Abstract
This study documents the identity of a calcium- regulated membrane guanylate cyclase transduction system in the photoreceptor-bipolar synaptic region. The guanylate cyclase is the previously characterized ROS-GC1 from the rod outer segments and its modulator is S100β. S100β senses increments in free Ca2+ and stimulates the cyclase. Specificity of photoreceptor guanylate cyclase activation by S100β is validated by the identification of two S100β-regulatory sites. A combination of peptide competition, surface plasmon resonance binding and deletion mutation studies has been used to show that these sites are specific for S100β and not for another regulator of ROS-GC1, guanylate cyclase-activating protein 1. One site comprises amino acids (aa) Gly962–Asn981, the other, aa Ile1030–Gln1041. The former represents the binding site. The latter binds S100β only marginally, yet it is critical for control of maximal cyclase activity. The findings provide evidence for a new cyclic GMP transduction system in synaptic layers and thereby extend existing concepts of how a membrane-bound guanylate cyclase is regulated by small Ca2+-sensor proteins.
Keywords: calcium/membrane guanylate cyclase/retinal synapse/ROS-GC1/S100β
Introduction
Visual perception is a complex process involving transformation of the patterns of light and darkness received by the retinal photoreceptors into the images of shape defined with depth and color intensity in the visual cortex of the brain. The process involves flow of information from the photoreceptor to the bipolar and ganglion retinal neurons. Each of these neurons acts as an intermediate ‘processing-unit’. The information flows in the form of action potentials, causing hyper- or de-polarization in the membranes of the neurons. The outer segments of the rods (or cones) (rod outer segment, ROS) are the sites where the light signal is generated and converted into an electrical signal. The biochemical process involved in the conversion is termed phototransduction (reviewed in Pugh et al., 1997; Koch et al., 2002). Two critical molecules of phototransduction are Ca2+ and cyclic GMP; they are interlocked. The captured photon causes a decline in the level of cyclic GMP, closure of the cyclic GMP-gated cation channel, and hyperpolarization of the photoreceptor plasma membrane. This results in the fall of intracellular Ca2+ from ∼500 nM in the dark-adapted photoreceptor cell to <100 nM and in the activation of a ROS membrane guanylate cyclase (ROS-GC). Increased synthesis of cyclic GMP along with the inactivation of the phototransduction cascade leads to the reopening of the cyclic GMP-gated channels and, thus, restoration of the dark current of the photoreceptors. The identity of native ROS-GC has been revealed by its direct purification from the bovine ROS (Koch, 1991; Margulis et al., 1993), the site of phototransduction; by its subsequent cloning from the bovine retina based on the sequences of four peptides (Goraczniak et al., 1994); and by its Ca2+-dependent reconstitution (Duda et al., 1996a). This original form of ROS-GC has been renamed ROS-GC1 (retGC1, GC-E; Shyjan et al., 1992; Yang et al., 1995) with the discovery of a new form, ROS-GC2 (Goraczniak et al., 1998) (retGC2, GC-F; Lowe et al., 1995; Yang et al., 1995). So far, most of the biochemical work has focused on ROS-GC1.
Reconstitution studies with recombinant ROS-GC1 have indicated that it can sense changes in free Ca2+ concentrations through either of the two Ca2+-binding proteins named guanylate cyclase-activating proteins (GCAPs) 1 and 2 (Duda et al., 1996a; Krishnan et al., 1998; Dizhoor and Hurley, 1999). Each GCAP binds to a specific module in ROS-GC1, which resides in the intracellular region of the cyclase (Duda et al., 1996a; Krishnan et al., 1998; Lange et al., 1999; Sokal et al., 1999; Krylov and Hurley, 2001). In the current phototransduction model, GCAPs and ROS-GC1 form a permanent complex in native ROS (reviewed in Koch et al., 2002). GCAPs sense light-dependent Ca2+ fluctuations and, in turn, cause proportionate changes in ROS-GC1 activity. In this way, three pivotal components of phototransduction—Ca2+, GCAPs and ROS-GC1—are physiologically connected. In the dark phase, free Ca2+ concentration is ∼500 nM, Ca2+ is bound to GCAP and the cyclase is in its basal state. Increasing levels of photoillumination intensity cause graded decrements of Ca2+. These cause concomitant increments in the cyclase activity, which, in turn, adjust the cyclic GMP concentration in the photoreceptor cell.
Until recently, the ROS-GC1 signaling pathway was considered to be exclusively present in ROS, where it is linked with phototransduction. However, two lines of evidence suggest that this concept needs revision. First, there is immunocytochemical evidence that a ROS-GC is present in the synaptic layers of the retina (Liu et al., 1994; Cooper et al., 1995). These layers have been collectively termed the plexiform layers, denoted by the term fraction ‘P’ (Redburn and Thomas, 1979). The immunohistochemical studies, however, made no differentiation between whether the detected cyclase was ROS-GC1 or ROS-GC2 (Liu et al., 1994; Cooper et al., 1995). Secondly, S100β, purified from the retina (Pozdnyakov et al., 1995) and then cloned from the bovine retinal library (Pozdnyakov et al., 1997), stimulated ROS-GC1 at micromolar (>250 nM) Ca2+ concentrations (Pozdnyakov et al., 1995; Margulis et al., 1996). This Ca2+-regulated feature of ROS-GC1 regulation was subsequently confirmed through studies in the heterologous expression system of COS cells in which the recombinant ROS-GC1 and S100β were the sole interacting components (Duda et al., 1996b). S100β stimulated ROS-GC1 from submicromolar to the micromolar Ca2+ range, with a K½ activation of ∼1 µM (Duda et al., 1996b).
It was postulated, that Ca2+-dependent regulation of ROS-GC1 by S100β occurs in retinal synaptic regions, where it is involved in synapse function (Pozdnyakov et al., 1995; Duda et al., 1996b; Margulis et al., 1996). A first step towards deciphering a possible role of ROS-GC1 therein would be to demonstrate co-localization of these two proteins and to show that ROS-GC1 activity is modulated by Ca2+ in retinal synaptosomal preparations. Specificity of ROS-GC1 activation by S100β is a second crucial task to investigate. Identification of a specific target site in ROS-GC1 for S100β would argue in favor of a S100β/ROS-GC1 signaling pathway in synaptosomal preparations. These questions are addressed in the present paper and evidence is provided that (i) ROS-GC1 and S100β are co-localized in synaptosomal fraction; (ii) ROS-GC1 responds to Ca2+-spikes via S100β signaling; and (iii) target sites of S100β can be specifically mapped to two short regions in the C-terminal domain of ROS-GC1.
Results
Preparation of photoreceptor-bipolar synaptic region, P1, of the retina is devoid of the ROS and expresses ROS-GC1
In order to demonstrate that the Ca2+-sensitive ROS-GC1 transduction system is present in the particulate P1 region, it is important to show that the P1 fraction is not contaminated with ROS, the original residential site of the cyclase. The rhodopsin kinase antibody probe was used to address this issue. The results demonstrate that there is no immunoreactive band in the lane containing the P1 membrane fraction (Figure 1A). The antibody, however, yielded an immunoreactive band of expected mobility in the lanes containing the retinal extract and the ROS fraction. Consistent with existing knowledge, the ROS region of the retinal neurons contains rhodopsin kinase; however, it is not present in the P1 fraction. Thus, the P1 preparation is not contaminated with ROS and is suitable to explore the presence of ROS-GC1.
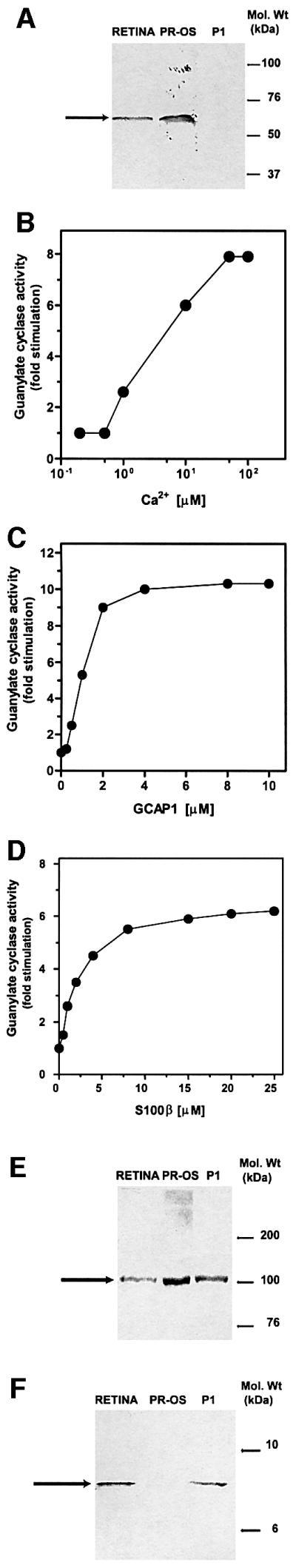
Fig. 1. Characterization of a membrane guanylate cyclase present in the P1 fraction. (A) Purity of the fraction. Membrane fractions from whole retina (RETINA), photoreceptor outer segments (PR-OS) and P1 synaptosomal fraction (P1) were isolated as described in Materials and methods, and subjected to western blot analysis using an antibody probe for rhodopsin kinase (specific for photoreceptor outer segments). Electrophoresis, transfer and antibody probing were carried out exactly as described previously (Venkataraman et al., 2000). Molecular size markers are given at the right. One hundred micrograms of membrane protein were loaded in each lane. The immunoreactive band is indicated by an arrow. (B–D) Biochemical analyses. Membranes of P1 fraction were assayed for guanylate cyclase activity in the presence of incremental concentrations of Ca2+ (B); GCAP1 at 10 nM Ca2+ (C); and S100β at 10 µM Ca2+ (D). The experiments were carried out in triplicate and repeated three times with separate membrane preparations. The results are from one representative experiment. (E and F) Immuno logical analyses. Western blot analysis with membrane fractions from whole retina (RETINA), photoreceptor outer segments (PR-OS) and P1 fraction (P1) was performed with ROS-GC1 antibody [100 µg protein/lane, (E)] or S100β antibody [50 µg protein/lane, (F)]. Molecular size markers are given at the right. The positions of the immunoreactive bands are indicated by arrows.
The P1 membrane fraction was systematically subjected to four tests. Test 1 was to determine the guanylate cyclase activity, which was ∼1 nmol of cyclic GMP/mg of protein/min. This is a cumulative value representing all putative guanylate cyclases present in the fraction. To assess whether any of this activity represented ROS-GC1, the fraction was exposed to increasing concentrations of free Ca2+ and the guanylate cyclase activity was measured. There was a dose-dependent stimulation of the cyclase activity (Figure 1B). The maximal stimulation was ∼8-fold over the basal value and was achieved at ∼50 µM Ca2+. The EC50 value for Ca2+ was ∼5 µM, which is comparable to the Ca2+ concentration of 2–4 µM found in rod synapses (Rieke and Schwartz, 1996). These results show that the P1 membrane fraction contains a Ca2+-dependent guanylate cyclase transduction system.
Test 2 was designed to determine the form of the Ca2+-dependent membrane guanylate cyclase present in the P1 membrane fraction. The three known Ca2+-dependent membrane guanylate cyclases—ROS-GC1, ROS-GC2 and GC-D (ONE-GC)—can be differentiated biochemically (Koch, 1991; Margulis et al., 1993; Goraczniak et al., 1994, 1998; Lowe et al., 1995; Duda et al., 2001). The ROS-GCs are stimulated by Ca2+-free GCAPs (reviewed in Koch et al., 2002) but ONE-GC is not (Duda et al., 2001). ROS-GC1 is stimulated by both GCAPs, while ROS-GC2 is stimulated only by GCAP2.
Ca2+-free GCAP1 stimulated the P1 membrane guanylate cyclase in a dose-dependent fashion, with an EC50 of ∼1 µM (Figure 1C). The total stimulation was ∼10-fold over the basal level. These results suggest the presence of ROS-GC1 in the P1 fraction.
Test 3 was using reconstitution experiments. Earlier reconstitution studies with recombinant ROS-GC1 have indicated that at micromolar Ca2+ concentration, S100β stimulates the cyclase with an EC50 value of ∼1 µM (Duda et al., 1996b). When the P1 membranes were reconstituted with S100β, guanylate cyclase activity was stimulated in a dose-dependent fashion with an EC50 of ∼1 µM (Figure 1D). Thus, by this criterion, the P1 membrane guanylate cyclase is also ROS-GC1.
Test 4 was carried out to establish the direct biochemical presence of ROS-GC1 in the P1 membrane fraction. Western blot analysis of the fraction was carried out with a ROS-GC1-specific antibody according to a previous protocol (Venkataraman et al., 2000). Membrane preparations from the whole retina and purified ROS (PR-OS) served as positive controls. A single immunoreactive band was detected in the lane containing P1 membranes (Figure 1E, lane P1). A band was identified with identical mobility (∼118 kDa, as expected for ROS-GC1) to the single immunoreactive band observed in the positive controls (Figure 1E, RETINA and PR-OS compared with P1).
Thus, four independent criteria establish the expression of ROS-GC1 in native P1 region of the bovine retina. Preliminary evidence indicates that other components of the ROS-GC transduction system, ROS-GC2 and GCAP1, are also present in the P1 layer (see Supplementary data available at The EMBO Journal Online). Their significance, however, remains to be determined.
As well as ROS-GC1, the P1 membrane fraction contains S100β
Previous studies have established that the retinal neurons contain S100β (reviewed in Donato, 2001) and that S100β is one of the Ca2+ modulators of recombinant ROS-GC1 (Duda et al., 1996b). To determine whether S100β is a natural modulator of ROS-GC1, the presence of S100β in the P1 layer was investigated. Western blot analyses (Figure 1F, lane P1) showed the presence of a S100β antibody-reacting band in P1 membrane. An immunoreactive band of identical mobility was also observed with the retinal membranes (Figure 1F, RETINA, positive control). No antibody reaction was observed in the lane containing ROS (Figure 1F, PR-OS). Thus, ROS-GC1 and S100β are both present in the P1 layer.
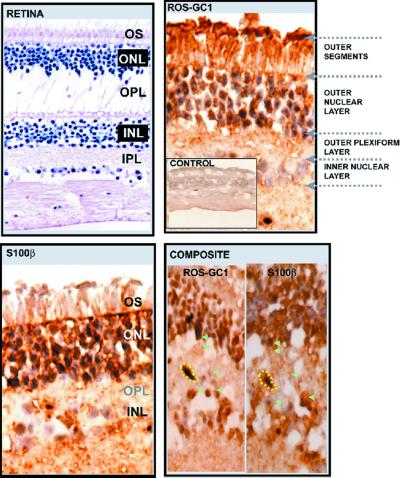
Immunocytochemical studies show that in certain regions of the P1 fraction ROS-GC1 and S100β co-localize
In order to determine whether ROS-GC1 and S100β are co-localized in the P1 layer, immunolocalization of the two proteins was carried out on serial cryosections of the retina. The results are presented in Figure 2: the RETINA panel presents a paraffin section stained to depict retinal morphology, the ROS-GC1 and S100β panels depict staining of retinal frozen sections with the antibodies against the respective proteins, and the COMPOSITE panel presents a side-by-side comparison of ROS-GC1 and S100β expression in an isolated region of the retina. The retinal layers are indicated in the ROS-GC1, RETINA and S100β panels. No significant staining is observed in the control panel, provided as an inset in the ROS-GC1 panel. Specific and intense staining (brown color) is observed in both ROS-GC1 and S100β panels. However, there is a distinct difference in the localization of the two proteins: expression of ROS-GC1 is widespread throughout all the layers depicted, but far lower expression of S100β in the outer segments is observed (compare outer segment layers of ROS-GC1 with S100β). Expression of both proteins is detected in the photoreceptor cell bodies, the bipolar cell bodies as well as the P1 synaptosomal region. In the COMPOSITE panel, an identical region from serial sections separately stained for ROS-GC1 (ROS-GC1 in COMPOSITE panel) and S100β (S100β in COMPOSITE panel) is presented. A blood vessel (marked) serves as the reference point. Green arrowheads in these panels (ROS-GC1 and S100β in COMPOSITE panel) indicate identical regions on serial sections, a photoreceptor cell body, two bipolar cell bodies and the synaptic regions between these cells, which stain positive for the two molecules. Thus, the expression of ROS-GC1 is more general in the P1 region but that of S100β is limited. In some areas, ROS-GC1 and S100β co-localize.
Fig. 2. Immunohistochemical analysis of co-localization of ROS-GC1 and S100β in the retinal outer plexiform layer. Consecutive cryosections of the retina were subjected to immunohistochemical analyses to localize ROS-GC1 and S100β. Pre-immune serum was used as a control. Results with the different antibodies are shown in their respective panels. The RETINA panel is provided to depict the retinal morphology and is a paraffin section stained with hematoxylin and eosin. Positive staining is in brown. The nuclei appear blue. In the CONTROL panel, inset in the ROS-GC1 panel, no specific staining is observed, as opposed to the ROS-GC1 and S100β panels. The COMPOSITE panel contains two segments: ROS-GC1 and S100β. Block arrowheads indicate identical locations of staining and are colored green. A blood vessel is marked (dashed yellow) as a reference point. The outer segments (OS), outer nuclear layer (ONL), outer plexiform layer (OPL) and inner nuclear layer (INL) are labeled in the ROS-GC1 panel and are denoted by abbreviations in the RETINA and S100β panels.
The C-terminal region of ROS-GC1, amino acids 733–1054, binds S100β
Direct interaction between ROS-GC1 and S100β was verified by cross-linking experiments. Washed ROS membranes were reconstituted with S100β and cross-linked by bis-(sulfosuccinimidyl)suberate (BS3). The cross-linked complex, ROS-GC1 dimer:S100β, was detected by both ROS-GC1 and S100β antibodies on western blots (see Supplementary data).
Previous experiments have suggested that S100β affects the cyclase activity by its interaction at the C-terminus of ROS-GC1, amino acids (aa) 731–1054; deletion of the extracellular (aa 8–409) or kinase homology (aa 447–730) domains, individually or together, does not affect regulation by S100β (Duda et al., 1996b). To support this conclusion, direct binding was measured by surface plasmon resonance (SPR) spectroscopy.
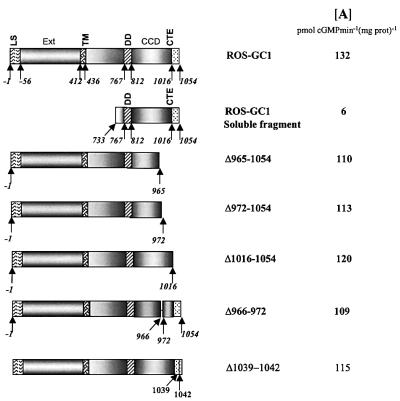
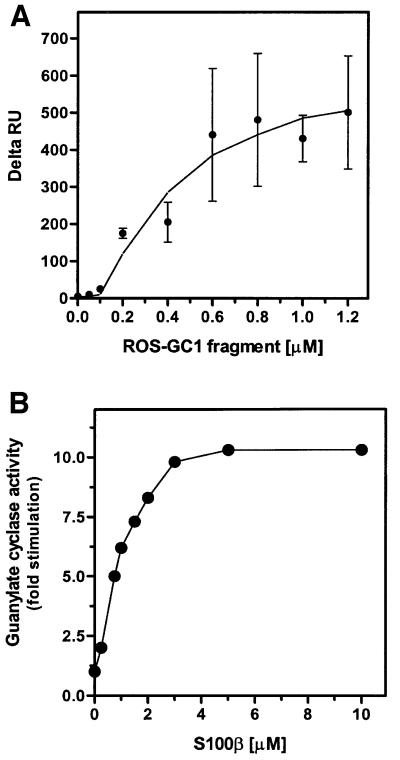
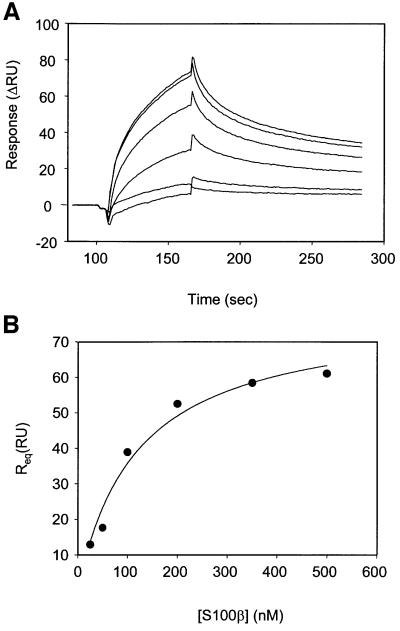
The ROS-GC1 fragment, aa 733–1054 (Figure 3), was expressed in bacteria and purified to homogeneity. The fragment is biologically active: it contains intrinsic guanylate cyclase activity and is stimulated significantly by S100β in the presence of free Ca2+ (Duda et al., 2000). Thus, this fragment was most suitable for direct binding studies with S100β. Binding of the fragment to immobilized S100β was observed as an increase in resonance units (RU) and the maximal amplitude was determined. Amplitudes of control recordings (with recoverin instead of S100β) were subtracted from recordings obtained with S100β. Corrected maximal amplitudes (Figure 4A, Delta RU) are shown as a function of the fragment concentration. Half-maximal binding (EC50) occurred at 395 ± 51 nM of the ROS-GC1 fragment (four to six determinations of three independent data sets), which is comparable to the ∼800 nM EC50 value for S100β observed for the stimulation of ROS-GC1 (Figure 4B). The results demonstrate that S100β interaction with ROS-GC1 is direct, the interacting domain resides in the ROS-GC1 fragment between aa 733–1054 and the interaction results in a physiologically meaningful stimulation of the cyclase.
Fig. 3. Schematic representation of ROS-GC1 and its deletion mutants. The following abbreviations denote the predicted domains: LS, leader sequence; Ext, extracellular domain; TM, transmembrane domain; DD, dimerization domain; CCD, cyclase catalytic domain; CTE, C-terminal extension. Numbers indicated correspond to the mature protein. Specific basal guanylate cyclase activity of ROS-GC1 and of the deletion mutants expressed in COS cells is given in the right-hand column [A].
Fig. 4. (A) Binding of S100β to ROS-GC1 soluble fragment aa 733–1054. S100β was immobilized on a CM5 sensor chip (BIAcore) and the soluble ROS-GC1 fragment was supplied in the mobile phase at concentrations between 50 and 1200 nM in running buffer. Control recordings were obtained with a recoverin-coated sensor surface. Maximal amplitude of each recording was determined and the amplitude of the corresponding control recording was subtracted to yield Delta RU. Half-maximal binding was at 395 ± 51 nM. (B) Effect of S100β on ROS-GC1 activity. COS cells were transfected with ROS-GC1 cDNA and their membranes were prepared as described in Materials and methods. These were recombined with incremental concentrations of S100β in the presence of 100 µM Ca2+ and assayed for guanylate cyclase activity. The experiment was carried out in triplicate and repeated several times for reproducibility. The results presented are from one typical experiment. Error bars are within the size of the symbols.
Mapping of the S100β-modulated domain in ROS-GC1
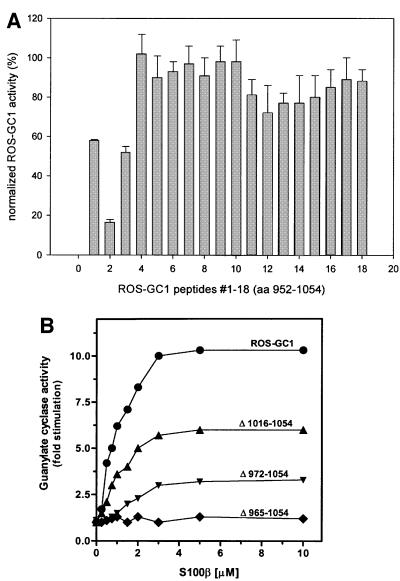
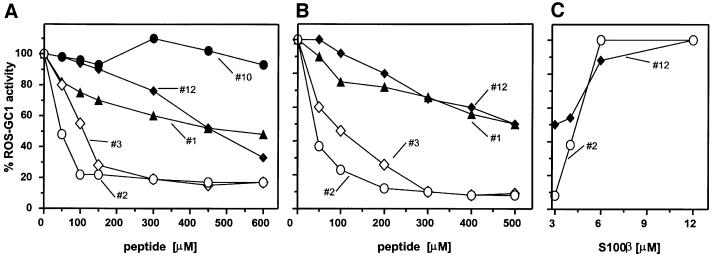
Within the aa 733–1054 region, the highly conserved residues 733–964 constitute the putative dimerization and catalytic domains. The aa 965–1054 segment is the least conserved and therefore was the focus of further analyses. Two complementary approaches were used. First, overlapping peptides encompassing this region were screened for their effects on S100β-dependent stimulation of ROS-GC1. Washed ROS membranes were reconstituted with 5–6 µM S100β and 1 mM CaCl2. Activation of the native cyclase was 6- to 7-fold, as reported previously (Duda et al., 1996b; Margulis et al., 1996). Each peptide was individually tested for its efficiency in preventing the S100β-dependent activation of ROS-GC1. The results were calculated as a percentage of the maximal stimulated activity (Figure 5A). Their analysis revealed that peptide 2 (aa 962–981) inhibited the cyclase activity by >90%; the two neighboring peptides, 1 and 3 (aa 952–971 and aa 972–991, respectively), also showed significant, although not as prominent, inhibition of 50–60%. A second region of inhibitory peptides was centered on peptide 12 (aa 1029–1040), yet the decrease in the S100β-dependent ROS-GC1 stimulation did not exceed 25–30% (Figure 5A). These results implicate two ROS-GC1 regions, one covering aa 952–991 (domains of peptides 1–3) and the second corresponding to aa 1029–1040 (domain of peptide 12), in mediating S100β-dependent stimulation of ROS-GC1 activity.
Fig. 5. Mapping the S100β-modulated regions. (A) The effect of synthetic peptides (ROS-GC1 aa 952–1054) on S100β-dependent stimulation of ROS-GC1. Washed ROS membranes were reconstituted with 6 µM S100β and 1 mM Ca2+. Peptides were added at a final concentration of 0.5 mg/ml and guanylate cyclase activity was assayed. Incubations with S100β, but without peptide, were set to 100%. (B) Effect of S100β on the cyclase activity of ROS-GC1 and its deletion mutants. COS cells were individually transfected with ROS-GC1 or the deletion mutant (Δ1016–1054, Δ972–1054 and Δ965–1054) cDNA, and the cell particulate fractions were prepared (see Materials and methods). These were recombined with incremental concentrations of S100β in the presence of 100 µM Ca2+ and assayed for guanylate cyclase activity. The experiment was carried out in triplicate and repeated three times. The results presented are from one typical experiment. Error bars are within the size of the symbols.
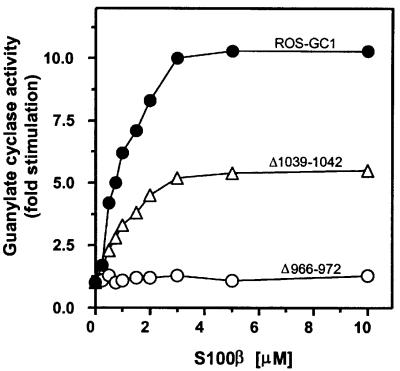
In the second approach, deletion mutation studies were conducted. Three ROS-GC1 deletion mutants—Δ965– 1054, Δ972–1054 and Δ1016–1054 (depicted in Figure 3)—were constructed, expressed in COS cells and tested for basal and S100β-dependent guanylate cyclase activities at a fixed free Ca2+ concentration of 100 µM. Identically treated membranes expressing wild-type ROS-GC1 acted as controls. The basal activity of all the mutants was comparable to that of the wild-type ROS-GC1 (Figure 3), indicating that all the cyclases were equally expressed and the deleted fragments have no influence on the structural integrity of the catalytic module. The wild-type cyclase responded to S100β in a dose-dependent fashion. S100β at ∼4 µM activated the enzyme maximally (7.5-fold), with an EC50 of ∼0.8 µM (Figure 5B). How ever, the mutants varied in their responses to S100β: mutant Δ965–1054 completely lost the S100β-modulated cyclase activity; and mutant Δ972–1054 was minimally responsive to S100β (Figure 5B), losing 75% of its maximal activity. Furthermore, there was an ∼2.5-fold change in the S100β EC50 value, from 0.8 to 2 µM (Figure 5B). Thus, the S100β-modulated domain resides within the aa 965–1054 segment of ROS-GC1 and the fragment aa 966–971 can partially restore the S100β-modulation of the cyclase, which had been lost in the Δ965–1054 mutant. The Δ1016–1054 mutant exhibited a 50% reduction in the cyclase activity at saturating S100β concentration (Figure 5B), but there was no change in the EC50 value of S100β (Figure 5B). Through these two approaches, two regions, one centered at aa 962–981 and the other at aa 1029–1040, were indicated as necessary for S100β signaling of ROS-GC1 activation.
Peptide competition experiments
To verify the above conclusions on the S100β-regulatory site and to narrow down the regulatory site further, peptide competition experiments were performed with washed ROS membranes and COS cells expressing ROS-GC1. The membranes were incubated with increasing concentrations of selected peptides in the presence of S100β and Ca2+ and the effect on S100β-dependent cyclase activity was determined. Both strategies gave very similar results (Figure 6A and B). Peptides 2 and 3 inhibited S100β-stimulated cyclase activity by 80–90%. Peptide 2 inhibited with an IC50 value of 50 µM, with the maximal inhibition at 100 µM; peptide 3, with an IC50 of 100 µM and the maximal inhibition at 300 µM; and peptide 1, with an IC50 of 300 µM and the maximal inhibition of ∼50% at 500 µM. Therefore, the region 962–981 (peptide 2) must represent the critical region of the S100β interaction with ROS-GC1. Reversal of the inhibition by peptide 2 with an excess of S100β indicates that this region is the binding site for S100β (Figure 6C).
Fig. 6. Inhibition of ROS-GC1 activity as a function of the peptide concentrations. Membranes containing ROS-GC1 were incubated with indicated concentrations of the peptide in the presence of S100β and Ca2+. Experiments were carried out in triplicate and repeated at least twice for reproducibility. The data shown are from a representative experiment. (A) Effect on native ROS-GC1. Photoreceptor outer segments were used as a source of ROS-GC1. Membranes were incubated with 6 µM S100β and 1 mM Ca2+. Peptide 10, which did not inhibit S100β-stimulated ROS-GC1 activity, served as the control. (B) Effect on recombinant ROS-GC1. Membranes from COS cells expressing ROS-GC1 were used for the assay with 3 µM S100β and 100 µM Ca2+. (C) Effect of excess S100β. Incremental concentrations of S100β were added to reaction mixture containing 300 µM peptide 2 or 500 µM peptide 12 with 3 µM S100β (conditions of maximal inhibition) and assayed as in (B).
The ROS-GC1 segment aa 962–981 binds S100β
To assess whether the ROS-GC1 region aa 962–981 binds directly to S100β, SPR analyses were performed. Peptide 2 (aa 962–981) was immobilized on a sensor chip and incremental concentrations of S100β were supplied in the mobile phase. A representative set of sensorgrams (Figure 7A) and its transformation to Req versus S100β concentrations (Figure 7B) are presented. Half-maximal binding was at 198 ± 109 nM (calculated from six independent data sets). This value is comparable to that determined for the ROS-GC1 fragment aa 733–1054 (395 ± 51 nM; Figure 4A). Thus, the aa 962–981 region of ROS-GC1 defines the S100β-binding domain.
Fig. 7. SPR analysis of S100β binding to peptide 2. (A) Peptide 2 was immobilized and S100β was supplied in the mobile phase at different concentrations (25, 50, 100, 200, 350 and 500 nM) and the association and dissociation phase was recorded. Blank runs were subtracted. (B) Plot of Req versus concentration of S100β yielded an EC50 value of 120 nM.
A recent study proposed an amino acid motif, +OXOoXOO (+, basic; X, variable; O, hydrophobic; o, hydrophilic), as a general S100β target site (McClintock and Shaw, 2000). The sequence of the proposed motif is derived from the results of a bacteriophage random peptide display library screening (Ivanenkov et al., 1995), followed by a DDBJ/EMBL/GenBank search (Ivanenkov et al., 1995; McClintock and Shaw, 2000). Scanning of peptide 2 identifies the region 966RIHVNRS972 as conforming to the proposed motif. To determine whether this region is indeed critical in S100β signaling, deletion mutants and binding assays were utilized. Mutant Δ966–972 (depicted in Figure 3) was analyzed for its response to S100β, and failed to respond at all concentrations tested (Figure 8). Thus, this region is critical for S100β regulation.
Fig. 8. Effect of S100β on the cyclase activity of the ROS-GC1 mutants Δ966–972 and Δ1039–1042. The Δ966–972 and Δ1039–1042 mutants were constructed, COS cells were individually transfected with ROS-GC1 or the mutant cDNA, and the cell particulate fractions were prepared (see Materials and methods). These were recombined with incremental concentrations of S100β in the presence of 100 µM Ca2+ and assayed for guanylate cyclase activity. The experiment was carried out in triplicate and repeated three times. The results presented are from one representative experiment. Error bars are within the size of the symbols.
Is this region sufficient for binding of S100β? Peptide Arg966–Ser972 did not bind S100β, nor did it compete for binding with the aa 962–981 region in SPR analyses; in reconstitution experiments, it fails to inhibit S100β-dependent stimulation significantly (see Supplementary data). This is in contrast to the results obtained with peptide 2 (aa 962–981), which houses the Arg966–Ser972 region and is effective in all three assays (Figures 5–7; Supplementary data). These results demonstrate that the 966RIHVNRS972 motif is indispensable in S100β signaling of ROS-GC1 activation; however, the flanking regions (present in peptide 2) are necessary to create the complete binding site.
A second S100β-regulatory site
The results of peptide competition and deletion/expression experiments indicated that an additional ROS-GC1 region, aa 1030–1041 (region of peptide 12), is also involved in the regulation by S100β. Competition experiments show that peptide 12 inhibits S100β-stimulated ROS-GC1 activity in a dose-dependent manner. The inhibitory effect of this peptide is, however, small when compared with that of peptide 2: the respective IC50 values are ∼300 and 50 µM; the maximal inhibition is 50–60% at 500 µM and 80–90% at 100 µM (Figure 6A and B). The inhibition by peptide 12 can be reversed by further addition of S100β (Figure 6C). These results indicate that the region (aa 1030–1041) is a low-affinity binding site for S100β. The functional role of this site was assessed as follows.
Analysis of this region reveals the presence of a cluster of four basic amino acid residues, 1039RRQK1042. To test whether this basic cluster might be a secondary attachment point for the acidic S100β, a deletion mutant, Δ1039–1042 (Figure 3), was examined for its S100β-dependent features. The deletion resulted in a 50% loss of maximal stimulated activity (Figure 8). This response is identical to that of the Δ1016–1054 mutant (Figure 5B). Loss of S100β-stimulated ROS-GC1 activity by 50% with Δ1016–1042 and Δ1039–1042 mutants and 50% inhibition of this activity by peptide 12 (aa 1030–1041) indicate that the aa 1030–1041 region, albeit of low binding affinity, controls the maximal activation of ROS-GC1 by S100β, and the aa 1039–1042 region within this segment mediates this effect.
The S100β-regulatory site of ROS-GC1 does not overlap with the GCAP1-regulatory site
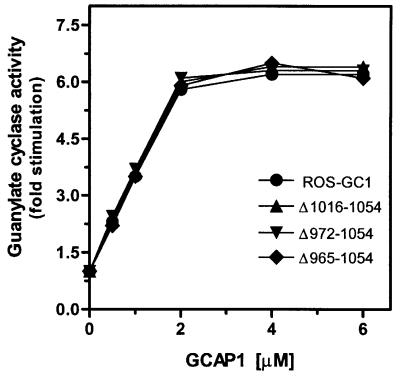
A unique feature of ROS-GC1 is that it responds to two opposite Ca2+ signals: low (nanomolar) and high (micromolar). To determine the specificity of the recognition site for the high Ca2+ signal mediated by S100β, all the ROS-GC1 deletion mutants were tested for their responses to GCAP1 that mediate the low Ca2+ signal. At 10 nM Ca2+, activation of the mutants by GCAP1 was identical to that of wild-type ROS-GC1 (Figure 9). Thus, the deletions affected ROS-GC1 regulation by S100β exclusively and had no effect on regulation by GCAP1. Therefore, the S100β and GCAP1 regulatory sites of ROS-GC1 are distinct and do not interfere with one other.
Fig. 9. Effect of GCAP1 on the cyclase activity of ROS-GC1 and its deletion mutants. Membranes of COS cells expressing ROS-GC1 or its deletion mutants were reconstituted with the indicated concentrations of GCAP1 in the presence of 10 nM Ca2+ and assayed for guanylate cyclase activity. The experiment was carried out in triplicate and repeated twice. The results presented are from one representative experiment. Error bars are within the size of the symbols.
Discussion
The present comprehensive study defines a new paradigm of Ca2+ signaling in the vertebrate retinal neurons. It shows the presence of a Ca2+-dependent ROS-GC1 transduction system in the photoreceptor-bipolar neurons. In contrast to what happens in ROS, where increments in free Ca2+ inhibit ROS-GC1 activity, in the photoreceptor-bipolar neurons free Ca2+ increments stimulate the activity. The reversal in cyclase operation is caused by the sub stitution of GCAP in ROS with S100β in the photoreceptor-bipolar neurons. This study provides the molecular description of this process.
The regulation of ROS-GC1 by S100β is specific and occurs through defined contact sites
Consistent with previous studies (Duda et al., 1996b), the binding domain of S100β is located in the intracellular region of the cyclase—at its C-terminus, downstream from the catalytic domain. In the present study, the possible target sites in ROS-GC1 have been narrowed down to two stretches of short amino acid sequences: Arg966–Ser972 and Arg1039–Lys1042. The first represents a direct binding site, as demonstrated by SPR, and harbors a partial consensus S100β recognition site (McClintock and Shaw, 2000). The second is a low-affinity site, essential for maximal cyclase activation. This interpretation is based on the observation that a peptide representing the motif competes only minimally with the activation of cyclase by S100β, yet deletion of this site leads to a significant decrease of activation by S100β.
Thus, identification of specific target sites in ROS-GC1 for S100β, together with the fact that the proteins interact directly and co-localize in P1 fraction, indicates that this interaction is of functional relevance.
Predictions on the functional relevance of the transduction system
What are the downstream targets of the cyclic GMP that is synthesized by ROS-GC1 in response to Ca2+-bound S100β? There is no definite answer to this question, given the complete lack of precedence upon which any discussion can be based; however, some possibilities can be envisioned. The simplest one is based on the presumption that the function of cyclic GMP in the photoreceptor-bipolar neurons is similar to that in ROS, i.e. it regulates the activity of the cyclic nucleotide-gated (CNG) channels. In this situation, Ca2+ spikes, resulting in accelerated cyclic GMP production, will cause depolarization in the membranes of the photoreceptor-bipolar neurons. In this prediction, the photoreceptor-bipolar neuron contains a CNG channel, and the channel is linked to ROS-GC1.
In fact, a CNG channel is present in cone synaptic terminals (Rieke and Schwartz, 1994). There, it controls exocytosis and is under the control of nitric oxide (NO) (Savchenko et al., 1997), probably via a NO–soluble guanylate cyclase signaling pathway (Koch et al., 1994; Blute et al., 1998; Margulis et al., 1998). If the same CNG channel is regulated by the soluble and the ROS-GC1 guanylate cyclases, this poses a conceptual problem. Why is the channel regulated by two guanylate cyclases, which sense Ca2+ through different mechanisms? There are two possible explanations: (i) the two cyclases operate at different time intervals, producing short spikes of cyclic GMP; (ii) synthesis of cyclic GMP is spatially restricted. The role of spatial restriction of cyclic GMP on Ca2+ homeostasis has recently been described in human epithelial cells (Zolle et al., 2000), but it remains a challenging task to demonstrate a spatial restriction of cyclic GMP effects in photoreceptor synaptic terminals. However, photoreceptor cells show a strict compartmentalization of their Ca2+-extrusion mechanisms (Krizaj and Copenhagen, 1998). Photoreceptor synaptic terminals house a plasma membrane Ca2+ ATPase as well as a caffeine-sensitive intracellular Ca2+ store (Morgans et al., 1998; Krizaj et al., 1999). Glutamate release at synaptic terminals is under the control of this caffeine-sensitive Ca2+ release from intracellular stores. Synthesis of cyclic GMP by S100β/ROS-GC1 could be spatially restricted and, thus, have a limited effect on the Ca2+ ATPase or the caffeine-sensitive Ca2+-release channel.
Another possibility is that the target of the cyclic GMP produced via the Ca2+-S100β/ROS-GC1 transduction system is cyclic GMP-dependent protein kinase. Type 1 and type 2 forms of this kinase have been localized in the mouse retina (Gamm et al., 1995). However, data are lacking on the co-existence of ROS-GC1 and cyclic GMP-dependent protein kinase in this tissue. Thus, no functional linkage between the ROS-GC1 transduction system and PKG can be made.
In summary, this study has established the presence of an original Ca2+-dependent S100β/ROS-GC1 signal transduction system in the photoreceptor-bipolar region of the neurons. The operational principles of this system at the molecular level have been defined.
Materials and methods
Antibodies
Characterization of highly specific antibodies raised against ROS-GC1 has been described previously (Venkataraman et al., 2000). Antibodies against S100β and rhodopsin kinase were obtained commercially and a polyclonal antibody against denatured S100β was raised in rabbits, because the commercial antibody was not sensitive enough to detect the antigen on western blots. The antiserum was tested for specificity through the enzyme-linked immunosorbent assay (ELISA) and by western blotting (Duda et al., 2001).
Synthesis of oligopeptides
Peptides encompassing the region Val952–Lys1054 in bovine photoreceptor guanylate cyclase ROS-GC1 were synthesized in two batches. Peptides covering the region Val952–Gln1022 were synthesized and purified according to Zoche et al. (1996). These peptides were 20 aa long and overlapped by 10 aa with the preceding one. Peptides covering the region Asp1020–Lys1054 were 12 aa long, overlapped with the preceding one by 10 aa, and were synthesized using a Multipin peptide synthesis kit (Chiron Mimotopes) according to the manufacturer’s protocol with the modifications made by Schrem et al. (1999). For site-directed immobilization in SPR experiments, peptides were synthesized as a variant with an additional Cys at the N-terminus to allow thiol coupling to the surface.
SPR spectroscopy
Sensorgrams were recorded with a BIAcore system using BIAcore control software 1.2. For immobilization, S100β was dissolved in 0.05 M sodium formate buffer pH 4.0 containing 1 mM CaCl2 and coupled to the sensor surface via cysteine thiol groups as described previously (Lange and Koch, 1997; Weitz et al., 1998). Immobilized recoverin was used as a control (Lange and Koch, 1997). Immobilization levels varied from 1 to 4 ng/mm2. The running buffer for interaction experiments contained 10 mM HEPES pH 7.5, 150 mM NaCl, 20 mM MgCl2, 2 mM CaCl2 and 0.005% Tween-20. The flow rate was set to 5 µl/min. The ROS-GC1 fragment (aa 733–1054) was dissolved in running buffer at varying concentrations (50–1200 nM) and flushed over the S100β-coated flow cell. Maximal amplitudes of binding signals were corrected by subtraction of non-specific binding to a control surface (immobilized recoverin). Details of data analysis were as described previously (Koch, 2000). SPR experiments employing peptides were performed in 10 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2 and 2 mM CaCl2 as running buffer. Peptides were immobilized as described previously (Weitz et al., 1998) and S100β was supplied in the mobile phase (25–2000 nM) using a flow rate of 40 µl/min. Regeneration was performed by short pulses (1 min) of 10 mM EGTA and 40 mM octylglucoside. Control recordings were performed over surfaces with immobilized cysteine or immobilized peptides, that did not bind S100β. These blank runs were subtracted from positive binding signals. Values of EC50 were obtained from a plot of Req (resonance signal at equilibrium) versus concentration of S100β. In cases where Req values were not reached during the time course of a sensorgram, Req values were obtained by extrapolation using a curve fitting program (BIAevaluation 3.1).
Construction of ROS-GC1 deletion mutants
Expression and purification of the aa 733–1054 fragment of ROS-GC1 is described in Duda et al. (2000). The ROS-GC1 deletion mutants, Δ1016–1054, Δ972–1054 and Δ965–1054, were constructed by introducing the TGA (stop) codon at ROS-GC1 position 1016, 972 or 965, respectively; regions 966–972 and 1039–1042 were deleted by ‘looping out’. The mutations were introduced using the QuickChange kit (Stratagene) and were verified by sequencing.
Expression studies
COS-7 cells were transfected with the wild-type recombinant or mutant ROS-GC1 expression constructs by the calcium phosphate co-precipitation technique (Sambrook et al., 1989). Sixty hours after transfection, cells were washed twice with 50 mM Tris–HCl pH 7.5/10 mM buffer, scraped into 2 ml of cold buffer, homogenized, centrifuged for 15 min at 5000 g and washed several times with the same buffer. The resulting pellet represented crude membranes.
Preparation of retinal P1 fraction
The isolation of P1 fraction from the retina was carried out as described previously (Redburn and Thomas, 1979; Margulis et al., 1998). Briefly, bovine retinas were dissected out in ice-cold buffer A (0.32 M sucrose, 10 mM Tris–HCl pH 7.4, and 1 mM phenylmethylsulfonyl fluoride; four retinas per 10 ml of the buffer) and vortexed for 5 s; the tubes were then allowed to stand and the supernatant was aspirated to remove ROS. This procedure was repeated three more times. The retinas were then hand homogenized with five strokes in a Thomas Teflon homogenizer and centrifuged at 150 g for 10 min to remove cell debris and nuclei. The supernatant was then centrifuged at 800 g for 10 min to obtain the pellet containing the P1 fraction. The pellet was washed twice in buffer A and stored at –150°C until use.
Guanylate cyclase activity assay
Membrane fractions were assayed for guanylate cyclase activity as described previously (Nambi et al., 1982; Paul et al., 1987; Duda et al., 1996a,b).
Peptide competition experiments
Native ROS-GC1. Washed ROS were prepared from fresh bovine retinas as described previously (Koch, 1991; Frins et al., 1996). The predominant cyclase in the ROS preparation is ROS-GC1 (K.-W.Koch, T.Duda and R.K.Sharma, manuscript in preparation). Ten microliters of washed ROS membranes were incubated with 5–6 µM S100β, 1 mM CaCl2 and increasing amounts of peptides for 5 min at 30°C. Activity of ROS-GC1 was determined by a high-performance liquid chromatography assay using a nucleotide separation and quantitation system (Koch, 1991; Frins et al., 1996).
Recombinant ROS-GC1. Membranes of COS cells expressing ROS-GC1 or its deletion mutants were recombined with 4 µM S100β and increasing concentrations of peptides. Guanylate cyclase activity was assayed in the presence of 100 µM Ca2+ as described above.
Western blotting
Western blotting and analyses were carried out according to previously described protocols (Venkataraman et al., 2000; Duda et al., 2001).
Immunohistochemical analyses
Cryosections of the retina were prepared and used for immunohistochemical analyses as described previously (Venkataraman et al., 2000). The tissue was fixed, cryoprotected and sectioned at 4 µm (Leica cryostat). Staining and detection were carried out as described previously (Venkataraman et al., 2000; Duda et al., 2001). Digital images were processed using commercially available software (ImagePro Plus; Phase3 Imaging Systems and Adobe Photoshop). Controls included detection reactions carried out under identical conditions except that either primary antibody was omitted or was replaced by pre-immune serum. Staining was insignificant in both cases.
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Joan Sharma for editorial assistance. This study was supported by USPHS awards EY 10828 (to R.K.S.) and HL 58151 (to T.D.), and Deutsche Forschungsgemeinschaft (DFG) grant Ko948/5-3 (to K.-W.K.), and by the facilities provided by UMDNJ-SOM.
References
- Blute T.A., Velasco,P. and Eldred,W.D. (1998) Functional localization of soluble guanylate cyclase in turtle retina: modulation of cGMP by nitric oxide donors. Vis. Neurosci., 15, 485–498. [DOI] [PubMed] [Google Scholar]
- Cooper N., Liu,L., Yoshida,A., Pozdnyakov,N., Margulis,A. and Sitaramayya,A. (1995) The bovine rod outer segment guanylate cyclase, ROS-GC, is present in both outer segment and synaptic layers of the retina. J. Mol. Neurosci., 6, 211–222. [DOI] [PubMed] [Google Scholar]
- Dizhoor A.M. and Hurley,J.B. (1999) Regulation of photoreceptor membrane guanylyl cyclases by guanylyl cyclase activator proteins. Methods, 19, 521–531. [DOI] [PubMed] [Google Scholar]
- Donato R. (2001) S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol., 33, 637–668. [DOI] [PubMed] [Google Scholar]
- Duda T., Goraczniak,R., Surgucheva,I., Rudnicka-Nawrot,M., Gorczyca,W.A., Palczewski,K., Baehr,W. and Sharma,R.K. (1996a) Calcium modulation of bovine photoreceptor guanylate cyclase. Biochemistry, 35, 8478–8482. [DOI] [PubMed] [Google Scholar]
- Duda T., Goraczniak,R.M. and Sharma,R.K. (1996b) Molecular characterization of S100A1–S100B protein in retina and its activation mechanism of bovine photoreceptor guanylate cyclase. Biochemistry, 35, 6263–6266. [DOI] [PubMed] [Google Scholar]
- Duda T., Venkataraman,V., Jankowska,A., Lange,C., Koch,K.-W. and Sharma,R.K. (2000) Impairment of the rod outer segment membrane guanylate cyclase dimerization in a cone–rod dystrophy results in defective calcium signaling. Biochemistry, 39, 12522–12533. [DOI] [PubMed] [Google Scholar]
- Duda T., Jankowska,A., Venkataraman,V., Nagele,R.G. and Sharma,R.K. (2001) A novel calcium-regulated membrane guanylate cyclase transduction system in the olfactory neuroepithelium. Biochemistry, 40, 12067–12077. [DOI] [PubMed] [Google Scholar]
- Frins S., Bonigk,W., Muller,F., Kellner,R. and Koch,K.-W. (1996) Functional characterization of a guanylyl cyclase-activating protein from vertebrate rods. Cloning, heterologous expression and localization. J. Biol. Chem., 271, 8022–8027. [DOI] [PubMed] [Google Scholar]
- Gamm D.M., Francis,S.H., Angelotti,T.P., Corbin,J.D. and Uhler,M.D. (1995) The type II isoform of cGMP-dependent protein kinase is dimeric and possesses regulatory and catalytic properties distinct from the type I isoforms. J. Biol. Chem., 270, 27380–27388. [DOI] [PubMed] [Google Scholar]
- Goraczniak R.M., Duda,T., Sitaramayya,A. and Sharma,R.K. (1994) Structural and functional characterization of the rod outer segment membrane guanylate cyclase. Biochem. J., 302, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goraczniak R.M., Duda,T. and Sharma,R.K. (1998) Calcium modulated signaling site in type 2 rod outer segment membrane guanylate cyclase (ROS-GC2). Biochem. Biophys. Res. Commun., 245, 447–453. [DOI] [PubMed] [Google Scholar]
- Ivanenkov V.V., Jamieson,G.A.,Jr, Gruenstein,E. and Dimlich,R.V. (1995) Characterization of S-100β binding epitopes. Identification of a novel target, the actin capping protein, CapZ. J. Biol. Chem., 270, 14651–14658. [DOI] [PubMed] [Google Scholar]
- Koch K.-W. (1991) Purification and identification of photoreceptor guanylate cyclase. J. Biol. Chem., 266, 8634–8637. [PubMed] [Google Scholar]
- Koch K.-W. (2000) Identification and characterization of calmodulin binding sites in cGMP-gated channel using surface plasmon resonance spectroscopy. Methods Enzymol., 315, 785–797. [DOI] [PubMed] [Google Scholar]
- Koch K.-W., Lambrecht,H.G., Haberecht,M., Redburn,D. and Schmidt,H.H. (1994) Functional coupling of a Ca2+/calmodulin-dependent nitric oxide synthase and a soluble guanylyl cyclase in vertebrate photoreceptor cells. EMBO J., 13, 3312–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K.-W., Duda,T. and Sharma,R.K. (2002) Photoreceptor specific guanylate cyclases in vertebrate phototransduction. Mol. Cell. Biochem., 230, 97–106. [PubMed] [Google Scholar]
- Krishnan A., Goraczniak,R.M., Duda,T. and Sharma,R.K. (1998) Third calcium-modulated rod outer segment membrane guanylate cyclase transduction mechanism. Mol. Cell. Biochem., 178, 251–259. [DOI] [PubMed] [Google Scholar]
- Krizaj D. and Copenhagen,D.R. (1998) Compartmentalization of calcium extrusion mechanisms in the outer and inner segments of photoreceptors. Neuron, 21, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D., Bao,J.X., Schmitz,Y., Witkovsky,P. and Copenhagen,D.R. (1999) Caffeine-sensitive calcium stores regulate synaptic transmission from retinal rod photoreceptors. J. Neurosci., 19, 7249–7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylov D.M. and Hurley,J.B. (2001) Identification of proximate regions in a complex of retinal guanylyl cyclase 1 and guanylyl cyclase-activating protein-1 by a novel mass spectrometry-based method. J. Biol. Chem., 276, 30648–30654. [DOI] [PubMed] [Google Scholar]
- Lange C. and Koch,K.-W. (1997) Calcium-dependent binding of recoverin to membranes monitored by surface plasmon resonance spectroscopy in real time. Biochemistry, 36, 12019–12026. [DOI] [PubMed] [Google Scholar]
- Lange C., Duda,T., Beyermann,M., Sharma,R.K. and Koch,K.-W. (1999) Regions in vertebrate photoreceptor guanylyl cyclase ROS-GC1 involved in Ca2+-dependent regulation by guanylyl cyclase-activating protein GCAP-1. FEBS Lett., 460, 27–31. [DOI] [PubMed] [Google Scholar]
- Liu X., Seno,K., Nishizawa,Y., Hayashi,F., Yamazaki,A., Matsumoto,H., Wakabayashi,T. and Usukura,J. (1994) Ultrastructural localization of retinal guanylate cyclase in human and monkey retinas. Exp. Eye Res., 59, 761–768. [DOI] [PubMed] [Google Scholar]
- Lowe D.G., Dizhoor,A.M., Liu,K., Gu,Q., Spencer,M., Laura,R., Lu,L. and Hurley,J.B. (1995) Cloning and expression of a second photoreceptor-specific membrane retina guanylyl cyclase (RetGC), RetGC-2. Proc. Natl Acad. Sci. USA, 92, 5535–5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis A., Goraczniak,R.M., Duda,T., Sharma,R.K. and Sitaramayya,A. (1993) Structural and biochemical identity of retinal rod outer segment membrane guanylate cyclase. Biochem. Biophys. Res. Commun., 194, 855–861. [DOI] [PubMed] [Google Scholar]
- Margulis A., Pozdnyakov,N. and Sitaramayya,A. (1996) Activation of bovine photoreceptor guanylate cyclase by S100 proteins. Biochem. Biophys. Res. Commun., 218, 243–247. [DOI] [PubMed] [Google Scholar]
- Margulis A., Pozdnyakov,N., Dang,L. and Sitaramayya,A. (1998) Soluble guanylate cyclase and nitric oxide synthase in synaptosomal fractions of bovine retina. Vis. Neurosci., 15, 867–873. [DOI] [PubMed] [Google Scholar]
- McClintock K.A. and Shaw,G.S. (2000) A logical sequence search for S100B target proteins. Protein. Sci., 9, 2043–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans C.W., El Far,O., Berntson,A., Wassle,H. and Taylor,W.R. (1998) Calcium extrusion from mammalian photoreceptor terminals. J. Neurosci., 18, 2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambi P., Aiyar,N.V. and Sharma,R.K. (1982) Adrenocorticotropin-dependent particulate guanylate cyclase in rat adrenal and adrenocortical carcinoma: comparison of its properties with soluble guanylate cyclase and its relationship with ACTH-induced steroidogenesis. Arch. Biochem. Biophys., 217, 638–646. [DOI] [PubMed] [Google Scholar]
- Paul A.K., Marala,R.B., Jaiswal,R.K. and Sharma,R.K. (1987) Coexistence of guanylate cyclase and atrial natriuretic factor receptor in a 180-kD protein. Science, 235, 1224–1226. [DOI] [PubMed] [Google Scholar]
- Pozdnyakov N., Yoshida,A., Cooper,N.G., Margulis,A., Duda,T., Sharma,R.K. and Sitaramayya,A. (1995) A novel calcium-dependent activator of retinal rod outer segment membrane guanylate cyclase. Biochemistry, 34, 14279–14283. [DOI] [PubMed] [Google Scholar]
- Pozdnyakov N., Goraczniak,R., Margulis,A., Duda,T., Sharma,R.K., Yoshida,A. and Sitaramayya,A. (1997) Structural and functional characterization of retinal calcium-dependent guanylate cyclase activator protein (CD-GCAP): identity with S100β protein. Biochemistry, 36, 14159–14166. [DOI] [PubMed] [Google Scholar]
- Pugh E.N. Jr, Duda,T., Sitaramayya,A. and Sharma,R.K. (1997) Photoreceptor guanylate cyclases: a review. Biosci. Rep., 17, 429–473. [DOI] [PubMed] [Google Scholar]
- Redburn D.A. and Thomas,T.N. (1979) Isolation of synaptosomal fractions from rabbit retina. J. Neurosci. Methods, 1, 235–242. [DOI] [PubMed] [Google Scholar]
- Rieke F. and Schwartz,E.A. (1994) A cGMP-gated current can control exocytosis at cone synapses. Neuron, 13, 863–873. [DOI] [PubMed] [Google Scholar]
- Rieke F. and Schwartz,E.A. (1996) Asynchronous transmitter release: control of exocytosis and endocytosis at the salamander rod synapse. J. Physiol., 493, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook M.J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Savchenko A., Barnes,S. and Kramer,R.H. (1997) Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide. Nature, 390, 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrem A., Lange,C., Beyermann,M. and Koch,K.-W. (1999) Identification of a domain in guanylyl cyclase-activating protein 1 that interacts with a complex of guanylyl cyclase and tubulin in photoreceptors. J. Biol. Chem., 274, 6244–6249. [DOI] [PubMed] [Google Scholar]
- Shyjan A.W., de Sauvage,F.J., Gillett,N.A., Goeddel,D.V. and Lowe,D.G. (1992) Molecular cloning of a retina-specific membrane guanylyl cyclase. Neuron, 9, 727–737. [DOI] [PubMed] [Google Scholar]
- Sokal I., Haeseleer,F., Arendt,A., Adman,E.T., Hargrave,P.A. and Palczewski,K. (1999) Identification of a guanylyl cyclase-activating protein-binding site within the catalytic domain of retinal guanylyl cyclase 1. Biochemistry, 38, 1387–1393. [DOI] [PubMed] [Google Scholar]
- Venkataraman V., Nagele,R., Duda,T. and Sharma,R.K. (2000) Rod outer segment membrane guanylate cyclase type 1-linked stimulatory and inhibitory calcium signaling systems in the pineal gland: biochemical, molecular and immunohistochemical evidence. Biochemistry, 39, 6042–6052. [DOI] [PubMed] [Google Scholar]
- Weitz D., Zoche,M., Muller,F., Beyermann,M., Korschen,H.G., Kaupp,U.B. and Koch,K.-W. (1998) Calmodulin controls the rod photoreceptor CNG channel through an unconventional binding site in the N-terminus of the β-subunit. EMBO J., 17, 2273–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.B., Foster,D.C., Garbers,D.L. and Fulle,H.J. (1995) Two membrane forms of guanylyl cyclase found in the eye. Proc. Natl Acad. Sci. USA, 92, 602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoche M., Bienert,M., Beyermann,M. and Koch,K.-W. (1996) Distinct molecular recognition of calmodulin-binding sites in the neuronal and macrophage nitric oxide synthases: a surface plasmon resonance study. Biochemistry, 35, 8742–8747. [DOI] [PubMed] [Google Scholar]
- Zolle O., Lawrie,A.M. and Simpson,A.W. (2000) Activation of the particulate and not the soluble guanylate cyclase leads to the inhibition of Ca2+ extrusion through localized elevation of cGMP. J. Biol. Chem., 275, 25892–25899. [DOI] [PubMed] [Google Scholar]