Abstract
Recently, corticosteroid hormone-induced factor (CHIF) and the γ-subunit, two members of the FXYD family of small proteins, have been identified as regulators of renal Na,K-ATPase. In this study, we have investigated the tissue distribution and the structural and functional properties of FXYD7, another family member which has not yet been characterized. Expressed exclusively in the brain, FXYD7 is a type I membrane protein bearing N-terminal, post-translationally added modifications on threonine residues, most probably O-glycosylations that are important for protein stabilization. Expressed in Xenopus oocytes, FXYD7 can interact with Na,K-ATPase α1–β1, α2–β1 and α3–β1 but not with α–β2 isozymes, whereas, in brain, it is only associated with α1–β isozymes. FXYD7 decreases the apparent K+ affinity of α1–β1 and α2–β1, but not of α3–β1 isozymes. These data suggest that FXYD7 is a novel, tissue- and isoform-specific Na,K-ATPase regulator which could play an important role in neuronal excitability.
Keywords: brain Na,K-ATPase modulator/FXYD7/FXYD proteins/Xenopus oocytes
Introduction
A widespread mechanism that mediates the regulation of the activity and/or expression of ion transporters or channels involves their interaction with small single-span membrane polypeptides. Best defined examples are the cardiac K+ channel which is regulated by IsK (Suessbrich and Busch, 1999), the sarcoplasmic reticulum Ca2+-ATPase which is regulated by phospholamban (Simmerman and Jones, 1998) and sarcolipin (Odermatt et al., 1998), or the yeast H+-ATPase which is associated with Pmp (Navarre et al., 1992). Based on sequence homology, a new family of proteins has recently been defined (Sweadner and Rael, 2000) which are related to but distinct from those mentioned above. The so-called FXYD proteins share a signature sequence comprising the FXYD motif in the N-terminus and conserved glycine and serine residues in the transmembrane domain. The FXYD family consists of seven members (FXYD1–7; Sweadner and Rael, 2000) including ‘phospholemman’ (PLM; Palmer et al., 1991; FXYD1), the ‘γ-subunit’ of Na,K-ATPase (Forbush et al., 1978; Mercer et al., 1993; FXYD2), ‘mammary tumor marker 8’ (MAT-8; Morrison et al., 1995; FXYD3), ‘corticosteroid hormone-induced factor’ (CHIF; Attali et al., 1995; FXYD4), ‘related to ion channel’ (RIC; Fu and Kamps, 1997; FXYD5), phosphohippolin (Yamaguchi et al., 2001; FXYD6) and FXYD7, a protein not yet studied. Members of the FXYD family are widely distributed in mammalian tissues, with prominent expression of phospholemman in the heart (Palmer et al., 1991), of γ-subunits in various segments of the nephron (Pu et al., 2001), and of CHIF in the distal colon and the renal collecting duct (Shi et al., 2001).
Phospholemman (Palmer et al., 1991), the γ-subunit (Béguin et al., 1997) and CHIF (Béguin et al., 2001) were shown to be type I proteins exposing their C-terminus to the cytoplasm. Phospholemman and CHIF, but not γ-subunits, adopt this membrane orientation after cleavage of a signal sequence. Phospholemman is a target for protein kinase A and C phosphorylation (Palmer et al., 1991), whereas the γ-subunit (Arystharkova et al., 2002) and CHIF (Béguin et al., 2001) are subjected to as yet unidentified co- or post-translational modifications. A common feature of several FXYD proteins is their ability to induce ion-specific conductances when overexpressed in Xenopus oocytes (Moorman et al., 1992; Attali et al., 1995; Minor et al., 1998), but the physiological relevance of this observation is still obscure. On the other hand, it is now well established that the γ-subunit is a component of the renal Na,K-ATPase which regulates its K+, Na+ and ATP affinities (Béguin et al., 1997; Thérien et al., 1997; Pu et al., 2001; Arystarkhova et al., 2002). Significantly, cases of human primary hypomagnesemia have been linked to a heterozygous mutation in the γ-subunit (Meij et al., 2000). Recently, we demonstrated that not only the γ-subunit but also CHIF can associate with the Na,K-ATPase and modulate its transport properties. The γ-subunit decreases and CHIF increases the apparent Na+ affinity of the Na,K-ATPase (Béguin et al., 2001). These opposite effects of CHIF and the γ-subunit are likely to be of physiological relevance for the fine regulation of Na+ reabsorption along the nephron.
In view of these data, we speculated that not only the γ-subunit and CHIF but also other FXYD proteins could associate with and regulate the Na,K-ATPase activity in a tissue-specific way.
The Na,K-ATPase is a ubiquitous membrane protein that uses the energy of ATP hydrolysis to maintain Na+ and K+ gradients across the cell membrane. The Na+ gradient plays an essential role in providing the energy for the activity of Na+-dependent transporters as well as in specialized cellular functions such as muscle contraction or propagation of action potentials in excitable tissues. Together with the Na+ gradient, the K+ gradient essentially is involved in preserving the cell volume and the membrane potential, and its maintenance is of crucial importance for normal electrical signaling and to prevent potential pathologies (for references see D’Ambrosio et al., 2002). Na,K-ATPase consists of a catalytic α-subunit which contains the cation-, ATP- and cardiac glycoside-binding sites, and a β-subunit which is required for the structural and functional maturation of the α-subunit (for a review see Geering, 2001). Four α and three β isoforms have been identified which show a different tissue distribution (for a review see Blanco and Mercer, 1998) and which may produce Na,K-ATPase isozymes with different transport properties (Crambert et al., 2000).
In view of the central role of Na,K-ATPase in physiological and pathophysiological processes, the elucidation of the mechanisms that are involved in the regulation of Na,K-ATPase activity and expression becomes an important issue. Regulation of Na,K-ATPase mediated by direct interaction with small membrane proteins such as the γ-subunit and CHIF represents a novel mechanism that is complementary to short- and long-term regulation by different hormones. In the present study, we have identified and characterized FXYD7 as a new brain-specific modulator of Na,K-ATPase activity which is likely to play a crucial role in the re-uptake of extracellular K+ after action potentials in neurons.
Results
Tissue and cellular distribution of FXYD7
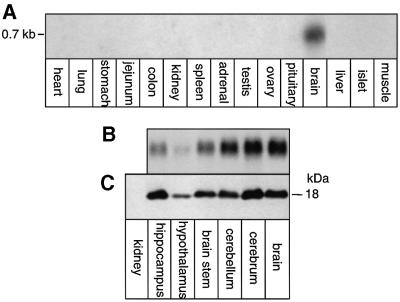
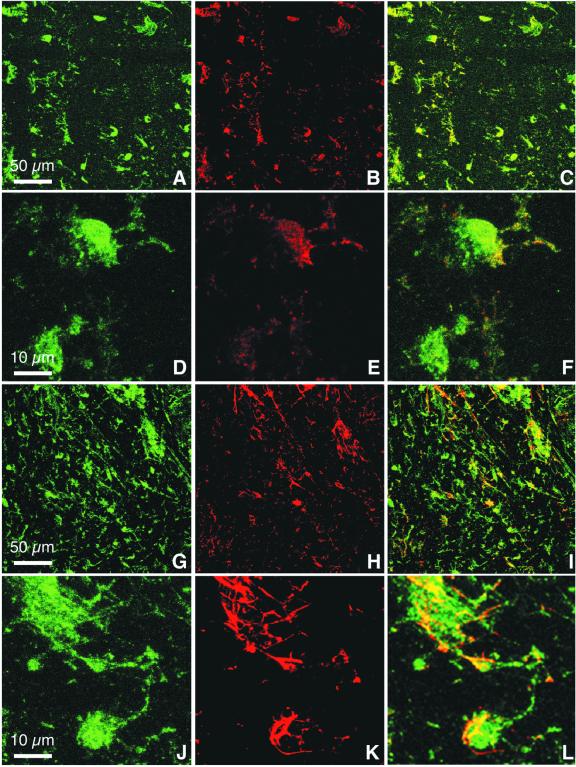
The FXYD7 mRNA and protein distribution was investigated in different rat tissues by northern and western blot analysis. As shown in Figure 1A, a strong signal corresponding to a 0.7 kb mRNA could be revealed in the brain, but not in other tissues. Within the brain, FXYD7 mRNA (Figure 1B) and the corresponding protein (Figure 1C) levels were high in various regions and lowest in the hypothalamus. Immunofluorescence microscopy on sections of rat brain (Figure 2A–L) showed co-localization of FXYD7 with synaptophysin (Figure 2C and F) as well as with glial fibrillary acidic protein (GFAP; Figure 2I and L), indicating the presence of FXYD7 in both neurons and astroglial cells. However, FXYD7 appeared to be expressed predominantly in neurons, as suggested by the intense immunoreactivity of FXYD7 (Figure 2G) compared with GFAP immunostaining (Figure 2H) and by the modest co-localization of FXYD7 and GFAP (Figure 2I). Nevertheless, at higher magnifications, both glial and neuronal FXYD7 could clearly be revealed. Figure 2L (bottom) shows an FXYD7-positive cell (probably a neuron) which is wrapped by an FXYD7-positive astrocyte.
Fig. 1. FXYD7 is expressed specifically in brain. A 10 µg aliquot of total RNA from different rat tissues (A) or rat brain regions (B) was migrated on agarose gels and transferred onto nitrocellulose prior to hybridization with an FXYD7 cDNA probe. A 50 µg aliquot of protein from microsomes of rat brain regions or rat kidney (C) was subjected to SDS–Tricine gel electrophoresis and western blot analysis with an FXYD7 antibody.
Fig. 2. FXYD7 is present in both neurons and glial cells. Double immunostaining of rat brain sections with an affinity-purified N-terminal FXYD7 antibody and with either synaptophysin, a neuronal marker, or GFAP, an astroglial marker. Confocal analysis shows FXYD7 immunoreactivity in green (A, D, G and J), and synaptophysin (B and E) and GFAP (H and K) in red. Co-localization appears in yellow (C, F, I and L). Shown are low (200×) (A–C and G–I) and high (1000×) (D–F and J–L) magnifications. Similar results were obtained with a C-terminal FXYD7 antibody.
Biosynthesis and processing of FXYD7
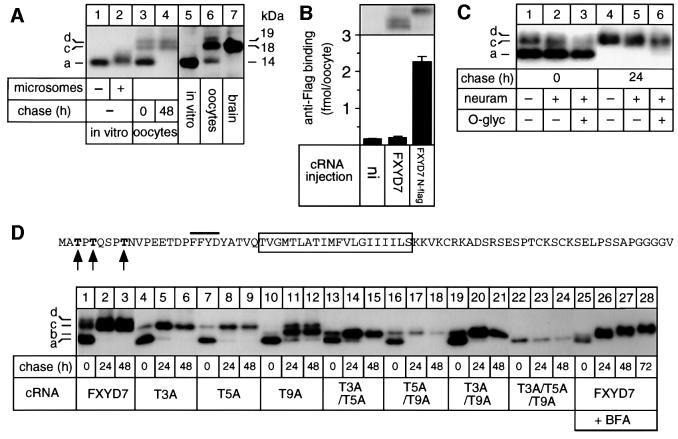
Based on the ‘inside positive’ rule (von Heijne and Gavel, 1988), it could be predicted that FXYD7 is a type I membrane protein similar to other characterized FXYD proteins. According to sequence analysis, FXYD7 should adopt the type I membrane orientation without cleavage of a signal sequence, similar to the γ-subunit. The lack of a cleavable signal sequence was confirmed by the observation that FXYD7 exhibited a similar molecular mass of ∼14 kDa when synthesized in vitro in a reticulocyte lysate in the absence of membranes (Figure 3A, lanes 1 and 5) or when expressed in Xenopus oocytes after a 24 h pulse period (lane 3, 14 kDa band). Moreover, the type I membrane orientation was verified by testing anti-Flag binding to intact oocytes expressing an N-terminally epitope-flagged FXYD7 (FXYD7 N-flag). Anti-Flag binding was observed in oocytes expressing FXYD7 N-flag but not in oocytes expressing epitope-deficient FXYD7 (Figure 3B), indicating that FXYD7 is targeted to the cell surface with the N-terminus exposed to the extracytoplasmic side.
Fig. 3. FXYD7 is a post-translationally modified, type I protein. (A) Mouse FXYD7 cRNA was translated and labeled with 35[S]methionine in a reticulocyte lysate in the absence (lane 1) or presence (lane 2) of canine pancreatic membranes, or injected into Xenopus oocytes prior to metabolic labeling with 35[S]methionine for 24 h (lane 3) followed by a 48 h chase period (lane 4). After denaturing immunoprecipitations with an FXYD7 antibody of in vitro translated samples or digitonin extracts of oocytes, samples were subjected to SDS–Tricine gel electrophoresis and revealed by fluorography. In addition, proteins from in vitro translation assays (lane 5), from microsomes of FXYD7 cRNA-injected oocytes (50 µg) (lane 6) or from rat brain microsomes (50 µg) (lane 7) were migrated on SDS–Tricine gels and subjected to western blot analysis with an FXYD7 antiserum (1:5000). (B) Oocytes were injected or not (ni) with FXYD7 or N-flag FXYD7 cRNA (2 ng). Upper panel: 3 days after injection, microsomes were prepared from metabolically labeled oocytes, immunoprecipitated with an FXYD7 antibody and the immunoprecipitates migrated on SDS–Tricine gels. Lower panel: 3 days after cRNA injection, intact oocytes were subjected to a radioimmunolabeling assay using a 125I-labeled FLAG antibody. (C) Oocytes were injected with FXYD7 cRNA, metabolically labeled for 6 h and subjected to a 48 h chase. Microsomes were immunoprecipitated with an FXYD7 antibody, and the immunoprecipitates bound to Sepharose beads were treated with or without O-glycosidase and/or neuraminidase as described in Materials and methods, before gel migration. (D) Oocytes were injected with wild-type or threonine mutant FXYD7 cRNA, metabolically labeled for 6 h and subjected to 24 and 48 h chase periods in the absence (lanes 1–24) or presence of BFA (lanes 25–28). Microsomes were immunoprecipitated under denaturing conditions with an FXYD7 antibody. a = no modified threonine (14 kDa), b = one modified threonine (15 kDa), c = two modified threonines (18 kDa) and d = three modified threonines (19 kDa). In the sequence of FXYD7, the bar indicates the position of the FXYD motif, the boxed region indicates the transmembrane segment, and the arrowheads indicate Thr3, Thr5 and Thr9, which were subjected to mutations.
Addition of microsomes to reticulocyte lysates occasionally led to a small shift in the molecular mass of FXYD7 (Figure 3A, lanes 1 and 2) suggestive of a co-translational modification depending on the batch of rough microsomes. When expressed in oocytes, FXYD7 appeared as three species after a 24 h pulse: the core protein of 14 kDa and two slower migrating bands of ∼18 and 19 kDa (lane 3). Only the higher molecular mass doublet persisted after a 48 h chase period (lane 4). These results suggest that in oocytes, FXYD7 is subjected to specific post- but not co-translational modifications. Significantly, FXYD7 detected in the brain (lane 7) appeared exclusively as a band of 18 kDa, which also corresponds to the most prominent FXYD7 species in oocytes, as revealed by western blot analysis (lane 6). This result indicates that FXYD7 is processed similarly in oocytes and in the native tissue.
Since an N-terminally truncated FXYD7 protein was expressed as a single band in oocytes (data not shown), we searched for possible protein modifications in the extracytoplasmic N-terminus. There is no consensus site for N-glycosylation in this region and, consistently, peptide-N-glycosidase F (PNGase F) treatment had no effect on the gel migration of FXYD7 (data not shown). On the other hand, a search in the NetOGlyc database (Hansen et al., 1998) predicted mucin-type N-acetylgalactosamine (GalNAc) O-glycosylation acceptor sites at Thr3, Thr5 and Thr9 (Figure 3C) with a score close to 1. Biochemical analysis of O-glycosylation partially confirmed this prediction. Indeed, extensive treatment with neuraminidase or neuraminidase plus O-glycosidase of oocyte-expressed, immunoprecipitated FXYD7 had some effect on the electrophoretic mobility of the higher molecular mass doublet observed after a 6 h pulse (Figure 3C, lanes 1–3) or a 24 h chase (lanes 4–6). On the other hand, neither treatment with galactosidase, glucosaminidase and/or N-acetylhexosaminidase, nor incubation of oocytes with phenyl-N-acetyl-α-d-galactosamide, an inhibitor of N-acetyl-neuraminic acid incorporation (Rettig et al., 1992), abolished the appearance of multiple FXYD7 bands (data not shown).
To verify and characterize the implication of Thr3, Thr5 and Thr9 in the processing of FXYD7, we replaced these residues with alanine, either alone or in combination. As shown in Figure 3D, wild-type FXYD7 was processed progressively from the 14 kDa core protein present after a 6 h pulse (lane 1, band a) to the slower migrating doublet visible after 24 or 48 h chase periods (lanes 3 and 4, bands c and d). The T3A (lanes 4–6) and the T5A (lanes 7–9) mutants produced band (a), corresponding to the core protein during the pulse, and the lower (c) but not the upper (d) band of the doublet during the chase periods. On the other hand, the T9A (lanes 10–12) mutant mainly appeared as a new species (b) with a molecular mass of ∼15 kDa. Double T3A/T5A (lanes 13–15), T5A/T9A (lanes 16–18) or T3A/T9A (lanes 19–21) mutants produced only band (b), and concomitant mutations of Thr3, Thr5 and Thr9 (lanes 22–24) led to the production of a 14 kDa species corresponding to the predicted core protein. From these data, we conclude that band (a) is the core FXYD7 protein with no modifications, whereas band (b) bears one, band (c) two and band (d) three modified sites. Since none of the single or double mutants produced band (d), corresponding to the slowest migrating FXYD7 species, it is likely that it is not produced by a progressive but by an original modification of one site. Interestingly, in contrast to the similar expression after the pulse and a 24 h chase period of the single and the double T3A/T5A and T3A7T9A mutants, the expression of the triple mutant (compare lanes 22 and 23) and the double T5A/T9A mutant (compare lanes 16 and 17) was decreased after a 24 h chase period, indicating that modifications on Thr5 and/or Thr9 are necessary and sufficient for the stable cellular expression of FXYD7.
To characterize the progression of FXYD7 processing, we made use of brefeldin A (BFA), a drug which, in somatic cells, causes Golgi vesiculation, redistribution of most of the Golgi into the endoplasmic reticulum (ER) and a block of protein transport from the ER out of the mixed ER–Golgi complex (Lippincott-Schwartz et al., 1989). In Xenopus oocytes, we have shown that, in the presence of BFA, proteins remain in their core glycosylated, though trimmed form (Geering et al., 1996), indicating that in these cells, BFA may lead to redistribution of cis-Golgi compartments but not of medial/trans-Golgi compartments involved in complex type glycosylation. FXYD7 synthesized in oocytes in the presence of BFA was processed progressively from the core protein (a) (Figure 3C, lane 25) to band (b) and (c) (lanes 26–28) containing one and two modified sites, respectively. In the presence of BFA, band (d) never appeared, in contrast to FXYD7 synthesized in the absence of BFA (compare lanes 1–3 with lanes 25–28). From the data obtained in in vitro translations and in oocytes in the absence or presence of BFA, we conclude that FXYD7 is post-translationally modified on two N-terminal threonine residues in the ER or in a cis-Golgi compartment. In Xenopus oocytes, a third threonine is likely to be modified in a more distal cellular compartment, but this modification is not observed in FXYD7 expressed in brain (Figure 3A, lane 7). At present, we cannot decide definitively which of the N-terminal threonines in FXYD7 are targets for early or late modifications.
Association of FXYD7 with Na,K-ATPase
The specific association of FXYD7 with Na,K-ATPase was investigated by co-expressing FXYD7 in Xenopus oocytes together with rat Na,K-ATPase α1–β1, α2–β1, α3–β1, α1–β2 or α2–β2 isozymes, all expected to be present in the brain (for a review see Blanco and Mercer, 1998). After metabolic labeling and various chase periods, microsomes were prepared and subjected to immunoprecipitation under non-denaturing conditions with α or FXYD7 antibodies. As shown in Figure 4A, an FXYD7 antibody efficiently co-immunoprecipitated all α–β1 isozymes with FXYD7 even after long chase periods (lanes 1–9). On the other hand, co-immunoprecipitation of α–β2 isozymes was very poor (lanes 10–15), despite a similar expression of all α isoforms over prolonged chase periods (Figure 4B, lanes 1–15) which reflects stable production of α–β1 and α–β2 isozymes. Likewise, an α antibody co-immunoprecipitated FXYD7 with α–β1 but not with α–β2 isozymes (data not shown). These results indicate that FXYD7 can indeed associate specifically with Na,K-ATPase isozymes but only if they contain β1 isoforms.
Fig. 4. FXYD7 associates specifically with Na,K-ATPase α–β1 isozymes after co-expression in Xenopus oocytes and with α1–β1 isozymes in the native brain tissue. Oocytes were injected with FXYD7 cRNA (0.4 ng) together with rat Na,K-ATPase α isoforms (α1, α2 or α3; 7–10 ng) cRNAs and rat β1 (1 ng) or human β2 (1 ng) cRNAs. After injection, oocytes were metabolically labeled for 24 h and subjected to 24, 72 and 120 h chase periods. Microsomes were immunoprecipitated under non-denaturing conditions with an FXYD7 antibody (A) and under denaturing conditions with an Na,K-ATPase α antibody (B). Indicated are the positions of Na,K-ATPase α-subunits, fully glycosylated (fg) β-subunits and FXYD7. * = artifactual bands only observed in FXYD7 immunoprecipitations under non-denaturing conditions. (C) Microsomes from rat brain or kidney were either loaded directly on an SDS–Tricine gel (lanes 1 and 3) or first immunoprecipited under non-denaturing conditions with an Na,K-ATPase α antibody (lanes 2 and 4). After western blotting, FXYD7 was revealed with an FXYD7 antibody. (D) Western blot analysis of kidney and brain microsomes, which were either loaded directly on a 5–13% polyacrylamide gel (50 µg; lanes 1, 3, 6, 8, 11 and 13) or subjected first to non-denaturing immunoprecipitations with an FXYD7 antibody (lanes 2, 4, 7, 9, 12 and 14) or with a pre-immune serum (control, lanes 5, 10 and 15). After protein transfer, the blot was probed with isoform-specific α1 (lanes 1–5), α2 (lanes 6–10) or α3 (lanes 11–15) antibodies. The position of α-subunits is indicated. * = non-specific band probably due to heavy chains of antibodies.
Association of FXYD7 with Na,K-ATPase was observed not only in oocytes but also in native brain tissue. Western blot analysis confirmed the absence of FXYD7 in the kidney (Figure 4C, lanes 3 and 4) and its presence in the brain (lane 1) where it could be co-immunoprecipitated with an Na,K-ATPase α antibody (lane 2). To check for Na,K-ATPase isozyme-specific interactions of FXYD7 in the brain, we made use of Na,K-ATPase α isoform-specific antibodies (Figure 4D). As expected, Na,K-ATPase α1 (lane 1), but not α2 (lane 6) or α3 (lane 11) isoforms could be detected in kidney microsomes, whereas all three α isoforms were present in brain (lanes 3, 8 and 13). As revealed by western blot analysis with Na,K-ATPase α isoform-specific antibodies, an FXYD7 antibody co-immunoprecipitated α1–β (lane 4) but not α2–β (lane 9) or α3–β (lane 14) complexes from brain microsomes. Together with the results obtained in oocytes, these data suggest that in brain, only α1–β1 complexes are associated with FXYD7.
Functional effects of FXYD7
Since FXYD7 is able to reach the plasma membrane when expressed without Na,K-ATPase in Xenopus oocytes (Figure 3B), we first tested whether FXYD7 may induce an ionic conductance either by itself or as a modulator of an endogenous, oocyte ion transporter, as reported for other FXYD proteins (Attali et al., 1995; Moorman et al., 1992; Minor et al., 1998). In the presence of extracellular K+, Na+, Ca2+ and Cl–, the current–voltage relationship measured between –70 and +10 mV of oocytes expressing FXYD7 was similar to that of non-injected oocytes (Table I). Moreover, no current activation was observed during a long (5 s) depolarization step (Table I).
Table I. Conductance properties of non-injected (ni) oocytes and oocytes injected with FXYD7 cRNA.
| Gm (µS) | I0.5 s (μA) | I4 s (µA) | |
|---|---|---|---|
| ni | 8.9 ± 3.3 | 2.7 ± 0.9 | 3.4 ± 1 |
| FXYD7 | 10.4 ± 3.5 | 3.1 ± 0.7 | 3.7 ± 0.8 |
The membrane conductance (Gm) is the slope of the I–V curve between –70 and +10 mV. I0.5 s and I4 s are the current values recorded 0.5 and 4 s after the start of a 5 s depolarization from –80 to +20 mV. Values are means ± SE of 8–10 oocytes from two different batches.
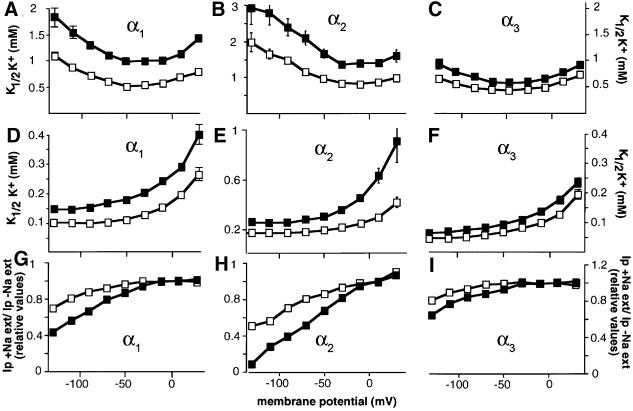
The effects of FXYD7 on Na,K-ATPase function were investigated by electrophysiological measurements of Na,K-ATPase transport properties in Xenopus oocytes expressing α1–β1, α2–β1 or α3–β1. As shown in Figure 5, FXYD7 had a significant effect on the apparent affinity for extracellular K+ of Na,K-ATPase α1–β1 complexes, which are most probably the physiologically relevant interaction partners of FXYD7 in the brain. After association of α1–β1 complexes with FXYD7, the K1/2K+ value increased nearly 2-fold over a wide potential range when measured in the presence of external Na+ (Figure 5A). The effect of FXYD7 on the apparent K+ affinity was also observed when measured in the absence of external Na+ (Figure 5D). The similar FXYD7 effect on the K+ activation kinetics with and without external Na+ suggests a modification of the intrinsic affinity of the external K+-binding site. Moreover, in FXYD7-associated α1–β1 complexes, the Na,K-ATPase pump activity was inhibited more strongly by the presence of external Na+ at high negative membrane potentials (Figure 5G), suggesting that the translocation and the release of Na+ are also affected by the presence of FXYD7.
Fig. 5. FXYD7 modulates the transport properties of Na,K-ATPase. Oocytes were injected with FXYD7 cRNA (2 ng) together with rat Na,K-ATPase α isoforms (α1, α2* or α3*, 7–10 ng; * = oubain resistant) and rat β1 (1 ng) cRNAs. The voltage dependence of the external K+ activation constant (K1/2 K+) of Na,K-ATPase isozymes was measured in the presence (A–C) or absence (D–F) of 90 mM external Na+. FXYD7 had a significant (P < 0.05) effect on the K+ activation of α1–β1 and α2–β1 but not of α3–β1 isozymes over the whole potential range in the presence and absence of external Na+. The effect of external Na+ (G–I) on ouabain-sensitive currents (Ip) was determined by comparing Ip in the presence of external Na+ (Ip +Na+ ext) with those in its absence (Ip –Na+ ext). Ip was set arbitarily to 1 at 10 mV. Open squares, Na,K-ATPase alone; closed squares, Na,K-ATPase + FXYD7. Data are means ± SE of 20–40 oocytes from 4–6 different batches.
Despite the apparent lack of FXYD7 association with α2–β and α3–β isozymes in the brain, FXYD7 interacted with α2–β1 and α3–β1 complexes in Xenopus oocytes. We therefore also tested the regulatory effect of FXYD7 on the transport properties of these Na,K-ATPase isozymes. FXYD7 influenced the apparent K+ affinity of α2–β1 isozymes in a way similar to that of α1–β1 isozymes (Figure 5B, E and H; Table II) but it had no significant effect on the K+ activation of α3–β1 isozymes (Figure 5C, F and I; Table II). Finally, in contrast to the apparent K+ affinity, FXYD7 did not affect the affinity for intracellular Na+ of α1–β1, α2–β1 or α3–β1 isozymes (Table II).
Table II. Na,K-ATPase properties in oocytes expressing or not FXYD7 wild-type or T3A/T5A/T9A mutant.
| Imax (nA) |
K1/2 K+ (–50 mV) (mM) |
K1/2 Na+ (mM) | ||
|---|---|---|---|---|
| With ext Na+ | No ext Na+ | |||
| α1–β1 | 369 ± 31 | 0.51 ± 0.02 | 0.11 ± 0.06 | 7.8 ± 0.8 |
| α1–β1 + FXYD7 | 236 ± 9 | 0.99 ± 0.04** | 0.18 ± 0.06** | 7.7 ± 0.5 |
| α1–β1 + FXYD7 T3A/T5A/T9A | 200 ± 16 | 0.91 ± 0.02** | ND | 8.3 ± 1.2 |
| α2–β1 | 247 ± 21 | 0.97 ± 0.03 | 0.19 ± 0.07 | 10.6 ± 0.7 |
| α2–β1 + FXYD7 | 132 ± 20 | 1.67 ± 0.13** | 0.29 ± 0.01** | 9.8 ± 0.5 |
| α3–β1 | 242 ± 15 | 0.43 ± 0.03 | 0.07 ± 0.03 | 12.1 ± 0.8 |
| α3–β1 + FXYD7 | 158 ± 13 | 0.57 ± 0.02* | 0.10 ± 0.02 | 11.1 ± 0.5 |
Values are means ± SE of 9–40 oocytes of 2–6 animals. *P < 0.05, **P < 0.01.
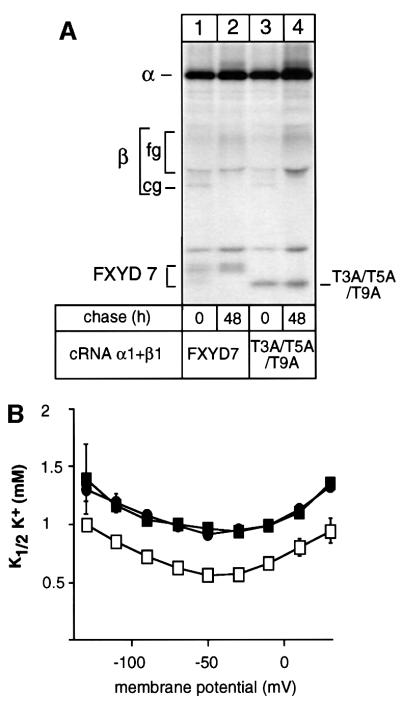
To assess the question of whether the identified post-translational processing of FXYD7 is important for the modulatory effect on Na,K-ATPase, we co-expressed Na,K-ATPase α1–β1 isozymes with either wild-type FXYD7 or the T3A/T5A/T9A mutant and compared the K+ and Na+ effect of the two peptides. The T3A/T5A/T9A mutant was able to form stable and functionally active complexes with Na,K-ATPase (Figure 6A), which were expressed at the cell surface and which exhibited similar functional characteristics to Na,K-ATPase associated with wild-type FXYD7 (Figure 6B; Table II).
Fig. 6. Threonine modifications of FXYD7 are not involved in the association with Na,K-ATPase or in the K+ effect of FXYD7 on Na,K-ATPase. (A) Oocytes were injected with wild-type FXYD7 or the triple threonine mutant (T3A/T5A/T9A) cRNAs (0.4 ng) together with rat Na,K-ATPase α1 (10 ng) and rat β1 (1 ng) cRNAs. After injection, oocytes were metabolically labeled for 24 h and subjected to a 48 h chase period. Microsomes were immunoprecipitated under non-denaturing conditions with an FXYD7 antibody. Indicated are the positions of Na,K-ATPase α-subunits, core (cg) and fully (fg) glycosylated β-subunits, and of wild-type and mutant FXYD7. (B) Oocytes were injected with wild-type FXYD7 or the triple threonine mutant (T3A/T5A/T9A) cRNAs (2 ng) together with rat Na,K-ATPase α1 (10 ng) and rat β1 (1 ng) cRNAs. Three days after injection, K+ activation constants (K1/2 K+) were determined in the presence of 90 mM external Na+. Open squares, Na,K-ATPase alone; closed squares, Na,K-ATPase + FXYD7; closed circles, Na,K-ATPase + T3A/T5A/T9A mutant. Data are means ± SE of 10–13 oocytes from two different batches.
Discussion
FXYD7 is the most recently disclosed member of the FXYD family (Sweadner and Rael, 2000) and has not been characterized yet as a protein. In this study, we have determined the membrane topology, processing and tissue distribution of FXYD7 and have identified it as a novel, tissue- and isoform-specific Na,K-ATPase regulator.
FXYD7 is a post-translationally modified type I protein
Our results first confirm that FXYD7 is a type I protein which, similarly to the γ-ubunit (Béguin et al., 1997), but in contrast to CHIF (Béguin et al., 2001) and phospholemman (Palmer et al., 1991), lacks a cleavable signal sequence.
FXYD7 synthesized in Xenopus oocytes, or expressed in situ in the brain, is subjected to chemical modifications on Thr3, Thr5 and Thr9. In the brain, FXYD7 is modified on two threonines, probably Thr5 and Thr9 or Thr3 and Thr9, whereas in oocytes, FXYD7 can, at least partially, be modified on three threonines. Though all three threonines are predicted to represent putative O-glycosylation sites, and neuraminidase and O-glycosidase had some effect on the electrophoretic mobility of FXYD7, we could not identify the nature of FXYD7 modifications definitively. Since addition of O-linked sugars is a stepwise process which occurs mainly in different Golgi compartments (for a review see Van den Steen et al., 1998), our finding that FXYD7 is processed post-translationally is compatible with O-glycosylation. Further experiments are needed to identify the particular sugar composition and/or the presence of other types of modifications.
Co- or post-translational processing is a common feature of all studied FXYD proteins (Palmer et al., 1991; Béguin et al., 2001; Arystarkhova et al., 2002). However, the nature of the modifications is not known and may in some cases be cell-type specific. Similarly to FXYD7 detected in brain, native γa and γb splice variants and CHIF expressed in kidney exhibit a higher molecular mass than those synthesized in vitro in the absence of microsomes (Béguin et al., 2001). On the other hand, only CHIF and FXYD7 but not γ splice variants are modified after expression in Xenopus oocytes. Since the stability of FXYD7 (this study) and CHIF (Béguin et al., 2001), expressed in Xenopus oocytes in the absence of Na,K-ATPase, correlates with the presence of chemical modifications, and the instability of γ-subunits with the absence of modifications (Béguin et al., 1997, 2001), it is possible that processing is of general importance for the cellular expression of FXYD proteins. In contrast to the postulated influence of post-translational modifications of γ splice variants on the γ-subunit’s functional effects on Na,K-ATPase (Arystarkhova et al., 2002), our results show that post-translational modifications of FXYD7 are not required for its effect on the K+ affinity of Na,K-ATPase nor do they influence the Na+ affinity of Na,K-ATPase. Further experiments are necessary to reveal the role of modifications of FXYD7, e.g. in the intracellular routing or differential targeting to the plasma membrane.
FXYD7 is brain specific and associated with Na,K-ATPase α1–β1 isozymes
Similarly to γ-subunits and CHIF, FXYD7 specifically assembles with Na,K-ATPase after co-expression in Xenopus oocytes, and is distributed and associated with Na,K-ATPase in a tissue-specific pattern. This supports our hypothesis that FXYD proteins may be a family of cell-type specific Na,K-ATPase regulators. Whereas CHIF (Shi et al., 2001) and γ-subunits (Pu et al., 2001) are expressed mainly in distal colon and/or specific nephron segments, FXYD7 is brain specific, present in both neurons and glial cells.
Interestingly, when expressed in Xenopus oocytes, FXYD7 can interact with α1–β1, α2–β1 and α3–β1 Na,K-ATPase isozymes but not with α–β2 isozymes. This result raises the interesting possibility that the β1 isoform is involved directly or indirectly in the specific association of FXYD proteins with Na,K-ATPase. Despite the interaction with all three Na,K-ATPase α–β1 isozymes in Xenopus oocytes, our results indicate that in brain, FXYD7 is only associated with α1–β or, in view of the results obtained in oocytes, possibly only with α1–β1 isozymes. We cannot entirely exclude that complexes of FXYD7 with α2–β1 isozymes are not detected due to the low abundance of these Na,K-ATPase isozymes. On the other hand, α3–β1 isozymes are probably the main Na,K-ATPase isozymes in neurons, and the absence of FXYD7 interaction may imply tissue-specific factors. Since FXYD7 can be expressed at the plasma membrane independently of its association with Na,K-ATPase, it is possible that FXYD7 and Na,K-ATPase do not associate co-translationally but only post-translationally at the cell surface. If this is the case, specific interaction of FXYD7 with α1–β1 isozymes may be achieved by their similar intracellular routing and cell surface distribution which, in astrocytes and neurons, differ from that of α2–β and α3–β isozymes (Juhaszova and Blaustein, 1997).
Functional effects of FXYD7 on Na,K-ATPase
Significantly, despite the ability of FXYD7 to associate with both neuronally expressed α1–β1 and α3–β1 Na,K-ATPase isozymes, only α1–β1 transport properties were influenced by FXYD7 after co-expression in Xenopus oocytes. This result may highlight the exquisite, functional specificity of FXYD7 proteins in cells that express different Na,K-ATPase isozymes, as is the case in neurons.
Apparently, all FXYD proteins characterized so far, such as the γ-subunit, CHIF and FXYD7, influence the transport properties of the ‘housekeeping’ α1–β1 isozymes in the native tissue where they are expressed. However, the functional effects of the different FXYD proteins are clearly distinct and are likely to be adjusted to tissue-specific physiological demands. The γ-subunit decreases and CHIF increases the Na+ affinity of Na,K-ATPase α1–β1 isozymes (Béguin et al., 2001), permitting an appropriate fine control of Na+ reabsorption in different segments of the nephron. Significantly, the brain-specific FXYD7 does not influence the Na+ activation of Na,K-ATPase α1–β1 isozymes, which is the rate-limiting step in the Na,K pump activity, but decreases its apparent affinity for external K+ ∼2-fold. By the association of FXYD7, the α1–β1 isozyme acquires a K+ affinity similar to that of the intrinsically low K+ affinity α2–β2 isozyme (Crambert et al., 2000). Possibly, association of FXYD7 with α1–β1 complexes could account for the lower apparent K+ affinity observed for α1–β1 from axolemma compared with that of α1–β1 complexes expressed in HeLa cells (Thérien et al., 1996).
What could be the physiological relevance of the K+ effect of FXYD7 on Na,K-ATPase α1–β1 isozymes in the K+ homeostasis of the nervous system? Extracellular K+ concentrations ([K+]o) increase significantly during neuronal activity and, to prevent K+-mediated perturbations of the excitability of neurons, K+ must be cleared rapidly from the extracellular space (for a recent review see Walz, 2000). Indeed, imbalances in K+ extrusion and re-uptake frequently have been associated with abnormal neuronal function (for references see D’Ambrosio et al., 2002). The implication of Na,K-ATPase in the recovery of [K+]o is well established, and it has been suggested recently that Na,K-ATPase determines the rate of recovery of baseline [K+]o during high frequency firing and is responsible for the post-activity extracellular K+ undershoot (D’Ambrosio et al., 2001). Experimental evidence has been provided that both glial cells and neurons contribute to the recovery of [K+]o, implicating not only glial low K+ affinity Na,K-ATPase α2–β2 isozymes, but also neuronal and possibly glial α1–β isozymes (Ransom et al., 2000; D’Ambrosio et al., 2002). In such a model, association of FXYD7 with glial and neuronal Na,K-ATPase α1–β isozymes may be of importance. With a K1/2 K+ of <1 mM, the α1–β1 isozyme would transport at a rate close to maximum in the presence of a ‘resting’ K+ concentration of ∼4 mM (D’Ambrosio et al., 2002), and an increase in the [K+]o related to neuronal activity would not result in any significant increase in the rate of cellular K+ uptake. In contrast, an FXYD7-associated α1–β1 complex, with a lower apparent affinity for K+, would not transport at a maximal rate under resting conditions and would still have the capacity to increase its rate at higher [K+]o following intense neuronal activity. Moreover, FXYD7-associated α1–β1 isozymes may have a protective effect in preventing an excessive K+ undershoot after sustained neuronal stimulation. Of course, at present, we cannot entirely exclude that, alternatively or in addition to the observed K+ effect on α1–β1 Na,K-ATPase isozymes, FXYD7 may be involved in other regulatory mechanisms of the expression or function of Na,K-ATPase isozymes in neurons and glial cells.
In conclusion, in the present study, we have characterized the tissue distribution, biosynthesis and processing of FXYD7, a member of the FXYD family of small proteins which so far has not been studied, and have identified it as a modulator of brain Na,K-ATPase. These results extend the evidence that most if not all members of the FXYD family are involved in the fine regulation of Na,K-ATPase in various tissues and act in an operationally distinct way adapted to cell-specific physiological demands.
Materials and methods
Cloning and site-directed mutagenesis
An expressed sequence tag (EST) clone corresponding to mouse FXYD7 (mc25f09.r1, DDBJ/EMBL/GenBank accession No. w30353) was obtained from ATCC and verified by sequencing. The insert was excised by digestion with EcoRI and NotI and subcloned into pcDNA3 cut with the same enzymes. It was then excised by digestion with EcoRI and XhoI and subloned into a modified pBluescript II SK vector between 5′ and 3′ sequences of Xenopus β-globin. Point mutations were introduced into the mouse FXYD7 cDNA by the PCR-based method. FXYD7 was tagged with a flag epitope at the N-terminus (N-flag FXYD7) as previously described for the γ-subunit (Béguin et al., 1997). The mutations were confirmed by DNA sequencing. cDNAs of rat Na,K-ATPase α1, α2*, α3* (* = ouabain resistant) and rat β1 subunits (kindly provided by J.Lingrel), human β2 (Crambert et al., 2000), and rat epithelial Na+ channel (rENaC) α-, β- and γ-subunits (kindly provided by B.C.Rossier) were subcloned into the pSD5 vector.
Northern blot
Total RNA was extracted from various rat tissues, run on a 1% denaturing formaldehyde agarose gel and blotted onto nylon membranes. Membranes were hybridized with a nick-translated 32P-labeled, full-length FXYD7 cDNA as a probe under standard conditions.
FXYD7 antibodies
For the preparation of specific FXYD7 antibodies, segments of the mouse cDNA encoding an N-terminal (Ala2–Asp21) or a C-terminal region (Arg52–Val80) were amplified by PCR and subcloned into the pGEX-4T1 vector (Pharmacia Biotech). FXYD7–GST fusion proteins were prepared and used for immunization of rabbits (Cocalico Biologicals, Inc.). Affinity purification of antibodies was carried out by absorption on a fusion protein consisting of a maltose-binding protein fused to the C- or N-terminal regions of FXYD7 used for immunization. The C-terminal FXYD7 antibody was used in non-denaturing and denaturing immunoprecipitations and in western blot analysis. Affinity-purified N- and C-terminal FXYD7 antibodies were used for immunostaining and confocal microscopy.
In vitro translation
In vitro translation of FXYD7 cRNA in the presence or absence of canine pancreatic microsomal membranes was performed according to the manufacturer’s instructions (Promega).
Protein expression in Xenopus oocytes and radioimmunolabeling
Stage V–VI oocyte were obtained from Xenopus laevis as described (Geering et al., 1996). In vitro synthetized cRNAs encoding rat Na,K-ATPase α1, α2* or α3* isoforms, rat Na,K-ATPase β1-subunit, human Na,K-ATPase β2-subunit, mouse wild-type or mutant FXYD7, or rENaC α-, β- and γ-subunits were injected into oocytes in different combinations as described in the figure legends. To study protein expression and association, oocytes were incubated in modified Barth’s solution (MBS) containing 0.7–1 mCi/ml [35S]methionine for 6 or 24 h and subjected to 24–120 h chase periods in MBS containing 10 mM cold methionine. After various pulse–chase periods, digitonin extracts or microsomes were prepared as described (Geering et al., 1996). Radioimmunolabeling with 125I-labeled FLAG antibodies was performed as previously described (Béguin et al., 1997) on intact non-injected oocytes or on oocytes injected with wild-type FXYD7 or N-flag FXYD7 cRNA.
Brain and kidney microsome preparation
Rat brain microsomes were obtained as previously described (Uldry et al., 2001). Briefly, brain homogenates were prepared in a solution containing 30 mM NaCl, 5 mM dithiothreitol (DTT), 5 mM phenylmethylsulfonyl fluoride (PMSF), 10 µg/ml aprotinin, 10 mM Tris–HCl pH 7.5, layered onto a 41% sucrose cushion and centrifuged at 9500 g for 60 min. The sample layer was diluted in homogenization buffer and centrifuged at 40 000 g for 30 min. The pellets were resuspended in phosphate-buffered saline (PBS) containing 1 mM PMSF. Rat kidney microsomes were prepared as previously described (Béguin et al., 2001). Briefly, kidney microsomes were obtained by differential centrifugation, freeze/thawed twice and sonicated for 4 s before solubilization in buffer containing 0.5% CHAPS, 20 mM Tris–HCl pH 7.4, 100 mM NaCl, 1 mM PMSF and 5 µg/ml each of leupeptin, pepstatin and antipain.
Immunoprecipitation and western blot analysis
FXYD7 or rat Na,K-ATPase α isoforms expressed in Xenopus oocytes, brain or kidney were immunoprecipitated with a C-terminal FXYD7 antibody or with a previously characterized α antibody (Béguin et al., 1997), respectively, under denaturing or non-denaturing conditions (Geering et al., 1996). Immunoprecipitates were resolved on 5–13% SDS–polyacrylamide gels or SDS–Tricine gels (prepared according to the manufacturer’s instructions) and tranferred occasionally onto nitrocellulose and revealed by either FXYD7 antibodies or rat Na,K-ATPase α1, α2 and α3 isoform-specific antibodies (kindly provided by T.Pressley; Pressley, 1992) and chemiluminescence detection (ECL, Amersham). Immunoprecipitated proteins from metabolically labeled oocytes were revealed by fluorography.
Glycosidase treatment and inhibition of glycosylation
To verify whether N-terminal threonine residues are O-glycosylated as predicted by the NetOGlyc database (Hansen et al., 1998), we treated microsomes of FXYD7-expressing oocytes according to the manufacturer’s instructions with PNGase F, sialidase A, endo-O-glycosidase, β(1–4) galactosidase and/or glucosaminidase contained in the ProZyme Glycopro Deglycosylation Kit (Promega). Alternatively, immunoprecipitates of FXYD7 immobilized on protein A–Sepharose were washed twice with buffer A containing 50 mM sodium acetate pH 5, 9 mM CaCl2, 150 mM NaCl and 100 µg/ml bovine serum albumin (BSA) and incubated with or without neuraminidase (Roche, 25 mU) for 3 h at 37°C. Sample buffer was added and aliquots were loaded directly on SDS–Tricine gels. Moreover, after incubation with or without neuraminidase, samples of Sepharose beads were washed twice with 50 mM Tris phosphate buffer pH 7, and incubated with or without O-glycosidase (1 mU, Roche) overnight at 37°C before gel electrophoresis. FXYD7 immobilized on protein A–Sepharose resuspended in 50 mM Na-phosphate buffer pH 7 was also treated with jack bean N-acetylhexosaminidase (3 U, Calbiochem) overnight at 37°C. As an alternative method to explore the presence of O-linked sugars, we treated oocytes injected with FXYD7 cRNA overnight with 1 mM phenyl-N-acetyl-α-d-galactosamide (Roche), an inhibitor of incorporation of N-acetyl-neuraminic acid (Rettig et al., 1992), and incubated them for 24 h in the presence of the inhibitor and 35[S]methionine, before microsome preparation and immunoprecipitation of FXYD7.
Immunostaining and confocal microscopy
Rat brain slices were frozen on dry ice and kept at –80°C for further analysis. Acetone-fixed 10 µm cryostat sections were double-labeled with polyclonal affinity-purified antibodies against FXYD7 (7 µg/ml) and with monoclonal antibodies against either synaptophysin (Roche Chemicals, 1:25) or GFAP (Sigma, 1:200). Non-specific sites were blocked with a mix of normal goat serum and normal donkey serum (1:20 in PBS, with 0.1% Triton X-100). Revelation of polyclonal antibodies was performed using the avidin–biotin system [biotinylated goat anti-rabbit IgG (1:100, Vector) and avidin (DCS) bound to fluorescein isothiocyanate (FITC) (1:50)], and visualization of monoclonal antibodies was performed with donkey anti-mouse IgG bound to fluorochrome Cy3 (1:250, Milan Analytical). Sections were analyzed on a Leica confocal microscope.
Electrophysiology
Measurements of a putative channel activity induced by FXYD7 were performed 3 days after injection of 2 ng of FXYD7 cRNA into Xenopus oocytes. The conductance was examined over a –130 to +50 mV potential range with a series of ten 200 ms voltage steps in a solution containing 80 mM NaCl, 2.5 mM NaHCO3, 4 mM MgCl2, 1 mM KCl, 4 mM CaCl2 and 20 mM Na-HEPES pH 7.4. Current activation was also measured 0.5 and 4 s after the start of a 5 s depolarization from –80 to +20 mV.
Electrophysiological measurements were performed 3 days after Xenopus oocyte injection with rat α1–β1, α2*–β1 or α3*–β1 cRNA alone or together with wild-type or mutant FXYD7 cRNA, by using the two-electrode voltage clamp technique. Measurements of the apparent external K+ affinity were carried out as described previously (Béguin et al., 2001) in the presence of 1 µM ouabain which inhibits the endogenous, oocyte Na,K pump, but not the expressed ouabain-resistant rat Na,K-ATPase isozymes. The maximal Na,K pump current and the apparent K+ affinity (K1/2 K+), measured in the presence or absence of external Na+, were obtained by fitting the Hill equation to the data using a Hill coefficient of 1.6 or 1, respectively (Jaisser et al., 1994). The effect of extracellular Na+ (90 mM) was determined by comparing the ouabain (2 mM)-sensitive current induced by 10 mM K+ (in the presence of external Na+) or by 5 mM K+ (in the absence of external Na+).
Measurements of the apparent Na+ affinity of Na,K-ATPase were performed as described previously (Hasler et al., 1998) in oocytes co-expressing rat Na,K-ATPase α1, α2* or α3* cRNA with β1 cRNA and with rat epithelial Na+ channel α, β and γ cRNAs in the presence or absence of FXYD7. The Hill equation was fitted to the experimental data by using a Hill coefficient of 3.
Statistical analysis was performed by paired and unpaired Student’s t-tests.
Acknowledgments
Acknowledgements
We thank Sophie Roy and Danièle Schaer for technical assistance, T.Pressley for antibodies against Na,K-ATPase α1, α2 and α3 isoforms, and B.C.Rossier and J.Lingrel for cDNA probes. We are very grateful to Paul Honegger for helpful discussion and suggestions. This work was supported by grants from the Swiss National Fund for Scientific Research Nos 31-53721.98 and 31-64793.01, and from the Roche Research Foundation.
References
- Arystarkhova E., Donnet,C., Asinovski,N.K. and Sweadner,K.J. (2002) Differential regulation of renal Na,K-ATPase by splice variants of the γ subunit. J. Biol. Chem., 277, 10162–10172. [DOI] [PubMed] [Google Scholar]
- Attali B., Latter,H., Rachamim,N. and Garty,H. (1995) A corticosteroid-induced gene expressing an ‘IsK-like’ K+ channel activity in Xenopus oocytes. Proc. Natl Acad. Sci. USA, 92, 6092–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P., Wang,X.Y., Firsov,D., Puoti,A., Claeys,D., Horisberger,J.D. and Geering,K. (1997) The γ subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO J., 16, 4250–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P., Crambert,G., Guennoun,S., Garty,H., Horisberger,J.-D. and Geering,K. (2001) CHIF, a member of the FXYD protein family, is a regulator of Na,K-ATPase distinct from the γ-subunit. EMBO J., 20, 3993–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco G. and Mercer,R.W. (1998) Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol., 275, F633–F650. [DOI] [PubMed] [Google Scholar]
- Crambert G., Hasler,U., Beggah,A.T., Yu,C., Modyanov,N.N., Horisberger,J.D., Lelievre,L. and Geering,K. (2000) Transport and pharmacological properties of nine different human Na,K-ATPase isozymes. J. Biol. Chem., 275, 1976–1986. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R., Gordon,D.S. and Winn,H.R. (2002) Differential role of KIR channel and Na+/K+-pump in the regulation of extracellular K+ in rat hippocampus. J. Neurophysiol., 87, 87–102. [DOI] [PubMed] [Google Scholar]
- Forbush B. III, Kaplan,J.H. and Hoffman,J.F. (1978) Characterization of a new photoaffinity derivative of ouabain: labeling of the large polypeptide and of a proteolipid component of the Na,K-ATPase. Biochemistry, 17, 3667–3676. [DOI] [PubMed] [Google Scholar]
- Fu X. and Kamps,M. (1997) E2a-Pbx1 induces aberrant expression of tissue-specific and developmentally regulated genes when expressed in NIH 3T3 fibroblasts. Mol. Cell. Biol., 17, 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering K. (2001) The functional role of β subunits in oligomeric P-type ATPases. J. Bioenerg. Biomembr., 33, 425–438. [DOI] [PubMed] [Google Scholar]
- Geering K., Beggah,A., Good,P., Girardet,S., Roy,S., Schaer,D. and Jaunin,P. (1996) Oligomerization and maturation of Na,K-ATPase: functional interaction of the cytoplasmic NH2-terminus of the β subunit with the α subunit. J. Cell Biol., 133, 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J.E., Lund,O., Tolstrup,N., Gooley,A.A., Williams,K.L. and Brunak,S. (1998) NetOglyc: prediction of mucin type O-glycosylation sites based on sequence context and surface accessibility. Glycoconj. J., 15, 115–130. [DOI] [PubMed] [Google Scholar]
- Hasler U., Wang,X., Crambert,G., Béguin,P., Jaisser,F., Horisberger,J.D. and Geering,K. (1998) Role of β-subunit domains in the assembly, stable expression, intracellular routing and functional properties of Na,K-ATPase. J. Biol. Chem., 273, 30826–30835. [DOI] [PubMed] [Google Scholar]
- Jaisser F., Jaunin,P., Geering,K., Rossier,B.C. and Horisberger,J.D. (1994) Modulation of the Na,K-pump function by β subunit isoforms. J. Gen. Physiol., 103, 605–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhaszova M. and Blaustein,M.P. (1997) Na+ pump low and high ouabain affinity α subunit isoforms are differently distributed in cells. Proc. Natl Acad. Sci. USA, 94, 1800–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan,L.C., Bonifacino,J.S. and Klausner,R.D. (1989) Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell, 56, 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meij I.C. et al. (2000) Dominant isolated renal magnesium loss is caused by misrouting of the Na+,K+-ATPase γ-subunit. Nature Genet., 26, 265–266. [DOI] [PubMed] [Google Scholar]
- Mercer R.W., Biemesderfer,D., Bliss,D.P., Collins,J.H. and Forbush,B. (1993) Molecular cloning and immunological characterization of the γ-polypeptide, a small protein associated with the Na,K-ATPase. J. Cell Biol., 121, 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor N.T., Sha,Q., Nichols,C.G. and Mercer,R.W. (1998) The γ subunit of the Na,K-ATPase induces cation channel activity. Proc. Natl Acad. Sci. USA, 95, 6521–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman J.R., Palmer,C.J., John,J.E.,III, Durieux,M.E. and Jones,L.R. (1992) Phospholemman expression induces a hyperpolarization-activated chloride current in Xenopus oocytes. J. Biol. Chem., 267, 14551–14554. [PubMed] [Google Scholar]
- Morrison B.W., Moorman,J.R., Kowdley,G.C., Kobayashi,Y.M., Jones,L.R. and Leder,P. (1995) Mat-8, a novel phospholemman-like protein expressed in human breast tumors, induces a chloride conductance in Xenopus oocytes. J. Biol. Chem., 270, 2176–2182. [DOI] [PubMed] [Google Scholar]
- Navarre C., Ghislain,M., Leterme,S., Ferroud,C., Dufour,J.-P. and Goffeau,A. (1992) Purification and complete sequence of a small proteolipid associated with the plasma membrane H+-ATPase of Saccharomyces cerevisiae. J. Biol. Chem., 267, 6425–6428. [PubMed] [Google Scholar]
- Odermatt A., Becker,S., Khanna,V.K., Kurzydlowski,K., Leisner,E., Pette,D. and MacLennan,D.H. (1998) Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem., 273, 12360–12369. [DOI] [PubMed] [Google Scholar]
- Palmer C.J., Scott,B.T. and Jones,L.R. (1991) Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J. Biol. Chem., 266, 11126–11130. [PubMed] [Google Scholar]
- Pressley T.A. (1992) Phylogenetic conservation of isoform-specific regions within α-subunit of Na+-K+-ATPase. Am. J. Physiol., 262, C743–C751. [DOI] [PubMed] [Google Scholar]
- Pu H.X., Cluzeaud,F., Goldshlegger,R., Karlish,S.J.D., Farman,N. and Blostein,R. (2001) Functional role and immunocytochemical localization of the γa and γb forms of the Na,K-ATPase γ subunit. J. Biol. Chem., 276, 20370–20378. [DOI] [PubMed] [Google Scholar]
- Ransom C.B., Ransom,B.R. and Sontheimer,H. (2000) Activity-dependent extracellular K+ accumulation in rat optic nerve: the role of glial and axonal Na+ pumps. J. Physiol., 522, 427–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig W.J., Garin-Chesa,P., Healey,J.H., Su,S.L., Jaffe,E.A. and Old,L.J. (1992) Identification of endosialin, a cell surface glycoprotein of vascular endothelial cells in human cancer. Proc. Natl Acad. Sci. USA, 89, 10832–10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Levy-Holzman,R., Cluzeaud,F., Farman,N. and Garty,H. (2001) Membrane topology and immunolocalization of CHIF in kidney and intestine. Am. J. Physiol., 280, F505–F512. [DOI] [PubMed] [Google Scholar]
- Simmerman H.K. and Jones,L.R. (1998) Phospholamban: protein structure, mechanism of action and role in cardiac function. Physiol. Rev., 78, 921–947. [DOI] [PubMed] [Google Scholar]
- Suessbrich H. and Busch,A.E. (1999) The IKs channel: coassembly of IsK (minK) and KvLQT1 proteins. Rev. Physiol. Biochem. Pharmacol., 137, 191–226. [DOI] [PubMed] [Google Scholar]
- Sweadner K.J. and Rael,E. (2000) The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence and expression. Genomics, 68, 41–56. [DOI] [PubMed] [Google Scholar]
- Thérien A.G., Nestor,N.B., Ball,W.J. and Blostein,R. (1996) Tissue-specific versus isoform-specific differences in cation activation kinetics of the Na,K-ATPase. J. Biol. Chem., 271, 7104–7112. [DOI] [PubMed] [Google Scholar]
- Thérien A.G., Goldshleger,R., Karlish,S.J. and Blostein,R. (1997) Tissue-specific distribution and modulatory role of the γ subunit of the Na,K-ATPase. J. Biol. Chem., 272, 32628–32634. [DOI] [PubMed] [Google Scholar]
- Uldry M., Ibberson,M., Horisberger,J.D., Chatton,J.Y., Riederer,B.M. and Thorens,B. (2001) Identification of a mammalian H+-myo-inositol symporter expressed predominantly in the brain. EMBO J., 20, 4467–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Steen P., Rudd,P.M., Dwek,R.A. and Opdenakker,G. (1998) Concepts and principles of O-linked glycosylation. Crit. Rev. Biochem. Mol. Biol., 33, 151–208. [DOI] [PubMed] [Google Scholar]
- vonHeijne G. and Gavel,Y. (1988) Topogenic signals in integral membrane proteins. Eur. J. Biochem., 174, 671–678. [DOI] [PubMed] [Google Scholar]
- Walz W. (2000) Role of astrocytes in the clearance of excess extracellular potassium. Neurochem. Int., 36, 291–300. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F., Yamaguchi,K., Tai,Y., Sugimoto,K. and Tokuda,M. (2001) Molecular cloning and characterization of a novel phospholemman-like protein from rat hippocampus. Mol. Brain Res., 86, 189–192. [DOI] [PubMed] [Google Scholar]