Abstract
The N-terminal domain of the largest subunit of the Saccharomyces cerevisiae origin recognition complex (Orc1p) functions in transcriptional silencing and contains a bromo-adjacent homology (BAH) domain found in some chromatin-associated proteins including Sir3p. The 2.2 Å crystal structure of the N-terminal domain of Orc1p revealed a BAH core and a non-conserved helical sub-domain. Mutational analyses demonstrated that the helical sub-domain was necessary and sufficient to bind Sir1p, and critical for targeting Sir1p primarily to the cis-acting E silencers at the HMR and HML silent chromatin domains. In the absence of the BAH domain, ∼14–20% of cells in a population were silenced at the HML locus. Moreover, the distributions of the Sir2p, Sir3p and Sir4p proteins, while normal, were at levels lower than found in wild-type cells. Thus, in the absence of the Orc1p BAH domain, HML resembled silencing of genes adjacent to telomeres. These data are consistent with the view that the Orc1p–Sir1p interaction at the E silencers ensures stable inheritance of pre-established Sir2p, Sir3p and Sir4p complexes at the silent mating type loci.
Keywords: chromatin/gene expression/inheritance/mating type/origin recognition complex
Introduction
Epigenetic silencing, the heritable repression of transcription of genes within chromatin domains, is a highly conserved phenomenon observed from yeast to human. In the yeast Saccharomyces cerevisiae, genes within the silent mating type loci HML and HMR, and genes near telomeres exist in transcriptionally silenced, epigenetic states of chromatin (Loo and Rine, 1995). Unlike at telomeres, where silencing varies among cells in a genetically identical population, repression of the HM loci occurs in all cells in a population and requires specific cis-acting DNA sequences called the E and I ‘silencers’ that flank the HMR and HML genes. Each of the silencers contains two or more binding sites for three different DNA-binding proteins: the origin recognition complex (ORC), Rap1p and Abf1p. Deletion of the HMR-E silencer causes loss of silencing at HMR, whereas removal of the HMR-I silencer has little effect on HMR silencing. In contrast, the HML-E and HML-I silencers function equivalently (for a review, see Loo and Rine, 1995).
Repression of the HM loci requires additional trans-acting factors, most notably the four silent information regulator (Sir) proteins Sir1p, Sir2p, Sir3p and Sir4p that includes the conserved NAD-dependent histone deacetylase Sir2p (Guarente, 1999; Shore, 2000; Dutnall and Pillus, 2001; Gasser and Cockell, 2001). Although no sequence orthologs of Sir1p, Sir3p or Sir4p have been identified in metazoans, they appear to function like heterochromatin protein 1 (HP1) in higher eukaryotes. Deletion of either the SIR2, SIR3 or SIR4 gene abolishes silencing at HM loci, whereas removal of SIR1 results in partial derepression of the HML locus. The partial derepression is the result of co-existence of two genetically identical, but phenotypically distinct populations of cells (Pillus and Rine, 1989). The Sir proteins are targeted to the silenced loci by interacting with silencer-bound proteins such as Rap1p, Abf1p and ORC (Loo and Rine, 1995; Guarente, 1999).
ORC is a six-protein complex that functions in many aspects of DNA metabolism, including initiation of DNA replication and transcriptional silencing (Bell and Stillman, 1992; Bell et al., 1993; Fox et al., 1997; Dillin and Rine, 1998). The N-terminal region of the largest subunit of ORC, Orc1p, is required for transcriptional silencing at the HM loci but is dispensable for DNA replication. The silencing domain of Orc1p shares ∼50% amino acid identity with the N-terminal region of Sir3p (Bell et al., 1995). Unlike Sir3p, however, the Orc1p domain interacts with Sir1p (Triolo and Sternglanz, 1996), and in Drosophila, the corresponding region of dORC1 interacts with HP1 (Pak et al., 1997). In all species, this region of Orc1p contains a bromo-adjacent homology (BAH) domain, which is also present in Sir3p and other chromatin-associated proteins such as mammalian DNA-(cytosine-5)-methyltransferases, components of the yeast RISC chromatin-remodeling complex and histone deacetylase complexes (Callebaut et al., 1999; Figure 1A). Here we report the structure and function of the N-terminal region of Orc1p, and show that it recruits Sir1p to the E silencers to initiate formation of silent chromatin. Localization of the other SIR proteins across the silent HMR and HML loci is partially dependent on Orc1p–Sir1p interaction.
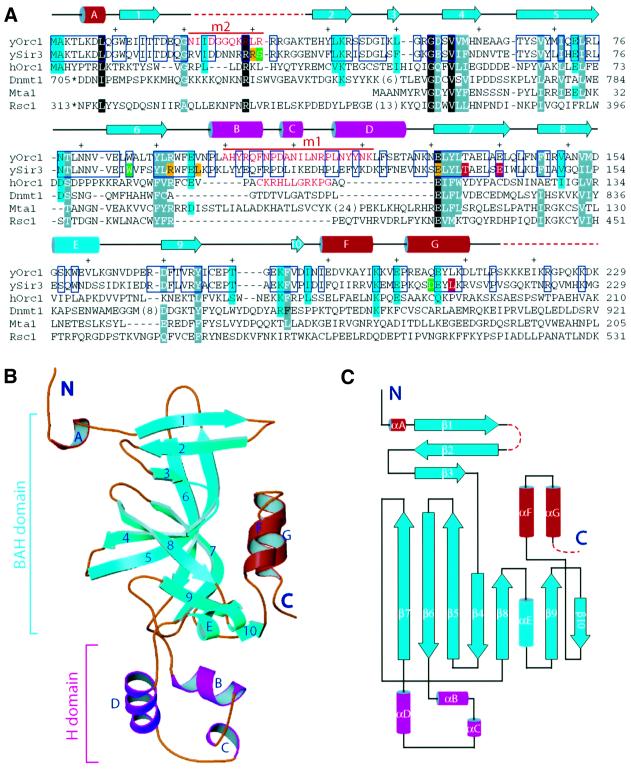
Fig. 1. The structure of the N-terminal domain of Orc1p. (A) Structure-guided sequence alignment of the N-terminal region of S.cerevisiae Orc1p (yOrc1) with the BAH domain-containing region of Sir3p (ySir3), human Orc1p (hOrc1), DNA-(cytosine-5)-methyltransferase 1 (Dnmt1), the human metastasis-associated protein 1 (Mta1) and S.cerevisiae Rsc1. The amino acids shown in white letters on a black background are invariant; white letters on a gray background indicate that similar amino acids are found in at least five proteins. Amino acids similar among yOrc1, ySir3 and hOrc1 are highlighted in cyan, and amino acids identical between yOrc1 and ySir3 are in blue rectangles. Green highlights the position of SIR3 mutants suppressing histone H4 and Rap1 mutations (Johnson et al., 1990; Liu and Lustig, 1996). Residues highlighted in yellow and red are class I and class II Sir3p mutants, respectively, which enhance the sir1 mating-defective phenotype (Stone et al., 2000). Secondary structural elements are colored as in (C) and shown above the sequences. Every 10 aa are indicated with a + sign. Residues shown in red were removed in the orc1m1 and orc1m2 mutants of yOrc1. In the orc1m1 mutant, the amino acids shown in red were replaced by the amino acids from hOrc1, also shown in red. (B) The crystal structure is shown in a ribbon representation. (C) Topology diagram showing the fold of the structure. The core of the structure consists mainly of β-strands and is colored cyan. The H domain is shown in magenta, and N- and C-terminal helices are shown in red. β-strands are numbered consecutively and α-helices are labeled alphabetically from the N- to the C-terminus.
Results
Structure of the BAH region of Orc1p
The Orc1pN235 region [amino acids (aa) 1–235] was crystallized and the structure solved to 2.2 Å resolution using the multi-wavelength anomalous diffraction (MAD) method (see Supplementary table 1 available at The EMBO Journal Online). There were two molecules per asymmetric unit in the crystal unit cell, although purified Orc1pN235 existed as a monomer in solution. Two regions were disordered in the structure: the first was from aa 18 to 36 (aa 23–36 in the second molecule in the asymmetric unit) and the second was in the C-terminus extending beyond aa 215 (Figure 1A). A truncated variant including aa 1–219 (Orc1pN219) crystallized similarly and a mercurial derivative was used for MAD phasing (Supplementary figure 1).
The structure has an elongated shape consisting of two domains: a large domain comprised of mainly β-strands and a small helical domain (Figure 1B and C). The BAH domain originally defined by sequence analysis contained β3–β10 and αE, but we now extend the definition to include β1–β3 because β1–β10 and αE form the core of the structure (shown in cyan in Figure 1B and C). In the BAH domain, β1–β3 forms a separate antiparallel sheet packed against β4, β8, the loop preceding β8, and one side of the long antiparallel β5 and β6 strands. The loop connecting β1 and β2 is disordered in the structure and is dispensable for Orc1p-dependent silencing (see below). β7–β10 form a consecutive β-sheet, with β7–β9 forming a distorted barrel comprising a mixture of parallel and antiparallel β-strands (Figure 1C). There is no channel running through the β-barrel as it is filled with hydrophobic residues inside. The β-barrel is capped on one end by β10 and two C-terminal helices, αF and αG. Almost all of the highly conserved residues identified have important structural roles for the folding of the BAH domain (Callebaut et al., 1999; Figure 1A).
The small, non-conserved helical domain, referred to as the H domain hereafter, is inserted between β6 and β7 (Figure 1B and C). Helices αB and αD are approximately perpendicular to each other. A one-turn helix, αC, is found in only one of the protein molecules in the asymmetric unit; the other molecule has weaker electron density in this region, but it is clear that an α-helix is not present. This difference can be attributed to stabilization of αC by crystal packing interactions. A number of interactions stabilize the positioning of the H domain with respect to the BAH domain. Major interactions include: (i) the ring of Phe105 is tightly packed between Tyr175 and Phe184; (ii) the side chain of Gln104 makes two hydrogen bonds with the main-chain carbonyl and amide of Phe184; (iii) the Nδ atom of Asn128 makes a hydrogen bond with the ring nitrogen of Trp158; and (iv) a hydrogen bond between the main-chain amide of Asn97 and the carbonyl of Gly181. The amino acids participating in these interactions are conserved between Orc1p and Sir3p (Bell et al., 1995), suggesting that the domain architecture of the N-terminal region of Orc1p and Sir3p should be the same.
The H domain is required for the silencing function of Orc1p
To probe the structure of Orc1pN235, a series of structure-guided mutations were introduced to identify amino acids critical for silencing and Sir1p binding. Since the protein sequences in the H domain are most divergent among all the BAH domain-containing sequences including human Orc1, yeast Orc1p and Sir3p, we made a yeast ORC1 mutant, designated orc1m1, by substituting a portion of the H domain of yeast Orc1p (aa 100–129) with the corresponding region of human Orc1 (aa 98–108; Figure 1A, red amino acids). Moreover, the amino acids between β1 and β2 (aa 21–35) that were disordered in the structure were replaced with four alanines to make the orc1m2 allele (Figure 1C). These substitutions should cause minimal perturbation to the core BAH domain structure.
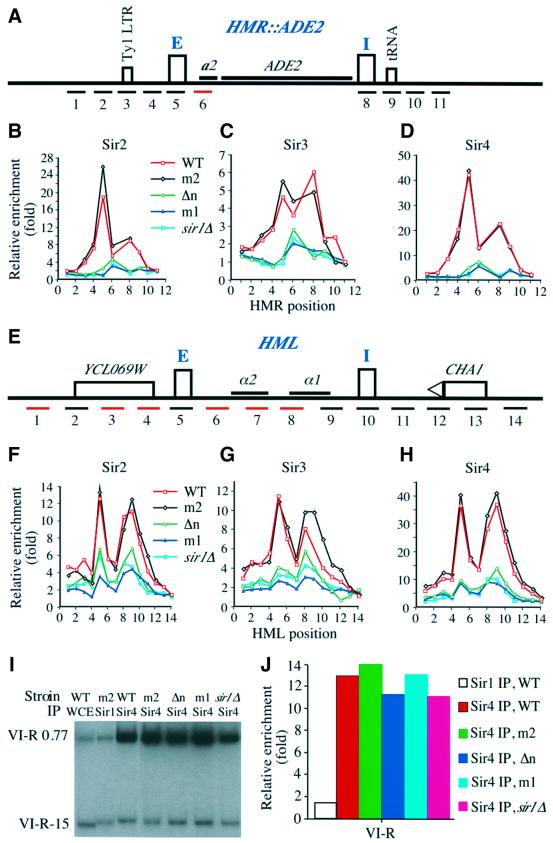
To assay their effect on silencing at the HMR locus, we used a colony color assay that utilized the ADE2 gene integrated into the HMR locus in a strain background containing a mutant version of the normal ADE2 gene (Figure 2A). In wild-type cells the ADE2 gene at HMR was repressed, resulting in dark-pink yeast colonies. In contrast, when the ADE2 gene at HMR was derepressed due to mutations, for example in the SIR1 gene, yeast colonies were white (Sussel et al., 1993; Figure 2B). Genes encoding wild-type ORC1, orc1m1, orc1m2 or a gene encoding Orc1p lacking the N-terminal 235 aa (orc1Δn) were individually integrated at the LEU2 locus as the only copy of ORC1 in strains harboring hmr::ADE2. The colony color of the orc1m2 mutant was dark pink, indistinguishable from that of the wild type, indicating that this mutant did not affect silencing. The colony color of the orc1m1 and orc1Δn strains was white and indistinguishable from sir1Δ mutant colonies, indicating that the ADE2 gene at HMR was derepressed in these orc1 mutants. Several site-specific orc1 mutants were made by substituting selected surface residues with an alanine and tested for their effect on HMR silencing (Supplementary table 2). Interestingly, some mutants displayed pink/white-sectoring colonies, suggesting that two transcriptional states (repressed and derepressed) of the ADE2 gene co-existed in these mutant cells.
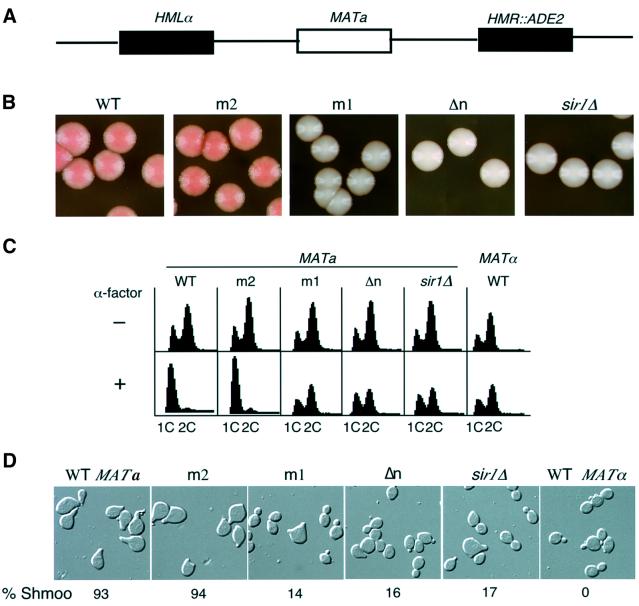
Fig. 2. Mutations at the N-terminus of Orc1p decreased HMR and HML repression. (A) A schematic representation of mating type loci of yeast strains used for the silencing assays. (B) A qualitative colony color assay for silencing of the ADE2 gene at the HMR locus. Wild-type ORC1 (WT) or orc1 mutants, orc1m1 (m1), orc1m2 (m2) and orc1Δn (Δn) or sir1 mutant (sir1Δ) cells were assayed for repression of the ADE2 gene. (C) A FACS analysis of yeast cells before and after α-factor confrontation assay. Fractions of yeast cells at early logarithmic phase were either collected for FACS analysis (upper panels, –) or incubated with 10 µg/ml α-factor for 3 h before harvesting for FACS analysis (lower panels, +). The genotype of each strain was the same as in (B). 1C and 2C refer to the DNA content of yeast cells. (D) Images of cells after α-factor confrontation. Cells were counted under the microscope and the percentage of shmoo-cells from one representative experiment is shown.
HML silencing in the orc1 mutants was monitored by following the response of cells to α-factor, a mating pheromone. In the presence of α-factor, wild-type haploid MATa cells arrested in G1 phase with a 1C DNA content and formed typical elongated shmoo morphologies (Figure 2C and D). In contrast, wild-type MATα cells or mutant MATa cells that co-expressed both the a and α genes due to derepression of the α genes at HML were resistant to α-factor because they continued to progress through the cell cycle and did not have the shmoo phenotype (Figure 2C and D). A majority of orc1m2 mutant cells arrested with a 1C DNA content after exposure to α-factor, indicating that in this mutant the α gene at HML remained silenced. Under the same conditions, orc1m1, orc1Δn and sir1Δ cells exhibited little difference in DNA content with or without α-factor (Figure 2C).
The derepression of HML genes in sir1Δ cells was known to be an epigenetic phenomenon because two populations of cells co-existed after α-factor treatment (Pillus and Rine, 1989): ∼20% of sir1Δ cells arrested with a shmoo morphology and 80% of the cells were resistant to α-factor and continued to bud. Therefore, the morphology of the ORC1 mutant cells was monitored in response to α-factor. Over 90% of wild-type or orc1m2 cells showed the shmoo morphology in response to α-factor (Figure 2D). In contrast, ∼80% of orc1m1 or orc1Δn cells were resistant to α-factor, and 14–20% of cells arrested as shmoos. Thus, like sir1Δ, two populations of cells co-existed in orc1 mutants. Moreover, the repressed or derepressed states in orc1m1 mutants were inherited for many generations, but the repressed state was less stable than the repressed state in wild-type cells (data not shown).
The H domain of yeast Orc1p confers the binding specificity with Sir1p
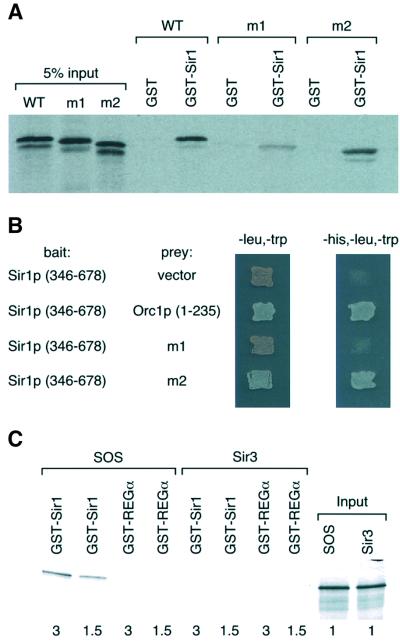
The N-terminus of Orc1p interacts with the C-terminus of Sir1p (Triolo and Sternglanz, 1996; Gardner et al., 1999). Since the orc1m1 mutant displayed silencing defects at the HM loci similar to those of sir1Δ, we tested whether the Orc1p-m1 protein bound to the Sir1p-C terminus (Sir1p-C) using an in vitro GST pull-down assay. Sir1p-C interacted with the in vitro translated Orc1pN235 (aa 1–235), as well as the N-terminus of the protein containing the orc1m2 mutation (Figure 3A). Under the same conditions, the interaction between Sir1p-C and the Orc1p-m1 protein was significantly reduced (Figure 3A). This interaction result was confirmed using a two-hybrid assay (Figure 3B). Thus, the silencing-defective orc1 mutant exhibited reduced binding to Sir1p.
Fig. 3. Mutations in the BAH domain of Orc1p reduce its affinity to Sir1p. (A) The orc1m1 mutant reduced its affinity for Sir1p in vitro. Equal amounts of GST, or GST–Sir1 (346–678) proteins were used to pull down in vitro translated [35S]methionine-labeled Orc1p N-terminus (WT), or two mutants with mutations at the Orc1p N-terminus (m1 and m2). The proteins were resolved on an SDS–polyacrylamide gel and visualized by autoradiography. (B) Interactions between Sir1p and Orc1 or mutants determined by the yeast two-hybrid interaction system. After transforming a yeast strain (AH106) with relevant plasmids, yeast cells were patched onto yeast synthetic complete media lacking leucine and tryptophan for selection of plasmids, or media lacking histidine, leucine and tryptophan to test interactions. (C) GST–Sir1p interacted with an SOS (Sir3-Orc1 H domain Swap) mutant. GST–Sir1 or GST–REGα was used to pull down in vitro translated [35S]methionine-labeled Sir3p or SOS mutant. The number below indicates the relative amounts of in vitro translated proteins used to perform the binding assay.
The N-terminal 215 residues of Orc1p and Sir3p have 49% sequence identity (Figure 1A). The N-terminus of Orc1p binds Sir1p, and yet no interaction between Sir3p and Sir1p has been detected (Triolo and Sternglanz, 1996). Since the H domain sequences are relatively less conserved between Sir3p and Orc1p (∼35% sequence identity) and the H domain of yeast Orc1p is required for its binding with Sir1p, we tested whether the H domain confers the Sir1p-binding specificity of Orc1p. The H domain sequence of Sir3p (aa 96–130) was replaced with that of yeast Orc1p in the full-length Sir3p to make a Sir3p–Orc1p–Swap (SOS) mutant protein that was tested for its interaction with Sir1p. As expected, GST–Sir1p did not bind Sir3p (Figure 3C), but it bound to the SOS protein efficiently under the same conditions. Thus, the H domain of yeast Orc1p confers its binding specificity for Sir1p.
Chromatin association of Sir1p in wild-type ORC1 or orc1 BAH-domain mutants
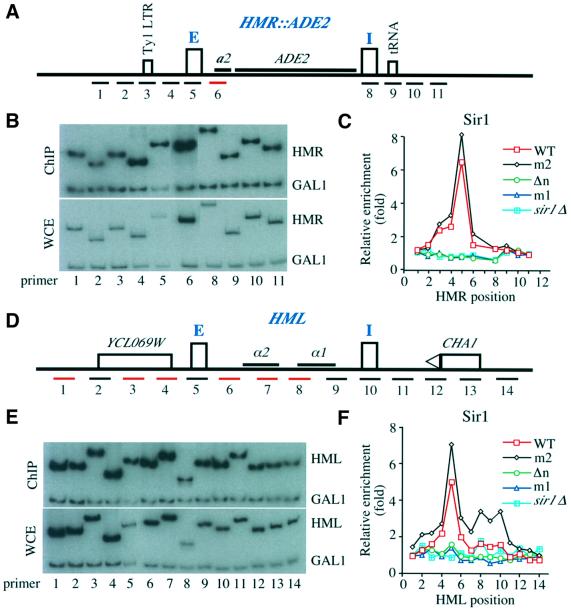
There are a number of possible explanations for the similarity of the phenotype between the sir1Δ and orc1 BAH-domain mutants, but one of the most likely is that these orc1 mutations disrupt recruitment of Sir1p to the silencers by Orc1p. We therefore tested whether orc1 mutations affected Sir1p localization to the HM loci using chromatin immunoprecipitation (ChIP) assays (Hecht et al., 1996). Sir1p was tagged with the HA epitope in yeast strains containing wild-type ORC1 or orc1 mutants, orc1m2, orc1m1 and orc1Δn, and chromatin fragments were precipitated with anti-Sir1p-HA antibodies. A sir1 strain was used as a control. To analyze DNA co-precipitated with Sir1p, a total of 10 primer sets across the HMR locus, each designed to amplify ∼400 bp of DNA (Figure 4A), and 14 non-overlapping primer sets across an ∼8 kb region surrounding HML (Figure 4D), were used in quantitative PCRs in the presence of [32P]dATP. DNA from whole-cell extracts (WCE) was compared with DNA co-precipitated with anti-Sir1p antibodies (Figure 4B and E). To account for variations in amplification with different primer sets, differences in DNA sampling or in loading, multiplex PCR was used to normalize the enrichment of each HMR or HML fragment to the background precipitation of a GAL1 gene fragment, which should be constant between samples (Figure 4B and E).
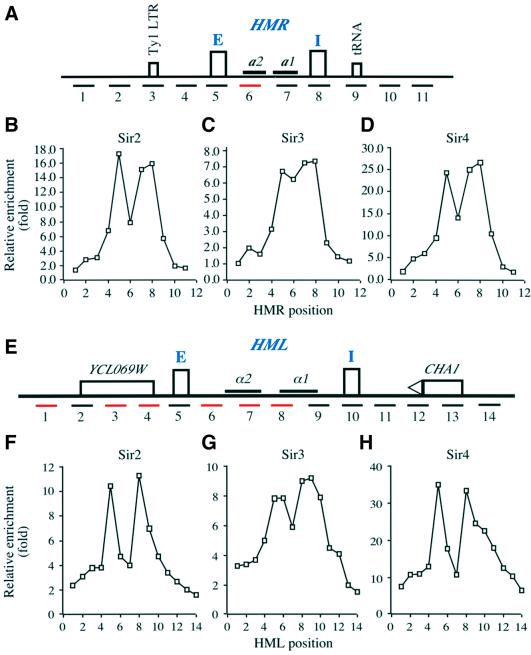
Fig. 4. Localization of Sir1p in WT ORC1 or orc1 mutant cells at the HM loci. (A) A schematic representation of the HMR locus with an integrated ADE2 gene. E and I silencers are represented by rectangles and the approximate position of the DNA fragment amplified by each PCR primer set is labeled with a number. A Ty1 LTR at position 3 and a tRNA gene located at site 9 are represented by rectangles. (B) An example of polyacrylamide gel analysis of PCR products. Multiplex PCR using primer sets shown in (A) and a primer set against a GAL1 fragment was performed in the presence of [32P]dATP using DNA from a Sir1p immunoprecipitation (ChIP, upper panel) or whole cell extract (WCE). (C) Localization of Sir1p at the HMR locus in wild-type ORC1 (WT, squares), orc1 mutants orc1m2 (m2, diamonds), orc1Δn (Δn, circles) and orc1m1 (m1, triangles), and sir1 (sir1Δ, filled squares) cells. The relative enrichment of each HMR fragment by Sir1p ChIP is plotted against the position of the PCR primer pair. (D) A schematic representation of the HML locus. Two silencers, E and I, are represented by rectangles and the two silenced genes α1 and α2 are also shown. The relative location of the CHA1 gene and an open reading frame YCL069W is shown. The approximate location of each DNA fragment amplified by each PCR primer is represented by a number and red lines represent non-unique primer sets. (E) An example of PCR fragments from multiplex PCR using DNA from Sir1p ChIP or WCE resolved on polyacrylamide gels. (F) Location of Sir1p at the HML locus in wild-type ORC1 or orc1 mutants. The relative enrichment (y-axis) of each HML DNA fragment from the Sir1p ChIP is plotted against primer location. All the strains except sir1 mutant strain contained Sir1-3HA.
As shown in Figure 4C and F, in wild-type cells Sir1p was predominantly localized at the E silencer of both the HMR and HML silent loci (red lines). Surprisingly, little Sir1p was detected at the I silencer of each locus, even though both I silencers contain an ORC-binding site. We also observed the predominant localization of Sir1p at the native HMR locus (Supplementary figure 2A), suggesting that the ADE2 gene at the HMR locus did not affect Sir1p localization. Since the tagged Sir1p protein was not fully functional, as revealed by the sensitive colony color assay as described in Figure 2A (see Supplementary note 2), we tested whether Sir1-3HA affected localization of Sir2p and Sir4p preferentially at the HMR I silencer. Within experimental error, we could not detect any significant changes in the localization profiles of Sir2p and Sir4p at the HMR locus by the HA epitope at the Sir1p C-terminus (Supplementary figure 3). Thus, we conclude that Sir1p was predominantly localized at the E silencers of both HMR and HML loci.
In the orc1m2 mutant, Sir1p localization at both HMR and HML was very similar to that observed in the wild-type ORC1 strain (Figure 4C and F, black line), consistent with our data that this mutant did not affect the silencing of these loci. The orc1m1 and orc1Δn mutants, however, reduced Sir1p binding to the E silencers at both HML and HMR to a background level similar to that in a sir1Δ strain (Figure 4C and F). Thus, the defects in HM silencing in the orc1 strains appear to be due to their inability to recruit Sir1p to the E silencers. This interpretation is consistent with previous results (Gardner and Fox, 2001), except that we found that Sir1p localized only at the E silencers.
Localization of Sir2p, Sir3p and Sir4p at the HM loci
Prompted by the unexpected result that Sir1p was predominantly localized at the E silencers of the HMR and HML loci, we analyzed the localization of Sir2p, Sir3p and Sir4p in MATα cells at the these two loci. In contrast to the restricted localization of Sir1p, Sir2p and Sir4p were detected at both the E and I silencers of the HMR locus (Figure 5B and D). Between the E and I silencers, lower amounts of Sir2p and Sir4p were detected at site 6 (see relative location in Figure 5A). Since primer pair 6 could anneal to three different locations in a MATα strain (see Supplementary table 4 and figure 4), it was possible that the apparent reduced binding of Sir2p and Sir4p detected with this primer pair was a normalization artifact.
Fig. 5. Localization of Sir2p, Sir3p and Sir4p proteins at the HMR and HML loci. (A) A schematic representation of the wild-type HMR locus. The relevant information is labeled as in Figure 4A. (B–D) Localization of Sir2p (B), Sir3p (C) and Sir4p (D) at the HMR silent chromatin domain. The relative enrichment of each DNA fragment at the HMR locus shown in (A) from each SIR ChIP is plotted against primer pair location. (E) A schematic representation of the HML locus as described in Figure 4D. (F–G) Localization of Sir2p (F), Sir3p (G) and Sir4p (H) at the HML locus. The relative enrichment of each HML fragment shown in (A) by each Sir ChIP is plotted against primer pair location.
Even though the amount of Sir2p and Sir4p decreased sharply to the left of the HMR-E silencer and to the right of the HMR-I silencer, significant amounts of Sir2p and Sir4p could still be detected at site 2 to the left of the HMR-E silencer and at site 9 to the right of the HMR-I silencer. Thus, Sir2p and Sir4p localized to a region of ∼4.3 kb (between sites 2 and 9) encompassing the whole HMR locus. Sir3p also bound to the same region.
The HMR silent domain, spanning an ∼4 kb region, is refractory to digestion by endonucleases (Loo and Rine, 1994). The ChIP assay clearly showed that Sir2p, Sir3p and Sir4p bound to the whole HMR region. Recently, a tRNA gene at site 9 (Figure 5A) was shown to be the primary determinant of a barrier element that prevented spreading of silent chromatin rightward (Donze et al., 1999; Donze and Kamakaka, 2001). As shown in Figure 5B–D, significant amounts of Sir2p, Sir3p and Sir4p proteins were localized at this site, but Sir proteins were undetected beyond this site. The left boundary element at HMR is less well defined. Since there was no sharp transition from Sir2p-, Sir3p- and Sir4p-bound sites to unbound sites to the left of the HMR-E silencer (Figure 5B–D), it was difficult to define the exact DNA sequence serving as the HMR left boundary element. We noticed that the DNA at site 3, which binds low levels of Sir2p, Sir3p and Sir4p, contained a Ty1 LTR. It has been shown that a Ty1 LTR between the HMR-I silencer and the tRNA serves as the HMR right boundary element (Donze et al., 1999).
The localization of Sir2p, Sir3p and Sir4p at the HML locus is shown in Figure 5F–H, respectively. Sir2p, Sir3p and Sir4p localization patterns were similar to each other, as in the case at the HMR locus. All three protein levels peaked at the E silencer, decreased sharply around the α2 gene and peaked again around the α gene. To the left of the E silencer, Sir2p, Sir3p and Sir4p levels decreased but significant amounts still bound to the far left site (site 1, Figure 5E), which is ∼2.3 kb away from the HML-E silencer and contains a hypothetical gene of unknown function. To the right of the HML-I silencer, the amounts of Sir2p, Sir3p and Sir4p decreased gradually, significant amounts were still detected over the CHA1 gene, which is ∼2 kb away from the HML-I silencer. These experiments demonstrated that Sir2p, Sir3p and Sir4p localized to a silent HML chromatin domain spanning at least 8 kb.
The broad localization of the Sir2p–4p proteins is unlikely to be due to inefficient shearing of the DNA in the ChIP procedure, as the same samples gave sharp localization of Sir1p. We therefore favor the conclusion that the lack of a sharp transition at the boundaries of HML was due to a lack of boundary elements near the E and I silencers. A 1.5 kb fragment to the left of the HML-E silencer or to the right of the HML-I silencer exhibited no barrier activity, whereas a boundary element was present in a 1.5 kb fragment to the right of the HMR-E silencer (Bi et al., 1999). Interestingly, the CHA1 promoter possesses robust barrier activity only in the presence of serine in the medium (Donze and Kamakaka, 2001). In S.cerevisiae, utilization of the amino acids serine and threonine as the sole nitrogen source was dependent on the CHA1 gene, whose expression was induced by serine/threonine. Sir4p is required for full repression of the CHA1 gene (Moreira and Holmberg, 1998). As shown in Figure 5H, Sir4p was clearly present at the CHA1 gene. Whether Sir2p and Sir3p proteins were also present was not clear since the relative enrichments of Sir2p and Sir3p at the sites containing the CHA1 gene were within experimental error.
Effect of orc1 BAH domain mutations on the localization of Sir2p, Sir3p and Sir4p at the HM loci
The localization of Sir2p, Sir3p and Sir4p in ORC1 wild-type and mutant strains was investigated in the hmr:: ADE2 background so that a direct comparison could be made with the localization of Sir1p (Figure 6, compare with Figure 4). The localization of Sir2p, Sir3p and Sir4p in wild-type and orc1m2 strains was virtually identical, consistent with the data shown in Figure 2 that orc1m2 did not affect HMR silencing. In contrast, in orc1m1, orc1Δn and sir1Δ mutant cells, no significant amounts of Sir2p, Sir3p and Sir4p were detected over the entire HMR silent chromatin domains (Figure 6B–D). Thus, sir1 and orc1 mutations that eliminated HMR silencing also eliminated binding of Sir2p, Sir3p and Sir4p proteins to the modified HMR locus.
Fig. 6. Effect of orc1 and sir1 mutations on the localization of Sir2p, Sir3p and Sir4p at the HM loci. (A) A schematic representation of the altered HMR locus as described in Figure 4A. (B–D) Localization of Sir2p (B), Sir3p (C) and Sir4p (D) at the HMR chromatin domain in ORC1 (WT, squares), orc1m2 (m2, diamonds), orc1Δn (Δn, circles), orc1m1 (m1, triangles) and sir1 (sir1Δ, filled squares) cells. (E) A schematic representation of the HML locus as described in Figure 4D. (F–H) localization of Sir2p (F), Sir3p (G) and Sir4p (H) at the HML chromatin domain in ORC1 (WT, squares), orc1m2 (m2, diamonds), orc1Δn (Δn, circles), orc1m1 (m1, triangles) and sir1 (sir1Δ, filled squares) cells. (I) Localization of Sir4p at the right end of chromosome VI (VI-R) in wild-type ORC1 or ORC1 mutants (m1, m2, Δn) or sir1Δ. The precipitated DNA or DNA from WCE was analyzed by multiplex PCR using one primer set located at 0.77 kb (VI-R 0.77) away from the right end of the telomere and another 15 kb (VI-R 15) away. (J) Relative enrichment of Sir1p and Sir4p localization at the telomere VI-R in ORC1 WT (WT) or orc1 mutant cells (m1, m2 and Δn) or sir1Δ cells.
Sir2p, Sir3p and Sir4p localization at the HML locus in wild-type ORC1, orc1 mutants and sir1Δ cells is shown in Figure 6F–H, respectively. In the ORC1 cells, the localization profiles of Sir2p, Sir3p and Sir4p were similar to those observed in wild-type MATα cells shown in Figure 5, even though MATa cells were used in the new experiments. Again, the orc mutants significantly reduced Sir protein binding to the locus. Unlike the situation at HMR, some Sir proteins were detected at HML in a pattern that precisely resembled the wild-type pattern, although at reduced levels. As shown in Figure 2, ∼20% of the orc mutant cells were repressed and ∼80% of the cells were derepressed at the HML locus. It is possible that the detected amount of normally distributed Sir proteins derived from the 20% of cells in which HML remained repressed. These results also reveal a fundamental difference between HMR and HMR silencing following deletion of SIR1 or the ORC1 BAH domain.
We also tested the effect of orc1 BAH-domain mutants on Sir4p binding to the right end of chromosome VI. Two PCR primer sets were used: one 0.77 kb and the other 15 kb away from the right telomere of chromosome VI. In agreement with published results (Strahl-Bolsinger et al., 1997), Sir4p bound preferentially to a chromosomal fragment 0.77 kb away from the telomere, but not to a fragment 15 kb away (Figure 6I and J). Moreover, the orc1 mutants m1 and Δn and sir1Δ did not affect Sir4p binding (Figure 6I and J). Thus, the orc1 mutations did not globally affect silencing, but reduced silencing only at the HM loci.
Discussion
The structural and mutation data presented here showed that the N-terminal domain of Orc1p has a novel structure. No significant homology with any known structures can be found. We showed that a small non-conserved H domain was necessary and sufficient for Sir1p binding to Orc1p, and it was required for the interaction between Sir1p and the cis-acting E silencers at both HM loci. We discuss below the implications of these results in understanding the molecular mechanism of transcriptional silencing at the silent mating-type loci and the function of the BAH domain.
Orc1p–Sir1p interaction in transcriptional silencing
The BAH domain is found in a number of chromatin-associated proteins. It is often present in conjunction with other well-defined domains that are involved in chromatin function, such as bromo-domains that bind acetyl-lysines of N-terminal histone tails, PHD fingers and methyl-DNA-binding domains (Callebaut et al., 1999). The structure suggests that the BAH domain has at least two functions. First, it can serve as a scaffold for harboring specific protein–protein interaction modules. Secondly, a number of Sir3 mutations that affect silencing mapped to its core BAH domain, suggesting a direct role for the BAH domain in interacting with key components of chromatin, such as histones. In the case of Orc1p, its BAH domain supports and positions the associated H domain that is required for interaction with Sir1p. It is unlikely that the H domain can form a defined structure independently as this region has great variations in amino acid length and sequence among different BAH domain-containing proteins. We have shown here that the H domain of yeast Orc1p, the region differing significantly among the BAH domain-containing proteins, confers binding specificity for Sir1p. These results provided an explanation for the striking difference between Orc1p and Sir3p in their ability to interact with Sir1p despite their overall high degree of sequence homology at their N-termini. It is likely that the H domains in other BAH domain-containing proteins also provide binding specificity for their cognate partners.
It was shown previously that Sir1p, Sir2p, Sir3p and Sir4p bound to the HMR and HML loci (Strahl-Bolsinger et al., 1997; Gardner and Fox, 2001). Unexpectedly, we found that Sir1p was restricted predominantly to the E silencers of both HMR and HML loci. In contrast, Sir2p–4p were located throughout the silenced regions, although with reduced binding in the middle of the HM loci (see Supplementary note 3). Little Sir1p was detected at HMR-I and HML-I silencers, even though they both contain ORC-binding sites. Since disruption of the Orc1p–Sir1p interaction prevented localization of Sir1p to the silent HM loci, Orc1p–Sir1p interaction is required for targeting Sir1p to the E silencer, but this interaction is not sufficient. It is possible that stable retention of Sir1p on chromatin, and hence stable repression in all cells, requires the presence of ORC and Rap1p-binding sites, both of which are known to bind to the E silencers (Shore and Nasmyth, 1987; Buchman et al., 1988; Bell et al., 1993). In support of this idea, mutations in Rap1p or removal of the ORC-binding site at HMR also reduced Sir1p binding to chromatin (Gardner and Fox, 2001).
Gardner and Fox (2001) reported that Sir1p bound to the whole HMR locus in a manner similar to Sir3p. This difference in Sir1p localization at the HMR locus could be due to the strain background used, since in their strain a DNA fragment ∼800 bp in length encompassing the HMR-E silencer was replaced by a synthetic silencer. Thus, it is possible that this deletion altered the resolution of Sir1p within the silent locus. The fact that we observed that Sir1p was localized predominantly at the E silencers of both HMR and HML loci in both wild-type and orc1m2 mutant provides confidence that Sir1p was predominantly localized at the E silencer. Nonetheless, it is possible that Sir1p binds to the other regions of HMR and HML loci at such low abundance that it is beyond the detection limit of the ChIP assay under our conditions.
Insights into the function of the highly homologous BAH domain of Sir3p
The N-terminal domain of Orc1p shares a high degree of sequence identity with that of Sir3p (Figure 1A) and the two domains are functionally interchangeable for mating-type silencing when tethered to the rest of the Orc1p or Sir3p proteins (Bell et al., 1995; Stone et al., 2000). Because of the high degree of similarity between the BAH domains of Orc1p and Sir3p, previous mutational studies of the Sir3p domain provided insights into the function of this conserved domain (Johnson et al., 1990; Stone et al., 2000).
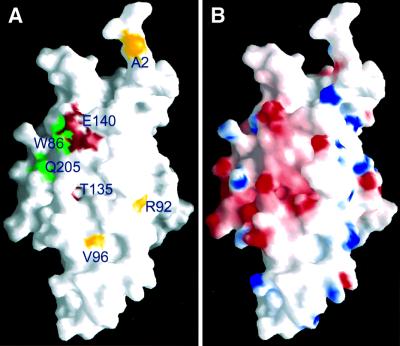
A number of Sir3p mutants previously known to affect silencing were mapped onto the structure of the Orc1pN235 protein (Figure 7A). Among these, three different mutants of Sir3p, Sir3p (W86R), Sir3p (D205N) and Sir3p (S31L), suppressed mating defects of mutations in the N-terminal tail of histone H4 (Johnson et al., 1990; Liu and Lustig, 1996; Stone et al., 2000). Sir3p (W86R) and Sir3p (D205N) restored repression of the silent mating type loci when Lys16 of histone H4 was changed to a glycine or glutamine. They also suppressed mutations of Arg17 and His18 of H4 to glycines. Sir3p (D205N) and Sir3p (S31L) also suppressed mutations at the C-terminal domain of Rap1p in telomeric silencing, as well as the H4 mutations in HML silencing. Sir3p (W86R) and Sir3p (D205N) are located in a generally negatively charged region (Figure 7; Q205 and W86 in Orc1p, respectively) of the BAH domain in Orc1p (and Sir3p) and these mutations both change the area to a less negatively charged environment. Sir3p (S31L) is located in the disordered loop connecting β1 and β2, and its location cannot be inferred. This region is not well conserved between Sir3p and Orc1p, and a deletion of 15 amino acids in this region of Orc1p (m2 mutant) showed no silencing defects at the HM loci.
Fig. 7. Grasp surface representation of the structure. (A) The location of Sir3p mutants mapped onto the Orc1pN235 structure (with the Orc1p amino acids labeled). As in Figure 1A, green indicates the position of mutants that suppress histone H4 mutations. Red indicates class II and yellow indicates class I sir3 mutants that enhance the sir1mutant defect (Stone et al., 2000). (B) Electrostatic potential distribution on the Orc1pN235 surface. Red indicates negative (–15 KBT), white indicates neutral (0 KBT) and blue indicates positive (+15 K KBT) charges, where KB is the Boltzmann constant and T is the temperature.
Other SIR3 mutants shown in Figure 7A enhance the sir1 mating-defective phenotype (Stone et al., 2000), and many of the mutated amino acids are conserved between Orc1p and Sir3p. One mutant, Sir3p (E140K), is located adjacent to Sir3p (W86R) and Sir3p (D205N) (Figure 7A) and it follows the same pattern of decreasing negative electric charges on the protein surface. It is interesting that Sir3p (W86R) and Sir3p (D205N) are located in a negatively charged environment, as the mutations they suppress are located in a positively charged stretch of amino acids (Lys16-Arg17-His18-Arg19) of histone H4. One possibility is that this positively charged region of the H4 tail interacts with the negatively charged area of Sir3p where W86 and D205 are located. For example, an acetylated Lys16 may interact with the hydrophobic tryptophan, and the rest of the positively charged residues in the H4 tail could interact with the negatively charged surface around Asp205 of Sir3p. The N-terminus of Sir3p can then present the bound Lys16-acetylated H4 tails to Sir2p, which has been shown to deacetylate Lys16 in an NAD-dependent manner (Shore, 2000; Dutnall and Pillus, 2001). The Sir3p (W86R) and Sir3p (D205N) mutants may lose the ability to bind Lys16-acetylated H4 tail, and Sir2p can access the free N-terminal tail of H4 to deacetylate Lys16. This model is inconsistent with a previous study showing that the N-terminal tail of histone H4 interacts with the C-terminal region of Sir3p (Grunstein, 1998). The C-terminal domain, however, may interact with histone H4 when Lys16 is deacetylated. Indeed, an acetylated N-terminal tail of histone H4 has reduced binding to the C-terminal domain of Sir3p (aa 510–970; Carmen et al., 2001). The possibility of two types of Sir3p–H4 interaction, one involving acetylated H4 with the BAH domain and the other involving hypoacetylated H4 with the C-terminal domain of Sir3p, may reflect two different functions for Sir3p.
Mechanisms of transcriptional silencing at the HM loci
The Sir2p, Sir3p and Sir4p proteins are essential for transcriptional silencing at both telomeres and the silent mating type HM loci, whereas Sir1p functions exclusively in HM silencing (Loo and Rine, 1995). When Sir1p was absent, a population of genetically identical cells exhibited two different gene expression states at HML, with ∼80% of the cells expressing HMLα genes and the other 20% with silenced HMLα genes (Pillus and Rine, 1989). This led to the proposal that Sir1p was involved in the establishment, but not the maintenance, of silencing. However, an alternative model in which Sir1p is required for the stable inheritance of a preformed Sir2p, Sir3p and Sir4p-mediated silencing complex over many generations cannot be excluded (Gardner and Fox, 2001).
Targeting Sir1p to the HMR-E silencer can promote silencing in the absence of an ORC-binding site (Chien et al., 1993). Here, we show that Sir1p binding is restricted at the E silencers, thus providing additional evidence that it is the location of Sir1p at the E silencer that is critical for its function. Since ∼20% of sir1 or orc1 N-terminal BAH-domain mutant cells still possessed a silent HML locus, this suggests either that factors other than Sir1p and Orc1p can also support normal silencing, albeit at a lower frequency. It is likely that there are multiple pathways that cooperate to produce stable silencing, including pathways involving PCNA and Rap1p, in combination with Sir1p and the N-terminus of Orc1p. In support of this idea, several Orc1 mutant alleles (Supplementary table 2), PCNA mutant alleles (Zhang et al., 2000) and RAP1 alleles (Sussel et al., 1993) showed a sectoring phenotype when assayed with the ADE2 gene integrated at the HMR locus, suggesting that these proteins are involved in some aspects of establishment or stable inheritance of silencing at the HMR locus. In addition, targeting of Sir3p and Sir4p to the HMR-E silencer in the absence of ORC and Rap1p-binding sites can still establish silencing, even in the absence of Sir1p (Marcand et al., 1996; Moretti and Shore, 2001). This idea could also explain the paradox that Sir1p cannot bind to the BAH domain of Sir3p, and yet the BAH domain of Sir3p can substitute for the BAH domain of Orc1p in silencing function (Bell et al., 1995). It is possible that the BAH domain of Sir3p, when tethered to Orc1p, promotes silencing in a Sir1p independent manner, possibly via an interaction with Rap1p.
We show here that in the absence of Sir1p, the distribution of Sir2p, Sir3p and Sir4p binding at HML was the same as that found in wild-type cells, but the levels of chromatin-bound Sir2p, Sir3p and Sir4p were ∼20% of those found in wild-type cells. The same results were obtained when Orc1p BAH domain mutations were examined. The normal distribution, but reduced levels of Sir2p, Sir3p and Sir4p, correlated with an unstable, epigenetic state of silenced gene expression found at HMLα in these mutants. Thus, in many respects, the HML locus in the absence of Sir1p or the Orc1p BAH domain resembles the epigenetic state of gene expression at telomeres where Raplp is a dominant cis-acting component.
In contrast to what was observed at HML, at the modified hmr::ADE2 locus, elimination of Sir1p or the Orc1p-N-terminus reduced Sir2p–4p binding to undetectable levels. This could be due to the presence of the foreign ADE2 gene (but see note 2 in Supplementary data). More likely, it may reflect fundamental differences between HML and HMR that have been observed previously (Ehrenhofer-Murray et al., 1997).
In the light of these observations, we suggest that silenced chromatin containing Sir2p, Sir3p and Sir4p is assembled and maintained by DNA replication-coupled chromatin assembly mediated by CAF-1, Asf1p and PCNA in combination with Rap1p, all of which are known to play important roles in establishment and inheritance of the silent states of gene expression at the HM loci and at telomeres (Shibahara and Stillman, 1999; Tyler et al., 1999; Verreault, 2000; Zhang et al., 2000; Sharp et al., 2001). In the absence of Sir1p or the Orc1p BAH domain, these silenced states are either inefficiently established or, more likely, are mitotically unstable over many generations. Once the Sir2p-, Sir3p- and Sir4p- containing chromatin is established, we suggest that the role of Orc1p in the ORC complex is to bind to the silencers at the HM loci and via its BAH and H domains interact with histone tails and Sir1p. According to this view, Sir1p would lock in the BAH domain–histone tail interaction and, thereby, indirectly lock in the existing Sir proteins. Future studies on the relationship among these proteins in silencing using specific alleles and biochemical studies should be able to test this model.
Materials and methods
Protein preparation, purification, crystallization and crystallographic analysis
The Orc1pN235 protein, containing the first 235 aa of S.cerevisiae Orc1p, was produced in Escherichia coli using a T7 expression vector, pET15b (Novagen). His-tagged Orc1pN235 was purified using Ni-NTA (Qiagen), hydroxyapatite and ion-exchange columns. The His tag was then removed by thrombin digestion. Purified protein was concentrated to ∼30 mg/ml for crystallization. Crystals were grown at 16°C by hanging-drop vapor diffusion in 0.1 M Tris pH 7.5, 28% polyethylene glycol monomethyl ether (PEGMME)-2000, 0.2 M magnesium acetate, 10 mM MnCl2 and 10% 2-methyl-2,4-pentanediol (MPD). A shorter, untagged version of the protein containing the first 219 aa of Orc1p, Orc1pN219, was produced using a pET11a vector. Expressed Orc1pN219 contains four extra amino acids, MHMT, at the N-terminus to mimic the N-terminus of Orc1pN235 that resulted from thrombin digestion of the His-tagged protein. Orc1pN219 was purified using hydroxyapatite and ion-exchange columns. The best diffracting Orc1pN219 crystals were grown at 4°C in 50 mM Tris pH 8.3, 0.2 M NaCl, 10 mM MgCl2, 10 mM MnCl2, 28% PEG400 and 10% MPD. A mercurial derivative of the Orc1pN219 crystal was prepared by soaking it in mother liquor supplemented with 2 mM HgCl2 for 30 days. Both the Orc1pN235 and Orc1pN219 crystals have P21212 symmetry and similar cell dimensions. The Orc1pN219 was solved to 2.6 Å resolution by MAD phasing using the HgCl2 derivative. The MAD structure was then used as the starting model for refinement of the 2.2 Å resolution structure of Orc1pN235. More detailed description of procedures and statistics of the crystallographic analysis can be found in Supplementary figure 1, table 1 and note 1.
Yeast strains and plasmid constructions
All the yeast strains used in this study were derivatives from a parental strain W303-1 (leu2-3,112 ura3-1 his3-11,15 trp1-1 ade2-1 can1-100), and they are listed in Supplementary table 3. Standard yeast media were used. To test HMR silencing, a yeast strain AIAy19 (MATa, W303-1, orc1::hisG), pSPB16 (ORC1 ARS CEN URA3) from Dr Stephen Bell was used to cross with ZGY009 (MATα, cac1::LEU2 hmr::ADE2) to generate ZGY120 (MATa, orc1::hisG, pSPB16 hmr::ADE2). To generate yeast strains containing orc1 mutations, each of the orc1 mutant plasmids in the parental plasmid pSPB50 was cut with BstEII and transformed into ZGY120. The transformants with the ORC1 or mutants integrated at the LEU2 locus were verified by colony PCR. To select for loss of pSPB16, yeast colonies containing ORC1 or mutants integrated at the LEU2 locus were selected on FOA-containing media.
See Supplementary data for detailed procedures for antibody production, two-hybrid interaction and GST pull-down assays.
Yeast silencing assays
The HMR silencing assay was performed as described previously (Zhang et al., 2000). To assay HML silencing, yeast cells were grown to an early logarithmic phase. A fraction of the cells was harvested and fixed for FACS analysis. The remaining cells were incubated at 30°C in the presence of 10 µg/ml α-factor for 3 h and then fixed for FACS analysis or differential interference contrast (DIC) image analysis. To prepare yeast cells for DIC microscopy, α-factor treated cells were washed once with H2O, fixed with cold methanol (–20°C) for 5 min, washed twice with PBS and resuspended in PBS.
Chromatin immunoprecipitation (ChIP) assays
The ChIP assay was performed essentially as described previously (Zou and Stillman, 2000). Briefly, after cross-linking and shearing DNA to an average size of <0.7 or 0.5 kb, yeast lysates were cleared by centrifugation. The resulting lysates were then divided into equal parts (lysates from a 50 ml yeast culture were used for one precipitation), mixed with antibodies to either Sir1p, Sir2p, Sir3p or Sir4p, and incubated for at least 6 h at 4°C. Then, 50 µl of protein G–Sepharose beads were added and incubated for 1 h at 4°C. After the beads were extensively washed, the precipitated DNA was heated to 65°C to reverse the cross-linking and purified according to the procedures described previously (Zou and Stillman, 2000). Detailed procedures to quantify the relative enrichment of each HM fragment over the GAL1 fragment are presented in Supplementary procedures.
Atomic coordinates
Atomic coordinates have been deposited in the Protein Data Bank under ID code 1M4Z.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Steve Bell, Rolf Sternglanz, Jasper Rine, Danesh Moazed, James Broach and Catherine Fox for plasmids and yeast strains used in this study, and Michael Grunstein for providing sequence information of some of the PCR primers. We thank Ying Zhang for technical help, Andrei Chabes and Liz Murchison for reading drafts of the paper, members of Stillman and Xu laboratories for helpful discussion, and staff at beamlines X12C and X26C of the National Synchrotron Light Source at Brookhaven National Laboratory for assistance in data collection. This work was supported in part by the W.M.Keck foundation (R.-M.X.) and grants from the National Institutes of Health (CA13106 and GM45436 to B.S. and GM 63716 to R.-M.X.). Z.Z. is supported by a postdoctoral fellowship from the Cancer Research Fund of the Damon Runyon Foundation (DRG1549).
References
- Bell S.P. and Stillman,B. (1992) ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature, 357, 128–134. [DOI] [PubMed] [Google Scholar]
- Bell S.P., Kobayashi,R. and Stillman,B. (1993) Yeast origin recognition complex functions in transcription silencing and DNA replication. Science, 262, 1844–1849. [DOI] [PubMed] [Google Scholar]
- Bell S.P., Mitchell,J., Leber,J., Kobayashi,R. and Stillman,B. (1995) The multidomain structure of Orc1p reveals similarity to regulators of DNA replication and transcriptional silencing. Cell, 83, 563–568. [DOI] [PubMed] [Google Scholar]
- Bi X., Braunstein,M., Shei,G.J. and Broach,J.R. (1999) The yeast HML I silencer defines a heterochromatin domain boundary by directional establishment of silencing. Proc. Natl Acad. Sci. USA, 96, 11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A.R., Lue,N.F. and Kornberg,R.D. (1988) Connections between transcriptional activators, silencers, and telomeres as revealed by functional analysis of a yeast DNA-binding protein. Mol. Cell. Biol., 8, 5086–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut I., Courvalin,J.C. and Mornon,J.P. (1999) The BAH (bromo-adjacent homology) domain: a link between DNA methylation, replication and transcriptional regulation. FEBS Lett., 446, 189–193. [DOI] [PubMed] [Google Scholar]
- Carmen A.A., Milne,L. and Grunstein,M. (2002) Acetylation of the yeast histone H4 N-terminus regulates its binding to heterochromatin protein SIR3. J. Biol. Chem., 277, 4778–4781. [DOI] [PubMed] [Google Scholar]
- Chien C.T., Buck,S., Sternglanz,R. and Shore,D. (1993) Targeting of SIR1 protein establishes transcriptional silencing at HM loci and telomeres in yeast. Cell, 75, 531–541. [DOI] [PubMed] [Google Scholar]
- Dillin A. and Rine,J. (1998) Roles for ORC in M phase and S phase. Science, 279, 1733–1737. [DOI] [PubMed] [Google Scholar]
- Donze D. and Kamakaka,R.T. (2001) RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in Saccharomyces cerevisiae. EMBO J., 20, 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze D., Adams,C.R., Rine,J. and Kamakaka,R.T. (1999) The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes Dev., 13, 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutnall R.N. and Pillus,L. (2001) Deciphering NAD-dependent deacetylases. Cell, 105, 161–164. [DOI] [PubMed] [Google Scholar]
- Ehrenhofer-Murray A.E., Rivier,D.H. and Rine,J. (1997) The role of Sas2, an acetyltransferase homologue of Saccharomyces cerevisiae, in silencing and ORC function. Genetics, 145, 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C.A., Ehrenhofer-Murray,A.E., Loo,S. and Rine,J. (1997) The origin recognition complex, SIR1, and the S phase requirement for silencing. Science, 276, 1547–1551. [DOI] [PubMed] [Google Scholar]
- Gardner K.A. and Fox,C.A. (2001) The Sir1 protein’s association with a silenced chromosome domain. Genes Dev., 15, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner K.A., Rine,J. and Fox,C.A. (1999) A region of the Sir1 protein dedicated to recognition of a silencer and required for interaction with the Orc1 protein in Saccharomyces cerevisiae. Genetics, 151, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S.M. and Cockell,M.M. (2001) The molecular biology of the SIR proteins. Gene, 279, 1–16. [DOI] [PubMed] [Google Scholar]
- Grunstein M. (1998) Yeast heterochromatin: regulation of its assembly and inheritance by histones. Cell, 93, 325–328. [DOI] [PubMed] [Google Scholar]
- Guarente L. (1999) Diverse and dynamic functions of the Sir silencing complex. Nat. Genet., 23, 281–285. [DOI] [PubMed] [Google Scholar]
- Hecht A., Strahl-Bolsinger,S. and Grunstein,M. (1996) Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature, 383, 92–96. [DOI] [PubMed] [Google Scholar]
- Johnson L.M., Kayne,P.S., Kahn,E.S. and Grunstein,M. (1990) Genetic evidence for an interaction between SIR3 and histone H4 in the repression of the silent mating loci in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 87, 6286–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. and Lustig,A.J. (1996) Genetic analysis of Rap1p/Sir3p interactions in telomeric and HML silencing in Saccharomyces cerevisiae. Genetics, 143, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo S. and Rine,J. (1994) Silencers and domains of generalized repression. Science, 264, 1768–1771. [DOI] [PubMed] [Google Scholar]
- Loo S. and Rine,J. (1995) Silencing and heritable domains of gene expression. Annu. Rev. Cell Dev. Biol., 11, 519–548. [DOI] [PubMed] [Google Scholar]
- Marcand S., Buck,S.W., Moretti,P., Gilson,E. and Shore,D. (1996) Silencing of genes at non-telomeric sites in yeast is controlled by sequestration of silencing factors at telomeres by Rap 1 protein. Genes Dev., 10, 1297–1309. [DOI] [PubMed] [Google Scholar]
- Moreira J.M. and Holmberg,S. (1998) Nucleosome structure of the yeast CHA1 promoter: analysis of activation-dependent chromatin remodeling of an RNA-polymerase-II-transcribed gene in TBP and RNA pol II mutants defective in vivo in response to acidic activators. EMBO J., 17, 6028–6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P. and Shore,D. (2001) Multiple interactions in Sir protein recruitment by Rap1p at silencers and telomeres in yeast. Mol. Cell. Biol., 21, 8082–8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak D.T., Pflumm,M., Chesnokov,I., Huang,D.W., Kellum,R., Marr,J., Romanowski,P. and Botchan,M.R. (1997) Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell, 91, 311–323. [DOI] [PubMed] [Google Scholar]
- Pillus L. and Rine,J. (1989) Epigenetic inheritance of transcriptional states in S. cerevisiae. Cell, 59, 637–647. [DOI] [PubMed] [Google Scholar]
- Sharp J.A., Fouts,E.T., Krawitz,D.C. and Kaufman,P.D. (2001) Yeast histone deposition protein Asf1p requires Hir proteins and PCNA for heterochromatic silencing. Curr. Biol., 11, 463–473. [DOI] [PubMed] [Google Scholar]
- Shibahara K. and Stillman,B. (1999) Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin. Cell, 96, 575–585. [DOI] [PubMed] [Google Scholar]
- Shore D. (2000) The Sir2 protein family: a novel deacetylase for gene silencing and more. Proc. Natl Acad. Sci. USA, 97, 14030–14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D. and Nasmyth,K. (1987) Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell, 51, 721–732. [DOI] [PubMed] [Google Scholar]
- Stone E.M., Reifsnyder,C., McVey,M., Gazo,B. and Pillus,L. (2000) Two classes of sir3 mutants enhance the sir1 mutant mating defect and abolish telomeric silencing in Saccharomyces cerevisiae. Genetics, 155, 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl-Bolsinger S., Hecht,A., Luo,K. and Grunstein,M. (1997) SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev., 11, 83–93. [DOI] [PubMed] [Google Scholar]
- Sussel L., Vannier,D. and Shore,D. (1993) Epigenetic switching of transcriptional states: cis- and trans-acting factors affecting establishment of silencing at the HMR locus in Saccharomyces cerevisiae. Mol. Cell. Biol., 13, 3919–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triolo T. and Sternglanz,R. (1996) Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature, 381, 251–253. [DOI] [PubMed] [Google Scholar]
- Tyler J.K., Adams,C.R., Chen,S.R., Kobayashi,R., Kamakaka,R.T. and Kadonaga,J.T. (1999) The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature, 402, 555–560. [DOI] [PubMed] [Google Scholar]
- Verreault A. (2000) De novo nucleosome assembly: new pieces in an old puzzle. Genes Dev., 14, 1430–1438. [PubMed] [Google Scholar]
- Zhang Z., Shibahara,K. and Stillman,B. (2000) PCNA connects DNA replication to epigenetic inheritance in yeast. Nature, 408, 221–225. [DOI] [PubMed] [Google Scholar]
- Zou L. and Stillman,B. (2000) Assembly of a complex containing Cdc45p, replication protein A, and Mcm2p at replication origins controlled by S-phase cyclin-dependent kinases and Cdc7p-Dbf4p kinase. Mol. Cell. Biol., 20, 3086–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]