Abstract
Drosophila has three membrane-tethered epidermal growth factor (EGF)-like proteins: Spitz, Gurken and Keren. Spitz and Gurken have been genetically confirmed to activate the EGF receptor, but Keren is uncharacterized. Spitz is activated by regulated intracellular translocation and cleavage by the transmembrane proteins Star and the protease Rhomboid-1, respectively. Rhomboid-1 is a member of a family of seven similar proteins in Drosophila. We have analysed four of these: all are proteases that can cleave Spitz, Gurken and Keren, and all activate only EGF receptor signalling in vivo. Star acts as an endoplasmic reticulum (ER) export factor for all three. The importance of this translocation is highlighted by the fact that when Spitz is cleaved by Rhomboids in the ER it cannot be secreted. Keren activates the EGF receptor in vivo, providing strong evidence that it is a true ligand. Our data demonstrate that all membrane-tethered EGF ligands in Drosophila are activated by the same strategy of cleavage by Rhomboids, which are ancient and widespread intramembrane proteases. This is distinct from the metalloprotease-induced activation of mammalian EGF-like ligands.
Keywords: Gurken/Keren/Rhomboid/Spitz/Star
Introduction
The epidermal growth factor (EGF) receptors are a family of receptor tyrosine kinases essential for the control of many cellular processes, including proliferation, survival and differentiation (Adamson, 1990; Schweitzer and Shilo, 1997; Domínguez et al., 1998; Sibilia et al., 1998). In Drosophila, EGF receptor signalling is used repeatedly and in many different contexts throughout growth and development, highlighting the need for stringent regulation of receptor activity (Schweitzer and Shilo, 1997; Wasserman and Freeman, 1997). Genetic and molecular studies have revealed that the production of an active EGF ligand by the signal-sending cell is a key regulatory step in receptor activation. In most contexts, the main activating ligand of the EGF receptor in Drosophila is Spitz, a membrane-tethered EGF ligand that resembles several of the mammalian EGF receptor ligands, including transforming growth factor-α (TGF-α) (Rutledge et al., 1992; Freeman, 1994; Tio et al., 1994; Schweitzer et al., 1995; Tio and Moses, 1997). Like its mammalian counterparts, Spitz is translated as a transmembrane molecule with an extracellular EGF domain. Full-length Spitz is unable to signal and must be processed into an active, soluble form. Rhomboid-1 and Star, first implicated by genetic analysis, are the primary positive regulators of Spitz activation in the signal-sending cell (Ruohola-Baker et al., 1993; Sturtevant et al., 1993; Freeman, 1994; Bang and Kintner, 2000; Wasserman et al., 2000; Lee et al., 2001; Urban et al., 2001; Klämbt, 2002; Tsruya et al., 2002).
In the absence of Star, Spitz is confined to the endoplasmic reticulum (ER) whereas Rhomboid-1 is in the Golgi apparatus (Lee et al., 2001). Star relocalizes Spitz from the ER to the Golgi, where Spitz is cleaved within its transmembrane domain by Rhomboid-1, an intramembrane serine protease (Urban et al., 2001). Drosophila EGF receptor activation is thus controlled by the regulated intracellular trafficking and proteolytic activation of its ligand, Spitz (Lee et al., 2001; Urban et al., 2001; Tsruya et al., 2002). This mechanism defines a new pathway for growth factor release in which the cleavage occurs intracellularly and is a form of ‘regulated intramembrane proteolysis’ (RIP) (Brown et al., 2000; Huppert and Kopan, 2001). This is in contrast to all other known examples of growth factor activation, which use cell surface metalloproteases to release the active growth factor domains by a cleavage in the juxtamembrane region (Arribas et al., 1996; Black and White, 1998).
Regulation of Drosophila EGF receptor ligand activation is made more complex by the fact that there are seven Rhomboids and three membrane-tethered EGF ligands (Guichard et al., 2000; Wasserman et al., 2000; Ghiglione et al., 2002). For example, despite its key role in the proteolytic cleavage of Spitz, there are several contexts where the Rhomboid-1 protease is not essential for EGF receptor activation. In the developing Drosophila eye, Spitz activation is required for the recruitment of all cell types, while Rhomboid-1 is completely dispensable in this process (Freeman et al., 1992). Instead, genetic analysis has shown that Rhomboid-3, a close homologue of Rhomboid-1, functions as an eye-specific Rhomboid to activate EGF receptor signalling (Wasserman et al., 2000). Recently, Rhomboid-2 (also called Brother of Rhomboid; Guichard et al., 2000) has been shown to trigger cleavage of Gurken in cell culture (Ghiglione et al., 2002).
As well as Rhomboid-1-independent EGF receptor activation, there are several examples of Rhomboid-1-dependent EGF receptor signalling that do not depend on Spitz, most notably in the developing eye and wing (Simcox, 1997; Domínguez et al., 1998; Nagaraj et al., 1999). These imply the existence of a missing EGF receptor ligand, predicted to be a substrate for Rhomboid-1. There are two other membrane-tethered EGF receptor ligands in Drosophila: Gurken and Keren. Gurken function is restricted to oogenesis where it is required for polarizing the egg by signalling from the oocyte to the overlying EGF receptor-expressing follicle cells (Schüpbach, 1987; Neuman-Silberberg and Schüpbach, 1993; Gonzalez-Reyes et al., 1995). Recent evidence suggests that, like Spitz, Gurken requires post-translational activation to signal (Ghiglione et al., 2002). Consistent with a possible role in Gurken processing, Rhomboid-2 is expressed exclusively in the oocyte (Guichard et al., 2000).
The third apparent membrane-tethered EGF receptor ligand, Keren (previously also called Spitz-2; Baonza et al., 2001) or Gritz (Flybase reference 0126856), is highly similar to Spitz and was identified originally by the Drosophila Genome Project. It is the best candidate for the Rhomboid-dependent ligand required for eye and wing development since it is the only other membrane-tethered EGF receptor ligand apparent in the Drosophila genome. However, there is no mutant allele to prove its biological function. Furthermore, although the missing ligand is predicted to be Rhomboid dependent (Wasserman et al., 2000), nothing is known about Keren processing.
The multiplicity of Rhomboids and potential ligands in Drosophila implies that there is potential for complexity in EGF receptor signal processing. We have addressed four questions that arise from this possibility. First, do other Drosophila Rhomboids share Rhomboid-1’s protease activity? Secondly, do all participate in EGF receptor signalling, or do they activate other pathways? Thirdly, do the different combinations of ligands, Rhomboids and Star produce different outcomes, indicating a potential for complex combinatorial control of EGF receptor signalling? Fourthly, is Keren a genuine ligand for the EGF receptor and thereby likely to be the missing ligand? To begin to answer these questions, we have analysed systematically the properties of all combinations of four Rhomboids and all three membrane-tethered EGF receptor ligands in the Drosophila EGF signal activation pathway. Our results show that all four Rhomboids have similar proteolytic activity and that all membrane-tethered ligands are substrates for the Rhomboid proteases. Although not required for ligand release from the cell in every Rhomboid–ligand combination, we found that Star acts as an ER export factor for all three ligands. Our data also suggest that at least these four Rhomboids are dedicated to regulation of EGF receptor activity in Drosophila and that Keren is indeed an effective EGF receptor ligand. These results indicate a common mechanism for activating all membrane-tethered EGF receptor ligands in Drosophila but demonstrate the potential for a complex system of regulation.
Results
Rhomboids 2, 3 and 4 are proteases that promote EGF receptor signalling
Rhomboids 1–4 form a group of similar proteins. Within the seven Rhomboid-like molecules in Drosophila, Rhomboids 1–4 are more closely related to each other than they are to any of the remaining three Rhomboids (Figure 1A). Rhomboids 6 and 7 are more divergent, although they both retain the residues required for the serine protease activity of Rhomboid-1 (Urban et al., 2001). Rhomboid-5 has similarities to the others but does not contain the catalytic residues. Because of their similarity to each other and as we have been unable to identify full-length cDNAs for Rhomboids 5, 6 or 7, we focused our analysis on Rhomboids 1–4.
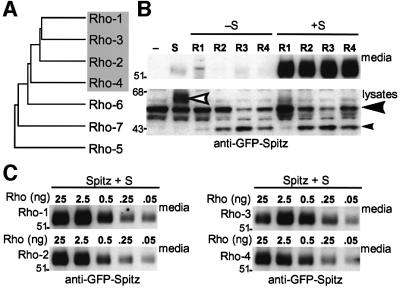
Fig. 1. Drosophila Rhomboids 1–4 are proteases that can cleave Spitz. (A) A dendrogram illustrating the sequence relationship between the seven Drosophila Rhomboids. Rhomboids 1–4 are most similar to each other. (B) GFP-tagged Spitz was cleaved by Rhomboids 1–4 in a Star-dependent manner when transiently expressed in COS cells and analysed by western blotting; the cleaved N-terminus of Spitz accumulated in the medium in all four cases. Note that Rhomboids 2, 3 and 4 could cleave Spitz intracellularly in the absence of Star (small arrowhead, full-length Spitz indicated by large arrowhead: compare the relative levels of full-length and cleaved Spitz in the presence of each Rhomboid), but this cleaved product was not secreted. The white arrowhead shows the hyperglycosylated form of Spitz caused by Star expression in the absence of Rhomboid (Lee et al., 2001). (C) Rhomboid levels in the Spitz cleavage assay were reduced by decreasing the amount of transfected rhomboid DNA (shown in ng) (Urban et al., 2001). All four Rhomboids cleaved and secreted Spitz at equivalent levels, even when they became limiting.
Rhomboids 1–4 catalyse Spitz proteolysis. We tested whether Rhomboids 1–4 all had proteolytic activity against Spitz, the known substrate for Rhomboid-1 (Lee et al., 2001; Urban et al., 2001) (Figure 1B). Spitz was cleaved efficiently by all Rhomboids tested, albeit with some significant differences. Our cell culture assay allowed us to distinguish Spitz cleaved in cell lysates from that which had been secreted into the medium. The amount of cleaved Spitz detected in cells varied with different Rhomboids; no or very little intracellular cleaved Spitz was detected in cells with Rhomboid-1, while cleaved intracellular Spitz was readily detected with Rhomboids 2–4. This is most apparent by comparing the relative levels of full-length and cleaved Spitz in cell lysates (Figure 1B; large and small arrowheads, respectively). In the presence of Star, the amount of secreted Spitz present in the medium was the same for all core Rhomboids, even when they were made limiting by reducing their levels of expression (Figure 1C), indicating that all four Rhomboids have similar levels of proteolytic activity against Spitz.
Star is not essential for cleavage by Rhomboids 2, 3 and 4. Star regulates Spitz cleavage by Rhomboid-1 by transporting Spitz to the Golgi apparatus (Lee et al., 2001). Strikingly, although Star was essential for ligand secretion into the culture medium in each case, it did not affect the ability of Rhomboids 2, 3 and 4 to catalyse Spitz cleavage (Figure 1B). The extensive O-linked glycosylation that is diagnostic of transit through the Golgi apparatus (and which increases the apparent molecular weight of Spitz) (Lee et al., 2001) was not present in cell lysates [Figure 1B; compare the size of secreted green fluorescent protein (GFP)–Spitz in the media (∼55 kDa) with the size of the cleaved intracellular GFP–Spitz (∼43 kDa)]. Therefore, in contrast to Rhomboid-1, Rhomboids 2, 3 and 4 caused the accumulation of an intracellular cleaved Spitz that was not transported past the trans-Golgi network and thus not secreted.
Rhomboids 2–4 promote EGF receptor signalling in Drosophila. The ability of Rhomboids 1–4 to catalyse Spitz cleavage in the tissue culture assay suggested that all may be involved in activating the EGF receptor in vivo. This has been clearly demonstrated for Rhomboid-1, was genetically determined in the case of Rhomboid-3, and was proposed for Rhomboid-2 (Ruohola-Baker et al., 1993; Sturtevant et al., 1993; Wasserman and Freeman, 1997; Guichard et al., 2000; Wasserman et al., 2000; Lee et al., 2001; Ghiglione et al., 2002). To investigate this further, we compared the potential activity of Rhomboids 2–4 in vivo by overexpressing them in developing Drosophila tissues. In all cases examined, Rhomboids 2–4 caused similar phenotypes to Rhomboid-1, consistent only with EGF receptor hyperactivation. When expressed in the developing wing, for example, all core Rhomboids produced ectopic and thickened vein phenotypes similar to those observed for Rhomboid-1 (Figure 2). This phenotype was modified predictably by mutations in other members of the EGF receptor pathway (data not shown). Furthermore, as in cell culture assays, all four Rhomboids were synergistic with the co-expression of Star (e.g. Rhomboid-4 shown in Figure 2). In all cases, UAS Rhomboids 1 and 3 produced consistently strong wing phenotypes, whereas Rhomboids 2 and 4 were weaker. Similar results were obtained in the eye, follicle cells of the ovary and the embryo (data not shown). Importantly, no other phenotypes were observed in eyes, wings or embryos expressing Rhomboids, suggesting that they do not affect any other pathways. If, for example, the previously uncharacterized Rhomboids 2 or 4 caused the activation of other signalling pathways, their ectopic expression would lead to additional phenotypes. These observations confirm that Rhomboids 2–4 contain the same proteolytic activity as Rhomboid-1; furthermore, the absence of phenotypes associated with other pathways strongly suggests that Rhomboids 1–4 are all dedicated to regulating EGF receptor signalling.
Fig. 2. Analysis of Rhomboid 1–4 activity in Drosophila. Rhomboids 1–4 were ectopically expressed in wings using the MS1096-Gal4 driver and caused EGF receptor hyperactivation phenotypes, including thick veins and blisters. Multiple transgenic lines were isolated in each case: Rhomboids 2 and 4 produced a spectrum of phenotypes (each indicated with three panels), which included phenotypes identical to Rhomboids 1 and 3, but their average phenotypes were significantly weaker. While the expression of Star and the weakest Rhomboid-4 transgene (which was X-linked and examined in females) yielded only subtle effects, if any, their co-expression resulted in strongly synergistic phenotypes (bottom row).
These data demonstrate that Rhomboids 1–4 all share proteolytic activity against the ligand Spitz. The next question we addressed was whether the other Drosophila membrane-tethered ligands, Keren and Gurken, were also substrates for any of Rhomboids 1–4.
Keren processing by Rhomboids and Star
Keren is highly similar to Spitz. We identified a new Spitz-like gene as a cDNA submitted to GenBank by the Berkeley Drosophila cDNA sequencing project (DDBJ/EMBL/GenBank accession No. AA990660). With the subsequent completion of the Drosophila genome sequence, this gene has been annotated as Keren (Gadfly CG8056) and is the only previously unknown membrane-tethered EGF-like molecule identified by the Drosophila genome project. Keren has been referred to previously as Spitz-2 and Gritz. Like Spitz and Gurken, Keren has a single extracellular EGF repeat and a single transmembrane domain. The amino acid sequence of Keren is more closely related to Spitz than to Gurken (49% identity, 55% similarity to Spitz; 30% identity, 37% similarity to Gurken, Figure 3A). While all three ligands were predicted to have N- and O-linked glycosylation signals, Spitz contains a 10 residue insert in its N-terminus, which contains an additional O-linked glycosylation site. Consistent with this, Spitz is the only ligand to be hyperglycosylated in the presence of Star (compare Figure 1B, white arrow, with Figures 3B and 5A), although deletion of the insert does not fully abolish hyperglycosylation (data not shown).
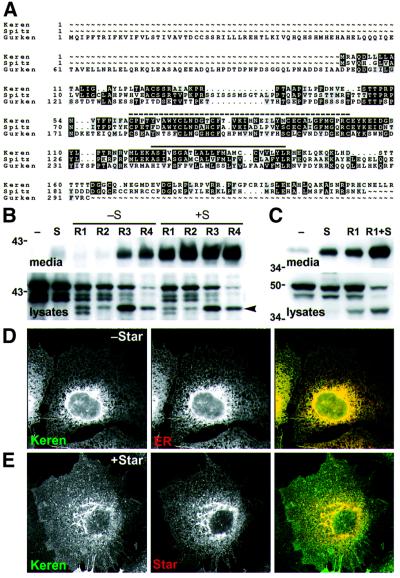
Fig. 3. Keren processing by Star and Rhomboids 1–4. (A) A GCG pileup alignment of Keren, Gurken and Spitz. A dashed line indicates the locations of the EGF domains. Transmembrane domains predicted by TMHMM (Krogh et al., 2001) are indicated with a solid line. (B) Western blots of conditioned medium and lysates from COS cells transfected with Keren with or without Star and/or Rhomboids 1–4. GFP–Keren was expressed alone (–) or in the presence (+S) or absence (–S) of Star with each of the four Rhomboids (R1–R4). Keren was cleaved and secreted efficiently by all four Rhomboids in the presence of Star; it was also cleaved and secreted at a lower level by Rhomboids 3 and 4 in the absence of Star. An intracellular cleaved product (arrowhead) was produced by all four Rhomboids. (C) Processing of Keren by Star and Rhomboid-1 in S2 cells: compared with Spitz, there is a higher level of cleavage and secretion triggered by Star or Rhomboid-1 alone, but the presence of Rhomboid-1 and Star together enhanced processing. (D and E) Intracellular localization of Keren. Cells were transfected with GFP–Keren with or without Myc-Star. The localization of each protein was detected by immunofluorescence. (D) GFP–Keren co-localized with an antibody against an endogenous ER marker (αPDI). (E) Co-expression of Star with GFP–Keren caused Keren to be exported from the ER and accumulate in the Golgi apparatus and at the cell surface.
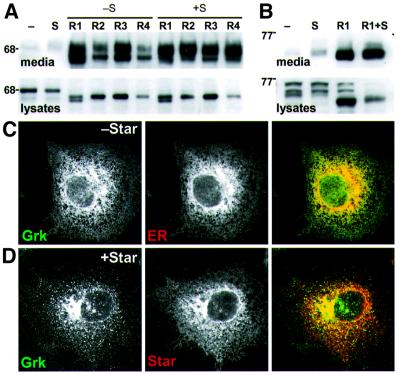
Fig. 5. Gurken processing by Star and Rhomboids 1–4. (A) Western blots of conditioned medium and lysates from COS cells transfected with Gurken with or without Star and/or Rhomboids 1–4. GFP–Gurken was expressed alone (–) or in the presence (+S) or absence (–S) of Star with each of the four Rhomboids (R1–R4). In COS cells, Gurken was cleaved and secreted by all four Rhomboids, independently of Star; all four also caused intracellular cleavage. (B) Processing of Gurken in S2 cells was similar to that in COS cells. (C and D) Intracellular localization of Gurken. Cells were transfected with GFP–Gurken and/or Myc-Star. The localization of each protein was detected by immunofluorescence. (C) GFP-tagged Gurken co-localized with an antibody against an endogenous ER marker (αPDI). (D) Co-expression of Star with GFP–Gurken caused Gurken to be exported from the ER and accumulate in the Golgi apparatus but, in contrast to Spitz and Keren, not at the cell surface.
Rhomboids 1–4 cleave Keren. Keren’s high similarity to Spitz suggested that it might also be a substrate for Rhomboids 1–4, and we have confirmed this: Rhomboids 1–4 all cleaved Keren in a mammalian tissue culture assay. There was, however, an interesting distinction between Keren and Spitz: Star was not essential for Keren secretion in every case (Figure 3B). A significant amount of Keren was secreted in the presence of Rhomboids 3 and 4 alone. Despite this, Star always enhanced the secretion of cleaved Keren, implying that it can interact with Keren. Another difference between Keren and Spitz was that cleaved but non-secreted Keren accumulated in cells in the presence of any of Rhomboids 1–4. Recall that this was the case for Spitz cleaved by Rhomboids 2–4, but not for Rhomboid-1. This discrepancy could be explained if Keren was a better substrate than Spitz for Rhomboids 1–4 or as a consequence of the higher expression levels of Keren. Keren processing by Rhomboid-1 in Drosophila S2 cells was similar but not identical to that observed in COS cells: in S2 cells, a small amount of Keren was secreted in the absence of additional Star and Rhomboid-1 (Figure 3C), but the amount increased in the presence of Star or Rhomboid-1 alone and, as in mammalian cells, was significantly enhanced in the presence of both Rhomboid-1 and Star (Figure 3C).
Keren is localized to the ER. A key to understanding the regulation of Spitz activation by Rhomboid-1 and Star was the observation that the ligand was restricted to the ER in the absence of Star (Lee et al., 2001). We therefore examined the intracellular localization of Keren in COS cells. Like Spitz, Keren was only detectable in the ER; it exhibited characteristic perinuclear and reticular staining and co-localized with the ER marker protein disulfide isomerase (PDI) (Figure 3D).
Star exports Keren out of the ER. Star’s role in Spitz activation is to export Spitz from the ER to the Golgi apparatus where it encounters the proteolytic activity of Rhomboid-1. Star also promoted the release of Keren into the medium, suggesting its role was similar to that in Spitz processing. To test this directly, we co-expressed Star and Keren in COS cells. In the presence of Star, Keren was no longer detectable in the ER and was now entirely in the Golgi apparatus and plasma membrane (Figure 3E). This relocalization of Keren was very similar to the relocalization observed for Spitz, suggesting that Keren also needs to be relocalized to the Golgi apparatus for efficient processing and secretion.
Keren can activate EGF receptor signalling in vivo
To test the prediction that Keren is a genuine EGF receptor ligand, we misexpressed it in developing Drosophila tissues. By analogy to similar experiments with Spitz, we expressed either full-length, membrane-tethered Keren (mKeren), or a truncated form that corresponds to the extracellular, secreted form of Keren (sKeren). In most contexts, full-length Spitz is unable to signal when misexpressed because Star and Rhomboid-1 activity limit its activation, while a truncated form of Spitz, missing its transmembrane domain and C-terminus, signals in a Rhomboid-1- and Star-independent manner (Schweitzer et al., 1995).
In contrast to Spitz, ectopic expression of mKeren activated the EGF receptor pathway in both eyes and wings. For example, when expressed in the developing wing, mKeren produced wing phenotypes similar to misexpression of other positively acting members of the EGF receptor pathway, ranging from thickened and ectopic wing veins to blistering (Figure 4B and C). In many cases, the activation was so strong that the entire wing was converted to vein-like material (Figure 4C). These results indicate that either Keren has membrane-tethered, juxtacrine activity or that it is processed and secreted, possibly by Rhomboids 3 or 4 (see Discussion), which would be consistent with the results obtained in the cell culture assay.
Fig. 4. Ectopic expression of Keren in wings and the ventral epidermis. (A) Wild-type wing. (B and C) Full-length UAS-Keren expressed in wings with the MS1096-Gal4 driver produced a range of EGF receptor hyperactivity phenotypes: thickened wing veins and extra wing vein material. (D–F) Expression of secreted Spitz in the embryo with the arm-Gal4 driver produced typical EGF receptor overactivation phenotypes in the ventral epidermis (Szüts et al., 1997; Payre et al., 1999). Wild-type denticle belts have a characteristic arrangement of six rows (D); overexpression of sSpi (E) or sKer (F) caused the formation of extra denticles.
To test whether the activity of mKeren represented the full potential phenotype of ectopic Keren, or whether proteolytic activation had the potential to activate it further, we examined the effects of sKeren. This form was even more potent than mKeren, causing lethality even when driven by tissue-specific drivers. However, in the embryo, where mKeren had a weak effect, ubiquitous misexpression of sKeren caused lethality and resulted in significantly widened denticle belts and a reduction in naked cuticle in the ventral epidermis (Figure 4F), identical to that caused by the misexpression of sSpitz (Figure 4E) (O’Keefe et al., 1997; Szüts et al., 1997). The greater potency of sKeren therefore suggests that Keren is proteolytically activated in vivo.
Together, these results indicate that Keren is a genuine ligand for the EGF receptor, being able to activate the receptor pathway in vivo. They also suggest that Keren is activated by Rhomboid proteases and Star, although it remains possible that the membrane-tethered form of the ligand has some juxtacrine activity.
Gurken processing by Rhomboids and Star
Rhomboids 1–4 cleave Gurken. The improved signalling ability of a secreted form of Gurken and the recent observation that Rhomboid-2 is required for Gurken activity in a wing misexpression assay (Guichard et al., 2000) raise the possibility that Gurken may also be processed by Rhomboid proteases. To investigate this further, we analysed Gurken processing by Rhomboids 1–4 in the mammalian cell culture assay. As with Spitz and Keren, we found that all core Rhomboids could indeed cleave Gurken, although the role of Star was more variable: in some experiments, Star appeared to have very little influence (Figure 5A and B), while in others it significantly enhanced secretion. In fact, Gurken cleavage appeared very efficient: unlike the other two ligands, only the cleaved form was seen in cell lysates. This parallels the complete cleavage of Gurken deduced in oocytes (Ghiglione et al., 2002) and suggests that Gurken may be the best substrate for Rhomboids 1–4.
In COS cells, Gurken was secreted in response to each of Rhomboids 1–4 in the absence of Star. Although Star improved secretion of Gurken for Rhomboids 2 and 4, it had only a minor effect on Gurken secretion in the presence of Rhomboids 1 and 3 (Figure 5A). Gurken cleavage by Rhomboid-1 in S2 cells was similar to that observed in COS cells (Figure 5B).
Gurken is localized to the ER. To determine if Gurken processing might also be regulated by its intracellular localization, we expressed GFP–Gurken in COS cells. Like Spitz and Keren, Gurken was confined to the ER and could not be detected in the Golgi apparatus (Figure 5C).
Star exports Gurken out of the ER. Despite the more limited effect of Star on Gurken secretion, co-expression of Star caused the export of Gurken from the ER to the Golgi apparatus, implying that its trafficking can be regulated similarly to Spitz and Keren. Unlike for Spitz and Keren, however, in the absence of Rhomboids, Star did not cause full-length Gurken to relocate to the plasma membrane (Figure 5D). Instead, relocalized Gurken remained confined to the Golgi apparatus. This suggests that Gurken may lack signals required for Golgi to plasma membrane transport, or may contain signals for Golgi retention. In either case, it suggests that post-Golgi secretion of Gurken may be a regulated process.
Subcellular localization of Rhomboids 1–4
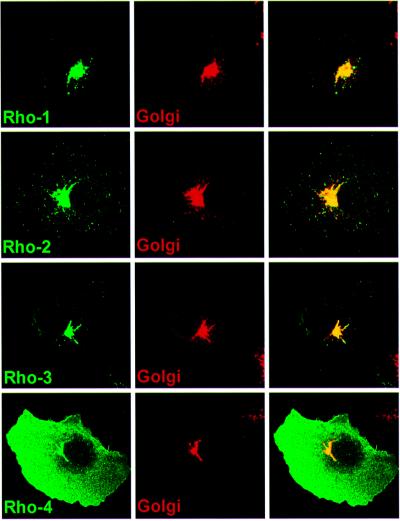
Rhomboid-1 is a Golgi-localized protein and its proteolytic activity for Spitz is confined to this compartment; it does not cleave Spitz to any significant degree in the ER. To determine whether the amount of Spitz cleavage in the absence of Star correlated with the subcellular localization of Rhomboids 1–4 in COS cells, we examined their intracellular localization by immunofluorescence (Figure 6). Rhomboids 2 and 3 were indistinguishable from Rhomboid-1: they were observed solely in the Golgi apparatus. In contrast, Rhomboid-4 was present at high levels at the plasma membrane, as well as in the Golgi apparatus. None of Rhomboids 1–4 were detectable in the ER.
Fig. 6. Intracellular localization of HA-tagged Rhomboids 1–4 in COS cells. Rhomboids 2 and 3 could only be detected in the Golgi apparatus like Rhomboid-1, and co-localized with the known Golgi marker p115. Rhomboid-4 displayed a pronounced cell surface distribution, although it was also detected in the Golgi apparatus, but not in the ER.
ER-cleaved Spitz cannot be secreted
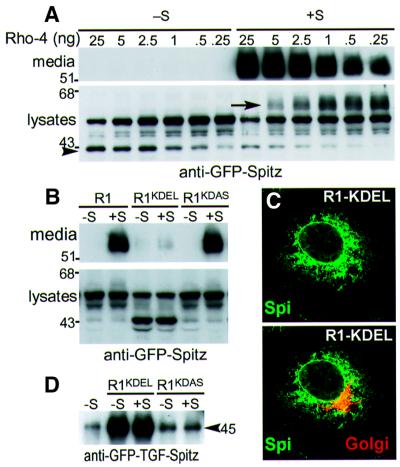
Unlike Spitz cleavage by Rhomboid-1, all other Rhomboids led to substantial intracellular accumulation of cleaved Spitz and concomitant reduction of the full-length form. The distinct glycosylation signature of Spitz allows its location to be inferred (Lee et al., 2001): the cleaved ligand appeared to be in a pre-trans-Golgi compartment. This intracellular cleavage suggests that there might be a low but functional amount of Rhomboid in the ER, perhaps in transit through the ER on its way to the Golgi apparatus. This was not an artefact of overexpression, as reducing Rhomboid-4 by 1000-fold still resulted in intracellular Spitz cleavage in the absence of Star (Figure 7A). Since the intracellularly cleaved Spitz was not secreted, these results imply that Spitz is not secreted when cleaved prior to reaching the Golgi apparatus.
Fig. 7. The subcellular site of Spitz cleavage determines whether Spitz is secreted. (A) A limiting dilution series of Rhomboid-4 resulted in efficient intracellular Spitz cleavage in the absence of Star (arrowhead), but this form was not secreted (as detected by western blotting). Note that in the presence of Star, full-length Spitz became hyperglycosylated (arrow) when it passed through the Golgi apparatus without being cleaved (Lee et al., 2001) as a result of limiting Rhomboid-4 expression. (B) Rhomboid-1 targeted to the ER by a KDEL signal in its C-terminus cleaved Spitz in the absence of Star, but this cleaved product was not secreted, even in the presence of Star. Rhomboid-1 carrying the mutated ER retention signal KDAS behaved as wild-type Rhomboid-1. (C) GFP–Spitz (green) was confined to the ER even in the presence of Rhomboid-1–KDEL, when ∼50% was cleaved, see (B). Note that there was no co-localization of GFP–Spitz with a Golgi marker (red). (D) When the N-terminus of Spitz was replaced by TGF-α (junction at the last cysteine of the EGF repeat), this chimeric protein (GFP–TGF–Spi) was cleaved by Rhomboid-1–KDEL, and the cleaved form (of ∼45 kDa) was secreted from cells efficiently.
To address this further, we artificially introduced Rhomboid-1 to the ER by fusing it to the ER retrieval signal KDEL (Munro and Pelham, 1987). As expected, when expressed in COS cells, Rhomboid-1–KDEL was now present in the ER (Urban et al., 2001). This caused the intracellular accumulation of cleaved Spitz, but this soluble Spitz was retained in the ER and not secreted, as detected by western blotting (Figure 7B), nor could it be seen in the Golgi apparatus (Figure 7C). These results imply that Spitz that has been cleaved in the ER does not readily exit to the Golgi apparatus. In contrast, when the Spitz extracellular domain was replaced with that of TGF-α in a chimeric protein, the ER-cleaved form was readily secreted (Figure 7D), implying that the luminal domain of Spitz can be retained specifically in the ER. Interestingly, the presence of Star did not facilitate the secretion of the ER-cleaved Spitz (Figure 7B), suggesting that Rhomboid-cleaved Spitz cannot be transported efficiently by Star. These results highlight the importance of regulated subcellular localization in the activation and secretion of Spitz. Note, however, that we and others (Pascall et al., 2002) have found that an artificial form of Spitz, which is truncated in the extracellular region between the membrane and the EGF domain, can have its secretion enhanced by Star, albeit with considerable variability between experiments and cell types. This suggests that there may be additional complexity in the regulation of secretion of soluble ligands which may depend in part on the exact nature of their C-terminus (S.Urban and M.Freeman, unpublished data).
Discussion
A common mechanism activates all membrane-tethered EGF receptor ligands in Drosophila
Activation of Spitz, the principal EGF receptor ligand in Drosophila, occurs through its regulated intracellular trafficking by Star and intramembrane proteolysis by Rhomboid-1 (Lee et al., 2001; Urban et al., 2001; Tsruya et al., 2002). Recent evidence suggests that Gurken may also be activated by proteolytic release (Ghiglione et al., 2002). We have addressed four initial questions that arise from the presence of multiple Rhomboids and potential ligands in Drosophila by characterizing the activation of Spitz, Keren and Gurken by Star and the proteases Rhomboids 1–4. First, we found that Rhomboids 1–4 all have proteolytic activity and efficiently catalysed the proteolysis of Keren, Gurken and Spitz. Secondly, Rhomboids 1–4 promoted only EGF receptor signalling when misexpressed in vivo, indicating that they are specific to the EGF receptor pathway and do not affect any other pathways. Thirdly, ligand release for different Rhomboid–ligand combinations had different requirements for Star, and the three ligands were cleaved by Rhomboids 1–4 with varying efficiency, suggesting that each combination of Rhomboid and ligand may produce different signalling intensities in vivo. Fourthly, we found that Keren was a new activating ligand of the EGF receptor pathway and could be processed in a Rhomboid/Star-dependent manner, strongly suggesting that it is indeed the predicted missing ligand.
Thus, coupled with previous genetic evidence, the activation of all Drosophila membrane-tethered EGF receptor ligands appears to use a common mechanism involving subcellular transport by Star and intramembrane proteolysis by Rhomboid proteases. This mechanism is distinct from the activation of most previously characterized membrane-tethered growth factors, in which ligands are cleaved at the cell surface by metalloproteases such as TACE (Arribas et al., 1996; Peschon et al., 1998). The conservation of Rhomboids throughout all branches of life (Wasserman et al., 2000) suggests that this EGF activation mechanism in Drosophila may represent an ancient and widespread signal-sending strategy.
The secretion of Rhomboid-cleaved Spitz is determined by its intracellular localization
Although Star is required to export Spitz from the ER to the Golgi apparatus, where Spitz is cleaved by Rhomboid-1, our observation that ER-cleaved Spitz cannot be secreted refines this model: only the form cleaved in the Golgi apparatus appears to be competent for secretion. This may guard against inappropriate ligand activation by Rhomboid proteases in the absence of Star, which could occur while the proteases are en route from the ER to the Golgi apparatus. This would explain why Spitz can be cleaved intracellularly by Rhomboids 2–4 without Star, but is not secreted concomitantly. Since the TGF-α extracellular domain is readily secreted from the ER, Spitz appears either to contain signals in its N-terminal domain for ER retention, or to lack signals for ER export.
The inability of Star to interact with a KDEL– Rhomboid-cleaved form of Spitz emphasizes Star’s predominant role as an ER export factor for full-length Spitz rather than as a secretion factor for Rhomboid-cleaved Spitz. The more limited role of Star in Keren and Gurken processing may suggest that these ligands are cleaved and secreted more readily from the ER, or are able to cycle between the ER and the Golgi apparatus where they can be cleaved by Golgi-localized Rhomboids. Current evidence does not allow us to distinguish between these two models.
Keren is a new Drosophila EGF receptor ligand
Genetic experiments suggest that, in addition to Spitz, there is another Rhomboid- and Star-dependent EGF receptor ligand. Specifically, in the Drosophila eye, loss of both Rhomboid-1 and Rhomboid-3 mimics the excess apoptosis phenotype observed in Egfr– clones, whereas spitz– clones have different survival characteristics (Domínguez et al., 1998; Wasserman et al., 2000; Baker and Yu, 2001). Similarly, in the wing, the EGF receptor, Rhomboid-1 and Star are required for vein formation, whereas Spitz is not (Sturtevant et al., 1993; Simcox, 1997; Nagaraj et al., 1999). The identification of Keren, the only other membrane-tethered EGF-like protein identified in Drosophila (apart from Gurken, whose function is confined to the germline), led us to speculate that this was the predicted missing ligand in the eye and wing (Wasserman et al., 2000; Baonza et al., 2001).
Consistent with the profile of the missing ligand, our analysis now shows that, like Spitz, Keren can be processed in a Rhomboid- and Star-dependent manner and can ectopically activate the EGF receptor pathway in vivo. Note that we could not detect significant levels of Keren mRNA in any developing tissues by in situ hybridization, but RT–PCR on RNA from staged embryos, larvae and adults indicates that it is expressed at a low level, perhaps ubiquitously (J.R.Lee and M.Freeman, unpublished data). Despite these similarities, Keren does not behave identically to Spitz. The most striking difference was that Keren did not require Star for low levels of secretion in the presence of Rhomboid-3 and Rhomboid-4 and was readily secreted from S2 cells in the presence of either Star or Rhomboid-1 alone. Consistent with this observation, Keren was able to activate the EGF receptor when expressed as a full-length precursor, suggesting that, unlike for Spitz, Keren processing is not limited by Rhomboid-1 and Star activity alone. Because it appears to be cleaved efficiently by all core Rhomboids, perhaps the full-length form can be activated by a low level, ubiquitously expressed Rhomboid in a Star-independent manner. Of the two Rhomboids that can activate Keren in the absence of Star, Rhomboid-4 may be the best candidate as it may be expressed ubiquitously at low levels (S.Urban and M.Freeman, unpublished data), whereas Rhomboid-3 expression is detected only in the eye (Wasserman et al., 2000). An alternative is that Keren could participate in juxtacrine signalling, although, given its ER localization, this would be expected to be limited by Star expression. A detailed genetic analysis of a null allele of Keren (as yet unavailable) will be required to fully understand Keren function and processing.
Gurken processing by Rhomboid proteases and Star
Gurken function is restricted to oogenesis where it is required to polarize both major axes of the egg (Schüpbach, 1987). Recent evidence strongly suggests that Gurken undergoes proteolytic processing in vivo: Gurken is released from the oocyte and is internalized by follicle cells (Peri et al., 1999), exists exclusively in a cleaved form in oocytes (Ghiglione et al., 2002), and an uncleavable mutant form is inactive (Queenan et al., 1999; C.Bökel, personal communication). Our observations demonstrate that Gurken can be processed directly by Rhomboid proteases 1–4. In our tissue culture assay, Rhomboid protease activity was required for Gurken cleavage and secretion in all cases. Although Rhomboid-1 is not required in the female germline, the specific expression of Rhomboid-2 in the early oocyte suggests that a Rhomboid might have a role in Gurken processing (Guichard et al., 2000; Ghiglione et al., 2002). Consistent with this suggestion, both groups have demonstrated recently that Rhomboid-2 has the potential to activate Gurken in vivo.
We have also shown directly that Star can translocate Gurken from the ER to the Golgi apparatus in cell culture and, in some cases, enhance Gurken secretion. Consistent with this, Ghiglione et al. (2002) demonstrated a role for Star in Gurken activation in vivo using antisense Star constructs. Together, these results strongly suggest that Gurken activity, like that of Spitz, is at least partially regulated by Star-dependent ER to Golgi transport. The regulation of Gurken activity, however, also depends on the transmembrane protein Cornichon (Roth et al., 1995; Queenan et al., 1999). Recent evidence in yeast and Drosophila suggests that Cornichon is an ER export factor (Powers and Barlowe, 1998; C.Bökel, personal communication), raising the question of the relative roles and significance of Star and Cornichon.
Role of the Rhomboid protease family in Drosophila
All animal species examined contain more than one Rhomboid, with Drosophila itself having seven Rhomboids; why? In principle, each could have a distinct substrate and thus function in different pathways. Current evidence suggests that this is not the case for Rhomboids 1–4; when ectopically expressed in developing Drosophila tissues, they caused phenotypes restricted to EGF receptor hyperactivation, suggesting that their activity is limited to this pathway. Consistent with this prediction, all four core Rhomboids were proteolytically active, and processed the three Drosophila membrane-tethered EGF ligands Spitz, Keren and Gurken, each with high efficiency. Never theless, until loss-of-function mutants are available for all rhomboid genes, their respective roles in regulating EGF receptor signalling cannot be determined.
Current data suggest two possible explanations for the multiplicity of Drosophila Rhomboids. First, several members appear to have tissue-specific functions. For example, Rhomboid-3 acts specifically in the developing eye (Wasserman et al., 2000). This duplication of function may reflect the complexity of regulation of EGF receptor activation. Rhomboid-1 is the principal determinant of EGF receptor activity and is regulated transcriptionally. The full gamut of transcriptional control may be too difficult to achieve in a single rhomboid gene. Partitioning this transcriptional regulation among multiple genes of similar biochemical activity could solve this problem. Note that although there is also a requirement for Star, which exists only as a single gene in Drosophila, its expression is not as restricted as that of Rhomboid-1 (Kolodkin et al., 1994), and its requirement is not absolute, as certain Rhomboids have the ability to release EGF receptor ligands in the absence of Star.
A second reason for the multiplicity of Rhomboids is suggested by their distinct characteristics: although all four cleave EGF ligands, they have different abilities to elicit cleavage and secretion of ligands in the absence of Star. As such, multiple Rhomboid–ligand combinations may result in distinct signalling characteristics such as intensity, duration or range.
Finally, the present analysis has been limited to Rhomboids 1–4. Rhomboid-5 lacks residues necessary for proteolysis, but the sequences of Rhomboids 6 and 7 suggest that they are proteases, and a key question will be whether these more distant members of the family are also dedicated to the EGF receptor pathway or whether they have distinct functions.
Materials and methods
Drosophila stocks
UAS-rho-1 and UAS-rho-3 flies are described elsewhere (Wasserman et al., 2000). The following stocks were generated using standard techniques: UAS-mKer, UAS-sKer, UAS-rho-2 and UAS-rho-4. Other lines used were arm-GAL4 (Sanson et al., 1996), MS1096 (Capdevila and Guerrero, 1994) and UAS-Star (Golembo et al., 1996).
Phenotypic analysis
Cuticle preparations were performed as previously described (Szüts et al., 1997).
Sequence analysis
Pileup (Genetics Computer Group, WI) was used to align Spitz, Gurken and Keren in Drosophila. We aligned the seven Rhomboid sequences with Clustal W (Thompson et al., 1994) using default parameters, and conducted a UPGMA analysis, as implemented by PAUP* 4.b10 (Sinauer Associates, Sunderland, MA).
Constructs
Full-length Keren, secreted Keren (truncated at residue 112), Rhomboid-2 and Rhomboid-4 were cloned into the pUAST vector for fly transformation. A BsiWI site was introduced by PCR mutagenesis between residues 27 and 28 of Keren and 28 and 29 of Gurken, allowing the EGFP open reading frame (Clontech) to be inserted. Haemagglutinin (HA)-tagged Rhomboid-1 and Myc-tagged Star have been described elsewhere (Lee et al., 2001). GFP–TGF–Spitz is a chimera that resembles TGF-α:Spi-TMC (Lee et al., 2001) except that the junction is at the last cysteine of the EGF domain, giving it a Spitz juxtamembrane domain. A triple HA tag was inserted after the start methionine of Rhomboids 2, 3 and 4.
Cell culture
All constructs for mammalian tissue culture were cloned into pcDNA3.1 (Invitrogen). Constructs for Drosophila S2 tissue culture were cloned into pRmHa3 (Bunch et al., 1988) and induced with 250 µM CuSO4. The cleavage assay has been described previously (Lee et al., 2001). Briefly, COS cells were transfected in 35 mm culture wells; 24–30 h post-transfection, the medium was replaced with serum-free medium; this was harvested 24 h later and cells were lysed in SDS sample buffer. After induction, lysates and conditioned medium were prepared from S2 cells as previously described (Lee et al., 2001). GFP was detected by western blotting with a rabbit polyclonal antibody (gift of Rob Arkowitz).
Immunofluorescence
Preparation and staining of fixed transfected cells was described in Lee et al. (2001). The following primary antibodies were used: mouse anti-Myc 9E10 (Santa Cruz Biotechnology) at 1:250, rat anti-HA (Roche) at 1:500, rabbit anti-PDI (Calbiochem) at 1:250 and mouse anti-p115 (Transduction Labs; a mammalian cell Golgi marker) at 1:250. Alexa Fluor 568 (red)- and Alexa Fluor 488 (green)-conjugated secondary antibodies from Molecular Probes were used at 1:500. All images were collected on a Radiance 2001 confocal microscope (Bio-Rad).
Acknowledgments
Acknowledgements
We thank Joseph Parker for help with sequence analysis, and Christian Bökel for helpful discussions and sharing unpublished data. S.U. was partly supported by an NSERC (Canada) scholarship, an external research studentship of Trinity College, Cambridge and a Junior Research Fellowship from Christ’s College, Cambridge. J.R.L. was partly supported by an NSERC (Canada) scholarship and the Cambridge Commonwealth Trust.
References
- Adamson E.D. (1990) Developmental activities of the epidermal growth factor receptor. Curr. Top. Dev. Biol., 24, 1–29. [DOI] [PubMed] [Google Scholar]
- Arribas J., Coodly,L., Vollmer,P., Kishimoto,T.K., Rose-John,S. and Massagué,J. (1996) Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J. Biol. Chem., 271, 11376–11382. [DOI] [PubMed] [Google Scholar]
- Baker N.E. and Yu,S.Y. (2001) The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell, 104, 699–708. [DOI] [PubMed] [Google Scholar]
- Bang A.G. and Kintner,C. (2000) Rhomboid and Star facilitate presentation and processing of the Drosophila TGF-α homolog Spitz. Genes Dev., 14, 177–186. [PMC free article] [PubMed] [Google Scholar]
- Baonza A., Casci,T. and Freeman,M. (2001) A primary role for the EGF receptor in ommatidial spacing in the Drosophila eye. Curr. Biol., 11, 396–404. [DOI] [PubMed] [Google Scholar]
- Black R.A. and White,J.M. (1998) ADAMs: focus on the protease domain. Curr. Opin. Cell Biol., 10, 654–659. [DOI] [PubMed] [Google Scholar]
- Brown M.S., Ye,J., Rawson,R.B. and Goldstein,J.L. (2000) Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell, 100, 391–398. [DOI] [PubMed] [Google Scholar]
- Bunch T.A., Grinblat,Y. and Goldstein,L.S.B. (1988) Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids Res., 16, 1043–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J. and Guerrero,I. (1994) Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J., 13, 4459–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez M., Wasserman,J.D. and Freeman,M. (1998) Multiple functions of the EGF receptor in Drosophila eye development. Curr. Biol., 8, 1039–1048. [DOI] [PubMed] [Google Scholar]
- Freeman M. (1994) The spitz gene is required for photoreceptor determination in the Drosophila eye where it interacts with the EGF receptor. Mech. Dev., 48, 25–33. [DOI] [PubMed] [Google Scholar]
- Freeman M., Kimmel,B.E. and Rubin,G.M. (1992) Identifying targets of the rough homeobox gene of Drosophila: evidence that rhomboid functions in eye development. Development, 116, 335–346. [DOI] [PubMed] [Google Scholar]
- Ghiglione C., Bach,E.A., Paraiso,Y., Carraway,K.L.,III, Noselli,S. and Perrimon,N. (2002) Mechanism of activation of the Drosophila EGF receptor by the TGFα ligand Gurken during oogenesis. Development, 129, 175–186. [DOI] [PubMed] [Google Scholar]
- Golembo M., Raz,E. and Shilo,B.Z. (1996) The Drosophila embryonic midline is the site of Spitz processing and induces activation of the EGF receptor in the ventral ectoderm. Development, 122, 3363–3370. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A., Elliott,H. and St Johnston,D. (1995) Polarization of both major body axes in Drosophila by gurken–torpedo signalling. Nature, 375, 654–658. [DOI] [PubMed] [Google Scholar]
- Guichard A., Roark,M., Ronshaugen,M. and Bier,E. (2000) brother of rhomboid, a rhomboid-related gene expressed during early Drosophila oogenesis, promotes EGF-R/MAPK signaling. Dev. Biol., 226, 255–266. [DOI] [PubMed] [Google Scholar]
- Huppert S. and Kopan,R. (2001) Regulated intramembrane proteolysis takes another twist. Dev. Cell, 1, 590–592. [DOI] [PubMed] [Google Scholar]
- Klämbt C. (2002) EGF receptor signalling: roles of star and rhomboid revealed. Curr. Biol., 12, R21–R23. [DOI] [PubMed] [Google Scholar]
- Kolodkin A.L., Pickup,A.T., Lin,D.M., Goodman,C.S. and Banerjee,U. (1994) Characterization of Star and its interactions with sevenless and EGF receptor during photoreceptor cell development in Drosophila. Development, 120, 1731–1745. [DOI] [PubMed] [Google Scholar]
- Krogh A., Larsson,B., von Heijne,G. and Sonnhammer,E.L. (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol., 305, 567–580. [DOI] [PubMed] [Google Scholar]
- Lee J.R., Urban,S., Garvey,C.F. and Freeman,M. (2001) Regulated intracellular ligand transport and proteolysis controls EGF signal activation in Drosophila. Cell, 107, 161–171. [DOI] [PubMed] [Google Scholar]
- Munro S. and Pelham,H.R. (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell, 48, 899–907. [DOI] [PubMed] [Google Scholar]
- Nagaraj R., Pickup,A.T., Howes,R., Moses,K., Freeman,M. and Banerjee,U. (1999) Role of the EGF receptor pathway in growth and patterning of the Drosophila wing through the regulation of vestigial. Development, 126, 975–985. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg F.S. and Schüpbach,T. (1993) The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGFα-like protein. Cell, 75, 165–174. [PubMed] [Google Scholar]
- O’Keefe L., Dougan,S.T., Gabay,L., Raz,E., Shilo,B.Z. and DiNardo,S. (1997) Spitz and Wingless, emanating from distinct borders, cooperate to establish cell fate across the Engrailed domain in the Drosophila epidermis. Development, 124, 4837–4845. [DOI] [PubMed] [Google Scholar]
- Pascall J.C., Luck,J.E. and Brown,K.D. (2002) Expression in mammalian cell cultures reveals interdependent, but distinct, functions for Star and Rhomboid proteins in the processing of the Drosophila transforming-growth-factor-α homologue Spitz. Biochem. J., 363, 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payre F., Vincent,A. and Carreno,S. (1999) ovo/svb integrates Wingless and DER pathways to control epidermis differentiation. Nature, 400, 271–275. [DOI] [PubMed] [Google Scholar]
- Peri F., Bökel,C. and Roth,S. (1999) Local Gurken signaling and dynamic MAPK activation during Drosophila oogenesis. Mech. Dev., 81, 75–88. [DOI] [PubMed] [Google Scholar]
- Peschon J.J. et al. (1998) An essential role for ectodomain shedding in mammalian development. Science, 282, 1281–1284. [DOI] [PubMed] [Google Scholar]
- Powers J. and Barlowe,C. (1998) Transport of axl2p depends on erv14p, an ER-vesicle protein related to the Drosophila cornichon gene product. J. Cell Biol., 142, 1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan A.M., Barcelo,G., Van Buskirk,C. and Schüpbach,T. (1999) The transmembrane region of Gurken is not required for biological activity, but is necessary for transport to the oocyte membrane in Drosophila. Mech. Dev., 89, 35–42. [DOI] [PubMed] [Google Scholar]
- Roth S., Neuman-Silberberg,F.S., Barcelo,G. and Schüpbach,T. (1995) Cornichon and the EGF receptor signaling process are necessary for both anterior–posterior and dorsal–ventral pattern formation in Drosophila. Cell, 81, 967–978. [DOI] [PubMed] [Google Scholar]
- Ruohola-Baker H., Grell,E., Chou,T.B., Baker,D., Jan,L.Y. and Jan,Y.N. (1993) Spatially localized rhomboid is required for establishment of the dorsal–ventral axis in Drosophila oogenesis. Cell, 73, 953–965. [DOI] [PubMed] [Google Scholar]
- Rutledge B.J., Zhang,K., Bier,E., Jan,Y.N. and Perrimon,N. (1992) The Drosophila spitz gene encodes a putative EGF-like growth factor involved in dorsal–ventral axis formation and neurogenesis. Genes Dev., 6, 1503–1517. [DOI] [PubMed] [Google Scholar]
- Sanson B., White,P. and Vincent,J.P. (1996) Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature, 383, 627–630. [DOI] [PubMed] [Google Scholar]
- Schüpbach T. (1987) Germline and soma cooperate during oogenesis to establish the dorsoventral pattern of egg shell and embryo in Drosophila melanogaster. Cell, 49, 699–707. [DOI] [PubMed] [Google Scholar]
- Schweitzer R. and Shilo,B.-Z. (1997) A thousand and one roles for the Drosophila EGF receptor. Trends Genet., 13, 191–196. [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Shaharabany,M., Seger,R. and Shilo,B.-Z. (1995) Secreted Spitz triggers the DER signalling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes Dev., 9, 1518–1529. [DOI] [PubMed] [Google Scholar]
- Sibilia M., Steinbach,J.P., Stingl,L., Aguzzi,A. and Wagner,E.F. (1998) A strain-independent postnatal degeneration in mice lacking the EGF receptor. EMBO J., 17, 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox A. (1997) Differential requirement for EGF-like ligands in Drosophila wing development. Mech. Dev., 62, 41–50. [DOI] [PubMed] [Google Scholar]
- Sturtevant M.A., Roark,M. and Bier,E. (1993) The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes Dev., 7, 961–973. [DOI] [PubMed] [Google Scholar]
- Szüts D., Freeman,M. and Bienz,M. (1997) Antagonism between EGFR and Wingless signalling in the larval cuticle of Drosophila. Development, 124, 3209–3219. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins,D.G. and Gibson,T.J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res., 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tio M. and Moses,K.M. (1997) The Drosophila TGFα homolog Spitz acts in photoreceptor recruitment in the developing retina. Development, 124, 343–351. [DOI] [PubMed] [Google Scholar]
- Tio M., Ma,C. and Moses,K. (1994) Spitz, a Drosophila homolog of transforming growth factor-α, is required in the founding photoreceptor cells of the compound eye facets. Mech. Dev., 48, 13–23. [DOI] [PubMed] [Google Scholar]
- Tsruya R., Schlesinger,A., Reich,A., Gabay,L., Sapir,A. and Shilo,B.Z. (2002) Intracellular trafficking by Star regulates cleavage of the Drosophila EGF receptor ligand Spitz. Genes Dev., 16, 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S., Lee,J.R. and Freeman,M. (2001) Drosophila Rhomboid-1 defines a family of putative intramembrane serine proteases. Cell, 107, 173–182. [DOI] [PubMed] [Google Scholar]
- Wasserman J.D. and Freeman,M. (1997) Control of EGF receptor activation in Drosophila. Trends Cell Biol., 7, 431–436. [DOI] [PubMed] [Google Scholar]
- Wasserman J.D., Urban,S. and Freeman,M. (2000) A family of rhomboid-like genes: Drosophila rhomboid-1 and roughoid/rhomboid-3 cooperate to activate EGF receptor signalling. Genes Dev., 14, 1651–1663. [PMC free article] [PubMed] [Google Scholar]