Abstract
While an important role for the POU domain transcription factor Oct-6 in the developing peripheral nerve has been well established, studies into its exact role in nerve development and regeneration have been hampered by the high mortality rate of newborn Oct-6 mutant animals. In this study we have generated a Schwann cell-specific Oct-6 allele through deletion of the Schwann cell-specific enhancer element (SCE) in the Oct-6 locus. Analysis of mice homozygous for this allele (ΔSCE allele) reveals that rate-limiting levels of Oct-6 in Schwann cells are dependent on the SCE and that this element does not contribute to Oct-6 regulation in other cell types. We demonstrate a Schwann cell autonomous function for Oct-6 during nerve development as well as in regenerating nerve. Additionally, we show that Krox-20, an important regulatory target of Oct-6 in Schwann cells, is activated, with delayed kinetics, through an Oct-6-independent mechanism in these mice.
Keywords: enhancer/glia/myelin/Oct-6/POU domain
Introduction
Over the years, a considerable research effort has focused on how the myelination programme in Schwann cells is regulated. This cellular differentiation programme is characterized by dramatic metabolic and morphological changes, including polarization of the cell by deposition of a basal lamina, the production of massive amounts of cell membrane, incorporating myelin-specific lipids and proteins, and the spiralling of these lamellae around the axon (reviewed in Mezei, 1993; Garbay et al., 2000). The myelin organelle further matures into structurally and functionally distinct domains of compact and non-compact myelin such as the Schmidt–Lantermann incisures and paranodal loops (Arroyo and Scherer, 2000; Peles and Salzer, 2000; Pedraza et al., 2001). The synthesis and maintenance of myelin is an exquisitely sensitive process, as demonstrated by the many, inherited or acquired, demyelinating and dysmyelinating diseases such as Guillain-Barré Syndrome and the hereditary motor and sensory neuropathies. If we are to understand the interactions between glial cells and neurones that shape and maintain the functionally mature histo-architecture of the nerve or lead to pathogenesis, it is important to elucidate the molecular basis of the myelination programme.
Myelination involves the coordinate and sequential activation of sets of genes whose expression is controlled by transcription factors that are modulated during Schwann cell differentiation. While many transcription factors are known to be present in premyelinating and myelinating Schwann cells, two transcription factors have gained prominence in recent years for their important role in regulation of the myelination programme (Wegner, 2000; Topilko and Meijer, 2001). These transcription factors are the zinc finger protein Krox20 (Egr-2) and the POU-homeodomain protein Oct-6/SCIP/Tst-1 (referred to as Oct-6 in this paper) (Monuki et al., 1989; Meijer et al., 1990; Suzuki et al., 1990; Topilko et al., 1994). Both genes are dynamically expressed within the Schwann cell lineage, during development as well as during nerve regeneration, and their regulated expression depends on continued axonal contact (Scherer et al., 1994; Zorick et al., 1996). Genetic and cell biological studies have revealed that these transcription factors act in a genetic cascade (Topilko and Meijer, 2001). In promyelin Schwann cells, Oct-6 expression is strongly increased in response to an unknown axonal contact-related signal and subsequently activates a set of genes that includes Krox-20. Induction of high-level Krox-20 expression leads to the activation of an additional set of genes including the major myelin genes and those involved in lipid metabolism (Nagarajan et al., 2001). Oct-6 is strongly down-regulated after the peak of myelination. A third transcription factor, Sox-10, is expressed throughout the development of the Schwann cell lineage and possibly interacts with both Oct-6 and Krox-20 in regulating their target genes (Kuhlbrodt et al., 1998).
Further study into the role of Oct-6 in nerve development and regeneration is hampered by the fact that Oct-6 knock-out mice die shortly after birth because of breathing insufficiency, most likely caused by a defect in migration and differentiation of neurones in the brainstem (Bermingham et al., 1996). To circumvent this problem of early postnatal lethality, one would have to generate a viable Schwann cell-specific allele for Oct-6. Such a mouse would be of great value, allowing studies into the role of Oct-6 in nerve regeneration and allowing study of Oct-6 protein domains, target genes and potential Oct-6 redundant proteins.
Recently, we have identified putative regulatory elements within the Oct-6 locus using DNase I hypersensitivity mapping. Eight hypersensitive sites were mapped within a region of 35 kb. Using a deletion mapping approach in transgenic mice, we characterized a major cis-acting element within the Oct-6 locus on which intracellular signalling pathways converge to activate Oct-6 gene expression (Mandemakers et al., 2000). This element, the Oct-6 Schwann cell enhancer or SCE, is characterized by two DNase I hypersensitive sites. The SCE was shown to be sufficient to drive regulated expression within the Schwann cell lineage of transgenic mice. However, endogenous Oct-6 gene expression is not restricted to the Schwann cell lineage but is also expressed in the developing nervous system and skin. Expression is particularly high in the hippocampus, cortex, superior colliculus and brainstem nuclei, such as those of the hypoglossus and facial nerves (He et al., 1989; Alvarez-Bolado et al., 1995). No consistent transgene expression was observed in any of these brain regions in mice carrying a β-galactosidase reporter gene under the control of the SCE.
Based on these results, we hypothesized that deletion of the SCE from its normal chromosomal context would result in a Schwann cell-specific Oct-6 null allele. To test this hypothesis, we have generated mice homozygous for this deletion allele, the ΔSCE allele, and found that Oct-6 gene expression is affected in the Schwann cell lineage but not in any other cell type examined. These results demonstrate that the SCE is the decisive cis-regulating element governing Schwann cell-specific expression of the gene and that the SCE does not contribute to other aspects of the Oct-6 expression pattern. Consequently, these mice, which are viable, have allowed us to study, for the first time, the role of Oct-6 in regeneration. Our results demonstrate that activation of Oct-6 gene expression in reactive Schwann cells in regenerating nerves depends on the SCE and that the temporally correct activation of the myelination programme requires Oct-6. Also, our results demonstrate that the peripheral nerve phenotype observed in Oct-6 mutant animals results from a loss of function of Oct-6 in Schwann cells and not in neurones. Additionally, we provide evidence that Oct-6 protein levels are rate limiting in the differentiation of promyelin Schwann cells into myelinating cells, demonstrating the importance of precise quantitative expression during development and regenerative processes. Furthermore, we show that Krox-20 gene expression is activated in these mice with delayed kinetics, involving an Oct-6-independent mechanism.
Results
Deletion of the Oct-6 SCE through gene targeting
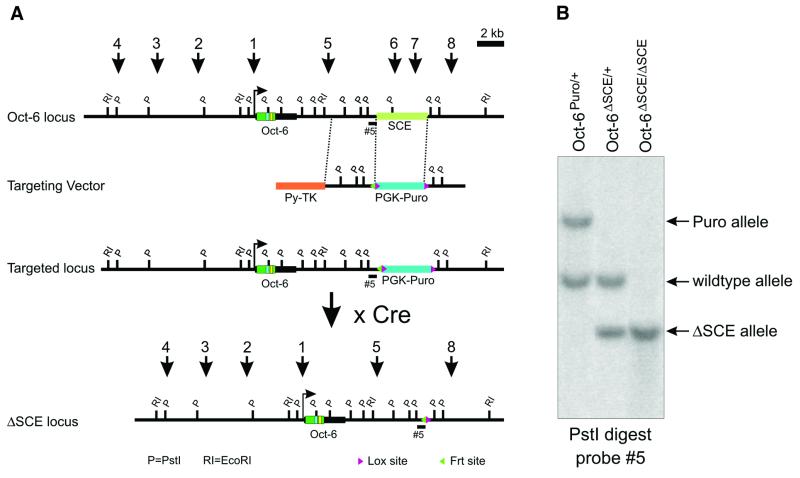
To delete the 4.3 kb SCE, a gene targeting vector was constructed in which the SCE was replaced with a puromycin selection cassette (Figure 1A). A negative selection cassette was introduced (Py-TK) flanking the 5′ homologous region, allowing counterselection of randomly integrated targeting constructs. The puromycin selection cassette was flanked by LoxP sites, which allowed deletion of the puromycin gene and its regulatory sequences from the targeted allele using Cre recombinase (Le and Sauer, 2001). Of the 141 embryonic stem (ES) cell clones that were puromycin resistant and ganciclovir insensitive, four were found to contain a homologous recombination event, as judged by Southern blot analysis (Figure 1B). Of those four, three had additional random integrations of the targeting cassette and were discarded. The one correctly targeted ES cell clone had a correct number of chromosomes and was used to generate chimeric mice. Chimeric animals were mated to Zp3-Cre transgenic female animals (D.Drabek). Zp3-Cre transgenic animals express high levels of the Cre recombinase in the oocyte, resulting in the removal of the puromycin cassette on the paternal chromosome in the zygote. Offspring in which the puromycin cassette was removed and the Zp3-Cre transgene was absent were identified using Southern blot analysis. These mice were used for further analysis.
Fig. 1. Deletion of the 4.3 kb SCE from the Oct-6 locus. (A) Gene targeting scheme for the Oct-6 SCE. The SCE is indicated with a light green bar and is located ∼12 kb downstream of the Oct-6 gene CAP site. The intronless Oct-6 transcription unit is indicated with a thick black line and the open reading frame is dark green with the POU-specific domain and POU homeodomain highlighted in blue and yellow, respectively. The relative positions of the mapped DNase I HSSs are indicated by numbered arrows. The SCE contains HSS6 and HSS7. In the targeting vector, a puromycin selection gene driven by the pgk-1 promoter replaces the 4.3 kb HpaI–MscI fragment containing the SCE. LoxP sites (red triangles) flank the selection cassette, while an FRT site (green triangle) is present directly 5′ of the 5′ LoxP site. The orange box represents the counterselection cassette containing the HSV TK gene plus promoter linked to a polyoma virus enhancer. The probe used to identify the correctly targeted allele at the 5′ end is indicated (5). The locus after the predicted homologous recombination event and removal of the puromycin cassette by Cre recombinase is shown. (B) Southern analysis of mice carrying the targeted allele before and after removing the selection cassette. Using probe 5, the targeted allele is identified by a 3.5 kb PstI fragment, while a 1.8 kb band identifies the wild-type allele. Removal of the selection cassette is demonstrated by the appearance of a 1.1 kb PstI fragment with probe 5.
Deletion of the SCE results in loss of Oct-6 expression in the Schwann cell lineage but not in other lineages
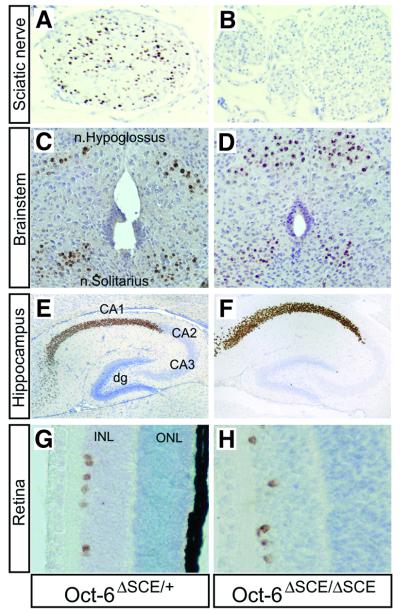
Adult mice heterozygous for the targeted allele, Oct-6ΔSCE/+, were intercrossed and offspring were genotyped. All three genotypes were represented in the offspring of these intercrosses at the expected Mendelian frequencies (out of 122 pups, 38 were Oct-6ΔSCE/ΔSCE, 54 were Oct-6ΔSCE/+ and 30 were wild type). To examine whether Oct-6 expression in Schwann cells of the developing nerve was affected by the homozygous deletion of the SCE, we collected sciatic nerves from Oct-6ΔSCE/+ and Oct-6ΔSCE/ΔSCE pups at day 8 after birth and processed them for immunohistochemistry. While large numbers of Oct-6-positive Schwann cell nuclei were observed in nerves of Oct-6ΔSCE/+ animals, no Oct-6-positive nuclei were observed in the nerve of Oct-6ΔSCE/ΔSCE animals (Figure 2A and B). Thus, homozygous deletion of the SCE results in a strong reduction of Oct-6 expression to levels beyond detection in our immunohistochemistry experiment.
Fig. 2. The ΔSCE allele affects Oct-6 expression in the Schwann cell lineage only. (A) A brown precipitate identifies Oct-6 protein-expressing Schwann cell nuclei in transverse sections of sciatic nerve of Oct-6ΔSCE/+ mice at P8. (B) None of the Schwann cells in the sciatic nerve of Oct-6ΔSCE/ΔSCE mice expresses high levels of Oct-6 at this stage. In contrast, Oct-6 expression is not affected in the brainstem of Oct-6ΔSCE/ΔSCE mice, in particular the nucleus hypoglossus or nucleus solitarius [compare (C) and (D); P8]. Also, neurones in the hippocampal CA1 field express high levels of Oct-6 and this expression is not affected by the deletion of the SCE [compare (E) and (F); P8]. Oct-6 is expressed in a subset of neurones in the inner (INL) but not the outer (ONL) nuclear layer of the developing retina (P8). Although we made no further attempt to identify these neurones, their position within the nuclear layer corresponds to amacrine neurones. Again, expression of Oct-6 in these neurones is not affected by deletion of the SCE [compare (G) and (H)]. All paraffin sections are counterstained with haematoxylin.
As mentioned above, Oct-6ΔSCE/ΔSCE and Oct-6ΔSCE/+ genotypes were found among the offspring of heterozygote crosses at the expected Mendelian ratios. In contrast, heterozygous crosses between mice carrying an insertional null allele for Oct-6 (the βgeo allele) produced only a few offspring alive at 10 days post-partum, and homozygous for the null allele (Bermingham et al., 1996; Jaegle et al., 1996). It was found that most Oct-6βgeo/βgeo animals die of respiratory distress shortly after birth (Bermingham et al., 1996). This high incidence of lethality in newborn Oct-6 null mice was attributed to a disorganization or reduction in cell number of cervical motor neurone groups of the phrenic nucleus and possibly medullar nuclei involved in breathing regulation, such as the nucleus tractus solitarius. Thus, the apparent lack of respiratory distress in neonatal Oct-6ΔSCE/ΔSCE mice suggests that the function of these nuclei is not affected by the deletion of the SCE. We therefore examined whether Oct-6 expression in a number of brain regions was affected by deletion of the SCE. We first examined Oct-6 expression in the medulla of the same animals as presented in Figure 2A and B. As can be seen in Figure 2C and D, Oct-6 is highly expressed in a subset of neurones in the nuclei of the hypoglossal nerve (XII) and the solitary tract. The identity of these Oct-6-positive neurones was confirmed by, in addition to anatomical criteria, immunostaining with antibodies directed against choline acetyltransferase, a general marker for cholinergic neurones (data not shown). Oct-6 expression in these neurones was not affected by the deletion of the SCE. In addition, we found that Oct-6 is normally expressed in neurones of the CA1 field of the hippocampus, putative amacrine neurones in the inner nuclear layer of the retina, superior colliculus and the skin (Figure 2E–H; data not shown). In fact we have not observed a tissue or cell type other than Schwann cells in which deletion of the SCE affects Oct-6 expression. These results demonstrate that deletion of the SCE from its normal genomic context leads to a severe reduction of Oct-6 gene expression in Schwann cells, while expression in other tissues is not affected, thus providing a plausible explanation for the viability of ΔSCE homozygous animals. Thus, the SCE is required for Schwann cell-specific expression of the Oct-6 gene, but does not contribute to regulation of the gene in other cell types.
Developmental delay in peripheral nerve development
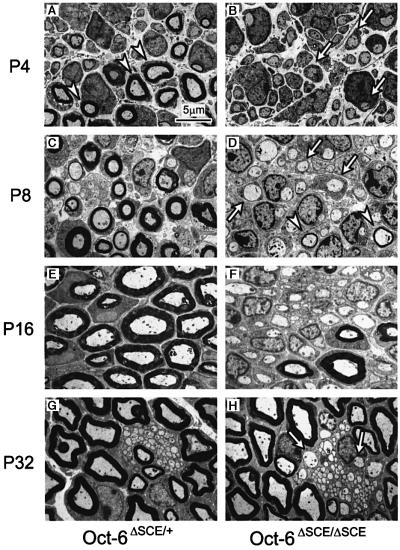
Mice homozygous for complete loss-of-function alleles show delayed peripheral nerve development with Schwann cells transiently arrested at the promyelin stage of differentiation. It has been assumed that this developmental delay results from loss of Oct-6 function in the Schwann cell lineage. However, as Oct-6 is widely expressed during embryonic development throughout the neurectoderm, it is possible that part of the phenotype results from loss of Oct-6 function in neurones or their precursors (Alvarez-Bolado et al., 1995; Zwart et al., 1996). Analysis of peripheral nerve development in ΔSCE homozygous animals should resolve this issue, as in these animals Oct-6 expression is selectively lost in the Schwann cell lineage only. Therefore, we examined electron microscopically the developmental maturation of the sciatic nerve in Oct-6ΔSCE/+ and Oct-6ΔSCE/ΔSCE animals at different postnatal stages (Figure 3). In the sciatic nerve of heterozygous animals at postnatal day 4 (P4), many Schwann cells are actively myelinating, with significant numbers of cells still at the promyelin stage (Figure 3A). Four days later, at P8, most, if not all, prospective myelinating cells have progressed beyond the promyelin stage and are actively engaged in elaborating myelin around their associated axon (Figure 3C). In contrast, in nerves of animals homozygous for the SCE deletion, a majority of Schwann cells are found in a promyelin configuration during the first week of postnatal life (Figure 3B and D). Only in the second week are increasing numbers of myelinating cells observed (Figure 3F). By 4.5 weeks of postnatal development, most myelinating Schwann cells have elaborated myelin, although few promyelin figures are still observed at this time, especially around groups of non-myelinated low-calibre fibres (arrows in Figure 3H). Thus, while most prospective myelinating Schwann cells in heterozygous nerves have initiated myelination by P8, the vast majority of such cells in homozygous animals only do so between P16 and P32. These results suggest that the delay in nerve development, as observed in Oct-6βgeo/βgeo and Oct-6ΔSCE/ΔSCE mice, results primarily from loss of Oct-6 function in Schwann cells and not in neurones.
Fig. 3. Schwann cell differentiation is delayed at the promyelin stage in developing nerves of Oct-6ΔSCE/ΔSCE mice relative to heterozygous mice. (A–H) Representative electron micrographs of transverse sections through the sciatic nerve of homozygous and heterozygous animals at different postnatal stages of development. While condensed myelin figures are abundantly present at P4 in heterozygous animals [arrowheads in (A)], such myelin figures are only appearing at P8 in homozygous animals [arrowheads in (D)]. Most Schwann cells at P4 and P8 in homozygous animals are morphologically (and molecularly; see Figure 2) at the promyelin stage [arrows in (B) and (D)]. Promyelin figures are still found at P32 in mutant animals [arrows in (H)], while in heterozygous animals all myelin-competent Schwann cells are at later stages of myelination.
The SCE deletion is a Schwann cell-specific Oct-6 hypomorphic mutation
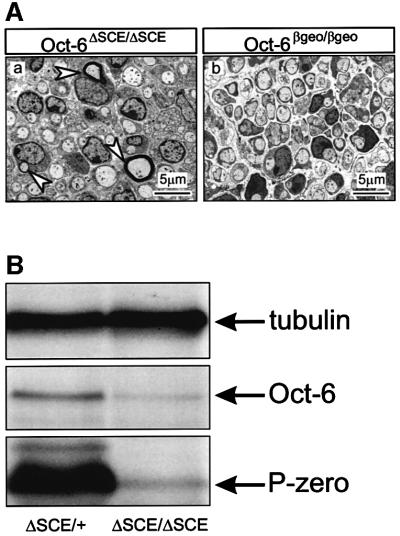
The developmental delay in peripheral nerves of mice homozygous for the ΔSCE allele appears slightly milder than that observed in mice homozygous for the βgeo allele (full knock out). This is particularly evident at P8 (Figure 4A). At this stage, no myelin figures are observed in the nerves of Oct-6βgeo/βgeo mice, while few myelin figures are present in the nerves of Oct-6ΔSCE/ΔSCE mice. This difference in severity of peripheral nerve phenotype could be due to non-Schwann cell autonomous or systemic effects of the Oct-6 βgeo allele that add to the Schwann cell autonomous effect. Alternatively, it is possible that the ΔSCE allele is a hypomorphic Oct-6 allele characterized by low-level residual expression of Oct-6 protein not detected in our immunohistochemistry experiments (Figure 2A). We therefore examined Oct-6 expression at P8 in nerves of animals heterozygous or homozygous for the ΔSCE allele using the more sensitive western blotting technique (Figure 4B; see also Figure 6). Low amounts of Oct-6 protein are observed in P8 nerve extracts of Oct-6ΔSCE/ΔSCE mice, while Oct-6βgeo/βgeo mice do not express Oct-6 (data not shown). It is, therefore, likely that the ΔSCE allele is a strong hypomorphic allele.
Fig. 4. The ΔSCE allele is a hypomorphic allele of Oct-6. (A) Comparison of sciatic nerve morphology in Oct-6ΔSCE/ΔSCE (a) and Oct-6βgeo/βgeo (b) mice at P8 reveals that a minority of Schwann cells in Oct-6ΔSCE/ΔSCE mice have progressed to form compact myelin (arrowheads). In contrast, all Schwann cells are still at the promyelin stage of differentiation in sciatic nerve of Oct-6βgeo/βgeo mice. (B) Mice homozygous for the ΔSCE allele express strongly reduced levels of Oct-6 in Schwann cells of the developing nerve at P8. Western blot experiments showing low levels of Oct-6 protein in nerve extracts from Oct-6ΔSCE/ΔSCE animals. The amounts of protein loaded per lane were similar, as demonstrated by the similar intensities of the α-tubulin immunoreactive band. In accordance with the delayed myelination status of sciatic nerve in Oct-6ΔSCE/ΔSCE animals, low levels of P-zero protein are detected at this stage. Nerve development in heterozygous mice is normal and myelinating Schwann cells express high levels of P-zero.
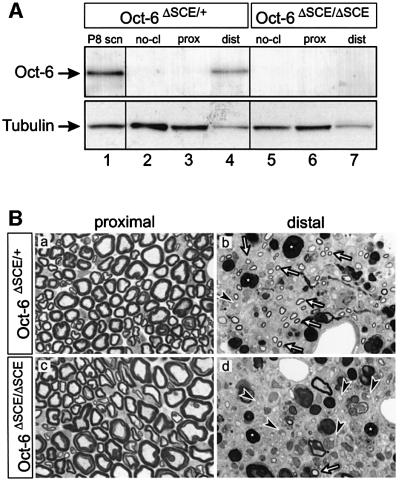
Fig. 6. Oct-6 regulation and function in the regenerating nerve. (A) Reactivation of Oct-6 expression is mediated through the SCE. Western blot analysis of sciatic nerves 12 days after crush lesion from adult Oct-6ΔSCE/+ (lanes 2–4) and Oct-6ΔSCE/ΔSCE mice (lanes 5–7). Nerves were divided in a proximal part (prox, lanes 3 and 6) and a distal part (dist, lanes 4 and 7) of equal length. Controls included are the undamaged nerves, contralateral to those operated on (no-cl, lanes 2 and 5), and developing sciatic nerve at P8 (P8 scn). Oct-6 is not expressed in Schwann cells of the adult nerve, while at P8, Schwann cells express high levels of Oct-6. Tubulin served as loading control. However, tubulin expression is reduced in the distal part of the regenerating nerve because of incomplete regeneration. (B) Schwann cell myelination is delayed in regenerating nerves of Oct-6ΔSCE/ΔSCE mice. Regenerating nerves, 12 days post-operation, were examined by light microscopy. Nerves were embedded in plastic and semi-thin sections were cut at 5 mm proximal (a and c) and 3 mm distal (b and d) to the lesion site. Sections were stained with ppd. In both genotypes, much myelin debris is still present (asterisk in b and d). Many regenerating fibres in the Oct-6ΔSCE/ΔSCE mouse are ensheathed by Schwann cells that have not elaborated compact myelin yet (arrowheads in b and d). In contrast, many compact myelin figures are found surrounding the regenerating fibres in Oct-6ΔSCE/+ mice (arrows in b and d).
Krox-20 activation is delayed in Schwann cells of Oct-6ΔSCE/ΔSCE mice
Schwann cell differentiation is arrested at the promyelin stage in Oct-6 and Krox-20 null mice. However, this differentiation arrest is transient in Oct-6 mutant mice, while the arrest is permanent in Krox-20 null mice, although these mice die before 3 weeks of age. Previously, we have shown that one important target of Oct-6 regulation in myelinating Schwann cells is the zinc-finger transcription factor Krox-20 (Ghislain et al., 2002). In particular, we have shown that Krox-20 is not expressed in Schwann cells during the first week of postnatal development in Oct-6 null mice. One could speculate that the failure to initiate myelination on schedule in Oct-6 mutant animals results from a failure to activate Krox-20 gene expression. The transient nature of the differentiation block in Oct-6 mutant animals then suggests that Krox-20 is activated at a later stage in an Oct-6-independent manner.
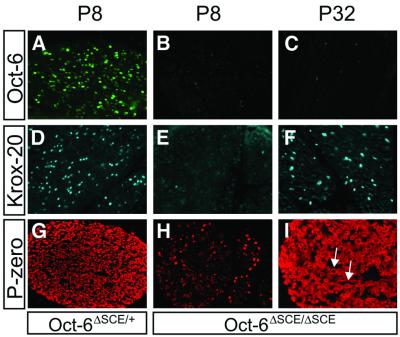
To address this question, we collected sciatic nerves of Oct-6ΔSCE/+ and Oct-6ΔSCE/ΔSCE mice at different postnatal stages, and examined the temporal expression of Oct-6 and Krox-20 and myelin protein P-zero by immunohistochemistry (Figure 5). P-zero is the major myelin protein in peripheral myelin and its accumulation in the compacting myelin sheath provides a convenient measure for the progression of myelin formation in the developing nerve (Greenfield et al., 1973). We first confirmed that Schwann cells in P8 Oct-6ΔSCE/+ nerves express high levels of Oct-6, while no Oct-6 expression was observed in Schwann cells of Oct-6ΔSCE/ΔSCE nerves (Figure 5A). Also, in agreement with our previous observations, Krox-20 expression is undetectable in P8 Oct-6ΔSCE/ΔSCE nerves, while Schwann cells in Oct-6ΔSCE/+ nerves do express Krox-20 at this stage (Ghislain et al., 2002). In addition, P-zero protein expression is severely reduced in Schwann cells of P8 nerves of Oct-6ΔSCE/ΔSCE animals. However, at P32, Krox-20 is expressed in Schwann cells in nerves of Oct-6ΔSCE/ΔSCE animals and extensive myelination is evident by the high level of P-zero immunoreactivity, showing characteristic ring structures in transverse sections (arrows in Figure 5I). Thus, in the absence of Oct-6 function, Krox-20 expression is eventually activated at the time extensive myelination is observed.
Fig. 5. Oct-6 expression is lost in Schwann cells of mice homozygous for the ΔSCE allele. (A) Homozygous deletion of the SCE results in loss of Oct-6 expression in Schwann cells of the developing nerve and delayed appearance of Krox-20 and the major myelin protein P-zero. Transverse sections of paraffin-embedded sciatic nerves at P8 or P32 from Oct-6ΔSCE/+ and Oct-6ΔSCE/ΔSCE mice were incubated with antibodies against Oct-6, Krox-20 or P-zero. P-zero immunoreactivity reveals the typical ring-like myelin structures indicated by arrows in (I).
Nerve regeneration
Oct-6 gene expression is strongly increased in reactive Schwann cells during nerve regeneration (Scherer et al., 1994; Zorick et al., 1996). Previous work has suggested that the SCE is sufficient to mediate this reactivation of Oct-6 gene expression during regeneration (Mandemakers et al., 2000). However, in these experiments, the SCE was coupled to the Oct-6 promoter and upstream region. It is, therefore, possible that activation of Oct-6 gene expression in reactive Schwann cells is mediated through elements outside the SCE, such as the promoter. To assess whether the SCE is also necessary for reactivation of Oct-6 gene expression and, if so, whether Oct-6 function is required in reactive Schwann cells in regenerating nerves, we first comparatively examined Oct-6 expression in regenerating nerves of Oct-6ΔSCE/+ and Oct-6ΔSCE/ΔSCE animals. Oct-6 is highly expressed in Schwann cells of the regenerating distal nerve stump 8 days after axotomy (Scherer et al., 1994; Zorick et al., 1996; Mandemakers et al., 2000; Figure 6A). In contrast, Oct-6 expression is not detectable at this stage in the regenerating nerve of Oct-6ΔSCE/ΔSCE mice (Figure 6A). Thus, the SCE is also required for reactivation of Oct-6 gene expression during regeneration.
We next examined how nerve regeneration at the morphological level was affected in the absence of Oct-6 reactivation. The sciatic nerves of Oct-6ΔSCE/+ or Oct-6ΔSCE/ΔSCE animals were crush lesioned at mid-femoral level. The extent of regeneration was assessed by serial sectioning and microscopic analysis of the regenerating nerves. At 12 days post-transection, many regenerating axon fibres were seen at 3 mm distal of the lesion. In addition, we observed many myelin ovoids that had not been cleared yet by macrophages or had not been autophagocytosed (arrowheads in Figure 6B, b and d). The degree of degeneration and axonal ingrowth of the distal nerve stumps was similar in Oct-6ΔSCE/+ and Oct-6ΔSCE/ΔSCE nerves. Many regenerating nerve fibres are being actively myelinated in the Oct-6ΔSCE/+ nerves, as demonstrated by the many thin compact myelin figures (arrow in Figure 6B, b). In contrast, myelination of regenerating fibres in Oct-6ΔSCE/ΔSCE nerves was much less advanced at this stage. Many fibres that had not yet progressed beyond the promyelin stage of ensheathment and those that were myelinated had thinner myelin sheaths. These observations indicate that, as in development, myelination is delayed in the absence of Oct-6 gene function.
Discussion
In the work described here, we have generated a viable and Schwann cell-specific Oct-6 knock-out mouse through deletion of the SCE. Analysis of this mouse allowed us to address questions related to the regulation and function of Oct-6 during peripheral nerve development and regeneration.
Deletion of the SCE results in a Schwann cell-specific hypomorphic Oct-6 allele
An Oct-6 allele in which the SCE is deleted was created through homologous recombination in ES cells and removal of the LoxP-flanked puromycin cassette by Cre recombinase. The selection cassette was removed as such cassettes have frequently been found to interfere with expression from the targeted locus (see for example McDevitt et al., 1997). Oct-6 expression was found to be severely reduced in the Schwann cell lineage of mice homozygous for the ΔSCE allele. These low residual levels of Oct-6 expression could be visualized only by western blotting (Figure 4) and electrophoretic mobility shift assays (not shown), and not by immunohistochemistry (Figures 2 and 3). We estimated that the residual level of Oct-6 expressed in the sciatic nerve at P8 is 5–10% of wild-type Oct-6 levels. These low levels of Oct-6 are not sufficient to sustain normal differentiation of Schwann cells, as Oct-6ΔSCE/ΔSCE mice exhibit a peripheral nerve phenotype that is only slightly less severe than that observed in Oct-6βgeo/βgeo mice.
Expression of Oct-6 in neurones in the hippocampus, brainstem and retina was not affected by the deletion of the SCE. These neurones express high levels of Oct-6 at the correct developmental time point. In fact, we have not found any cell lineage that normally expresses Oct-6 (apart from the Schwann cell lineage) in which Oct-6 expression was affected by deletion of the SCE. Thus, Oct-6 expression in these cell types is under the control of additional elements within the Oct-6 locus not requiring interaction with hypersensitive site (HSS) 6 and/or HSS7 within the SCE. Other regulatory elements may include some of the HSSs that we have mapped previously (see Figure 1; Mandemakers et al., 2000).
The fact that Oct-6 expression is selectively lost in Schwann cells while expression is not affected in neurones of homozygous ΔSCE mice helps to resolve the long-standing question of cell autonomy of the peripheral nerve phenotype in Oct-6 mutant animals. Our results now unequivocally demonstrate that this phenotype results from a loss of Oct-6 function in Schwann cells and not in neurones.
Furthermore, it has been suggested previously that the high incidence of neonatal death of mice carrying a null allele (βgeo allele) of the Oct-6 gene is caused primarily by migration or differentiation defects in neurones involved in breathing regulation, and not by defects in peripheral nerves as a consequence of delayed Schwann cell differentiation. The fact that the ΔSCE allele does not affect Oct-6 expression in these neurones and that Oct-6ΔSCE/ΔSCE animals are viable with no evidence of breathing problems, but with the same Schwann cell differentiation defect as in Oct-6βgeo/βgeo mice, strongly supports the notion that neonatal death in Oct-6βgeo/βgeo mice does indeed result from a neuronal defect, as originally suggested by Bermingham et al. (1996).
Function of the Oct-6 Schwann cell enhancer
Why is expression of Oct-6 from the ΔSCE allele not completely lost in Schwann cells? Traditionally, enhancers have been thought to function by increasing the rate of transcription initiation from a linked promoter. In recent years, it has been shown that in some cases enhancers not so much influence the rate of transcription initiation, but instead increase the chance that a linked promoter is activated. In this probabilistic model, enhancers are thought to function through a mechanism that involves modifications to the local chromatin configuration or relocation to an active centre within the nucleus (Fiering et al., 2000; Hume, 2000). This model predicts that an enhancer increases the percentage of cells in a population expressing the gene. In transgenic mice experiments, such a mechanism might explain the often observed variegated expression of the transgene (Elliott et al., 1995; Milot et al., 1996). The low level of Oct-6 expression we observed in the developing nerve of Oct-6ΔSCE/ΔSCE animals could thus result from either a small number of Schwann cells expressing the gene at normal levels, or a very low expression in most Schwann cells. We did not observe individual Schwann cells expressing normal levels of Oct-6 in P8 Oct-6ΔSCE/ΔSCE nerves (see Figure 2B: a field containing >100 nuclei). Therefore, it appears that the SCE functions as a classical enhancer in Schwann cells by modulating the rate or the frequency of transcription of the linked gene.
The reduced levels of Oct-6 expressed in Schwann cells of Oct-6ΔSCE/ΔSCE mice result in a slightly less severe peripheral nerve phenotype than that observed in Oct-6βgeo/βgeo mice, which do not express Oct-6 at all. In particular, we found that the number of Schwann cells that have entered the myelinating phase of differentiation at P8 is lower in Oct-6βgeo/βgeo mice than in Oct-6ΔSCE/ΔSCE mice. These observations suggest that the level of Oct-6 determines the rate at which a Schwann cell progresses through the promyelin stage of differentiation. This suggests that increased levels of Oct-6 might result in an increased rate of differentiation of Schwann cells, potentially resulting in early onset of myelination and hypermyelination. Weinstein et al. (1995) have previously shown that expression of a mutant form of the Oct-6 protein (ΔSCIP) under the control of the P-zero promoter in Schwann cells of transgenic animals results in early onset of myelination and hypermyelination. Although these results were initially interpreted differently, involving a dominant-negative action of the ΔSCIP protein, more recent interpretation suggests that the protein acts as a dominant positive (Wu et al., 2001). This reinterpretation strongly suggests that the levels of Oct-6 are rate limiting in Schwann cell differentiation. Such transcription factor dose-dependent differentiation has also been demonstrated for a number of other systems, including the haematopoietic system (McDevitt et al., 1997; Vivian et al., 1999). For example, it has been demonstrated that 80% reduction in Gata-1 expression levels results in a decreased rate or efficiency of red blood cell maturation.
How would the rate of Schwann cell differentiation depend on the level of Oct-6 protein? It is possible that high levels of Oct-6 are needed to saturate all potential binding sites in the cis-acting elements of target genes. Lower levels of Oct-6 would then result in lower transcription rates of these targets and a longer time for the differentiation programme to complete. One potential target of Oct-6 is the Krox-20 gene. The relevant Krox-20 myelination-associated enhancer (MSE; myelinating Schwann cell element) contains at least one high-affinity Oct-6 binding site, and several lower-affinity binding sites (Ghislain et al., 2002). Although the relevance of these binding sites for Krox-20 enhancer function has not been assessed genetically, it is possible that full Krox-20 enhancer activation depends on maximum occupancy of the Oct-6 binding sites. In addition, high levels of Oct-6 protein might be required for efficient interaction with other proteins, such as Sox-10 (Kuhlbrodt et al., 1998). As these types of interaction are often of low affinity, high protein concentrations are needed. Following activation, Krox-20 expression is maintained through a mechanism that does not involve Oct-6.
We found that Krox-20 expression is activated through an Oct-6-independent mechanism in Schwann cells of ΔSCE homozygous animals, albeit with a delay of 10–14 days (Figure 5). Although it is not known whether this delayed activation is mediated through the Krox-20 MSE, it is possible that an ‘Oct-6-like’ function, activated after the first week of postnatal development, is involved in Krox-20 activation. Recently, a potential candidate for this function has been postulated (Wu et al., 2001). Brn-5, a class VI POU domain gene, is expressed at higher levels in advanced stages of nerve development and expression is not dependent on Oct-6. Although the optimal DNA binding sequence for Brn-5 differs from that of Oct-6, both factors can bind to the octamer and octamer-related sequences present in the MSE (Rhee et al., 1998). If Brn-5 does indeed serve an Oct-6-redundant function in Schwann cell differentiation, it is expected that expression of Brn-5, from a transgenic construct controlled by the Oct-6 SCE, will result in a substantial alleviation of the delayed myelination phenotype in an Oct-6 mutant background. These experiments are currently under way.
Myelination is delayed in regenerating nerves in the absence of Oct-6
Using a nerve lesion paradigm that allows regeneration, we have shown that reactivation of Oct-6 gene expression in reactive Schwann cells requires the SCE and that Oct-6 is important, as it is in development, for the progression of Schwann cell differentiation. Both in developing and regenerating nerves, myelination is delayed in the absence of Oct-6. It is, therefore, most likely that the transcriptional programme regulated by Oct-6 is the same in Schwann cells during development as well as during regeneration. Furthermore, we did not observe differences between the two genotypes in the extent and numbers at which regenerating axons enter the distal nerve stump. Also, the extent of clearance of myelin debris did not differ between the two genotypes. Therefore, the ΔSCE allele has no obvious effect on Wallerian degeneration. Whether the delayed myelination in Oct-6 mutant nerves results in reduced functional recovery of the regenerated nerve is not known.
Results presented here and elsewhere could be helpful in the development of strategies to improve peripheral nerve regeneration in several ways (Gondre et al., 1998; Mandemakers et al., 2000). First, the SCE would be an excellent choice for inclusion in gene therapy vehicles to express neurotrophic factors such as BDNF and GDNF in Schwann cells during a tight window of nerve regeneration. These factors have proven beneficial for regeneration and functional recovery (Xu et al., 1995; Menei et al., 1998; Terenghi, 1999; Ramer et al., 2000). The inclusion of the SCE in such vectors will alleviate complications that arise from continued administration of these factors to the lesioned nerve. Secondly, regenerated axons generally have a lower calibre, thinner myelin sheath and shorter internodes (Beuche and Friede, 1985). Based on the observation that Oct-6 protein levels are limiting in Schwann cell differentiation, we hypothesize that increased Oct-6 levels will increase the rate and extent of myelination of Schwann cells, resulting in restoration of myelin thickness and axonal diameter to near normal. We are currently testing this hypothesis.
In conclusion, we have generated a novel Schwann cell-specific allele of Oct-6 through deletion of the major Schwann cell-specific regulatory element, the SCE. Analysis of these mice reveals a Schwann cell autonomous function for Oct-6 in nerve development and regeneration. We have further shown that Krox-20 is activated in Schwann cells of these mice through a mechanism that does not involve Oct-6. This new mouse mutant, together with the possibility to generate transgenic mice expressing genes selectively in the Schwannn cell lineage, provides a unique and excellent genetic system to address future questions related to the transcriptional targets of Oct-6, potential Oct-6 redundant functions in Schwann cell development, the study of functional domains of the Oct-6 protein and the role of Oct-6 in nerve regeneration.
Materials and methods
Targeting of the Oct-6 SCE
A genomic clone encompassing the 4.3 kb HpaI–MscI Schwann cell enhancer fragment was subcloned from cosmid clone pTBE 6Cos. From this clone, a 3.2 kb MscI–HpaI fragment, containing homologous genomic sequences 5′ of the SCE, was cloned behind the negative selection cassette py-TK. This selection cassette consists of the herpes simplex virus (HSV) thymidine kinase gene including its own promoter and a variant polyoma virus enhancer. A second clone was generated containing the puromycin resistance gene driven by the phosphoglycerate kinase-1 (PGK) promoter. This clone was flanked on both sites by LoxP sites that have the same orientation. An FRT site was introduced immediately 5′ of the 5′ LoxP site. This also introduces a unique SwaI site at the 5′ end of this clone. Downstream of the 3′ LoxP site, a unique SnaBI site was used to introduce a 2.8 kb MscI fragment containing the 3′ genomic homologous region. The entire fragment, encompassing the FRT–LoxP–PGK–puromycin–LoxP–3′ 2.8 kb MscI, was excised as a SwaI–NotI fragment and cloned in the HpaI–NotI-linearized Py-TK plasmid. This resulted in the targeting vector, as depicted in Figure 1A. The targeting vector was first linearized using NotI before electroporation into E14 ES cells. Electroporation and selection of cells in which homologous recombination had occurred were carried out as described. G418-resistant and ganciclovir-insensitive ES cell clones were screened for homologous recombination by Southern blotting of genomic DNA digested with PstI using a 32P-labelled DNA probe derived from the 5′ homologous region (see Figure 1A). Chimeric mice were generated by injection of ES cells from the correctly targeted clone into C57BL/6 blastocyst embryos. Chimeric males were crossed with FVB females and offspring were genotyped by Southern blotting of tail DNA digested with PstI using the probe described above. Offspring carrying the chromosome with the targeted SCE allele were identified (see Figure 1B) and subsequently crossed to mice carrying the Zp3-Cre transgene to obtain offspring in which the puromycin cassette was removed. These mice were then inter-crossed to obtain mice homozygous for the deleted SCE allele (see Figure 1B).
Immunohistochemistry and western blotting
For western analysis, nerves were isolated and directly lysed in loading buffer, followed by sonication and heating in a boiling water bath. Equal amounts of nerve extracts were resolved on a 12.5% SDS–PAGE gel and transferred to a PVDF membrane (Millipore) by electroblotting. Membranes were blocked with 3% bovine serum albumin (BSA), 0.05% Tween-20 in phosphate-buffered saline (PBS) for 1 h at room temperature. Primary antibodies were diluted in blocking buffer and incubated overnight at room temperature. Primary antibodies used were an Oct-6 rabbit polyclonal antiserum used at 1:300 dilution (Jaegle et al., 1996), a P-zero mouse monoclonal (clone P07; Archelos et al., 1993) used at 1:1000 dilution, a tubulin antibody (Sigma T-6793) and a rabbit Krox-20 antibody. Filters were subsequently washed five times in 0.5% Tween-20 in PBS and incubated with secondary antibodies conjugated with horseradish peroxidase or alkaline phosphatase (Dako) for 1 h in blocking buffer. Following five washes in 0.5% Tween-20 in PBS, the antigens were visualized by luminol (in the case of horseradish peroxidase) or NBT/BCIP (in the case of alkaline phosphatase) detection methods.
For immunohistochemistry, paraffin sections were dewaxed in xylene and rehydrated in a descending series of alcohol. Sections were blocked in 1% BSA, 0.05% Tween-20/PBS for 2 h at room temperature. Primary antibodies were diluted in blocking buffer and incubated overnight at room temperature. Secondary antibodies used were Oregon Green-conjugated goat anti-rabbit IgGs (Molecular Probes) and Texas Red-conjugated goat anti-mouse IgGs (Molecular Probes). Cell nuclei were visualized by DAPI staining.
Electron microscope analysis
Wild-type and mutant littermates (P4–P32) were anaesthetized with pentobarbital and perfused transcardially, first with PBS followed by 3% paraformaldehyde/1% glutaraldehyde in 100 mM cacodylate buffer pH 7.3. Sciatic nerves were dissected out and immersion fixed overnight in the same fixative or formalin at 4°C. Nerves were then rinsed in 100 mM cacodylate and postfixed in 1% osmium tetroxide/ferricyanide in 100 mM cacodylate overnight at 4°C. Following dehydration through an ascending alcohol series, nerves were embedded in Epon resin as described. One-micrometre sections were cut, mounted on a microscope slide and stained with paraphenylenediamine (ppd; Estable-Puig et al., 1965). Sections were examined under an Olympus BX40 light microscope and photographed using an Olympus DP50 digital camera. For electron microscopy, sections were cut at 50–60 nm and mounted on grids. Sections were contrasted with lead acetate and uranyl citrate, and examined using a Philips CM100 transmission electron microscope. Photographs were taken using a Megaview II digital camera.
Animal surgery
Young adult mice were anaesthetized by inhalation of halothane and placed on a heating pad. The sciatic nerve in the left leg was exposed and crushed for two times 15 s at the midfemoral level using No. 5 biology forceps. Animals were killed 12 days after the operation and the lesioned and contralateral nerves were isolated for western analysis. For light and electron microscope analysis, animals were perfused as described in the previous section.
Acknowledgments
Acknowledgements
The authors wish to thank Sjaak Philipsen, David Whyat, Elaine Dzierzak and Ben Barres for their critical comments on the manuscript. Juan José Archelos (University of Graz) is thanked for the monoclonal antibody against P-zero protein (clone P07). Hans van den Berg’s assistance in animal surgery procedures is greatly acknowledged. Dubi Drabek generated the Zp3-Cre transgenic mouse. This work was funded by grants from the Dutch research council (ALW 805-17.281 and MW 903-42-195) and the European Community (Biomed 2 PLp62069).
References
- Alvarez-Bolado G., Rosenfeld,M.G. and Swanson,L.W. (1995) Model of forebrain regionalization based on spatiotemporal patterns of POU-III homeobox gene expression, birthdates and morphological features. J. Comp. Neurol., 355, 237–295. [DOI] [PubMed] [Google Scholar]
- Archelos J.J., Roggenbuck,K., Schneider-Schaulies,J., Linington,C., Toyka,K.V. and Hartung,H.P. (1993) Production and characterization of monoclonal antibodies to the extracellular domain of P0. J. Neurosci. Res., 35, 46–53. [DOI] [PubMed] [Google Scholar]
- Arroyo E.J. and Scherer,S.S. (2000) On the molecular architecture of myelinated fibers. Histochem. Cell Biol., 113, 1–18. [DOI] [PubMed] [Google Scholar]
- Bermingham J.R., Scherer,S.S., O’Connell,S., Arroyo,E., Kalla,K.A., Powell,F.L. and Rosenfeld,M.G. (1996) Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev., 10, 1751–1762. [DOI] [PubMed] [Google Scholar]
- Beuche W. and Friede,R.L. (1985) A new approach toward analyzing peripheral nerve fiber populations. II. Foreshortening of regenerated internodes corresponds to reduced sheath thickness. J. Neuropathol. Exp. Neurol., 44, 73–84. [DOI] [PubMed] [Google Scholar]
- Elliott J.I., Festenstein,R., Tolaini,M. and Kioussis,D. (1995) Random activation of a transgene under the control of a hybrid hCD2 locus control region/Ig enhancer regulatory element. EMBO J., 14, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estable-Puig J.F., Bauer,W.C. and Blumberg,J.M. (1965) Technical note: paraphenylenediamine staining of osmium-fixed plastic embedded tissue for light and phase microscopy. J. Neuropathol. Exp. Neurol., 24, 531–536. [Google Scholar]
- Fiering S., Whitelaw,E. and Martin,D.I. (2000) To be or not to be active: the stochastic nature of enhancer action. BioEssays, 22, 381–387. [DOI] [PubMed] [Google Scholar]
- Garbay B., Heape,A.M., Sargueil,F. and Cassagne,C. (2000) Myelin synthesis in the peripheral nervous system. Prog. Neurobiol., 61, 267–304. [DOI] [PubMed] [Google Scholar]
- Ghislain J., Desmarquet-Trin-Dinh,C., Jaegle,M., Meijer,D., Charnay,P. and Frain,M. (2002) Characterisation of cis-acting sequences reveals a biphasic, axon-dependent regulation of Krox20 during Schwann cell development. Development, 129, 155–166. [DOI] [PubMed] [Google Scholar]
- Gondre M., Burrola,P. and Weinstein,D.E. (1998) Accelerated nerve regeneration mediated by Schwann cells expressing a mutant form of the POU protein SCIP. J. Cell Biol., 141, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S., Brostoff,S., Eylar,E.H. and Morell,P. (1973) Protein composition of myelin of the peripheral nervous system. J. Neurochem., 20, 1207–1216. [DOI] [PubMed] [Google Scholar]
- He X., Treacy,M.N., Simmons,D.M., Ingraham,H.A., Swanson,L.W. and Rosenfeld,M.G. (1989) Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature, 340, 35–41. [DOI] [PubMed] [Google Scholar]
- Hume D.A. (2000) Probability in transcriptional regulation and its implications for leukocyte differentiation and inducible gene expression. Blood, 96, 2323–2328. [PubMed] [Google Scholar]
- Jaegle M., Mandemakers,W., Broos,L., Zwart,R., Karis,A., Visser,P., Grosveld,F. and Meijer,D. (1996) The POU factor Oct-6 and Schwann cell differentiation. Science, 273, 507–510. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K., Herbarth,B., Sock,E., Enderich,J., Hermans-Borgmeyer,I. and Wegner,M. (1998) Cooperative function of POU proteins and SOX proteins in glial cells. J. Biol. Chem., 273, 16050–16057. [DOI] [PubMed] [Google Scholar]
- Le Y. and Sauer,B. (2001) Conditional gene knockout using Cre recombinase. Mol. Biotechnol., 17, 269–275. [DOI] [PubMed] [Google Scholar]
- Mandemakers W., Zwart,R., Jaegle,M., Walbeehm,E., Visser,P., Grosveld,F. and Meijer,D. (2000) A distal Schwann cell-specific enhancer mediates axonal regulation of the Oct-6 transcription factor during peripheral nerve development and regeneration. EMBO J., 19, 2992–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt M.A., Shivdasani,R.A., Fujiwara,Y., Yang,H. and Orkin,S.H. (1997) A ‘knockdown’ mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc. Natl Acad. Sci. USA, 94, 6781–6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer D., Graus,A., Kraay,R., Langeveld,A., Mulder,M.P. and Grosveld,G. (1990) The octamer binding factor Oct6: cDNA cloning and expression in early embryonic cells. Nucleic Acids Res., 18, 7357–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menei P., Montero-Menei,C., Whittemore,S.R., Bunge,R.P. and Bunge,M.B. (1998) Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur. J. Neurosci., 10, 607–621. [DOI] [PubMed] [Google Scholar]
- Mezei C. (1993) Myelination in the peripheral nerve during development. In Griffin,J.W., Low,P.A. and Poduslo,J.F. (eds), Peripheral Neuropathy. W.B.Saunders Co., Philadelphia, PA, pp. 267–281.
- Milot E. et al. (1996) Heterochromatin effects on the frequency and duration of LCR-mediated gene transcription. Cell, 87, 105–114. [DOI] [PubMed] [Google Scholar]
- Monuki E.S., Weinmaster,G., Kuhn,R. and Lemke,G. (1989) SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron, 3, 783–793. [DOI] [PubMed] [Google Scholar]
- Nagarajan R., Svaren,J., Le,N., Araki,T., Watson,M. and Milbrandt,J. (2001) EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron, 30, 355–368. [DOI] [PubMed] [Google Scholar]
- Pedraza L., Huang,J.K. and Colman,D.R. (2001) Organizing principles of the axoglial apparatus. Neuron, 30, 335–344. [DOI] [PubMed] [Google Scholar]
- Peles E. and Salzer,J.L. (2000) Molecular domains of myelinated axons. Curr. Opin. Neurobiol., 10, 558–565. [DOI] [PubMed] [Google Scholar]
- Ramer M.S., Priestley,J.V. and McMahon,S.B. (2000) Functional regeneration of sensory axons into the adult spinal cord. Nature, 403, 312–316. [DOI] [PubMed] [Google Scholar]
- Rhee J.M., Gruber,C.A., Brodie,T.B., Trieu,M. and Turner,E.E. (1998) Highly cooperative homodimerization is a conserved property of neural POU proteins. J. Biol. Chem., 273, 34196–34205. [DOI] [PubMed] [Google Scholar]
- Scherer S.S., Wang,D.Y., Kuhn,R., Lemke,G., Wrabetz,L. and Kamholz,J. (1994) Axons regulate Schwann cell expression of the POU transcription factor SCIP. J. Neurosci., 14, 1930–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Rohdewohld,H., Neuman,T., Gruss,P. and Scholer,H.R. (1990) Oct-6: a POU transcription factor expressed in embryonal stem cells and in the developing brain. EMBO J., 9, 3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenghi G. (1999) Peripheral nerve regeneration and neurotrophic factors. J. Anat., 194, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topilko P. and Meijer,D. (2001) Transcription factors that control Schwann cell development and myelination. In Jessen,K.R. and Richardson,W.D. (eds), Glial Cell Development: Basic Principles and Clinical Relevance. Oxford University Press, Oxford, UK, pp. 223–244.
- Topilko P., Schneider-Maunoury,S., Levi,G., Baron-Van Evercooren,A., Chennoufi,A.B., Seitanidou,T., Babinet,C. and Charnay,P. (1994) Krox-20 controls myelination in the peripheral nervous system. Nature, 371, 796–799. [DOI] [PubMed] [Google Scholar]
- Vivian J.L., Gan,L., Olson,E.N. and Klein,W.H. (1999) A hypomorphic myogenin allele reveals distinct myogenin expression levels required for viability, skeletal muscle development and sternum formation. Dev. Biol., 208, 44–55. [DOI] [PubMed] [Google Scholar]
- Wegner M. (2000) Transcriptional control in myelinating glia: flavors and spices. Glia, 31, 1–14. [DOI] [PubMed] [Google Scholar]
- Weinstein D.E., Burrola,P.G. and Lemke,G. (1995) Premature Schwann cell differentiation and hypermyelination in mice expressing a targeted antagonist of the POU transcription factor SCIP. Mol. Cell. Neurosci., 6, 212–229. [DOI] [PubMed] [Google Scholar]
- Wu R., Jurek,M., Sundarababu,S. and Weinstein,D.E. (2001) The POU gene Brn-5 is induced by neuregulin and is restricted to myelinating Schwann cells. Mol. Cell. Neurosci., 17, 683–695. [DOI] [PubMed] [Google Scholar]
- Xu X.M., Guenard,V., Kleitman,N., Aebischer,P. and Bunge,M.B. (1995) A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp. Neurol., 134, 261–272. [DOI] [PubMed] [Google Scholar]
- Zorick T.S., Syroid,D.E., Arroyo,E., Scherer,S.S. and Lemke,G. (1996) The transcription factors SCIP and Krox-20 mark distinct stages and cell fates in Schwann cell differentiation. Mol. Cell. Neurosci., 8, 129–145. [DOI] [PubMed] [Google Scholar]
- Zwart R., Broos,L., Grosveld,G. and Meijer,D. (1996) The restricted expression pattern of the POU factor Oct-6 during early development of the mouse nervous system. Mech. Dev., 54, 185–194. [DOI] [PubMed] [Google Scholar]