Abstract
To investigate the roles of retinoic acid (RA) receptors (RARs) in the physiology of epidermis that does not express RARβ, conditional spatio-temporally controlled somatic mutagenesis was used to selectively ablate RARα in keratinocytes of RARγ-null mice. Keratinocyte proliferation was maintained in adult mouse epidermis lacking both RARα and RARγ, as well as in RARβ-null mice. All RAR-mediated signalling pathways are therefore dispensable in epidermis for homeostatic keratinocyte renewal. However, topical treatment of mouse skin with selective retinoids indicated that RXR/RARγ heterodimers, in which RXR transcriptional activity was subordinated to that of its RARγ partner, were required for retinoid-induced epidermal hyperplasia, whereas RXR homodimers and RXR/RARα heterodimers were not involved. RA-induced keratinocyte proliferation was studied in mutant mice in which RXRα, RXRα and RARα, RARγ, or RXRα and RARγ genes were specifically disrupted in either basal or suprabasal keratinocytes. We demonstrate that the topical retinoid signal is transduced by RXRα/RARγ heterodimers in suprabasal keratinocytes, which, in turn, stimulate proliferation of basal keratinocytes via a paracrine signal that may be heparin-binding EGF-like growth factor.
Keywords: conditional somatic mutagenesis/heparin-binding EGF-like growth factor/nuclear receptor/paracrine control/synthetic retinoids
Introduction
The initial observations of abnormal keratinization of various epithelia in vitamin A-deficient rats (Wolbach and Howe, 1925) and humans (Frazier and Hu, 1931) were followed by numerous pharmacological studies with vitamin A derivatives (retinoids), which led to the development of retinoid therapy for several skin diseases (Livrea, 2000). When applied topically on adult skin, retinoic acid (RA, the biologically active metabolite of vitamin A) generates an epidermal hyperplasia that results from an hyperproliferation of basal keratinocytes leading, upon their vectorial migration towards the skin surface, to thickening of the differentiated suprabasal (spinous and granular) layers (Fisher and Voorhees, 1996). RA treatment also decreases the cohesiveness of the stratum corneum, impairing the adult skin barrier and increasing trans-epidermal water loss (Elias et al., 1981). Similar effects were induced by synthetic retinoids (Chen et al., 1995; Thacher et al., 1997). Although retinoid pharmacological effects have been more studied in skin than in any other tissue, the skin cell-type(s) in which RA regulates gene expression and the network of RA-responsive target genes remain largely unknown.
Retinoids exert their highly pleiotropic effects through two groups of nuclear receptors (NRs), the retinoic acid receptors (RARα, β and γ) and the retinoid X receptors (RXRα, β and γ), which belong to the NR superfamily of ligand-dependent transcriptional regulators. RARs bind all trans- and 9cis-RA stereo-isomers, whereas RXRs interact exclusively with 9cis-RA. RARs and RXRs act through binding to cis-acting response elements located in the regulatory regions of retinoid-responsive genes, and RARs, like NRs for thyroid hormone (TRs), vitamin D3 (VDR), peroxisome proliferators (PPARs) and several orphan receptors, require heterodimerization with RXRs to function in vitro and in vivo (Kastner et al., 1995; Chambon, 1996; Morriss-Kay and Ward, 1999).
RXRα, RXRβ, RARα and RARγ are expressed in epidermis, and RXRα and RARγ are the predominant receptors (Fisher and Voorhees, 1996). However, their precise function in mediating retinoid effects on epidermis are unknown. Mouse transgenic expression of a dominant-negative (dn) RARα that subverts wild-type RAR functions has suggested that RARs could be involved in keratinocyte differentiation and RA-induced hyperproliferation (Imakado et al., 1995; Saitou et al., 1995; Xiao et al., 1999). Furthermore, expression of a dnRXRα in epidermal suprabasal layers indicated that RXRα could also be involved in retinoid-induced cell proliferation in adult mouse skin (Feng et al., 1997). However, as dn receptors could artefactually repress the expression of genes that are not ‘normal’ targets of co-repressor-associated unliganded RXR/RAR heterodimers (Chen and Evans, 1995), and/or interfere through sequestration with a variety of signalling pathways mediated by NRs that heterodimerize with RXRs (Mangelsdorf et al., 1995), the abnormalities exhibited by these transgenic models may not reflect the possible physiological roles of RA in epidermis homeostasis.
Targeted gene disruption through homologous recombination has been used extensively to investigate the physiological functions of retinoid receptors in the mouse. These genetic studies have indicated that RARα is apparently dispensable for epidermal homeostasis, whereas RARγ is involved in minor aspects of granular keratinocyte differentiation (our unpublished data). However, due to functional redundancy amongst retinoid receptors, some functions of RARα and/or RARγ in epidermis could have been overlooked (Kastner et al., 1995, 1997; Mascrez et al., 1998 and references therein). Unfortunately, compound germ-line disruptions of RARα and RARγ lead to lethality before embryonic day (E)11.5 (Wendling et al., 2001), thus precluding any epidermis analysis. Similarly, the possible effect of RXRα knockout on mouse epidermis could not be assessed, as this muta tion is lethal at E14.5, i.e. at the onset of epidermal morphogenesis. We have recently circumvented these limitations by using the Cre/loxP technology and a tamoxifen-inducible Cre-ERT recombinase (Metzger and Chambon, 2001) in order to selectively abrogate RXRα expression in adult mouse keratinocytes. This approach allowed us to demonstrate that RXRα is dispensable for epidermal morphogenesis, but is involved in the control of hair cycling, most probably through RXRα/VDR heterodimers (Li et al., 2000, 2001). We also found that RXRα, heterodimerized with other NRs, could be implicated in the control of interfollicular keratinocyte proliferation and differentiation (Li et al., 2000, 2001). To further investigate the skin function of RARα, RARγ and RXRα, we produced mice selectively lacking them in epidermal keratinocytes.
Results
RAR-mediated signalling is dispensable for homeostatic epidermal proliferation
As RARβ was not detected in epidermis (Fisher et al., 1994; see below) and epidermis of RARβ-null mice is apparently normal (Ghyselinck et al., 1997), the cell-autonomous requirement of the RAR-mediated signalling pathway in epidermis homeostasis can be studied in mutant mice selectively lacking RARα and RARγ in keratinocytes. To generate such mutants, RARαL2/L2 mice (Figure 1A; Chapellier et al., 2002a) were first crossed with RARγ+/– heterozygotes (Lohnes et al., 1993), and subsequently with K5-Cre-ERT(tg/0) transgenic mice that express the tamoxifen-inducible Cre recombinase specifically in basal keratinocytes (Indra et al., 1999). These crosses generated RARαL2/L2, RARαL2/L2/RARγ–/– and K5-Cre-ERT(tg/0)/RARαL2/L2/RARγ–/– animals. These mice were subjected to the tamoxifen (TAM) treatment known to permanently ablate RXRα in epidermis and hair follicle (i.e. RXRαep–/– mice; Li et al., 2000). Excision at the RARαL2/L2 locus in TAM-treated mice harbouring the K5-Cre-ERT transgene was efficient, permanent and selective, as RARα L2 alleles were converted into L– alleles in tail epidermis, no RARα L2 allele could be recovered in tail epidermis, even 12 months after TAM treatment, and no excision could be detected in dermis (thus yielding RARαep–/– mice; Figure 1B; data not shown). To check whether RARβ could be aberrantly expressed in epidermis upon RARα and/or RARγ ablation, RARβ RNA levels were determined in skin samples. RARβ1/3 isoforms were not detected, whereas similar levels of RARβ2 isoform were present in control and RARαep–/–/RARγ–/– mice (Figure 1C). As RARβ is selectively expressed in dermis (Redfern and Todd, 1992; our unpublished data), these data show that it is neither upregulated in dermis nor ectopically expressed in epidermis of RARαep–/–/RARγ–/– mice, which therefore exhibit a ‘panRAR-null’ epidermis (panRARep–/– mice).
Fig. 1. Conditional mutagenesis of RARα in epidermis and homeostatic keratinocyte proliferation. (A) Schematic drawing of wild-type RARα locus (+), floxed L2 and excised (L–) alleles. Black boxes stand for exons 7–9 (E7–9). Restriction sites and location of the 3′ probe are indicated. Sizes of restriction fragments are in kilobases (kb). H, HpaI; S, SacI. Arrowhead flags represent loxP sites. (B) Efficiency of Cre-ERT-mediated RARα gene disruption. Fourteen-week-old mice (genotypes as indicated) received TAM (5 days, 1 mg/day), and were treated again 2, 4 and 6 weeks later. RARα L2 and L– alleles were identified on tail epidermis genomic DNA, before and 6 months after TAM injection. M: DNA ladder. (C) RARβ expression in skin samples. RNAse protection assay was performed on total RNA (20 µg) from control (RARαL2/L2) and mutant mice (RARγ–/– and RARαep–/–/RARγ–/–), 6 and 12 months after TAM administration. A tRNA sample and total RNAs from RA-treated F9 teratocarcinoma cells were used as controls. Histone H4 protection was included for quantitation of the RNA samples. (D–F) Representative skin semi-thin sections, 12 months after TAM administration (genotypes as indicated). (G–I) Representative skin sections labelled with BrdU (white colour), showing proliferation of basal keratinocytes 12 months after TAM administration (genotypes as indicated). Sections were counterstained with DAPI (blue colour). Arrows point to the dermal-epidermal junction. hf, hair follicles. Scale bar (in F and I): 50 µm.
Topical RA alters epidermal cell differentiation (Fisher and Voorhees, 1996). However, histology of skin biopsies did not reveal any alteration in panRARep–/– mice (compare Figure 1D with F; and see below), and there was no alteration of keratin 5, 6, 10 and 13 expression (immunohistochemical data not shown). Moreover, electron microscopy of epidermis from panRARep–/– mice did not reveal abnormalities other than those exhibited by RARγ–/– mice (data not shown), indicating that RARα does not compensate for the loss of RARγ during keratinocyte differentiation in adult mice.
About 1% of basal keratinocytes were BrdU positive in epidermis of 12-month-old RARαL2/L2 mice (Figure 1G). This low labelling index relative to that observed in younger adults (5 ± 2%, see below and Table I) is a normal feature of epidermal ageing (Engelke et al., 1997). Similarly, in 12-month-old RARγ–/– and panRARep–/– animals, 1% of basal keratinocytes were BrdU positive (Figure 1H and I). Thus, homeostatic keratinocyte proliferation is maintained for >1 year in absence of RARα, β and γ in epidermis.
Table I. Overview of retinoid effects on proliferation and gene expression in the epidermis.
| Genotype | Treatment | Epidermal status | % BrdU-positive nuclei | % Ki67-positive nuclei | CRABPII mRNA abundance | HB-EGF mRNA abundance |
|---|---|---|---|---|---|---|
| Wild type | none | Resting (control) | 5 ± 2 | 6 ± 2 | + | + |
| Wild type or wild-type-likea | RA | RA-stimulated | 54 ± 6 | 56 ± 5 | ++++ | ++++ |
| Wild type | RA + BMS493 | Antagonism of RA stimulation | ND | 6 ± 3 | + | + |
| Wild type | BMS649 (SR11237) | Activation of RXR signalling | ND | 10 ± 4 | + | + |
| Wild type | BMS753 | Activation of RARα signalling | ND | 7 ± 2 | ND | ND |
| Wild type | BMS753 + BMS649 | Activation of RARα and RXR signalling | ND | 9 ± 3 | ND | ND |
| Wild type | BMS961 | Activation of RARγ signalling | ND | 35 ± 6 | +++ | +++ |
| Wild type | BMS961 + BMS649 | Activation of RARγ and RXR signalling | ND | 51 ± 8 | ++++ | ++++ |
| RARα–/– | RA | RARα-null | 50 ± 4 | ND | ++++ | ++++ |
| K5-Cre-ERT(tg/0)/RXRαL2/L2 | RA | RXRα-null in all layers (RXRαep–/–) | 12 ± 4 | ND | ++ | ++ |
| K5-Cre-ERT(tg/0)/RXRαL2/L2/RARα–/– | RA | RXRα-null in all layers and RARα-null (RXRαep–/–/RARα–/–) | 13 ± 4 | ND | ND | ND |
| RARγ–/– | RA | RARγ-null | 12 ± 3 | 14 ± 3 | + | + |
| K5-Cre-ERT(tg/0)/RXRαL2/L2/RARγ–/– | RA | RXRα-null in all layers and RARγ-null (RXRαep–/–/RARγ–/–) | 7 ± 2 | ND | + | + |
| CMV-Cre-ERT(tg/0)/RARγL3/L3 | RA | RARγ-null in granular layer only (RARγsb–/–) | ND | 13 ± 4 | + | + |
aWild-type-like include RXRαL2/L2; CMV-Cre-ERT(tg/0)/RARγ+/L3 and CMV-Cre-ERT(tg/0)/RXRα+/L2; +, basal level of expression; ++ to ++++, increasing level of expression; ND, not determined.
Pharmacological evidence that RXR/RARγ heterodimers are involved in RA-induced epidermal hyperplasia
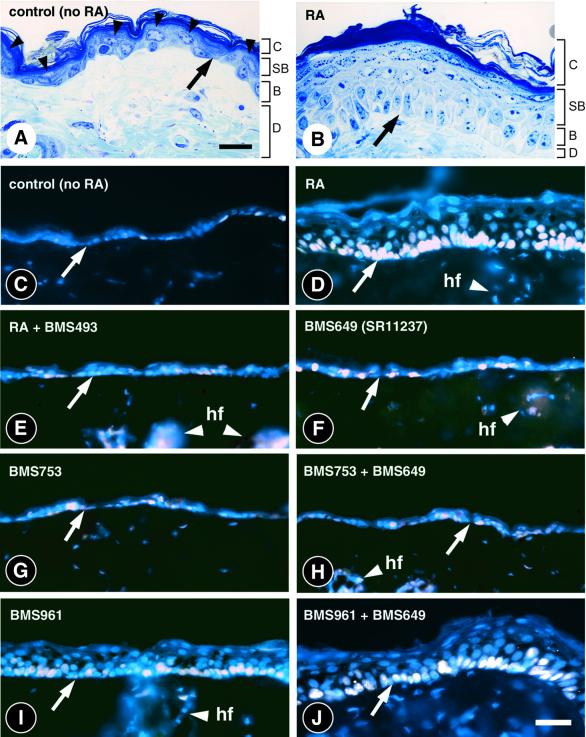
Dorsal epidermis of 2- to 3-month-old mice consists of (i) an irregular layer of basal keratinocytes, (ii) suprabasal layers formed by scattered spinous and granular keratinocytes (arrowheads) and (iii) several rows of cornified keratinocytes (Figure 2A). Topical RA treatment causes epidermal thickening due to basal cell hyperproliferation (Fisher and Voorhees, 1996). Accordingly, suprabasal layers were thickened in RA-treated wild-type mice, due to marked increases in numbers of spinous and granular keratinocytes; mitotic figures were frequent in basal keratinocytes (Figure 2B), and the number of basal keratinocytes expressing the proliferation marker Ki67 or incorporating BrdU was ∼10-fold higher in RA- than in vehicle-treated epidermis (56 ± 5% versus 6 ± 2%; Figure 2C and D; Table I; data not shown). Simultaneous administration of the panRAR antagonist BMS493 prevented RA-induced epidermal hyperplasia, demonstrating that keratinocyte proliferation resulted from RAR activation (Figure 2E; Table I). Interestingly, a panRAR antagonist application on its own had no apparent effect on keratinocyte proliferation (data not shown).
Fig. 2. Histology and proliferative response of wild-type skin upon topical retinoid treatment. Dorsal skin was treated for four consecutive days with 40 nmol (in 400 µl acetone) of RA (B and D) or synthetic retinoids (E–J), as indicated. Controls (A and C) were treated with acetone vehicle. (A and B) Histology of control and RA-treated epidermis. Semi-thin sections (2-µm thick) were stained with toluidine blue. Arrowheads point to the spinous and granular keratinocytes in control epidermis. (C–J) Skin sections showing the proliferation marker Ki67 (white colour) and counterstained with DAPI (blue colour). Arrows point to the dermal-epidermal junction. RA, retinoic acid; BMS493, panRAR-selective antagonist; BMS649 (SR11237), panRXR-selective agonist; BMS753, RARα-selective agonist; BMS961, RARγ-selective agonist. B, basal layer; C, cornified layer; D, dermis; hf, hair follicle; SB, suprabasal layers (spinous and granular keratinocytes). Scale bar: 15 µm in (A) and (B); 25 µm in (C)–(I).
To determine the potency of RAR isotypes in mediating retinoid-induced epidermal hyperplasia, wild-type mice were treated topically with selective ligands for RARs or RXRs. On their own or in association (Figure 2H), the panRXR agonist BMS649 (Figure 2F) and the RARα-selective agonist BMS753 (Figure 2G) did not alter the percentage of proliferating, Ki67-positive, basal keratinocytes (Table I). In contrast, the number of proliferating keratinocytes was strongly increased by the RARγ-selective agonist BMS961 (Figure 2I). However, the potency of this latter retinoid was below that of RA, while co-administration of BMS961 and BMS649 fully reproduced the effects of RA (compare Figure 2D, I and J; Table I).
Altogether, these observations indicate that RA-induced skin hyperplasia most probably involves RXR/RARγ heterodimers, in which RXR transcriptional activity is subordinated to that of RARγ, i.e. liganded RXR is inactive unless its RAR partner is itself liganded (Chambon, 1996). However, these pharmacological experiments do not reveal which cells are retinoid primary targets in epidermis and/or even in dermis, which is known to secrete various growth factors involved in epidermal cell proliferation (Werner and Smola, 2001). We therefore analysed RA-induced epidermal hyperplasia in mice carrying RAR- and RXR-null mutations selectively in their epidermis.
Genetic evidence that RXRα/RARγ heterodimers are the functional units responsible for RA-induced epidermal hyperplasia
RXRαL2/L2 mice were crossed with either RARα+/– or RARγ+/– mice. These animals were further mated with K5-Cre-ERT(tg/0) transgenic mice (see above) to generate RXRαL2/L2, RARα–/–, RARγ–/–, K5-Cre-ERT(tg/0)/RXRαL2/L2, K5-Cre-ERT(tg/0)/RXRαL2/L2/RARα–/– and K5-Cre-ERT(tg/0)/RXRαL2/L2/RARγ–/– animals, which were treated with TAM at 14 weeks of age (Figure 3A). The efficacy of RXRα gene excision in keratinocytes was quantified after separation of epidermis from dermis. Whereas no RXRα L– excised allele was found in epidermis before TAM administration (Figure 3B, left panel), this treatment resulted in almost 100% L– alleles in mice carrying the K5-Cre-ERT transgene (yielding RXRαep–/– mice), but had no effect in those lacking the transgene (Figure 3B, right panel). The RXRα L– allele was never detected in the dermis (data not shown). No RXRα protein was detected by immunohistochemistry in the interfollicular epidermis and hair follicles of RXRαep–/– mice at D11 (compare Figure 3C with D). Thus, TAM administration efficiently and selectively abolishes RXRα expression in epidermis of mice bearing the K5-Cre-ERT transgene and the RXRα L2 alleles. Note that the RXRα-ablated epidermis did not exhibit any interfollicular hyperplasia shortly after the TAM treatment (Figure 3D), in agreement with our previous report showing that hyperproliferation is a late event occurring 10–12 weeks after RXRα ablation (Li et al., 2000).
Fig. 3. Conditional mutagenesis of RXRα in epidermis, and RA-induced proliferative response. (A) Scheme of the experimental protocol. Intraperitoneal injection of TAM (1 mg) was performed from day 1 (D1) to day 4 (D4). A tail biopsy was made on day 11 to check for RXRα gene disruption. RA (40 nmol) was topically applied on skin for 4 days (D12–D15). On day 16 (D16), an injection of BrdU (50 mg/kg) was given 2 h before skin sampling. (B) Efficiency of RXRα gene disruption in mice bearing the K5-Cre-ERT transgene (as indicated). RXRα L2 and L– alleles were identified on tail epidermis genomic DNA before and after TAM administration. M: DNA ladder. (C and D) Immunohistochemical detection of RXRα on skin sections from control (RXRαL2/L2) and mutant (RXRαep–/–) mice. (E–J) Representative skin sections labelled with BrdU (brown colour), showing epidermis thickness and basal keratinocyte proliferation in mutants (genotypes as indicated). Arrows point to the dermal–epidermal junction. hf, hair follicles. Scale bar: 25 µm in (C) and (D); 50 µm in (E)–(J).
Cell proliferation was quantified by counting BrdU-labelled basal keratinocytes (see Table I). In ‘control’ mice (RXRαL2/L2), about half (54 ± 6%) of the basal keratinocytes were BrdU positive after RA treatment (Figure 3E; note that 5 ± 2% of the basal keratinocytes were BrdU positive in vehicle-treated mice). RA-induced epidermal thickening and cell hyperproliferation in RARα–/– mice was similar to that of controls (Figure 3F, 50 ± 4%), while it was much reduced in RARγ–/– mice (Figure 3G, 12 ± 3%) and in RXRαep–/– mice (Figure 3H, 12 ± 4%). Ablation of RXRα in epidermis of RARα–/– mice (i.e. RXRαep–/–/RARα–/– mice) did not further decrease RA-induced proliferation (Figure 3I, 13 ± 4%). In contrast, RA treatment had almost no effect in RXRαep–/–/RARγ–/– mice (Figure 3J, 7 ± 2%). Thus, RXRα/RARγ, but not RXRα/RARα, heterodimers appear to be required within the epidermis to mediate RA-induced hyperplasia. Note that the percentage of proliferating keratinocytes in vehicle-treated mice (∼6%) is below that observed in RA-treated RARγ–/– or RXRαep–/– epidermis (∼13%), but is similar to that of RA-treated RXRαep–/–/RARγ–/– mice (∼7%). As RARα and RXRβ are also expressed in epidermis (Fisher et al., 1994; Li et al., 2000), these observations suggest some functional redundancy (see Introduction) between RARγ and RARα, and between RXRα and RXRβ, even though, on their own, disruption of either the RARα gene (see above) or the RXRβ gene (Kastner et al., 1996) did not affect the RA-induced proliferation (data not shown). Disruption of the RARβ gene (Ghyselinck et al., 1997), which is expressed in dermal fibroblasts but not in epidermis (see above), did not perturb RA-induced epidermal hyperproliferation (data not shown).
To determine in which epidermal cell layer RA exerts its primary effect (i.e. basal or suprabasal), we next ablated RARγ and RXRα in suprabasal layers.
Genetic evidence that RARγ is required in suprabasal cell layers for RA-induced epidermal hyperplasia
RARγL3/L3 mice (Figure 4A; Chapellier et al., 2002b) were crossed with CMV-Cre-ERT(tg/0) transgenic mice, in which TAM administration can induce Cre-mediated recombination in suprabasal layers but not in the basal layer (Brocard et al., 1997), to produce ‘control’ (CMV-Cre-ERT(tg/0)/RARγ+/L3) and experimental (CMV-Cre-ERT(tg/0)/RARγL3/L3) animals. At 14 weeks of age, all mice were treated with TAM (Figure 4B) and RARγ gene disruption was quantified. No RARγ L– excised allele was found before TAM treatment (Figure 4C, left panel). As expected from the selective expression of the CMV-Cre-ERT transgene in suprabasal (sb), but not in basal keratinocytes (Brocard et al., 1997), this treatment resulted only in a partial conversion of RARγ L3 alleles into L2Neo (in which the neo gene was not removed; see Figure 4A) and L– alleles (Figure 4C, middle panel). Importantly, no RARγ L– and L2Neo alleles could be detected a few weeks after the end of TAM treatment (data not shown), proving that no disruption of the RARγ gene had occurred in basal keratinocytes (see also below; Figure 4H). Thus, TAM administration to CMV-Cre-ERT(tg/0)/RARγL3/L3 mice effectively resulted in selective disruption of the RARγ gene in epidermal suprabasal layers, yielding RARγsb–/– mice.
Fig. 4. Conditional mutagenesis of RARγ and RA-induced proliferative response in mice lacking RARγ in suprabasal layers (RARγsb–/– mice). (A) Schematic drawing of RARγ wild-type (+), L3, partially excised L2Neo and fully excised L– alleles. Sizes of NsiI fragments obtained for each allele are in kilobases (kb). Black boxes, exons 7–13 (E7–13); neo, neomycin gene; H, HpaI; N, NsiI. Arrowhead flags represent loxP sites. (B) Experimental protocol. Injection of TAM (1 mg) was performed from day 1 (D1) to day 3 (D3), then every second day for 10 days. A tail biopsy was made on day 9 to check for RARγ gene disruption. RA (40 nmol) was then applied topically on skin for 4 days (D10–D13). On day 14 (D14), the skin was sampled. (C) Efficiency of RARγ gene disruption in mice bearing the CMV-Cre-ERT transgene. RARγ wild-type (+), L3, L2Neo and L– alleles were detected on tail epidermis and dermis genomic DNA before and after TAM administration. Note that the minute amount of RARγ excised L– allele present in dermis most probably originates from contaminating epidermal keratinocytes (Li et al., 2000). M: DNA ladder. (D–F) Representative skin sections showing RA-induced basal keratinocyte proliferation (white colour, Ki67 signal; blue colour, DAPI nuclear staining). RA-induced epidermal proliferation in RARγ–/– mice is illustrated in (F). (G–I) Immunohistochemical detection of RARγ on back skin sections from control [(G), RARγsb+/–] and mutant [(H), RARγsb–/–] mice, after RA treatment. An RARγ–/– skin sample was used as negative control (I). Arrows point to the dermal–epidermal junction. hf, hair follicles. Scale bars (in F and I): 50 µm.
In RA-treated ‘control’ mice, the epidermis was markedly thickened and about half (56 ± 5%) of basal keratinocytes expressed Ki67 (Figure 4D), instead of 6 ± 2% in vehicle-treated mice (Figure 2C; Table I). In contrast, epidermal proliferation was lower in RA-treated RARγsb–/– mice (Figure 4E, 13 ± 4%; Table I), a situation similar to that observed in RARγ–/– mice (Figure 4F, 14 ± 3%; Table I). Following RA treatment, the RARγ protein was easily detected by immunohistochemistry throughout the hyperplastic epidermal layers and in hair follicles of ‘control’ mice (Figure 4G). As expected, RARγ was also detected in basal and hair follicle keratinocytes of RARγsb–/– mice, but not in suprabasal keratinocytes (Figure 4H; data not shown). It was also not detected in epidermis of RA-treated RARγ–/– mice (Figure 4I). Importantly, RA-induced epidermal hyperproliferation in RARγsb–/– mice was restored to ‘control’ levels a few weeks after the end of TAM treatment (data not shown), clearly demonstrating that RARγ disruption was restricted to suprabasal cells. Altogether, these results indicate that RARγ expressed in suprabasal cells is required to transduce the retinoid signal that triggers proliferation of basal keratinocytes.
Persistence of the RXRα protein in epidermal suprabasal cells in which the RXRα gene is disrupted
To determine whether RXRα could be the heterodimeric partner of RARγ in suprabasal cells, RXRαL2/L2 mice were crossed with CMV-Cre-ERT(tg/0) transgenic mice, to produce ‘control’ (CMV-Cre-ERT(tg/0)/RXRα+/L2) and ‘experimental’ (CMV-Cre-ERT(tg/0)/RXRαL2/L2) mice. Before TAM administration, no RXRα L– allele was found in epidermis (Figure 5A, left panel), whereas this treatment induced conversion of RXRα L2 alleles into L– alleles in ‘experimental’ mice (Figure 5A, right panel). The remaining L2 alleles originated from the basal keratinocytes, as this species was absent in DNA from an RXRαep–/– mouse skin sample. Importantly, as in the case for the RARγL3/L3 gene (see above), no disrupted RXRαL2/L2 gene could be detected a few weeks after the end of TAM treatment (data not shown), indicating that the RXRα gene was not disrupted in the basal cell layer.
Fig. 5. Conditional mutagenesis of RXRα in epidermis suprabasal layers, and RA-induced proliferative response in RXRαsb–/– mice. (A) Efficiency of RXRα gene disruption in mice bearing the CMV-Cre-ERT transgene as compared with the K5-Cre-ERT transgene (as indicated). The experimental protocol is the same as in Figure 4B. RXRα wild-type (+), L2 and L– alleles were detected on tail epidermis genomic DNA before and after TAM administration. M: DNA ladder. (B and C) Representative back skin sections labelled with BrdU (brown colour), showing RA-induced epidermal thickening and increased basal keratinocytes proliferation. (D and E) Immuno-histochemical detection of RXRα on skin sections from control [(D), RXRαsb+/–] and experimental [(E), RXRαsb–/–] mice, after RA treatment. Arrowheads indicate nuclei of suprabasal keratinocytes expressing RXRα. Arrows point to the dermal–epidermal junction. hf, hair follicles. Scale bar (in D): 50 µm.
Thus, TAM administration successfully induced disruption of the floxed RXRα gene in suprabasal, but not basal, keratinocytes of mice bearing the CMV-CreERT transgene, yielding RXRαsb–/– mice. However, RA-induced proliferation was similar in TAM-treated ‘control’ and ‘experimental’ (RXRαsb–/–) mice (Figure 5B and C, 54 ± 6% and 47 ± 8%, respectively), and unexpectedly RXRα was readily detected by immunohistochemistry throughout epidermis, including in suprabasal keratinocytes (arrowheads in Figure 5E) of both RA-treated ‘control’ and RXRαsb–/– mice (compare Figure 5D with E). Thus, the RXRα protein was still present in epidermis suprabasal cells of experimental mice, even though TAM administration efficiently induced Cre-mediated disruption of the RXRα L2 alleles in these cells. This indicates that there is little turnover of the RXRα protein upon differentiation of basal into suprabasal cells.
RA-induced epidermal hyperplasia is associated with induction of HB-EGF expression
Altogether, the above observations suggest that disruption of RARγ in suprabasal keratinocytes impairs a paracrine signalling system mediating the effects of RA on basal keratinocyte proliferation. Epidermal growth is modulated by several factors, including epidermal growth factor (EGF), TGFα, heparin-binding EGF-like growth factor (HB-EGF), amphiregulin, epiregulin, heregulin and β-cellulin (Jost et al., 2000). Expression of HB-EGF was previously shown to be selectively induced by RA in epidermis suprabasal layers (Stoll and Elder, 1998; Xiao et al., 1999). To investigate whether the epidermal response to synthetic retinoids (see Figure 2) could be correlated with activation of the HB-EGF signalling pathway, HB-EGF mRNA levels were determined (Figure 6A). The panRAR antagonist BMS493 abolished the RA-induced increase of HB-EGF mRNA, while the increase observed upon treatment with the RARγ agonist, BMS961, was potentiated by co-administration of the panRXR agonist BMS649, which, on its own, did not induce HB-EGF mRNA expression. Thus, retinoid-induced epidermis hyperplasia is correlated with an increased expression of HB-EGF mRNA in the epidermis. Furthermore, the ability of RA to induce HB-EGF mRNA was severely impaired whenever RARγ expression was abrogated either in epidermis or selectively in suprabasal keratinocytes (i.e. in RARγ–/–, RXRαep–/–/RARγ–/– and RARγsb–/– mice), but was unaffected in mice lacking RARα (Figure 6B). Expression of HB-EGF mRNA was also significantly reduced, but not abolished, in mice lacking RXRα in epidermis (RXRαep–/– animals), most probably due to some functional redundancy between RXRα and RXRβ, even though no decrease of HB-EGF transcripts was observed in the skin of RXRβ–/– mice (data not shown). In contrast, selective disruption of the RXRα gene in suprabasal keratinocytes (RXRαsb–/– mice) had no effect on HB-EGF mRNA levels, in keeping with persistence of the RXRα protein (see above).
Fig. 6. Expression of HB-EGF, CRABPII, RARβ2 and β-actin mRNA in whole back skin following topical application of retinoids. (A) Northern blot analysis of total RNA (25 µg) before (control) and after retinoid administration, as indicated. RA, retinoic acid; BMS493, panRAR antagonist; BMS961, RARγ agonist; BMS649 (SR11237), panRXR agonist. (B) Northern blot analysis of total RNA (25 µg) from control (RXRαL2/L2) and mutant mice (genotypes as indicated) before (–) and after (+) RA administration.
The cellular retinoic acid-binding protein II (CRABPII), which is expressed in suprabasal keratinocytes, is considered as an indicator of RA activity in the epidermis (Fisher and Voorhees, 1996). Variations in levels of CRABPII mRNA in response to retinoid treatments and in RARγ and RXRα mutants strictly paralleled those of HB-EGF mRNA (Figure 6). It is therefore likely that, within the RAR/RXR heterodimers controlling RA target gene expression in suprabasal cells (e.g. HB-EGF and CRABPII), RARγ plays a crucial role, whereas RXRα can, to some extent, be functionally replaced by RXRβ.
Interestingly, RARβ2 mRNA, which is not or barely detectable in skin under normal conditions (Fisher et al., 1994; see Figure 6B, lanes 1 and 2), but is known to be RA-inducible in dermal fibroblasts (Redfern and Todd, 1992; Tsou et al., 1994), was present in all RA-treated samples (Figure 6B), except in those from mice harbouring a germ-line RARγ-null mutation (RARγ–/– and RXRαep–/–/RARγ–/– mice). This observation suggests that (i) topical RA treatment was efficient down to the dermis and (ii) induction of RARβ2 expression by RA in the dermis is mediated through heterodimers involving RARγ.
Discussion
Based on their expression patterns (Fisher and Voorhees, 1996) and on results from overexpression of dn retinoid receptors in epidermis of transgenic mice (see Introduction), it has been proposed that RARs and RXRs (notably RARγ and RXRα) play important physiological functions in epidermis. To gain insight into the role of retinoid receptors, and more generally of RA signalling in epidermis, we have investigated here the phenotype of mutant mice in which RARα, RARγ and/or RXRα have been selectively disrupted in this tissue.
RARs are dispensable for homeostatic self-renewal of epidermal keratinocytes
The skin of RARα-null and RARβ-null mutants appears normal, while skin of RARγ-null mutants exhibit only minor defects of granular keratinocyte differentiation (Lohnes et al., 1993; Lufkin et al., 1993; Ghyselinck et al., 1997; our unpublished results). Functional redundancy between RARα and RARγ (see Introduction), which, in contrast to RARβ, are both expressed in epidermis (Fisher et al., 1994), could have accounted for the paucity of alterations in epidermis of RARα- and RARγ-null mice. Such a functional redundancy could not be assessed from examination of RARα/RARγ-null mice, as they die before the onset of epidermis formation (Wendling et al., 2001). The inducible conditional somatic mutagenesis strategy used here has allowed us to generate mice selectively lacking both RARα and RARγ in adult epidermis. As RARβ is not ectopically expressed in the epidermis of these mice, they actually display a ‘panRAR-null’ epidermis fully devoid of RARα, β and γ. As this panRAR-null epidermis is similar to that of a RARγ-null mice (our unpublished results), we conclude that there is little (and possibly no) redundancy between RARα and RARγ for adult keratinocyte proliferation and differentiation. Moreover, as the complete absence of RARs in basal keratinocytes does not alter their homeostatic proliferation, our study demonstrates for the first time that self-renewal of epidermal keratinocytes does not require an epidermal RAR-dependent signalling pathway.
We have previously shown that RXRα ablation in basal keratinocytes results notably in hair follicle degeneration and hyperproliferation of interfollicular keratinocytes (Li et al., 2000, 2001). These features most probably reflect a role of RXRα/VDR heterodimers in hair follicle cycling, and the involvement of other heterodimeric partners of RXRα in the control of interfollicular keratinocytes proliferation (Li et al., 2000, 2001). Such possible partners known to be expressed in epidermis include RARs (Fisher et al., 1994), TRs (Billoni et al., 2000) and PPARs (Peters et al., 2000; Michalik et al., 2001). As RARγ-null and RARα/RARγ-null keratinocytes cultured in vitro have been shown to be refractory to RA-induced growth arrest (Goyette et al., 2000), RARs could have been the partners of RXRα that mediate an anti-proliferative effect in resting (i.e. unstimulated) epidermis. However, our present data demonstrate that ablation of the RARs in keratinocytes does not result in epidermal hyperplasia, ruling out the possibility that RAR/RXRα heterodimers could be critically involved in controlling the keratinocyte proliferation under physiological conditions in vivo. Interestingly, mutant mice expressing an RXRα lack ing its AF-2 ligand-dependent activation function do not exhibit an epidermal interfollicular hyperplasia (B.Mascrez, N.B.Ghyselinck, M.Mark, D.Metzger, M.Li and P.Chambon, unpublished observations). Therefore, basal keratinocyte homeostatic proliferation might be controlled through heterodimers comprising a transcriptionally inactive (unliganded) RXRα and a nuclear receptor other than a RAR.
As essential components of the vitamin A metabolic machinery are expressed in keratinocytes (Niederreither et al., 2002), RA could be synthesized in the epidermis. However, the observations that (i) a RXR-specific agonist has no effect on its own on keratinocyte proliferation (Thacher et al., 1997; our present data), and (ii) the panRAR antagonist BMS493 does not reduce the homeostatic rate of basal keratinocyte proliferation (data not shown), indicate that endogenous RA available for activation of RARs is either lacking, or present at very low levels in resting epidermis. Along the same lines, it is noteworthy that RA is undetectable in human epidermis (Vahlquist, 1982).
The RA signal inducing epidermal hyperproliferation is transduced through RARγ/RXRα heterodimers in suprabasal keratinocytes
Although RARs are apparently not involved in the control of homeostatic epidermal proliferation under resting conditions, our pharmacological and genetic data demonstrate that topical treatment of skin with retinoids results in a marked increase in keratinocyte proliferation that is mediated in suprabasal keratinocytes through RARγ/RXR heterodimers. In human and mouse epidermis, RARγ represents 90% of the RARs, the remaining 10% being RARα (Fisher et al., 1994; our unpublished results). The fact that RARγ-null epidermis exhibits some RA-induced increased proliferation raised the possibility that RARα could be involved in a RA-triggered cell proliferation pathway distinct from that involving RARγ. However, RA-induced hyperproliferation of RARα-null epidermis is identical to that of wild-type epidermis, and hyperproliferation is not induced by a RARα-selective agonist. Thus, the residual hyperproliferation observed in RARγ-null epidermis probably results from a functional redundancy with RARα, which is artefactually generated by the knockout of RARγ and similar to that previously seen in RARγ-null cells in vitro (Taneja et al., 1996; see Introduction for further references). Similarly, the slight increase in proliferation still observed upon RA-stimulation of epidermis lacking RARγ selectively in the suprabasal layers (RARγsb–/– mice; Table I) could also reflect a redundancy with RARα. Therefore, RARγ is the main, if not the only, RAR-mediating RA-induced keratinocyte hyperproliferation in wild-type mice. Likewise, the residual RA-induced proliferation increase in RXRα-null epidermis most probably reflects a functional redundancy with RXRβ (Li et al., 2000).
In any event, the striking synergism between RARγ- and RXRα-null mutations strongly supports the conclusion that RXRα/RARγ heterodimers are the functional units mediating RA-induced epidermal hyperplasia. The alternative possibility that two different converging pathways could be involved in the generation of keratinocyte hyperproliferation, the first one requiring RARγ and the other one RXRα, is unlikely: (i) the RXR-specific agonist BMS649 has no effect on its own, indicating that RA acts neither through RXR homodimers nor through heterodimers in which RXR activity is not subordinated to that of its partner (e.g. RXR/PPAR heterodimers); and (ii) the effect of the RARγ-selective agonist is potentiated by the RXR-selective agonist. It is noteworthy that our present study also demonstrates that the subordination of the ligand-dependent RXR activity to its liganded-RAR partner that was previously evidenced in vitro (Roy et al., 1995; Taneja et al., 1996), also occurs in the animal.
Paradoxically, RA induces opposite responses in keratinocytes depending on whether they are studied in vivo or cultured in vitro. For example, RA treatment induces keratinocyte growth-arrest in vitro (Goyette et al., 2000) and has no effect on CRABPII expression (Fisher and Voorhees, 1996). In contrast, RA stimulates keratinocyte proliferation and increases transcription of the CRABPII gene in vivo. We have shown here that these RA effects are transduced by RARγ present in epidermis suprabasal cell layers. Thus, the failure of RA to increase proliferation and CRABPII expression in cultured keratinocytes may just reflect their similarity with basal keratinocytes. This raises the interesting possibility that the RARγ-dependent pathway involved in inhibition of keratinocyte proliferation in vitro (Goyette et al., 2000) may also operate in basal keratinocytes in vivo, in order to finely tune pathophysiological epidermal responses to RA signalling, for example during skin wound healing (see below).
The RA signal transduced by RARγ/RXRα heterodimers in suprabasal keratinocytes generates a paracrine signal inducing hyperproliferation of basal keratinocytes
As cell proliferation only takes place in the basal layer, our finding that RARγ/RXRα heterodimers present in suprabasal keratinocytes are required for RA-induced epidermal hyperplasia demonstrates that retinoids induce, through these heterodimers, the synthesis of a paracrine signal in suprabasal keratinocytes, which in turn causes hyperproliferation of basal keratinocytes. This is in keeping with previous data showing that expression of a dnRARα in suprabasal layers abrogate RA-induced hyperplasia (Xiao et al., 1999). However, as an excess of dnRAR may, through binding to RXRs, interfere with the function of both RARs and a number of other heterodimeric partners of RXRs (e.g. PPARs), this previous study could not reveal which RXR/NR heterodimer generates the RA-induced hyperproliferation signal in suprabasal cells.
Several growth factors released from both the epidermis and the dermis affect proliferation of basal keratinocytes through binding to epidermal growth factor receptors that are located in basal keratinocyte membranes and belong to the ErbB family (Piepkorn et al., 1998; Jost et al., 2000; Werner and Smola, 2001). Amongst the ligands for the ErbB isotypes that are expressed in epidermis, HB-EGF appears to be the only one regulated by RA (Stoll and Elder, 1998; Xiao et al., 1999). Furthermore, its mRNA is exclusively expressed in suprabasal keratinocytes (Xiao et al., 1999), whereas mature HB-EGF proteins are localized only in epidermal basal cells (Downing et al., 1997). Our present data demonstrate that RXRα/RARγ heterodimers play a key role in RA-induced HB-EGF expression in suprabasal cells, although they do not reveal whether these heterodimers act directly, or rather through a RA-responsive factor, on this expression. Our study also underlines a positive correlation between expression of HB-EGF and RA-induced proliferation (Table I), further supporting the possibility that HB-EGF could be ‘the’ paracrine factor that is synthesized in the suprabasal layers and mediates RA-induced hyperplasia (Xiao et al., 1999). We note, however, that RA does not increase HB-EGF expression in RARγ–/– and RARγsb–/– mice, whereas it still exerts some proliferative effect on their epidermis (Table I). Thus, HB-EGF may not be the only signalling molecule involved in RA-induced proliferation. Interestingly, a recent study has shown that expression of keratinocyte growth factor is stimulated by RA in cultured gingival fibroblasts (Mackenzie and Gao, 2001).
In conclusion, our data indicate that a retinoid-inducible RARγ/RXRα heterodimer-mediated pathway, which functions in epidermal suprabasal cells to synthesize a paracrine signal triggering hyperproliferation of basal keratinocytes, is dispensable for their homeostatic proliferation in resting skin. It is tempting to speculate that this pathway could be required under stress conditions, such as wound healing. In this respect, it is noteworthy that: (i) vitamin A deficiency causes delayed wound healing, whereas pre-treatment with vitamin A or RA improves epidermal regeneration (Hunt, 1986); (ii) HB-EGF secretion is increased in response to epidermal injury (Marikovsky et al., 1993; McCarthy et al., 1996); (iii) RA-induced HB-EGF expression occurs prior to the onset of basal cell layer proliferation (Xiao et al., 1999). Thus, the possibility exists that the beneficial actions of retinoids during wound healing could be mediated through activation of RARγ/RXRα heterodimers, which at the very least may control HB-EGF signalling in suprabasal keratinocytes.
Materials and methods
Mice and treatments
RARα- and RARγ-null, floxed RXRα, RARα and RARγ, and CMV-Cre-ERT and K5-Cre-ERT transgenic lines have been described previously (Lohnes et al., 1993; Lufkin et al., 1993; Brocard et al., 1997; Indra et al., 1999; Li et al., 2000; Chapellier et al., 2002a,b). Tail epidermis was separated from dermis as described previously (Li et al., 2000). Each experiment (two to three females of each genotype) was repeated three times. Neither acetone nor TAM treatments affected epidermal proliferation.
Histochemistry and proliferation analysis
For histology, skin samples were fixed with 2.5% glutaraldehyde (0.1 M cacodylate pH 7.2). RXRα and RARγ immunolocalization were as described previously (Ghyselinck et al., 1997; Li et al., 2001). Proliferating cells were detected using an anti-BrdU antibody, revealed by peroxidase activity and counterstained with Harris haematoxylin. The proliferation marker Ki67 was detected by immunohistochemistry (Novocastra’s protocol) on frozen sections post-fixed in 2% paraformaldehyde, and counterstained with 0.01% DAPI. The number of either BrdU- or Ki67-positive and total basal keratinocytes were counted on five areas from three animals of each genotype. The mean ± SD percentage of proliferating over total basal cells (>200) was then estimated.
Supplementary data
More details of Materials and methods are available as supplementary data at The EMBO Journal Online, or on request.
Acknowledgments
Acknowledgements
We are grateful to C.Suzi (Bristol-Myers Squibb) for synthetic retinoids. We thank B.Féret, B.Bondeau, I.Tilly, B.Weber, O.Wendling and C.Dennefeld for excellent technical assistance, as well as the staff of the animal facility. We are indebted to A.Dierich and E.Blondelle for embryonic stem cell culture. This work was supported by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Collège de France, the Association pour la Recherche sur le Cancer, the Fondation pour la Recherche Médicale, the Human Frontier Science Program, the Ministère de l’Éducation Nationale de la Recherche et de la Technologie and the EEC (CT 97-3220).
References
- Billoni N., Buan,B., Gautier,B., Gaillard,O., Mahé,Y.F. and Bernard,B.A. (2000) Thyroid hormone receptor β1 is expressed in the human hair follicle. Br. J. Dermatol., 142, 645–652. [DOI] [PubMed] [Google Scholar]
- Brocard J., Warot,X., Wendling,O., Messaddeq,N., Vonesch,J.L., Chambon,P. and Metzger,D. (1997) Spatio-temporally controlled site-specific somatic mutagenesis in the mouse. Proc. Natl Acad. Sci. USA, 94, 14559–14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P. (1996) A decade of molecular biology of retinoic acid receptors. FASEB J., 10, 940–954. [PubMed] [Google Scholar]
- Chapellier B., Mark,M., Garnier,J.M., LeMeur,M., Chambon,P. and Ghyselinck,N.B. (2002a) A conditional floxed (loxP-flanked) allele for the Retinoic Acid Receptor alpha (RARα) gene. Genesis, 32, 87–89. [DOI] [PubMed] [Google Scholar]
- Chapellier B., Mark,M., Garnier,J.M., Dierich,A., Chambon,P. and Ghyselinck,N.B. (2002b) A conditional floxed (loxP-flanked) allele for the Retinoic Acid Receptor gamma (RARγ) gene. Genesis, 32, 95–98. [DOI] [PubMed] [Google Scholar]
- Chen J.D. and Evans,R.M. (1995) A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature, 377, 454–457. [DOI] [PubMed] [Google Scholar]
- Chen S. et al. (1995) Retinoic acid receptor γ mediates topical retinoid efficacy and irritation in animal models. J. Invest. Dermatol., 104, 779–783. [DOI] [PubMed] [Google Scholar]
- Downing M.T., Brigstock,D.R., Luquette,M.H., Crissman-Combs,M. and Besner,G.E. (1997) Immunohistochemical localization of heparin-binding epidermal growth factor-like growth factor in normal skin and skin cancers. Histochem. J., 29, 735–744. [DOI] [PubMed] [Google Scholar]
- Elias P.M., Fritsch,P.O., Lampe,M., Williams,M.L., Brown,B.E., Nemanic,M. and Grayson,S. (1981) Retinoid effects on epidermal structure, differentiation and permeability. Lab. Invest., 44, 531–540. [PubMed] [Google Scholar]
- Engelke M., Jensen,J.M., Ekanayake-Mudiyanselage,S. and Proksch,E. (1997) Effects of xerosis and ageing on epidermal proliferation and differentiation. Br. J. Dermatol., 137, 219–225. [DOI] [PubMed] [Google Scholar]
- Feng X., Peng,Z.H., Di,W., Li,X.Y., Rochette-Egly,C., Chambon,P., Voorhees,J.J. and Xiao,J.H. (1997) Suprabasal expression of a dominant-negative RXRα mutant in transgenic mouse epidermis impairs regulation of gene transcription and basal keratinocyte proliferation by RAR-selective retinoids. Genes Dev., 11, 59–71. [DOI] [PubMed] [Google Scholar]
- Fisher G.J. and Voorhees,J.J. (1996) Molecular mechanisms of retinoid actions in skin. FASEB J., 10, 1002–1013. [DOI] [PubMed] [Google Scholar]
- Fisher G.J., Talwar,H.S., Xiao,J.H., Datta,S.C., Reddy,A.P., Gaub,M.P., Rochette-Egly,C., Chambon,P. and Voorhees,J.J. (1994) Immuno logical identification and functional quantitation of retinoic acid and retinoid X receptor proteins in human skin. J. Biol. Chem., 269, 20629–20635. [PubMed] [Google Scholar]
- Frazier C.N. and Hu,C.K. (1931) Cutaneous lesions associated with a deficiency in vitamin A in man. Arch. Intern. Med., 48, 507–514. [Google Scholar]
- Ghyselinck N.B., Dupé,V., Dierich,A., Messaddeq,N., Garnier,J.M., Rochette-Egly,C., Chambon,P. and Mark,M. (1997) Role of the retinoic acid receptor beta (RARβ) during mouse development. Int. J. Dev. Biol., 41, 425–447. [PubMed] [Google Scholar]
- Goyette P., Feng-Chen,C., Wang,W., Seguin,F. and Lohnes,D. (2000) Characterization of retinoic acid receptor-deficient keratinocytes. J. Biol. Chem., 275, 16497–16505. [DOI] [PubMed] [Google Scholar]
- Hunt T.K. (1986) Vitamin A and wound healing. J. Am. Acad. Dermatol., 15, 817–821. [DOI] [PubMed] [Google Scholar]
- Imakado S. et al. (1995) Targeting expression of a dominant-negative retinoic acid receptor mutant in the epidermis of transgenic mice results in loss of barrier function. Genes Dev., 9, 317–329. [DOI] [PubMed] [Google Scholar]
- Indra A.K., Warot,X., Brocard,J., Bornert,J.M., Xiao,J.H., Chambon,P. and Metzger,D. (1999) Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ERT and Cre-ERT2 recombinases. Nucleic Acids Res., 27, 4324–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M., Kari,C. and Rodeck,U. (2000) The EGF-receptor—an essential regulator of multiple epidermal functions. Eur. J. Dermatol., 10, 505–510. [PubMed] [Google Scholar]
- Kastner P., Mark,M. and Chambon,P. (1995) Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell, 83, 859–869. [DOI] [PubMed] [Google Scholar]
- Kastner P. et al. (1996) Abnormal spermatogenesis in RXR β mutant mice. Genes Dev., 10, 80–92. [DOI] [PubMed] [Google Scholar]
- Kastner P., Mark,M., Ghyselinck,N., Krezel,W., Dupé,V., Grondona, J.M. and Chambon,P. (1997) Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development, 124, 313–326. [DOI] [PubMed] [Google Scholar]
- Li M., Indra,A.K., Warot,X., Brocard,J., Messaddeq,N., Kato,S., Metzger,D. and Chambon,P. (2000) Skin abnormalities generated by temporally controlled RXRα mutations in mouse epidermis. Nature, 407, 633–636. [DOI] [PubMed] [Google Scholar]
- Li M., Chiba,H., Warot,X., Messaddeq,N., Gérard,C., Chambon,P. and Metzger,D. (2001) RXRα ablation in skin keratinocytes results in alopecia and epidermal alterations. Development, 128, 675–688. [DOI] [PubMed] [Google Scholar]
- Livrea M.A. (2000) Vitamin A and Retinoids: An Update of Biological Aspects and Clinical Applications. Birkhauser Verlag, Basel, Switzerland.
- Lohnes D., Kastner,P., Dierich,A., Mark,M., LeMeur,M. and Chambon,P. (1993) Function of retinoic acid receptor γ in the mouse. Cell, 73, 643–658. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Lohnes,D., Mark,M., Dierich,A., Gorry,P., Gaub,M.P., LeMeur,M. and Chambon,P. (1993) High postnatal lethality and testis degeneration in retinoic acid receptor α mutant mice. Proc. Natl Acad. Sci. USA, 90, 7225–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie I.C. and Gao,Z. (2001) Keratinocyte growth factor expression in human gingival fibroblasts and stimulation of in vitro gene expression by retinoic acid. J. Periodontol., 72, 445–453. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D.J. et al. (1995) The nuclear receptor superfamily: the second decade. Cell, 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikovsky M., Breuing,K., Liu,P.Y., Eriksson,E., Higashiyama,S., Farber,P., Abraham,J. and Klagsbrun,M. (1993) Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc. Natl Acad. Sci. USA, 90, 3889–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascrez B., Mark,M., Dierich,A., Ghyselinck,N.B., Kastner,P. and Chambon,P. (1998) The RXRα ligand-dependent activation function 2 (AF-2) is important for mouse development. Development, 125, 4691–4707. [DOI] [PubMed] [Google Scholar]
- McCarthy D.W., Downing,M.T., Brigstock,D.R., Luquette,M.H., Brown,K.D., Abad,M.S. and Besner,G.E. (1996) Production of heparin-binding epidermal growth factor-like growth factor (HB-EGF) at sites of thermal injury in pediatric patients. J. Invest. Dermatol., 106, 49–56 [DOI] [PubMed] [Google Scholar]
- Metzger D. and Chambon,P. (2001) Site- and time-specific gene targeting in the mouse. Methods, 24, 71–80. [DOI] [PubMed] [Google Scholar]
- Michalik L. et al. (2001) Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)α and PPARβ mutant mice. J. Cell Biol., 154, 799–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss-Kay G.M. and Ward,S.J. (1999) Retinoids and mammalian development. Int. Rev. Cytol., 188, 73–131. [DOI] [PubMed] [Google Scholar]
- Niederreither K., Fraulob,V., Garnier,J.M., Chambon,P. and Dollé,P. (2002) Differential expression of retinoic-acid-synthesizing (RALDH) enzymes during fetal development and organ differentiation in the mouse. Mech. Dev., 110, 165–171. [DOI] [PubMed] [Google Scholar]
- Peters J.M., Lee,S.S., Li,W., Ward,J.M., Gavrilova,O., Everett,C., Reitman,M.L., Hudson,L.D. and Gonzalez,F.J. (2000) Growth, adipose, brain and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor β(δ). Mol. Cell. Biol., 20, 5119–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepkorn M., Pittelkow,M.R. and Cook,P.W. (1998) Autocrine regulation of keratinocytes: the emerging role of heparin-binding, epidermal growth factor-related growth factors. J. Invest. Dermatol., 111, 715–721. [DOI] [PubMed] [Google Scholar]
- Redfern C.P.F. and Todd,C. (1992) Retinoic acid receptor expression in human skin keratinocytes and dermal fibroblast in vitro. J. Cell Sci., 102, 113–121. [DOI] [PubMed] [Google Scholar]
- Roy B., Taneja,R. and Chambon,P. (1995) Synergistic activation of retinoic acid (RA)-responsive genes and induction of embryonal carcinoma cell differentiation by an RA receptor alpha (RARα)-, RARβ-, or RARγ-selective ligand in combination with a retinoid X receptor-specific ligand. Mol. Cell. Biol., 15, 6481–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M., Sugai,S., Tanaka,T., Shimouchi,K., Fuchs,E., Narumiya,S. and Kakizuka,A. (1995) Inhibition of skin development by targeted expression of a dominant negative retinoic acid receptor. Nature, 374, 159–162. [DOI] [PubMed] [Google Scholar]
- Stoll S.W. and Elder,J.T. (1998) Retinoid regulation of heparin-binding EGF-like growth factor gene expression in human keratinocytes and skin. Exp. Dermatol., 7, 391–397. [DOI] [PubMed] [Google Scholar]
- Taneja R., Roy,B., Plassat,J.L., Zusi,C.F., Ostrowski,J., Reczek,P.R. and Chambon,P. (1996) Cell-type and promoter-context dependent retinoic acid receptor (RAR) redundancies for RAR β2 and Hoxa-1 activation in F9 and P19 cells can be artefactually generated by gene knockouts. Proc. Natl Acad. Sci. USA, 93, 6197–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacher S.M., Standeven,A.M., Athanikar,J., Kopper,S., Castilleja,O., Escobar,M., Beard,R.L. and Chandraratna,R.A. (1997) Receptor specificity of retinoid-induced epidermal hyperplasia: effect of RXR-selective agonists and correlation with topical irritation. J. Pharmacol. Exp. Ther., 282, 528–534. [PubMed] [Google Scholar]
- Tsou H.C., Lee,X., Si,S.P. and Peacocke,M. (1994) Regulation of retinoic acid receptor expression in dermal fibroblasts. Exp. Cell Res., 211, 74–81. [DOI] [PubMed] [Google Scholar]
- Vahlquist A. (1982) Vitamin A in human skin: I. detection and identification of retinoids in normal epidermis. J. Invest. Dermatol., 79, 89–93. [DOI] [PubMed] [Google Scholar]
- Wendling O., Ghyselinck,N.B., Chambon,P. and Mark,M. (2001) Roles of retinoic acid receptors in early embryonic morphogenesis and hindbrain patterning. Development, 128, 2031–2038. [DOI] [PubMed] [Google Scholar]
- Werner S. and Smola,H. (2001) Paracrine regulation of keratinocyte proliferation and differentiation. Trends Cell Biol., 11, 143–146. [DOI] [PubMed] [Google Scholar]
- Wolbach S.B. and Howe,P.R. (1925) Tissue changes following deprivation of fat-soluble A vitamin. J. Exp. Med., 42, 753–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J.H., Feng,X., Di,W., Peng,Z.H., Li,L.A., Chambon,P. and Voorhees,J.J. (1999) Identification of heparin-binding EGF-like growth factor as a target in intercellular regulation of epidermal basal cell growth by suprabasal retinoic acid receptors. EMBO J., 18, 1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]