cells inside the seminiferous tubules. Specifically, late pachytene and meiotically dividing spermatocytes account for almost 90% of the annexin V–FITC-positive cells, suggesting that the majority of the differentiating germ cells die in a stage-specific manner, leading to a 58% reduction in sperm count, as compared with Hsf2+/+ animals. In Hsf2–/– females, meiotic defects are illustrated by a high number of abnormal eggs detected in naturally occurring ovulation or superovulation experiments. The ovaries of Hsf2–/– females with ovulatory abnormalities exhibit a lower number of follicles at each stage, and large cystic hemorrhagic follicles were observed in the ovaries of females that failed to have progeny. The high number of retarded or resorbed embryos in the litters of Hsf2–/– females, before E9.5, is suggestive of aneuploidy and consistent with meiotic disturbances.
Inactivation of a number of mammalian genes implicated in meiosis results in phenotypes similar to that of the Hsf2–/– males (Baudat et al., 2000; Roeder and Bailis, 2000; Romanienko and Camerini-Otero, 2000; Yuan et al., 2000; Tay and Richter, 2001). In these studies, meiotic problems in females were evident due to a severely reduced number of follicles in ovaries. The meiotic phenotype of Msh5–/– females is associated with estrus cycle defects and giant cysts in the ovaries, similar to that of Hsf2–/– females. Hsf2–/– ovaries therefore exhibit the stigmata of meiotic problems.
Alteration of spermatogenesis with no impact on male fertility has been reported previously. Even in cases of considerable impairment of spermatogenesis, in CPEB null mice, matings of ∼20% of the CPEB null males with fertile females resulted in offspring, although the males produce six times fewer sperm than wild type and only 2% of sperm is motile (Tay and Richter, 2001).
Function of HSF2 in meiosis
Recent studies have demonstrated the existence of a ‘pachytene checkpoint’, the triggerring of which at late meiotic prophase leads to the elimination of defective germ cells by apoptosis (Roeder and Bailis, 2000). We observed structural defects in the SC of Hsf2–/– mice; 16.5% of the mid-pachytene spermatocytes had loop-like configurations between the lateral elements of SCs. This number is most probably an underestimate due to the limits of resolution. The lack of HSF2 may lead to disturbances in the expression of structural components of the SC or in the enzymatic activities required for synapsis/desynapsis or, alternatively, in genetic recombination preceding synapsis.
In addition to late pachytene spermatocytes, a large number of spermatocytes die at the meiotic M phase. Apoptosis may be due to premature separation of the centromere regions of the homologous chromosomes. Occasionally, we observed spermatocytes with defective synapsis at the centromere region. This is a potent mechanism for induction of unpaired univalent chromosomes that causes cell cycle arrest at the first meiotic division metaphase, which eventually leads to the elimination of arrested cells (Mahadevaiah et al., 2000). Alternatively, HSF2 may participate in regulation of M phase-specific molecules such as protein phosphatase 2A (PP2A) that negatively regulates entry into M phase in Xenopus egg extracts (Lee, 1995) and has also been implicated in regulation of microtubule dynamics and centrosome function (Snaith et al., 1996; Tournebize et al., 1997). In somatic tissue culture cells, PP2A activity has been shown to be controlled by HSF2 (Hong and Sarge, 1999).
We did not detect HSF2 in the adult ovary, but HSF2 was detected by immunohistochemistry in the primordial germ cells (data not shown). This implies that the meiotic defects in Hsf2–/– females may occur at the first meiotic division, i.e. during embryogenesis. This could explain why >43% of the ovulated eggs are fragmented or even lack the first polar body.
Hormone response disturbance in Hsf2–/– females
Hormonal treament of pre-pubescent females rescued a normal number of eggs ovulated in all the Hsf2–/– females. This suggests that anovulatory pubescent Hsf2–/– females naturally lack a sufficient hormonal stimulus, or do not possess an adequate ovulatory competence to respond to such a stimulus (appropriate levels of FSH- and LH-Rs). Hsf2–/– females with marked ovulatory defects exhibit abnormally elevated (50–60 times) levels of LH-Rs. Currently, we have no interpretation for these extremely high levels of LH-R mRNA, except that they are a sign of ovarian disturbance. In preliminary vaginal smear experiments, some Hsf2–/– pubescent females showed lengthening of and, later, total absence of the estrus cycle. The same females exhibited shortening of the luteal period (suggestive of non-functional corpora lutea) in pseudopregnancy experiments after mating with vasectomized males. Hypertrophy of the seminal vesicles, which was observed systematically in males, is suggestive of hormonal disturbance (Qian et al., 2001) and was occasionally associated with hydronephros. Whether these hormone response alterations are a consequence of the meiotic problems remains to be established. The presence of a high number of abnormal (or apoptotic) follicles due to meiotic defects in Hsf2–/– ovaries could therefore alter the dialog between follicular cells and the oocyte (Erickson and Shimasaki, 2000) and, as a consequence, the hormone response and the function of the hypothamalo-pituitary– ovarian axis. Histological analysis of pituitaries did not reveal any alterations, but HSF2 was expressed in some hypothalamic neurons, and lack of HSF2 could lead to hormone release problems.
In contrast to HSF1, necessary for the development of pre-implantation embryos (Christians et al., 2000) and involved in placentation (Xiao et al., 1999), HSF2 is dispensable for embryonic development, and Hsf2–/– females do not seem to have implantation or placentation problems. Drosophila HSF is necessary for oogenesis (Jedlicka et al., 1997), and HSF2 may have retained part of the ancestral function of this unique HSF. In Drosophila, Hsps are not targets of HSF during development. We could not detect significant differences between Hsf2–/– or Hsf2+/+ individuals for the expression of several Hsps: HSP25, HSP84, HSP86, HSP70, GRP78, HSJ2, APG1 and APG2, by semi-quantitative RT–PCR or western blots on testis or embryonic brains. The target genes of these HSFs during development remain to be identified.
Brain abnormalities in Hsf2–/– animals
Adult Hsf2–/– mice systematically exhibit brain abnormalities characterized by the enlargement of the lateral and third ventricles. Since the choroid plexus, involved in the production of the cerebrospinal fluid, is a site of strong HSF2 expression (Figure 3A), we cannot rule out the possibility that this phenomenon is due to hydrocephaly. Since no cortex compression is observed, but rather specific reduction in the volume of hippocampus and striatum, we favor the hypothesis of a proliferative defect or an increased apoptosis rate, in agreement with HSF2 expression during embryonic life in proliferative cells of the vz. In adult brain, HSF2 was expressed in some discrete cells of the ependymal layer, which is considered as a source of stem cells in the adult brain (Momma et al., 2000).
Materials and methods
Construction of the targeting vector
A fragment of the Hsf2 gene containing exon 4, the following intron and the beginning of exon 5 (Manuel et al., 1999) was fused in-phase with the β-geo gene. A further region of homology was added at the 3′ end to increase the frequency of recombination.
ES cell culture, electroporation and screening
CK35 ES cells derived from the 129/SV Pasteur strain were electroporated and selected by geneticin at 600 µg/ml (Sigma). Genomic DNA extraction and Southern blot analysis were performed as described previously (Sarig et al., 1999) with an external probe corresponding to exon 4, and generated by PCR (Table I).
Table I. PCR primers.
| Gene or RNA | PCR primers | PCR product | |
|---|---|---|---|
| Southern blotprobe | HSF2 (exon 4) | 5′-gcgGTTTCATCTTCAAAACCAGAGG-3′5′-TCTGAAAGCCTGGACTCAATAG-3′ | 119 bp |
| PCR forgenotyping | Primer 1[end of intron preceding exon 5;Manuel et al. (1999)–beginning of exon 5 nucleotides614–636 of the cDNA; Sarge et al. (1991)] | 5′-GAGTGAGAATGAATCCCTTTGGAAGG-3′ | |
| Primer 2 | 5′-GCTGGAAGCTTCTTACCTTCCG-3′ | 95 bp | |
| (end of exon 5, nucleotides 685–689 and16 first nucleotides of the following intron;reverse orientation) | |||
| Primer 3 | 5′-ATGTGCTGCAAGGCGATTAAGTTGG-3′ | 132 bp | |
| [beginning of the lacZ portion of the β-geo gene;Kalnins et al. (1983)] | |||
| RT–PCR | LH receptor | 5′-CTTATACATAACCACCATACCAG-3′ | 517 bp |
| 5′-ATCCCAGCCACTGAGTTCATTC-3′ | |||
| HSF2 | 5′-ATCCCTTTGGAAGGAGGTGT-3′ | 114 bp | |
| 5′-TCACAAGTTGATTATTCTGAACCAA-3′ | |||
| Recombinant chimeric β-geo | 5′-ATCCCTTTGGAAGGAGGTGT-3′ | 290 bp | |
| 5′-GACAGTATCGGCCTCAGGAA-3′ | |||
| GAPDH | Xu et al. (1999) | 300 bp | |
| Actin | 5′-TGTTACCAACTGGGACGACA-3′ | 396 bp | |
| 5′-CTCTCAGCTGTGGTGAA-3′ |
Generation and screening of Hsf2 mutant mice
The presence of the Hsf2 disrupted allele was detected by PCR analysis of genomic DNA on the offspring of the female chimera using a mixture of three oligonucleotides (Table I).
HSF2 detection by western blots and EMSA
Embryo extracts were prepared and western blot analysis and EMSA performed as described in Rallu et al. (1997). Anti-mouse HSF2 rabbit polyclonal antibody was used at a dilution of 1:10 000 (Sarge et al., 1993). Monoclonal antibody against HSF1 (Ab-4; Neomarkers) was used at 1:100.
Morphological analysis of the testes
Testes of wild-type and Hsf2–/– mice were isolated immediately after euthanasia by cervical dislocation. One of the two testes was fixed with Bouin’s fixative at room temperature for 24 h, embedded in paraffin, cut into 5 µm sections and stained with hematoxylin and eosin. The diameter of a total of 100 tubule cross-sections was measured from five Hsf2+/+ and Hsf2–/– mice. The second of the two testes was used for apoptosis and immunohistochemical assays.
Preparation and fixation of stage-specific monolayers of testicular cells
Isolation of defined stages of spermatogenesis was performed according to Parvinen and Vanha-Perttula (1972). Testes were isolated and decapsulated as described in Parvinen et al. (1993). For apoptosis assays and immunofluorescence, all stages of spermatogenesis were collected by cutting a short 1–3 mm segment of the seminiferous tubule from a specific stage. Segments were placed on microscope slides and covered with a coverslip to form a monolayer of cells. The squash slide was immersed in liquid nitrogen, the coverslip was removed, and the cells were fixed with ice-cold methanol:acetone (3:1) for 15 min and air dried for 60 min.
Sperm count
The epididymes of Hsf2+/+ (n = 5) and Hsf2–/– mice (n = 5) were isolated, rinsed with saline solution and placed in a Petri dish containing 3 ml of M16 medium (Sigma). The sperm were released from the epididymis into the medium by applying pressure with forceps. After removal, the sperm were allowed to capacitate for 30 min before counting with a cell counter.
Apoptosis assays
Seminiferous tubules were dissociated by incubation for 30 min at 32°C with periodic agitation in Hank’s balanced salt solution (HBSS) without Ca2+ and Mg2+ containing 25 mM HEPES, 1 mg/ml collagenase (type VIII; Sigma) and 1% bovine serum albumin (BSA). The tubules were dissociated by pipeting and the cell suspension was passed through a 100 µm silk membrane. Cells were centrifuged for 5 min at 500 g and washed with HBSS with 25 mM HEPES and 1% BSA. A 500 µl aliquot of a 1 × 106 cell suspension of fresh cells was stained with annexin V–FITC (Alexis) by mixing 500 µl of binding buffer (2.5 mM HEPES–NaOH pH 7.4, 35 mM NaCl, 0.625 mM CaCl2), 2 µl of annexin V and 1 µl/ml propidium iodide (Molecular Probes) and analyzed with a FACScan flow cytometer (Becton Dickinson) using the FL1 detector at 488 nm excitation with an argon laser. Stained cells were mixed and counted to 20 000 events. Stage-specific monolayers of testicular cells were prepared as described above and stained with annexin V–FITC using the ApoAlert kit (Clontech). TUNEL assays were performed using the Apoptag kit according to the manufacturer’s instructions (Intergen Company).
Immunofluorescence and microscopy
Anti-SCP3 antibodies (a generous gift from Dr Christer Höög; Yuan et al., 2000) were used at 1:800 dilution. The centromere regions of meiotic chromosomes were stained with human anti-centromere sera (Crest, 1:400). A population of 500 annexin V–FITC-positive Hsf2–/– cells at early stages of apoptosis were cell cycle categorized into three groups: pachytene spermatocytes, meiotically dividing spermatocytes or other cell type. A total of 200 mid-pachytene cells were scored for SC defects in five Hsf2+/+ and five Hsf2–/– mice.
Statistical analysis
Student’s t-test was used with 95% confidence interval.
Superovulation experiments and in vitro culture of one-cell stage embryos
Superovulation experiments and egg recovery from ampulla were performed as described (Hogan et al., 1994). In vitro culture of one-cell stage embryos was performed at 37°C by incubation in M16 medium (Sigma).
β-galactosidase staining and histological analysis
Whole embryos were fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). Pre-implantation embryos were fixed in 0.2% glutaraldehyde; 2% PFA. Frozen sections (16 µm) of the X-gal-stained E7.5 embryos were performed after cryoprotection of the embryos in 15% sucrose and 7% gelatin (Merck 1.04070 Mikrobiologie). X-gal-stained E13.5 embryos (Sarig et al., 1999) were sectioned by vibratome (200 µm) after embedding in gelatin/ovalbumin (0.5%; 30%). For adult animals, testes were fixed in 4% PFA after removal of the tunica albicans and soft tearing of the seminiferous tubules. X-gal-stained testes were embedded in paraffin and cut in 10 µm sections which were counterstained with eosin. Adult mice were perfused through the heart with 4% PFA for 20 min, dissected brains were sectioned by vibratome after embedding in gelatin/ovalbumin, and the 200 µm sections were stained with X-gal. Ovaries were fixed in Bouin fixative and embedded in paraffin, then cut in 6 µm sections on a microtome and stained with hematoxylin and eosin.
HSF2 immunocytochemistry
Paraffin (10 µm) or frozen (50 µm) sections of immersion-fixed neural tubes were incubated with anti-HSF2 polyclonal antibodies (Fiorenza et al., 1995) at 1:300–1:100 dilutions (Rallu et al., 1997).
BrdU labeling
BrdU (20 mg/kg) was injected intraperitoneally at E12.5 and E15.5, and pregnant females were sacrificed 2 or 3 h after. BrdU staining was carried out as described in Takahashi et al. (1992) on 50 µm frozen sections of immersion-fixed neural tubes at both stages.
Semi-quantitative RT–PCR and RT–PCR detection of HSF2
Total RNA was isolated from ovaries by using Trizol reagent (Life Technologies). A 2 µg aliquot of total RNA and 1 µg of oligo(dT) primers (Promega) were annealed. Reverse transcription was carried out with M-MLV reverse transcriptase as described by the manufacturer (Life Technologies). Aliquots of 0.5 µl and 4-fold serial dilutions were used in a 25 µl PCR mixture containing 200 µM of each deoxynucleotide (Promega), 500 nM of each primer, 2.5 µl of 10× Expand HF buffer with 15 mM MgCl2, and 0.87 U of Expand High Fidelity enzyme mix (Roche). PCR conditions were 94°C for 5 min, then 94°C for 45 s, 61°C for 1 min, 72°C for 45 s for 23–25 cycles and 72°C for 7 min.
An 18 µl aliquot of the reaction was run on a 1% agarose gel in 1× TBE and photographed on a UV transilluminator using a digital camera. The relative levels of mRNAs in different samples were determined by quantitating the intensity of the ethidium bromide-stained bands using NIH Image, and calculating the fold dilution of the starting material that would be required to obtain similar signal intensities.
Primers used for the molecular characterization of the Hsf2 KO are described in Table I.
Acknowledgments
Acknowledgements
We thank Dr Philippe Soriano for the gift of plasmid pSAβgeo, Jacqueline Barra and Charles Babinet for injecting the recombinant ES clones, Yvan Lallemand for help in the first breeding of mice, Nadine Binart and Aurélie Lucas for advice on LH receptor detection, Christer Höög for kindly providing the anti-SCP3 antibody, Henri Blomster, Andrey Mikhailov, Thomas Söderström and Cindy Lasnier for technical help, and Michael Dresser for discussions and critical comments on the manuscript. The work was supported by grants to L.S. from The Academy of Finland, The Sigrid Juselius Foundation and The Finnish Cancer Organizations, and grants to M.M. and V.M. (nos 9293 and 5939) and a fellowship to M.M. from the Association pour la Recherche sur le Cancer. T.-P.A. was supported by The Turku Graduate School of Biomedical Sciences and Y.C. by the Association Franco-Chinoise pour la Recherche Scientifique et Technique and the French Embassy in China.
References
- Alastalo T.P., Lonnstrom,M., Leppa,S., Kaarniranta,K., Pelto-Huikko,M., Sistonen,L. and Parvinen,M. (1998) Stage-specific expression and cellular localization of the heat shock factor 2 isoforms in the rat seminiferous epithelium. Exp. Cell Res., 240, 16–27. [DOI] [PubMed] [Google Scholar]
- Baudat F., Manova,K., Pui Yuen,J., Jasin,M. and Keeney,S. (2000) Chromosone synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol. Cell, 6, 989–998. [DOI] [PubMed] [Google Scholar]
- Christians E., Michel,E., Adenot,P., Mezger,V., Rallu,M., Morange,M. and Renard,J.P. (1997) Evidence for the involvement of mouse heat shock factor 1 in the atypical expression of hsp70.1 heat shock gene during mouse zygotic genome activation. Mol. Cell. Biol., 17, 778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians E., Davis,A.A., Thomas,S.D. and Benjamin,I.J. (2000) Maternal effect of Hsf1 on reproductive success. Nature, 407, 693–694. [DOI] [PubMed] [Google Scholar]
- Clermont Y. (1972) Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonia renewal. Physiol. Rev., 52, 198–236. [DOI] [PubMed] [Google Scholar]
- Eddy E.M. (1999) Role of heat shock protein HSP70-2 in spermatogenesis. Rev. Reprod., 4, 23–30. [DOI] [PubMed] [Google Scholar]
- Erickson G.F. and Shimasaki,S. (2000) The role of the oocyte in folliculogenesis. Trends Endocrinol. Metab., 11, 193–198. [DOI] [PubMed] [Google Scholar]
- Fiorenza M.T., Farkas,T., Dissing,M., Kolding,D. and Zimarino,V. (1995) Complex expression of murine heat shock transcription factors. Nucleic Acids Res., 23, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich G. and Soriano,P. (1991) Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev., 5, 1513–1523. [DOI] [PubMed] [Google Scholar]
- Henriksen K. and Parvinen,M. (1998) Stage-specific apoptosis of male germ cells in the rat: mechanism of cell death studied by supravital squash preparations. Tissue Cell, 30, 692–701. [DOI] [PubMed] [Google Scholar]
- Hogan B., Beddington,R., Costantini,F. and Lucy,E. (1994) Manipulating the Mouse Embryo. A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 131–132.
- Hong Y. and Sarge,K.D. (1999) Regulation of protein phosphatase 2A activity by heat shock transcription factor 2. J. Biol. Chem., 274, 12967–12970. [DOI] [PubMed] [Google Scholar]
- Jedlicka P., Mortin,M.A. and Wu,C. (1997) Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J., 16, 2452–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins A., Otto,K., Rüther,U. and Müller-Hill,B. (1983) Sequence of the lacZ gene of Escherichia coli. EMBO J., 2, 593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.H. (1995) The role of protein phosphatase type-2A in the Xenopus cell cycle: initiation of the G2/M transition. Semin. Cancer Biol., 6, 203–209. [DOI] [PubMed] [Google Scholar]
- Mahadevaiah S.K., Evans,E.P. and Burgoyne,P.S. (2000) An analysis of meiotic impairment and of sex chromosome associations throughout meiosis in XYY mice. Cytogenet. Cell Genet., 89, 29–37. [DOI] [PubMed] [Google Scholar]
- Manuel M., Sage,J., Mattei,M., Morange,M. and Mezger,V. (1999) Genomic structure and chromosomal localization of the mouse Hsf2 gene and promoter sequences. Gene, 232, 115–124. [DOI] [PubMed] [Google Scholar]
- Mathew A. and Morimoto,R.I. (1998) Role of the heat-shock response in the life and death of proteins. Ann. N. Y. Acad. Sci., 851, 99–111. [DOI] [PubMed] [Google Scholar]
- McMillan D.R., Xiao,X., Shao,L., Graves,K. and Benjamin,I.J. (1998) Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J. Biol. Chem., 273, 7523–7528. [DOI] [PubMed] [Google Scholar]
- Mezger V., Rallu,M., Morimoto,R.I., Morange,M. and Renard,J.P. (1994a) Heat shock factor 2-like activity in mouse blastocysts. Dev. Biol., 166, 819–822. [DOI] [PubMed] [Google Scholar]
- Mezger V., Renard,J.P., Christians,E. and Morange,M. (1994b) Detection of heat shock element-binding activities by gel shift assay during mouse preimplantation development. Dev. Biol., 165, 627–638. [DOI] [PubMed] [Google Scholar]
- Momma S., Johansson,C.B. and Frisen,J. (2000) Get to know your stem cells. Curr. Opin. Neurobiol., 10, 45–49. [DOI] [PubMed] [Google Scholar]
- Nakai A., Suzuki,M. and Tanabe,M. (2000) Arrest of spermatogenesis in mice expressing an active heat shock transcription factor 1. EMBO J., 19, 1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvinen M. and Vanha-Perttula,T. (1972) Identification and enzyme quantitation of the stages of the seminiferous epithelial wave in the rat. Anat. Rec., 174, 435–449. [DOI] [PubMed] [Google Scholar]
- Parvinen M., Toppari,J. and Lähdetie,J. (1993) Transillumination phase contrast microscopic techniques for evaluation of male germ cell toxicity and mutagenicity. In Chapin,R.E. and Heindel,J.J. (eds), Methods in Toxicology, 3. Academic Press, New York, NY, pp. 142–165.
- Pirkkala L., Nykänen,P. and Sistonen,L. (2001) Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J., 15, 1118–1131. [DOI] [PubMed] [Google Scholar]
- Qian Y.M., Sun,X.J., Tong,M.H., Li,X.P. and Richa Song,W. (2001) Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology, 142, 5342–5350. [DOI] [PubMed] [Google Scholar]
- Rallu M., Loones,M., Lallemand,Y., Morimoto,R., Morange,M. and Mezger,V. (1997) Function and regulation of heat shock factor 2 during mouse embryogenesis. Proc. Natl Acad. Sci. USA, 94, 2392–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G.S. and Bailis,J.M. (2000) The pachytene checkpoint. Trends Genet., 16, 395–403. [DOI] [PubMed] [Google Scholar]
- Romanienko P.J. and Camerini-Otero,R.D. (2000) The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol. Cell, 6, 975–987. [DOI] [PubMed] [Google Scholar]
- Sarge K.D., Zimarino,V., Holm,K., Wu,C. and Morimoto,R.I. (1991) Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes Dev., 5, 1902–1911. [DOI] [PubMed] [Google Scholar]
- Sarge K.D., Murphy,S.P. and Morimoto,R.I. (1993) Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity and nuclear localization and can occur in the absence of stress. Mol. Cell. Biol., 13, 1392–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarge K.D., Park-Sarge,O.K., Kirby,J.D., Mayo,K.E. and Morimoto,R.I. (1994) Expression of heat shock factor 2 in mouse testis: potential role as a regulator of heat-shock protein gene expression during spermatogenesis. Biol. Reprod., 50, 1334–1343. [DOI] [PubMed] [Google Scholar]
- Sarig R., Mezger-Lallemand,V., Gitelman,I., Davis,C., Fuchs,O., Yaffe,D. and Nudel,U. (1999) Targeted inactivation of Dp71, the major non-muscle product of the DMD gene: differential activity of the Dp71 promoter during development. Hum. Mol. Genet., 8, 1–10. [DOI] [PubMed] [Google Scholar]
- Schalk J.A., Dietrich,A.J., Vink,A.C., Offenberg,H.H., van Aalderen,M. and Heyting,C. (1998) Localization of SCP2 and SCP3 protein molecules within synaptonemal complexes of the rat. Chromosoma, 107, 540–548. [DOI] [PubMed] [Google Scholar]
- Sheldon L.A. and Kingston,R.E. (1993) Hydrophobic coiled-coil domains regulate the subcellular localization of human heat shock factor 2 [published erratum appears in Genes Dev., 1994, 8, 386]. Genes Dev., 7, 1549–1558. [DOI] [PubMed] [Google Scholar]
- Snaith H.A., Armstrong,C.G., Guo,Y., Kaiser,K. and Cohen,P.T. (1996) Deficiency of protein phosphatase 2A uncouples the nuclear and centrosome cycles and prevents attachment of microtubules to the kinetochore in Drosophila microtubule star (mts) embryos. J. Cell Sci., 109, 3001–3012. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nowaskowski,R.S. and Caviness,V.S. (1992) BrdU as an S-phase marker for quantitative studies of cytokinetic behaviour in the murine cerebral ventricular zone. J. Neurocytol., 21, 185–197. [DOI] [PubMed] [Google Scholar]
- Tay J. and Richter,J.D. (2001) Germ cell differentiation and synaptonemal complex formation are disrupted in CPEB knockout mice. Dev. Cell, 1, 201–213. [DOI] [PubMed] [Google Scholar]
- Tournebize R., Andersen,S.S., Verde,F., Doree,M., Karsenti,E. and Hyman,A.A. (1997) Distinct roles of PP1 and PP2A-like phosphatases in control of microtubule dynamics during mitosis. EMBO J., 16, 5537–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engeland M., Nieland,L.J., Ramaekers,F.C., Schutte,B. and Reutelingsperger,C.P. (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry, 31, 1–9. [DOI] [PubMed] [Google Scholar]
- Vermes I., Haanen,C., Steffens-Nakken,H. and Reutelingsperger,C. (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods, 184, 39–51. [DOI] [PubMed] [Google Scholar]
- Voss A.K., Thomas,T. and Gruss,P. (2000) Mice lacking HSP90β fail to develop a placental labyrinth. Development, 127, 1–11. [DOI] [PubMed] [Google Scholar]
- Walker M.Y. and Hawley,R.S. (2000) Hanging on to your homolog: the roles of pairing, synapsis and recombination in the maintenance of homolog adhesion. Chromosoma, 109, 3–9. [DOI] [PubMed] [Google Scholar]
- Wu C. (1995) Heat shock transcription factors: structure and regulation. Annu. Rev. Cell Dev. Biol., 11, 441–469. [DOI] [PubMed] [Google Scholar]
- Xiao X., Davis,A.A., McMillan,D.R., Zuo,X.X., Curry,B.B., Richardson,J.A. and Benjamin,I.J. (1999) Growth retardation, female infertility, placental insufficiency and susceptibility to endotoxemia in mice lacking HSF1. EMBO J., 18, 5943–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Liguori,G., Persico,M.G. and Adamson,E.D. (1999) Abrogation of the Cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development, 126, 483–494. [DOI] [PubMed] [Google Scholar]
- Yuan L., Liu,J.-G., Zhao,J., Brundell,E., Daneholt,B. and Höög,C. (2000) The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis and male fertility. Mol. Cell, 65, 73–83. [DOI] [PubMed] [Google Scholar]