Abstract
In Xenopus embryos, cell cycle elongation and degradation of Cdc25A (a Cdk2 Tyr15 phosphatase) occur naturally at the midblastula transition (MBT), at which time a physiological DNA replication checkpoint is thought to be activated by the exponentially increased nucleo-cytoplasmic ratio. Here we show that the checkpoint kinase Chk1, but not Cds1 (Chk2), is activated transiently at the MBT in a maternal/zygotic gene product-regulated manner and is essential for cell cycle elongation and Cdc25A degradation at this transition. A constitutively active form of Chk1 can phosphorylate Cdc25A in vitro and can target it rapidly for degradation in pre-MBT embryos. Intriguingly, for this degradation, however, Cdc25A also requires a prior Chk1-independent phosphorylation at Ser73. Ectopically expressed human Cdc25A can be degraded in the same way as Xenopus Cdc25A. Finally, Cdc25A degradation at the MBT is a prerequisite for cell viability at later stages. Thus, the physiological replication checkpoint is activated transiently at the MBT by developmental cues, and activated Chk1, only together with an unknown kinase, targets Cdc25A for degradation to ensure later development.
Keywords: Cdc25A/Chk1/midblastula transition/replication checkpoint/Xenopus
Introduction
In eukaryotic cells, cell cycle checkpoints responding to damaged or unreplicated DNA elicit signalling pathways that ultimately inhibit cyclin-dependent kinases, thereby providing time for repair or replication (for reviews see Weinert, 1998; Zhou and Elledge, 2000). In vertebrates, upstream elements of the signalling pathways include the phosphatidylinositol 3-kinase family member ATM and its related kinase ATR (for a review see Abraham, 2000). The checkpoint kinases Chk1 and Cds1 (or Chk2) act downstream of ATR and ATM, respectively, and directly target regulators of the cell cycle (see Abraham, 2000; Walworth, 2000). Typically, at the G2 DNA damage/replication checkpoint, Chk1 and Cds1 phosphorylate and inhibit Cdc25C, a Tyr15 phosphatase of the cyclin B-dependent kinase Cdc2, thereby arresting the cell cycle at the G2 phase (see Zhou and Elledge, 2000; Walworth, 2001). More recently, it has also been shown that in response to DNA damage induced by γ radiation during S phase, human Cds1 phosphorylates Cdc25A, a cyclin E–Cdk2 Tyr15 phosphatase needed for S phase (Hoffmann et al., 1994; Draetta and Eckstein, 1997), and targets it for degradation, thereby enforcing the S phase checkpoint (Falck et al., 2001). UV radiation (Mailand et al., 2000) or replication blocks (Molinari et al., 2000) can also induce the degradation of human Cdc25A, although in neither case is it known whether Cds1 or Chk1 directly phosphorylates the phosphatase and targets it for degradation.
Cell cycle checkpoints have generally been defined as non-essential pathways that delay the cell cycle in response to environmentally induced defects in the DNA (see Weinert, 1998). Indeed, in fission yeast, both Rad3 (a homologue of ATR) and Chk1 are non-essential under normal conditions (see Zhou and Elledge, 2000). Interestingly, however, recent genetic studies in mice show that ATR and Chk1 are both essential for normal cell proliferation at the blastocyst stage (Brown and Baltimore, 2000; Liu et al., 2000; Takai et al., 2000). Moreover, in Drosophila, both Mei-41 (a homologue of ATR) and Grapes (a homologue of Chk1) are essential for normal cell cycle progression at the midblastula stage (Fogarty et al., 1997; Sibon et al., 1997, 1999). These results would suggest either that the metazoan ATR–Chk1 checkpoint pathway is induced specifically in early embryos by some developmental cue(s) (Sibon et al., 1999; Walworth, 2000) or that the pathway functions constitutively throughout development except for the earliest stages (Zhou and Elledge, 2000; Canman, 2001). This important issue remains unsolved, probably because of the technical problems (in both Drosophila and mice) in obtaining a sufficient number of well-synchronized embryos (for biochemical analysis) as well as in measuring the activity of ATR or Chk1 protein (see Abraham, 2000; Kumagai and Dunphy, 2000). In addition, probably for the same reasons, the direct physiological targets of Chk1 are not known in Drosophila or mouse embryos.
The amphibian Xenopus is an excellent model system to analyse cell cycle regulation biochemically in early development. In this species, as in many other species including Drosophila, the cell cycles after fertilization are very rapid, consisting mostly of S and M phases, and are elongated rather abruptly at the midblastula transition (MBT) by the appearance of the G2 (and G1) phase and the lengthening of S phase (Newport and Kirschner, 1982; Howe et al., 1995). This cell cycle elongation at the MBT, which is accompanied by inhibitory Tyr15 phosphorylation of Cdc2 (Hartley et al., 1996) and initiation of zygotic transcription (Newport and Kirschner, 1982), is most probably triggered by the exponentially increased nucleo-cytoplasmic ratio (Newport and Kirschner, 1982), which has long been thought to titrate or deplete some maternal (replication) factor(s) (Newport and Dasso, 1989) and, thereby, to activate a physiological DNA replication checkpoint (Dasso and Newport, 1990). Moreover, and intriguingly, Xenopus Cdc25A protein, which is synthesized after fertilization and contributes to the rapid pre-MBT cell cycles, is vastly degraded at the MBT (Kim et al., 1999), reminiscent of the degradation of human Cdc25A in response to the environmentally induced DNA damage/replication checkpoint (see above).
By manipulating Chk1 or Cds1 activity in Xenopus embryos, here we addressed whether the physiological replication checkpoint is truly activated at the MBT and, if so, how the checkpoint is regulated at this transition, and whether such a checkpoint is involved in the degradation of Cdc25A at the MBT. Our results show that physiological replication checkpoint signalling is induced transiently at the MBT in a maternal/zygotic gene product-regulated manner and involves ATR and Chk1 (but not Cds1), and that Cdc25A degradation is an integral component of the ATR–Chk1 replication checkpoint pathway at the MBT and involves another unknown kinase. On the basis of the present as well as previous results, we suggest that the physiological replication checkpoint in early embryos may be activated by the DNA structures that are generated when maternal (replication) factors are depleted to be replaced by zygotic factors. We also discuss the possibility that the unknown kinase (which phosphorylates Cdc25A at Ser73) might have a more general role in cell cycle progression and checkpoints.
Results
Requirement for Chk1, but not Cds1, in cell cycle elongation at the MBT
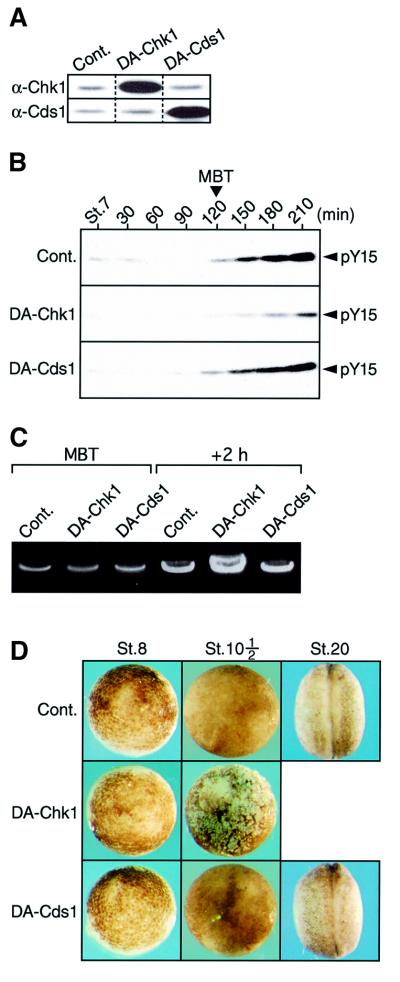
In Xenopus, the MBT occurs after 12 rapid and synchronous divisions or ∼6 h after fertilization (Newport and Kirschner, 1982), and the checkpoint kinases Chk1 and Cds1 are both stably present throughout early embryogenesis (Nakajo et al., 1999; Gotoh et al., 2001; see also Figure 2A). First, we examined whether cell cycle elongation (or the physiological replication checkpoint) at the MBT would require the function of endogenous Chk1 or Cds1. For this, we microinjected fertilized eggs with mRNA encoding either kinase-dead Xenopus Chk1 (DA-Chk1) or Cds1 (DA-Cds1); kinase-dead Chk1 or Cds1 proteins can serve as dominant-negative mutants (Nakajo et al., 1999; Chehab et al., 2000; see also Supplementary figure 1 available at The EMBO Journal Online). Both DA-Chk1 and DA-Cds1 proteins were expressed 25- to 30-fold over endogenous levels at the MBT (or at the Nieuwkoop–Faber stage 8½) (Figure 1A), with no appreciable effect on the rapid pre-MBT cell divisions (see Figure 1D). Under these conditions, inhibitory Tyr15 phosphorylation of Cdc2, an indicator of cell cycle elongation (Okamoto et al., 2002) or of presumptive checkpoint activation (Kappas et al., 2000), occurred normally at the MBT in DA-Cds1-expressing embryos as well as in control GST-expressing embryos, but, notably, was significantly (∼1 h) retarded in DA-Chk1-expressing embryos (Figure 1B). Tyr15 phosphorylation of Cdk2 (a primary substrate of Cdc25A) was also strongly inhibited at the MBT by overexpression of DA-Chk1 but not DA-Cds1 (not shown). Consistent with these results, 2 h after the MBT, the DNA content per embryo was considerably (∼3-fold) higher in DA-Chk1-expressing embryos than in control and DA-Cds1-expressing embryos (Figure 1C). Thus, these results would indicate that the Chk1-inhibited embryos performed one or two more rounds of the rapid (∼30 min) cell cycle even after the MBT. Eventually, while control and Cds1-inhibited embryos developed essentially normally at least until the late neurula stage, Chk1-inhibited embryos invariably died at the early gastrula stage (stage 10½) with a dramatic disruption of intercellular contacts (characteristic of embryonic apoptosis; Anderson et al., 1997) (Figure 1D). We obtained essentially similar results even with 10-fold overexpression of DA-Chk1 or with injection of neutralizing anti-Xenopus Chk1 antibody (200 ng/embryo; Nakajo et al., 1999) (our unpublished data). Thus, most certainly, Chk1 but not Cds1 was essential for cell cycle elongation at the MBT and for cell viability shortly after the MBT. These results suggest strongly that the physiological DNA replication checkpoint occurs at the MBT and involves Chk1 but not Cds1 (see also below).
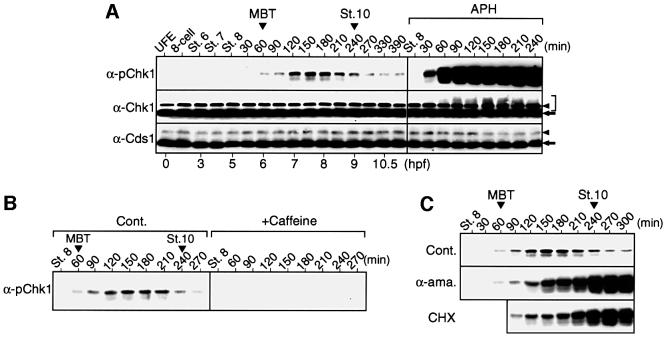
Fig. 2. Activating phosphorylation of Chk1 and its regulation during early embryogenesis. (A) Normally developing embryos at the indicated stages or times (in minutes after stage 8) and embryos treated with aphidicolin (APH) from stage 8 were analysed by immunoblotting using anti-human Chk1 phospho-Ser345 antibody (α-pChk1, which can recognize ATR-phosphorylated Ser342 of Xenopus Chk1; see Materials and methods), anti-Xenopus Chk1 antibody (α-Chk1) or anti-Xenopus Cds1 antibody (α-Cds1). Hours post-fertilization (hpf) are shown at the bottom. Authentic non-phosphorylated Chk1 or Cds1 protein, background protein and size-shifted Chk1 protein are indicated by the arrow, arrowhead and bracket, respectively. (B) Stage 7½ embryos were mock treated (Cont.) or treated with caffeine and analysed by immunoblotting using α-pChk1 at the indicated stage or times (in minutes). (C) Fertilized eggs were left uninjected (Cont.) or injected with α-amanitin (α-ama.), and embryos were treated with cycloheximide (CHX) 30 min after the MBT; they were analysed by immunoblotting using α-pChk1 at the indicated stages or times (in minutes after stage 8).
Fig. 1. Requirement for Chk1, but not Cds1, in cell cycle elongation at the MBT. (A) One-cell embryos injected with 15 ng of mRNA encoding either GST (Cont.), DA-Chk1 or DA-Cds1 were cultured and, at the MBT, were analysed by immunoblotting using anti-Chk1 (α-Chk1) or anti-Cds1 (α-Cds1) antibodies. (B–D) Embryos injected with mRNAs as in (A) were analysed for Cdc2 Tyr15 phosphorylation (pY15) at 30 min intervals after Nieuwkoop–Faber stage 7 (St.7; early blastula) (B), for the DNA content at the MBT and 2 h after the MBT (see Materials and methods) (C), and for the external morphology at the indicated stages (D).
Transient activation of Chk1 at the MBT
Because Chk1 apparently played an essential role at the MBT, we asked whether it underwent any regulation at that time. To this end, we subjected one-cell to midgastrula stage embryos to immunoblot analysis by using an antibody that can specifically recognize ATR-mediated (and hence activating) phosphorylation of Chk1 (see Guo et al., 2000; Liu et al., 2000). (For the characterization of this antibody, see Materials and methods.) Interestingly, Chk1 underwent the diagnostic activating phosphorylation principally in embryos from the MBT to the initial gastrula stage (stage 10) (or for ∼3 h, during the first two post-MBT cell cycles; Howe et al., 1995), with a peak modification at the late blastula stage (Figure 2A, top left). As expected, this modification was considerably weaker than that in embryos treated with the potent replication checkpoint inducer aphidicolin (Figure 2A, top right), and was abolished by pre-treatment of the embryos with the ATR inhibitor caffeine (Figure 2B). Probably because of its weak (though significant) activation, the total Chk1 protein did not show visible upward size shifts (indicative of activation; Kumagai and Dunphy, 2000) at the MBT or during the following short period, although it did show (smeared) size shifts in aphidicolin-treated embryos (Figure 2A, middle). In contrast, Cds1 did not show any appreciable size shifts (associated with activation; Guo and Dunphy, 2000) either in normal embryos or in aphidicolin-treated embryos (Figure 2A, bottom), consistent with it playing no essential role at the MBT (Figure 1) or at the aphidicolin-induced replication checkpoint (Guo and Dunphy, 2000). Thus, these results indicate that Chk1, but not Cds1, undergoes ATR-mediated, weak and transient activation at the MBT and during the following short period.
The period of Chk1 modification observed during normal development, or that from the MBT to stage 10 (Figure 2A, top), corresponded to the period that should be called the maternal/zygotic transition (MZT) (Edgar, 1995; Howe et al., 1995), during which maternal (replication) factors are thought to be depleted and replaced by new zygotic factors (Dasso and Newport, 1990; Sible et al., 1998). We therefore asked whether new zygotic gene products played a role in the regulation of Chk1 modification during the MZT. For this, we specifically inhibited either new zygotic transcription (by α-amanitin) or protein synthesis (by cycloheximide) after the MBT. Both types of inhibition had no effect on the initial Chk1 modification at the MBT but did cause rather a progressive and strong Chk1 modification after the late blastula stage (Figure 2C, middle and bottom). Thus, apparently, the attenuation of Chk1 modification that normally occurred after the late blastula stage (Figure 2A and C, top) was dependent on new zygotic gene products. This intriguing result could imply that new zygotic gene products replace maternal products (whose depletion at the MBT potentially activates the replication checkpoint; Dasso and Newport, 1990), thereby attenuating the checkpoint and hence the Chk1 modification (see Discussion). At any rate, these results show that Chk1 regulation during the MZT involves new zygotic gene products.
Requirement for Chk1 in Cdc25A degradation at the MBT
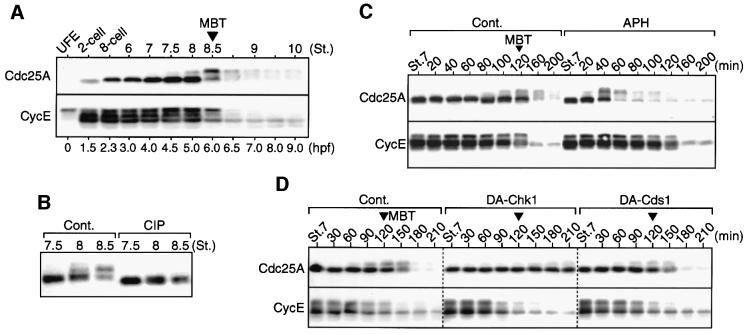
Having determined that Chk1 is most probably involved in the physiological DNA replication checkpoint at the MBT, we next asked whether Cdc25A protein underwent any checkpoint-dependent regulation at the MBT. Xenopus Cdc25A protein is synthesized only after fertilization, accumulates during cleavage and is degraded at the MBT (Kim et al., 1999); interestingly, cyclin E, a partner of Cdk2 (Hoffmann et al., 1994), is also degraded largely at the MBT (Howe and Newport, 1996). Initially, we found that Cdc25A, but not cyclin E, undergoes prominent upward size shifts just before its degradation (Figure 3A), or at the time when Chk1 modification is initiated (see Figure 2A). These size shifts were sensitive to treatment of the protein with alkaline phosphatase, implying that they were due to phosphorylation (Figure 3B). To assess whether the degradation of Cdc25A or cyclin E at the MBT was dependent on the activation of the replication checkpoint or Chk1, first we treated early blastula embryos with aphidicolin (as we did earlier to activate endogenous Chk1 prematurely; Figure 2A). This treatment did induce very rapid size shifts and degradation of Cdc25A (very similar to those at the MBT), but not of cyclin E, in the pre-MBT embryos (Figure 3C), indicating that Cdc25A degradation can be induced by the replication checkpoint. We then inhibited the function of endogenous Chk1 (and also Cds1) at the MBT by overexpressing dominant-negative mutants in the manner described earlier (see Figure 1). Interestingly, degradation as well as size shifts of Cdc25A were strongly retarded in Chk1- but not Cds1-inhibited embryos, whereas cyclin E degradation occurred normally in both types of embryo (Figure 3D). Degradation of Cdc25A (Figure 3C) as well as Tyr15 phosphorylation of Cdc2 (see Kappas et al., 2000) in response to aphidicolin was also prevented strongly by overexpression of the dominant-negative Chk1 mutant (see Supplementary figure 2). Thus, these results strongly suggest that Cdc25A degradation (as well as cell cycle elongation) at the MBT is dependent on the physiological replication checkpoint or Chk1 activity, while cyclin E degradation is not (consistent with it occurring by a timing mechanism; Howe and Newport, 1996).
Fig. 3. Requirement for Chk1 in Cdc25A degradation at the MBT. (A) Unfertilized eggs (UFE) and embryos at the indicated stages were analysed by immunoblotting using anti-Cdc25A or anti-cyclin E antibodies. (B) Extracts from the embryos at the indicated stages were mock treated (Cont.) or treated with calf intestine alkaline phosphatase (CIP) and analysed for Cdc25A as in (A). (C) Stage 7 blastula embryos were mock treated (Cont.) or treated with aphidicolin (APH) and, at the indicated times (in minutes), were analysed for Cdc25A or cyclin E. (D) Embryos overexpressing either GST (Cont.), DA-Chk1 or DA-Cds1 as in Figure 1A were analysed for Cdc25A or cyclin E at 30 min intervals after stage 7.
Rapid induction of phosphorylation and degradation of Cdc25A by activated Chk1
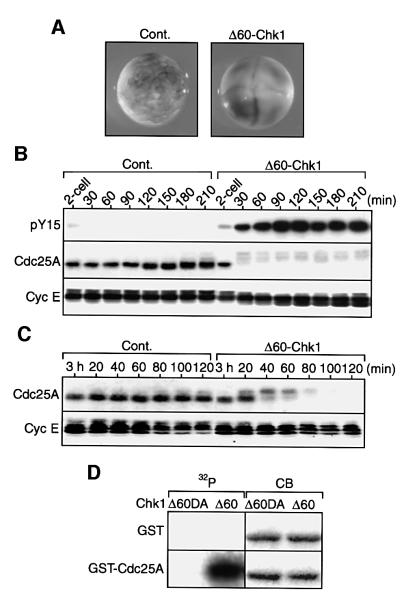
Because Chk1 apparently was activated and required for Cdc25A degradation at the MBT, we addressed whether (activated) Chk1 could directly induce Cdc25A degradation at that time. To assess this, first we injected two-cell embryos with mRNA encoding a C-terminal 60 amino acid-deleted, constitutively active form of Xenopus Chk1 (Δ60-Chk1; Oe et al., 2001). These embryos, but not control embryos (expressing GST), ceased to divide at the four-cell stage (Figure 4A) with high Tyr15 phosphorylation of Cdc2 (Figure 4B, top), indicating that activated Chk1 could induce MBT-like events prematurely. (Wild-type Chk1 also induced cleavage arrest and Cdc2 Tyr15 phosphorylation, but much less efficiently than Δ60-Chk1; not shown, but see Kappas et al., 2000.) Interestingly, Cdc25A levels, although they increased continuously in control embryos, did remain low (with prominent upward size shifts or phosphorylation) in Δ60-Chk1-expressing embryos (Figure 4B, middle); cyclin E levels, however, were not affected (Figure 4B, bottom). Thus, Chk1 either inhibited the synthesis of Cdc25A or targeted it for degradation (probably via phosphorylation) during its continuous synthesis. To distinguish between these two possibilities, we next injected Δ60-Chk1 mRNA into (unfertilized) eggs that had been artificially activated with calcium ionophore and then cultured for 3 h. In these activated eggs (undergoing essentially normal DNA replication but not cytokinesis), Cdc25A was present at considerable levels (as in normal early blastula embryos; Figure 3A) (Figure 4C, left) and the injected mRNA was distributed successfully throughout the egg cytoplasm. The results showed that Cdc25A, but not cyclin E, was shifted in size very rapidly and degraded only after expression of Δ60-Chk1 (Figure 4C, right). Thus, (activated) Chk1 specifically could induce the degradation (as well as phosphorylation) of Cdc25A in pre-MBT embryos (just as aphidicolin treatment could; Figure 3C), suggesting that Chk1 directly induces the degradation of Cdc25A at the MBT.
Fig. 4. Rapid induction of Cdc25A degradation and in vitro phosphorylation of Cdc25A by Chk1. (A) Both blastomeres of a two-cell embryo were injected with 2 ng of mRNA encoding either GST (Cont.) or Δ60-Chk1, cultured for 3 h and photographed. (B) Two-cell embryos injected with mRNAs as in (A) were cultured, collected at 30 min intervals and analysed for Cdc2 Tyr15 phosphorylation (pY15), Cdc25A or cyclin E. (C) Unfertilized eggs that had been activated with calcium ionophore (A23187) and cultured for 3 h were injected with 4 ng of mRNA encoding either GST (Cont.) or Δ60-Chk1, collected at 20 min intervals and analysed for Cdc25A or cyclin E. (D) GST protein or GST–Cdc25A fusion protein, 2 µg of each, was incubated with [γ-32P]ATP and either Δ60-Chk1 (Δ60) or kinase-dead Δ60-Chk1DA (Δ60DA) protein (see Materials and methods), subjected to SDS–PAGE, stained with Coomassie Blue (CB) and then autoradiographed (32P).
The above results suggest that Chk1 might directly phosphorylate Cdc25A (and then target it for degradation). To test this possibility, we incubated bacterially pro duced GST–Cdc25A fusion protein with [γ-32P]ATP and Δ60-Chk1 protein (which was immunoprecipitated from activated eggs). Δ60-Chk1, but not its kinase-dead version (Δ60-Chk1DA), strongly phosphorylated GST–Cdc25A, but not GST alone (Figure 4D, left). Moreover, as compared with the bulk of non-phosphorylated GST– Cdc25A (stained with Coomassie Blue; Figure 4D, right), the Δ60-Chk1-phosphorylated GST–Cdc25A showed clear size shifts, similar to endogenous Cdc25A in Δ60-Chk1-expressing eggs. Thus, it seems very likely that Chk1 directly phosphorylates Cdc25A (to target it for degradation), although yet another kinase could also contribute to this phosphorylation.
Chk1-independent and -dependent phosphorylation sites required for Cdc25A degradation
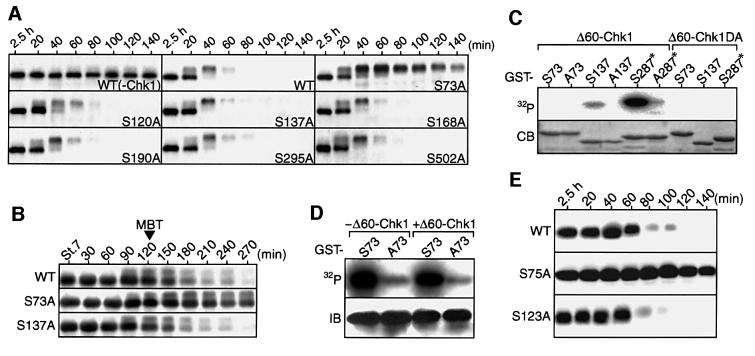
We intended to identify the possible Chk1 phosphorylation site(s) in Cdc25A that would be required for Cdc25A degradation. Xenopus Cdc25A has seven serine residues that lie in the consensus Chk1 phosphorylation motif (Arg-X-X-Ser; Chen et al., 2000) and are conserved in human Cdc25A (Okazaki et al., 1996). We therefore constructed seven Cdc25A mutants, each with a single Ser→Ala substitution, expressed them (by injection of mRNA) in activated eggs, and then tested their stability after expression of Δ60-Chk1 as described above (see Figure 4C). In the absence of Δ60-Chk1 expression, all the mutants as well as wild-type Cdc25A were very stable in activated eggs (not shown, but see Figure 5A for wild-type Cdc25A). After Δ60-Chk1 expression, however, most of the mutants (S120A, S137A, S168A, S190A, S295A and S502A) were rapidly degraded similarly to wild-type Cdc25A, but, intriguingly, one mutant (S73A) was very stable (although shifted in size probably due to phosphorylation at yet other sites) (Figure 5A). Importantly, when expressed in normally fertilized eggs, S73A Cdc25A was also much more stable than wild-type Cdc25A (and other Cdc25A mutants) at the MBT (Figure 5B). Thus, seemingly, among the seven candidate serine residues, only Ser73 was essential for the Chk1-induced degradation of Cdc25A.
Fig. 5. Identification of a phosphorylaion site that is required for Cdc25A degradation. (A) Activated eggs were injected with 1.5 ng of mRNA encoding either Myc-tagged wild-type Cdc25A (WT) or each of the Myc-tagged Ser→Ala mutants (SAs), reinjected 2.5 h later with 3 ng of Δ60-Chk1 mRNA, and analysed by immunoblotting using anti-Myc antibody at 20 min intervals. For WT Cdc25A, eggs expressing no Δ60-Chk1 (–Chk1) were also analysed. (B) Fertilized eggs were injected with 1 ng of mRNA encoding (Myc-tagged) wild-type (WT), S73A or S137A Cdc25A and, at 30 min intervals after stage 7, were analysed as in (A). (C) GST–Cdc25A peptide fusion proteins (GST–S73, –A73, –S137 or –A137; see text) or GST–Cdc25C peptide fusion proteins (GST–S287* or –A287*), 2 µg of each, were incubated with [γ-32P]ATP and either Δ60-Chk1 or kinase-dead Δ60-Chk1DA protein, subjected to SDS–PAGE, stained with Coomassie Blue (CB) and then autoradiographed (32P). (D) Activated eggs were injected (+) or not (–) with 3 ng of Δ60-Chk1 mRNA and 1.5 h later reinjected with 20 ng of GST–S73 or GST–A73 proteins with or without [γ-32P]ATP. One hour later, the GST fusion proteins were immunoprecipitated with polyclonal anti-GST antibody and then subjected to SDS–PAGE for either autoradiography (32P) or immunoblotting with monoclonal anti-GST antibody (IB). (E) Activated eggs were injected with 1.5 ng of mRNA encoding either (Myc-tagged) wild-type human Cdc25A (WT) or its Ser→Ala mutants (S75A or S123A), reinjected 3 h later with 2 ng of Δ60-Chk1 mRNA, and analysed as in (A).
We then tested whether Ser73 could be phosphorylated directly by Chk1. For this, we subjected either Ser73-containing GST–Cdc25A peptide (residues 44–103) fusion protein (GST–S73) or its alanine-substituted version (GST–A73) to in vitro Chk1 kinase assays as described above (see Figure 4D). As a positive control for Chk1 substrate, we used GST–Xenopus Cdc25C peptide (residues 254–316) fusion protein (GST–S287*) in which Ser287 is known to be a Chk1 phosphorylation site (Kumagai et al., 1998). As expected, GST–S287*, but not its alanine-substituted version (GST–A287*), was strongly phosphorylated by Δ60-Chk1 (Figure 5C). Very unexpectedly, however, neither GST–S73 nor GST–A73 was phosphorylated appreciably by Δ60-Chk1, suggesting that Ser73 was not a Chk1 phosphorylation site. This raised the important question of whether Ser73 underwent any phosphorylation in vivo. To answer this question, we injected either GST–S73 or GST–A73 protein (together with [γ-32P]ATP) into activated eggs. Strikingly, GST–S73 but not GST–A73 was phosphorylated very strongly in activated eggs (Figure 5D, left), and this phosphorylation was not increased further by the presence of Δ60-Chk1 (Figure 5D, right). (GST–S73, but not GST–A73, was also strongly phosphorylated in pre-MBT embryos; data not shown.) Thus, somewhat surprisingly, but intriguingly, Ser73 was phosphorylated most probably by a kinase(s) distinct from Chk1 in eggs or embryos (well before the MBT), this prior phosphorylation apparently being essential for the Chk1-induced degradation of Cdc25A (Figure 5A and B).
A recent study shows that human Cds1 phosphorylates (human) Cdc25A at Ser123 and thereby targets it for degradation in response to DNA damage (Falck et al., 2001). Interestingly however, as shown above (Figure 5A and B), Ser137, which corresponded to Ser123 of human Cdc25A, was not essential for Cdc25A degradation in Δ60-Chk1-expressing eggs or at the MBT, although it was phosphorylated appreciably by Chk1 in vitro (Figure 5C). Therefore, we also tested whether human Cdc25A could be degraded in Δ60-Chk1-expressing eggs and, if so, whether its Ser123 (a Cds1 phosphorylation site) or Ser75 (a residue corresponding to Ser73 of Xenopus Cdc25A) was required for that degradation. Very similarly to Xenopus Cdc25A, human Cdc25A was degraded quite efficiently by Δ60-Chk1 expression and, for this degradation, Ser123 was not essential, while, notably, Ser75 was (Figure 5E). Thus, although formally Chk1 could modulate some other factor that regulates Cdc25A stability, these results, together with the earlier results (Figures 3D and 4B–D), would suggest that Chk1 phosphorylates Cdc25A for degradation at some other site(s) besides the Cds1 phosphorylation site (or Ser137) (see Discussion).
Taken together, the present results suggest that Cdc25A initially (or probably constitutively) is phosphorylated at Ser73 by an unknown kinase(s), then phosphorylated at some specific site(s) by Chk1 at the MBT, and then rapidly degraded. Moreover, they suggest that the mechanisms for targeting Cdc25A for degradation at the DNA replication checkpoint are well conserved in vertebrates.
Requirement of Cdc25A degradation for cell viability in later development
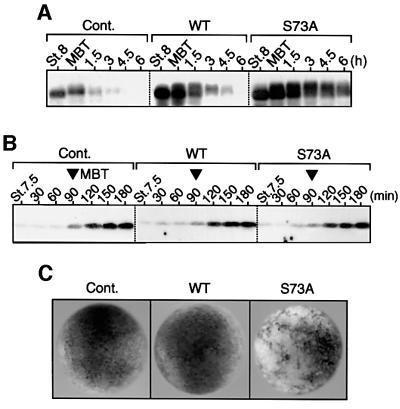
Finally, we addressed the important question of whether Cdc25A degradation at the MBT would be required for cell cycle elongation or cell viability at or after the MBT. For this, we expressed either wild-type Cdc25A or degradation-resistant S73A Cdc25A (∼3-fold over endogenous levels) ectopically in blastula embryos. In these embryos, not only S73A Cdc25A but also wild-type Cdc25A was present at higher levels than endogenous Cdc25A (at the MBT) even 2 h after the MBT (Figure 6A), but neither Cdc25A had an appreciable effect on the timing of Tyr15 phosphorylation of Cdc2 at the MBT (Figure 6B). This unexpected result was presumably not due to inactivation of (overexpressed) Cdc25A at the MBT since, in human cells, the replication checkpoint does not seem to inhibit Cdc25A phosphatase activity itself (see Molinari et al., 2000). Therefore, most probably (endogenous) Cdc25A degradation per se was not essential for cell cycle elongation at the MBT. (For the probable reasons for this, see Discussion.) Notably, however, while the embryos expressing wild-type Cdc25A developed essentially normally, those embryos expressing S73A Cdc25A died at the late gastrula stage with apoptotic phenotypes (Figure 6C). At the midgastrula stage (or 6 h after the MBT), S73A Cdc25A but not wild-type Cdc25A was still present at levels comparable with endogenous Cdc25A at the MBT (see Figure 6A). Thus, these results suggest that Cdc25A degradation at the MBT, although not required for cell cycle elongation at this transition, is required for cell viability at later stages.
Fig. 6. Effects of ectopic expression of Cdc25A on cell cycle elongation and development after the MBT. (A–C) One-cell embryos uninjected (Cont.) or injected with 1.2 ng of mRNA encoding either wild-type (WT) or degradation-resistant (S73A) Cdc25A were cultured and analysed for Cdc25A at the indicated stages or times (A), for Tyr15 phosphorylation of Cdc2 (B) and for the external morphology at the late gastrula stage (stage 12) (C).
Discussion
Our study shows clearly that Chk1, but not Cds1, is essential for cell cycle elongation or the physiological DNA replication checkpoint at the MBT and for cell viability shortly after the MBT in Xenopus (Figure 1). As shown previously by genetic studies, Chk1 is also essential for normal cell proliferation at the MBT in Drosophila (Fogarty et al., 1997; Sibon et al., 1997) and at the blastocyst stage in mice (Liu et al., 2000; Takai et al., 2000). In these organisms, however, it is not known whether Chk1 undergoes any regulation at the relevant stages of development. Our results in Xenopus do show, however, that Chk1 undergoes ATR-mediated phosphorylation (and hence activation; Guo et al., 2000; Liu et al., 2000) weakly and transiently at the MBT or, more accurately, during the MZT (Figure 2). This result, which is the first direct demonstration of a physiological Chk1 activation in metazoan development, would imply that replication checkpoint signalling in early embryos is induced, rather than constitutive, and intrinsically weak. Notably, however, we consistently observed very low basal levels of Chk1 modification even in post-MZT embryos (see Figure 2A, top; see also Figure 7A), which could imply that constitutive replication checkpoint signalling occurs at low basal levels in normal cells (see Zhou and Elledge, 2000; Canman, 2001). At present, we do not know whether such basal signalling is essential for later development, although, in Drosophila, ATR or Chk1 has been shown to be non-essential for post-embryonic development (Sibon et al., 1997, 1999).
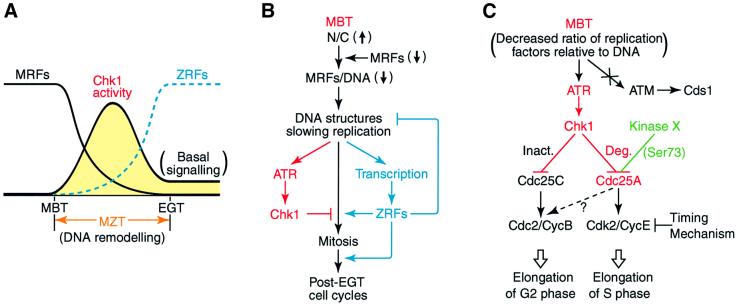
Fig. 7. Models for the regulation of Chk1 and Cdc25A at the Xenopus MBT. (A and B) Depletion model for the mechanism of the transient activation of Chk1 during the MZT. At the MBT, an abrupt decrease in the ratio of some maternal replication factor(s) (MRFs) relative to DNA occurs probably due to both the exponentially increased nucleo-cytoplasmic (N/C) ratio and the depletion or degradation of MRFs, thereby generating the DNA structures that slow DNA replication. This replicational stress would activate the DNA replication checkpoint that involves ATR and Chk1. However, the DNA remodelling or the slowed replication would also activate zygotic transcription, producing zygotic replication factors (ZRFs) that replace MRFs. This replacement by ZRFs, which should occur by the end of the MZT or the early gastrula transition (EGT), could generate normal DNA structures that attenuate DNA replication checkpoint signalling to basal (and constitutive) levels. Thus, during the MZT, Chk1 would be activated only transiently when (the total) replication factors are scarce, with its activity peaking at the late blastula stage (A), and would function to prevent premature mitosis until normal DNA structures are formed by the appearance of ZRFs (B). The ZRFs, together with other zygotic cell cycle regulators, would also function for the post-EGT cell cycles. (C) Model for the regulation of Cdc25A and the cell cycle at the MBT. At the MBT, the DNA replication checkpoint activates Chk1, but not Cds1, via the activation of ATR. Activated Chk1 then phosphorylates Cdc25A, probably at multiple sites and targets it for degradation (Deg.); for this degradation, however, Cdc25A also requires prior phosphorylation at Ser73 by an unknown kinase X. Cdc25A degradation itself is not essential for cell cycle elongation at the MBT, however, probably because, at this transition, Chk1 would also inhibit (Inact.) Cdc25C (and activate Wee1) to elongate the G2 phase while maternal cyclin E is degraded by the timing mechanism to elongate the S phase. However, Cdc25A degradation at the MBT is required for cell viability at later stages.
What would activate the ATR–Chk1 checkpoint pathway at the MBT in Xenopus? In metazoans, the ATR–Chk1 pathway responds primarily to the DNA structures that are generated by stalled or slowed replication (Abraham, 2000; Canman, 2001). Therefore, a plausible signal for activating the pathway at the MBT would be the DNA structures that are generated when the rate of DNA replication slows at the MBT (Vassetzky et al., 2000), probably due to the exponentially increased nucleo-cytoplasmic ratio (Newport and Kirschner, 1982) or the depletion of some maternal (replication) factor (Newport and Dasso, 1989; Dasso and Newport, 1990) (see Figure 7A and B). Such DNA structures might occur only transiently during the MZT, however, since the depleted maternal factors could soon be replaced (if not entirely) by new zygotic factors by the end of the MZT or the early gastrula transition (EGT) (Howe et al., 1995; Sible et al., 1998). Consistent with this idea, Chk1 modification occurred only transiently during the MZT, peaking at the late blastula stage (Figure 2A), and its attenuation from the late blastula stage did require new zygotic transcription or protein synthesis—hence, new zygotic gene products—after the MBT (Figure 2C). Thus, it seems very likely that the ATR–Chk1 checkpoint pathway is activated by the DNA structures generated when maternal (replication) factors are being depleted but not yet (fully) replaced by zygotic factors (Figure 7A), and that the pathway acts to prevent premature mitosis until normal DNA structures are formed (or showed replication is completed) by the replacement with zygotic factors (Figure 7B). (Formally, the responsible zygotic factor could be a specific inhibitor, e.g. some phosphatase, of ATR or Chk1, although we believe that this possibility is less likely.) Given this ‘depletion’ model, the transient activation of Chk1 might occur in a single post-MBT cell cycle (cycle 13 or 14), although the duration of Chk1 modification we observed seemed to cover two cycles (perhaps due to the asynchronous cell divisions after the MBT) (see Figure 2A). Moreover, the very strong, sustained Chk1 modification in zygotic transcription-inhibited embryos (Figure 2C) could be a result of the extreme DNA structures generated by the complete loss of some replication factor(s), and would directly explain the long-known inability of such transcription-inhibited embryos to undergo mitosis shortly after the MBT (Newport and Kirschner, 1982).
The MZT for cell cycle control as well as the reprogramming of DNA structures (which prepares for zygotic transcription) occurs universally in early embryogenesis, albeit at different stages depending on the species (for reviews see Edgar, 1995; Thompson et al., 1998). Thus, based on the present results as well as the previous results from Drosophila (Fogarty et al., 1997; Sibon et al., 1999) and mice (Brown and Baltimore, 2000; Liu et al., 2000), the metazoan ATR–Chk1 pathway may universally be activated and play an essential role in early embryogenesis, principally during the MZT or the DNA remodelling. In this context, it is noteworthy that Cds1, which is activated by ATM responding primarily to double-stranded DNA breaks (Abraham, 2000), is not essential for early Xenopus embryogenesis (Figure 1B–D). This is, however, consistent with the observations that mice lacking ATM are viable (Barlow et al., 1996).
Our results show that Chk1, but not Cds1, is essential for Cdc25A phosphorylation and degradation at the MBT (Figure 3), and suggest that Chk1 directly phosphorylates Cdc25A and targets it for degradation at this physiological replication checkpoint (Figure 4; see also Figure 7C). Our findings contrast with the direct involvement of Cds1, not Chk1, in human Cdc25A degradation in response to ionizing radiation (which causes double-stranded breaks) (Falck et al., 2001), but, intriguingly, can explain how the same human protein is targeted for degradation in response to UV radiation (in which case Chk1 seems to be involved) (Mailand et al., 2000) or replication blocks (Molinari et al., 2000). Interestingly, however, the essential Cds1 phosphorylation site (Ser123) of human Cdc25A and its corresponding site (Ser137) in Xenopus Cdc25A, although phosphorylated by Chk1 in vitro (Figure 5C), are both dispensable for their Chk1-induced degradation in eggs (Figure 5A, B and E). Therefore, Chk1 may phosphorylate Cdc25A (for degradation) at some other site(s) besides the Cds1 phosphorylation site. In this regard, our recent study shows that combined mutations of the consensus Chk1 phosphorylation sites (including Ser137 but excluding Ser73; see Figure 5A) can prevent Chk1-induced degradation of Cdc25A. Thus, it seems that Chk1 phosphorylates Cdc25A for degradation at multiple sites including the Cds1 phosphorylation site.
Importantly, for its Chk1-induced degradation, Xenopus (as well as human) Cdc25A apparently requires prior phosphorylation at Ser73 (Ser75 for human Cdc25A) by an unknown kinase X (Figure 5). In human cells, it is not known whether Ser75 phosphorylation of Cdc25A (by kinase X) is required for its Cds1-induced degradation (Falck et al., 2001). However, this does seem to occur, at least in Xenopus eggs, since Ser73 (Ser75) apparently is required for Cdc25A degradation in eggs in which endogenous Cds1 is activated (by DNA templates with double-stranded ends; see Guo and Dunphy, 2000; our unpublished data). Moreover, Ser73 (Ser75) phosphorylation may underlie the intrinsic instability of Cdc25A during the normal (somatic) cell cycle (see Mailand et al., 2000; Molinari et al., 2000), since Ser73 (Ser75) seems to be phosphorylated constitutively in eggs and embryos at various stages (Figure 5D; our unpublished results) and is also required for the instability of Cdc25A even well after the MBT (see Figure 6A). When overexpressed in normal cells, human Cdc25A is oncogenic (see Draetta and Eckstein, 1997), so a tight regulation of its stability (perhaps by kinase X) may be important for suppressing tumours.
Interestingly, Ser216 of human Cdc25C and its corresponding site (Ser309) of Cdc25B are also phosphorylated (and inhibited) by an unknown kinase(s) throughout interphase of the normal cell cycle (Peng et al., 1997; Bulavin et al., 2001). Notably, these serine residues lie in the same Chk1 phosphorylation or 14-3-3 binding motif (Leu/Met-X-Arg-X-X-Ser) as Ser73 (Ser75) of Cdc25A. Therefore, kinase X or its related kinase(s) might negatively regulate all the Cdc25 family members throughout interphase (and might link the normal cell cycle with the DNA damage/replication checkpoint; Peng et al., 1997; Bulavin et al., 2001). A possible candidate for kinase X could be C-TAK1, as this kinase is constitutively active and can phosphorylate human Cdc25C at Ser216 (Peng et al., 1998). In any case, our study is the first to demonstrate that Chk1, only together with an unknown kinase X, targets Cdc25A for degradation at the DNA replication checkpoint (Figure 7C).
As can be deduced from our experiments (Figure 6B), Chk1-induced Cdc25A degradation per se is not likely to be essential for cell cycle elongation at the MBT. This could occur, however, because at the MBT, Chk1 would also inhibit Cdc25C (our unpublished data; see also Kappas et al., 2000) and could even activate the Cdc25-antagonizing kinase Wee1 (Lee et al., 2001) (see Figure 7C). (Note also that at the MBT, maternal cyclin E, a partner of the Cdc25A substrate Cdk2, is vastly degraded; Figure 3A; Hartley et al., 1996.) Nevertheless, Cdc25A degradation at the MBT is required for cell viability at later gastrula stages (Figure 6C), perhaps allowing normal regulation of zygotic cyclin E/A–Cdk2 complexes. Thus, Cdc25A degradation at the MBT may be to ensure normal cell proliferation at later stages, rather than to elongate the cell cycle at the MBT. In this regard, it should be noted that Chk1, which apparently has multiple targets, is absolutely required for cell cycle elongation at the MBT and hence is essential for cell viability at significantly earlier stages than is Cdc25A degradation alone (compare with Figure 1D).
In summary, the physiological DNA replication checkpoint is activated transiently at the MBT (or, more accurately, during the MZT) in Xenopus, and Chk1, only together with an unknown kinase, targets Cdc25A for degradation to ensure later development. Our findings imply that physiological replication checkpoint signalling in early embryos is induced by developmental cues, and that Cdc25A regulation is an integral component of the ATR–Chk1 DNA replication checkpoint pathway (whether physiological or environmental) in vertebrate cells and involves another as yet unidentified kinase.
Materials and methods
Embryos
Embryos were prepared, cultured, staged and microinjected as described (Sagata et al., 1989). In some experiments, unfertilized eggs were activated artificially by treatment with the calcium ionophore A23187 (1 µg/ml; Sigma), fertilized eggs were injected with α-amanitin (400 pg/egg; Nacalai Tesque), early blastula embryos (at stage 7 or 8) were treated with aphidicolin (100 µg/ml; Sigma) or caffeine (10 mM; Nacalai Tesque), and embryos 30 min after the MBT were treated with cycloheximide (300 µg/ml; Sigma).
DNA constructs and in vitro transcription
cDNAs encoding a dominant-negative Asp148→Ala mutant (DA-Chk1) or a constitutively active mutant (Δ60-Chk1) of Xenopus Chk1 have been described (Nakajo et al., 1999; Oe et al., 2001). A cDNA encoding a dominant-negative Asp340→Ala Cds1 mutant (DA-Cds1) was made by site-directed mutagenesis of Xenopus Cds1 (a gift from H.Takisawa). cDNAs encoding Cdc25A mutants were all prepared by site-directed mutagenesis of Xenopus Cdc25A (Okazaki et al., 1996). A cDNA encoding human Cdc25A was a gift from H.Okayama. All constructs were subcloned into the pT7G (UK II+) vector (Oe et al., 2001); in some constructs, the encoded protein was tagged with a human c-Myc epitope at its N-terminus. In vitro transcription of the cDNAs was performed as described (Nakajo et al., 1999).
GST fusion proteins
cDNAs encoding a full-length Cdc25A protein, a Cdc25A peptide (residues 44–103 or 120–168) or a Cdc25C peptide (residues 254–316) were subcloned into the pGEX-3X plasmid vector (Amersham Pharmacia Biotech), and the GST–fusion proteins were prepared by standard methods.
Immunoblotting and immunoprecipitation
Routinely, proteins equivalent to one egg or embryo were analysed by immunoblotting using anti-Xenopus Cdc25A antibody (Okazaki et al., 1996), anti-Xenopus cyclin E1 antibody (a gift from T.Kishimoto), anti-Cdc2 phospho-Tyr15 antibody (New England Biolabs), anti-Xenopus Chk1 antibody (Nakajo et al., 1999), anti-human c-Myc epitope antibody (Santa Cruz) or anti-human Chk1 phospho-Ser345 antibody (Cell Signaling), essentially as described (Oe et al., 2001). The anti-human Chk1 phospho-Ser345 antibody can recognize ATR-phosphorylated Ser342 of Xenopus Chk1 (Nakajo et al., 1999) (or Ser344 of another Chk1 clone; Guo et al., 2000), as demonstrated by its ability to detect specifically the wild-type (but not S342A) form of Xenopus Chk1 in aphidicolin- (but not aphidicolin plus caffeine-) treated embryos (our unpublished data). Artificially activated eggs were injected with 20 ng of the GST fusion protein and 5 µCi of [γ-32P]ATP (6000 Ci/mmol), cultured, immunoprecipitated with anti-GST antibody (Santa Cruz) and analysed as described (Oe et al., 2001).
Measurement of the DNA content
Total DNA extracted from one embryo was electrophoresed on a 1% agarose gel and stained with ethidium bromide as described (Okamoto et al., 2002).
Phosphatase treatment
Embryo extracts prepared in the presence of 100 nM okadaic acid (Wako) were incubated in the absence or presence of alkaline phosphatase (Takara) and analysed as described (Oe et al., 2001).
In vitro kinase assays
GST fusion proteins were incubated with [γ-32P]ATP and Δ60-Chk1 protein (immunoprecipitated from eggs overexpressing Δ60-Chk1) and analysed essentially as described previously (Oe et al., 2001).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank T.Kishimoto for anti-Xenopus cyclin E antibody, H.Takisawa for a Xenopus Cds1 cDNA and anti-Xenopus Cds1 antibody, and H.Okayama for a human Cdc25A cDNA. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan.
References
- Abraham R.T. (2000) Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev., 15, 2177–2196. [DOI] [PubMed] [Google Scholar]
- Anderson J.A., Lewellyn,A.L. and Maller,J.L. (1997) Ionizing radiation induces apoptosis and elevates cyclin A1–Cdk2 activity before but not after the midblastula transition in Xenopus. Mol. Biol. Cell, 8, 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow C. et al. (1996) Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell, 86, 159–171. [DOI] [PubMed] [Google Scholar]
- Brown E.J. and Baltimore,D. (2000) ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev., 14, 397–402. [PMC free article] [PubMed] [Google Scholar]
- Bulavin D.V., Higashimoto,Y., Popoff,I.J., Gaarde,W.A., Basrur,V., Potapova,O., Appella,E. and Fornace,A.J. (2001) Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature, 411, 102–107. [DOI] [PubMed] [Google Scholar]
- Canman C.E. (2001) Replication checkpoint: preventing mitotic catastrophe. Curr. Biol., 11, R121–R124. [DOI] [PubMed] [Google Scholar]
- Chehab N.H., Malikzay,A., Apple,M. and Halazonetis,T.D. (2000) Chk2/hCds1 functions as a DNA damage checkpoint in G1 by stabilizing p53. Genes Dev., 14, 278–288. [PMC free article] [PubMed] [Google Scholar]
- Chen P. et al. (2000) The 1.7 Å crystal structure of human cell cycle checkpoint kinase Chk1: implication for Chk1 regulation. Cell, 100, 681–692. [DOI] [PubMed] [Google Scholar]
- Dasso M. and Newport,J.W. (1990) Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell, 61, 811–823. [DOI] [PubMed] [Google Scholar]
- Draetta G. and Eckstein,J. (1997) Cdc25 protein phosphatases in cell proliferation. Biochim. Biophys. Acta, 1332, M53–M63. [DOI] [PubMed] [Google Scholar]
- Edgar B. (1995) Diversification of cell-cycle controls in developing embryos. Curr. Opin. Cell Biol., 7, 815–824. [DOI] [PubMed] [Google Scholar]
- Falck J., Mailand,N., Syljuåsen,R.G., Bartek,J. and Lukas,J. (2001) The ATM–Chk–Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature, 410, 842–847. [DOI] [PubMed] [Google Scholar]
- Fogarty P., Campbell,S.D., Abu-Shumays,R., Phalle,B.S, Yu,K.R., Uy,G.L. Goldberg,M.L. and Sullivan,W. (1997) The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr. Biol., 7, 418–426. [DOI] [PubMed] [Google Scholar]
- Gotoh T., Ohsumi,K., Matsui,T., Takisawa,H. and Kishimoto,T. (2001) Inactivation of the checkpoint kinase Cds1 is dependent on cyclin B–Cdc2 kinase activation at the meiotic G2/M-phase transition in Xenopus oocytes. J. Cell Sci., 114, 3397–3406. [DOI] [PubMed] [Google Scholar]
- Guo Z. and Dunphy,W.G. (2000) Response of Xenopus Cds1 in cell-free extracts to DNA templates with double-stranded ends. Mol. Biol. Cell, 11, 1535–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Kumagai,A., Wang,S.X. and Dunphy,W.G. (2000) Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev., 14, 2745–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley R.S., Rempel,R.E. and Maller,J.L. (1996) In vivo regulation of the early embryonic cell cycle in Xenopus. Dev. Biol., 173, 408–419. [DOI] [PubMed] [Google Scholar]
- Hoffmann I., Draetta,G. and Karsenti,E. (1994) Activation of the phosphatase activity of human cdc25A by a cdk2–cyclin E dependent phosphorylation at the G1/S transition. EMBO J., 13, 4302–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J.A. and Newport,J.W. (1996) A developmental timer regulates degradation of cyclin E1 at the midblastula transition during Xenopus embryogenesis. Proc. Natl Acad. Sci. USA, 93, 2060–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J.A., Howell,M., Hunt,T. and Newport,J.W. (1995) Identification of a developmental timer regulating the stability of embryonic cyclin A and a new somatic A-type cyclin at gastrulation. Genes Dev., 9, 1164–1176. [DOI] [PubMed] [Google Scholar]
- Kappas N.C., Savage,P., Chen,K.C., Walls,A.T. and Sible,J.C. (2000) Dissection of the XChk1 signaling pathway in Xenopus laevis embryos. Mol. Biol. Cell, 11, 3101–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Li,C. and Maller,J.L. (1999) A maternal form of the phosphatase Cdc25A regulates early embryonic cell cycles in Xenopus laevis. Dev. Biol., 212, 381–391. [DOI] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy,W.G. (2000) Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol. Cell, 6, 839–849. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Guo,Z., Emami,K.H., Wang,S.X. and Dunphy,W.G. (1998) The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J. Cell Biol., 142, 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Kumagai,A. and Dunphy,W.G. (2001) Positive regulation of Wee1 by Chk1 and 14-3-3 proteins. Mol. Biol. Cell, 12, 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. et al. (2000) Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev., 14, 1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Mailand N., Falck,J., Lukas,C., Sylijuasen,R.G., Welcker,M., Bartek,J. and Lukas,L. (2000) Rapid destruction of human Cdc25A in response to DNA damage. Science, 288, 1425–1429. [DOI] [PubMed] [Google Scholar]
- Molinari M., Mercurio,C., Dominguez,J., Goubin,F. and Draetta,G.F. (2000) Human Cdc25A inactivation in response to S phase inhibition and its role in preventing premature mitosis. EMBO rep., 1, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajo N., Oe,T., Uto,K. and Sagata,N. (1999) Involvement of Chk1 kinase in prophase I arrest of Xenopus oocytes. Dev. Biol., 207, 432–444. [DOI] [PubMed] [Google Scholar]
- Newport J.W. and Dasso,M. (1989) On the coupling between DNA replication and mitosis. J. Cell Sci. (Suppl.), 12, 149–160. [DOI] [PubMed] [Google Scholar]
- Newport J.W. and Kirschner,M.W. (1982) A major developmental transition in early Xenopus embryos: I. Characterization and timing of cellular changes at the midblastula stage. Cell, 30, 675–686. [DOI] [PubMed] [Google Scholar]
- Oe T., Nakajo,N., Katsuragi,Y., Okazaki,K. and Sagata,N. (2001) Cytoplasmic occurrence of the Chk1/Cdc25 pathway and regulation of Chk1 in Xenopus oocytes. Dev. Biol., 229, 250–261. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Nakajo,N. and Sagata,N. (2002) The existence of two distinct Wee1 isoforms in Xenopus: implications for the developmental regulation of the cell cycle. EMBO J., 21, 2472–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Hayashida,K., Iwashita,J., Harano,M., Furuno,N. and Sagata,N. (1996) Isolation of a cDNA encoding the Xenopus homologue of mammalian Cdc25A that can induce meiotic maturation of oocytes. Gene, 178, 111–114. [DOI] [PubMed] [Google Scholar]
- Peng C.Y., Graves,P.R., Thoma,R.S., Wu,Z., Shaw,A.S. and Piwnica-Worms,H. (1997) Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science, 277, 1501–1505. [DOI] [PubMed] [Google Scholar]
- Peng C.Y., Graves,P.R., Ogg,S., Thoma,R.S., Byrnes,M.J., Wu,Z.Q., Stephenson,M.T. and Piwnica-Worms,H. (1998) C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ., 9, 197–208. [PubMed] [Google Scholar]
- Sagata N., Watanabe,N., Vande Woude,G.F. and Ikawa,Y. (1989) The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature, 342, 512–518. [DOI] [PubMed] [Google Scholar]
- Sible J.C., Erikson,E., Hendrickson,M., Maller,J.L. and Gautier,J. (1998) Developmental regulation of MCM replication factors in Xenopus laevis. Curr. Biol., 8, 347–350. [DOI] [PubMed] [Google Scholar]
- Sibon O.C.M., Stevenson,V.A. and Theurkauf,W.E. (1997) DNA-replication checkpoint control at the Drosophila midblastula transition. Nature, 388, 93–97. [DOI] [PubMed] [Google Scholar]
- Sibon O.C.M., Laurencon,A., Hawley,R.S. and Theurkauf,W.E. (1999) The Drosophila ATM homologue mei-41 has an essential checkpoint function at the midblastula transition. Curr. Biol., 9, 302–312. [DOI] [PubMed] [Google Scholar]
- Takai H. et al. (2000) Aberrant cell cycle checkpoint function and early embryonic death in Chk1(–/–) mice. Genes Dev., 14, 1439–1447. [PMC free article] [PubMed] [Google Scholar]
- Thompson E.M., Legouy,E. and Renard,J.P. (1998) Mouse embryos do not wait for the MBT: chromatin and RNA polymerase remodeling in genome activation at the onset of development. Dev. Genet., 22, 31–42. [DOI] [PubMed] [Google Scholar]
- Vassetzky Y., Hair,A. and Mechali,M. (2000) Rearrangement of chromatin domains during development in Xenopus. Genes Dev., 14, 1541–1552. [PMC free article] [PubMed] [Google Scholar]
- Walworth N.C. (2000) Cell-cycle checkpoint kinases: checking in on the cell cycle. Curr. Opin. Cell Biol., 12, 697–704. [DOI] [PubMed] [Google Scholar]
- Walworth N.C. (2001) DNA damage: Chk1 and Cdc25, more than meets the eye. Curr. Opin. Genet. Dev., 11, 78–82. [DOI] [PubMed] [Google Scholar]
- Weinert T. (1998) DNA damage and checkpoint pathways: molecular anatomy and interactions with repair. Cell, 94, 555–558. [DOI] [PubMed] [Google Scholar]
- Zhou B.-S.S. and Elledge,S.J. (2000) The DNA damage response: putting checkpoints in perspective. Nature, 408, 433–439. [DOI] [PubMed] [Google Scholar]