Abstract
Siderophores are small iron-binding molecules that are synthesized and secreted in the iron-free form by microorganisms. Saccharomyces cerevisiae takes up iron bound to siderophores by two separate systems, one of which requires the ARN family of sidero phore–iron transporters. Arn1p and Arn3p are expressed in endosome-like intracellular vesicles. Here we present evidence that, in the absence of its specific substrate, ferrichrome, Arn1p is sorted directly from the Golgi to the endosomal compartment and does not cycle to the plasma membrane. When cells are exposed to ferrichrome at low concentrations, Arn1p stably relocalizes to the plasma membrane. At higher concentrations of ferrichrome, Arn1p relocalizes to the plasma membrane and rapidly undergoes endocytosis. Plasma membrane localization of Arn1p occurs only in the presence of its specific substrate, and not in the presence of other siderophores. Despite expression of Arn1p on the plasma membrane, mutant strains with defects in endocytosis exhibit reduced uptake of ferrichrome–iron. Thus, siderophores influence the trafficking of the Arn transporters within the cell and this trafficking is important for transporter function.
Keywords: endocytosis/exocytosis/iron/siderophore/yeast
Introduction
Iron is an essential nutrient for virtually every organism because many cellular processes rely on proteins that incorporate iron-containing prosthetic groups. Iron is particularly useful in prosthetic groups because it can occupy multiple redox states in an intracellular environment. In an aerobic, extracellular milieu, however, iron is largely present in the oxidized, ferric form, which is only sparingly soluble at neutral pH and forms precipitates of oxyhydroxides. Thus, iron, despite being a very abundant metal, has very low bioavailability. Because of its low bioavailability and its importance to many cellular processes, organisms have developed sophisticated and highly regulated systems for the acquisition and utilization of iron.
The budding yeast Saccharomyces cerevisiae has two separate, high-affinity systems, as well as low-affinity systems, devoted to the uptake of iron. The high-affinity systems are expressed under conditions of iron deprivation and are under the control of Aft1p, the major iron-dependent transcription factor in yeast (Yamaguchi-Iwai et al., 1995). The first system consists of a two-step process that begins with reduction of ferric iron to ferrous iron at the plasma membrane by metalloreductases encoded by FRE1, FRE2 and FRE3 (Dancis et al., 1990; Georgatsou and Alexandraki, 1994; Yun et al., 2001). Three additional genes, FRE4, FRE5 and FRE6, exhibit sequence similarity to FRE2 and are regulated by Aft1p, but have not yet been shown to encode reductase activity (Martins et al., 1998). The reduced iron is then taken up through a transporter complex that contains a multi-copper oxidase encoded by FET3 (Askwith et al., 1994) and an iron permease encoded by FTR1 (Stearman et al., 1996). Substrates for this system include iron salts, low-affinity iron chelates and iron–siderophore chelates (Yun et al., 2000b). Siderophores are low-molecular-weight organic compounds that specifically bind ferric iron with exceptionally high affinity. These compounds are synthesized and secreted in the iron-free form by microorganisms. They bind and thereby solubilize the iron, allowing the iron–siderophore chelate to be captured by cellular transport systems (Neilands, 1995). Most bacteria and fungi synthesize, secrete and take up at least one type of siderophore, yet have the capacity to take up other types of siderophores secreted by other microorganisms (Byers and Arceneaux, 1998). Although S.cerevisiae does not synthesize or secrete siderophores, this species is capable of taking up iron from a variety of siderophores secreted by other microorganisms (Lesuisse et al., 1987; Neilands, 1995). Iron–siderophore chelates are also the substrates for the second high-affinity system of iron uptake, which relies on the transporters encoded by ARN1, ARN2/TAF1, ARN3/SIT1 and ARN4/ENB1 (Lesuisse et al., 1998; Heymann et al., 1999, 2000a,b; Yun et al., 2000a,b). These genes are part of a homologous subfamily of the major facilitator superfamily of transporters, which facilitate the transport of small molecules (sugars, organic acids, amino acids, drugs, etc.) (Goffeau et al., 1997). The individual Arn transporters exhibit specificity for different siderophores of the hydroxamate and catecholate classes.
The high-affinity ferrous iron transport system is expressed on the plasma membrane (Stearman et al., 1996; Askwith and Kaplan, 1998; Yun et al., 2000a). In contrast, Arn1p and Arn3p are located in multiple, punctate intracellular vesicles that resemble the endosomal compartments of yeast. Very little, if any, of these proteins is detected on the plasma membrane (Yun et al., 2000a,b). The Arnp-containing vesicles, when separated by density gradient centrifugation, co-migrate with vesicles that contain Pep12p, a late endosomal protein (Becherer et al., 1996). The mechanism by which intracellular Arn transporters facilitate the uptake of extracellular siderophore– iron is unknown.
In order to determine what role the trafficking of the Arn transporters plays in the uptake of siderophore–iron, we have used strains with defects in protein-sorting pathways to analyze both the location of the Arn transporters and the function of the siderophore uptake system. Using this strategy, we report that the siderophore substrates of the Arn transporters influence the trafficking of the transporters within the cell, and that proper trafficking of the transporters is necessary for siderophore uptake.
Results
Sorting of Arn1p in the absence of siderophores
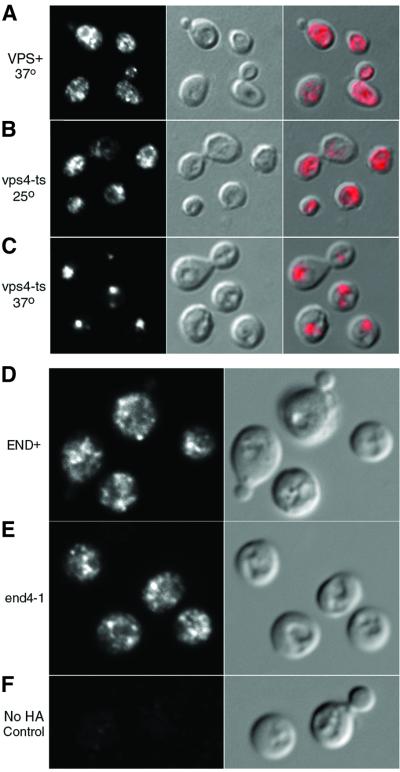
Arn1p is predominantly located in endosome-like intracellular vesicles. In yeast, plasma membrane proteins are internalized into endocytic vesicles that enter the early endosome. There, endocytic cargo is sorted for recycling to the plasma membrane or delivery to the vacuole via the late endosome (Shaw et al., 2001). VPS4 encodes an AAA-ATPase that is required for exit from the late endosome, as both anterograde (late endosome-to- vacuole) and retrograde (late endosome-to-Golgi) traffic is impaired in vps4 mutants (Babst et al., 1997). Resident proteins of the late endosome are typically mislocalized in vps4 mutants and we questioned whether Arn1p would also exhibit mislocalization. We transformed the vps4-ts strain and the congenic VPS+ parent strain with a low-copy-number plasmid containing ARN1-HA under the control of its native promoter (pArn1-HA) and grew the cells in iron-poor medium at 25°C (the permissive temperature) to induce the expression of Arn1p. Aliquots of the VPS+ and vps4-ts cultures were shifted to 37°C (the restrictive temperature) prior to fixation of all cultures and preparation for indirect immunofluorescence (Figure 1). In the VPS+ strain, Arn1p was detected in numerous punctate, vesicular structures that are distributed throughout the cell (Figure 1A). This pattern of fluorescence was present at both the restrictive temperature and the permissive temperature (data not shown), and also in the vps4-ts strain at the permissive temperature (Figure 1B). At the restrictive temperature, however, Arn1p was no longer present in numerous, punctate structures, but instead was detected in one or two large, bright structures (Figure 1C, fluorescence panel) that were located adjacent to the vacuole. This mislocalization of Arn1p in the vps4-ts strain provided additional evidence that Arn1p was either confined to the late endosomal compartment or was cycling through the late endosomal compartment.
Fig. 1. Mislocalization of Arn1p in a vps4-ts strain and localization to the endosome in END+ and end4-1 strains. (A–C) Strains SEY6210 (VPS+) and MBY3 (vps4-ts) were transformed with pArn1-HA and grown in iron-poor medium at 25°C. Aliquots of culture were shifted to 37°C (A and C) for 1 h or grown at 25°C (B) prior to fixation and preparation for indirect immunofluorescence. Mouse monoclonal HA.11 was the primary antibody and Cy3-conjugated donkey anti-mouse was the secondary antibody. Images are in sets of three: fluorescence on the left, differential interference contrast (DIC) in the center and the merged image on the right. (D and E) Congenic RH144–3D (END+; D) and RH268–1C (end4-1; E) strains were transformed with pMetArn1-HA and grown in iron-poor medium at 22°C. Cells were shifted to methionine-free, iron-poor medium and cultured at 37°C for 2 h prior to fixation and preparation for indirect immunofluorescence microscopy. A wild-type strain that did not carry an HA-tagged allele was treated identically, as a control (F). Images are in pairs with fluorescence on the left and DIC on the right.
In order for intracellular Arn1p to come into contact with Fe-ferrichrome (FC), its substrate, the transporter must either be expressed on the plasma membrane or FC must be imported to the endosome. We considered that the intracellular location of Arn1p may only represent the steady-state distribution of the transporter, and that Arn1p may be expressed transiently on the plasma membrane before being rapidly internalized. In this pattern of trafficking, Arn1p would be expected to accumulate on the plasma membrane if the internalization step were blocked. To test this possibility, we investigated the localization of Arn1p in a mutant strain bearing a temperature-sensitive allele of END4/SLA2. Mutations in END4/SLA2 lead to defects in the internalization step of both fluid-phase and receptor-mediated endocytosis at the restrictive temperature (Raths et al., 1993). The end4-1 strain and the congenic parent strain were transformed with a plasmid containing an HA epitope-tagged Arn1p under the control of the inducible MET3 promoter (pMetArn1-HA). These cells were grown at the permissive temperature, then shifted to the restrictive temperature and placed in medium that induced expression of Arn1p. The use of the inducible promoter enabled us to observe the localization of only the Arn1p that was synthesized after the shift to the restrictive temperature and the introduction of the defect in endocytosis. The localization of Arn1p in the end4-1 mutant at the restrictive temperature was not different from that of the congenic parent strain, as Arn1p was localized to intracellular vesicles in both strains (Figure 1D and E). The defect in endocytosis was confirmed by observing that internalization of the lipophilic dye FM4-64 (Vida and Emr, 1995) was delayed in the end4-1 strain at the restrictive temperature (data not shown). The punctate fluorescence signal was confirmed to be specific to Arn1p by observing the absence of signal in a strain that did not express the HA tag (Figure 1F). These results suggested that either newly synthesized Arn1p was sorted to the plasma membrane and internalized in an END4-independent manner, or that Arn1p was not sorted to the plasma membrane, either directly from the Golgi apparatus or after sorting to a post-Golgi compartment.
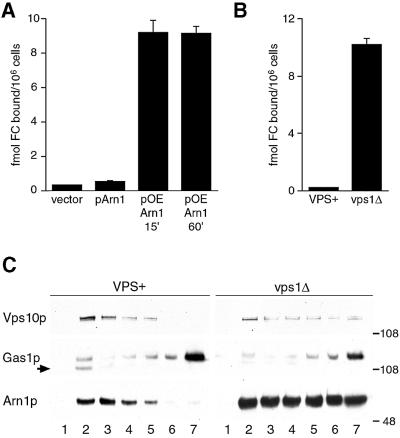
Integral membrane proteins that reach the Golgi are subsequently either directed into secretory vesicles destined for the plasma membrane or sorted to intracellular vesicles of the vacuolar protein-sorting pathway. Two pathways of sorting from the Golgi to the vacuole have been described in yeast (Bryant and Stevens, 1998). The direct pathway is characterized by the sorting of the vacuolar membrane protein alkaline phosphatase and the indirect pathway by the soluble vacuolar hydrolase carboxypeptidase Y (CPY). The CPY protein-sorting pathway and the endocytic pathway intersect in an organelle variously referred to as the post-Golgi/pre-vacuolar/late endosomal compartment (Wendland et al., 1998). The data presented in Figure 1 suggested that newly synthesized Arn1p might be sorted directly from the Golgi to the late endosomal compartment. VPS1 encodes a dynamin homolog that is thought to be required for the formation of vesicles that carry cargo from the late Golgi to the late endosome (Nothwehr et al., 1995). In the absence of Vps1p, proteins that are targeted to the late endosome spill over into the secretory pathway, arrive on the plasma membrane and undergo endocytosis. Both secretion and endocytosis are normal in vps1 mutants. We tested whether deletion of Vps1p would result in aberrant sorting and mislocalization of Arn1p to the plasma membrane by developing an assay that measures the capacity of Arn proteins on the plasma membrane to bind radiolabeled FC, a specific transport substrate for Arn1p (Figure 2A and B).
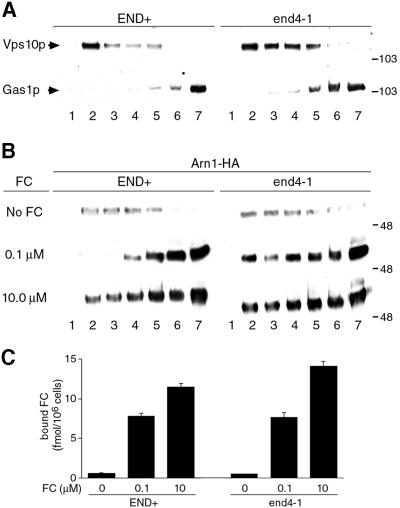
Fig. 2. Mislocalization of Arn1p to the plasma membrane in a vps1Δ strain. (A and B) Binding of ferrichrome to Arn1p expressed on the plasma membrane. (A) Strain CWY101, in which all four Arn transporters have been deleted, was transformed with the vector pRS316 (vector), the low-copy-number pArn1-HA (pArn1) to reconstitute endogenous levels of Arn1p, or the high-copy-number pOE-Arn1-HA (pOE Arn1). Cells were grown to mid-log phase in iron-poor medium, then membrane transport and uptake systems were inactivated by incubation with NaN3 and KF at 0°C. The capacity of Arn1p that is expressed on the plasma membrane to bind [55Fe]ferrichrome at 0°C was measured. (B) Strain RH144–3D (VPS+) and the congenic strain CWY102 (vps1Δ) were grown to mid-log phase in rich medium and cell surface binding of [55Fe]ferrichrome was measured. (C) Expression of Arn1p in both plasma membrane and intracellular vesicle fractions in a vps1 deletion strain. Strain RH144-3D (VPS+) and the congenic strain CWY102 (vps1Δ) were transformed with pArn1-HA and grown to mid-log phase in rich medium. Cells were lysed, membranes were collected and then separated on discontinuous sucrose gradients. Fractions were collected from the top and subjected to SDS–PAGE and western blotting using antibodies directed against Vps10p to detect late-Golgi membranes (Vps10p), Gas1p to detect plasma membrane (Gas1p) and the HA epitope to detect Arn1p-HA (Arn1p). The arrow indicates an endoplasmic reticulum-derived, immature form of Gas1p. Molecular weight standards are indicated in kDa.
Overexpression of Arn1p from a high-copy-number plasmid, pOE-Arn1-HA (50- to 100-fold overexpression), resulted in the mislocalization of Arn1p to membranes throughout the cell, including the plasma membrane, by indirect immunofluorescence (data not shown; J.Kaplan, personal communication). We questioned whether Arn1p, when expressed on the plasma membrane, would exhibit FC-binding activity when both membrane transport and FC uptake were inhibited. A strain from which all four Arn transporters had been deleted was transformed with different plasmids, grown in iron-poor medium to induce the expression of plasmid-encoded Arn1p, and the FC-binding activity of intact cells was measured. The ARN deletion strain did not exhibit significant FC-binding activity when transformed with an empty vector, nor when transformed with the low-copy-number pArn1-HA (Figure 2A). When this strain was transformed with the high-copy-number pOE-Arn1-HA, however, a significant amount of [55Fe]FC was bound to the cells, and the binding was saturated within 15 min. These data suggested that when Arn1p was expressed on the plasma membrane, it specifically bound FC, and provided a quantitative indicator of Arn1p plasma membrane localization. In Figure 2B, a VPS1 deletion strain and its congenic parent strain were grown in rich medium and assayed for FC-binding activity. While the VPS+ parent strain did not exhibit FC binding, the vps1Δ strain exhibited significant binding of FC to the plasma membrane, indicating that some fraction of the Arn protein expressed in the vps1Δ strain was mislocalized to the plasma membrane.
To confirm that the observed FC-binding activity in the vps1Δ strain represented Arn1p mislocalization, we analyzed the distribution of Arn1p-containing membranes by equilibrium sedimentation in sucrose step gradients designed to separate intracellular vesicles from plasma membranes (Bagnat et al., 2001). Membranes isolated from the vps1Δ strain and the congenic parent strain carrying pArn1-HA were sedimented on gradients, the gradients were fractionated, and the fractions were subjected to western blotting with antibodies directed against Vps10p, a marker of late Golgi vesicles (Cooper and Stevens, 1996), Gas1p, a plasma membrane protein (Horvath et al., 1994), and the HA epitope, to detect Arn1p-HA (Figure 2C). In the VPS+ parent strain, Vps10p (intracellular vesicles) was primarily detected in fractions 2–5, while Gas1p (plasma membranes) was primarily detected in fractions 5–7. An immature form of Gas1p was also present in membranes derived from endoplasmic reticulum present in fraction 1 (Sutterlin et al., 1997). Arn1p was also detected in fractions 2–5 in the VPS+ strain, confirming the localization of Arn1p to intracellular vesicles. In the vps1Δ strain, however, while the distribution of Gas1p in fractions 5–7 remained unchanged, both Vps10p and Arn1p were detected in fractions 5–7 as well as in fractions 2–5. These data suggested that when membrane transport from the Golgi to post-Golgi vesicles was blocked, as in the vps1Δ strain, a portion of the Arn1p was mislocalized to the plasma membrane. Together, these data support a model in which newly synthesized Arn1p is sorted directly from the Golgi to a post-Golgi compartment.
Effects of altered Arn1p sorting on ferrichrome uptake activity
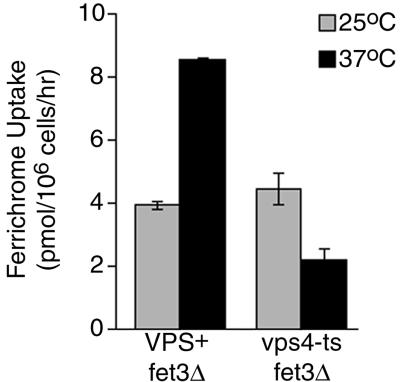
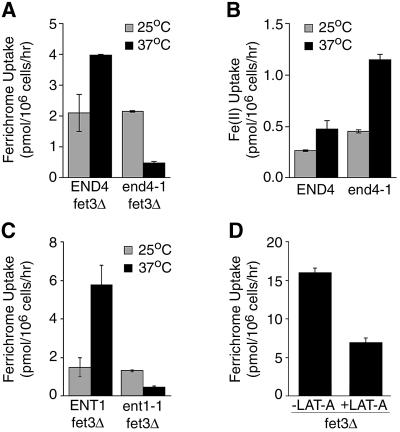
We investigated whether missorting of Arn1p would affect the FC–iron uptake activity of the transporter by measuring the uptake of FC–iron in both the vps4-ts strain and the vps1Δ strain. In order to measure the uptake of FC–iron exclusively through the ARN-dependent system, the high-affinity ferrous iron uptake system was inactivated by deletion of FET3 in the vps4-ts and the VPS+ strains. Uptake of FC–iron was measured at the permissive and restrictive temperatures in these strains and the results are given in Figure 3. The VPS+ fet3Δ strain and the vps4-ts fet3Δ strain exhibited similar levels of FC–iron uptake at the permissive temperature. However, after shifting to the restrictive temperature, the VPS+ fet3Δ strain exhibited a higher level of FC–iron uptake, while the vps4-ts fet3Δ strain had a lowered level of uptake, which was 25% of that of the VPS+ strain. These data suggested that when exit from the late endosome was blocked, as in the vps4-ts strain at 37°C, ARN-dependent FC–iron uptake activity was inhibited. In the vps1Δ strain, uptake of FC–iron was higher than in the VPS+ strain (data not shown), indicating that Golgi-to-late endosome trafficking was not an essential part of the FC–iron uptake process.
Fig. 3. Loss of the uptake of ferrichrome–iron in the vps4-ts strain. Congenic strains CWY106 (VPS+ fet3Δ) and CWY105 (vps4-ts fet3Δ) were grown to mid-log phase in rich media (YPD). Strains were either grown continuously at 25°C or shifted to 37°C for 1 h prior to the measurement of uptake of [55Fe]ferrichrome at the indicated temperatures, as described in Materials and methods. Experiments were repeated twice; data from a representative experiment are shown.
Trafficking of Arn1p in the presence of ferrichrome
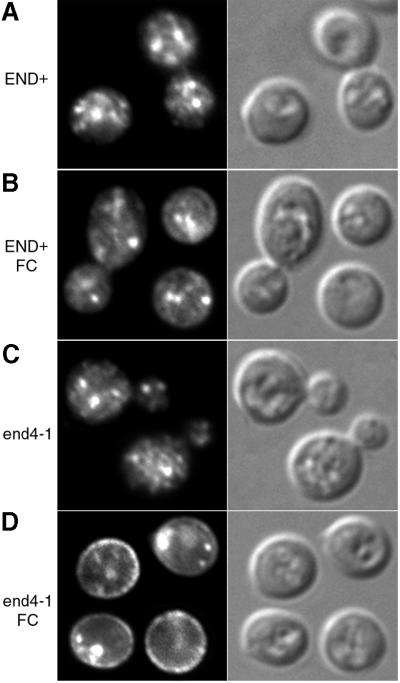
The preceding immunofluorescence experiments examined the sorting of Arn1p under conditions where Arn1p was expressed, but was not transporting FC–iron. We considered that the presence of the transport substrate of Arn1p might alter its localization. We therefore examined the localization of Arn1p in the end4-1 and END+ strains in the presence of FC. Strains were transformed with pMetArn1-HA and grown at the permissive temperature under conditions of iron deprivation. Then cells were simultaneously transferred to medium to induce Arn1p-HA expression, treated with FC, and shifted to the restrictive temperature for 2 h before immunofluorescence imaging. Addition of FC to cultures of the END+ strain resulted in a very subtle increase in Arn1p signal at the periphery of the cell (Figure 4A versus B), but no major change in the overall pattern of fluorescence. When FC was added to cultures of the end4-1 strain, however, Arn1p fluorescence at the periphery of the cell was markedly increased, although some residual intracellular signal remained (Figure 4C versus D). Pre-incubation of the end4-1 strain at 37°C did not prevent relocalization of Arn1p. These data suggested that FC induced the relocalization of Arn1p to the plasma membrane, and that accumulation of Arn1p on the plasma membrane was enhanced when endocytosis was blocked.
Fig. 4. Plasma membrane localization of Arn1p in the end4-1 strain in the presence of ferrichrome. Strains RH144-3D (END+) and RH268-1C (end4-1) were transformed with pMetArn1-HA and grown in iron-poor medium at 22°C. Cells were shifted to methionine-free, iron-poor medium at 37°C without (A and C) or with (B and D) 10 µM ferrichrome (FC) and grown for two additional hours prior to preparation for immunofluorescence microscopy. Images are in pairs with fluorescence on the left and DIC on the right.
Concentration dependence of relocalization
Localization of Arn1p to the plasma membrane occurred in the presence of FC at concentrations at or above the Km for transport (0.9 µM) (Yun et al., 2000b), but primarily in the end4-1 strain, rather than in the END+ strain. In order to determine what effect lower concentrations of FC might have on Arn1p localization, we performed immunofluorescence on the END+ strain carrying pMetArn1-HA after growth in decreasing concentrations of FC. Exposure to FC at 10.0 and 1.0 µM resulted in very little apparent localization of Arn1p to the plasma membrane (Figure 5A and B). However, exposure to 0.1 and 0.01 µM FC resulted in a marked relocalization of Arn1p to the plasma membrane in a significant number of cells (Figure 5C and D). Treatment of cells with 0.001 µM FC did not result in a change in localization of Arn1p (Figure 5E). These data suggested that relocalization to the plasma membrane could occur at substrate concentrations well below the Km for transport, and that localization of Arn1p to the plasma membrane, followed by endocytosis, primarily occurred at substrate concentrations greater than or equal to the Km. We confirmed that this relocalization involved fusion of exocytic vesicles with the plasma membrane by observing the localization of Arn1p in a sec1-1 strain that was transformed with pArn1-HA and treated with FC at 0.1 µM. The sec1-1 strain exhibits a temperature-sensitive defect in the fusion of exocytic vesicles with the plasma membrane (Schekman et al., 1983). FC at 0.1 µM failed to induce relocalization of Arn1p to the plasma membrane in the sec1-1 strain (Figure 5F), suggesting that the appearance of Arn1p at the periphery of the cell required fusion of exocytic vesicles.
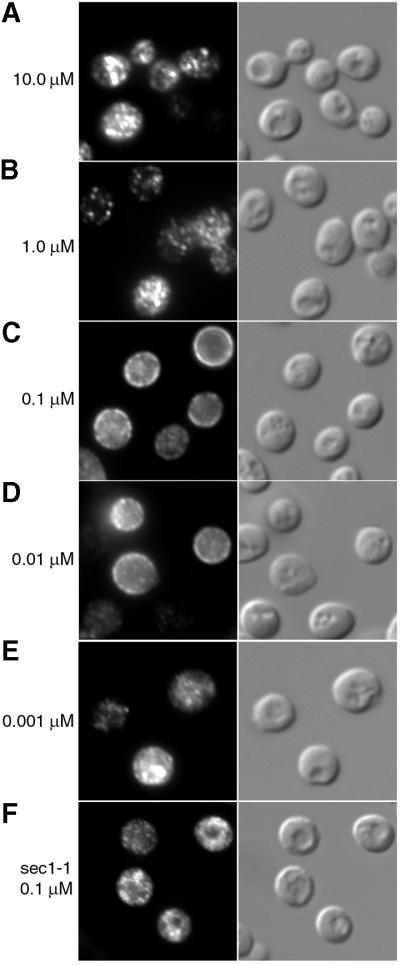
Fig. 5. Concentration dependence of ferrichrome-induced plasma membrane localization of Arn1p. Parent strain RH144-3D carrying pMetArn1-HA (A–E) and SEY5017 (sec1-1) carrying pArn1-HA (F) were grown in iron-poor medium at 22°C to mid-log phase. Cells were then transferred to methionine-free, iron-poor medium and exposed to ferrichrome at the indicated concentrations for 2 h prior to fixation and preparation for immunofluorescence microscopy. In (F), the sec1-1 strain was shifted to 37°C and exposed to 0.1 µM ferrichrome for 2 h prior to fixation and preparation for immunofluorescence microscopy. The identically treated parent strain (SEY6210, congenic to sec1-1) exhibited a fluorescence pattern identical to that shown in (C).
To confirm that the FC-induced peripheral localization of Arn1p seen by immunofluorescence represented relocalization of the transporter to the plasma membrane, we again analyzed the distribution of Arn1p-containing membranes between intracellular vesicles and plasma membranes by equilibrium sedimentation, fractionation and western blotting. In Figure 6, the END+ and the end4-1 strains were transformed with pArn1-HA, grown in iron-poor medium, and shifted to the restrictive temperature in the presence of no FC, 0.1 µM FC or 10.0 µM FC. These concentrations were chosen because they produced differing patterns of Arn1p immunofluorescence in the END+ strain. Membranes were isolated from cells, fractionation was carried out as in Figure 2C, and western blotting was performed to detect markers of intracellular vesicles and plasma membranes (Figure 6A) and the Arn1 transporter (Figure 6B). Again, in the END+ strain without FC, intracellular vesicles (Vps10p) were detected by western blotting in fractions 2–5, while plasma membranes (Gas1p) were detected in fractions 5–7 (Figure 6A). Western blots from the end4-1 strain without FC exhibited a distribution of the membrane marker proteins that was similar to that of the END+ strain, but the membranes were consistently less well separated, which may reflect an alteration in the buoyant density of these membranes in the end4-1 strain. In the END+ strain in the absence of FC, Arn1p co-localized with intracellular vesicles in fractions 2–5 (Figure 6B), but in the presence of 0.1 µM FC, virtually all of the Arn1p was detected with the plasma membranes in fractions 5–7. At 10.0 µM FC, Arn1p was detected both in fractions containing plasma membranes and in fractions containing intracellular vesicles. These data are consistent with low concentrations of FC leading to a stable relocalization of Arn1p to the plasma membrane and higher concentrations of FC leading to a cycling of Arn1p between the plasma membrane and endosomal compartments. In the end4-1 strain in the absence of ferrichrome, Arn1p was largely present in fractions 2–5, but in the presence of both low and high concentrations of FC, Arn1p was detected both in fractions containing plasma membranes and in fractions containing intracellular vesicles. Expression levels of Arn1p were noted to be higher in the presence of FC, both in the END+ and end4-1 strains, and this effect was also present when Arn1p was expressed under the control of a heterologous promoter (C.C.Philpott, unpublished observations). These data suggested that FC may alter both the intracellular trafficking and the abundance of Arn1p.
Fig. 6. Detection of Arn1p in the plasma membrane after exposure to ferrichrome. (A and B) Redistribution of Arn1p to fractions containing plasma membranes after exposure to ferrichrome. Strains RH144-3D (END+) and RH268-1C (end4-1) carrying pArn1-HA were grown in iron-poor medium at 22°C. Cells were then exposed to no ferrichrome, 0.1 µM or 10 µM ferrichrome and shifted to 37°C for two additional hours. Cells were lysed, membranes were collected and fractionated on sucrose step gradients, and the fractions subjected to SDS–PAGE and western blotting. In (A), lysates from cells not treated with ferrichrome were used and arrows indicate the distribution of late Golgi vesicles (Vps10p) and plasma membranes (Gas1p). In (B), the distribution of Arn1p was detected using anti-HA antibody. Protein molecular weight standards in kDa are indicated. (C) Binding of ferrichrome by Arn transporters localized to the plasma membrane. Strains RH144-3D (END+) and RH268-1C (end4-1) expressing only endogenous Arn transporters were grown and treated with ferrichrome as in (A) and (B). The binding of radiolabeled ferrichrome to the surface of the cells was measured as described in Materials and methods.
Further evidence of the relocalization of Arn1p to the plasma membrane in the presence of FC was obtained by measuring the capacity of whole cells to bind radiolabeled [55Fe]FC on the cell surface (Figure 6C). Cells of the END+ and end4-1 strains, expressing only the endogenous Arn transporters, were grown and treated with FC as in Figure 6A and B, then washed and assayed for [55Fe]FC-binding activity as in Figure 2A. In the absence of FC in the culture medium, neither strain exhibited a significant amount of FC-binding activity. However, when cells were grown in media containing FC, a significant amount of FC-binding activity was detected on the cell surface, and this binding activity was maximal in the end4-1 strain grown in the presence of 10 µM FC. By immunofluorescence, the localization of Arn1p to the plasma membrane in the END+ strain appeared to be stronger at lower concentrations of FC and weaker at higher concentrations (Figure 5C versus A). Paradoxically, treatment with both lower and higher amounts of FC resulted in the detection of similar amounts of Arn1p on the plasma membrane, as measured by fractionation and western blotting (Figure 6B) and by surface binding of FC (Figure 6C). This apparent discrepancy occurred because treatment with the lower concentration of FC resulted in an increase in the total amount of Arn1p, with the vast majority of the protein localized to the plasma membrane. Treatment with the higher concentration of FC resulted in a greater increase in the total amount of Arn1p, but with a relatively lower proportion of the protein localized to the plasma membrane.
Substrate specificity of Arn1p relocalization
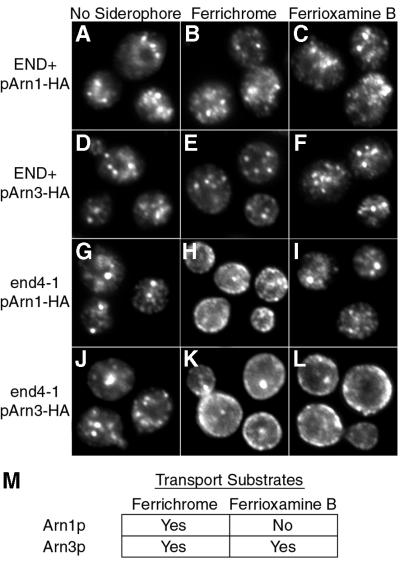
The Arn transporters exhibit specificity for different siderophore substrates. Arn1p facilitates the uptake of FC and a group of related siderophores, including ferrichrome A, ferrirubin and ferrirhodin (Heymann et al., 2000b; Yun et al., 2000b; Lesuisse et al., 2001). A homologous transporter, Arn3p, facilitates the uptake of both FC and a structurally distinct siderophore, ferrioxamine B (FOB), which is not a substrate for Arn1p (Lesuisse et al., 1998; Yun et al., 2000a). We questioned whether the relocalization of Arn1p to the plasma membrane could be stimulated only by FC, the specific substrate, or by FOB, a siderophore that is not a substrate for Arn1p. The END+ and end4-1 strains were transformed with pArn1-HA and pArn3-HA, grown in iron-poor medium at 22°C to induce expression of Arn proteins, then simultaneously shifted to 37°C and exposed to either FC or FOB. Cells were then fixed and indirect immunofluorescence was performed. In the END+ strain, FC and FOB induced very little change in the immunofluorescence patterns of Arn1p and Arn3p (Figure 7A versus B and C, and D versus E and F). In the end4-1 strain, however, FC induced the plasma membrane localization of both Arn1p and Arn3p (Figure 7G versus H, and J versus K), whereas FOB induced the plasma membrane localization of only Arn3p (Figure 7I versus L). Both the apo-siderophore and the holo-siderophore were found to induce plasma membrane localization. Ferric iron alone, however, did not (data not shown). Thus, both Arn1p and Arn3p localized to the plasma membrane after exposure to siderophore, but only the specific transport substrate appeared to induce this localization.
Fig. 7. Substrate-specific relocalization of Arn1p. Strains RH144-3D (END+; A–F) and RH268-1C (end4-1; G–L) were transformed with either pArn1-HA (A–C, G–I) or pArn3-HA (D–F, J–L) and grown at 22°C in iron-poor medium to mid-log phase. Cultures were shifted to 37°C, supplemented with no siderophore (No Siderophore; A, D, G and J), 10 µM ferrichrome (Ferrichrome; B, E, H and K) or 10 µM desferrioxamine B (Ferrioxamine B; C, F, I and L), and grown for an additional 2 h. Cells were then prepared for immunofluorescence microscopy. (M) Substrates of Arn1p and Arn3p. Siderophores taken up by Arn1p and Arn3p, as determined in siderophore–iron uptake assays, are indicated.
Requirement of endocytosis for siderophore–iron uptake
Our experimental data suggested that Arn1p cycled between the endosome and the plasma membrane when the cells were exposed to higher concentrations of FC. In this model, the translocation of FC-bound iron across the membrane and into the cytoplasm could occur when Arn1p was at the plasma membrane, or translocation could occur after endocytosis. To distinguish between these possibilities, we measured the uptake of FC-bound iron through the ARN-dependent uptake system in the END+ and end4-1 strains at the permissive and restrictive temperatures. In order to measure the uptake of FC–iron exclusively through the Arn-dependent system, the high-affinity ferrous transport system was again inactivated by the deletion of FET3 in the END+ and end4-1 strains. The END+ fet3Δ strain exhibited an increased level of uptake of FC–iron when shifted from 25 to 37°C (Figure 8A). In contrast, the end4-1 fet3Δ strain exhibited a lowered level of uptake of FC–iron when shifted from 25 to 37°C, which was 12% of that measured for the END+ fet3Δ strain. Uptake of uncomplexed, ferrous iron through the FET3-dependent system was increased in both the END+ and end4-1 strains when cells were shifted from 25 to 37°C (Figure 8B), indicating that the ferrous iron uptake system was fully functional when endocytosis was inhibited. These data suggested that endocytosis was an important step in the ARN-dependent process of FC uptake.
Fig. 8. Diminished uptake of ferrichrome–iron in yeast defective in endocytosis. (A–C) Strains of the indicated genotype were grown in rich medium (YPD) to mid-log phase. Cells were either grown continuously at 25°C or shifted to 37°C for 1 h prior to the measurement of ferrichrome–iron uptake (A and C) or ferrous iron uptake (B) at the indicated temperatures. (D) Strain CPY119 (fet3Δ) was grown in rich medium to mid-log phase and cells were treated with either 200 µM latrunculin-A (+LAT-A) or an equal volume of DMSO (–LAT-A) for 15 min prior to harvesting and measurement of ferrichrome–iron uptake. LAT-A or DMSO was also included in the uptake assay buffer.
In order to confirm this observation, we examined the uptake of FC–iron in another strain with a temperature-sensitive defect in endocytosis and in the presence of the actin-depolymerizing drug latrunculin-A (LAT-A). ENT1 and ENT2 encode homologs of mammalian epsin, which is required for endocytosis (Chen et al., 1998). An ent1Δ ent2Δ strain carrying the conditional ent1-1 allele exhibits a temperature-sensitive delay in the internalization of FM4-64 and in the degradation of Ste6p, the a-factor transporter whose degradation is dependent on endocytosis (Wendland et al., 1999). In a pattern similar to the END+ and end4-1 strains, the ENT+ fet3Δ strain exhibited a higher level of uptake of FC–iron when shifted from the permissive to the restrictive temperature, while the ent1-1 fet3Δ strain exhibited a markedly lower level of uptake of FC–iron at the restrictive temperature (Figure 8C). LAT-A is a potent inhibitor of the actin cytoskeleton and actin-dependent processes, such as endocytosis (Ayscough, 1998). In Figure 8D, a FET3-deleted strain was treated with LAT-A prior to the measurement of FC–iron uptake. LAT-A-treated cells exhibited a 57% reduction in FC–iron uptake when compared with untreated cells. These data supported the hypothesis that endocytosis is an important step in siderophore–iron uptake.
Discussion
Sequestration of Arn transporters
The findings presented in this study indicated that the yeast siderophore transporters exhibited distinct intracellular trafficking patterns that were regulated in part by their siderophore substrates. When no siderophore was present outside the cell, the Arn transporters were synthesized and sorted directly to endosomes, where they remained sequestered. Why would the cell maintain these transporters in an intracellular location? One possibility is that large transporters with multiple membrane-spanning domains can potentially act as receptors or low-affinity transporters for compounds not needed by and potentially harmful to the cell. The Escherichia coli outer membrane receptor for FC, FhuA, also enables the transport of multiple bacteriophages, the bactericidal protein colicin M and the antibiotic albomycin (Ferguson et al., 1998). Sequestration of the Arn transporters in the endosome minimizes their opportunity to act as transporters for potentially toxic compounds.
Control of Arnp trafficking: a model
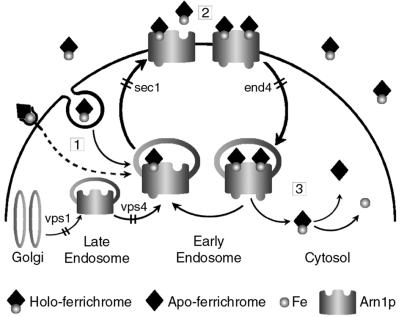
Extracellular siderophore triggered the relocalization of Arn1p and Arn3p, a process that was integral to the function of the transporters. Figure 9 illustrates a model of FC uptake in yeast that incorporates the experimental evidence presented in this study. In step 1, a signal indicating the presence of extracellular FC is transmitted to Arn1p in the early endosome. This signal may be FC itself, either the apo or holo form, which is internalized through endocytosis. Internalized FC can then bind to a high-affinity site on the Arn transporter, which leads to the exocytosis of Arn1p. Alternatively, FC may interact with a receptor at the plasma membrane and activate a signal transduction pathway that results in Arn1p relocalization. Although endocytosis mutants were capable of sensing extracellular FC, we cannot conclude that the sensing mechanism does not involve endocytosis. Both the end4-1 strain and LAT-A-treated cells exhibited low, residual levels of endocytosis. As we do not know the rate-limiting step in the relocalization process, a low level of endocytosis might be sufficient. Further quantitative analysis of the signaling process may clarify this issue.
Fig. 9. Proposed model for trafficking of Arn1p in the presence of ferrichrome. In step 1, the presence of extracellular ferrichrome is signaled to Arn1p as it cycles between the early and late endosome, which results in translocation of Arn1p to the plasma membrane. Ferrichrome may gain access to the endosome via fluid-phase endocytosis and directly bind Arn1p. Alternatively, receptor-mediated signaling events may indicate the presence of ferrichrome to the endosomal Arn1p. In step 2, Arn1p binds a second molecule of ferrichrome and subsequently undergoes endocytosis. In step 3, the ferrichrome–iron chelate is transported to the cytosol, where it undergoes reduction and release of iron. Binding of ferrichrome to each of the two sites may result in conformational changes in the transporter that affect sorting. Mutant strains used in this study are noted; the trafficking steps that are blocked by the mutations are indicated by a double bar.
In step 2, FC binds to a second site on the Arn transporter that is specific for the ferric form of the siderophore. This binding triggers endocytosis of the Arn transporter with bound siderophores. Our data suggested that Arn1p might contain two binding sites for FC. First, kinetic measurements of FC uptake through Arn1p indicated that the Michaelis constant (Km) is ∼1 µM (Yun et al., 2000b). Significant uptake of FC occurred near and above this concentration of substrate, implying that an FC binding site with micromolar affinity existed on Arn1p. Micromolar concentrations of FC were also associated with endosomal cycling of Arn1p. However, we also observed that nanomolar concentrations of FC induced stable plasma membrane localization, and preliminary data indicate the presence of an FC binding site on Arn1p with nanomolar affinity, despite the fact that no appreciable uptake of FC occurs at nanomolar concentrations (Y.Kim, unpublished observations). Therefore, the most likely explanation is that Arn1p contains two FC binding sites.
Although a previous report indicated that endocytosis did not play a role in the uptake of siderophore–iron (Lesuisse et al., 2001), our data clearly suggested that endocytosis was an important component of the uptake process. Not only did Arn1p exhibit altered localization when endocytosis was inhibited, but the uptake of FC–iron was reduced in two mutant strains that exhibit specific defects in endocytosis and when the drug LAT-A inhibited endocytosis. Because defects in endocytosis were associated with both accumulation of Arn1p on the plasma membrane and inhibition of FC–iron uptake, we propose that Arn1p binds the FC–iron chelate at the plasma membrane and undergoes endocytosis prior to cytosolic uptake of the chelate. In step 3, the FC–iron chelate is translocated across the endosomal membrane, and the Arn protein (with FC occupying the high-affinity binding site) is free to return to the plasma membrane. Cytosolic FC–iron chelates can then either dissociate through the activities of cytosolic reductases, or be degraded and thereby release the iron.
The model presented here indicates that binding of FC to the luminal face of the transporter, either in the early endosome or on the plasma membrane, results in re-sorting of the transporter. Sorting of integral membrane proteins between membrane-bound compartments is generally controlled through the interactions of cis-acting elements on the cytoplasmic face of the protein with trans-acting factors in the sorting machinery. We propose that binding of FC on the luminal face of Arn1p results in conformational changes on the cytoplasmic face of Arn1p that alter the interactions of these cis-acting elements with the sorting machinery. In mammalian cells, tyrosine-based sorting signals and dileucine motifs have been identified as critical for interaction with the sorting machinery (Kirchhausen, 2000). In yeast, however, these signals are less well defined. Clearly, ubiquitin is an important internalization and sorting signal (Hicke, 2001), and the role of ubiquitin in the sorting of Arn transporters has not been defined. Preliminary data indicate, however, that sorting signals are present in the cytosolic C-terminal tail of Arn1p (C.C.Philpott and Y.Kim, unpublished observations).
Although endosomal recycling is well described in mammalian cells, evidence for a recycling pathway in yeast has emerged more recently. Localization of the chitin synthase Chs3p to the nascent septum in large budded cells is achieved through a process of endocytosis and incorporation into exocytic vesicles destined for the bud neck (Ziman et al., 1996; Holthuis et al., 1998). Furthermore, the a-factor receptor, Ste3p, was shown to undergo recycling to the plasma membrane following receptor-mediated endocytosis (Chen and Davis, 2000), and the exocytic SNARE Snc1p marks a recycling pathway between the plasma membrane and the early endosome (Lewis et al., 2000). In this study, we observed that uptake of FC-bound iron was inhibited by mutations in VPS4, which lead to defects in the exit of cargo vesicles from the late endosome. Because Arn protein trafficking (and ARN-dependent FC uptake) involved an endosome-to-plasma membrane translocation step, the observed defect in FC uptake may indicate a role for Vps4p in a late endosome-to-early endosome cycling pathway for Arn1p. In the absence of FC, Arn1p may cycle between the early and late endosome. When Arn1p encounters FC in the early endosome, the transporter may then begin cycling between the early endosome and the plasma membrane.
Regulated exocytosis and endocytosis: a general mechanism for control of transporter activity
Studies in yeast and mammalian systems indicate that regulated exocytosis and endocytosis of membrane transporters constitutes an important system for the regulation of transporter activity. In yeast, Gap1p, a general amino acid permease, is differentially sorted according to the quality of the available nitrogen source. In the presence of a poor nitrogen source, such as urea, Gap1p is sorted from the late Golgi to the plasma membrane, where it is functional. In the presence of a rich nitrogen source, such as glutamate, the permease is sorted from the late Golgi, through the endosome/prevacuolar compartment, to the vacuole, where it is degraded (Roberg et al., 1997). Shifting from a poor nitrogen source to a rich one results in endocytosis of Gap1p and degradation in the vacuole. Ubiquitylation of Gap1p has a role in both the endocytosis of Gap1p and its cycling between the late endosome and late Golgi (Helliwell et al., 2001). Thus, the regulated exocytosis and endocytosis of membrane transport proteins may constitute a general mechanism to control the activity of membrane transport systems in eukaryotic organisms.
Materials and methods
Yeast strains, plasmids and growth conditions
The yeast strains used in this study are listed in Table I. For deletion of FET3, the HIS3 gene in the plasmid pΔFet3-HIS was replaced with a PCR-generated URA3 gene, and the resulting plasmid was linearized with Apa I and used to transform RH144-3D, RH268-1C, SEY6210, MBY3 and BWY514. Disruption of FET3 was confirmed by PCR. Construction of pMetArn1-HA, pArn1-HA and pArn3-HA was described previously (Yun et al., 2000a,b). These plasmids containing an Arn protein carrying a C-terminal HA tag have been shown to fully complement deletion strains in both growth assays and siderophore–iron uptake assays. The plasmid pOE-Arn1-HA contains the HA epitope-tagged version of Arn1p under the control of the endogenous ARN1 promoter in the 2-µ-based plasmid pRS426.
Table I. Strains used in this study.
| Strain | Relevant genotype | Reference |
|---|---|---|
| RH144-3D | MATa his4 leu2 ura3 bar1-1 | Raths et al. (1993) |
| RH268-1C | MATa his4 leu2 ura3 bar1-1 end4-1 | Raths et al. (1993) |
| SEY6210 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-901 lys2-801 suc2-Δ9 | Robinson et al. (1988) |
| MBY3 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-901 lys2-801 suc2-Δ9 vps4::TRP1 pRS413 vps4-ts | Babst et al. (1997) |
| BWY514 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-901 lys2-801 suc2-Δ9 ent1::LEU2 ent2::HIS3 pent1-1 | Wendland et al. (1999) |
| SEY5017 | MATa leu2-3,112 ura3-52 suc2-Δ9 sec1-1 | Cowles et al. (1997) |
| CWY101 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 arn1Δ::HISG arn2Δ::HISG arn3Δ::HISGarn4Δ::HISG | Yun et al. (2000a) |
| CWY102 | MATa his4 leu2 ura3 bar1-1 vps1::LEU2 | This study |
| CWY103 | MATa his4 leu2 ura3 bar1-1 fet3Δ::URA3 | This study |
| CWY104 | MATa his4 leu2 ura3 bar1-1 end4-1 fet3Δ::URA3 | This study |
| CWY105 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-901 lys2-801 suc2-Δ9 vps4::TRP1 pRS413 vps4-ts fet3Δ::URA3 | This study |
| CWY106 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-90 lys2-801 suc2-Δ9 fet3Δ::URA3 | This study |
| YKY101 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-901 lys2-801 suc2-Δ9 ent1::LEU2 ent2::HIS3 pent1-1 fet3Δ::URA3 | This study |
| CPY119 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 fet3::HIS3 | Yun et al. (2000a) |
Rich media (YPD) and defined media (SD) were prepared as described previously (Sherman, 1991). Iron-poor medium was prepared as described previously (Philpott et al., 1998) using iron- and copper-free SD medium supplemented with 25 mM MES pH 6.1, 1 µM copper sulfate, 1 mM ferrozine (an iron chelator) and 10 µM ferrous ammonium sulfate. All experiments were performed on logarithmically growing cells that had been cultured in rich medium or iron-poor medium for 16–24 h. Strains transformed with pMetArn1-HA were grown overnight at 22°C in iron-poor media, then washed, resuspended in methionine-free, iron-poor medium, treated with 10 µM FC and placed at either 22 or 37°C for 2 h. FC and FOB were purchased from Sigma and added to cultures as the apo (iron-free) form unless stated otherwise.
Fluorescence microscopy, iron uptake and FC binding assays
Cells were prepared for indirect immunofluorescence microscopy as described previously (Stearman et al., 1996), using affinity-purified mouse monoclonal HA.11 (Covance) as the primary antibody and Cy3-conjugated polyclonal anti-mouse IgG antiserum from donkey (Jackson ImmunoResearch) as the secondary antibody. Alternatively, cells were stained with FM4-64 as described previously (Vida and Emr, 1995). Cells were imaged using a Zeiss photomicroscope equipped with a 63×/1.4 N.A. objective and differential interference contrast optics. Images were acquired with a charge-coupled device camera (Princeton Instruments) using IP Labs software. Iron uptake assays were performed as described previously (Dancis et al., 1994; Yun et al., 2000b) using either 1.0 µM 55FeCl3, reduced in ascorbate, or 1.0 µM [55Fe]FC. FC binding assays were performed by growing cells to mid-log phase, then inactivating uptake and transport processes by incubation with 20 mM sodium azide and 20 mM potassium fluoride at 0°C. Cells were then washed and resuspended in ice-cold assay buffer (5% glucose, 1 mM bathophenanthroline disulfonate, 20 mM sodium azide, 20 mM potassium fluoride, 50 mM sodium citrate pH 6.5) at a concentration of 2 × 108 cells/ml. Cells (0.5 ml) were incubated with an equal volume of [55Fe]FC at a final concentration of 100 nM at 0°C for 15 min with shaking. Cells were filtered through glass microfiber filters, washed extensively and radioactivity measured in a scintillation counter.
Fractionation and western blotting
Fractionation of membranes was performed essentially as described previously (Bagnat et al., 2001). Briefly, 2 × 108 cells were subjected to glass bead lysis in 25 mM Tris pH 8.0, 5 mM EDTA, with protease inhibitors. Lysates were cleared of unbroken cells and cell walls by centrifugation at 500 g for 5 min and membranes were sedimented by centrifugation at 18 000 g for 30 min. Membranes were resuspended in 0.35 ml of 20% glycerol in buffer B (10 mM Tris pH 7.4, 0.2 mM EDTA, 1 mM DTT, with protease inhibitors) and 0.3 ml of membranes were layered on top of a sucrose step gradient (0.3 ml of 53% and 0.6 ml of 43% in buffer B). After centrifugation for 2 h at 100 000 g, six 0.2 ml fractions were collected from the top and the pelleted material was resuspended in 0.2 ml of 53% sucrose for the seventh fraction.
SDS–PAGE and western blotting were performed as described previously (Yun et al., 2000a) using HA.11 (Covance) at 1:2000 dilution for the detection of Arn1p-HA, anti-Vps10p antibody (Molecular Probes) at 1:500 dilution for the detection of Vps10p, and polyclonal rabbit antiserum directed against Gas1p at 1:5000 dilution (Horvath et al., 1994). Horseradish peroxidase-conjugated secondary antibodies (Amersham) coupled with enhanced chemiluminescence (Pierce) were used to detect immobilized protein–antibody complexes.
Acknowledgments
Acknowledgements
The authors would like to thank Jerry Kaplan, Robert Moore and Beverly Wendland for their critical reading of this manuscript, and for innumerable helpful discussions and technical suggestions. We also thank Jerry Kaplan, Beverly Wendland, Steven Nothwehr, Marcus Babst and Chris Mullins for generously providing strains.
References
- Askwith C.C. and Kaplan,J. (1998) Site-directed mutagenesis of the yeast multicopper oxidase Fet3p. J. Biol. Chem., 273, 22415–22419. [DOI] [PubMed] [Google Scholar]
- Askwith C., Eide,D., Van Ho,A., Bernard,P.S., Li,L., Davis-Kaplan,S., Sipe,D.M. and Kaplan,J. (1994) The FET3 gene of S.cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell, 76, 403–410. [DOI] [PubMed] [Google Scholar]
- Ayscough K. (1998) Use of latrunculin-A, an actin monomer-binding drug. Methods Enzymol., 298, 18–25. [DOI] [PubMed] [Google Scholar]
- Babst M., Sato,T.K., Banta,L.M. and Emr,S.D. (1997) Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J., 16, 1820–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnat M., Chang,A. and Simons,K. (2001) Plasma membrane proton ATPase pma1p requires raft association for surface delivery in yeast. Mol. Biol. Cell, 12, 4129–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherer K.A., Rieder,S.E., Emr,S.D. and Jones,E.W. (1996) Novel syntaxin homologue, Pep12p, required for the sorting of lumenal hydrolases to the lysosome-like vacuole in yeast. Mol. Biol. Cell, 7, 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant N.J. and Stevens,T.H. (1998) Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol. Mol. Biol. Rev., 62, 230–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B.R. and Arceneaux,J.E.L. (1998) Microbial iron transport: iron acquisition by pathogenic microorganisms. In Sigel,A. and Sigel,H. (eds), Iron Transport and Storage in Microorganisms, Plants, and Animals, Vol. 35. Marcel Dekker, New York, NY, pp. 37–66. [PubMed]
- Chen H., Fre,S., Slepnev,V.I., Capua,M.R., Takei,K., Butler,M.H., Di Fiore,P.P. and De Camilli,P. (1998) Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature, 394, 793–797. [DOI] [PubMed] [Google Scholar]
- Chen L. and Davis,N.G. (2000) Recycling of the yeast a-factor receptor. J. Cell Biol., 151, 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A.A. and Stevens,T.H. (1996) Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol., 133, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles C.R., Snyder,W.B., Burd,C.G. and Emr,S.D. (1997) Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO J., 16, 2769–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis A., Yuan,D.S., Haile,D., Askwith,C., Elde,D., Moehle,C., Kaplan,J. and Klausner,R.D. (1994) Molecular characterization of a copper transport protein in S.cerevisiae: an unexpected role for copper in iron transport. Cell, 76, 393–402. [DOI] [PubMed] [Google Scholar]
- Ferguson A.D., Hofmann,E., Coulton,J.W., Diederichs,K. and Welte,W. (1998) Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science, 282, 2215–2220. [DOI] [PubMed] [Google Scholar]
- Georgatsou E. and Alexandraki,D. (1994) Two distinctly regulated genes are required for ferric reduction, the first step of iron uptake in Saccharomyces cerevisiae. Mol. Cell. Biol., 14, 3065–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffeau A., Park,J., Paulsen,I.T., Jonniaux,J.L., Dinh,T., Mordant,P. and Saier,M.H.,Jr (1997) Multidrug-resistant transport proteins in yeast: complete inventory and phylogenetic characterization of yeast open reading frames with the major facilitator superfamily. Yeast, 13, 43–54. [DOI] [PubMed] [Google Scholar]
- Helliwell S.B., Losko,S. and Kaiser,C.A. (2001) Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J. Cell Biol., 153, 649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann P., Ernst,J.F. and Winkelmann,G. (1999) Identification of a fungal triacetylfusarinine C siderophore transport gene (TAF1) in Saccharomyces cerevisiae as a member of the major facilitator superfamily. Biometals, 12, 301–306. [DOI] [PubMed] [Google Scholar]
- Heymann P., Ernst,J.F. and Winkelmann,G. (2000a) A gene of the major facilitator superfamily encodes a transporter for enterobactin (Enb1p) in Saccharomyces cerevisiae. Biometals, 13, 65–72. [DOI] [PubMed] [Google Scholar]
- Heymann P., Ernst,J.F. and Winkelmann,G. (2000b) Identification and substrate specificity of a ferrichrome-type siderophore transporter (Arn1p) in Saccharomyces cerevisiae. FEMS Microbiol. Lett., 186, 221–227. [DOI] [PubMed] [Google Scholar]
- Hicke L. (2001) A new ticket for entry into budding vesicles—ubiquitin. Cell, 106, 527–530. [DOI] [PubMed] [Google Scholar]
- Holthuis J.C.M., Nichols,B.J. and Pelham,H.R.B. (1998) The syntaxin Tlg1p mediates trafficking of chitin synthase III to polarized growth sites in yeast. Mol. Biol. Cell, 9, 3383–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A., Sutterlin,C., Manning-Krieg,U., Movva,N.R. and Riezman,H. (1994) Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J., 13, 3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. (2000) Clathrin. Annu. Rev. Biochem., 69, 699–727. [DOI] [PubMed] [Google Scholar]
- Lesuisse E., Raguzzi,F. and Crichton,R.R. (1987) Iron uptake by the yeast Saccharomyces cerevisiae: involvement of a reduction step. J. Gen. Microbiol., 133, 3229–3236. [DOI] [PubMed] [Google Scholar]
- Lesuisse E., Simon-Casteras,M. and Labbe,P. (1998) Siderophore-mediated iron uptake in Saccharomyces cerevisiae: the SIT1 gene encodes a ferrioxamine B permease that belongs to the major facilitator superfamily. Microbiology, 144, 3455–3462. [DOI] [PubMed] [Google Scholar]
- Lesuisse E., Blaiseau,P.L., Dancis,A. and Camadro,J.M. (2001) Siderophore uptake and use by the yeast Saccharomyces cerevisiae. Microbiology, 147, 289–298. [DOI] [PubMed] [Google Scholar]
- Lewis M.J., Nichols,B.J., Prescianotto-Baschong,C., Riezman,H. and Pelham,H.R. (2000) Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell, 11, 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins L.J., Jensen,L.T., Simon,J.R., Keller,G.L., Winge,D.R. and Simons,J.R. (1998) Metalloregulation of FRE1 and FRE2 homologs in Saccharomyces cerevisiae [published erratum appears in J. Biol. Chem. (1998) 273, 30056]. J. Biol. Chem., 273, 23716–23721. [DOI] [PubMed] [Google Scholar]
- Neilands J.B. (1995) Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem., 270, 26723–26726. [DOI] [PubMed] [Google Scholar]
- Nothwehr S.F., Conibear,E. and Stevens,T.H. (1995) Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J. Cell Biol., 129, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott C.C., Rashford,J., Yamaguchi-Iwai,Y., Rouault,T.A., Dancis,A. and Klausner,R.D. (1998) Cell-cycle arrest and inhibition of G1 cyclin translation by iron in AFT1-1up yeast. EMBO J., 17, 5026–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raths S., Rohrer,J., Crausaz,F. and Riezman,H. (1993) end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J. Cell Biol., 120, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberg K.J., Rowley,N. and Kaiser,C.A. (1997) Physiological regulation of membrane protein sorting late in the secretory pathway of Saccharomyces cerevisiae. J. Cell Biol., 137, 1469–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.S., Klionsky,D.J., Banta,L.M. and Emr,S.D. (1988) Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol., 8, 4936–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R., Esmon,B., Ferro-Novick,S., Field,C. and Novick,P. (1983) Yeast secretory mutants: isolation and characterization. Methods Enzymol., 96, 802–815. [DOI] [PubMed] [Google Scholar]
- Shaw J.D., Cummings,K.B., Huyer,G., Michaelis,S. and Wendland,B. (2001) Yeast as a model system for studying endocytosis. Exp. Cell Res., 271, 1–9. [DOI] [PubMed] [Google Scholar]
- Sherman F. (1991) Getting started with yeast. In Guthrie,C. and Fink,G. (eds), Guide to Yeast Genetics and Molecular Biology, Vol. 194. Academic Press, New York, NY, pp. 3–20.
- Stearman R., Yuan,D.S., Yamaguchi-Iwai,Y., Klausner,R.D. and Dancis,A. (1996) A permease–oxidase complex involved in high-affinity iron uptake in yeast. Science, 271, 1552–1557. [DOI] [PubMed] [Google Scholar]
- Sutterlin C., Doering,T.L., Schimmoller,F., Schroder,S. and Riezman,H. (1997) Specific requirements for the ER to Golgi transport of GPI-anchored proteins in yeast. J. Cell Sci., 110, 2703–2714. [DOI] [PubMed] [Google Scholar]
- Vida T.A. and Emr,S.D. (1995) A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol., 128, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B., Emr,S.D. and Riezman,H. (1998) Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr. Opin. Cell Biol., 10, 513–522. [DOI] [PubMed] [Google Scholar]
- Wendland B., Steece,K.E. and Emr,S.D. (1999) Yeast epsins contain an essential N-terminal ENTH domain, bind clathrin and are required for endocytosis. EMBO J., 18, 4383–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y., Dancis,A. and Klausner,R.D. (1995) AFT1: a mediator of iron regulated transcriptional control in Saccharomyces cerevisiae. EMBO J., 14, 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C.W., Ferea,T., Rashford,J., Ardon,O., Brown,P.O., Botstein,D., Kaplan,J. and Philpott,C.C. (2000a) Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake. J. Biol. Chem., 275, 10709–10715. [DOI] [PubMed] [Google Scholar]
- Yun C.W., Tiedeman,J.S., Moore,R.E. and Philpott,C.C. (2000b) Siderophore–iron uptake in Saccharomyces cerevisiae. Identification of ferrichrome and fusarinine transporters. J. Biol. Chem., 275, 16354–16359. [DOI] [PubMed] [Google Scholar]
- Yun C.W., Bauler,M., Moore,R.E., Klebba,P.E. and Philpott,C.C. (2001) The role of the FRE family of plasma membrane reductases in the uptake of siderophore–iron in Saccharomyces cerevisiae. J. Biol. Chem., 276, 10218–10223. [DOI] [PubMed] [Google Scholar]
- Ziman M., Chuang,J.S. and Schekman,R.W. (1996) Chs1p and Chs3p, two proteins involved in chitin synthesis, populate a compartment of the Saccharomyces cerevisiae endocytic pathway. Mol. Biol. Cell, 7, 1909–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]