Abstract
Embryo dormancy in flowering plants is an important dispersal mechanism that promotes survival of the seed through time. The subsequent transition to germination is a critical control point regulating initiation of vegetative growth. Here we show that the Arabidopsis COMATOSE (CTS) locus is required for this transition, and acts, at least in part, by profoundly affecting the metabolism of stored lipids. CTS encodes a peroxisomal protein of the ATP binding cassette (ABC) transporter class with significant identity to the human X-linked adrenoleukodystrophy protein (ALDP). Like X-ALD patients, cts mutant embryos and seedlings exhibit pleiotropic phenotypes associated with perturbation in fatty acid metabolism. CTS expression transiently increases shortly after imbibition during germination, but not in imbibed dormant seeds, and genetic analyses show that CTS is negatively regulated by loci that promote embryo dormancy through multiple independent pathways. Our results demonstrate that CTS regulates transport of acyl CoAs into the peroxisome, and indicate that regulation of CTS function is a major control point for the switch between the opposing developmental programmes of dormancy and germination.
Keywords: ABC lipid transporter/adrenoleukodystrophy/Arabidopsis/dormancy/germination
Introduction
Seeds of most plant species exhibit dormancy, and will not germinate even under otherwise favourable environmental conditions until dormancy is lost through a period of after-ripening. This capacity for dormancy is considered an evolutionary adaptation that spreads germination through time, thereby increasing seedling survival from a single seed set (Baskin and Baskin, 1998). The transition from embryo dormancy to germination combines the activation of developmental programmes and catabolic metabolism (the mobilization of seed storage reserves), which result in radicle emergence, seedling establishment and subsequent photoautotrophic growth (Bewley, 1997). In Arabidopsis, many genes have been identified that regulate the transition from embryogenesis to seedling growth (Holdsworth et al., 1999; Koornneef et al., 2000). Several function to repress germination and maintain embryo dormancy. The transcription factors ABSCISIC ACID INSENSITIVE3 (ABI3), FUSCA3 (FUS3), and LEAFY COTYLEDON1 and 2 (LEC1,2) are major regulators of this process (Baumlein et al., 1994; Meinke et al., 1994; Nambara et al., 1995; Parcy et al., 1997; Lotan et al., 1998; Stone et al., 2001). Embryos carrying severe mutations at these loci exhibit phenotypes associated with seedlings, including an activated apical meristem and vascular development. Several studies have shown that post-germination gene expression patterns are activated in these mutants, including genes involved in storage reserve mobilization (West et al., 1994; Nambara et al., 2000). These data suggest that these loci have two distinct functions, simultaneously activating embryo maturation and repressing premature germination. Other loci that repress germination do not affect the transition between embryo and seedling. For example ABI1, ABI4 and ABI5, and RDO1 and RDO2 all enhance embryo dormancy (Koornneef et al., 1984; Finkelstein, 1994; Léon-Kloosterziel et al., 1996), and mutation of these loci leads to highly precocious germination, but there is no alteration in embryo anatomy or gross gene expression patterns during embryo development. Several loci also enhance seed dormancy through their effect on seed coat anatomy and/or chemical composition, mutant seeds show reduced dormancy presumably because the passage of environmental signals and chemicals that influence germination are enhanced (Debeaujon et al., 2000).
Mutations of loci associated with metabolism of storage reserves prior to photoautotrophic seedling growth also influence germination and post-germinative growth. In oil seeds, mobilization of storage lipids via peroxisomal β-oxidation and the glyoxylate cycle provide energy and carbon skeletons for germination and seedling establishment. A number of mutants have been identified with compromised β-oxidation capacity, as measured by insensitivity to the synthetic auxin 2,4-dichlorophenoxy butyric acid (2,4DB) (e.g. ped1–3) (Hayashi et al., 1998) or indole butyric acid (IBA) (e.g. pxa1) (Zolman et al., 2001). These mutants all germinate, as defined by radicle emergence, but fail to establish in the absence of exogenous sucrose. In contrast, plants with null mutations of the glyoxylate cycle enzyme isocitrate lyase are able to germinate, albeit with reduced establishment and survival during growth under compromised environmental conditions (Eastmond et al., 2000).
The COMATOSE (CTS) locus was identified genetically as having a very strong effect on the enhancement of germination (Russell et al., 2000). cts mutant seeds do not germinate, and exhibit a ‘forever dormant’ phenotype (Russell et al., 2000). This strong phenotype implicated CTS as a target for loci that repress germination, and suggests CTS is a major control point between dormancy and germination. In this paper, we describe the cloning of CTS, a homologue of the human adrenoleukodystrophy protein (ALDP), an ATP binding cassette (ABC) transporter associated with transport of very long chain fatty acids (VLCFAs) into the peroxisome. CTS is equivalent to the recently described PXA1/PED3 locus (Zolman et al., 2001; Hayashi et al., 2002). We show that mutation of the CTS gene results in profound alterations in seedling anatomy and biochemistry, that CTS interacts genetically with loci that repress germination and that expression of CTS is tightly associated with germination. These data suggest CTS is a major control point regulating germination potential. We propose a model to explain the relationship between the known effects of CTS on lipid mobilization and the ‘forever dormant’ phenotype of cts mutant alleles.
Results
The CTS gene encodes a homologue of the human X-linked ALDP
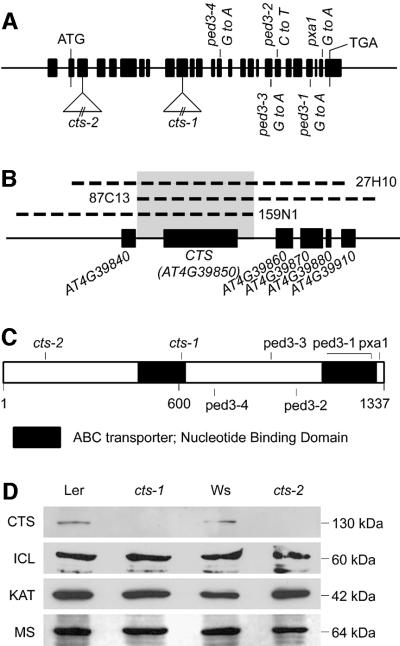
The CTS gene was identified using a combination of positional cloning and insertional mutagenesis. Pre viously, the cts-1 mutation had been mapped to the distal arm of chromosome IV, close to the marker DHS1 (Russell et al., 2000). Southern analysis of chromosomal organization in this region revealed a polymorphism associated with cts-1 within the predicted gene AT4G39850 (data not shown). Gene-specific PCR and PCR-based chromosome walking identified a chromosomal translocation associated with this polymorphism (Figure 1A). A second allele (designated cts-2, Figure 1A) was obtained by reverse genetics. This line contains a T-DNA insertion within the third exon of AT4G39850. The cts-1 and cts-2 mutants exhibited indistinguishable phenotypes, and did not complement one another. Three small overlapping genomic fragments delimiting a region 1.7 kb upstream to 480 bp downstream of the AT4G39850 gene complemented the cts-1 phenotype in transgenic plants (Figure 1B). In addition, western analysis with a CTS antibody directed against the C-terminal 225 amino acids of the protein shows that the CTS protein is undetectable in germinating seedlings in both cts-1 and cts-2 mutants (Figure 1D), whereas expression of other proteins associated with germination [malate synthase (MS), thiolase (KAT2) and iso-citrate lyase (ICL)] are unaffected. Together, these data confirm that the AT4G39850 predicted gene corresponds to the CTS locus. The predicted gene is incorrectly annotated; the correctly annotated version is deposited in DDBJ/EMBL/GenBank under accession No. AJ311341.
Fig. 1. CTS encodes an ALDP-related ABC transporter. (A) CTS gene structure. The positions of exons are indicated (boxes), and ATG and TGA codons. The cts-1 allele results from a translocation of 370 kb of chromosome V (positions 33.3–33.7 Mb) into exon 10 (data not shown). The cts-2 allele contains an insertion of T-DNA into exon 3 (codon Thr117). The positions of other known alleles in this gene are indicated (Zolman et al., 2001; Hayashi et al., 2002). (B) Positions of DNAs on chromosome IV complementing the cts-1 mutant phenotype. The CTS gene (AT4G39850) and adjacent open reading frames are shown (gene names; The Arabidopsis Genome Initiative, 2000). Clone numbers are indicated and the minimum overlapping region boxed. (C) Schematic of the CTS protein, indicating positions of two NBD and termination points of polypeptides corresponding to the cts-1 and cts-2 alleles. The most similar proteins in the SWISSPROT database to the amino terminal half of CTS (amino acids 1–690) are mouse ALD and human ALDP (Blast probability scores P = e-88). The most similar protein to the C-terminal half of CTS (691–1337), for which an experimental function has been assigned, is rat PMP70 (P = 1.1 e-69). Positions of other known alleles are indicated. (D) Western blot analysis of WT, cts-1 and cts-2. Protein extracts were from 3-day-old germinated seedlings (100 µg protein/lane). The western blot was probed for CTS, MS, ICL and KAT2 proteins.
The derived CTS amino acid sequence contained two highly conserved nucleotide binding domains (NBD) (Figure 1C), indicating that CTS encodes a full-size ABC transporter (Davies and Coleman, 2000). Both halves of the predicted protein show significant sequence similarity to the human X-linked ALDP, an ABC half-size transporter (Mosser et al., 1993). This protein and its homologues from yeast (Shani et al., 1995) are associated with transport of VLCFAs into the peroxisome (Hettema et al., 1996; Verleur et al., 1997) and VLCFA CoA-synthetase activity (Yamada et al., 1999). In humans, mutations of ALD result in metabolic abnormalities associated with degeneration of myelin sheaths, leading to neurological disorders, learning deficiencies, coma and early death (Moser et al., 1995; Smith et al., 1999; Ravid et al., 2000), although the exact biochemical function of the protein is still unclear. The Arabidopsis genome contains at least 129 ABC genes, of which 51 are full size (Sanchez-Fernandez et al., 2001). Of these, only CTS has significant similarity to ALDP. CTS corresponds to the recently reported gene PXA1/PED3 (Zolman et al., 2001; Hayashi et al., 2002). The relative positions of mutations for the different reported alleles within this gene and protein are shown in Figure 1A and C.
CTS is a peroxisomal protein
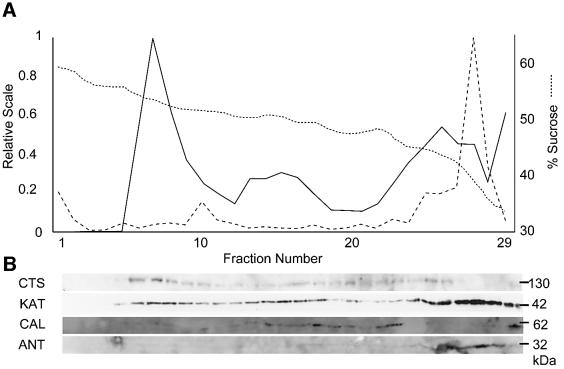
A rabbit polyclonal antibody was raised against a fragment of CTS corresponding to amino acids 1112–1337, affinity purified and used to localize the protein in organelles from rosette leaf tissue, which had been separated on a sucrose gradient. Catalase activity, a specific marker for peroxisomes (Figure 2), was found predominantly in fraction 7 (∼55% w/w sucrose), which corresponds to intact peroxisomes (Lopez-Huertas et al., 1995). KAT, an independent marker for peroxisomes, also showed a peak in fractions 7 and 8. Both catalase and thiolase are matrix proteins, and their presence in lighter fractions is due to a combination of organelle breakage and the intrinsic heterogeneity of peroxisomes (Volokita, 1991; Titorenko and Rachubinski, 2001). CTS protein closely follows the distribution of catalase and thiolase, and is most abundant in fraction 7. However, in contrast with the soluble peroxisomal matrix enzymes and consistent with its membrane localization, it is not detected in the lightest fractions (26–29). Markers for chloroplast (chlorophyll) and mitochondrial (adenine nucleotide translocator, ANT) membranes were resolved well from CTS, as was the ER marker calreticulin. Thus we conclude that CTS, like ALDP, is a peroxisomal protein.
Fig. 2. CTS is localized in the peroxisomes. Post-nuclear organellar pellet derived from leaf was separated on a sucrose density gradient (sucrose concentration, dotted line). Fractions (A) were assayed for catalase activity (continuous line), chlorophyll concentration (dashed line) and probed (B) by western blotting for CTS, KAT, ER-marker calreticulin (CAL) and ANT, a mitochondrial membrane marker. CTS co-localizes with intact peroxisomes (56% sucrose) corresponding to the first peak of catalase and thiolase.
cts mutants are defective in lipid mobilization and accumulate acyl CoAs
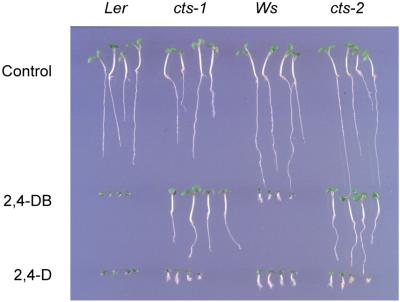
Seeds containing mutant cts-1 and cts-2 alleles were induced to germinate on sucrose media following removal of the surrounding testa and endosperm layers. Electron microscopic examination of cotyledons from cts-1 seedlings demonstrated a striking inability to break down lipid bodies. In comparison, lipid bodies were absent in the wild type (WT) (Figure 3). Peroxisomes were visible in both the WT and mutants, and there were no obvious differences in size or morphology. Cells from WT cotyledons were highly vacuolated (data not shown) with well-developed chloroplasts containing starch granules and granal stacks, whilst most cells from the cts-1 mutant contained small vacuoles, and chloroplast morphology was distorted. These observations show that in seedlings the cts-1 allele results in a severe block in lipid breakdown. This is confirmed by resistance of both cts-1 and cts-2 seedlings to 2,4DB. This compound is bioactivated by peroxisomal β-oxidation to 2,4-dichlorophenoxyacetic acid (2,4D), an auxin that results in stunted root growth (Hayashi et al., 1998; Germain et al., 2001; Zolman et al., 2001) (Figure 4). Growth of seedlings of both WT ecotypes and cts alleles were severely retarded on 2,4D, showing that they remain sensitive to auxin. However, for both mutant alleles, growth on media containing 2,4DB did not result in stunted roots, whereas WT seedlings were severely affected. Therefore β-oxidation of 2,4DB is compromised in cts-1 and cts-2.
Fig. 3. Loss of CTS in the cts-1 mutant leads to a striking failure of lipid breakdown in germinated cotyledons. Transverse electron micrograph sections were made from cotyledons germinated for 5 days in the presence of sucrose. WT and mutant seedling morphology is indistinguishable when grown under these conditions. In the WTs, lipid bodies are completely absent and mitochondria notably abundant (A, scale bar 2 µm) whilst in cts-1, lipid bodies persist and plastid morphology is abnormal (B, scale bar 2 µm). Similar peroxisomal morphology is observed in WT (C) and mutant cts-1 (D), scale bar 500 nm. Peroxisomes appear closely juxtaposed to lipid bodies in cts-1 (D). P, peroxisomes; LB, lipid bodies; M, mitochondria; C, chloroplast; V, vacuole.
Fig. 4. cts-1 and cts-2 mutants are defective in the bio-activation of 2,4DB by peroxisomal β-oxidation. Comparison of growth of WT and cts-1 and cts-2 seedlings on media with no added hormone (control) or media containing 2,4DB or 2,4D. Surrounding testa/endosperm layers were removed from seeds to facilitate germination of mutant seeds.
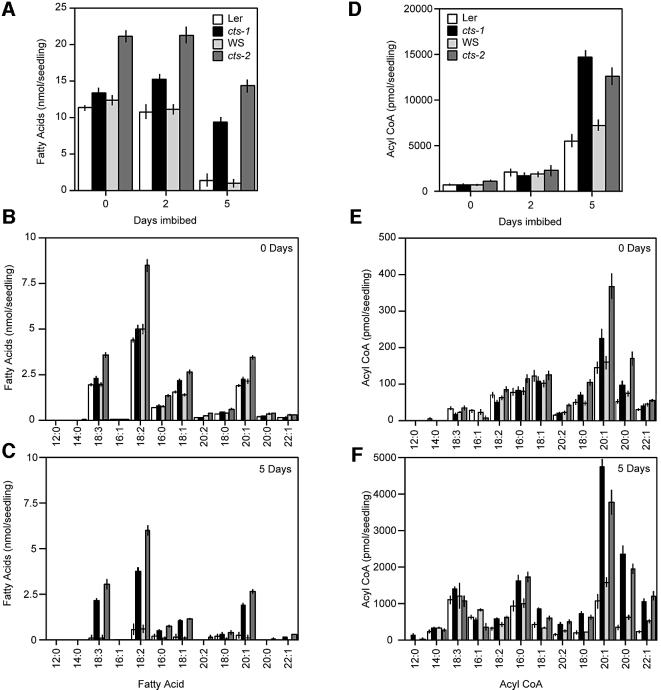
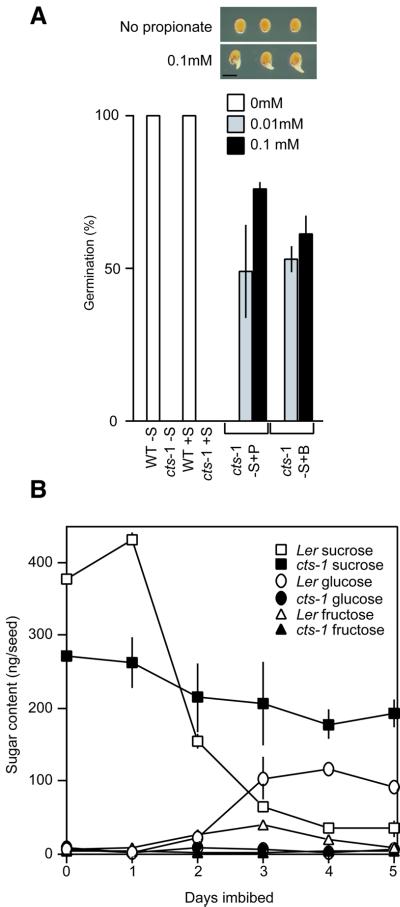
To provide further information on the specific nature of the block in lipid breakdown and hence the function of the CTS protein, the levels of triacyl glycerol (TAG) and acyl CoAs were measured in the mutants and corresponding WTs. All lines were germinated in the presence of 1% sucrose (and the testa of the cts mutants was ruptured to allow germination) to ensure that seedlings were at similar morphological stages of development. The summed changes in fatty acid content of extracted TAG from the two mutants and their corresponding WTs (Ler and WS for cts-1 and cts-2, respectively) indicate similar levels of TAG-derived fatty acids in imbibed seeds of the WTs and cts-1 on day 0 (Figure 5A) (higher apparent TAG levels in cts-2 reflects bigger seed size). TAG fatty acid levels only declined slightly after 2 days of germination, even in WT seedlings, presumably due to the presence of sucrose as an alternative energy source. However, by day 5, TAG-derived fatty acids had decreased by 88–95% in the WTs, but by only 28–33% in the two mutants, as expected from the persistence of lipid bodies (Figure 3). All TAG-derived fatty acid chain lengths were mobilized after 5 days of germination for both WTs, but both cts-1 and cts-2 mutants retained high levels of the same TAGs (Figure 5B and C).
Fig. 5. Profiles of TAG-derived fatty acids and acyl CoA levels in WTs and cts-1 and cts-2 mutants. (A) Comparison of TAG-derived fatty acids in the WTs and cts-1 and cts-2 mutants in seeds and seedlings 0, 2 and 5 days after sowing. (B) Profile of TAG-derived fatty acids in imbibed WT and mutant seeds by chain length. (C) As (B) but profile from 5-day-old seedlings. (D) Comparison of acyl CoA levels in WTs and cts-1 and cts-2 seedlings 0, 2 and 5 days after sowing. (E) Profile of acyl CoAs in imbibed WT and mutant seeds by chain length. (F) As (E) but profile from 5-day-old seedlings. All seeds were sown on media containing 1% sucrose and held at 4°C for 4 days before transfer to germination conditions. The testa of cts mutants was disrupted to allow germination. Seedlings were at similar morphological stages of development. Bars indicate SEM.
Total acyl CoAs increased in all lines over the period 0–5 days (Figure 5D), but this was much more dramatic in the two mutants. By day 5, some of the increase in both WT and mutant seedlings may reflect new lipid synthesis (e.g. for membrane biogenesis). However, the most striking observation is the retention of 20:1, and to a lesser extent 20:0 and 22:1, CoAs in seedlings of the two mutant alleles (Figure 5E and F). As C20 fatty acids are only very minor components of non-storage lipids in Arabidopsis, these data demonstrate that there is a severe block in carbon flux from stored TAGs during germination in the mutant alleles.
The β-oxidation pathway is functional in cts mutants, but carbohydrate metabolism is compromised
The data presented in Figures 3–5 demonstrate that there is a profound defect in fatty acid metabolism in seedlings containing the cts-1 and cts-2 mutant alleles, but do not indicate whether these alleles result in a defect in the transport of fatty acids or acyl CoAs into the peroxisome, or a defect in β-oxidation per se leading to an accumulation of acyl CoAs and subsequent inhibition of lipolysis [as in the kat2 (3-ketoacyl thiolase) mutant; Germain et al., 2001]. Both acyl CoA oxidase and thiolase activity were detectable in imbibed cts-1 seeds (data not shown). Furthermore, addition of propionate or butyrate (short chain fatty acids that can potentially enter seeds and partition across the peroxisome membrane) to the growth media greatly increased the germination potential of intact cts-1 seeds (Figure 6A). These results suggest that peroxisomal β-oxidation is functional in the cts-1 mutant, and the failure to metabolize TAG-derived acyl CoA is a consequence of a defect in transport into the peroxisome.
Fig. 6. Metabolic regulation of germination in cts-1 seeds. (A) Short chain fatty acids can rescue the cts-1 mutant phenotype. Analysis of germination potential of intact after-ripened Ler or cts-1 mutant seeds either with or without sucrose (S), propionate (P) and butyrate (B). Inset; typical germination of cts-1 seeds without (control) or with (0.1 mM) propionate. Bar represents 0.5 mm. Bars indicate SEM. (B) Sucrose mobilization is restricted in the cts-1 mutant. Carbohydrate analysis of intact after-ripened Ler and cts-1 seeds was carried out under germination conditions in the absence of exogenous sucrose. Germination was 98% for Ler seeds by day 2 followed by full establishment. By comparison, cts-1 germination was 1% by day 2 and 2% at day 5 with no further growth.
As other mutants defective in lipid reserve mobilization are able to germinate (Hayashi et al., 1998; Germain et al., 2001), and the energy for embryo expansion and radicle protrusion is believed to come from metabolism of endogenous carbohydrate (Bewley and Black, 1985), levels of sucrose, glucose and fructose were measured in the cts-1 mutant (Figure 6B). In dry seeds, sucrose levels were 27% lower in cts-1 (281 ± 7 ng per seed) than in the Ler WT (387 ± 33 ng per seed). Fructose levels were 20 ng per seed for cts-1 and 7 ng per seed for Ler, and glucose was at the limit of detection. By day 5 of imbibition, cts-1 seeds retained significant levels of sucrose (193 ng per seed) whilst in the germinated Ler seedlings, sucrose had fallen to 35 ng per seed. Fructose levels were similar (4 versus 9 ng per seed) but glucose levels were negligible in cts (6 ng per seed) compared with Ler (91 ng per seed). Thus the presence of the cts-1 mutation results in a modest decrease in sucrose content of the dry seed, but has a profound effect on its subsequent utilization.
CTS interacts with genes promoting dormancy
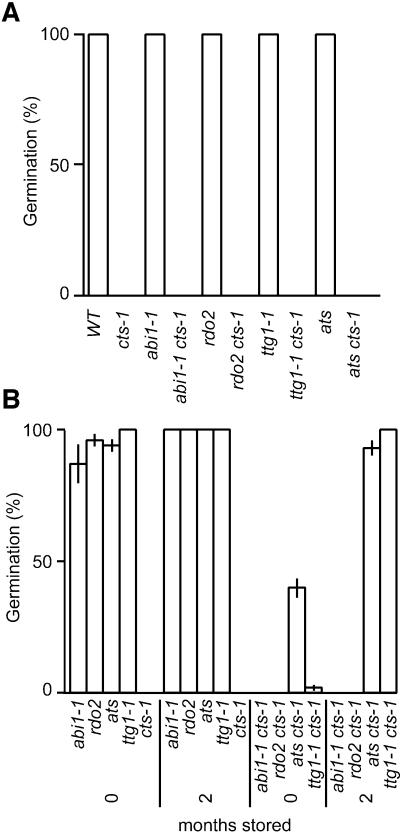
To define the relative position of CTS in the genetic pathway controlling germination, double mutants were generated with embryo (reduced dormancy2, rdo2), testa (altered testa shape, ats and transparent testa glabra 1 ttg1-1) and hormone-related (abscisic acid insensitive 1, abi1-1) mutants (Koornneef et al., 1984; Léon-Kloosterziel et al., 1996; Debeaujon et al., 2000). WT loci corresponding to these mutations promote embryo dormancy through independent pathways, therefore these mutants all increase germination potential of embryos. Seed germination was analysed following storage for 1 month (the time period required for WT seeds to completely after-ripen) in the absence of exogenous sucrose (Figure 7A). In all cases, single mutants (with the exception of cts-1) were completely non-dormant and germinated within 7 days of sowing. Conversely, all double mutant combinations tested showed the highly reduced germination potential of the single cts-1 allele. When freshly harvested seeds were plated on medium containing sucrose (Figure 7B), single mutants of abi1-1, rdo2, ats and ttg1-1 showed high germination potential that was markedly reduced by the presence of the cts-1 mutation in the double mutants. However, following dry storage of seed lots, the germination potential of the two testa mutants ats and ttg1-1 combined with cts-1 increased, but only in the presence of exogenous sucrose. In contrast, cts-1 rdo2 and cts1 abi1-1 double mutants failed to germinate regardless of whether seed was stored, or sucrose was supplied.
Fig. 7. Genetic interactions of CTS with loci that repress germination. Germination (%) of after-ripened seed single and double mutant combinations in the absence (A) or presence (B) of exogenous sucrose is shown. Germination was measured 7 days after seed sowing. Bars indicate SEM.
Expression of CTS reflects the germination potential of seeds
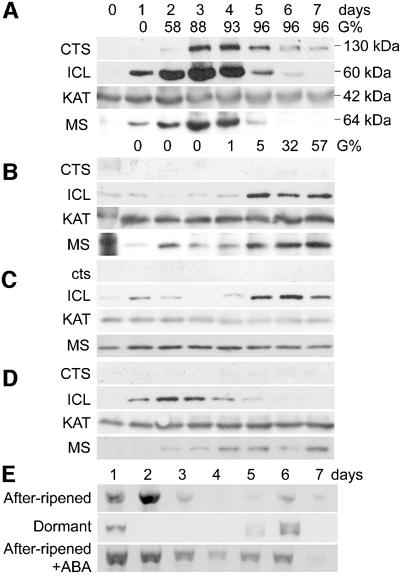
The expression pattern of CTS protein and RNA, and proteins associated with β-oxidation (KAT2) and the glyoxylate cycle (ICL, MS) were examined during germination of after-ripened non-dormant WT seedlings (Figure 8A). Strong induction of CTS and ICL occurred before and during radicle emergence and early seedling establishment. The cts-1 mutant (Figure 8C) was compared with freshly harvested dormant WT seed (Figure 8B) and WT seed treated with a germination-inhibiting concentration (10 µM) (Garciarrubio et al., 1997) of the hormone abscisic acid (ABA) (Figure 8D). In dormant seeds, a very low level of CTS expression was observable, after 6 days imbibition (Figure 8B). This may be because ecotype Ler has low dormancy levels (compare germination of after-ripened and dormant samples, Figure 8), and it is not possible to know a priori which seeds in sample populations are dormant, and which are about to germinate. However, this very low level of expression is consistent with genetic data (Figure 7) showing the requirement of CTS for radicle emergence. In ABA-treated seeds (Figure 8D), expression was repressed completely and seeds did not germinate. In imbibed seeds of the cts-1 mutant (Figure 8C), as expected, no CTS protein could be detected. In dormant WT seeds and in the cts-1 mutant, induction of ICL was diminished and delayed compared with germinating seeds. However, ICL induction was reduced but not delayed in timing in ABA-treated WT seeds (Figure 8D), which resembles the expression profile in germinating seeds. MS expression did not show the rapid induction observed in germinating seedlings, but a lower, more constitutive expression in cts-1 mutant, dormant and ABA-treated seeds. In all cases, expression of KAT2 protein was similar. Analysis of transcript levels by RT–PCR (Figure 8E) showed that in after-ripened seeds, maximum steady-state levels of CTS transcript precede the appearance of CTS protein by 1 day. In dormant seeds, weak expression is seen at days 5–6 around the time that some seeds in the sample begin to germinate (Figure 8B). In contrast, ABA-treated after-ripened seeds express CTS transcript from day 1 through to day 6, but do not synthesize detectable levels of CTS protein and do not germinate.
Fig. 8. Analysis of CTS RNA and protein expression. (A–D) Western analysis of seeds imbibed for 7 days, using antibodies for CTS, ICL, MS and KAT. In each case, sizes of proteins are indicated. (A) After- ripened seeds (germination percentage indicated; G%). (B) Dormant seeds (G% indicated). (C) cts-1 mutant seeds (no germination). (D) WT seeds imbibed with 10 µM ABA (no germination; this concentration of ABA inhibits germination; Garciarrubio et al., 1997). (E) RT–PCR analysis of CTS gene expression in imbibed seeds.
Discussion
CTS is a peroxisomal ABC transporter homologous to human ALDP
In this paper, we describe the cloning and characterization of COMATOSE, a locus that has a major role in the transition from embryo dormancy to germination. The CTS gene encodes a peroxisomal ABC transporter with a very high level of sequence similarity to the human X-linked ALDP. Of the 51 full-length ABC transporters present in the Arabidopsis genome, only CTS demonstrates significant similarity to ALDP. All other members of this gene family in humans [ALDP, PMP70, ALDR and PMP70R (Kamijo et al., 1992; Mosser et al., 1993; Lombard-Platet et al., 1996; Shani et al., 1997), and yeast PXA1/PAT2 and PXA2/PAT1 (Shani et al., 1995; Shani and Valle, 1996)], belong to the half-size transporter class and have been shown to form homo- and heterodimers. Studies in Saccharomyces cerevisiae have shown that the transporter is associated with ATP-dependent transport of oleoyl CoA into the peroxisome (Verleur et al., 1997). Acyl CoAs, unlike fatty acids, do not readily flip across lipid bilayers therefore active import of acyl CoAs by the transporter could regulate β-oxidation (Hettema and Tabak, 2000). The CTS gene is equivalent to the recently reported PXA1/PED3 (Zolman et al., 2001; Hayashi et al., 2002). These papers reported analyses of weaker alleles (see Figure 1A and later) at this locus, and suggested possible functions for this protein in fatty acid metabolism. Here we have presented data that argue for a central role of CTS in the control of long and very long chain acyl CoA transport into peroxisomes during germination, and suggest a second additional function for CTS in regulating the transition from embryo dormancy to germination.
CTS is a transporter of acyl CoAs with a broad substrate specificity
Lipid bodies are retained in cts-1 seedings. Analysis of TAG-derived fatty acids and acyl CoAs demonstrates that while some lipid is mobilized in the cts-1 and cts-2 mutants, catabolism is inhibited before β-oxidation, resulting in an increased acyl CoA pool. This is particularly pronounced for C20 and C22 acyl CoAs, which are predominantly TAG-derived. Collectively, these data argue strongly that the primary defect in the cts mutants is in transport of fatty acyl CoAs into peroxisomes. 18:2 and 18:3 CoAs do not accumulate to a similar extent, which may reflect their use in the synthesis of structural lipids by ER-mediated pathways. In contrast, C20:1 is not a component of structural lipids and may accumulate because it lacks a synthetic sink route. It is possible that the accumulation of 20:1 CoA, or depletion of free coenzyme A, results in the inhibition of lipolysis, and therefore the release of further fatty acids from storage TAG. The accumulation of acyl CoAs argues that these are the substrates of the CTS protein, and suggests that unlike X-ALD patients, cts mutants retain VLCFA synthetase activity. The substantial accumulation of long and very long chain acyl CoAs in the cts-1 and cts-2 mutants is consistent with the activation of these fatty acids by acyl CoA synthetases on the cytoplasmic side of the peroxisomal membrane, as reported for S.cerevisiae (Hettema et al., 1996), mammals (Mannaerts et al., 1982) and plants (Olsen and Lusk, 1994). The finding that all fatty acid chain lengths are mobilized in the WT and retained in both mutants argues that the transporter has broad substrate specificity with respect to acyl chain length. The resistance of mutants in CTS/PED3/PXA1 to 2,4DB suggests that the transporter is also responsible for the uptake of this synthetic auxin into peroxisomes. Acyl CoAs, 2,4DB and IBA are all amphipathic molecules, as are the substrates for many ABC transporters. The recently determined structure of the Escherichia coli lipid flippase MsbA suggests a general mechanism for the function of ABC transporters like CTS/ALDP (Chang and Roth, 2001).
CTS expression predicts germination potential
CTS is expressed before and during radicle emergence. Naturally dormant seeds or those that have been prevented from germinating by ABA treatment do not express detectable levels of CTS protein, although the mechanism is different as ABA treatment does not prevent appearance of CTS mRNA. ABA treatment does not completely block TAG breakdown in Arabidopsis seeds (S.L.Pritchard, W.L.Charlton, A.Baker aned I.A.Graham, submitted) and the reported 30% reduction of 20:1 is similar to the 28–33% reduction in TAG-derived fatty acids seen in the cts-1 and cts-2 mutants. This level of breakdown could be mediated by a different transporter or represent a low level of diffusion of free fatty acids through the peroxisome membrane. ABA treatment resulted in a different expression pattern for both ICL and MS in comparison to dormancy. This indicates that although superficially similar, ABA-treated seeds are in a developmentally different state to dormancy. The expression of CTS therefore appears to be required for breaking dormancy, and this is supported by the double mutant results (Figure 7). Freshly harvested cts-1 ats and ttg1-1 cts-1 double mutants are dormant, but after 2 months storage, this dormancy is removed. This indicates that CTS function is normally required to break dormancy, but this requirement is not needed after storage. This provides additional evidence to support the hypothesis (Russell et al., 2000) that the cts mutant seeds resemble WT seeds in the dormant state. Both expression data and genetic analysis show that germinating embryos require CTS activity during early seedling development, before radicle emergence and seedling establishment. The requirement for CTS can be bypassed by mutations such as abi3-4, fus3-3 and lec1-1, which lead to activation of post-germination development programmes in the embryo (Russell et al., 2000). As the embryos of these mutants are already photosynthetic, they can germinate and establish independently of CTS function in the absence of exogenous sucrose because they have already passed the embryo/seedling phase transition. This suggests that expression of CTS is a required linking function between after-ripening, germination potential and the removal of dormancy. Analysis of the biochemical function of CTS therefore provides an opportunity to define the control mechanisms that switch the embryo from dormancy to germination programmes.
Can the failure of lipid mobilization explain all the phenotypes associated with cts-1 and cts-2 mutants?
Both the pxa1 allele reported by Zolman et al. (2001) and the four alleles of ped3 described by Hayashi et al. (2002) have defects in β-oxidation as determined by resistance to 2,4DB or IBA (Figure 1A). Lipid bodies also persist in the ped3 mutants. Consistent with the observation that pxa1 affects vegetative growth (Zolman et al., 2001), we have also observed vegetative phenotypes for cts-1 and cts-2; these phenotypes are subtle and are only revealed under compromised growth conditions (S.Footitt, S.Slocombe, A.Baker and M.Holdsworth, manuscript in preparation).
pxa1 and the ped3 series alleles are all point mutations (Figure 1A). The pxa1 allele is a single base mutation that results in mis-splicing of the final intron and absence of the final 32 of 1337 amino acids. ped3-2 and ped3-4 are missense mutations, and ped3-1 and ped3-3 are nonsense mutations resulting in the loss of the last 95 and 330 amino acids, respectively. All these mutations lie in the second ABC transporter domain (Figure 1A). In contrast, the cts-1 and cts-2 alleles result from large insertions within the first ABC transporter domain, and cts-2 is almost certainly a null allele as the site of insertion is only 77 amino acids from the initiating methionine. cts-1 is also likely to be non-functional, as the large insertion disrupts the first ‘Walker B’ motif. Consistent with this, cts-1 and cts-2 have the strongest phenotypes of the described alleles, and are unable to germinate, either with or without exogenous sucrose. In contrast, ped3-1 and ped3-3 require sucrose for germination, while ped3-2, ped3-4 and pxa1 all germinate in the absence of sucrose but fail to establish (Zolman et al., 2001; Hayashi et al., 2002). In this regard, the weaker alleles behave like the kat2/ped1 mutants, which lack the major seedling-expressed thiolase (Hayashi et al., 1998; Germain et al., 2001). These mutants have an equally severe lipid breakdown defect to cts-1 and cts-2, but can still germinate, as can seeds of the wri mutant, which have very low lipid content (Focks and Benning, 1998). This demonstrates that a deficiency of storage lipids or a block in their metabolism is not enough to inhibit germination.
Flux through β-oxidation and gluconeogenesis during seed development could be required for the accumulation of carbohydrates required for germination. Measurements of sucrose levels in dry seeds of cts-1 suggest this explanation is unlikely, as cts-1 seeds have 73% of the sucrose of the corresponding WT. The non-germination phenotype of the strong cts alleles is therefore difficult to explain solely on the basis of a defect in lipid mobilization, and indicates another function for CTS as a biochemical promoter of germination potential. This is supported by both the genetic and expression studies (Figures 7 and 8). What is striking is the failure of the cts-1 mutant to utilize endogenous carbohydrate, although exogenous carbohydrate supports germination in combination with mutations affecting testa permeability or the rupture of the seed coat. Whether this is a direct effect of the cts mutation or a consequence of the cts mutation resulting in a dormant state in which carbohydrate metabolism is repressed is unknown at present. Interestingly, ABA treatment of seeds, which blocks germination and represses CTS expression (Figure 8), also results in accumulation of sucrose at the expense of glucose (S.L.Pritchard, W.L.Charlton, A.Baker and I.A.Graham, submitted).
How might these observations be explained? One possibility is that a product of lipid metabolism accumulates in the absence of CTS, and either signals to the embryo to remain dormant, or interferes directly with carbohydrate metabolism thereby blocking germination. However, the lipid breakdown and germination promotion functions are separated in the weak alleles represented by pxa1, ped3-2 and ped3-4, as these mutants are able to germinate in the absence of sucrose. In this case, it would be necessary to postulate that these alleles retain sufficient transport function to keep the inhibitor below threshold levels. Alternatively, CTS, like some other ABC transporters, might be a bifunctional protein. In mammals, the cystic fibrosis transconductance regulator and the sulfonylurea receptor are ABC transporters that interact with passive ion channels, modulating their activity (Bryan and Aguilar-Bryan, 1999). In Arabidopsis, the ABC transporter AtMRP5 is an organic ion transporter that probably transports auxin conjugates, but also alters the response of stomatal opening to glibenclamide (a sulfonylurea compound), suggesting that it too may control an ion channel in guard cells (Gaedeke et al., 2001). CTS could have an additional function to the transport of acyl CoAs, which involves the removal of dormancy and promotion of germination potential. Therefore, strong alleles are not able to transport fatty acyl CoAs, and in addition cannot break dormancy and initiate germination. Weak alleles might retain this second function but lack fatty acyl CoA transport activity. The availability of multiple alleles of CTS with different phenotypes should also allow the further testing of this model.
Conclusion
As well as a conserved function in lipid mobilization in the plant and animal kingdoms, our results show that CTS has a distinct function in germination, reflecting the differing relationships between metabolism and growth strategies in plants and animals. The results presented here strongly implicate CTS function as a major control point in the developmental switch between dormancy and germination in Arabidopsis, as well as a transporter of fatty acyl CoAs into the peroxisome. The expression of CTS is regulated in the embryo by embryo dormancy status (itself controlled by loci that control ABA sensitivity), indicating one major mechanism through which developmental ABA signals and dormancy repress germination.
Materials and methods
Plant material
Arabidopsis thaliana plants and seeds were grown and treated as described previously with a minimum day length of 16 h (Russell et al., 2000). Mutants were obtained from the Nottingham Arabidopsis Stock Centre. Double mutant combinations were generated by standard procedures.
Sequencing and complementation
The cts-2 mutant allele was obtained by PCR-based screening of the Wisconsin-α gene knockout lines for insertions in the CTS gene (Krysan et al., 1999). The sequence of the CTS RNA was obtained using the cDNA clone H1A6T7 (ABRC, Ohio State University). The cts-1 mutation was located by CAPS mapping (Konieczny and Ausubel, 1993) and genome walking (Universal genome Walker Kit, Clontech). Complementation of cts-1 was achieved using BAC clones 27H10, 155A23 and 159N1 (GeTCID, John Innes Centre) by introduction into Agrobacterium strain GV3101 (Koncz and Schell, 1986) and transformation of cts-1 plants using the floral dip method (Clough and Bent, 1998). Transformants were subjected to double selection based on their ability to germinate on kanamycin (50 µg/ml) in the absence of sucrose. Transformation was confirmed by PCR screening genomic DNA for CTS, cts-1 and NPTII genes.
Expression analysis
For expression analysis, seeds were plated on media as indicated in the figure legends. Fresh seeds (dormant) were harvested from yellow unopened siliques, after-ripened seeds (or stored cts-1/cts-2 seeds) were dry stored for 1 month at 24°C in the dark. Samples were taken daily from plates for 7 days for RT–PCR and western analysis. In the case of dormant seeds, all germinating individuals were removed before sample extractions. RNA was extracted using the Plant RNeasy extraction kit (Qiagen) using a modified extraction buffer [4 M guanine hydrochloride, 20 mM 2-(N-morpholino)ethanesulfonic acid, 0.5 M EDTA, 2 M NaCl, 2% (w/v) polyvinyl pyrollidone-360, 0.22 M β-mercaptoethanol pH 7.0]. Total RNA (1 µg) was DNase treated and reverse transcribed by standard methods. PCR using CTS gene-specific primers was performed and products visualized on 1% (w/v) agarose gels. Proteins were extracted from seed/seedling samples in 50 mM Tris pH 7.5, 1% (w/v) SDS, 2 mM PMSF and 0.01% (v/v) Sigma protease inhibitor cocktail. Proteins were resolved by SDS–PAGE (50 µg of total protein per lane) (Laemmli, 1970), transferred to nitrocellulose membranes and probed with antibodies for CTS, ICL, MS and KAT.
Germination phenotype analysis
Testa/endosperm layers were removed as described previously (Russell et al., 2000). After-ripened WT and mutant seeds with or without testa/endosperm were incubated on germination media ± 3% sucrose. Resistance to auxin herbicides was tested by germinating mutant seeds with testa/endosperm layers removed on sucrose-containing germination media ± 0.2 µg/ml 2,4DB or 0.05 µg/ml 2,4D. Propionic and butyric acid were added to germination media (0.01–1.0 mM pH 4.8) ± sucrose. Germination was denoted as emergence of the radicle from the testa.
Biochemical analysis
Sucrose density gradient and assay of fractions were performed as described previously (Lopez-Huertas et al., 1995) using Arabidopsis rosette leaf material. A polyclonal antibody was raised against a C- terminal fragment of CTS (amino acids 1112–1337) expressed in E.coli strain BL21(DE3)pLysS (Novagen) using a pET28b (Novagen) construct containing an NheI–BamHI fragment of cDNA clone H1A6T7 (DDBJ/EMBL/GenBank accession No. AJ311341). The antibody was affinity-purified using the recombinant fragment (Tugal et al., 1999). Acyl CoAs and total lipids were extracted from five replicated 3–10 mg tissue samples and analysed according to Larson and Graham (2001). An aliquot of the total lipid extract was used for TAG determination. A 1 ml 100 mg bed volume Bond-Elut (Varian, Surrey, UK) SPE column was prepared by elution with 2 × 1 ml methanol, 3 × 1 ml hexane, and then 100 µl sample loaded in hexane. TAGs were eluted with 1.5 ml 2:3 (v/v) chloroform:hexane, dried under vacuum, transmethylated to fatty acid methyl esters and analysed as described previously (Larson and Graham, 2001). Specificity for TAG separation was optimized so that the diacylglycerol and dipalmitin (Sigma) were excluded from the SPE eluate. Carbo hydrates were extracted in triplicate from 50 seed samples as described in Focks and Benning (1998). Levels of soluble sugars were determined based on an adaptation of the assay of Outlaw and Tarczynski (1985).
Electron microscopy
Cotyledons from seedlings germinated for 5 days on 1/2 MS medium supplemented with 1% (w/v) sucrose were processed for electron microscopy as described, omitting treatment with 3,3′-diaminobenzidine (Frederick and Newcomb, 1969).
Acknowledgments
Acknowledgements
We are grateful to Dr J.Lenton and Dr F.Theodoulou for discussions and comments on the manuscript. For technical assistance, we thank Annalisa Marchese (LARS) and Barbara Johnson (Leeds). We gratefully acknowledge the assistance of the BBSRC GARNet service (Dr Ian Bancroft) for the provision of genomic DNA screening. We are grateful for donations of antibodies directed against Arabidopsis KAT (Dr L.Rylott, University of York), tobacco calreticulin (Dr J.Denecke, University of Leeds) and maize ANT (Professor C.J.Leaver, Oxford University). We are grateful to Dr T.Newman (MSU-DOE Plant Research Laboratory, Michigan State University) for supplying cDNA clone H1A6T7 (DDBJ/EMBL/GenBank accession No. N95877). IACR-Long Ashton receives grant-aided support from the BBSRC. S.S. is funded by EU grant QLRT1999-00213 awarded to A.B., V.L. by a BBSRC PhD studentship, and S.K. by the BBSRC Cell Commitment and Determination Initiative awarded to M.H.
References
- Baskin C.C. and Baskin,J.M. (1998) Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination. Academic Press, London, UK.
- Baumlein H., Misera,S., Luerssen,H., Kolle,K., Horstmann,C., Wobus,U. and Muller,A.J. (1994) The FUS3 gene of Arabidopsis thaliana is a regulator of gene expression during late embryogenesis. Plant J., 6, 379–387. [Google Scholar]
- Bewley J.D. (1997) Seed germination and dormancy. Plant Cell, 9, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley J.D. and Black,M. (1985) Seeds, Physiology of Development and Germination. Plenum Press, New York, NY.
- Bryan J. and Aguilar-Bryan,L. (1999) Sulfonyl urea receptors: ABC transporters that regulate ATP-sensitive K+ channels. Biochim. Biophys. Acta, 1461, 285–303. [DOI] [PubMed] [Google Scholar]
- Chang G. and Roth,C.B. (2001) Structure of MsbA from E.coli: a homologue of the multidrug resistance ATP binding cassette (ABC) transporters. Science, 293, 1793–1800. [DOI] [PubMed] [Google Scholar]
- Clough S.J. and Bent,A.F. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J., 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Davies T. and Coleman,J. (2000) The Arabidopsis thaliana ATP-binding cassette proteins: an emerging superfamily. Plant Cell Environ., 23, 431–443. [Google Scholar]
- Debeaujon I., Léon-Kloosterziel,K.M. and Koornneef,M. (2000) Influence of the testa on seed dormancy, germination and longevity in Arabidopsis. Plant Physiol., 122, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond P.J., Germain,V., Lange,P.R., Bryce,J.H., Smith,S.M. and Graham,I.A. (2000) Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc. Natl Acad. Sci. USA, 97, 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R.R. (1994) Mutations at 2 new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J., 5, 765–771. [Google Scholar]
- Focks N. and Benning,C. (1998) wrinkled1: a novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol., 118, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S.E. and Newcomb,E.H. (1969) Cytochemical localization of catalase in leaf microbodies (peroxisomes). J. Cell Biol., 43, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedeke N. et al. (2001) The Arabidopsis thaliana ABC transporter atMRP5 controls root development and stomatal movement. EMBO J., 20, 1875–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciarrubio A., Legaria,J.P. and Covarrubias,A.A. (1997) Abscisic acid inhibits germination of mature Arabidopsis seeds by limiting the availability of energy and nutrients. Planta, 203, 182–187. [DOI] [PubMed] [Google Scholar]
- Germain V., Rylott,E.L., Larson,T.R., Sherson,S.M., Bechtold,N., Carde,J.P., Bryce,J.H., Graham,I.A. and Smith,S.M. (2001) Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid β-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J., 28, 1–12. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Toriyama,K., Kondo,M. and Nishimura,M. (1998) 2,4-dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell, 10, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Nito,K., Takei-Hoshi,R., Yagi,M., Kondo,M., Suenaga,A., Yamaya,T. and Nishimura,M. (2002) Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid β-oxidation. Plant Cell Physiol., 43, 1–11. [DOI] [PubMed] [Google Scholar]
- Hettema E.H. and Tabak,H.F. (2000) Transport of fatty acids and metabolites across the peroxisomal membrane. Biochim. Biophys. Acta, 1486, 18–27. [DOI] [PubMed] [Google Scholar]
- Hettema E.H., van Roermund,C.W.T., Distel,B., van den Berg,M., Vilela,C., Rodrigues-Posada,C., Wanders,R.J.A. and Tabak,H.F. (1996) The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acids into peroxisomes of Saccharomyces cerevisiae. EMBO J., 15, 3813–3822. [PMC free article] [PubMed] [Google Scholar]
- Holdsworth M., Kurup,S. and McKibbin,R. (1999) Molecular and genetic mechanisms regulating the transition from embryo development to germination. Trends Plant Sci., 4, 275–280. [Google Scholar]
- Kamijo K., Kamijo,T., Ueno,I., Osumi,T. and Hashimoto,T. (1992) Nucleotide-sequence of the Human 70 kDa peroxisomal membrane protein—a member of ATP-binding cassette transporters. Biochim. Biophys. Acta, 1129, 323–327. [DOI] [PubMed] [Google Scholar]
- Koncz C. and Schell,J. (1986) The promoter of Tl-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet., 204, 383–396. [Google Scholar]
- Konieczny A. and Ausubel,F.M. (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J., 4, 403–410. [DOI] [PubMed] [Google Scholar]
- Koornneef M., Reuling,G. and Karssen,C.M. (1984) The isolation and characterization of abscisic acid insensitive mutants of Arabidopsis thaliana. Physiol. Plant., 61, 377–383. [Google Scholar]
- Koornneef M., Alonso Blanco,C., Bentsink,L., Blankestijn-de Vries,H., Debeaujon,I., Hanhart,C.J., Léon-Kloosterzeil,K.M., Peeters,A.J.M. and Raz,V. (2000) The genetics of seed dormancy in Arabidopsis thaliana. In Viemont,J.-D. and Crabbe,J.J. (eds), Dormancy in Plants. CABI Publishing, New York, NY, pp. 365–374.
- Krysan P.J., Young,J.C. and Sussman,M.R. (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell, 11, 2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Larson T.R. and Graham,I.A. (2001) A novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J., 25, 115–125. [DOI] [PubMed] [Google Scholar]
- Léon-Kloosterziel K.M., Vandebunt,G.A., Zeevaart,J.A.D. and Koornneef,M. (1996) Arabidopsis mutants with a reduced seed dormancy. Plant Physiol., 110, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard-Platet G., Savary,S., Sarde,C.-O. and Mandel,J.-L. (1996) A close relative of the adrenoleukodystrophy (ALD) gene codes for a peroxisomal protein with a specific expression pattern. Proc. Natl Acad. Sci. USA, 93, 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Huertas E., Sandalio,L.M. and Delrio,L.A. (1995) Integral membrane polypeptides of pea leaf peroxisomes—characterization and response to plant stress. Plant Physiol. Biochem., 33, 295–302. [Google Scholar]
- Lotan T. et al. (1998) Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell, 93, 1195–1205. [DOI] [PubMed] [Google Scholar]
- Mannaerts G.P., van Veldhoven,P., van Broekhoven,A., Vandebroek,G. and Debeer,L.J. (1982) Evidence that peroxisomal acyl-CoA synthetase is located at the cytoplasmic side of the peroxisomal membrane. Biochem. J., 204, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke D.W., Franzmann,L.H., Nickle,T.C. and Yeung,E.C. (1994) Leafy cotyledon mutants of Arabidopsis. Plant Cell, 6, 1049–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser H.W., Smith,K.D. and Moser,A.B. (1995) X-linked Adrenoleukodystrophy. McGraw-Hill, New York, NY.
- Mosser J., Douar,A.M., Sarde,C.O., Kioschis,P., Feil,R., Moser,H., Poustka,A.M., Mandel,J.L. and Aubourg,P. (1993) Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature, 361, 726–730. [DOI] [PubMed] [Google Scholar]
- Nambara E., Keith,K., McCourt,P. and Naito,S. (1995) A regulatory role for the ABI3 gene in the establishment of embryo maturation in Arabidopsis thaliana. Development, 121, 629–636. [Google Scholar]
- Nambara E., Hayama,R., Tsuchiya,Y., Nishimura,M., Kawaide,H., Kamiya,Y. and Naito,S. (2000) The role of ABI3 and FUS3 loci in Arabidopsis thaliana on phase transition from late embryo development to germination. Dev. Biol., 220, 412–423. [DOI] [PubMed] [Google Scholar]
- Olsen J.A. and Lusk,K.R. (1994) Acyl CoA synthetase activity associated with rapeseed lipid body membranes. Phytochemistry, 36, 7–9. [Google Scholar]
- Outlaw W.H. and Tarczynski,M.C. (1985) Sucrose. In Bergmeyer, V.C.H. (ed.), Methods of Enzymatic Analysis 6, Metabolites 1: Carbohydrates. Wiley, pp. 96–103.
- Parcy F., Valon,C., Kohara,A., Misera,S. and Giraudat,J. (1997) The ABSCISIC ACID INSENSITIVE3, FUSCA3 and LEAFY COTYLEDON1 loci act in concert to control multiple aspects of Arabidopsis seed development. Plant Cell, 9, 1265–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid S., Diamond,A.S. and Eviatar,L. (2000) Coma as an acute presentation of adrenoleukodystrophy. Pediatr. Neurol., 22, 237–239. [DOI] [PubMed] [Google Scholar]
- Russell L., Larner,V., Kurup,S., Bougourd,S. and Holdsworth,M.J. (2000) The Arabidopsis COMATOSE locus regulates germination potential. Development, 127, 3759–3767. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez R., Davies,T.G.E., Coleman,J.O.D. and Rea,P.A. (2001) The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J. Biol. Chem., 276, 30231–30244. [DOI] [PubMed] [Google Scholar]
- Shani N. and Valle,D. (1996) A Saccharomyces cerevisiae homolog of the human adrenoleukodystrophy transporter is a heterodimer of two half ATP-binding cassette transporters. Proc. Natl Acad. Sci. USA, 93, 11901–11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani N., Watkins,P.A. and Valle,D. (1995) Pxa1, a possible Saccharomyces cerevisiae ortholog of the human adrenoleuko dystrophy gene. Proc. Natl Acad. Sci. USA, 92, 6012–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani N., Jiminez-Sanchez,G., Steel,G., Dean,M. and Valle,D. (1997) Identification of a fourth half ABC transporter in the human peroxisomal membrane. Hum. Mol. Genet., 6, 1925–1931. [DOI] [PubMed] [Google Scholar]
- Smith K. et al. (1999) X-linked adrenoleukodystrophy: genes, mutations and phenotypes. Neurochem. Res., 24, 521–535. [DOI] [PubMed] [Google Scholar]
- Stone S.L., Kwong,L.W., Yee,K.M., Pelletier,J., Lepiniec,L., Fischer,R.L., Goldberg,R.B. and Harada,J.J. (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc. Natl Acad. Sci. USA, 98, 11806–11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature, 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Titorenko V.I. and Rachubinski,R.A. (2001) The life cycle of the peroxisome. Nature Rev. Mol. Cell. Biol., 2, 357–368. [DOI] [PubMed] [Google Scholar]
- Tugal H.B., Pool,M. and Baker,A. (1999) Arabidopsis 22-kilodalton peroxisomal membrane protein. Nucleotide sequence analysis and biochemical characterization. Plant Physiol., 120, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleur N., Hettema,E.H., van Roermund,C.W.T., Tabak,H.F. and Wanders,R.J.A. (1997) Transport of activated fatty acids by the peroxisomal ATP-binding-cassette transporter Pxa2 in a semi-intact yeast cell system. Eur. J. Biochem., 249, 657–661. [DOI] [PubMed] [Google Scholar]
- Volokita M. (1991) The carboxy terminal end of glycolate oxidase directs a foreign protein to tobacco leaf peroxisomes. Plant J., 1, 361–366. [DOI] [PubMed] [Google Scholar]
- West M.A.L., Yee,K.M., Danao,J., Zimmerman,J.L., Fischer,R.L., Goldberg,R.B. and Harada,J.J. (1994) Leafy Cotyledon1 is an essential regulator of late embryogenesis and cotyledon identity in Arabidopsis. Plant Cell, 6, 1731–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Taniwaki,T., Shinnoh,N., Uchiyama,A., Shimozawa,N., Ohyagi,Y., Asahara,H. and Kira,J. (1999) Adrenoleukodystrophy protein enhances association of very long-chain acyl-coenzyme A synthetase with the peroxisome. Neurology, 52, 614–616. [DOI] [PubMed] [Google Scholar]
- Zolman B.K., Silva,I.D. and Bartel,B. (2001) The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid β-oxidation. Plant Physiol., 127, 1266–1278. [PMC free article] [PubMed] [Google Scholar]