Abstract
Gene expression profiles were examined in freshly isolated peripheral blood mononuclear cells (PBMC) from two independent cohorts (training and test sets) of glucocorticoid (GC)-sensitive (n = 64) and GC-resistant (n = 42) asthma patients in search of genes that accurately predict responders and nonresponders to inhaled corticosteroids. A total of 11,812 genes were examined with high-density oligonucleotide microarrays in both resting PBMC (106 patients) and cells treated in vitro with IL-1β and TNF-α combined (88 patients), with or without GC. A total of 5,011 genes were expressed at significant levels in the PBMC, and 1,334 of those were notably up-regulated or down-regulated by IL-1β/TNF-α treatment. The expression changes of 923 genes were significantly reversed in GC responders in the presence of GC. The expression pattern of 15 of these 923 genes that most accurately separated GC responders (n = 26) from the nonresponders (n = 18) in the training set, based on the weighted voting algorithm, predicted the independent test set of equal size with 84% accuracy. The expression accuracy of these genes was confirmed by real-time-quantitative PCR, wherein 11 of the 15 genes predicted GC sensitivity at baseline with 84% accuracy, with one gene predicting at 81% in an independent cohort of 79 patients. We conclude that we have uncovered gene expression profiles in PBMC that predict clinical response to inhaled GC therapy with meaningful accuracy. Upon validation in an independent study, these results support the development of a diagnostic test to guide GC therapy in asthma patients.
Pharmacogenomics is a discipline focused on examining the genetic basis for individual variations in response to therapeutics (1). Improved understanding of the genetic information that regulates the response of patients to drugs is critical for elucidating the molecular mechanisms involved and for the development of new and more specific therapeutic strategies (2-5). In this context, diseases such as asthma that are common in the general population and have a strong, but complex, genetic component, together with variable responsiveness to drugs, present ideal diseases to target for pharmacogenomic research (6-8). Improved treatment of asthma patients is desired because currently used drugs are not effective in all patients, allow relapse in a high percentage of patients, and sometimes have severe adverse effects. The ability to analyze the expression levels of thousands of genes in a single assay, using oligonucleotide microarrays, allows for a powerful screen of multiple molecular pathways simultaneously that may elucidate differential expression of genes that determine drug response (9, 10).
We used Affymetrix (Santa Clara, CA) high-density oligonucleotide microarrays to search for differences in mRNA expression in peripheral blood mononuclear cells (PBMC), freshly isolated from glucocorticoid (GC)-sensitive (GC-S) and GC-resistant (GC-R) asthma patients. The mRNAs were examined at baseline (resting PBMCs) and after treatment with a combination of IL-1β and TNF-α. In an attempt to further unveil genes that contribute to responsiveness of GC, we examined in vitro effects of GC treatment on gene expression in cells that were activated with IL-1β and TNF-α. The rationale for using this strategy is based on two well established concepts. First, the manifestations of asthma are, at least in part, channeled through the actions of IL-1β and TNF-α (11, 12). Second, the efficacy of GCs in asthma is, at least in part, through its effect on the expression of genes that are modulated by proinflammatory cytokines (12, 13). In this study, we show that GC responders can be separated from nonresponders with meaningful accuracy by using the expression levels of only a few genes.
Materials and Methods
Patients. The original patient list contained the names of >7,000 patients who attended the clinic of allergists practicing at the Allergy/Pulmonary Divisions of the National Hospital of Iceland from 1977 to 2001 (14). The diagnosis of asthma is based on standard diagnostic criteria outlined by the National Heart, Lung, and Blood Institute and the American Thoracic Society (ATS) (15, 16). Among asthma patients who met the inclusion criteria, 106 of both sexes, aged 18-75 years, were recruited, all of whom have physician-diagnosed asthma and were being treated with inhaled GCs. The phenotypes were confirmed by asthma specialists and included clinical assessment of response to both inhaled and oral GC therapy, pulmonary function tests, total IgE levels, and skin tests to 12 common aeroallergens, including animals, pollen, grass, mold, and dust mites. Unless baseline forced expiratory volume in first second (FEV1) was ≤75% predicted, a methacholine challenge (MCh) test was performed. The phenotype assessments, pulmonary function tests, and methacholine tests were performed according to ATS guidelines (16, 17). The patient participation rate in the study exceeded 95%. The study was approved by the Icelandic Data Protection Authority and the National Bioethics Committee. All patients signed informed consent, donated blood samples, and completed a detailed medical questionnaire and all tests necessary for accurate phenotyping. Personal identities of the patients were encrypted by the Data Protection Authority of Iceland (18). All blood and RNA samples were also coded in the same way.
Assessment of GC Responsiveness. All patients were categorized as either GC-S or GC-R based on standard clinical and laboratory parameters (19-21). The inclusion criteria for the GC-S patients enrolled included documentation of good clinical response to conventional doses of inhaled GC therapy (i.e., ≤1,000-μg inhalation of GC in 24 h) as measured by one or more of the following parameters: improvement in asthma symptoms, improved lung function tests (i.e., FEV1 and peak expiratory flow), reduced requirements for β-agonist rescue therapy, or reduced frequencies of pulmonary exacerbations when taking inhaled GC in recommended therapeutic doses. In addition, all of the GC-S participants had documented prompt reversal of at least one asthma exacerbation while taking inhaled GC (≤ 1,000 μg per day) with or without a 5-day course of oral GC.
In contrast, the GC-R patients had documentation of having failed to satisfy any of the criteria for sensitivity, and they did not experience improvement either clinically or by lung function measures from conventional therapeutic doses (i.e., ≤1,000 μg per day) of inhaled GC. In addition, the GC-R patients failed to demonstrate clinical or pulmonary function improvement on a 7- to 14-day course of oral GC (40-60 mg per day of prednisolon), a criterion widely accepted when defining GC resistance (20, 21).
Assessment of Gene Expression Using High-Density Oligonucleotide Microarrays. PBMC were isolated from the 106 asthma patients for the microarray studies by using a standardized Ficoll method (22). The blood was drawn in the morning from fasting patients who had not taken their inhaled steroids for at least 24 h. The PBMC were isolated within 3 h from blood drawing, counted, and stained with a FITC-conjugated anti-CD3 mAb, phycoerythrin-conjugated anti-CD19 mAb, phycoerythrin-conjugated anti-CD14 mAb, and FITCconjugated anti-CD4 and anti-CD8 mAbs. The cells were then examined by flow cytometry to determine the relative contributions of T cells, B cells, and monocytes. The cells were then divided into three treatment conditions (baseline, IL-1β/TNF-α treatment, and IL-1β/TNF-α in the presence of GC treatment) with ≈6 million cells per condition. In brief, the cells were exposed for 4 h to IL-1β (1 ng/ml) and TNF-α (5 ng/ml) combined or to media alone in the absence or presence of 1 h pretreatment with 10-6 M dexamethasone, and maintained at 37°C in a humidified atmosphere of 5% CO2/95% air in RPMI medium 1640. Total RNA used for the microarray expression analysis was extracted and purified by using TRIzol Reagent and QIA RNeasy spin columns, respectively (23). Thereafter, multiple gene mRNA expression was examined with the Affymetrix GeneChip technology, using the A arrays of the Human Genome U95 set, containing 11,812 different characterized sequences. Approximately 5 μg of total RNA was used for first- and second-strand cDNA synthesis. After purification, the cDNAs were in vitro-transcribed to cRNAs (23). The biotinylated cRNAs were subsequently fragmented and hybridized to the microarrays overnight according to the manufacturer's recommendations (23). Unbound probes were removed with high-stringency washing. The hybridized chips were stained by using a streptavidin-phycoerythrin complex and scanned. The scanned images were read with the Affymetrix MicroArray Suite, version 5.0 (mas5) software. The expression data were analyzed by using matlab. Expression values were defined by the standardized mas5 output. The raw data for all genes were normalized to the trimmed mean (98%) expression values of the chip, and the trimmed mean value for all chips was set at 500 (23). In addition, the genes were normalized to a set of 20 control (maintenance) genes that were found to be stable during the various treatments of the samples.
Assessment of Gene Expression Using TaqMan [Real-Time-Quantitative PCR (RT-PCR)]. RT-PCR was used to validate the predictive signals of genes that were isolated by the microarray approach, in a separate cohort of 79 patients. Patients were phenotyped, blood was collected, and total RNA from PBMC was isolated as described for the microarray cohort. Total RNA was treated with DNase I and subsequently reverse-transcribed by using the TaqMan Reverse Transcription Reagents kit (N808-0234) and random hexamers.
The final RNA concentration in the cDNA synthesis reaction was 20 ng/μl, and the thermal cycling conditions were 25°C for 10 min, 48°C for 30 min, and 95°C for 5 min. One microliter of the final cDNA was used as template for each 10-μl PCR, containing TaqMan Universal PCR Master Mix (1×), forward primers (900 nM), reverse primers (900 nM), and TaqMan probe (200 nM). TaqMan PCRs were carried out on 384-well plates on the Applied Biosystems PRISM 7900HT Sequence Detection System (95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min). All PCRs were run in duplicate for both of the predictive genes (i.e., target genes) and three control genes (i.e., ACTB, GAPD, and 18S). Standard cDNA generated from resting PBMC was loaded in duplicate onto each PCR plate and used for assay calibration, allowing for comparison between plates within the study. After PCR, quality control and normalization of the raw results were performed. First, the threshold cycle difference (CT) was examined for each duplicate, including standard cDNAs, and samples exhibiting (CT) > 1 were discarded in the analysis. Second, the average CT value for the target gene and the constant average CT value for the standard cDNA were used to calculate the calibrated expression quantity Q for each sample within, with Q = 2-(deltaCT), where (deltaCT) = CT(Target) - CT(Standard). Finally, Q values for target genes were normalized to the geometric mean of the sample-matched values for the three control genes (24).
Classification of Drug Response Phenotypes. The analysis was focused on the 923 genes that were significantly induced or down-regulated by the in vitro cytokine stimulation and were subsequently reversed in the GC-responder cohort by the GC treatment. A predictive classifier was then applied to these genes. Three separate approaches were taken to search for predictive gene signals: (i) baseline expression values alone, (ii) expression values in response to in vitro exposure to cytokines, and (iii) expression values in response to cytokines after GC pretreatment.
Prediction of an Independent Test Set. The GC-S and GC-R patient cohorts were each split into training and test sets of equal size. The training set for the first run was put together by taking the most resistant vs. most sensitive patients based on clinical judgment. This process was done to ensure that the genes selected were based on expression differences among the most extreme phenotypes (i.e., the most difficult vs. the easiest patients to treat). The predictive accuracy of these genes was subsequently confirmed by RT-PCR. A random split of patients into training and test sets was also performed multiple times with the highest predictive genes selected each time to analyze for variance in the predictive accuracy. A detailed description of the method used is given in Supporting Text, which is published as supporting information on the PNAS web site.
Microarray Data and Reagents. All microarray data are available on request and for a list of reagents, see Supporting Text.
Results
Characteristics of Patients. After screening through ≈1,200 medical records, 680 patients using inhaled GC qualified for the study. Of those, 492 were clinically determined to be GC-S and 88 GC-R (see Materials and Methods). One hundred and six patients (64 GC-S and 42 GC-R) were randomly recruited to participate in the microarray study. Sixteen nonatopic, nonasthmatic (control) subjects who were not using GC drugs were also enrolled for comparison. An additional 79 patients (40 GC-S and 39 GC-R) were recruited to participate in the RT-PCR validation study. Table 1 includes demographic information and results from lung function, MCh, total IgE, and skin prick tests for the patient cohorts. The patients were asked to stop taking their inhaled corticosteroids for at least 24 h before sample collection and none of the patients were taking oral steroids within a month before the study. All patients were carefully assessed for inhalation techniques. The GC-S patients were well controlled on a low- to medium-dose therapy and all were using ≤1,200 μg per day of inhaled GC (mean ≈419 μg per day) as a maintenance therapy. In contrast, the GC-R patients failed to improve in lung function after 1-2 weeks of therapy with 40-60 mg per day of oral prednisolon (Table 2, which is published as supporting information on the PNAS web site). The asthma severity level was slightly higher in the GC-R group than in the GC-S group, as reflected by lower baseline pulmonary function test values. The cohorts also matched unevenly for age and sex, but the difference was not significant. Likewise, prediction of GC response based on clinical parameters such as lung function, age, or sex alone was also not significant, suggesting these variables were not impacting the GC-S/GC-R prediction in a significant way. Fifty-five percent of the GC-S and 36% of the GC-R patients were atopic as defined by a positive skin test. No difference in smoking history was detected between the two groups (24%), and all of the smokers had <20-pack years with no current smoker. No difference was observed in response to short-acting β-agonist therapy between the GC-S and GC-R patients. No differences were observed between the groups in the ratio of T cells to B cells or monocytes in the isolated mononuclear cell fraction (Table 3, which is published as supporting information on the PNAS web site).
Table 1. Demographic data, lung function, MCh, and total IgE values in GC-S and GC-R patients.
| Asthma
|
|||
|---|---|---|---|
| Characteristics | GC-S | GC-R | Control |
| Training set | |||
| n | 32 | 21 | 16 |
| Mean age, yr | 45 ± 17 | 55 ± 19 | 34 ± 12 |
| Sex, male/female | 10/22 | 4/17 | 7/9 |
| Forced vital capacity (FVC) | |||
| % predicted | 83 ± 19 | 76 ± 17 | |
| Liters | 3.7 ± 1.1 | 3.0 ± 1.0 | |
| FEV1 | |||
| % predicted | 81 ± 20 | 73 ± 16 | |
| Liters | 3.1 ± 1.0 | 2.1 ± 0.9 | |
| FEV1/FVC ratio | 79 ± 10 | 72 ± 12 | |
| MCh20 (mg/liter) | 3.1 ± 1.4 | 0.6 ± 1.2 | |
| Positive skin tests, % | 51 | 31 | |
| Total IgE, geometric means | 74 units/liter | 37 units/liter | |
| Test set | |||
| n | 32 | 21 | 16 |
| Mean age, yr | 43 ± 17 | 54 ± 18 | 34 ± 12 |
| Sex, male/female | 10/22 | 3/18 | 7/9 |
| FVC | |||
| % predicted | 85 ± 18 | 79 ± 16 | |
| Liters | 3.9 ± 1.0 | 3.4 ± 0.9 | |
| FEV1 | |||
| % predicted | 84 ± 20 | 77 ± 17 | |
| Liters | 3.3 ± 1.0 | 2.3 ± 0.8 | |
| FEV1/FVC ratio | 79 ± 10 | 72 ± 12 | |
| MCh20 (mg/liter) | 3.7 ± 1.3 | 0.8 ± 1.1 | |
| Positive skin tests, % | 58 | 34 | |
| Total IgE, geometric means | 82 units/liter | 40 units/liter | |
| Cohort | |||
| n | 40 | 39 | |
| Mean age, yr | 39 ± 16 | 48 ± 16 | |
| Sex, male/female | 9/31 | 12/27 | |
| FVC | |||
| % predicted | 89 ± 16 | 80 ± 17 | |
| Liters | 4.0 ± 0.9 | 3.3 ± 0.9 | |
| FEV1 | |||
| % predicted | 86 ± 19 | 78 ± 16 | |
| Liters | 3.4 ± 0.9 | 2.5 ± 0.8 | |
| FEV1/FVC ratio | 78 ± 10 | 72 ± 11 | |
| MCh20 (mg/liter) | 4.7 ± 1.3 | 1.8 ± 1.1 | |
| Positive skin tests, % | 56 | 41 | |
| Total IgE, geometric means | 101 units/liter | 69 units/liter | |
The values expressed include the mean ± SD for the training and test sets used in the microarray analysis at baseline and the cohort used in the RT-PCR analysis.
Performance of the Predictive Classifier with Present-Call Selected Genes. The 5,011 genes that were called present by the mas5 software in at least 50% of the sample were used in the analysis. To determine whether the microarray expression signals could be used to predict GC responsiveness at baseline, a classifier was trained on all 5,011 genes, using the weighted voting algorithm as described. The accuracy of predicting the test sets amounted to 59% at best. A method of leave-one-out cross validation (LOOCV) was used to assess the accuracy of the complete data set in predicting GC responsiveness. As shown in Fig. 3, which is published as supporting information on the PNAS web site, a panel of 11 genes that most accurately distinguished GC-responders (n = 64) from nonresponders (n = 42) at baseline predicted the GC response phenotype with 60% accuracy.
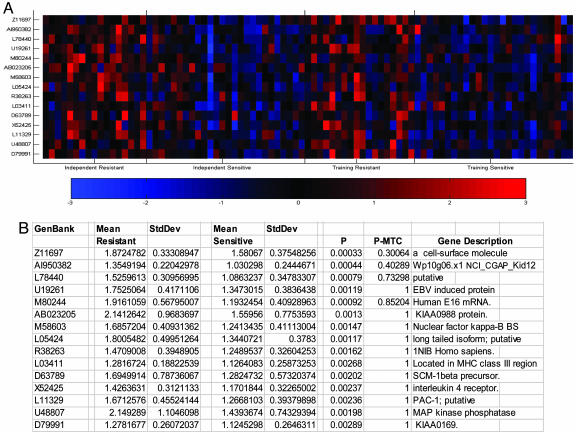
In view of these results, we postulated that using expression levels induced by the cytokines in the absence or presence of in vitro GC exposure might increase the potentiality to distinguish GC-S from GC-R patients. Accordingly, we next focused the analysis on genes whose average expression level across the sample was induced or down-regulated at least 1.5-fold from the baseline condition in response to IL-1β/TNF-α (n = 1,334) and then reversed in the GC-S cohort in the presence of GC treatment (n = 923). The latter gene list was then used to extract the fewest genes that most accurately differentiated between the most extreme GC-S and GC-R patients in the IL-1β/TNF-α treatment condition. When 87 patients who had complete microarray data for all three treatment conditions had been split into a training set and an independent test set of equal size, the patients phenotype of the test set could be predicted with an average accuracy of 84% by using 15 genes (Fig. 1). The accuracy of the classifier was determined by dividing the number of samples predicted correctly by the total number of samples tested. Student's t test, with and without Bonferroni multiple testing correction, was also performed to determine whether the difference between the means for the groups was significant, and the results are shown in Fig. 1B.
Fig. 1.
PBMC-derived gene expression profile predicting GC-R from GC-S asthma patients after exposure to IL-1β/TNF-α. (A) Differential expression of 15 genes that most accurately separated GC responders from nonresponders in the training set after cytokine treatment is shown. Genes were ranked by a metric similar to signal to noise and were considered the most differentially expressed genes according to the metric used. For each gene shown, red indicates a high level of expression relative to the mean; blue indicates a low level of expression relative to the mean. (B) Values expressed as mean ± SD are shown for the independent group. P values were obtained with a Student's t test with (P-MTC) or without (P) Bonferroni multiple testing correction. P < 0.05 is considered significant.
mRNA expression profiles were also examined in 16 nonasthmatic (control) subjects by using the above in vitro culturing approach to include additional nonasthmatic/nonatopic (control) subjects. Overall, the pattern of mRNA expression in the control subjects was similar to that of the GC-S patients (data not shown). Because >80% of individuals, in general, are GC-responders these results are in keeping with expectations.
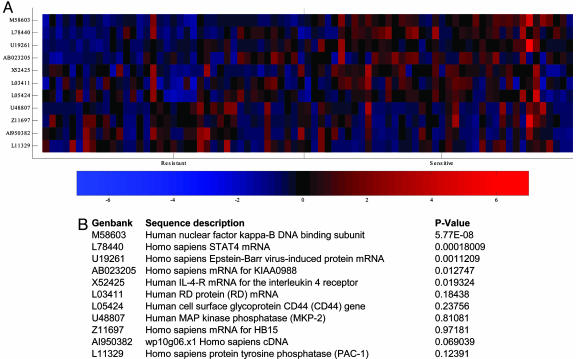
Predictive Accuracy of TaqMan Assays (RT-PCR). To further validate the efficacy of the gene array approach in selecting genes that accurately predict GC responders from nonresponders, quantitative PCR was successfully performed on 11 of the 15 genes (Fig. 1B) that gave the best signal in the analysis by using a training set with the most extreme phenotypes (three assays failed in design and one failed to give a signal). LOOCV was then performed on the TaqMan data, choosing from 1-11 genes. Given the complexity of the cytokine-induced conditions, our goal was to determine whether the baseline condition in the more sensitive RT-PCR assay could be used to make an accurate prediction. Indeed, the baseline values yielded a predictive accuracy of 84% by using all 11 genes. The quantitative PCR results are expressed in Fig. 2A. Student's t test was also done to test for differences in the means of the resistant and sensitive patient groups, and the associated P values can be seen in Fig. 2B. One gene (NFKB) predicted the GC responsiveness with 81.25% accuracy, and the corresponding confusion matrix for the LOOCV is presented in Table 4, which is published as supporting information on the PNAS web site.
Fig. 2.
PBMC-derived gene expression profile predicting GC-R from GC-S asthma patients by using RT-PCR. (A) Differential expression of 11 of the 15 best genes identified by the microarray approach based on TaqMan RT-PCR measurements in the baseline samples is shown. Genes were ranked by a metric similar to signal to noise and were considered the most differentially expressed genes according to the metric used. For each gene shown, red indicates a high level of expression relative to the mean; blue indicates a low level of expression relative to the mean. (B) Values are expressed as mean ± SD.
Discussion
Pharmacogenomics holds the promise that some day it will be possible to profile the variations in the genetic makeup that accurately predict drug responses, addressing both efficacy and safety issues (7, 8). As an early step in that process, this study determined whether GC-S asthma patients could be distinguished from GC-R patients by examining gene expression profiles in peripheral white blood cells; the results demonstrate that it can be done with clinically meaningful accuracy.
GCs are the most effective drugs available in asthma therapy (19). In sensitive individuals, inhalation of GCs at doses <1,000 μg per day has been shown to have relatively little capacity to activate transcription within PBMC at concentrations found in plasma, and their action is thought to occur mainly within the lung (25). This finding is in keeping with their relatively restricted systemic side effects at low or intermittent doses, whereas their repression of transcription factor activities, such as AP-1 and NF-κB, in the airways concurs with their clinical efficacy in GC-S patients (25). In contrast, GC-R patients may suffer serious side effects because of escalation of drug dose caused by hyporesponsiveness. GC resistance has been defined as the lack of a response to a prolonged course of high-dose (0.5-1.0 mg/kg per day) oral GC (20, 21). Because modern asthma therapy is largely centered on inhaled GC, a definition referring to GC-R asthma taking into account the inhalation route in the use of these drugs has been proposed (26).
In this study, we defined GC-R asthma as a condition where there is lack of improvement in morning prebronchodilator FEV1 values after a 1- to 2-week course of 40-60 mg per day of oral GC. In addition, the GC-R patients failed to improve clinically in response to conventional therapeutic doses of inhaled GC (19, 25, 26), and high doses of inhaled GC as maintenance therapy failed to suppress their airway inflammation as reflected by the MCh test results (Table 1). In contrast, most of the GC-R subjects demonstrated transient improvement in FEV1 values in response to bronchodilator therapy, further supporting the concept that these patients have persistent airway inflammation that is not alleviated by GC (Table 2).
To search for biomarkers of GC sensitivity in asthma, mRNA expression profiles were generated from PBMC to identify genes that accurately distinguish between GC-S and GC-R patients. The GC response phenotype was predicted with up to 60% accuracy at baseline. In an attempt to improve the predictive accuracy, we next examined gene expression profiles from cytokine-treated PBMC, and 923 genes showed profiles that were modulated by the in vitro IL-1β/TNF-α treatment and subsequently reversed by GC in the GC-S patients. When we searched for the fewest genes among those 923 that most accurately separated the GC response phenotype in the training set among the most extreme phenotypes, 15 genes were found that predicted the independent test set with 84% accuracy (Fig. 1). When RT-PCR was used to determine whether any one or combination of these 15 genes would distinguish GC-S from GC-R patients at baseline, 11 genes yielded reliable data and predicted the GC response phenotype of an independent cohort of 79 asthma patients with 84% accuracy. Notably, the NFKB gene alone predicted this phenotype with 81.25% accuracy.
Because these 15 genes were chosen from the patients with the most severe phenotypes we expected them to have better predictive accuracy than genes selected by randomly splitting the groups. This hypothesis was tested by selecting 10 equally sized training and test sets at random, wherein each training set was used to predict the corresponding independent test set by using 1-50 genes that best distinguished between the sensitive and resistant patients in the training set. The accuracy of applying random selection of patients in the training vs. test sets was 75% at best by using 30 genes in the IL-1β/TNF-α-treated test cohort. However, the accuracy improved to 79% with the LOOCV method. Taken together, these results demonstrate that pheno-type prediction based on microarray gene screening can be replicated in an independent patient cohort by using RT-PCR. This finding suggests that GC response genes identified by microarray screening could be used as molecular markers of GC responsiveness upon further validation in a multicenter patient cohort. It is also possible that genetic variations in one or more of these genes may together capture equivalent or better accuracy in distinguishing GC responders from nonresponders.
Several studies have reported association between asthma and the X chromosome (27-29). In our study, nearly 80% of the GC-R patients were females. Although many of these studies have demonstrated association between genes on the X chromosome and asthma, further genetic and epidemiological studies are needed to determine whether such an association truly exists. The observed difference in atopy status between the two groups is also noteworthy with fewer GC-R patients having atopy. Most reports indicate that ≈70% of patients in the United States and Europe are atopic (30), whereas ≈50% of Icelandic asthma patients have atopy (31). Because age, sex ratio, and atopy status of the RT-PCR cohort were more even in the GC-S and GC-R groups, it is unlikely that the predictive gene signals were skewed by these factors.
As reflected by the MCh20 values in Table 1, patients with GC-R asthma remain hyperresponsive despite chronic treatment with high doses of GC. These data are consistent ongoing inflammation of the airways as reported (32-36). In view of the potential heterogeneity and complexity of the mechanisms contributing to GC resistance and its potential impact on the natural history of chronic asthma, it is noteworthy that many of the genes in Fig. 1B encode transcription factors and cell signaling molecules that have been implicated in immune functions and asthma. Indeed, the finding that the gene encoding the NKκB DNA binding subunit (NFKB1) is the gene that confers the best prediction by the RT-PCR method is compelling. In this regard, NFκB is activated by extracellular signals and cell-to-cell interactions that are converted into intracellular activation signals through receptor molecules located in the cell membrane. A large number of genes are being translated after NFκB activation, including cytokines, chemokines, growth factors, cellular ligands, and adhesion molecules, many of which have been strongly associated with asthma. It is also common knowledge that the efficacy of GC drugs in asthma is, at least in part, related to their efficacy in inhibiting transcription factors such as NFκB. Thus, NFκB is an exciting candidate as one of the key culprit genes responsible for GC-R asthma. STAT-4 and IL-4-R also predicted well, and both are genes that have been strongly associated with asthma. Whether the genes in the predictive panel are only reactive or whether they play a regulatory role in GC responsiveness in asthma remains to be determined. It should be kept in mind that the response of PBMC to steroids may not reflect resistance to steroids in the cells of the airways.
Two forms of GC-R asthma have been reported, primary and acquired types (34-36). The acquired form (type I) has been associated with abnormally reduced GC receptor ligand and DNA binding affinity, whereas type II GC-R asthma has been associated with primary GC receptor binding abnormality. In both forms, there is lack of GC-mediated inhibition of expression and release of molecules in PBMC, including the cytokines, IL-13, and IL-4 (34-36). We observed reduced GC-mediated inhibition on the release of IL-13 in PBMC from GC-R compared with the GC-S patients (data not shown).
Although the majority of patients with asthma respond favorably to inhaled and systemic GCs, up to 1/3 of patients with difficult-to-control asthma have poor clinical responses to high doses of systemic GCs (37). Given the recent increase in asthma prevalence and severity worldwide, GC resistance has become a challenging health problem that is costly to the health-care system (38-41). Indeed, ≈2/3 of the annual $14 billion in health-care expenditure on asthma in the United States is used to treat the sickest 15% of asthma patients in the emergency room or hospital settings (40, 42, 43). Most of these patients are relatively resistant to GC therapy, so early identification of these patients would provide for an alternative therapy and could minimize serious side effects from long-term systemic GC therapy. Therefore, a test that would identify GC-R patients with >80% accuracy could both reduce the number of patients who would be getting a drug they do not respond to and allow these patients to be treated sooner with more effective therapy. Because up to 15% of asthma patients are GC-R, the application of such a test could reduce the number of steroid-resistant patients who would be receiving insufficient therapy from 15% to <3%, (i.e., by >80%). It would also encourage referral of these patients to asthma specialist centers where alternative therapy (such as rhuMAb-E25, IVIG, methotrexate, cyclosporin, and other immunomodulatory drugs) may be prescribed to reduce cost and minimize asthma morbidity (41-43). This test would also allow for a targeting of the more expensive asthma drugs to those patients who really need them, leading to more effective and less costly health-care management.
With the exception of certain tumor markers, there are no tests used in the clinic today to predict patient response to therapy. A few recent studies have attempted to find correlations between variation in certain candidate genes and clinical response to drugs such as albuterol, cholinesterase inhibitors, and statins (44-46). These studies report significantly increased relative risks but the corresponding penetrances are quite low and do not approach predictive accuracy in the mid-80s that we report here. The approach that we describe here has several advantages over the candidate gene approach. First, it represents a genomewide search for the most important genes correlating with response and is not restricted to an assessment of a limited set of genes. Second, the predictive accuracy of this system of expression information will likely be higher than that derived from genetic variation from a single gene. Comparable predictive accuracies with DNA-based tests will probably require the identification of several interacting genes whose variances together predict response.
In conclusion, in this study we compared gene expression profiles in freshly isolated PBMC from GC-R and GC-S asthma patients by using Affymetrix microarrays and determined that differential expression of a few genes could with high accuracy tell GC responders from nonresponders among asthma patients. The results show that: (i) when trained on baseline expression values (n = 106) the accuracy of the classifier in distinguishing GC responders from nonresponders with LOOCV was 60%; (ii) 1,334 genes were up-regulated or down-regulated by the in vitro treatment with cytokines and of those, 923 were reversed by GC treatment in the GC-S group; (iii) expression pattern of 15 of these 923 genes that most accurately separated a training set with the most extreme phenotypes, based on the weighted voting algorithm, predicted the test set with 84% accuracy; and (iv) the expression accuracy of these genes was confirmed by RT-PCR, with one gene predicting at 81% accuracy at baseline. This study characterizes gene expression profiles in freshly isolated PBMC that accurately differentiate between GC-R and GC-S patients and provides sufficient power to predict clinical response to GCs in asthma patients with >80% accuracy. Collectively, these results suggest that this pharmacogenomic approach may lead to the development of novel therapeutic strategies and diagnostic tests.
Supplementary Material
Acknowledgments
We thank the patients and the control subjects whose contributions have made this study possible; study coordinator Lovisa Gudmundsdottir; the Noatun blood drawing facility; and the deCODE bioinformatic software group for their valuable contributions. This study was sponsored by deCODE genetics.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PBMC, peripheral blood mononuclear cells; GC, glucocorticoid; GC-S, GC-sensitive; GC-R, GC-resistant; MCh, methacholine challenge; FEV1, forced expiratory volume in first second; RT-PCR, real-time-quantitative PCR; LOOCV, leave-one-out cross validation; FVC, forced vital capacity.
References
- 1.Cartwright, C. P. (2001) Exp. Rev. Mol. Diagn. 1, 371-376. [DOI] [PubMed] [Google Scholar]
- 2.Roses, A. D. (2002) Life Sci. 70, 1471-1480. [DOI] [PubMed] [Google Scholar]
- 3.Roses, A. D. (2001) Nature 405, 857-865. [DOI] [PubMed] [Google Scholar]
- 4.Grant, S. F. (2001) Trends Pharmacol. Sci. 22, 3-4. [DOI] [PubMed] [Google Scholar]
- 5.Marton, M. J., DeRisi, J. L., Bennett, H. A., Iyer, V. R., Meyer, M. R., Roberts, C. J., Stoughton, R., Burchard, J., Slade, D. & Dai, H. (1998) Nat. Med. 4, 1293-1301. [DOI] [PubMed] [Google Scholar]
- 6.McLeod, H. L. & Evans, W. E. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 101-121. [DOI] [PubMed] [Google Scholar]
- 7.Fenech, A. & Hall, I. P. (2002) Br. J. Clin. Pharmacol. 53, 3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall, I. P. (2002) Respir. Res. 3, 10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran, M. E. (1998) Curr. Opin. Biotechnol. 9, 565-572. [DOI] [PubMed] [Google Scholar]
- 10.Roses, A. D. (2000) Lancet 355, 1358-1361. [DOI] [PubMed] [Google Scholar]
- 11.Kim, M. H. & Agrawal, D. K. (2002) J. Asthma 39, 441-448. [DOI] [PubMed] [Google Scholar]
- 12.Hakonarson, H., Halapi, E., Whelan, R., Gulcher, R. J., Stefansson, K. & Grunstein, M. M. (2001) Am. J. Respir. Cell Mol. Biol. 25, 761-771. [DOI] [PubMed] [Google Scholar]
- 13.Vecchiarelli, A., Siracusa, A., Cenci, E., Puliti, M. & Abbritti, G. (1992) Clin. Exp. Allergy 22, 365-370. [DOI] [PubMed] [Google Scholar]
- 14.Hakonarson, H., Bjornsdottir, U. S., Halapi, E., Palsson, S., Adalsteinsdottir, E. A., Gislason, D., Finnbogason, G., Gislason, T., Kristjansson, K., Arnason, T., et al. (2002) Am. J. Hum. Genet. 71, 483-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health (1997) National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma, NIH Publication 97-4051 (U.S. Government Printing Office, Washington, DC).
- 16.American Thoracic Society (1994) Am. J. Respir. Crit. Care Med. 152, 107-1136. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society (2000) Am. J. Respir. Crit. Care Med. 161, 309-329. [DOI] [PubMed] [Google Scholar]
- 18.Gulcher, J. R., Kristjansson, K., Gudbjartsson, H. & Stefansson K. (2000) Eur. J. Hum. Genet. 8, 739-742. [DOI] [PubMed] [Google Scholar]
- 19.Barnes, P. J., Pedersen, S. & Busse, W. W. (1998) Am. J. Respir. Crit. Care Med. 157, S1-S53. [DOI] [PubMed] [Google Scholar]
- 20.Sher, E. R., Leung, D. Y., Surs, W., Kam, J. C., Zieg, G., Kamada, A. K. & Szefler, S. J. (1994) J. Clin. Invest. 93, 33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan, M. T., Leung, D. Y., Szefler, S. J. & Spahn, J. D. (1998) J. Allergy Clin. Immunol. 101, 594-601. [DOI] [PubMed] [Google Scholar]
- 22.Vries, T. J., Fourkour, A., Punt, C. J., Ruiter, D. J. & van Muijen, G. N. (2000) Melanoma Res. 10, 119-126. [PubMed] [Google Scholar]
- 23.de Wodicka, L., Dong, H., Mittmann, M., Ho, M. H. & Lockhart, D. J. (1997) Nat. Biotechnol. 15, 1359-1367. [DOI] [PubMed] [Google Scholar]
- 24.Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A. & Speleman, F. (2002) Genome Biol. 18, 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagliardo, R., Chanez, P., Vignola, A. M., Bousquet, J., Vachier, I., Godard, P., Bonsignore, G., Demoly, P. & Mathieu, M. (2000) Am. J. Respir. Crit. Care Med. 162, 1307-1322. [DOI] [PubMed] [Google Scholar]
- 26.Leung, D. V. & Chrousos, G. P. (2000) Am. J. Respir. Crit. Care Med. 162, 1-3. [DOI] [PubMed] [Google Scholar]
- 27.Kauppi, P., Laitinen, T., Ollikainen, V., Mannila, H., Laitinen, L. A. & Kere, J. (2000) Eur. J. Hum. Genet. 8, 788-792. [DOI] [PubMed] [Google Scholar]
- 28.Heinzmann, A., Mao, X. Q., Akaiwa, M., Kreomer, R. T., Gao, P. S., Ohshima, K., Umeshita, R., Abe, Y., Braun, S., Yamashita, T., et al. (2000) Hum. Mol. Genet. 9, 549-559. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed, S., Ihara, K., Sasaki, Y., Nakao, F., Nishima, S., Fujino, T. & Hara, T. (2000) Exp. Clin. Immunogenet. 17, 18-22. [DOI] [PubMed] [Google Scholar]
- 30.Eggleston, P. A. & Bush, R. K. (2001) J. Allergy Clin. Immunol. 107, S403-S405. [DOI] [PubMed] [Google Scholar]
- 31.The European Community Respiratory Health Survey Group (1997) Am. J. Respir. Crit. Care Med. 156, 1773-1780. [DOI] [PubMed] [Google Scholar]
- 32.Lane, S. J. & Lee, T. H. (1996) Am. J. Respir. Crit. Care Med. 154, S49-S51. [DOI] [PubMed] [Google Scholar]
- 33.Bamberger, C., Bamberger, A. M., de Castro, M. & Chrousos, G. P. (1995) J. Clin. Invest. 95, 2435-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sousa, A. R., Lane, S. J., Cidlowski, J. A., Staynov, D. Z. & Lee, T. H. (2000) J. Allergy Clin. Immunol. 105, 943-950. [DOI] [PubMed] [Google Scholar]
- 35.Lane, S. J. (1997) Br. J. Hosp. Med. 57, 94-398. [Google Scholar]
- 36.Chikanza, L. C. & Panayi, G. S. (1993) Eur. J. Clin. Invest. 23, 45-50. [DOI] [PubMed] [Google Scholar]
- 37.Leung, D. Y., Spahn, J. D. & Szefler, S. J. (1999) Allergy Asthma Proc. 20, 9-14. [DOI] [PubMed] [Google Scholar]
- 38.Woolcock, A. J. (1993) Eur. Respir. J. 6, 743-747. [PubMed] [Google Scholar]
- 39.Weiss, K. B., Gergen, P. J. & Hodgson, T. A. (1992) N. Engl. J. Med. 211, 862-866. [DOI] [PubMed] [Google Scholar]
- 40.Weiss, K. B. & Wagener, D. K. (1990) J. Am. Med. Assoc. 264, 1683-1687. [PubMed] [Google Scholar]
- 41.Smith, D. H., Malone, D. C., Lawson, K. A., Okamoto, L. J., Battista, C. & Saunders, W. B. (1997) Am. J. Respir. Crit. Care Med. 156, 787-793. [DOI] [PubMed] [Google Scholar]
- 42.Stanford, R., Mclaughlin, T. & Okamoto, L. (1999) Am. J. Respir. Crit. Care Med. 160, 211-215. [DOI] [PubMed] [Google Scholar]
- 43.Lang, D. M. & Polansky, M. (1994) N. Engl. J. Med. 331, 1542-1546. [DOI] [PubMed] [Google Scholar]
- 44.Drysdale, C. M., McGraw, D. W., Stack, C. B., Stephens, J. C., Judson, R. S., Nandabalan, K., Arnold, K., Ruano, G. & Liggett, S. B. (2000) Proc. Natl. Acad. Sci. USA 97, 10483-10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poirier, J. (1999) Mol. Diagn. 4, 335-341. [DOI] [PubMed] [Google Scholar]
- 46.Kuivenhven, J. A. (1988) N. Engl. J. Med. 338, 86-93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.